Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.107109
Revised: April 27, 2025
Accepted: June 11, 2025
Published online: July 24, 2025
Processing time: 128 Days and 5.2 Hours
Although thymopoietin (TMPO) has been elucidated to be overexpressed in cancers, its underlying mechanisms are not yet fully understood.
To investigate the expression and clinical significance of TMPO in papillary thyroid carcinoma (PTC).
Databases such as Gene Expression Omnibus, The Cancer Genome Atlas Pro
The TMPO protein was significantly overexpressed in PTC tissues, primarily localized in the cytoplasm and nuclear membrane. The mRNA level analysis showed mild overexpression of TMPO in PTC tissues, with a certain discriminatory value (area under the curve = 0.66). TMPO may promote cancer through involvement in cell adhesion, focal adhesion, leukocyte migration, and multiple cancer-related signaling pathways. Additionally, CRISPR gene knockout experiments confirmed that TMPO knockout significantly inhibited the proliferation of PTC cell lines, indicating its important role in tumor growth.
TMPO is overexpressed in PTC and may serve as a therapeutic target and molecular biomarker for PTC.
Core Tip: This study provides the first comprehensive analysis of thymopoietin (TMPO) in papillary thyroid cancer using global high-throughput sequencing datasets and multilevel analyses. This reveals that TMPO is significantly overexpressed in papillary thyroid cancer cells, with potential roles in promoting tumor proliferation, invasiveness, and immune interactions. Its inhibition via clustered regularly interspaced short palindromic repeats knockout significantly reduces cell proliferation, highlighting its potential as a therapeutic target.
- Citation: Song C, Pang YY, Lu SY, Li B, Li DM, He RQ, Qin DY, Li SD, Qv N, Chen YM, Chen G, He J, Jiang XB. Molecular mechanisms of thymopoietin in papillary thyroid cancer: Multiplatform gene expression data, gene knockout screening, and in-house immunohistochemistry. World J Clin Oncol 2025; 16(7): 107109
- URL: https://www.wjgnet.com/2218-4333/full/v16/i7/107109.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i7.107109
Thyroid cancer is the seventh most common tumor globally, with an incidence rate of three times higher in females than in males[1]. Papillary thyroid carcinoma (PTC) is the primary type of thyroid cancer, and its incidence has been increasing worldwide in recent years[2]. While this upward trend can partly be attributed to enhanced detection and diagnosis of small, indolent tumors[3], the significant increase in the incidence of advanced PTC and PTCs with diameters exceeding 5 centimeters has led to a rise in PTC mortality, reflecting a genuine increase in its incidence[4]. For most patients with advanced PTC, the combination of surgery, radioactive iodine ablation, and thyroid-stimulating hormone suppression therapy is highly effective. However, a small proportion of patients may progress to a state resistant to radioactive iodine ablation, which complicates treatment and poses a serious threat to patient survival[5]. Therefore, early screening and precise diagnosis are crucial for reducing the incidence of PTC and improving patient outcomes.
Recent studies have delved into the mechanisms of PTC, highlighting the essential roles of the tumor microenvironment, gene mutations, and signaling pathway abnormalities in tumor progression. Thymopoietin (TMPO), also known as lamina-associated polypeptide 2 (LAP2), is located on chromosome 12q22 and contains eight exons[6]. Through alternative splicing, this gene can encode six different TMPO protein isoforms (α, β, γ, δ, ε, and ζ), which share a common N-terminal domain[7]. Existing research indicates that TMPO-α can bind to cell cycle regulators and tumor suppressor protein pRb, affecting pRb localization and modulating tumor-suppressive activity[8-10]. However, there has been no specific study on the clinical value of TMPO in PTC.
Against this backdrop, this study aimed to analyze the differential expression and prognostic significance of TMPO in PTC through data mining of high-throughput databases from multiple global platforms and immunohistochemistry (IHC) on gene chips. Enrichment analysis was conducted to preliminarily explore the mechanisms of TMPO in the development and progression of PTC. The results suggest that TMPO is indispensable in the PTC process and has the potential to become a therapeutic target. Finally, clustered regularly interspaced short palindromic repeats (CRISPR) knockout screening and the Cancer Cell Line Encyclopedia (CCLE) database were utilized to further investigate the differential expression of the TMPO gene in cell lines and its potential impact on PTC cells. This study aimed to provide an effective molecular biomarker for the early diagnosis of PTC and to offer new strategies and targets for the clinical treatment of PTC.
High-throughput databases, such as Expression Omnibus and The Cancer Genome Atlas Program-Genotype-Tissue Expression, were screened to obtain PTC RNA-seq datasets from multiple platforms globally. Inclusion criteria were: (1) Human tissues rather than cell lines; (2) Samples containing both cancer and adjacent or normal thyroid tissues as controls, with the number of samples ≥ 3; (3) Complete expression data and clinical information; (4) Patients had not received any adjuvant treatments, such as chemotherapy or radiotherapy before surgery; and (5) Data types were miRNA or mRNA expression profiles. The datasets acquired were converted separately using log2 scaling and combined to remove batch effects. The relative expression values were transformed to a log2(x+1) scale and analyzed using the “limma-voom” package.
The TMPO protein expression status was explored based on The Human Protein Atlas (THPA) database. A 12-point staining scoring method was used, with staining intensity rated from 0 to 3, representing no staining, mild staining, moderate staining, and strong staining, respectively. The percentage of positive cells was scored as follows: Scores 0 to 4, representing < 5%, 5%-25%, 26%-50%, 51%-75%, and > 75% of positive cells, respectively. The protein expression level of TMPO was represented by the staining intensity score and the percentage of positive cell scores. The final IHC score was calculated by multiplying the percentage of positive tumor cells and the staining intensity.
In this study, we included tissue samples from 190 patients. Samples from 9 PTC patients confirmed at The First Affiliated Hospital of Guangxi Medical University were selected, with PTC tissues as the experimental group and corresponding peritumoral tissues as the control group. Additionally, 4 tissue microarrays (THC481, THC961, THC1021, and THC1501) were purchased from CHUN Fénomène Co., Ltd., Guilin, China, which contained 45 noncancerous thyroid tissue samples and 136 PTC tissue samples. The study was approved by The Ethics Committee of The First Affiliated Hospital of Guangxi Medical University, and all patients signed an informed consent form. IHC was used to detect the protein expression of TMPO in PTC tissues. During the experiment, antigen retrieval was performed using an ethylenediaminetetraacetic acid buffer, and a polyclonal antibody to LAP2 (an alias for TMPO) was used as the primary antibody, provided by Invitrogen, United States, at a dilution ratio of 1:10000 and incubated at 37 °C for 70 minutes. The secondary antibody was purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. (Product No. PV-6000) and incubated at room temperature for 25 minutes; the 3,3'-Diaminobenzidine chromogen, also from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., was used for 5 minutes.
This study employed a rigorous double-blind evaluation protocol in which two board-certified pathologists with over 10 years of diagnostic experience independently assessed the IHC results. For each tissue specimen, 10 random fields of view were selected, and the number of positive cells was quantified in 100 cells per field at the highest available magnification, using appropriate analytical tools. The scoring system utilized a 12-point scale, with the final score calculated as the product of the percentage of positive cells and the staining intensity. The percentage of positive cells was graded on a 0–4 scale: (1) 0: < 5%; (2) 1: 5%–25%; (3) 2: 26%–50%; (4) 3: 51%–75%; and (5) 4: > 75%. Staining intensity was scored from 0 to 3: (1) 0: No staining; (2) 1: Pale yellow; (3) 2: Light brown; and (4) 3: Dark brown[11-12]. Among the 190 evaluated specimens, initial scoring demonstrated complete concordance in 186 cases, while minor discrepancies (≤ 2-point difference) were observed in four cases. All discordant cases were resolved through a consensus review using a multiheaded microscope. The interrater reliability analysis yielded an intraclass correlation coefficient of 0.994, indicating excellent interobserver agreement according to the established criteria.
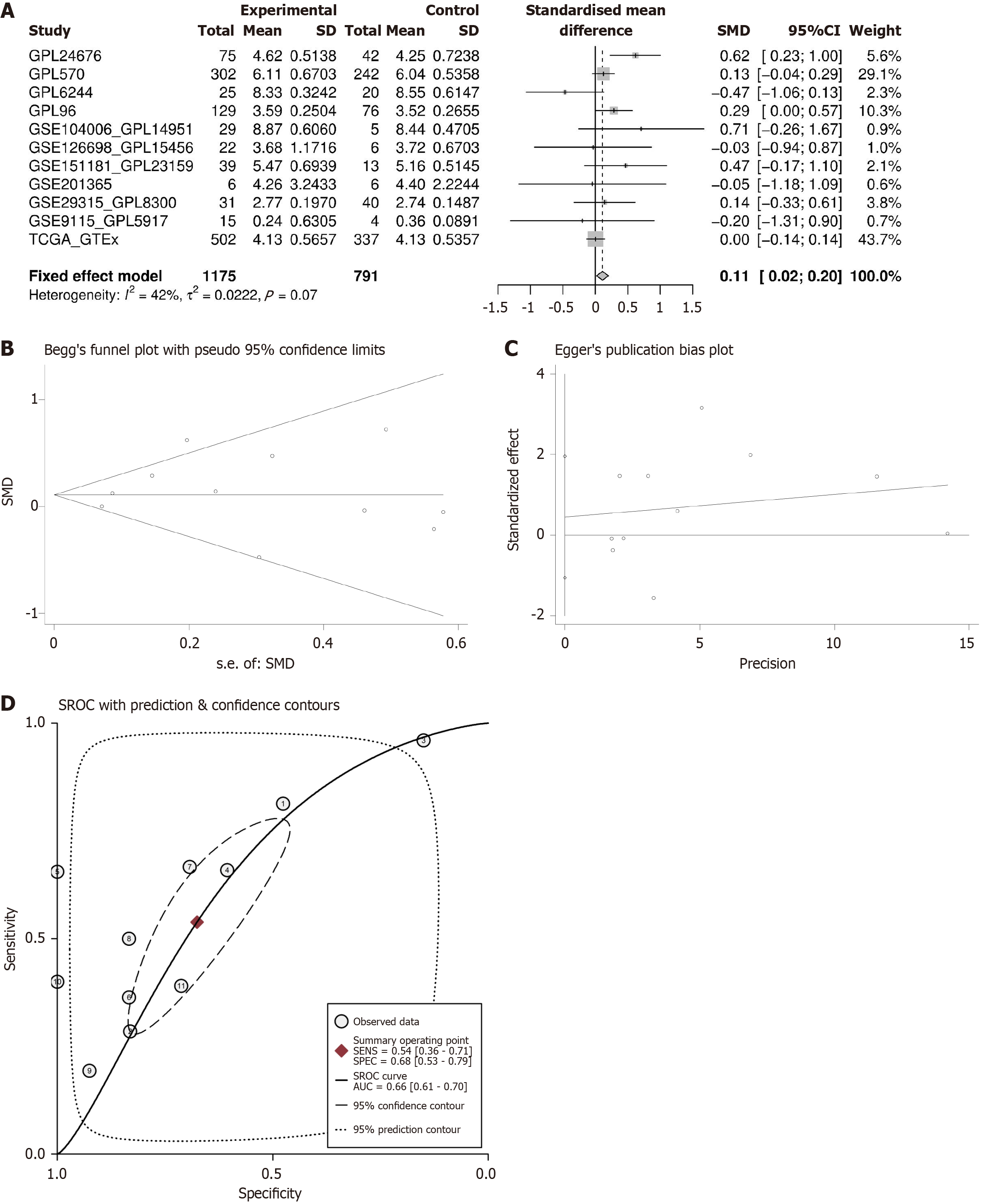
The standardized global mRNA expression matrix, after batch effect removal, was used to merge the standardized mean difference (SMD) of TMPO expression levels based on Stata v12.0 software. Sensitivity and specificity were calculated. The SMD values of 0.10–0.34, 0.35–0.64, 0.65–1.19, and ≥ 1.20 indicate low, moderate, high, and significant differences, respectively. A summary receiver operating characteristic (SROC) curve was plotted, and the area under the curve (AUC) value reflected the discriminatory capacity of TMPO in PTC. AUC values of 0.50–0.70 indicate low discriminatory capacity, 0.71–0.90 indicate moderate discriminatory capacity, and > 0.90 indicate high discriminatory capacity. Bias was evaluated through Begg and Egger tests, which determined the probability of the relationship between the standardized effect size and the variance and error.
Genes co-expressed with TMPO and positively correlated with it were screened. The samples were divided into high and low groups based on the median expression of TMPO. The “clusterProfiler” package was used to conduct Gene Set Enrichment Analysis to explore the Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways associated with TMPO.
The Search Tool for the Retrieval of Interacting Genes (STRING) database was used to analyze the functional interactions between TMPO-related proteins and to construct a protein–protein interaction (PPI) network to observe the correlations and connections between proteins. STRING was set to default settings. The cytohubba plugin was used to adjust the PPI network and identify the hub genes.
To further validate the expression of TMPO, we obtained the mRNA expression matrix of PTC cell lines from the CCLE database. mRNA expression analysis was performed using R (v4.0.3) and ggplot2 (v3.3.3), and the results were presented using a horizontal bar chart. To explicitly determine the effect of TMPO genes on the growth of PTC cells, the TMPO gene in PTC cell lines was knocked out using CRISPR technology, and the TMPO gene effect score was calculated using the CERES algorithm to evaluate the knockout effect of the TMPO gene in PTC cell lines. A negative score indicates inhibition of PTC cell growth after knockout of the TMPO gene.
Statistical analysis was conducted using R v4.2 and Stata v15.0 statistical software. The Wilcoxon signed-rank test was used to analyze the differences in TMPO expression in thyroid tissues globally. If the I² value was greater than 50% and the P value was less than 0.05, the SMD was calculated using a random-effects model; otherwise, a fixed-effects model was used. A P value < 0.05 was considered statistically significant.
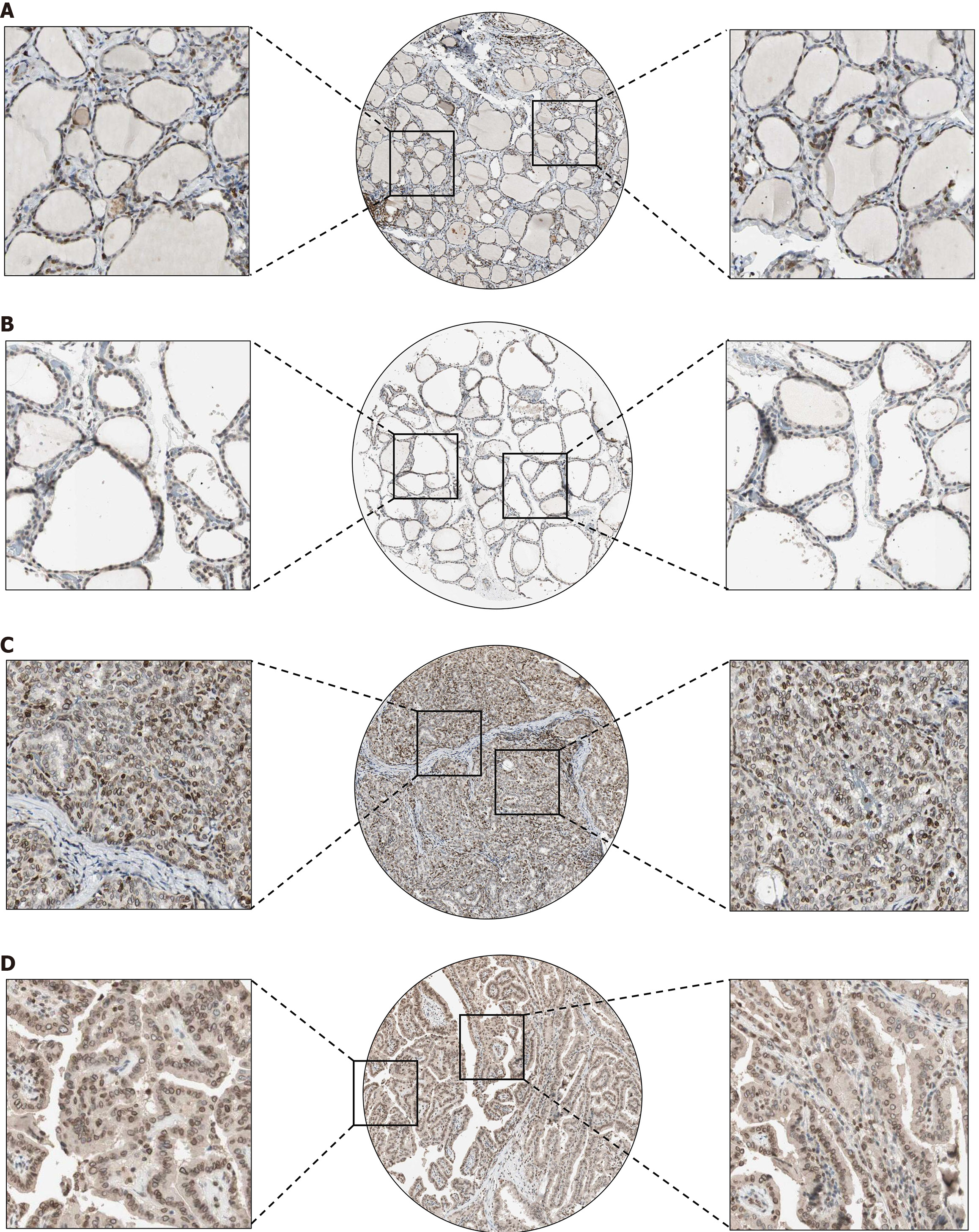
To verify the expression status of TMPO in PTC tissues at the protein level, this study conducted an in-depth analysis of its protein expression based on THPA. As shown in Figure 1, TMPO protein is expressed at low levels in normal thyroid cells, while its expression is significantly increased in PTC cells (P < 0.05). The differentially expressed positive staining signals are localized in the cytoplasm and nuclear membrane of PTC cells, with moderate expression intensity and staining density.
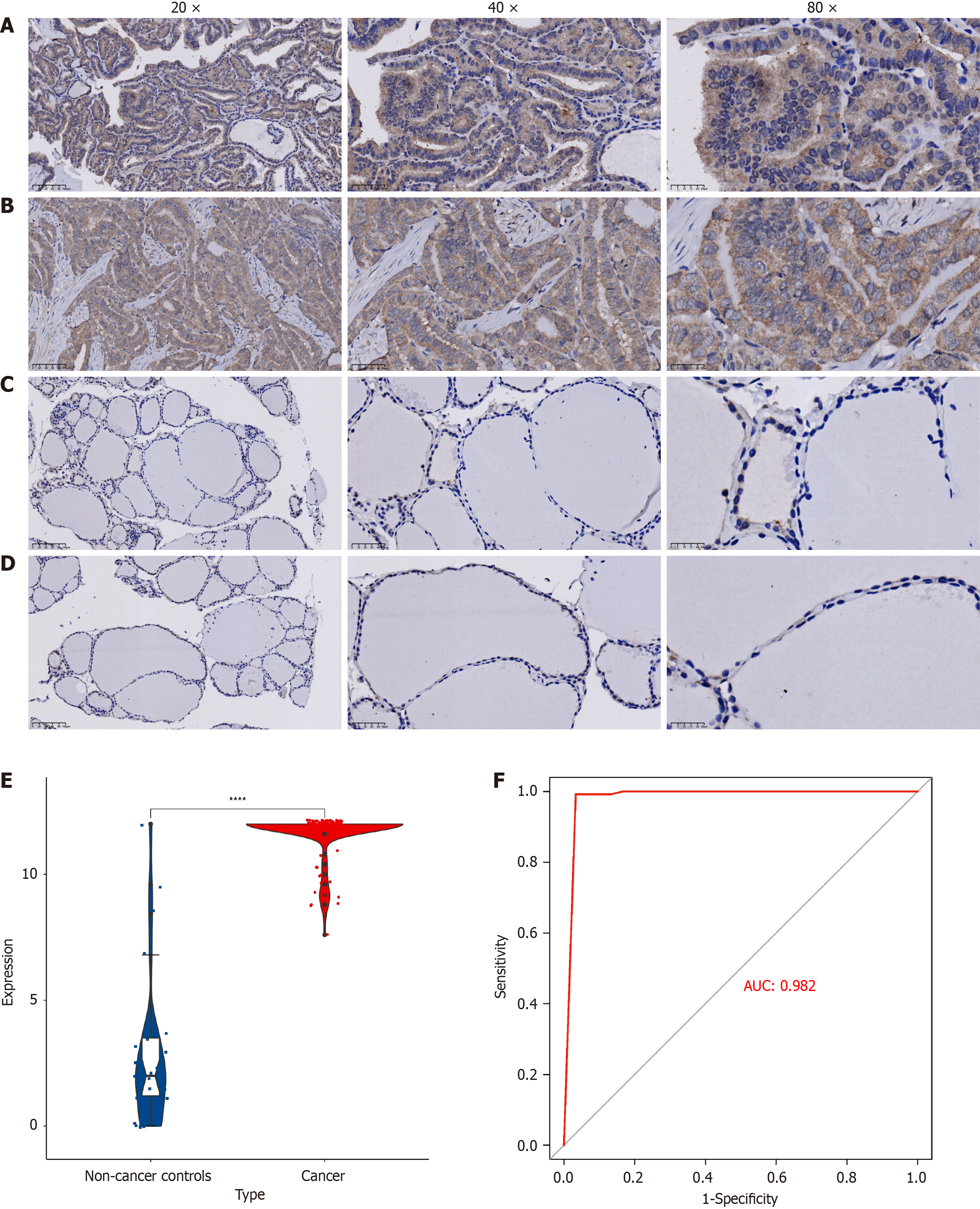
IHC results show that TMPO has strong staining intensity in PTC tissues, mainly concentrated in the nucleus, nuclear membrane, and cytoplasm (Figure 2A and B). In noncancerous thyroid tissues, the staining intensity of TMPO is either negative or weak (Figure 1C and D). Based on the IHC scoring, a violin plot was drawn (Figure 2E), which indicates that the expression level of TMPO in the PTC group is significantly higher than in the noncancerous thyroid group (P < 0.0001), and the IHC scoring of TMPO protein has strong discriminatory power between PTC tissues and noncancerous thyroid tissues (AUC = 0.982) (Figure 2A and B).
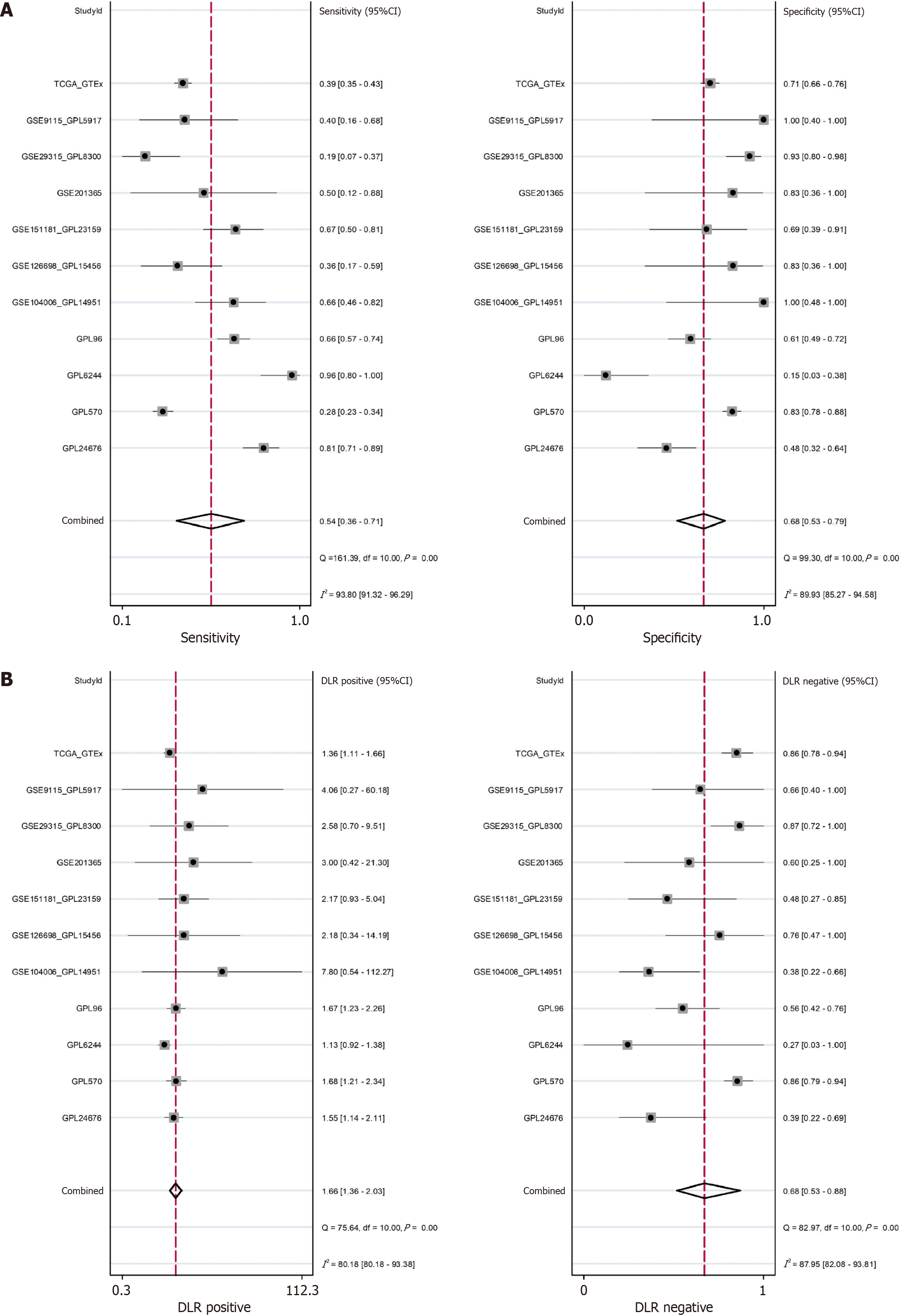
This study conducted a comprehensive analysis of 11 gene chip and high-throughput sequencing datasets from multiple centers globally, comparing the expression levels of the TMPO gene in 1175 PTC tissues and 791 healthy thyroid tissues. As shown in Figure 3A, TMPO exhibited mild overexpression in PTC tissues, with a SMD of 0.11 (95%CI: 0.02-0.20) and a P value of 0.07. Following Begg and Egger bias tests, no significant publication bias was detected (Figure 3B and C). Additionally, TMPO shows a certain discriminatory capacity for PTC. The SROC curve and sensitivity and specificity analysis results indicated an AUC of 0.66 (95%CI: 0.61–0.70), further validating its potential to distinguish thyroid cancer (Figure 3D). The sensitivity of TMPO is 0.54 (95%CI: 0.36-0.71), the specificity is 0.68 (95%CI: 0.53-0.79), the positive likelihood ratio is 1.66 (95%CI: 1.36-2.03), and the negative likelihood ratio is 0.68 (95%CI: 0.53-0.88) (Figure 4A and B).
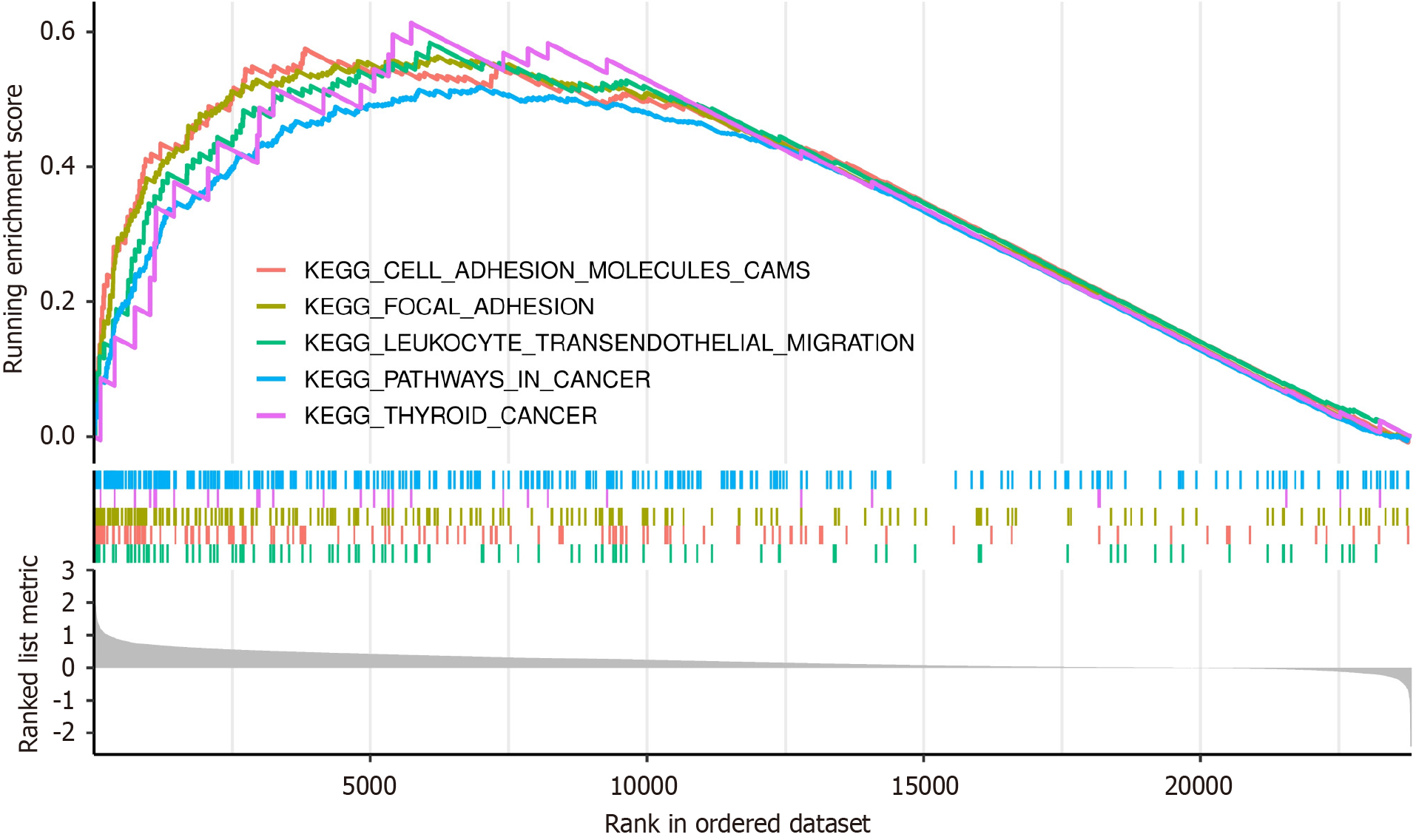
The mechanism by which TMPO exerts its procarcinogenic effects can be partially explained by its associated signaling pathways. This study collected TMPO-related genes and conducted gene enrichment and pathway analysis, revealing their involvement in several biological processes, such as cell adhesion, focal adhesion (a protein assembly involved in signal transduction), leukocyte transendothelial migration, and cancer-related signaling pathways (including cell proliferation, apoptosis, and metastasis). TMPO may promote cancer by participating in these related signaling pathways (Figure 5).
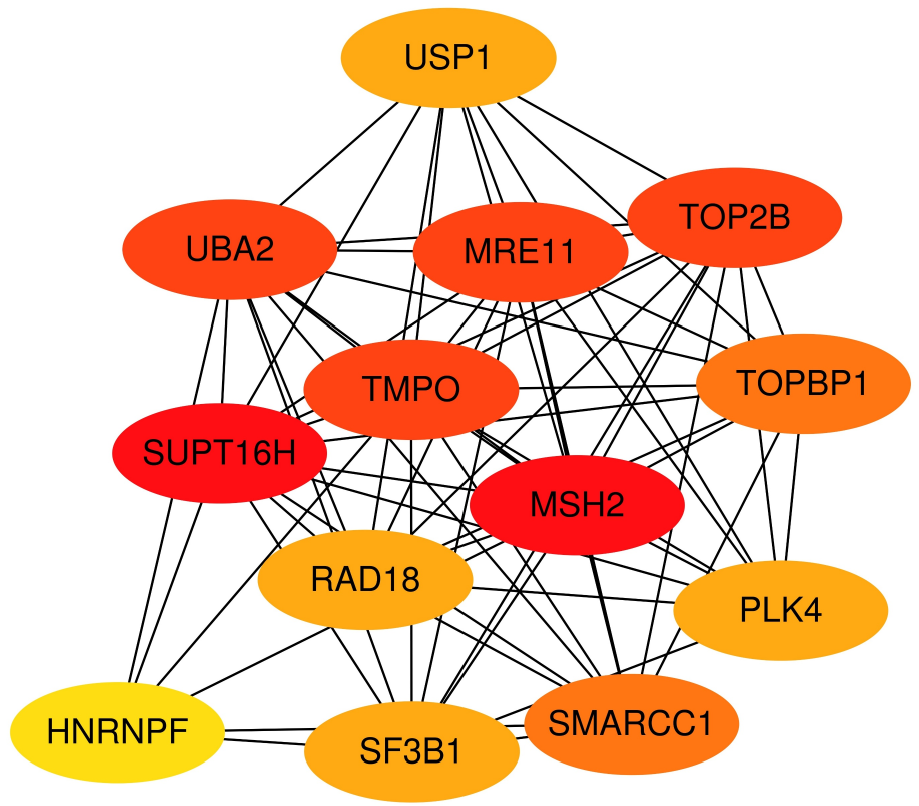
PPI network analysis of the differentially co-expressed genes was performed using the STRING database. Subsequently, the research team employed the igraph package to analyze the network topology, with a particular focus on the interaction relationships between the TMPO gene and pathway-related genes. Through systematic analysis of connection strength and topological characteristics among network nodes, the study identified five genes—UBA2, MRE11, SUPT16H, TOP2B, and MSH2—that exhibit direct and robust interactions with TMPO (Figure 6).
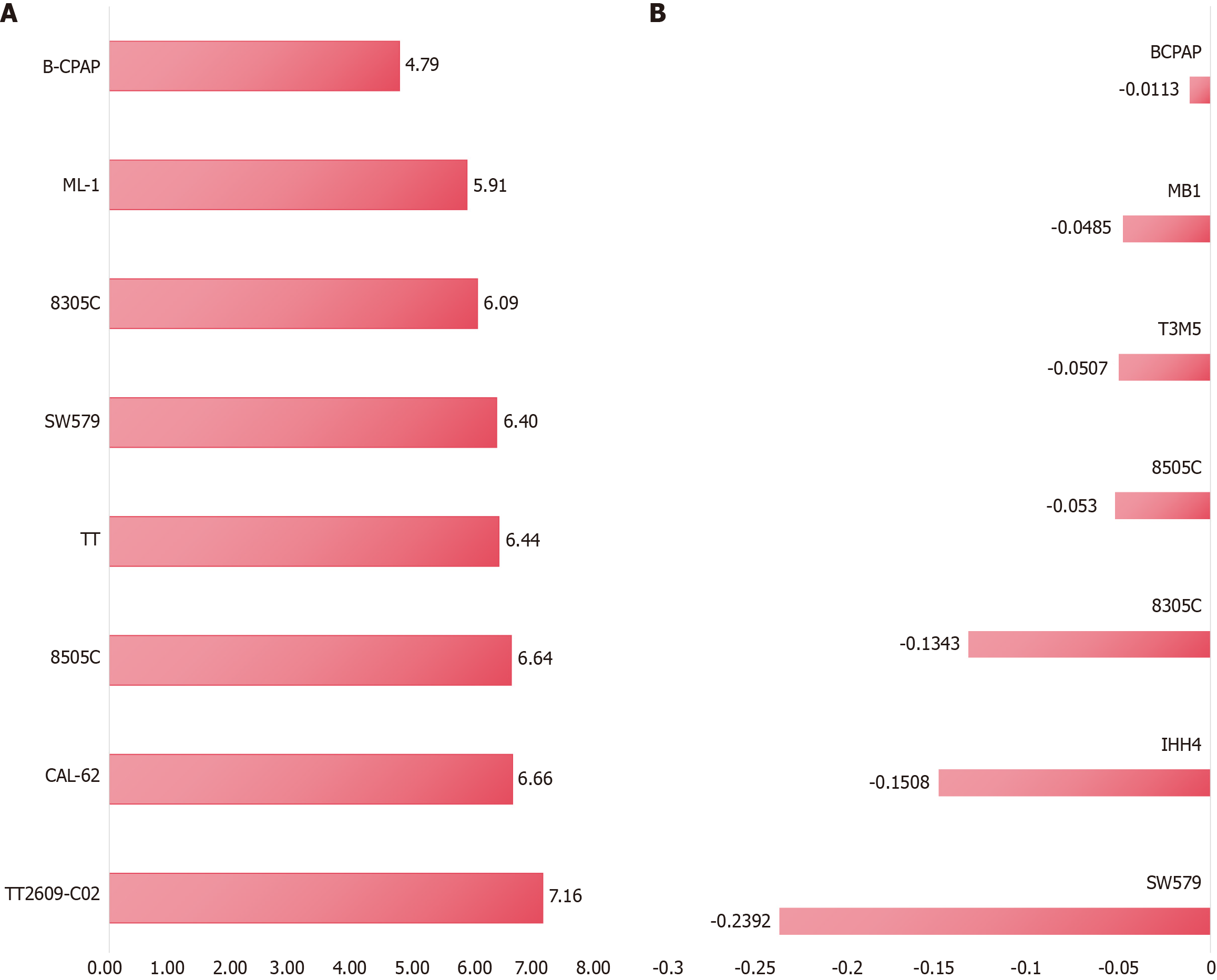
Using the CCLE dataset, this study evaluated the expression of TMPO in different PTC cell lines, which may aid in selecting appropriate cell lines for subsequent analysis or validation. As shown in Figure 7A, TMPO showed the highest expression in the B-CPAP, ML-1, 8305C, SW579, TT, 8505C, CAL-62, and TT2609-C02 cells. Furthermore, Crispr knockout screen technology was employed to assess the impact of TMPO on PTC cell proliferation. The gene effect scores for TMPO in seven PTC cell lines indicated that the growth of these cell lines was inhibited after knocking out the TMPO gene (Figure 7B).
Although the early detection rate of thyroid cancer has improved with the advancement of imaging technology and the widespread use of screening methods, the incidence of late-stage cases and large tumors has increased simultaneously. With the progress in sequencing technology and molecular targeted therapy, biomarkers are gradually becoming a key area of development in clinical diagnosis. TMPO, a protein that plays a crucial role in cell cycle regulation, may have significant implications for the development and progression of tumors. Existing studies have shown that TMPO exhibits different effects in various types of cancer. In glioblastoma, TMPO is significantly upregulated, and its knockdown promotes cell apoptosis[13]. In gastric cancer, high expression of TMPO-α is associated with cell invasion and is considered a prognostic marker[14]. In lung cancer, the overexpression of TMPO plays a key role in cancer cell movement, while TMPO-β is upregulated in pancreatic cancer, and its knockdown inhibits cell proliferation and migration[15,16]. In multiple myeloma, abnormally high expression of TMPO is associated with poor survival rates[17]. Additionally, in colorectal cancer, TMPO expression increases and affects NANOG expression through c-fos proto-oncogene phosphorylation, thereby promoting drug resistance[18]. Overall, TMPO has diverse functions in different tumors and may become an effective indicator for prognosis assessment, but its clinical value in PTC remains underexplored.
This study integrates global high-throughput sequencing datasets and, for the first time, explores the expression characteristics of TMPO in PTC through multilevel analysis, further investigating its potential mechanisms and clinical significance in PTC. At the protein level, the TMPO protein is significantly overexpressed in PTC cells, with positive staining signals primarily localized in the cytoplasm and the nuclear membrane. This suggests that it may not only participate in the stability of the cytoskeleton and nuclear-cytoplasmic material exchange but may also be involved in regulating gene expression and cell signaling, such as cell adhesion and cell–cell communication, thereby enhancing the invasiveness and metastatic potential of cancer cells. In pathway enrichment analysis, the association of TMPO with pathways such as leukocyte transendothelial migration further suggests that TMPO may play a crucial role in the interaction between tumors and the immune system. Furthermore, this study analyzed hub genes through PPI protein interaction networks and identified UBA2, MRE11, SUPT17H, TOP2B, and MSH2 as potential key interacting proteins of TMPO in PTC. This discovery has significant biological implications. UBA2, as a ubiquitin-like modifier activating enzyme, plays a crucial role in protein degradation and cell cycle regulation, and its interaction with TMPO may influence the proliferation and apoptosis processes in PTC cells[19-21]. MRE11 serves an essential function in DNA double-strand break repair, and its interaction with TMPO may be associated with maintaining genomic stability in PTC cells[22]. SUPT16H participates in transcriptional regulation, and its interaction with TMPO could potentially affect the gene expression profile of PTC cells[23-25]. TOP2B, as a topoisomerase, modulates DNA topological states during replication and transcription, and its interaction with TMPO may impact gene expression and cell cycle progression in PTC cells[26,27]. MSH2 is a critical protein in DNA mismatch repair, and its interaction with TMPO may be related to DNA repair capacity and genomic stability in PTC cells[28,29]. The identification of these key co-interacting proteins provides novel clues and targets for further elucidating the mechanistic role of TMPO in PTC. Future studies should validate these protein–TMPO interactions and their specific functions in PTC pathogenesis through in vitro cellular experiments and in vivo animal models, thereby offering new theoretical foundations and potential therapeutic targets for PTC diagnosis and treatment. The mRNA expression analysis of TMPO showed a mild overexpression in PTC tissues. The receiver operating characteristic curve analysis indicates that TMPO has a certain discriminatory ability in the diagnosis of PTC, with an AUC value of 0.66. These findings suggest that while TMPO demonstrates potential as a diagnostic biomarker, its moderate sensitivity (0.54) and specificity (0.68) may limit its clinical utility as a standalone test. The suboptimal diagnostic performance, as evidenced by the relatively low sensitivity and specificity values, highlights the necessity of incorporating TMPO into a multimarker panel. Such a combinatorial approach could potentially compensate for the individual limitations of each biomarker through synergistic effects, thereby improving overall diagnostic accuracy. Future studies should focus on identifying optimal biomarker combinations with TMPO to enhance both sensitivity and specificity for more reliable clinical applications. The observed discrepancy between strong TMPO protein expression (by IHC) and modest mRNA overexpression in this study can be explained from both molecular mechanism and technical perspectives. At the molecular level, TMPO may be subject to posttranscriptional regulation that enhances its mRNA translation efficiency. Additionally, TMPO protein could accumulate in the tumor microenvironment through deubiquitination or chaperone-mediated stabilization, leading to a prolonged half-life. Technically, IHC offers cell-type specificity that accurately reflects protein expression in tumor epithelial cells, whereas mRNA detection based on tissue homogenates is significantly affected by tumor heterogeneity. When TMPO is predominantly expressed in epithelial cells, low expression in stromal components (e.g., fibroblasts, immune cells) dilutes the overall signal, resulting in underestimated mRNA levels. This phenomenon is visually confirmed in Figures 1 and 2, where characteristically strong positivity is observed in tumor nests, while adjacent stromal areas remain essentially negative. These findings not only explain our experimental results but also emphasize the critical importance of considering tissue heterogeneity in tumor molecular profiling, providing methodological insights for future investigations. This study further evaluated the expression of TMPO and its impact on cell proliferation in multiple PTC cell lines using the CCLE dataset and CRISPR gene knockout technology. The results show that TMPO is highly expressed in multiple PTC cell lines and that its knockout significantly inhibits cell proliferation, indicating that TMPO plays a key role in tumor cell growth. Targeting and inhibiting TMPO may be a feasible therapeutic strategy, especially in patients with high TMPO expression.
This study elucidates the pivotal role of TMPO in PTC cells, particularly its tumor-promoting effects on proliferation. As a potential molecular target, TMPO demonstrates promising applications in the diagnosis and treatment of PTC. However, future research should not only further validate the in vivo functions of TMPO and the feasibility of its targeted inhibition strategies but also evaluate its potential variations and mechanisms of action across different genetic backgrounds or cell types. Furthermore, subsequent studies should prioritize investigating the potential value of TMPO in the prognostic assessment of PTC patients. By incorporating longer-term follow-up data, researchers should evaluate TMPO’s potential as a prognostic biomarker, thereby providing more valuable references for clinical decision-making.
This study, for the first time, comprehensively demonstrates the overexpression of TMPO in PTC tissues and its critical role in PTC cell growth based on global multicenter, multiplatform RNA-seq, gene chip data, and IHC. This suggests that TMPO may become a potential biomarker for the early diagnosis and treatment of PTC in clinical settings.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12562] [Article Influence: 6281.0] [Reference Citation Analysis (6)] |
| 2. | Bhattacharya S, Mahato RK, Singh S, Bhatti GK, Mastana SS, Bhatti JS. Advances and challenges in thyroid cancer: The interplay of genetic modulators, targeted therapies, and AI-driven approaches. Life Sci. 2023;332:122110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, Dal Maso L. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021;9:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 393] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 4. | Megwalu UC, Moon PK. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000-2018. Thyroid. 2022;32:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 198] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 5. | Pu W, Shi X, Yu P, Zhang M, Liu Z, Tan L, Han P, Wang Y, Ji D, Gan H, Wei W, Lu Z, Qu N, Hu J, Hu X, Luo Z, Li H, Ji Q, Wang J, Zhang X, Wang YL. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat Commun. 2021;12:6058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 6. | Harris CA, Andryuk PJ, Cline SW, Mathew S, Siekierka JJ, Goldstein G. Structure and mapping of the human thymopoietin (TMPO) gene and relationship of human TMPO beta to rat lamin-associated polypeptide 2. Genomics. 1995;28:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Berger R, Theodor L, Shoham J, Gokkel E, Brok-Simoni F, Avraham KB, Copeland NG, Jenkins NA, Rechavi G, Simon AJ. The characterization and localization of the mouse thymopoietin/lamina-associated polypeptide 2 gene and its alternatively spliced products. Genome Res. 1996;6:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Naetar N, Korbei B, Kozlov S, Kerenyi MA, Dorner D, Kral R, Gotic I, Fuchs P, Cohen TV, Bittner R, Stewart CL, Foisner R. Loss of nucleoplasmic LAP2alpha-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol. 2008;10:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Dorner D, Vlcek S, Foeger N, Gajewski A, Makolm C, Gotzmann J, Hutchison CJ, Foisner R. Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J Cell Biol. 2006;173:83-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ. Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell. 2002;13:4401-4413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 201] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Zhang HJ, Chen G, Chen SW, Fu ZW, Zhou HF, Feng ZB, Mo JX, Li CB, Liu J. Overexpression of cyclin-dependent kinase 1 in esophageal squamous cell carcinoma and its clinical significance. FEBS Open Bio. 2021;11:3126-3141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Huang WY, Chen G, Chen SW, Dang YW, Deng Y, Zhou HF, Kong JL, Zhang Y, Mo JX, Li CB, He J. The Indication of Poor Prognosis by High Expression of ENO1 in Squamous Cell Carcinoma of the Lung. J Oncol. 2021;2021:9910962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Zhang L, Wang G, Chen S, Ding J, Ju S, Cao H, Tian H. Depletion of thymopoietin inhibits proliferation and induces cell cycle arrest/apoptosis in glioblastoma cells. World J Surg Oncol. 2016;14:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Sun DP, Liew PL, Lin CC, Hung ST, Chen TC, Fang CL, Lin KY. Clinicopathologic and Prognostic Significance of Thymopoietin-α Overexpression in Gastric Cancer. J Cancer. 2019;10:5099-5107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kim HJ, Hwang SH, Han ME, Baek S, Sim HE, Yoon S, Baek SY, Kim BS, Kim JH, Kim SY, Oh SO. LAP2 is widely overexpressed in diverse digestive tract cancers and regulates motility of cancer cells. PLoS One. 2012;7:e39482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Parise P, Finocchiaro G, Masciadri B, Quarto M, Francois S, Mancuso F, Muller H. Lap2alpha expression is controlled by E2F and deregulated in various human tumors. Cell Cycle. 2006;5:1331-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Agnelli L, Forcato M, Ferrari F, Tuana G, Todoerti K, Walker BA, Morgan GJ, Lombardi L, Bicciato S, Neri A. The reconstruction of transcriptional networks reveals critical genes with implications for clinical outcome of multiple myeloma. Clin Cancer Res. 2011;17:7402-7412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 311] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Du X, Shi J. UBA2 promotes the progression of renal cell carcinoma by suppressing the p53 signaling. Ir J Med Sci. 2022;191:1555-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | He P, Sun X, Cheng HJ, Zou YB, Wang Q, Zhou CL, Liu WQ, Hao YM, Meng XW. UBA2 promotes proliferation of colorectal cancer. Mol Med Rep. 2018;18:5552-5562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ou Y, Luo H, Zhang Q, Du T, Liu R, Wang D, Chen J, Dong M, Wang Y, Yang Z, Wang X. UBA2 as a Prognostic Biomarker and Potential Therapeutic Target in Glioma. Front Biosci (Landmark Ed). 2024;29:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Zhang T, Yang H, Zhou Z, Bai Y, Wang J, Wang W. Crosstalk between SUMOylation and ubiquitylation controls DNA end resection by maintaining MRE11 homeostasis on chromatin. Nat Commun. 2022;13:5133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 23. | Espanola SG, Song H, Ryu E, Saxena A, Kim ES, Manegold JE, Nasamran CA, Sahoo D, Oh CK, Bickers C, Shin U, Grainger S, Park YH, Pandolfo L, Kang MS, Kang S, Myung K, Cooper KL, Yelon D, Traver D, Lee Y. Haematopoietic stem cell-dependent Notch transcription is mediated by p53 through the Histone chaperone Supt16h. Nat Cell Biol. 2020;22:1411-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Kari V, Shchebet A, Neumann H, Johnsen SA. The H2B ubiquitin ligase RNF40 cooperates with SUPT16H to induce dynamic changes in chromatin structure during DNA double-strand break repair. Cell Cycle. 2011;10:3495-3504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Zhou D, Wu Z, Park JG, Fiches GN, Li TW, Ma Q, Huang H, Biswas A, Martinez-Sobrido L, Santoso NG, Zhu J. FACT subunit SUPT16H associates with BRD4 and contributes to silencing of interferon signaling. Nucleic Acids Res. 2022;50:8700-8718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Austin CA, Cowell IG, Khazeem MM, Lok D, Ng HT. TOP2B's contributions to transcription. Biochem Soc Trans. 2021;49:2483-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Uusküla-Reimand L, Wilson MD. Untangling the roles of TOP2A and TOP2B in transcription and cancer. Sci Adv. 2022;8:eadd4920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 28. | Medina-Rivera M, Phelps S, Sridharan M, Becker J, Lamb NA, Kumar C, Sutton MD, Bielinsky A, Balakrishnan L, Surtees JA. Elevated MSH2 MSH3 expression interferes with DNA metabolism in vivo. Nucleic Acids Res. 2023;51:12185-12206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Thirumal Kumar D, Susmita B, Judith E, Priyadharshini Christy J, George Priya Doss C, Zayed H. Elucidating the role of interacting residues of the MSH2-MSH6 complex in DNA repair mechanism: A computational approach. Adv Protein Chem Struct Biol. 2019;115:325-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/