Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.107723
Revised: April 24, 2025
Accepted: June 7, 2025
Published online: July 24, 2025
Processing time: 116 Days and 0.4 Hours
Hepatocellular carcinoma (HCC) represents a major global health burden, ranking third as the leading cause of cancer-related mortality worldwide. This comprehensive review examines the substantial body of evidence linking modifiable lifestyle factors to HCC pathogenesis and clinical outcomes. We systematically evaluate dietary components, alcohol consumption patterns, tobacco use, physical activity levels, and emerging factors including metabolic disorders, psychological stress, and sleep disturbances. These factors collectively influence hepatocarcinogenesis through diverse biological mechanisms, including genotoxic damage, metabolic dysregulation, chronic inflammatory responses, and gut microbiome-mediated pathways. The accumulated data underscore the urgent need to inte
Core Tip: This review highlights key modifiable factors affecting hepatocellular carcinoma risk and outcomes. A diet that avoids aflatoxins while being rich in fiber, vegetables, coffee and tea provides protective benefits. Alcohol and tobacco cessation, along with regular exercise, significantly reduce risk and improve outcomes. Obesity, diabetes, poor mental health and sleep disorders worsen prognosis, particularly when interacting with viral hepatitis. These findings underscore the importance of personalized prevention strategies and the integration of lifestyle interventions into hepatocellular carcinoma management to address this global health challenge.
- Citation: Zhao H, Zhang XW, Li X. Lifestyle factors in hepatocellular carcinoma: From pathogenesis to prognosis. World J Clin Oncol 2025; 16(7): 107723
- URL: https://www.wjgnet.com/2218-4333/full/v16/i7/107723.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i7.107723
Liver cancer is one of the most common malignant tumors worldwide. According to the 2022 global cancer statistics, liver cancer causes around 865269 new cases and 757948 deaths annually, ranking as the sixth most diagnosed cancer and the third leading cause of cancer-related mortality[1]. Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, accounting for approximately 75%-85% of all cases[2]. The disease burden is particularly severe in East Asia and Africa. The prognosis of HCC remains extremely poor, with a 5-year survival rate of only around 20%, and the median survival for advanced-stage patients is only about two years[3]. This dismal outcome is attributed to the insidious onset of the disease, low early diagnosis rates (only 30% of cases are detected at a curable stage), and strong treatment resistance[4]. Therefore, further exploration of HCC pathogenesis, the development of novel biomarkers, and the optimization of treatment strategies remain critical research priorities.
Lifestyle factors play a significant role in both the risk of developing cancer and the outcome of cancer patients. Key modifiable behaviors, including tobacco use, alcohol consumption, dietary habits, physical activity levels, and sleep patterns, have been extensively studied in terms of their associations with cancer incidence, progression, and survival rates[5]. Multiple lifestyle factors, such as alcohol intake, dietary habits (e.g., aflatoxin contamination), sedentary behavior and obesity, are recognized as key modulators of HCC incidence and progression[6]. Emerging evidence further suggests that additional modifiable lifestyle elements may influence HCC pathogenesis and disease outcomes. For instance, higher dietary fiber and whole grain intake have been inversely associated with liver cancer risk[7,8]. Additionally, psychiatric disorders are linked to an elevated HCC incidence [standardized incidence ratio = 1.42, 95% confidence interval (CI): 1.28-1.57, P < 0.001][9]. Sleep patterns also appear relevant: Both short (< 5 hours) and prolonged (> 9 hours) nighttime sleep durations exhibit u-shaped associations with HCC risk, while daytime napping is associated with increased HCC incidence[10]. Given the established significance of these modifiable lifestyle factors, this review aims to provide a comprehensive and systematic evaluation of their critical roles in HCC risk modulation and treatment outcomes. By synthesizing current evidence, we seek to deliver actionable insights and evidence-based recommendations for HCC patients. These findings may serve as a practical reference for lifestyle modifications that could potentially contribute to HCC prevention, treatment optimization, and quality of life improvement in affected individuals.
Aflatoxin contamination: Aflatoxins, particularly aflatoxin B1 (AFB1), constitute a major global public health challenge due to their potent hepatocarcinogenic properties. These mycotoxins, produced by Aspergillus fungi, frequently contaminate dietary staples including rice, corn, peanuts, and spices. This contamination poses particularly severe risks in low-income regions where suboptimal food storage conditions prevail[11]. It is estimated that approximately 4.5 billion people worldwide are at risk of consuming AFB1-contaminated foods[12]. AFB1 exerts hepatocarcinogenic effects primarily through cytochrome P450-mediated metabolic activation, generating the reactive AFB1-8,9-epoxide that forms DNA adducts (e.g., AFB1-N7-guanine)[13]. These lesions drive characteristic TP53 mutations, notably G to T tran
Dietary carbohydrates: Dietary carbohydrates consumed by humans are classified into sugar (monosaccharides and disaccharides), and polysaccharides including digestible starches and indigestible dietary fibers. While sugars provide quick energy, complex carbohydrates from whole grains and fiber-rich foods offer sustained energy and health benefits. Sugars are rapidly digested and absorbed to provide immediate energy, whereas dietary fiber, although non-caloric, modulates sugar metabolism by slowing glucose absorption and improving glycemic control. Emerging evidence suggests that dietary carbohydrates play differential roles in HCC development. While refined sugars exhibit detrimental effects, dietary fiber and whole grains demonstrate protective potential. Large prospective cohort studies have revealed significant inverse associations between fiber intake and HCC risk. The National Institutes of Health- American Association of Retired Persons (NIH-AARP) prospective observational cohort study (n = 485717) in the United States found highest quintile fiber consumers had 31% lower HCC risk [hazard ratio (HR) = 0.69, 95%CI: 0.53-0.90][7]. Similarly, a meta-analysis of 2383 HCC cases demonstrated a 17% risk reduction per 10 g/day fiber intake (HR = 0.83, 95%CI: 0.76-0.91)[8]. Mechanistically, fiber may protect through improved glycemic control, reduced inflammation, and gut mi
Dietary fats: Dietary fats comprise different ratios of saturated and unsaturated fatty acids, including both monounsaturated and polyunsaturated varieties. Emerging evidence suggests differential effects of dietary fat subtypes on hepatocarcinogenesis. Large cohort studies reveal complex associations. While the Nurses’ Health Study and the Health Professionals Follow-up Study cohorts, which prospectively recruited 138483 healthy adults, found inverse associations for n-3 polyunsaturated fatty acids (PUFA) (HR = 0.63, 95%CI: 0.41-0.96) and n-6 PUFA (HR = 0.54, 95%CI: 0.34-0.86) with HCC risk[20], the Singapore Chinese Health Study (n = 63257) reported increased HCC risk with higher n-6 PUFA intake (Q4 vs Q1 HR = 1.49, 95%CI: 1.08-2.07), particularly in HBV/HCV-negative individuals (OR = 4.36, 95%CI: 1.59-11.94)[21]. The anti-HCC mechanisms of n-3 PUFAs may involve suppression of β-catenin and cyclooxygenase-2 pathways, anti-inflammatory effects via inhibition of interleukin-1 and tumor necrosis factor, and modulation of cell membrane lipid rafts, which regulate proliferation and apoptosis[20]. Saturated fats show more consistent harm, with the NIH-AARP study
Vitamins: Emerging evidence highlights the significant role of vitamins, particularly vitamin A and folate (vitamin B9), in HCC progression and survival. Vitamin A and its precursors demonstrate protective effects through multiple me
Fish: Growing evidence suggests that fish consumption, particularly n-3 PUFA-rich fish, is inversely associated with HCC risk in a dose-dependent manner. Several studies indicate that higher intake of n-3 PUFAs, eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid, is associated with a dose-dependent reduction in HCC incidence. A prospective study reported HRs of 0.64 (95%CI: 0.42-0.96) for n-3 PUFA-rich fish, 0.56 (95%CI: 0.36-0.85) for eico
Red meat and poultry: The relationship between red meat, poultry intake, and HCC risk remains inconsistent across studies. A large United States cohort study reported an almost two-fold HCC risk with higher processed red meat intake (3rdvs 1st tertile), while unprocessed red meat exhibited no association (HR = 1.06, 95%CI: 0.68-1.63)[32]. A case-control study of cirrhotic viral hepatitis patients found that N-acetyltransferase 2 rapid acetylators with high red meat intake dose-dependently increased HCC risk (OR = 3.89 for high intake; P-trend = 0.016), highlighting gene-diet interactions in hepatocarcinogenesis[33]. Conversely, a Chinese prospective cohort (n = 510048) observed no overall association between red meat, poultry, or fish and HCC, although rural residents showed a potential protective effect from poultry (P-interaction = 0.046)[34]. Recent substitution analyses found no significant link between replacing red meat with legumes and HCC risk (HR = 1.02, 95%CI: 0.96-1.08)[35]. Processed red meat may elevate HCC risk, particularly in genetically susceptible individuals, and unprocessed red meat and poultry show neutral or protective associations, respectively.
Vegetables and fruits: Accumulating evidence suggests that higher vegetable intake is associated with a reduced risk of HCC, while fruit consumption shows inconsistent associations. A meta-analysis of nine prospective cohort studies (1703 HCC cases) found a 39% lower HCC risk with high vegetable intake (95%CI: 0.50-0.75), with a 4% risk reduction per 100 g/day increase (P-trend < 0.001)[36]. This protective effect was particularly strong in males (50% risk reduction) but not significant in females. Similarly, the EPIC study (n = 486799) observed a 17% lower HCC risk per 100 g/day vegetable increase (HR = 0.83, 95%CI: 0.71-0.98), whereas fruit intake showed no association (HR = 1.01, 95%CI: 0.92-1.11)[37]. Specific vegetable subgroups, particularly cruciferous vegetables (e.g., broccoli, cauliflower, cabbage) and lettuce, appear to drive this protective effect. The NIH-AARP study (n = 485403) reported a 28% lower HCC risk with high total vegetable intake (HR = 0.72, 95%CI: 0.59-0.89), with cruciferous vegetables and lettuce showing the strongest inverse associations (P-trend < 0.005)[38]. Additionally, dietary antioxidants (e.g., flavanols) and high dietary fiber intake (particularly from cereals and vegetables) have been linked to reduced HCC risk. The EPIC cohort found that flavanol intake was inversely associated with HCC (HR = 0.62, 95%CI: 0.39-0.99)[39], while a United States prospective cohort study of health professionals (n = 125455) noted that whole grain and cereal fiber intake reduced HCC risk by 37% (HR = 0.63, 95%CI: 0.41-0.96)[40]. Therefore, current evidence strongly supports an inverse association between vegetable con
Coffee: Current research shows a consistent inverse association between coffee consumption and HCC risk. A Finnish randomized controlled study (27037 male smokers), originally designed to assess the effect of vitamin E on lung cancer risk, found that each daily cup of coffee reduced HCC risk by 18% (relative risk = 0.82, 95%CI: 0.73-0.93)[41]. No significant difference was observed between boiled vs filtered coffee, suggesting bioactive compounds remain effective regardless of preparation. Consistently, a meta-analysis of 32 studies involving 2492625 participants revealed that higher coffee intake was associated with a 47% reduction in HCC risk (relative risk = 0.53; 95%CI: 0.47-0.59), with similar protective effects observed across different study designs[42]. Another meta-analysis reported a 28% risk reduction per daily cup of coffee consumed (relative risk = 0.72; 95%CI: 0.66-0.79)[43]. The protective mechanisms of coffee against HCC appear multifaceted. Caffeine, a key bioactive component, functions as a non-selective adenosine receptor inhibitor, blocking adenosine-mediated immunosuppression and initiating antitumor immune responses[44]. Experimental studies demonstrate caffeine’s ability to inhibit HCC cell proliferation by activating the mitogen activated protein kinase/ERK/epidermal growth factor receptor signaling pathway[45]. The chemopreventive effects of coffee may also be attributed to its polyphenol content, which exhibits antioxidant and anti-inflammatory properties. These compounds may counteract dietary inflammatory patterns that increase HCC risk[46].
Tea: Tea consumption, particularly green tea, represents a promising dietary strategy for HCC prevention[47]. A comprehensive umbrella meta-analysis of observational studies established that high green tea consumption correlates with a 13% reduction in liver cancer risk, although the authors highlighted the necessity for more rigorous prospective studies to account for potential biases[48,49]. Subsequent meta-analyses encompassing over 2.4 million participants demonstrated a 20% decrease in HCC risk associated with green tea intake (relative risk = 0.80, 95%CI: 0.67-0.95), with consistent protective effects observed across both cohort and case-control study designs[42]. The Shanghai Women’s Health Study, a prospective cohort of 71841 middle-aged Chinese women, provided compelling longitudinal evidence, revealing that cumulative consumption exceeding 30 kg of dried tea leaves corresponded to a 44% lower HCC risk (adjusted HR = 0.56, 95%CI: 0.32-0.97). This protective association was even more pronounced among exclusive green tea drinkers (adjusted HR = 0.54, 95%CI: 0.30-0.98)[50]. While most evidence originates from Asian populations, the European EPIC study (n = 486799) corroborated these findings, demonstrating 72% and 59% risk reductions for the highest vs lowest quintiles of coffee and tea consumption, respectively[51]. The chemopreventive mechanisms of tea appear to operate through multiple bioactive compounds[47]. Theabrownin, a tea polyphenol, mediates tumor-inhibitory effects by activating the ataxia telangiectasia mutated-checkpoint kinase 2-p53 signaling axis and regulating c-Jun N-terminal kinase pathways, thereby inducing cellular senescence and apoptosis in HCC cells[52]. Similarly, epigallocatechin-3-gallate exhibits potent anti-HCC activity through three distinct mechanisms, suppression of osteopontin-mediated metastasis, enhancement of 5-fluorouracil chemosensitivity and induction of apoptosis via nuclear factor kappa B pathway inactivation[53-55].
The relationship between alcohol consumption and HCC has been well-established through multiple epidemiological and mechanistic studies. Alcohol-related HCC (A-HCC) accounts for approximately 19% of global liver cancer deaths, making it the third leading cause of HCC after HBV and HCV infections, and the primary cause in Europe[56]. Patients with alcohol-associated cirrhosis face a 1%, 3%, and 9% cumulative HCC incidence at 1, 5, and 10 years, respectively[57]. A-HCC is typically diagnosed at advanced stages, with only 24.5% receiving curative treatments vs 33.9% in non-A-HCC cases, leading to higher mortality (HR = 1.3; 95%CI: 1.1-1.5)[58]. The risk increases linearly with alcohol intake, with heavy drinkers (≥ 3 drinks/day) showing a 16% higher risk compared to non-drinkers[59], while former and always heavy drinkers face 3.2-fold and 5.5-fold increased risks, respectively[60]. Abstinence improves survival (5-year mortality: 52% vs 78% in active drinkers) but does not significantly enhance treatment access[61]. In addition, alcohol exacerbates HCC risk in metabolic disorders, with diabetics showing a 3.3-fold increased risk with heavy drinking vs non-drinking normoglycemic individuals[62]. Ethanol metabolism generates carcinogenic acetaldehyde and reactive oxygen species through antidiuretic hormone/cytochrome P450 2E1-mediated oxidation, causing DNA damage via N2-ethylidenedeoxyguanosine adduct formation and chromosomal instability. Concurrently, aldehyde dehydrogenase-mediated conversion of acetaldehyde to acetate fuels tumor bioenergetics and epigenetic regulation via histone acetylation (H3K9/27/56) of lipogenic genes (FASN, ACACA), promoting HCC survival under hypoxia[63]. Alcohol also induces immune dysfunction by suppressing natural killer-cell activity and promoting a tumor-permissive microenvironment rich in M2 macrophages and toll-like receptor 4-activated pathways[64]. Alcohol promotes HCC through direct genotoxicity, metabolic repro
Emerging evidence demonstrates significant associations between dietary patterns and HCC risk, with distinct protective effects observed across cultural contexts. A case-control study from Italy and Greece (518 HCC cases) revealed that high adherence to a Mediterranean diet reduced HCC risk by 50%, with particularly pronounced benefits in chronic hepatitis B/C patients[65]. The NIH-AARP study (n = 494942) found that higher Healthy Eating Index-2010 scores were associated with a 28% lower HCC risk (HR = 0.72, 95%CI: 0.53-0.97) and 43% reduced chronic liver disease mortality[66]. Similarly, the Alternative Healthy Eating Index-2010 demonstrated a 39% lower HCC risk for highest vs lowest adherence (HR = 0.61, 95%CI: 0.39-0.95) in United States cohorts[67]. The multiethnic cohort study confirmed these associations across racial groups, with alternate Mediterranean diet scores showing a 32% lower HCC risk overall (HR = 0.68, 95%CI: 0.51-0.90)[68]. In contrast, Chinese studies yielded culturally specific insights: Higher Chinese Healthy Eating Index scores were associated with 26% lower HCC-specific mortality (HR = 0.74, 95%CI: 0.56-0.98)[69] and 57% reduced primary liver cancer risk in case-control analyses (OR = 0.43, 95%CI: 0.38-0.50 per 5-point increase)[70]. The empirical dietary inflammatory pattern was associated with a two-fold HCC risk (HR = 2.03, 95%CI: 1.31-3.16)[71]. These findings consistently demonstrate that adherence to culturally rooted healthy dietary patterns can reduce HCC risk across diverse populations. Public health strategies promoting regionally tailored dietary guidelines may substantially impact HCC prevention, especially in high-risk groups.
A meta-analysis of 81 studies revealed current smokers had 55% higher HCC incidence (OR = 1.55, 95%CI: 1.46-1.65) and 29% greater mortality (OR = 1.29, 95%CI: 1.23-1.34) compared to non-smokers[72]. Subsequent research corroborated a 2.46-fold higher HCC risk in current smokers (HR = 2.46, 95%CI: 1.77-3.43)[60]. Effects were most pronounced in viral hepatitis patients[73,74]. Smoking promotes HCC through direct DNA damage/p53 inactivation[75], HBV-related immunosuppression (elevated viral load, impaired natural killer cells)[76], and inflammation/fibrosis (stellate cell activation, iron overload)[75]. It synergizes with viral hepatitis but not alcohol[60]. While some studies show mixed survival outcomes[77], the overwhelming body of evidence shows that tobacco use substantially elevates HCC risk and adversely impacts disease progression.
Current scientific evidence demonstrates a robust inverse association between physical activity and HCC risk. A meta-analysis of 14 prospective studies involving 6440 liver cancer cases revealed that high levels of physical activity were associated with a 25% reduction in HCC risk (HR = 0.75, 95%CI: 0.63-0.89)[78]. Another meta-analysis of 7 studies with 777662 participants confirmed this protective effect, showing 35% lower odds of HCC among more active individuals
Clinical studies highlight the therapeutic potential of physical activity in HCC management. Among patients receiving lenvatinib plus anti-programmed death-1 therapy, those maintaining regular activity had significantly longer overall survival (HR = 0.22), progression-free survival (HR = 0.16), and higher objective response rates (OR = 4.57) compared to sedentary patients[82]. Exercise interventions also improve various health parameters in HCC patients, including metabolic syndrome, muscle wasting, and quality of life[83]. Recent research indicates that supervised exercise programs are feasible and safe for HCC patients, with multiple studies demonstrating improvements in muscle mass and physical function[84]. These findings suggest that physical activity may serve as both a preventive measure and adjunct therapy for HCC.
The protective effects of physical activity against HCC operate through multiple interconnected biological mechanisms. At the molecular level, exercise activates critical tumor suppressor pathways, including p53-mediated upregulation of p27 to inhibit hepatocyte proliferation, while simultaneously enhancing adenosine monophosphate-activated protein kinase activity and suppressing mammalian target of rapamycin complex 1 signaling to block pro-growth pathways[85]. Regular physical activity also modulates circadian gene expression to degrade oncoproteins E2F transcription factor 1 and cellular myelocytomatosis oncogene[86], creating an unfavorable environment for tumor development. Beyond direct molecular effects, physical activity improves metabolic health by enhancing insulin sensitivity and mitigating obesity-related metabolic dysfunction, both established risk factors for HCC[87], with animal models demonstrating these benefits can occur independently of weight changes[88]. The immune system represents another key mediator, as exercise reduces transforming growth factor-β1 release and activates dopamine receptor signaling pathways, thereby stren
Obesity represents an independent risk factor, with meta-analyses showing an 89% higher HCC risk in obese individuals compared to normal-weight subjects[90]. Notably, men with a body mass index ≥ 35 kg/m2 face a 4.5-fold increased risk[91], with population-attributable fractions suggesting approximately 15.6% of HCC cases may be linked to overweight/obesity[92]. A nationwide prospective cohort study of 119316 health professionals found that higher dietary inflammatory (empirical dietary inflammatory pattern: HR = 2.03) and hyperinsulinemic patterns (empirical dietary index for hyperinsulinemia: HR = 1.61; empirical dietary index for insulin resistance: HR = 1.62) significantly increased HCC risk, with diabetes and adiposity partially mediating these associations[71]. The growing global prevalence of metabolic syndrome components suggests an increasing burden of metabolic-associated HCC, necessitating targeted interventions addressing insulin resistance, obesity, and associated inflammatory pathways for effective prevention strategies. The pathophysiological mechanisms connecting metabolic disorders to HCC are multifactorial. NAFLD, present in 70%-80% of obese individuals[93], serves as a critical intermediary, with studies confirming that NAFLD can progress to HCC even without cirrhosis[94]. Adipose tissue dysfunction contributes through altered adipokine secretion (increased leptin, decreased adiponectin) and chronic low-grade inflammation[19]. Leptin promotes angiogenesis and activates oncogenic pathways (c-Jun N-terminal kinase, protein kinase B, ERK) in hepatocytes[95], while insulin resistance drives carcinogenesis through hyperinsulinemia and inflammatory cascades[96,97]. Diabetes mellitus independently doubles HCC risk after adjusting for viral hepatitis and alcohol use[98]. These metabolic disturbances often synergize with other risk factors, obesity potentiates alcohol-related hepatocarcinogenesis[99].
A nationwide United States study of 11609 HCC patients found that 18.6% developed psychiatric diagnoses post-cancer detection, with depression (58.3%) and anxiety (53.0%) being most prevalent[100]. The relationship between psychological status and HCC appears bidirectional. Psychiatric patients demonstrate significantly elevated HCC risk, particularly those with substance-induced disorders[9]. Mendelian randomization studies further support causality, showing psychological distress increases HCC odds (OR = 1.006, P = 0.033) while social/leisure activities are protective (OR = 0.994, P = 0.035)[101]. Conversely, HCC diagnosis often triggers mental health challenges, with structural equation modeling revealing fear of progression mediates between psychological resilience/social support and psychosocial adjustment[102].
Mental health comorbidities significantly impact HCC outcomes. Multivariable analyses have demonstrated that psychiatric diagnoses worsen survival (HR = 1.10, 95%CI: 1.04-1.16), with dose-dependent effects (≥ 2 diagnoses: HR = 1.20, 95%CI: 1.08-1.32)[103]. Tumor-related psychiatric symptoms, particularly depression (49% contribution), substantially reduce health-related quality of life (β = -5.07, 95%CI: -10.01 to -0.13) independently of health behaviors[104]. In addition, nearly 20% of patients discontinue psychiatric medications post-HCC diagnosis[100]. Promisingly, interventions such as reminiscence therapy significantly reduce anxiety and improve global health status in elderly HCC patients[105]. These findings highlight the critical need for enhanced mental health screening in HCC patients, integrated psycho-oncological care models, and targeted interventions addressing fear of progression and treatment-related distress.
Sleep disorders, particularly short or disrupted sleep, are increasingly recognized as risk factors for chronic diseases, including HCC. Current evidence suggests a bidirectional relationship between sleep disorders and HCC, although direct causal mechanisms remain under investigation. A prospective study of 205 HCC patients post-hepatectomy identified sleep disorders as one of four key tumor-related psychiatric symptoms independently associated with decreased health-related quality of life[104]. Studies indicate that HCC patients frequently experience sleep disturbances, with 89.3% classified as poor sleepers (Pittsburgh sleep quality index > 5), compared to only 30.3% in healthy individuals[106]. Sleep disturbances in HCC are linked to worse clinical outcomes. Patients with poor sleep report higher symptom distress, depression, and reduced quality of life, particularly after treatments like transarterial chemoembolization[107]. The pathophysiological links may involve multiple mechanisms. Sleep deprivation further exacerbates HCC progression by impairing immune surveillance. Chronic sleep deprivation in murine models promotes tumor growth by reducing antitumor CD3+ T and natural killer cells while increasing immunosuppressive CD11b+ cells in the tumor microenvironment[108]. Psychosocial factors also play a role, as fear of cancer progression, which commonly disrupts sleep, was identified as a mediator between psychological resilience and psychosocial adjustment in HCC patients[102].
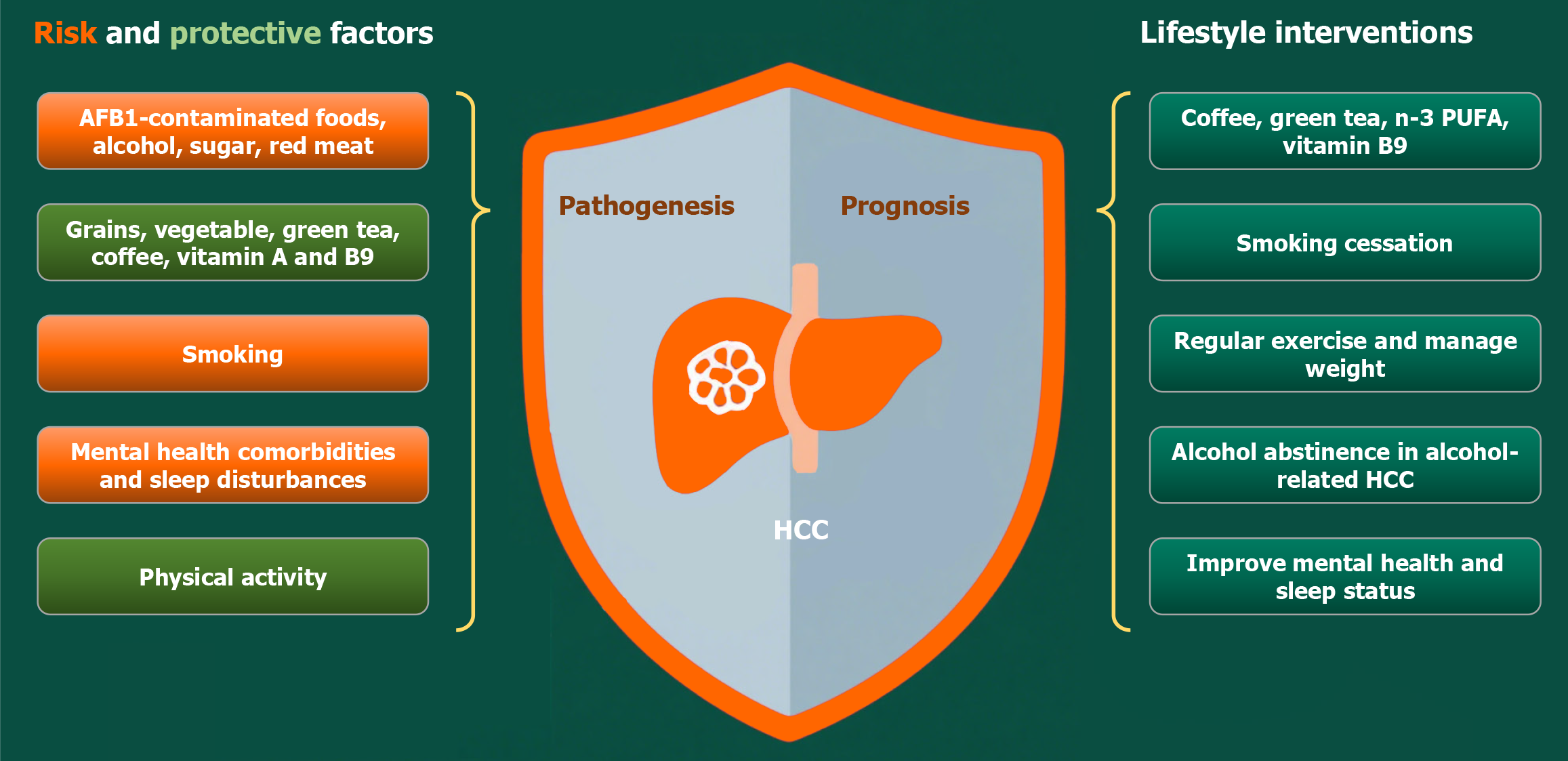
This review highlights the critical role of lifestyle factors in HCC prevention and management as shown in Figure 1. According to current evidence, key dietary strategies include avoiding aflatoxin exposure, increasing fiber and vegetable intake, and consuming coffee, green tea, and n-3 PUFA-rich fish, while limiting alcohol and processed red meats, as summarized in Table 1. Smoking cessation and regular physical activity may further reduce HCC risk, with exercise also improving treatment outcomes. Metabolic disorders, psychological stress, and poor sleep quality could exacerbate HCC progression, emphasizing the need for holistic interventions. Beyond the lifestyle factors discussed in this review, such as diet, smoking, metabolic health, physical activity, mental status, and sleep, emerging environmental exposures, including particulate matter, polycyclic aromatic hydrocarbons, heavy metals, industrial chemicals, and electromagnetic radiation, may influence HCC development and prognosis. Future studies should evaluate the roles of these emerging environmental exposures, refine lifestyle assessments with quantitative measures, explore diet-treatment interactions, and prioritize randomized controlled trials to establish causality between modifiable lifestyle factors and HCC risk. Additionally, personalized interventions must integrate genetic profiles and socioeconomic influences, particularly how income disparities and healthcare access mediate lifestyle behaviors and hepatocarcinogenesis. We believe that implementing these evidence-based strategies through public health initiatives and clinical practice will significantly reduce the burden of HCC and improve treatment outcomes.
| Strategies | Recommendation | Ref. |
| Avoid AFB1-contaminated foods1 | Strong | [12,16,17] |
| Consumption of grains | Strong | [7,8,40] |
| Consumption of vegetables | Strong | [7,38,40] |
| Consumption of n-3 PUFA-rich fish | Strong | [28,31] |
| Consumption of coffee | Moderate | [41,42,46] |
| Consumption of green tea | Moderate | [47,49,50] |
| Alcohol abstinence in A-HCC | Strong | [60-62] |
| Smoking cessation | Moderate | [72-74] |
| Regular exercise and manage weight | Strong | [81,82,86] |
| Improve mental health and sleep status | Moderate | [104-107] |
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12355] [Article Influence: 6177.5] [Reference Citation Analysis (6)] |
| 2. | Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 1432] [Article Influence: 1432.0] [Reference Citation Analysis (3)] |
| 3. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3086] [Article Influence: 771.5] [Reference Citation Analysis (61)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. 2025;82:315-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 321] [Article Influence: 321.0] [Reference Citation Analysis (6)] |
| 5. | Willyard C. Lifestyle: Breaking the cancer habit. Nature. 2011;471:S16-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer. 2020;20:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Liu X, Yang W, Petrick JL, Liao LM, Wang W, He N, Campbell PT, Zhang ZF, Giovannucci E, McGlynn KA, Zhang X. Higher intake of whole grains and dietary fiber are associated with lower risk of liver cancer and chronic liver disease mortality. Nat Commun. 2021;12:6388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Watling CZ, Wojt A, Florio AA, Butera G, Albanes D, Weinstein SJ, Huang WY, Parisi D, Zhang X, Graubard BI, Petrick JL, McGlynn KA. Fiber and whole grain intakes in relation to liver cancer risk: An analysis in 2 prospective cohorts and systematic review and meta-analysis of prospective studies. Hepatology. 2024;80:552-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Yip TC, Wong GL, Tse YK, Yuen BW, Luk HW, Lam MH, Li MK, Loo CK, Tsang OT, Tsang SW, Chan HL, Wing YK, Wong VW. High incidence of hepatocellular carcinoma and cirrhotic complications in patients with psychiatric illness: a territory-wide cohort study. BMC Gastroenterol. 2020;20:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Long L, Zhao L, Petrick JL, Liao LM, Huang T, Hakim A, Yang W, Campbell PT, Giovannucci E, McGlynn KA, Zhang X. Daytime napping, nighttime sleeping duration, and risk of hepatocellular carcinoma and liver disease-related mortality. JHEP Rep. 2023;5:100819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | Qin M, Lin L, Wang L, Zhang Y, Zhang L, Song Y, Chen J. Disease Burden Estimation of Hepatocellular Carcinoma Attributable to Dietary Aflatoxin Exposure in Sichuan Province, China. Nutrients. 2024;16:4381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Hamid AS, Tesfamariam IG, Zhang Y, Zhang ZG. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol Lett. 2013;5:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Zhu Q, Ma Y, Liang J, Wei Z, Li M, Zhang Y, Liu M, He H, Qu C, Cai J, Wang X, Zeng Y, Jiao Y. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct Target Ther. 2021;6:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Qi LN, Bai T, Chen ZS, Wu FX, Chen YY, De Xiang B, Peng T, Han ZG, Li LQ. The p53 mutation spectrum in hepatocellular carcinoma from Guangxi, China: role of chronic hepatitis B virus infection and aflatoxin B1 exposure. Liver Int. 2015;35:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Zhang W, He H, Zang M, Wu Q, Zhao H, Lu LL, Ma P, Zheng H, Wang N, Zhang Y, He S, Chen X, Wu Z, Wang X, Cai J, Liu Z, Sun Z, Zeng YX, Qu C, Jiao Y. Genetic Features of Aflatoxin-Associated Hepatocellular Carcinoma. Gastroenterology. 2017;153:249-262.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Chu YJ, Yang HI, Wu HC, Liu J, Wang LY, Lu SN, Lee MH, Jen CL, You SL, Santella RM, Chen CJ. Aflatoxin B(1) exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. 2017;141:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Chu YJ, Yang HI, Wu HC, Lee MH, Liu J, Wang LY, Lu SN, Jen CL, You SL, Santella RM, Chen CJ. Aflatoxin B(1) exposure increases the risk of hepatocellular carcinoma associated with hepatitis C virus infection or alcohol consumption. Eur J Cancer. 2018;94:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Fedirko V, Lukanova A, Bamia C, Trichopolou A, Trepo E, Nöthlings U, Schlesinger S, Aleksandrova K, Boffetta P, Tjønneland A, Johnsen NF, Overvad K, Fagherazzi G, Racine A, Boutron-Ruault MC, Grote V, Kaaks R, Boeing H, Naska A, Adarakis G, Valanou E, Palli D, Sieri S, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HBA, Siersema PD, Peeters PH, Weiderpass E, Skeie G, Engeset D, Quirós JR, Zamora-Ros R, Sánchez MJ, Amiano P, Huerta JM, Barricarte A, Johansen D, Lindkvist B, Sund M, Werner M, Crowe F, Khaw KT, Ferrari P, Romieu I, Chuang SC, Riboli E, Jenab M. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in Western Europeans. Ann Oncol. 2013;24:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Laguna JC, Alegret M, Roglans N. Simple sugar intake and hepatocellular carcinoma: epidemiological and mechanistic insight. Nutrients. 2014;6:5933-5954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Yang W, Sui J, Ma Y, Simon TG, Petrick JL, Lai M, McGlynn KA, Campbell PT, Giovannucci EL, Chan AT, Zhang X. High Dietary Intake of Vegetable or Polyunsaturated Fats Is Associated With Reduced Risk of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2020;18:2775-2783.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Koh WP, Dan YY, Goh GB, Jin A, Wang R, Yuan JM. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int. 2016;36:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Freedman ND, Cross AJ, McGlynn KA, Abnet CC, Park Y, Hollenbeck AR, Schatzkin A, Everhart JE, Sinha R. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Cancer Inst. 2010;102:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Fridén M, Warensjö Lemming E, Lind L, Vessby J, Rosqvist F, Risérus U. Substitutions of saturated fat intakes with other macronutrients and foods and risk of NAFLD cirrhosis and all-cause hepatocellular carcinoma: a prospective cohort study. Am J Clin Nutr. 2024;120:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Zhang DM, Luo Y, Yishake D, Liu ZY, He TT, Luo Y, Zhang YJ, Fang AP, Zhu HL. Prediagnostic dietary intakes of vitamin A and β-carotene are associated with hepatocellular-carcinoma survival. Food Funct. 2020;11:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Matsushima-Nishiwaki R, Okuno M, Takano Y, Kojima S, Friedman SL, Moriwaki H. Molecular mechanism for growth suppression of human hepatocellular carcinoma cells by acyclic retinoid. Carcinogenesis. 2003;24:1353-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Cui J, Gong M, He Y, Li Q, He T, Bi Y. All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1-6 cells through reversing EMT in vitro. Int J Oncol. 2016;48:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Fang AP, Liu ZY, Liao GC, Chen PY, Wang XY, Zhang DM, Luo Y, Long JA, Zhong RH, Zhou ZG, Xu YJ, Xu XJ, Ling WH, Chen MS, Zhang YJ, Zhu HL. Serum folate concentrations at diagnosis are associated with hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort study. Br J Nutr. 2019;121:1376-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Sawada N, Inoue M, Iwasaki M, Sasazuki S, Shimazu T, Yamaji T, Takachi R, Tanaka Y, Mizokami M, Tsugane S; Japan Public Health Center-Based Prospective Study Group. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology. 2012;142:1468-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Fedirko V, Trichopolou A, Bamia C, Duarte-Salles T, Trepo E, Aleksandrova K, Nöthlings U, Lukanova A, Lagiou P, Boffetta P, Trichopoulos D, Katzke VA, Overvad K, Tjønneland A, Hansen L, Boutron-Ruault MC, Fagherazzi G, Bastide N, Panico S, Grioni S, Vineis P, Palli D, Tumino R, Bueno-de-Mesquita HB, Peeters PH, Skeie G, Engeset D, Parr CL, Jakszyn P, Sánchez MJ, Barricarte A, Amiano P, Chirlaque M, Quirós JR, Sund M, Werner M, Sonestedt E, Ericson U, Key TJ, Khaw KT, Ferrari P, Romieu I, Riboli E, Jenab M. Consumption of fish and meats and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Oncol. 2013;24:2166-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Jayedi A, Shab-Bidar S. Fish Consumption and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Adv Nutr. 2020;11:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Gao M, Sun K, Guo M, Gao H, Liu K, Yang C, Li S, Liu N. Fish consumption and n-3 polyunsaturated fatty acids, and risk of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Causes Control. 2015;26:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Ma Y, Yang W, Li T, Liu Y, Simon TG, Sui J, Wu K, Giovannucci EL, Chan AT, Zhang X. Meat intake and risk of hepatocellular carcinoma in two large US prospective cohorts of women and men. Int J Epidemiol. 2019;48:1863-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Huang YS, Chern HD, Wu JC, Chao Y, Huang YH, Chang FY, Lee SD. Polymorphism of the N-acetyltransferase 2 gene, red meat intake, and the susceptibility of hepatocellular carcinoma. Am J Gastroenterol. 2003;98:1417-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Wang CR, Cai D, He K, Hu JJ, Dai X, Zhu Q, Zhong GC. Red Meat, Poultry, and Fish Consumption and the Risk of Liver Cancer: A Prospective Cohort Study of 0.5 Million Chinese Adults. Cancer Epidemiol Biomarkers Prev. 2025;34:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Bock N, Langmann F, Johnston LW, Ibsen DB, Dahm CC. The Association between the Substitution of Red Meat with Legumes and the Risk of Primary Liver Cancer in the UK Biobank: A Cohort Study. Nutrients. 2024;16:2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Guo XF, Shao XF, Li JM, Li S, Li KL, Li D. Fruit and vegetable intake and liver cancer risk: a meta-analysis of prospective cohort studies. Food Funct. 2019;10:4478-4485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Bamia C, Lagiou P, Jenab M, Aleksandrova K, Fedirko V, Trichopoulos D, Overvad K, Tjønneland A, Olsen A, Clavel-Chapelon F, Boutron-Ruault MC, Kvaskoff M, Katzke VA, Kühn T, Boeing H, Nöthlings U, Palli D, Sieri S, Panico S, Tumino R, Naccarati A, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Skeie G, Quirós JR, Agudo A, Chirlaque MD, Sanchez MJ, Ardanaz E, Dorronsoro M, Ericson U, Nilsson LM, Wennberg M, Khaw KT, Wareham N, Key TJ, Travis RC, Ferrari P, Stepien M, Duarte-Salles T, Norat T, Murphy N, Riboli E, Trichopoulou A. Fruit and vegetable consumption in relation to hepatocellular carcinoma in a multi-centre, European cohort study. Br J Cancer. 2015;112:1273-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Zhao L, Jin L, Petrick JL, Zeng H, Wang F, Tang L, Smith-Warner SA, Eliassen AH, Zhang FF, Campbell PT, Giovannucci E, Liao LM, McGlynn KA, Steck SE, Zhang X. Specific botanical groups of fruit and vegetable consumption and liver cancer and chronic liver disease mortality: a prospective cohort study. Am J Clin Nutr. 2023;117:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Zamora-Ros R, Fedirko V, Trichopoulou A, González CA, Bamia C, Trepo E, Nöthlings U, Duarte-Salles T, Serafini M, Bredsdorff L, Overvad K, Tjønneland A, Halkjaer J, Fagherazzi G, Perquier F, Boutron-Ruault MC, Katzke V, Lukanova A, Floegel A, Boeing H, Lagiou P, Trichopoulos D, Saieva C, Agnoli C, Mattiello A, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Engeset D, Skeie G, Argüelles MV, Molina-Montes E, Dorronsoro M, Tormo MJ, Ardanaz E, Ericson U, Sonestedt E, Sund M, Landberg R, Khaw KT, Wareham NJ, Crowe FL, Riboli E, Jenab M. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2013;133:2429-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Yang W, Ma Y, Liu Y, Smith-Warner SA, Simon TG, Chong DQ, Qi Q, Meyerhardt JA, Giovannucci EL, Chan AT, Zhang X. Association of Intake of Whole Grains and Dietary Fiber With Risk of Hepatocellular Carcinoma in US Adults. JAMA Oncol. 2019;5:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 41. | Lai GY, Weinstein SJ, Albanes D, Taylor PR, McGlynn KA, Virtamo J, Sinha R, Freedman ND. The association of coffee intake with liver cancer incidence and chronic liver disease mortality in male smokers. Br J Cancer. 2013;109:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Yu J, Liang D, Li J, Liu Z, Zhou F, Wang T, Ma S, Wang G, Chen B, Chen W. Coffee, Green Tea Intake, and the Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. Nutr Cancer. 2023;75:1295-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 43. | Tanaka K, Tamakoshi A, Sugawara Y, Mizoue T, Inoue M, Sawada N, Matsuo K, Ito H, Naito M, Nagata C, Kitamura Y, Sadakane A, Tsugane S, Shimazu T; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Coffee, green tea and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2019;49:972-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Shan L, Zhao N, Wang F, Zhai D, Liu J, Lv X. Caffeine in Hepatocellular Carcinoma: Cellular Assays, Animal Experiments, and Epidemiological Investigation. J Inflamm Res. 2024;17:1589-1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 45. | Okano J, Nagahara T, Matsumoto K, Murawaki Y. Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin Pharmacol Toxicol. 2008;102:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, Sudan S, Nicoll AJ. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients. 2021;13:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 47. | Yang JD, Malhi H. Green tea consumption: A potential chemopreventive measure for hepatocellular carcinoma? Hepatology. 2018;67:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Kim TL, Jeong GH, Yang JW, Lee KH, Kronbichler A, van der Vliet HJ, Grosso G, Galvano F, Aune D, Kim JY, Veronese N, Stubbs B, Solmi M, Koyanagi A, Hong SH, Dragioti E, Cho E, de Rezende LFM, Giovannucci EL, Shin JI, Gamerith G. Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv Nutr. 2020;11:1437-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 49. | Huang YQ, Lu X, Min H, Wu QQ, Shi XT, Bian KQ, Zou XP. Green tea and liver cancer risk: A meta-analysis of prospective cohort studies in Asian populations. Nutrition. 2016;32:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Li ZY, Tan YT, Liu DK, Gao LF, Li HL, Xiang YB. Cumulative consumption of tea is associated with lower risk of liver cancer: Updated results from the Shanghai Women's Health Study. Int J Cancer. 2023;152:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Bamia C, Lagiou P, Jenab M, Trichopoulou A, Fedirko V, Aleksandrova K, Pischon T, Overvad K, Olsen A, Tjønneland A, Boutron-Ruault MC, Fagherazzi G, Racine A, Kuhn T, Boeing H, Floegel A, Benetou V, Palli D, Grioni S, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita HB, Dik VK, Bhoo-Pathy N, Uiterwaal CS, Weiderpass E, Lund E, Quirós JR, Zamora-Ros R, Molina-Montes E, Chirlaque MD, Ardanaz E, Dorronsoro M, Lindkvist B, Wallström P, Nilsson LM, Sund M, Khaw KT, Wareham N, Bradbury KE, Travis RC, Ferrari P, Duarte-Salles T, Stepien M, Gunter M, Murphy N, Riboli E, Trichopoulos D. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. Int J Cancer. 2015;136:1899-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Xu J, Xiao X, Yan B, Yuan Q, Dong X, Du Q, Zhang J, Shan L, Ding Z, Zhou L, Efferth T. Green tea-derived theabrownin induces cellular senescence and apoptosis of hepatocellular carcinoma through p53 signaling activation and bypassed JNK signaling suppression. Cancer Cell Int. 2022;22:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Zapf MA, Kothari AN, Weber CE, Arffa ML, Wai PY, Driver J, Gupta GN, Kuo PC, Mi Z. Green tea component epigallocatechin-3-gallate decreases expression of osteopontin via a decrease in mRNA half-life in cell lines of metastatic hepatocellular carcinoma. Surgery. 2015;158:1039-1047; discussion 1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Yang XW, Wang XL, Cao LQ, Jiang XF, Peng HP, Lin SM, Xue P, Chen D. Green tea polyphenol epigallocatechin-3-gallate enhances 5-fluorouracil-induced cell growth inhibition of hepatocellular carcinoma cells. Hepatol Res. 2012;42:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Nishikawa T, Nakajima T, Moriguchi M, Jo M, Sekoguchi S, Ishii M, Takashima H, Katagishi T, Kimura H, Minami M, Itoh Y, Kagawa K, Okanoue T. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J Hepatol. 2006;44:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Ganne-Carrié N, Nahon P. Differences between hepatocellular carcinoma caused by alcohol and other aetiologies. J Hepatol. 2025;82:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Huang DQ, Tan DJH, Ng CH, Amangurbanova M, Sutter N, Lin Tay PW, Lim WH, Yong JN, Tang A, Syn N, Muthiah MD, Tan EXX, Dave S, Tay B, Majzoub AM, Gerberi D, Kim BK, Loomba R. Hepatocellular Carcinoma Incidence in Alcohol-Associated Cirrhosis: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 58. | Zeng RW, Ong CEY, Ong EYH, Chung CH, Lim WH, Xiao J, Danpanichkul P, Law JH, Syn N, Chee D, Kow AWC, Lee SW, Takahashi H, Kawaguchi T, Tamaki N, Dan YY, Nakajima A, Wijarnpreecha K, Muthiah MD, Noureddin M, Loomba R, Ioannou GN, Tan DJH, Ng CH, Huang DQ. Global Prevalence, Clinical Characteristics, Surveillance, Treatment Allocation, and Outcomes of Alcohol-Associated Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2024;22:2394-2402.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 59. | Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, Corrao G, Boffetta P, La Vecchia C. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2014;25:1526-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 60. | Aglago EK, Ramos I, Keski-Rahkonen P, Chatziioannou C, Freisling H, Fedirko V, Gunter MJ, Dahm CC, Langmann F, Bondonno N, Tjønneland A, Severi G, Truong T, Katzke V, Kaaks R, Bergmann M, Schulze MB, Masala G, Pala V, de Magistris MS, Di Girolamo C, Lukic M, Gram IT, Bonet C, Sánchez MJ, Chirlaque MD, Amiano P, Guevara M, Vermeulen R, Manjer J, Eriksson L, Key TJ, Mayen AL, Dossus L, Weiderpass E, Heath AK, Ferrari P, Jenab M. Alcohol and smoking habits in association with hepatocellular carcinoma risk. Int J Cancer. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 61. | Donati A, Henrion J, Regnier M, Deltenre P, Marot A. Abstinence is associated with better outcomes in patients with alcohol-related hepatocellular carcinoma: Results of an observational study. Clin Res Hepatol Gastroenterol. 2023;47:102225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | Cho EJ, Chung GE, Yoo JJ, Cho Y, Shin DW, Kim YJ, Yoon JH, Han K, Yu SJ. The association between alcohol consumption and the risk of hepatocellular carcinoma according to glycemic status in Korea: A nationwide population-based study. PLoS Med. 2023;20:e1004244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 63. | Fu Y, Maccioni L, Wang XW, Greten TF, Gao B. Alcohol-associated liver cancer. Hepatology. 2024;80:1462-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 64. | Zelber-Sagi S, Noureddin M, Shibolet O. Lifestyle and Hepatocellular Carcinoma What Is the Evidence and Prevention Recommendations. Cancers (Basel). 2021;14:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Turati F, Trichopoulos D, Polesel J, Bravi F, Rossi M, Talamini R, Franceschi S, Montella M, Trichopoulou A, La Vecchia C, Lagiou P. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 66. | Li WQ, Park Y, McGlynn KA, Hollenbeck AR, Taylor PR, Goldstein AM, Freedman ND. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology. 2014;60:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, Chong D, VoPham T, Meyerhardt JA, Wen D, Giovannucci EL, Chan AT, Zhang X. Dietary Patterns and Risk of Hepatocellular Carcinoma Among U.S. Men and Women. Hepatology. 2019;70:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 68. | Bogumil D, Park SY, Le Marchand L, Haiman CA, Wilkens LR, Boushey CJ, Setiawan VW. High-Quality Diets Are Associated With Reduced Risk of Hepatocellular Carcinoma and Chronic Liver Disease: The Multiethnic Cohort. Hepatol Commun. 2019;3:437-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | Luo Y, Zhang YJ, Zhang DM, Yishake D, Liu ZY, Chen MS, Wang F, Zhou ZG, Long JA, Zhong RH, Chen S, Lu XT, Li SY, He TT, Luo Y, Fang AP, Zhu HL. Association between dietary patterns and prognosis of hepatocellular carcinoma in the Guangdong liver cancer cohort study. Hepatol Res. 2020;50:1164-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Chen PY, Fang AP, Wang XY, Lan QY, Liao GC, Liu ZY, Zhang DM, Zhang YY, Chen YM, Zhu HL. Adherence to the Chinese or American Dietary Guidelines is Associated with a Lower Risk of Primary Liver Cancer in China: A Case-Control Study. Nutrients. 2018;10:1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Yang W, Sui J, Zhao L, Ma Y, Tabung FK, Simon TG, Lee DH, Zeng X, Nguyen LH, Meyerhardt JA, Chan AT, Giovannucci EL, Zhang X. Association of Inflammatory and Insulinemic Potential of Diet and Lifestyle with Risk of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30:789-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 72. | Abdel-Rahman O, Helbling D, Schöb O, Eltobgy M, Mohamed H, Schmidt J, Giryes A, Mehrabi A, Iype S, John H, Tekbas A, Zidan A, Oweira H. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 73. | Kolly P, Knöpfli M, Dufour JF. Effect of smoking on survival of patients with hepatocellular carcinoma. Liver Int. 2017;37:1682-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Li ZY, Shen QM, Tuo JY, Tan JY, Tan YT, Li HL, Xiang YB. A prospective cohort study of cigarette smoking, alcohol drinking and liver cancer incidence in Chinese men. J Dig Dis. 2022;23:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 75. | Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Khan Zahra TR, Janmeda P, Sharifi-Rad J, Daştan SD, Mahmud S, Docea AO, Calina D. Tobacco Smoking and Liver Cancer Risk: Potential Avenues for Carcinogenesis. J Oncol. 2021;2021:5905357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 76. | Wang YH, Chuang YH, Wu CF, Jan MC, Wu WJ, Lin CL, Liu CJ, Yang YC, Chen PJ, Lin SM, Tsai MH, Huang YW, Yu MW. Smoking and Hepatitis B Virus-Related Hepatocellular Carcinoma Risk: The Mediating Roles of Viral Load and Alanine Aminotransferase. Hepatology. 2019;69:1412-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Siegel AB, Conner K, Wang S, Jacobson JS, Hershman DL, Hidalgo R, Verna EC, Halazun K, Brubaker W, Zaretsky J, Moniodis A, Delgado-Cruzata L, Dove L, Emond J, Kato T, Brown RS Jr, Neugut AI. Smoking and hepatocellular carcinoma mortality. Exp Ther Med. 2012;3:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Baumeister SE, Leitzmann MF, Linseisen J, Schlesinger S. Physical Activity and the Risk of Liver Cancer: A Systematic Review and Meta-Analysis of Prospective Studies and a Bias Analysis. J Natl Cancer Inst. 2019;111:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | DiJoseph K, Thorp A, Harrington A, Schmitz KH, Chinchilli VM, Stine JG. Physical Activity and Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2023;68:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Lee J. Associations between Physical Activity and Liver Cancer Risks and Mortality: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2020;17:8943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | Luo X, Yang W, Ma Y, Simon TG, Meyerhardt JA, Chan AT, Giovannucci EL, Zhang X. Physical Activity and Risk of Hepatocellular Carcinoma Among U.S. Men and Women. Cancer Prev Res (Phila). 2020;13:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Liu XF, Zhu XD, Feng LH, Li XL, Xu B, Li KS, Xiao N, Lei M, Sun HC, Tang ZY. Physical activity improves outcomes of combined lenvatinib plus anti-PD-1 therapy in unresectable hepatocellular carcinoma: a retrospective study and mouse model. Exp Hematol Oncol. 2022;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Chen H, Zhou H, Wu B, Lu H, Zhang J, Zhang Y, Gu Y, Zhou G, Xiang J, Yang J. Physical activity and exercise in liver cancer. Liver Res. 2024;8:22-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 84. | Marcantei C, Couret A, King J, Mazeaud S, Armand A, Ennequin G. Effects of Exercise Training on Muscle Mass and Physical Function in Patients with Hepatocellular Carcinoma After Diagnosis: A Systematic Review. Dig Dis Sci. 2024;69:2667-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 85. | Özdemir BH, Özdemir AA. How Exercise Affects the Development and Progression of Hepatocellular Carcinoma by Changing the Biomolecular Status of the Tumor Microenvironment. Exp Clin Transplant. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Huyen VT, Echizen K, Yamagishi R, Kumagai M, Nonaka Y, Kodama T, Ando T, Yano M, Takada N, Takasugi M, Kamachi F, Ohtani N. Regular exercise suppresses steatosis-associated liver cancer development by degrading E2F1 and c-Myc via circadian gene upregulation. Genes Cells. 2024;29:1012-1025. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Saran U, Humar B, Kolly P, Dufour JF. Hepatocellular carcinoma and lifestyles. J Hepatol. 2016;64:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 88. | Arfianti A, Pok S, Barn V, Haigh WG, Yeh MM, Ioannou GN, Teoh NC, Farrell GC. Exercise retards hepatocarcinogenesis in obese mice independently of weight control. J Hepatol. 2020;73:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Devan AR, Pavithran K, Nair B, Murali M, Nath LR. Deciphering the role of transforming growth factor-beta 1 as a diagnostic-prognostic-therapeutic candidate against hepatocellular carcinoma. World J Gastroenterol. 2022;28:5250-5264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 90. | Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 91. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5355] [Article Influence: 232.8] [Reference Citation Analysis (2)] |
| 92. | Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 1231] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 93. | Karagozian R, Derdák Z, Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 94. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 95. | Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497-2507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 389] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 96. | Montella M, Crispo A, Giudice A. HCC, diet and metabolic factors: Diet and HCC. Hepat Mon. 2011;11:159-162. [PubMed] |
| 97. | Tokushige K, Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Shiratori K. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol. 2010;45:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 609] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 99. | Loomba R, Liu J, Yang HI, Lee MH, Lu SN, Wang LY, Iloeje UH, You SL, Brenner D, Chen CJ; REVEAL–HBV Study Group. Synergistic effects of family history of hepatocellular carcinoma and hepatitis B virus infection on risk for incident hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013;11:1636-45.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 100. | Patel MJ, Jones A, Jiang Y, Gowda P, VanWagner LB, Cotter TG, Seif El Dahan K, Louissaint J, Patel M, Rich NE, Singal AG, Lieber SR. Psychiatric disorders in patients with hepatocellular carcinoma: A large US cohort of commercially insured individuals. Aliment Pharmacol Ther. 2024;60:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Xu F, Dirsch O, Dahmen U. Causal relationship between psychological factors and hepatocellular carcinoma as revealed by Mendelian randomization. J Cancer Res Clin Oncol. 2024;150:100. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 102. | Li M, Yu B, He H, Li N, Gao R. Impact of psychological resilience and social support on psycho-social adjustment in postoperative patients with primary hepatocellular carcinoma: mediating effects of fear of progression. Front Psychol. 2024;15:1461199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 103. | Rice DR, Dalmacy D, Hyer JM, Diaz A, Tsilimigras DI, Pawlik TM. Impact of Psychiatric Illness on Survival among Patients with Hepatocellular Carcinoma. J Gastrointest Surg. 2021;25:3242-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Zhao FJ, Huo RR, Li FR, You XM. Associations of tumor-related psychiatric symptoms and healthy behaviors with dynamic quality of life after hepatocellular carcinoma hepatectomy. Support Care Cancer. 2024;32:589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 105. | Li T, Li B, Tan L, Lv B. Reminiscence Therapy as a Potential Method to Improve Psychological Health and Quality of Life in Elderly Hepatocellular Carcinoma Patients: A Randomized, Controlled Trial. Front Surg. 2022;9:873843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 106. | Huang TW, Cheung DST, Xu X, Loh EW, Lai JH, Su WW, Wu SS, Lin CC. Relationship Between Diurnal Cortisol Profile and Sleep Quality in Patients With Hepatocellular Carcinoma. Biol Res Nurs. 2020;22:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 107. | Chu TL, Yu WP, Chen SC, Peng HL, Wu MJ. Comparison of differences and determinants between presence and absence of sleep disturbance in hepatocellular carcinoma patients. Cancer Nurs. 2011;34:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Huang J, Song P, Hang K, Chen Z, Zhu Z, Zhang Y, Xu J, Qin J, Wang B, Qu W, Huang Z, Liang C. Sleep Deprivation Disturbs Immune Surveillance and Promotes the Progression of Hepatocellular Carcinoma. Front Immunol. 2021;12:727959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/