Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.107246
Revised: April 26, 2025
Accepted: June 18, 2025
Published online: July 24, 2025
Processing time: 125 Days and 20 Hours
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare heterogeneous primary malignant liver tumor containing both hepatocellular and cholangiocarcinoma features. The complex presentation of cHCC-CCA tends to be poorly investigated, and the information derived from traditional diagnostic techniques (histopathology and radiological imaging) is often not optimal. Since cHCC-CCA is usually difficult to diagnose due to complex histopathological features (edge learning) as excessive photos, hence, achieves treatment delays and poor prognosis, the incorporation of advanced artificial intelligence like edge learning is able to improve the patient’s outcome. Using artificial intelligence, particularly deep learning, has recently opened new doorways for the impro
Core Tip: Edge learning presents a novel approach in combined hepatocellular-cholangiocarcinoma diagnosis and classification, leveraging decentralized artificial intelligence for real-time processing and enhanced data privacy. Unlike traditional cloud-based artificial intelligence, edge learning enables on-site analysis of histopathological features and medical imaging (computed tomography, magnetic resonance imaging) while reducing latency and bandwidth usage. This review explores its technical integration, including federated learning, deep learning optimizations (convolutional neural networks, pruning, quantization), and privacy-preserving artificial intelligence frameworks. By overcoming challenges like diagnostic complexity and data security, edge learning enhances clinical decision-making, treatment planning, and diagnostic accuracy, offering a transformative potential in precision oncology and liver cancer management.
- Citation: Akbulut S, Colak C. Edge learning applications in the prediction and classification of combined hepatocellular-cholangiocarcinoma: A comprehensive narrative review. World J Clin Oncol 2025; 16(7): 107246
- URL: https://www.wjgnet.com/2218-4333/full/v16/i7/107246.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i7.107246
According to GLOBOCAN 2022 data, primary liver cancer is the sixth most frequently diagnosed cancer worldwide and ranks third among cancer-related deaths[1,2]. In 2022, 866136 new liver cancer cases and 758725 deaths were reported worldwide[1,2]. Liver cancer is more common in men than in women, with men accounting for approximately 69.35% of total cases[1]. Primary liver cancers are divided into three main types: Hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (CCA), and combined hepatocellular-CCA (cHCC-CCA)[1]. HCC is the most common subtype, accounting for 75%-90% of all primary liver cancers. It is usually associated with factors such as hepatitis B and C infections, alcohol consumption, and metabolic dysfunction-associated steatotic liver disease[1-3]. CCA is a subtype that originates from the biliary tract and is seen in 10%-20%. The rarer cHCC-CCA is a mixed tumor originating from both hepatocytes and bile duct cells and requires a different approach in terms of diagnosis and treatment[1,4]. The clinical course of cHCC-CCA is generally more aggressive, and its management becomes more complex with standard treatment methods because it has features of both HCC and CCA[1,3-5]. cHCC-CCA constitutes from 0.4% to 14.2% of all primary liver malignancies, varying by geographic location and diagnostic standards. The surveillance, epidemiology, and end results database estimates the incidence rate as 0.77%. The actual incidence is probably underestimated, as numerous instances are misdiagnosed as HCC or CCA. Importantly, cHCC-CCA carries a poorer overall prognosis compared to HCC or CCA alone[6]. cHCC-CCA has unique epidemiological and clinical characteristics. Research from Asia indicates a male majority in gender distribution, while studies from Western nations reveal no substantial gender disparity. Multiple factors have been associated with the onset of cHCC-CCA, including persistent infections with hepatitis B virus and hepatitis C virus, alcohol consumption, and primary sclerosing cholangitis. In contrast to HCC and CCA, cHCC-CCA can develop in both cirrhotic and non-cirrhotic livers. The median age upon diagnosis generally occurs between the ages of 60 and 70[6]. Table 1 summarizes the key histopathological and immunohistochemical features of HCC, CCA and cHCC-CCA. A better understanding of this rare tumor may be possible with combination of advanced imaging techniques, histopathological and immunohistochemical features and artificial intelligence-based diagnostic methods, thus enabling early diagnosis and development of targeted treatment strategies.
| Features | HCC | CCA | Combined HCC-CCA |
| Tumor cell morphology | Polygonal tumor cells, abundant clear to eosinophilic cytoplasm | Cuboidal/columnar cells, vacuolated or pale eosinophilic cytoplasm | Mixed hepatocytic and cholangiocytic features |
| Architecture | Trabecular, solid, pseudoglandular pattern | Glandular/tubular structures in desmoplastic stroma | Mixed trabecular and glandular patterns |
| Bile production | Present | Absent | Present (in HCC areas) |
| Mucin production | Absent | Frequent | Present in cholangiocarcinoma component (or in CCA areas) |
| Immunohistochemical markers | Hepatocyte paraffin antigen 1 +, arginase-1 +, glypican 3+ | Cytokeratin 7 +, cytokeratin 19 +, epithelial membrane antigen + | Co-expression of hepatocytic and biliary markers |
| Other histologic features | Steatosis, Mallory bodies, hyaline bodies | Marked fibrous stroma | Both hepatocytic and biliary differentiation within the same tumor |
cHCC-CCA represents a unique clinical entity within primary liver cancers, merging features of HCC and CCA. This duality complicates both diagnosis and treatment planning, as standard imaging modalities and histopathological techniques may not capture the tumor’s inherent heterogeneity effectively[6,7]. As a rare and aggressive liver cancer with an extremely poor prognosis, cHCC-CCA only affords surgical resection as the sole curative option. This means that diagnostic and treatment methods need to be improved even more. An option is to use machine learning and artificial intelligence to make it easier to tell the difference between cHCC-CCA and other types of cancer. In parallel, advances in deep learning have shown promise in enhancing image analysis and predictive modeling for various cancers[8]. However, centralized artificial intelligence systems that rely on data transfer to remote server’s face challenges such as high latency, increased risk of data breaches, and network constraints.
Edge learning mitigates these issues by enabling model training and inference on local devices, such as hospital imaging systems and mobile devices. This approach not only accelerates data processing but also maintains patient data privacy a crucial aspect in healthcare applications[9,10]. The proven success of edge learning in diverse healthcare settings indicates its potential value for combined HCC and CCA diagnosis although researchers have not applied it explicitly for this task. The analysis capabilities of edge learning have improved device monitoring in internet of medical things and it now enables efficient diagnostics with strict privacy rules including general data protection regulation and health insurance portability and accountability act for remote patient monitoring. The successful operation of edge learning systems indicates its potential to speed up cHCC-CCA differentiation processes by moving away from cloud-based systems with their latency issues[11,12].
Convolutional neural networks (CNNs) among conventional artificial intelligence models achieve high diagnostic accuracy (> 85%) when used in HCC diagnosis through radiological and histopathological data. The need for using centralized servers impedes data transfer speed and obstructs scalability duties along with privacy regulations. Edge learning solves these problems by distributing computation across devices or edge servers, which operate inside hospital networks. By implementing a localized approach, hospitals enhance information processing speed while maintaining onsite storage of medical data that protects it from breaches. Edge-learning frameworks support integration with current artificial intelligence systems because they increase the adaptability of complex functions such as HCC-CCA classification, which needs detailed analysis of image features[13-15].
The combination of edge learning allows healthcare systems to make advanced cHCC-CCA diagnostic capabilities possible that exceed traditional artificial intelligence capabilities. The subset of edge learning known as federated learning allows different institutions to work together on robust models while keeping their data separate thus ensuring confidentiality and model generalization. The exceptional feature gives significant benefits to diagnose rare cancers such as advanced cHCC-CCA because of limited available clinical data. Edge learning represents a privacy-aware path toward the deployment of oncology innovations at patient sites where it enables fast and accurate diagnoses[15,16].
Although its fraction is small in primary liver cancer, cHCC-CCA is also characterized by a notably high rate of misdiagnosis and unfavorable prognosis[3]. This situation highlights the imperative need to investigate new ways to address the issue such as edge learning that shows potential to improve diagnostic accuracy while improving patient care. This narrative review examines how edge learning can be applied to the prediction and classification of cHCC-CCA and discusses the technological advances that support its implementation. Additionally, the purpose of this review is to investigate how edge learning may be applied for cHCC-CCA to improve diagnosis accuracy, data privacy, and real-time clinical decision-making capability. It looks especially at the technological underpinnings, present uses, and future directions of edge learning in cancer. Answers to the following questions are sought in this review: (1) How can edge learning improve cHCC-CCA diagnosis? (2) How are the technical challenges and solutions in carrying out edge learning in the clinical practice? And (3) What further research directions could refine this technology known to the researcher further?
To condense a literature review that is systematic and rigorous in the space of artificial intelligence, edge computing, and medical oncology. The methodology followed the established guidelines for systematic review including a well-developed systematic search strategy, predefined eligibility criteria and the application of a multi stage screening process that would promote reproducibility and minimize bias.
The search was performed on three major scholarly sources, PubMed (biomedical), IEEE Xplore (technical and engineering), and ScienceDirect (multidisciplinary scientific) against this topic. The applications of these were selected for both capturing the clinical insights (e.g., oncology) and technological innovations (e.g., edge computing architectures).
The framework used the medical subject headings terms and free text terms to achieve best sensitivity and specificity. Primary search terms included: It involves ‘edge learning,’ a term for which it includes (edge computing, federated learning, and decentralized artificial intelligence frameworks); Taken together, these neoplasms were called ‘cHCC-CCA’ as well as related neoplasms (e.g., ‘primary liver cancer’, ‘mixed liver tumors’, etc.); Deep learning, CNNs, computer-aided diagnosis (subcategories: ‘Artificial intelligence’); ‘Medical imaging’ [modalities: Magnetic resonance imaging (MRI), computed tomography (CT), histopathology, radiomics].
Results were refined with the use of Boolean (AND/OR) and proximity filters. For the sake of focusing only on recent technological breakthroughs and emerging clinical paradigms, temporal scope was limited to articles published from January 2019 to May 2024. Original research articles, narrative reviews, and systematic reviews focusing on clinical applications, artificial intelligence diagnostic models, edge learning technologies, and the transdisciplinary integration of medical imaging and computer modeling were included.
Edge learning combines the edge computing principles with machine learning to perform local data processing. The primary advantages include.
Real-time processing: Edge devices can perform high-level computations locally near instantaneously to perform analysis or make decisions in image processing during such clinical procedures.
Enhanced data privacy: Thanks to the fact that sensitive patient data is stored on local devices, minimizing the risk of data breach and satisfying strict data protection rules, edge learning enhances privacy.
Bandwidth efficiency: Exchange of edges devices and central servers occurs with only lightweight updates or aggregated insights and no need for large data transfers from them to the center.
Current research attempts to optimize deep learning models for edge deployment. The development of lightweight CNNs with high accuracy is possible through techniques such as model pruning, quantization, and distortion learning[17-19].
Edge learning emerges as a new framework, which combines edge computing with machine learning for improving the data processing capability at network edge. This approach is effective in environments with the requirement of real time data analysis in cases like a healthcare and an internet of things (IoT) application. Key components of edge learning were summarized as follows.
End user devices: Smartphones, cameras and IoT devices that generate data can be considered as end user devices.
Edge learning servers: These servers are closer to the data source and they receive raw data and perform data processing in the initial phase by filtering out noise and extracting critical features from raw data as needed.
Deep learning clusters: Powerful cloud based resource, performing more complex computations using the processed data from edge servers such as running CNN and long short term memory networks are known as deep learning clusters[20,21].
The focus of edge learning research is to improve deep learning models for edge deployment.
Model pruning: Reducing neural network size through eliminating less significant weights (i.e., model pruning).
Quantization: Converting high precision weights to lower precision weights in order to reduce the model size and inference speed, without affecting the accuracy too much.
Distortion learning: Methods that improve model’s robustness with respect to input data variations.
The goal of these advancements is to produce lightweight CNNs with high accuracy and appropriate for deployment at the edge[18].
Clinically, cHCC-CCA offers several challenges: Histopathological heterogeneity: The tumor has a mixed cellular composition making the histological diagnosis difficult. Imaging complexity: Specifically, diagnosis relies on conventional imaging techniques like CT and MRI because they can devise uptake by tumor cells but cannot distinguish the hepatocellular and CCA components. Treatment planning: Because of different therapeutic strategies demanded for HCC and CCA components, precise classification is needed to ascertain suitable operations.
In order to overcome these challenges, there is a need to integrate advanced artificial intelligence techniques that are able to process multimodal data and thus improve the diagnostic accuracy as well as provide support for clinical decision[22].
Edge learning technologies operate within imaging devices to process ultrasound, CT and MRI images at their on-site locations. Optimized machine learning models can be employed for subsequent objectives.
Segment tumor regions: Dual segment tumor region differentiation examines hepatocellular and CCA structures in one lesion to generate immediate diagnostic outcomes.
Support intraoperative decisions: Real-time analysis speeds up tumor region classification during surgical interventions through continuous support to surgeons when time-sensitive procedures take place.
The processing of sensitive patient data locally makes edge learning a solution that improves data privacy standards. The collaborative model updating process through federated learning enables several clinical centers to work together without sharing their original data while simultaneously enhancing model applicability[23-25].
The implementation of edge/machine learning in the diagnosis and management of cHCC-CCA offers several direct clinical benefits.
Improved diagnostic accuracy: The detection capabilities of machine-learning radiomics analysis on MRI and CT characteristics showed promising success for differentiating cHCC-CCA from HCC and CCA. The early detection made possible because of this method proves essential for starting necessary treatment promptly.
Enhanced patient safety and data privacy: Processing data locally at the site where patients receive care improves their privacy protection. The protection of patient data security matters most in specific healthcare environments. Through its model, training method decentralized, efficient and privacy-enhanced federated edge learning allows medical devices from various institutions to build shared predictive models without disclosing their original data, which ensures privacy protection for healthcare systems handling cyber physical devices.
Personalized treatment planning: Rapid and accurate classification of cHCC-CCA supports tailored treatment strategies. Clinicians can better decide on therapeutic interventions by understanding the specific tumor composition, ultimately leading to more individualized patient care.
Operational efficiency: Edge learning reduces the delay inherent in centralized data processing. This efficiency not only benefits urgent clinical decision-making, such as during surgical procedures, but also minimizes the overall burden on hospital IT infrastructure.
Scalability in resource-limited settings: The reduced need for high-bandwidth data transfer makes edge learning an attractive option for remote or resource-constrained healthcare facilities, potentially expanding access to advanced diagnostic capabilities.
These clinical benefits underscore the potential of edge learning to revolutionize the diagnostic workflow for cHCC-CCA, ultimately leading to improved patient outcomes[23,24,26].
Several technical strategies have been developed to adapt deep learning models for edge deployment/computing in medical studies.
Model compression: Techniques such as pruning, quantization, and knowledge distillation work to create a model that is smaller and therefore uses less computational demands, to place the model on a resource constrained device without significant loss in accuracy.
Lightweight CNN architectures: Architectures that are tailored to extract useful features from medical images are explored for the purpose of accurate segmentation and classification.
Federated learning frameworks: Federated learning enables federating training such that data of multiple institutions can be trained on a shared model without violating data privacy. It has been shown across many healthcare settings to improve model performance across various populations using this approach.
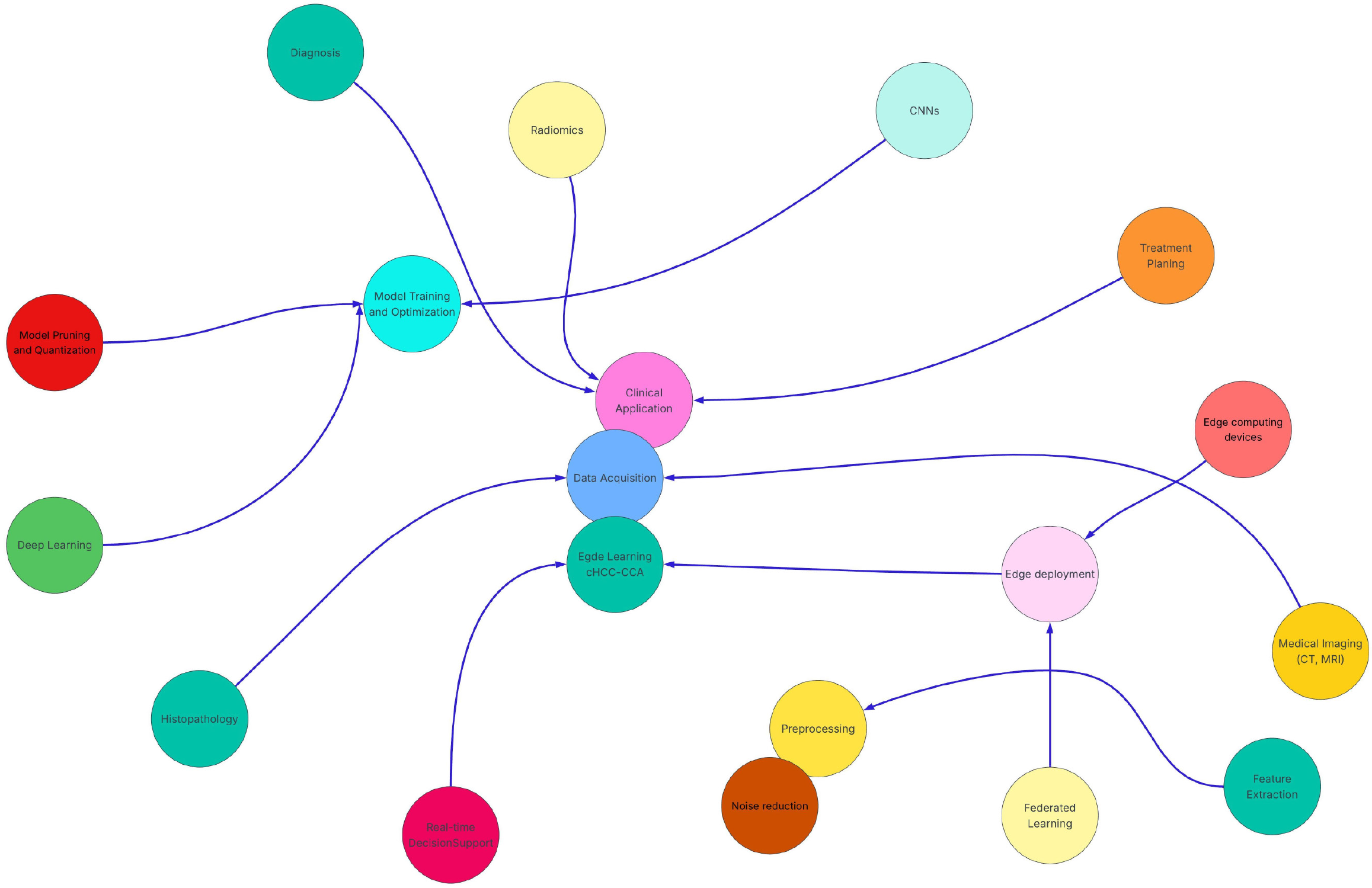
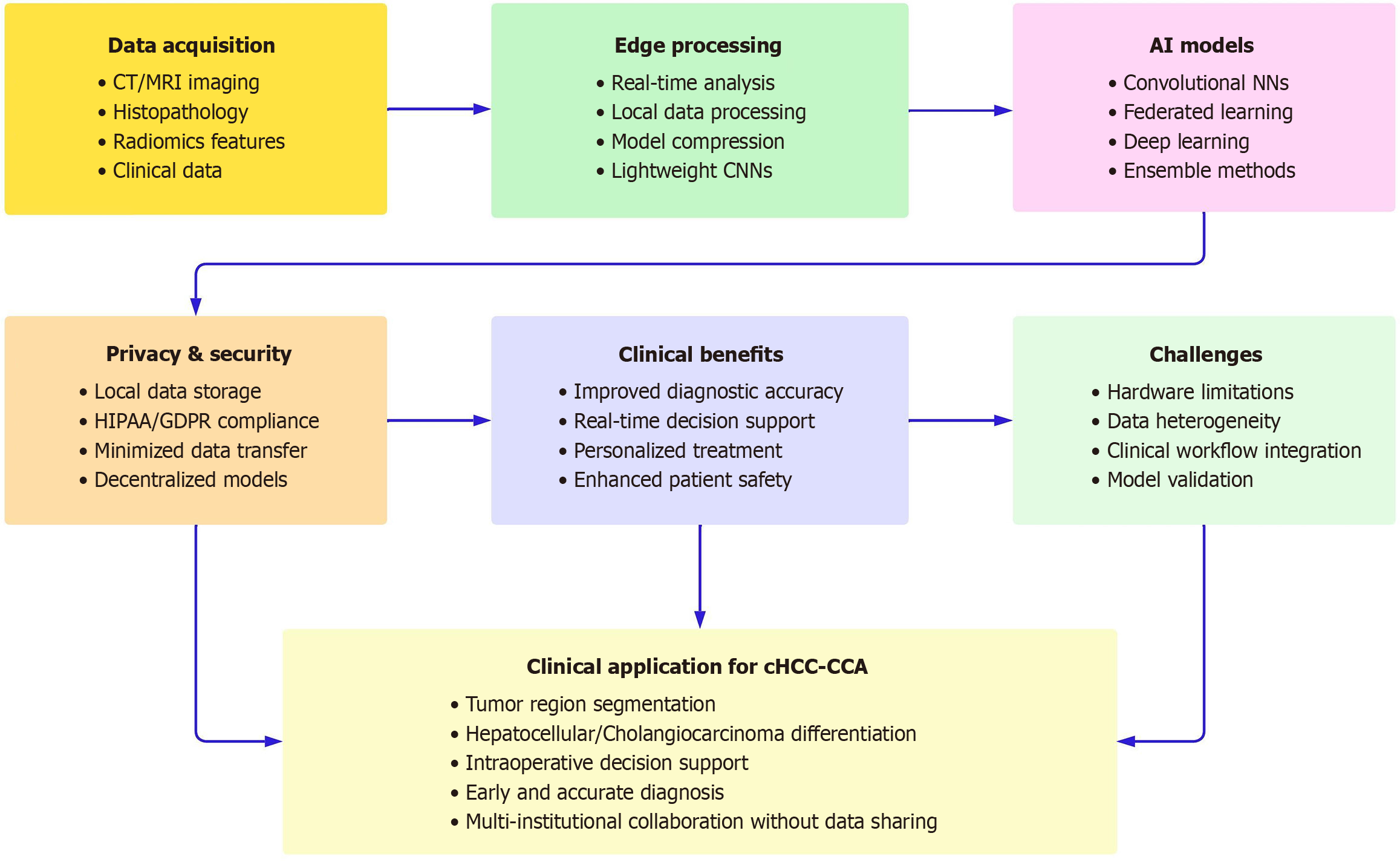
Overall, the use of model compression, lightweight CNN architectures and federated learning frameworks jointly allows for efficient, private and accurate deployment of deep learning models for edge computing in medical studies[27-29]. Neural network and conceptual framework diagram related to the use of edge learning applications in cHCC-CCA are shown in Figures 1 and 2, respectively.
Real time processing, enhanced privacy and reduce bandwidth are the advantages edge learning provides to predict and classify cHCC-CCA. However, it has several drawbacks to be fully realized in clinical practice. Hardware limitations, data heterogeneity, and integration into clinical workflow challenges are applicable for these studies. Future research should validate edge-learning trends on real large-scale clinical trials and attempt to incorporate multimodal data for making a more precise diagnosis.
Due to the limiting computational power of edge devices, not all types of deep learning models are suitable for deployment in edge devices. Pruning, quantization, and knowledge distillation are some of these methods that can help reduce the model size as well as decrease the computational requirements and thereby making it more suited for deployments in edge[30].
The performance of the model is highly dependent on a variety of sources of variability in imaging protocols and patient demographics across different institutions. To facilitate these issues, standardization efforts and sufficiently file thin federated learning frameworks are needed. By learning using multiple institutions in federated learning, models are trained over different datasets without sharing the sensitive data, while keeping the model generalization improved due to diversity of datasets[31].
An edge learning system’s implementation hinges on close collaboration between clinicians, data scientists, and engineers to ensure these systems work with existing diagnostic processes. Ensemble learning models based on edge computing, such as ensemble extreme learning machines, have been shown to improve diagnostic accuracy in various medical conditions and so are potentially admissible to clinical workflow[32].
Edge learning offers significant advantages for the prediction and classification of cHCC-CCA by enabling real-time processing, enhancing data privacy, and optimizing bandwidth. However, several challenges must be addressed to fully realize its potential in clinical practice. These challenges include hardware limitations, data heterogeneity, and integration into clinical workflows. Future research should focus on validating edge-learning approaches in large-scale clinical trials and exploring the integration of multimodal data to enhance diagnostic accuracy. However, the benefits of edge learning can be realized only by addressing the challenges of hardware limitations, data heterogeneity as well as workflow integration. Since these approaches need to be validated in large scale clinical trials, future research should focus on validating these techniques in large scale clinical trials and if possible, integrating the multimodal data including radiological images, histopathological data, and so on for more accurate diagnosis[5,33,34]. Centralized artificial in
These results will encourage put in place large-scale clinical trials and development of integration of multimodal data (radiological, histopathological, and genomic) to improve diagnostic accuracy in the future. Finally, case studies from across the different specialties would help to illustrate the applicability of edge learning in other domains in healthcare. Even though edge learning has great potential, more clinical trials that are large scale in number should be carried out to compare edge models with the conventional artificial intelligence study to prove that edge models are clearly superior in terms of diagnostic accuracy.
Consequently, edge-learning/computing has great potential to improve the prediction and classification of cHCC-CCA. Edge learning allows real-time analysis, protects patient data, and has the potential to reduce network demands enabling devices to overcome many of the challenges associated with the diagnosis and management of a complex liver ma
| 1. | Li Q, Ding C, Cao M, Yang F, Yan X, He S, Cao M, Zhang S, Teng Y, Tan N, Wang J, Xia C, Chen W. Global epidemiology of liver cancer 2022: An emphasis on geographic disparities. Chin Med J (Engl). 2024;137:2334-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 2. | Almohammadi NH. Liver cancer in Saudi Arabia: A registry-based nationwide descriptive epidemiological and survival analysis. Cancer Epidemiol. 2025;94:102731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Cutolo C, Dell'Aversana F, Fusco R, Grazzini G, Chiti G, Simonetti I, Bruno F, Palumbo P, Pierpaoli L, Valeri T, Izzo F, Giovagnoni A, Grassi R, Miele V, Barile A, Granata V. Combined Hepatocellular-Cholangiocarcinoma: What the Multidisciplinary Team Should Know. Diagnostics (Basel). 2022;12:890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Choi JH, Ro JY. Combined Hepatocellular-Cholangiocarcinoma: An Update on Pathology and Diagnostic Approach. Biomedicines. 2022;10:1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 5. | Calderaro J, Ghaffari Laleh N, Zeng Q, Maille P, Favre L, Pujals A, Klein C, Bazille C, Heij LR, Uguen A, Luedde T, Di Tommaso L, Beaufrère A, Chatain A, Gastineau D, Nguyen CT, Nguyen-Canh H, Thi KN, Gnemmi V, Graham RP, Charlotte F, Wendum D, Vij M, Allende DS, Aucejo F, Diaz A, Rivière B, Herrero A, Evert K, Calvisi DF, Augustin J, Leow WQ, Leung HHW, Boleslawski E, Rela M, François A, Cha AW, Forner A, Reig M, Allaire M, Scatton O, Chatelain D, Boulagnon-Rombi C, Sturm N, Menahem B, Frouin E, Tougeron D, Tournigand C, Kempf E, Kim H, Ningarhari M, Michalak-Provost S, Gopal P, Brustia R, Vibert E, Schulze K, Rüther DF, Weidemann SA, Rhaiem R, Pawlotsky JM, Zhang X, Luciani A, Mulé S, Laurent A, Amaddeo G, Regnault H, De Martin E, Sempoux C, Navale P, Westerhoff M, Lo RC, Bednarsch J, Gouw A, Guettier C, Lequoy M, Harada K, Sripongpun P, Wetwittayaklang P, Loménie N, Tantipisit J, Kaewdech A, Shen J, Paradis V, Caruso S, Kather JN. Deep learning-based phenotyping reclassifies combined hepatocellular-cholangiocarcinoma. Nat Commun. 2023;14:8290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Vij M, Veerankutty FH, Rammohan A, Rela M. Combined hepatocellular cholangiocarcinoma: A clinicopathological update. World J Hepatol. 2024;16:766-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Ye L, Schneider JS, Ben Khaled N, Schirmacher P, Seifert C, Frey L, He Y, Geier A, De Toni EN, Zhang C, Reiter FP. Combined Hepatocellular-Cholangiocarcinoma: Biology, Diagnosis, and Management. Liver Cancer. 2024;13:6-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Wang J, Wang S, Zhang Y. Deep learning on medical image analysis. CAAI Trans Intell Technol. 2025;10:1-35. [DOI] [Full Text] |
| 9. | Sheller MJ, Edwards B, Reina GA, Martin J, Pati S, Kotrotsou A, Milchenko M, Xu W, Marcus D, Colen RR, Bakas S. Federated learning in medicine: facilitating multi-institutional collaborations without sharing patient data. Sci Rep. 2020;10:12598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 10. | Lee D, Yoon SN. Application of Artificial Intelligence-Based Technologies in the Healthcare Industry: Opportunities and Challenges. Int J Environ Res Public Health. 2021;18:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 11. | Awad AI, Fouda MM, Khashaba MM, Mohamed ER, Hosny KM. Utilization of mobile edge computing on the Internet of Medical Things: A survey. ICT Express. 2023;9:473-485. [DOI] [Full Text] |
| 12. | Yang Y, Wang X, Ning Z, Rodrigues JJPC, Jiang X, Guo Y. Edge Learning for Internet of Medical Things and Its COVID-19 Applications: A Distributed 3C Framework. IEEE Internet Things M. 2021;4:18-23. [DOI] [Full Text] |
| 13. | Hamm CA, Wang CJ, Savic LJ, Ferrante M, Schobert I, Schlachter T, Lin M, Duncan JS, Weinreb JC, Chapiro J, Letzen B. Deep learning for liver tumor diagnosis part I: development of a convolutional neural network classifier for multi-phasic MRI. Eur Radiol. 2019;29:3338-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 14. | Yasaka K, Akai H, Abe O, Kiryu S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study. Radiology. 2018;286:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 423] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 15. | Liang F, Yu W, Liu X, Griffith D, Golmie N. Towards Edge-Based Deep Learning in Industrial Internet of Things. IEEE Internet Things J. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Guo Y, Liu F, Cai Z, Chen L, Xiao N. FEEL: A Federated Edge Learning System for Efficient and Privacy-Preserving Mobile Healthcare. ICPP’20: 49th International Conference on Parallel Processing; 2020 Aug 17-20; Edmonton, Canada. New York: Association for Computing Machinery, 2020: 1-11. [DOI] [Full Text] |
| 17. | Abreha HG, Hayajneh M, Serhani MA. Federated Learning in Edge Computing: A Systematic Survey. Sensors (Basel). 2022;22:450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Jouini O, Sethom K, Namoun A, Aljohani N, Alanazi MH, Alanazi MN. A Survey of Machine Learning in Edge Computing: Techniques, Frameworks, Applications, Issues, and Research Directions. Technologies. 2024;12:81. [DOI] [Full Text] |
| 19. | Liu Y, Xue J, Li D, Zhang W, Chiew TK, Xu Z. Image recognition based on lightweight convolutional neural network: Recent advances. Image Vision Comput. 2024;146:105037. [DOI] [Full Text] |
| 20. | Ghosh A, Grolinger K. Edge-Cloud Computing for IoT Data Analytics: Embedding Intelligence in the Edge with Deep Learning. IEEE Trans Ind Inf. 2020;. [DOI] [Full Text] |
| 21. | Erasto Muwanga K, Muwanguzi E. End User Security using Smart Devices with Ability to Access IoT services. IJISRT. 2024;. [DOI] [Full Text] |
| 22. | Qian Y, Lu Q, Rodriguez I, Vácha M, Min X, Ümütlü MR, Castrillon GA, Schreyer A, Haimerl M, Schoenberg SO, Ebert MP, Vellala A, Alaffita CR, Mora JAG, Guo Z, Hesser J, Weiss C, Froelich M, Sun Y, Teufel A. MRI imaging and machine learning based radiomics for detection of mixed HCC and CCA tumors: Still a need for liver biopsy? J Clin Oncol. 2025;43:531-531. [DOI] [Full Text] |
| 23. | Murugesan K, Sharaf R, Montesion M, Moore JA, Pao J, Pavlick DC, Frampton GM, Upadhyay VA, Alexander BM, Miller VA, Javle MM, Bekaii Saab TS, Albacker LA, Ross JS, Ali SM. Genomic Profiling of Combined Hepatocellular Cholangiocarcinoma Reveals Genomics Similar to Either Hepatocellular Carcinoma or Cholangiocarcinoma. JCO Precis Oncol. 2021;5:PO.20.00397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Liu X, Khalvati F, Namdar K, Fischer S, Lewis S, Taouli B, Haider MA, Jhaveri KS. Can machine learning radiomics provide pre-operative differentiation of combined hepatocellular cholangiocarcinoma from hepatocellular carcinoma and cholangiocarcinoma to inform optimal treatment planning? Eur Radiol. 2021;31:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Wang R, Lai J, Zhang Z, Li X, Vijayakumar P, Karuppiah M. Privacy-Preserving Federated Learning for Internet of Medical Things Under Edge Computing. IEEE J Biomed Health Inform. 2023;27:854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Lian Z, Yang Q, Wang W, Zeng Q, Alazab M, Zhao H, Su C. DEEP-FEL: Decentralized, Efficient and Privacy-Enhanced Federated Edge Learning for Healthcare Cyber Physical Systems. IEEE Trans Netw Sci Eng. 2022;9:3558-3569. [DOI] [Full Text] |
| 27. | Kaissis G, Ziller A, Passerat-palmbach J, Ryffel T, Usynin D, Trask A, Lima I, Mancuso J, Jungmann F, Steinborn M, Saleh A, Makowski M, Rueckert D, Braren R. End-to-end privacy preserving deep learning on multi-institutional medical imaging. Nat Mach Intell. 2021;3:473-484. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 28. | Yang J, Jiao L, Shang R, Liu X, Li R, Xu L. EPT-Net: Edge Perception Transformer for 3D Medical Image Segmentation. IEEE Trans Med Imaging. 2023;42:3229-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 29. | Larrakoetxea NG, Astobiza JE, López IP, Urquijo BS, Barruetabeńa JG, Rego AZ. Efficient Machine Learning on Edge Computing Through Data Compression Techniques. IEEE Access. 2023;11:31676-31685. [DOI] [Full Text] |
| 30. | Wang X, Jia W. Optimizing Edge AI: A Comprehensive Survey on Data, Model, and System Strategies. Available from: arXiv:2501.03265. [DOI] [Full Text] |
| 31. | Ganesh D, Ramanaiah O. Edge Federated Learning for Smart HealthCare Systems: Applications and Challenges. 2024 4th International Conference on Sustainable Expert Systems (ICSES); 2024 Oct 15-17; Kaski, Nepal. [DOI] [Full Text] |
| 32. | Vincent ACSR, Sengan S. Edge computing-based ensemble learning model for health care decision systems. Sci Rep. 2024;14:26997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Zhou Z, Chen X, Li E, Zeng L, Luo K, Zhang J. Edge Intelligence: Paving the Last Mile of Artificial Intelligence With Edge Computing. Proc IEEE. 2019;107:1738-1762. [DOI] [Full Text] |
| 34. | Kaissis GA, Makowski MR, Rückert D, Braren RF. Secure, privacy-preserving and federated machine learning in medical imaging. Nat Mach Intell. 2020;2:305-311. [RCA] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/