Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.107646
Revised: April 11, 2025
Accepted: May 28, 2025
Published online: June 24, 2025
Processing time: 84 Days and 22.5 Hours
Hepatocellular carcinoma (HCC), a leading cause of cancer mortality, faces diagnostic and therapeutic challenges due to its histopathological complexity and clinical heterogeneity. Pathomics, an emerging discipline that integrates artificial intelligence (AI) with quantitative pathology image analysis, aims to decode disease heterogeneity by extracting high-dimensional features from histopathological specimens. This review highlights how AI-driven pathomics has revolutionized liver cancer management through automated analysis of whole-slide images. Pathomics integrates deep learning with histopathological features to enable precise tumour classification (e.g., HCC vs cholangiocarcinoma), micro
Core Tip: Artificial intelligence (AI)-powered pathomics revolutionizes liver cancer management by decoding histopathological patterns through the use of deep learning models such as microvascular invasion (MVI)-AI diagnostic model and CHOWDER, which excel in detecting MVI, immune biomarkers, and prognostic features. The integration of multiomics data links tumour morphology with molecular pathways (e.g., EZH2 dysregulation and immune evasion). Key challenges persist in model generalizability, interpretability, and clinical integration. Future advancements will require cross-modal AI systems combining radiogenomics and liquid biopsy alongside standardized platforms to translate pathomics results into personalized therapeutic strategies for hepatocellular carcinoma and metastatic liver malignancies.
- Citation: Peng MH, Zhang KL, Guan SW, Lin Q, Yu HB. Advances and challenges in pathomics for liver cancer: From diagnosis to prognostic stratification. World J Clin Oncol 2025; 16(6): 107646
- URL: https://www.wjgnet.com/2218-4333/full/v16/i6/107646.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i6.107646
Cancer remains a critical challenge to global public health, accounting for one-sixth of all deaths worldwide and nearly one-quarter of fatalities attributed to noncommunicable diseases (NCDs). Recent estimates indicate 19.96 million new cancer cases and 9.74 million cancer-related deaths globally in 2022, with cancer now driving premature mortality more than any other NCD[1,2]. Hepatocellular carcinoma (HCC), the predominant form of liver cancer representing over 80% of primary liver malignancies, demonstrates particularly alarming global disparities[3,4]. It ranks among the top three causes of cancer mortality in 46 countries and appears in the top five in 90 nations, reflecting its disproportionate burden across diverse populations[5,6]. Despite decades of research advancing liver cancer screening, diagnostic imaging, and multimodal treatment[7,8], the overall prognosis remains poor. Persistent gaps in early risk prediction strategies and marked variability in treatment responses continue to hinder progress[9]. Although traditional histopathological eva
The emergence of artificial intelligence (AI) has opened transformative avenues to address these diagnostic challenges. By leveraging machine learning (ML) and deep learning (DL) architectures, AI has demonstrated remarkable ability in deciphering latent pathological patterns from multimodal medical datasets encompassing radiological, histological, and histopathological imaging. This technological breakthrough is revolutionizing precision oncology through three pivotal pathways: (1) Automated biomarker identification from complex feature spaces; (2) Development of prognostic pre
The core strengths of pathomics are its high throughput, objectivity and scalability. By automating the screening of benign samples[18], this system can optimize the pathology workflow and direct resources towards complex cases; the standardized analytical framework of pathomics reduces interobserver variation[19], and its remote diagnostic function[20] compensates for the geographic imbalance of medical resources. With the popularization of scanning equipment for whole-slide imaging[21], pathomics has gradually transformed from scientific research to clinical practice, marking the entry of liver cancer diagnosis and treatment into the new era of “digital intelligent pathology”.
In this review, the progress of AI and pathomics in the diagnosis, individualized treatment and prognosis of liver cancer was systematically described; the current technological bottlenecks were analyzed; and the potential for clinical translation and future development were explored, with the aim of providing a theoretical basis for the innovation of precision medicine in liver cancer. Table 1 lists studies included in the review.
| Ref. | References | Purpose of the study | Data source | Sample size | Major findings | External validation |
| The use of pathomics in the diagnosis and classification of liver cancer | ||||||
| Cheng et al[46], 2022 | 35202643 | Develop a deep learning system to improve histopathologic diagnosis of various liver lesions and tissues | Surgical and biopsy samples from 6 hospitals | 738 patients (providing 1115 whole-slide images) | The HnAIM system integrates ResNet50, InceptionV3, and Xception architectures, achieving an AUC of 93.5% for hepatic lesion classification | Yes (independent external validation cohort) |
| Aatresh et al[47], 2021 | 34053009 | Develop a deep learning framework for multi-subtype classification of hepatic carcinoma histopathology | KMC liver dataset (257 annotated slides) and TCGA-LIHC public cohort (300+ HCC samples) | Not mentioned | LiverNet: Lightweight ASPP architecture with 0.573M parameters achieving 90.93% accuracy in HCC grading | Not mentioned |
| Sun et al[48], 2020 | 31670686 | Develop a deep learning model for liver cancer histopathological image classification | Not mentioned | Not mentioned | Combine transfer learning and multi-instance learning to achieve high-precision classification of normal/abnormal liver histopathological images, addressing challenges of limited training samples and large-scale image processing | Not mentioned |
| Liao et al[49], 2020 | 32536036 | Deep learning model for HCC classification and mutation prediction | TCGA datasets (WSIs) and the West China Hospital Biospecimen Repository | TCGA (393 HCC vs 88 normal) and West China Hospital Biobank (455 HCC vs. 264 normal) | CNN model: Achieved AUC = 1.000 in WSI analysis, linking morphological features to gene mutations | Yes (Successfully tested on West China Hospital data) |
| Beaufrère et al[50], 2024 | 38379584 | Automated classification of primary liver cancer biopsy types | Biopsy samples (HE stained WSI) | 166 HE stained WSIs (90 training, 29 internal validation, 47 external validation) | Weakly supervised algorithm distinguishing HCC/iCCA subtypes and quantifying cHCC-CCA heterogeneity | Yes |
| Dong et al[51], 2022 | 35509058 | Classification of liver cancer differentiation grades | Histopathologic images of liver cancer | 73 hepatocellular carcinoma histopathological images from patients with varying differentiation grades | FuNet fusion strategy: Multimodal feature fusion with channel-space attention mechanism to enhance feature characterization | Not mentioned |
| Kiani et al[52], 2020 | 32140566 | Evaluating the Impact of AI assistants on the diagnosis of subtypes of hepatocellular carcinoma | HE stained WSI | Validation set 26 cases, test set 80 cases | Deep learning-assisted systems: Improving diagnostic consistency for pathologists, but false prediction bias needs to be addressed | Yes (independent test set of 80 cases validated) |
| Liu et al[53], 2023 | 37882066 | Building a faster RCNN model to identify PCCCL and CHCC | Case data of Beijing You'an Hospital | 151 cases | Faster RCNN: For rare PCCCL subtypes, the diagnostic accuracy is 96.2%, and a single case takes only 4 seconds to process | Not mentioned |
| The use of pathomics in the prediction of recurrence in liver cancer | ||||||
| Zhang et al[58], 2024 | 38488408 | Develop a deep learning model to improve the efficiency of MVI diagnosis | First affiliated hospital of Zhejiang University and TCGA database | 753 cases (internal) and 358 cases (external) | MVI-AIDM: Three-step simulation of pathology diagnostic process (localization, segmentation, classification) with 94.25% accuracy of MVI detection | Yes |
| Laurent-Bellue et al[59], 2024 | 38879083 | Construction of a deep learning model for predicting HCC recurrence after surgery | WSI of hepatic resection specimens and external hospital data | Internal 107 cases (680 WSIs), external 29 cases | ResNet34 model: Automatic quantification of invasive structures such as MVI and validation of correlation with recurrence risk | Yes |
| Chen et al[60], 2022 | 35349075 | Develop a deep learning model to assess microvascular invasion in HCC | Zhongshan first hospital, Dongguan People's Hospital and Southern Medical University Shunde Hospital | Internal 350 cases (2917 WSIs), external 120 cases (504 WSIs) | MVI-DL: Weakly supervised multiple exemplar learning framework with high AUC (0.871) despite single section or biopsy sample | Yes |
| Feng et al[61], 2021 | 34926264 | Develop a deep learning model for HCC diagnosis and classification | First Affiliated Hospital of Zhejiang University and TCGA database | 592 cases (training 137, testing 455), external validation 157 cases | Annotated noise optimization framework: Two-stage training strategy (feature filtering + dynamic label smoothing), segmentation accuracy 87.81%, diagnosis accuracy 98.77% | Yes |
| Qu et al[62], 2023 | 37031334 | Development of DPS to predict HCC recurrence after liver | HCC patient dataset | 380 cases | DPS: Based on ResNet-50 and DeepSurv network, C-index up to 0.827, associated NK cell infiltration | Not mentioned |
| The use of pathomics in prognosis and survival prediction of patients with liver cancer | ||||||
| Zhou et al[64], 2024 | 39640777 | Exploring the relationship between pathomics characteristics and EZH2 expression to predict HCC survival | TCGA database | 267 cases | Pathomics modeling: Prediction of EZH2 expression, high scores independently associated with poorer OS | Not mentioned |
| Jia et al[65], 2023 | 37450030 | Constructing a deep learning model to assess immune infiltration and prognosis of liver cancer | Xijing Hospital cohort, TCGA database | 100 WSIs (training set) | ResNet 101V2 model: Quantify TILs with AUC > 0.95, construct prognostic nomogram | Yes (TCGA and Xijing Hospital cohort cross-validation) |
| Saillard et al[66], 2020 | 32108950 | Constructing deep learning models to predict survival in HCC patients | Henri Mondor Hospital, TCGA database | Discovery set of 194 cases, validation set of 328 cases | CHOWDER model: Predicts HCC survival C-index up to 0.75-0.78 without annotation, identifies vascular and immunodeficiency features | Yes |
| Ding et al[67], 2024 | 38972973 | Interpretable modeling to resolve iCCA prognosis and morphological-molecular associations | Internal and external cohorts at research institutions | 373 cases (development set), validation set 381 cases (213 internal + 168 external) | Interpretable framework: Identify key prognostic markers such as tertiary lymphoid structure distribution and abnormal nuclear morphology, and validate the biological mechanism by multi-omics | Yes |
| Xie et al[68], 2022 | 35537220 | Development of quantitative morphological features to stratify ICC patient survival | Postoperative ICC patients (H&E stained whole-slide images) | 127 cases (78 in modeling set, 49 in test set) | Morphometric analysis framework: Construct IHC-free dependent prognostic model based on tumor architectural complexity and lymphocyte spatial topology (AUC = 0.68) | Not mentioned |
| Shi et al[69], 2021 | 32998878 | Development of deep learning model for HCC risk stratification | Zhongshan Hospital cohort (WSIs), TCGA database | Zhongshan cohort 1125 cases (2451 WSIs), TCGA 320 cases (320 WSIs) | TRS independently predicts prognosis, associates hepatic sinusoidal capillarization, FAT3 mutations and immune infiltration | Yes (TCGA cohort validation) |
| The use of pathomics in liver metastases | ||||||
| Chen et al[79], 2024 | 37822044 | Development of features to identify primary sites of liver metastases | WSIs of patients with liver metastases | 114 patients (175 WSIs) | Fusion model improves primary site identification with similar morphological features of primary and metastatic tumors | Not mentioned |
| Albrecht et al[80], 2023 | 37562657 | Development of HEPNET to differentiate ICC from colorectal liver metastases | Heidelberg University Hospital and University Medical Center Mainz | 456 cases (training), 115 cases (internal testing), 159 cases (external validation) | HEPNET system: Differentiation of iCCA from colorectal liver metastases, AUC = 0.997, reduced IHC dependence | Yes |
| Jang et al[81], 2023 | 38001649 | Development of deep learning models to distinguish HCC, CC and mCRC | WSIs for HCC, CC, mCRC (specific source not specified) | Not mentioned | Triple classification framework: Differentiate between HCC, iCCA and colorectal cancer metastasis with AUC > 0.995 | Yes (tested using external datasets) |
| Höppener et al[82], 2024 | 39471410 | Development of a deep learning algorithm to classify growth patterns of colorectal liver metastases | Erasmus MC and Radboud University Medical Center, The Netherlands | Development group 932 cases (3641 images), external validation 870 images | NIC algorithm: Automatic differentiation of fibrotic/non-fibrotic subtypes of colorectal liver metastases, AUC = 0.93-0.95, associated prognosis | Yes |
| Qi et al[83], 2023 | 37701575 | Development of a deep learning framework for automated analysis of CRLM tissue features | Not mentioned | Not mentioned | SOF: Quantification of 17 microenvironmental features including tumor necrosis rate, 5-year survival stratification difference improved to 22.5% | Yes (independent clinical cohort validation) |
| Xiao et al[84], 2022 | 35433467 | Development of a deep learning model to predict liver metastasis in colorectal cancer | Retrospectively collected data on colorectal cancer patients (unspecified institution) | 611 cases (428 in training group, 183 in validation group) | Deep learning nomogram: Combining pT/pN staging to predict the risk of liver metastasis (C-index = 0.81) and dynamically optimize the timing of chemotherapy | Not mentioned |
| The use of pathomics in image segmentation | ||||||
| Lal et al[86], 2021 | 33190012 | Development of deep learning network for Nuclei Segmentation of Liver Cancer Pathology Images | KMC liver dataset (Kasturba Medical College, India) | 80 images (KMC dataset) | NucleiSegNet: Residual block + attention decoder for efficient segmentation of complex adherent cell nuclei | Not mentioned |
| Rong et al[87], 2023 | 37100227 | Development of HD-Yolo for accelerated nuclear segmentation and tumor microenvironment quantification | Lung, liver, breast cancer tissue samples (unspecified specific institution/database) | Not mentioned | HD-Yolo algorithm: Optimizes the detection process, accelerates nuclear segmentation and enhances tumor microenvironment analysis | Not mentioned |
| Gu et al[88], 2025 | 39528162 | Development of a deep learning process to achieve whole cell segmentation of HE stained tissues | Hepatocellular carcinoma and normal liver tissue samples, 5 external datasets (liver/lung/oral disease) | Training set of 18 cases (7 cancerous + 11 normal), external test set of 5 datasets | CSGO framework: Nuclear membrane segmentation + post-processing algorithm, superior to Cellpose, supports TME whole cell morphology analysis | Yes (5 external datasets validation) |
| Jehanzaib et al[89], 2025 | 39265361 | Development of PathoSeg model to improve cancer tissue segmentation performance | Internal dataset (liver, prostate, breast cancer patients) | 82 full slices (from 62 patients) | PathoSeg model: Combined with synthetic data generation (PathopixGAN) to mitigate category imbalance. | Not mentioned |
| Hägele et al[90], 2024 | 39443575 | Development of a deep learning model to distinguish HCC and ICC | HE stained whole section images | 165 examples | Complementary labeling strategy: Weakly supervised segmentation of HCC and iCCA with balanced accuracy of 0.91 | Not mentioned |
| Chen et al[91], 2022 | 35787805 | Intelligent classification of differentiated liver cancer pathology images | Hepatocellular carcinoma samples from 74 patients in Xinjiang Medical University Cancer Hospital | 444 liver cancer pathology images | SENet model classifies multidifferentiated liver cancer pathology images with 95.27% accuracy | Not mentioned |
| Roy et al[92], 2021 | 33420322 | Development of a whole-slide tumor segmentation model for the liver | Not mentioned | Not mentioned | HistoCAE: Self-Encoder Reconstruction Strategy with Multi-Resolution Scaling to Improve Liver Tumor Segmentation Accuracy | Not mentioned |
| Khened et al[93], 2021 | 34078928 | Development of generic deep learning tissue analysis framework | Liver cancer public dataset | Not mentioned | DigiPathAI: Multi-model integration + uncertainty estimation, open source process supports segmentation to tumor load calculation. | Not mentioned |
| Combination of pathomics and transcriptomic data | ||||||
| Calderaro et al[94], 2023 | 38092727 | Improving diagnostic accuracy of mixed liver cancer | Not mentioned | 405 patients with cHCC-CCA | Deep learning framework: Self-supervised ResNet50+ attention MIL, AUROC = 0.99, spatial transcriptome validation of molecular subtypes | Not mentioned |
| Zeng et al[95], 2022 | 35143898 | Predicting activation of immune gene signatures in hepatocellular carcinoma | Not mentioned | Not mentioned | CLAM architecture: Multiscale analysis to predict immunogene activation status, AUC = 0.81-0.92, associated immune infiltration hotspots | Not mentioned |
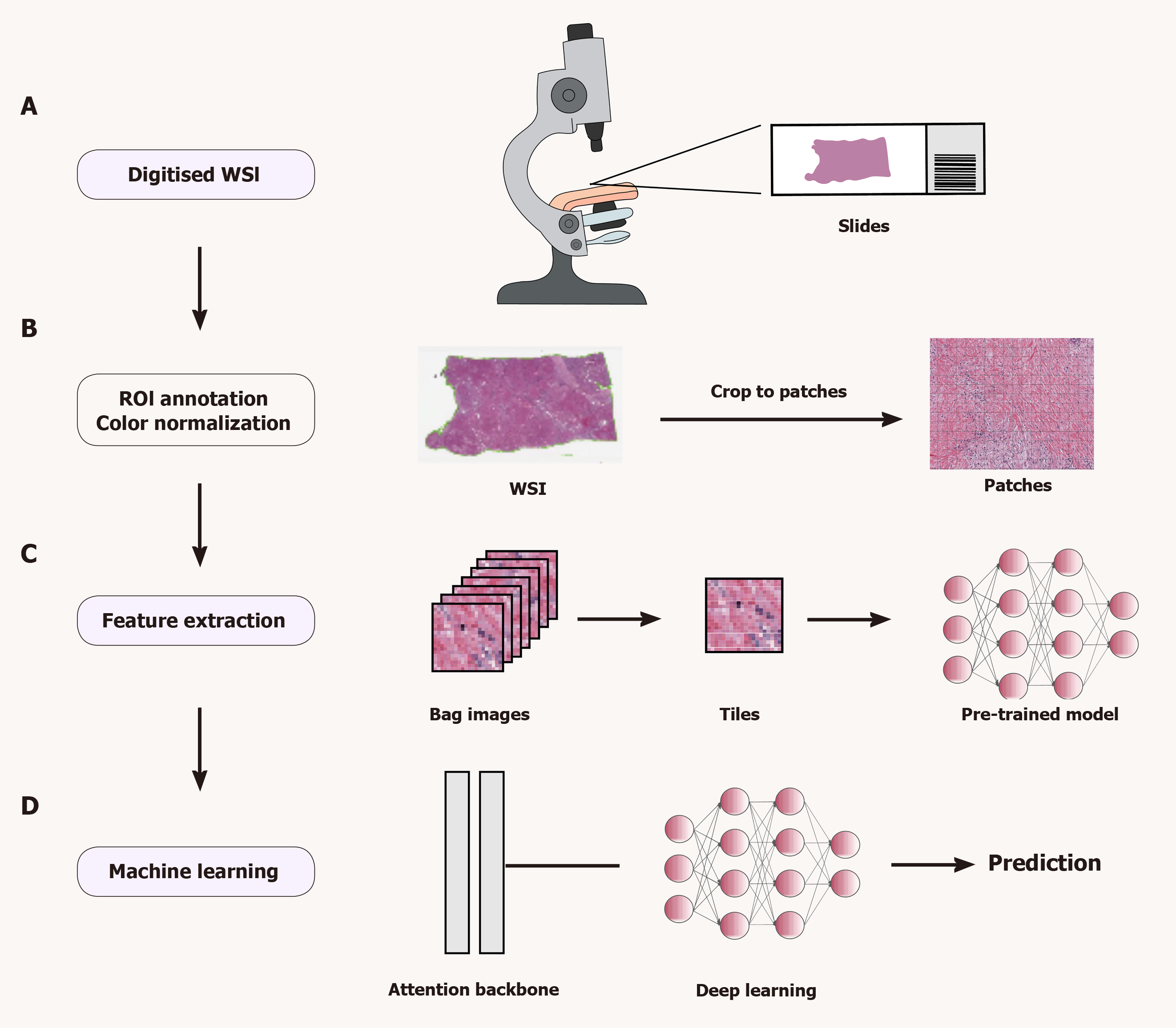
The pathomics analysis workflow consists of three main steps: Region of interest (ROI) selection, colour normalization, and extraction and analysis of pathomics features. First, after collecting and scanning pathology images, the ROIs are labelled manually or automatically. Second, DL features (low-, intermediate- and high-level features) and hand-designed features (morphological, textural, statistical and other features) are extracted from these images through a series of image preprocessing steps (e.g., ROI segmentation, meshing, block extraction and colour normalization). Finally, meaningful features are analysed by ML or DL algorithms, and disease states are classified or predicted according to different tasks. Figure 1 illustrates the pathomics workflow.
The initial step in pathomics analysis involves outlining ROIs on a whole-slide image (WSI) to identify areas that need to be processed or analyzed. Since the WSI contains a wealth of information, processing the entire WSI is computationally intensive and time consuming and may contain irrelevant or confusing information. Defining ROIs reduces computational costs and improves the quality of analysis by narrowing analysis to the most relevant areas of the image. In addition, defining ROIs allows for the extraction of representative and unique features, which can help identify, classify, or predict disease states. Therefore, effective ROI contouring and appropriate plot extraction are important factors to consider in pathological image analysis.
ROIs can be outlined manually or by automation. Professional pathologists generally use specialized software such as Qupath[22] and ASAP for manual outlining, which is accurate and flexible but time-consuming, subjective, and unrepeatable. Automated outlining methods utilize algorithms to achieve automatic or semiautomatic delineation of ROIs, which requires image preprocessing and the identification and location of ROIs using specific algorithms. Automation saves human resources, improves consistency and repeatability, and adapts to large-scale data processing. However, automated methods may not be able to effectively handle image quality differences, complex backgrounds, and variable target morphologies. To improve the performance and robustness of automated methods, tissue classifiers[23-25] for automatic classification have been proposed and have shown good overall performance. The different ROI outlining schemes have their own advantages and disadvantages. Choosing the right method for different needs is critical to achieve efficient and accurate ROI outlining.
During the preparation of liver tissue sections, colour differences in WSIs from different laboratories are inevitable, even when the same staining protocol is used, which limits the ability of the algorithm to generalize. Factors contributing to colour variation include differences in staining time, concentration and pH of the staining solution, and differences in staining platforms and scanner models[26]. Therefore, researchers have proposed various normalization techniques to reduce the effect of image colour variations on the trained model.
The purpose of feature extraction in pathomics is to transform complex, high-dimensional and diverse image data into simplified low-dimensional feature vectors. Traditional and DL methods have been developed for feature extraction. Traditional methods rely heavily on domain expertise to design and select suitable feature descriptors. These descriptors include first-order features (e.g., shape, size, texture, and colour distributions) and second-order features, such as colour histograms and grayscale co-occurrence matrices, which are derived from intermediate computational matrices and statistical formulations.
These hand-crafted features are commonly employed in traditional ML models, such as support vector machines (SVMs) and random forests, for tasks such as tumor classification and prognostic analysis[27-29]. However, these approaches depend heavily on prior domain expertise and may fail to capture nuanced, high-level patterns inherent in histopathological data. In recent years, DL methods have gained popularity for their ability to automatically learn feature representations using neural network models such as CNNs. These methods adaptively extract abstract and high-level features from many pathology images and optimize both features and classifiers. DL methods have surpassed traditional approaches in pathology image analysis[30], demonstrating the ability to identify discriminative features that may elude human experts. However, as model architectures increase in depth, the learned representations become increasingly abstract and suffer from limited interpretability at the individual feature level—a challenge that is compounded by the inherent complexity of neural networks[19]. Recent studies[31,32] have introduced hybrid methodologies that integrate handcrafted features with DL-derived representations. This combined approach has demonstrated superior detection accuracy compared with the use of either feature type alone, capitalizing on the complementary strengths of both manual feature engineering and data-driven hierarchical learning.
The high dimensionality and redundancy of features extracted from pathology images pose a significant risk of overfitting. To mitigate this risk, techniques such as feature selection and dimensionality reduction, which are critical for isolating the most discriminative and representative features while preserving model generalizability, are used. Standard dimensionality reduction techniques include principal component analysis (PCA) and linear discriminant analysis (LDA)[33,34]. PCA, an unsupervised learning method, reduces high-dimensional data to a low-dimensional subspace through a linear transformation that preserves the maximum variance of the original dataset. In contrast to unsupervised methods such as PCA, LDA is a supervised learning technique that projects data into a lower-dimensional space to maximize interclass separability. After completing feature selection and dimensionality reduction, a wide range of algorithms—from traditional ML models (e.g., logistic regression, decision trees, SVMs) to advanced DL architectures—can be used to analyze pathology images, enabling robust disease risk stratification and diagnostic prediction. Optimal algorithm selection hinges on data characteristics and task objectives: Decision trees are preferred for scenarios demanding model interpretability, whereas deep neural networks excel in high-accuracy prediction tasks where precision is paramount. Beyond predictive applications, analyzing correlations between selected features and clinical outcomes can elucidate latent pathomechanisms and inform therapeutic strategies, bridging the gap between computational analysis and actionable biomedical insights. Correlation analysis, cluster analysis, factor analysis and ML algorithms are the common analytical methods. By identifying relevant features and biomarkers, we can better understand the pathogenesis of diseases and develop effective treatment plans.
While DL has demonstrated exceptional performance in fields such as medical image analysis, disease prediction, and personalized treatment, its inherent "black box" nature—referring to the opaque internal decision-making logic of the models—remains one of the primary obstacles to its widespread adoption in clinical practice. Merely recognizing this limitation is insufficient, it is crucial to explore and apply advanced interpretability tools to bridge the gap between the predictive capabilities of DL models and their clinical trustworthiness. A variety of advanced interpretability techniques developed in recent years, such as Attention Maps, Gradient Weighted Class Activation Mapping (Grad-CAM), and SHapley Additive exPlanations (SHAP), provide powerful means to understand the decision-making process of DL models.
Attention mechanisms[35] and attention maps[36] were initially successful in the field of natural language processing and are now widely used in visual tasks such as medical image analysis. By mimicking the allocation of attention in human vision or cognition, these mechanisms allow the model to dynamically focus on the most relevant parts of the input data (e.g., medical images or electronic health record text) when making predictions. The generated attention maps can visualize regions or features that the model "focuses on" when making specific diagnoses or predictions (e.g., suspicious lesion areas in medical imaging, or critical descriptive terms in clinical text). This provides an intuitive explanation for the model's decision-making process, enabling researchers and clinicians to verify whether the model bases its judgments on medically logical characteristics.
Grad-CAM[37], a popular visualization technique specifically designed for CNNs, is particularly useful in medical image analysis. By leveraging gradient information from backpropagation, Grad-CAM calculates the importance of feature maps in the final convolutional layer for specific class predictions (e.g., "malignant tumors"). By combining these importance weights with feature maps, a coarse localization map can be generated (typically superimposed on the original image as a heatmap), highlighting the image regions that contribute most significantly to the final prediction. This enables physicians to visually identify the specific regions in the image (e.g., tumor areas, abnormal textures) that the model relies on for decision-making, thereby validating whether the diagnostic rationale aligns with clinical expertise and identifying potential causes of false positives or negatives.
SHAP[38] provides a model-agnostic interpretation framework based on Shapley values from game theory. Its core idea is to attribute the model's prediction output to the contribution values (SHAP values) of each input feature. These values quantify how each feature (e.g., patient age, specific gene expression levels, pixels/regions in medical images) drives the specific prediction outcome—either positively or negatively—relative to a baseline prediction. SHAP not only provides global feature importance rankings but also generates personalized explanations for individual cases, precisely identifying which factors and to what extent they influenced the prediction outcome for a specific patient. This granular attribution capability is particularly valuable for understanding individualized risk prediction, detecting complex interactions, and interpreting complex models that incorporate multimodal data (e.g., clinical, imaging, and omics data).
In summary, advanced interpretability tools such as Attention Maps, Grad-CAM, and SHAP have opened the "black box" of DL models. These tools not only enhance model transparency and foster clinicians' trust and acceptance of model outputs but also aid in model debugging, uncovering potential data biases, and even revealing novel biomarkers or disease mechanisms. In the medical field, the responsible development and deployment of DL technologies must incorporate these interpretability methods as a core component, ensuring the accuracy and reliability of the model's decision-making processes and ultimately serving the goal of improving patient care.
HCC and intrahepatic cholangiocarcinoma (iCCA) are the most prevalent subtypes of primary liver cancer. These malignancies occupy opposite ends of the primary liver cancer spectrum, exhibiting distinct risk factors, clinical outcomes, treatment approaches, and molecular/genetic profiles[39,40]. Combined HCC–cholangiocarcinoma (cHCC-CCA), a rare neoplasm with overlapping features of both HCC and iCCA, occupies an intermediate position between these two entities. Histological analysis remains the gold-standard diagnostic method for the confirmation of iCCA and cHCC-CCA, as histological analysis enables precise differentiation of the biphenotypic characteristics of these diseases[41-43]. However, most cases present ambiguous features and cannot be easily categorized as HCC or iCCA; therefore, methods for the accurate diagnosis and classification of primary liver cancer are urgently needed. With the rapid development of AI technology, pathomics shows promising potential in the field of precision diagnosis and treatment of liver cancer. WSIs[44,45] capture intricate histological details, including patterns imperceptible to human eyes. AI, particularly DL algorithms, can decode these complex morphological features through computational analysis of tiled WSIs, thereby leveraging latent information across multiple scales. Driven by advancements in AI technology, pathomics has emerged as a transformative tool, revolutionizing precision diagnosis and therapeutic strategies in liver cancer mana
To address the clinically important challenge of differentiating benign from malignant liver nodules, Cheng et al[46] developed the HnAIM DL system, which integrates the ResNet50, InceptionV3, and Xception networks into an ensemble architecture. This framework enables automated classification of hepatocellular lesions on histopathological slides from surgical and biopsy samples, achieving a 93.5% area under the curve (AUC) in external validation, which surpasses the diagnostic accuracy of pathologists with varying experience levels. This system not only visualizes the proportion of lesion distribution but also shortens the diagnostic time and provides a new perspective for the study of multistage carcinogenesis of the liver. However, the utility of this system in discriminating between well-differentiated HCC (WD-HCC) and precancerous lesions remains constrained by limitations in sample size and model interpretability.
Given the clear distinction between benign and malignant lesions, the study by Aatresh et al[47] focused on the refined diagnosis of liver cancer. The authors developed LiverNet, which uses an atrous spatial pyramid pooling module to grade HCC histological differentiation levels with 90.93% accuracy. With a streamlined architecture comprising only 0.5739M parameters, this lightweight framework demonstrates exceptional translational potential for clinical deployment, balancing diagnostic precision with computational efficiency. Similarly, Sun et al[48] strategically integrated transfer learning with multiexample learning to enable histopathological image classification using only slide-level labels, establishing a label-efficient paradigm to mitigate the scarcity of annotated data in computational pathology. Notably, Liao et al[49] developed a CNN-based platform leveraging datasets from The Cancer Genome Atlas (TCGA) and West China Hospital, achieving flawless diagnostic performance (AUC = 1.000) for WSI analysis. In addition to diagnostic precision, the study pioneered a transformative association model linking histomorphometric features to somatic mutations, thereby expanding the use of pathomics from traditional diagnostic frameworks to molecular subtyping in oncology research.
Advancements in diagnostic systems have driven histopathological subtyping of HCC as an emerging frontier in oncological research. Beaufrère et al[50] developed a weakly supervised DL model to differentiate HCC from iCCA using routinely stained histopathology slides. Their framework also quantified the compositional ratios within cHCC-CCA, providing an objective framework for tumour heterogeneity assessment and improving diagnostic precision for these challenging tumours. In contrast to single-modality approaches, Dong et al[51] developed the FuNet fusion strategy, which outperforms conventional single-model architectures in the histological subtyping of HCC through the synergistic integration of multimodal imaging features. The channel-spatial attention mechanism of FuNet effectively improves the feature characterization capability. Notably, Kiani et al[52] developed a DL-based decision support system to assist pathologists in differentiating HCC from CCA on haematoxylin–eosin (HE)-stained WSIs. Their study revealed a critical duality in AI-assisted diagnostics: While accurate model predictions significantly improved diagnostic concordance among pathologists (P = 0.000), erroneous predictions systematically introduced diagnostic bias. This finding underscores the need for a robust quality assurance framework. The Faster RCNN model developed by Liu et al[53] for diagnosing primary clear cell carcinoma of the liver (PCCCL), a rare subtype of HCC, highlights the indispensable role of histopathological analysis in identifying unique cancer subtypes. The model demonstrated exceptional diagnostic accuracy (96.2%) and ultrahigh efficiency, processing each case in just 4 s, thereby reinforcing its potential value for rapid, precise classification in complex histopathology workflows.
Despite significant advancements in existing research, three key challenges persist. First, most models are trained on single-center, small-sample datasets, highlighting the urgent need for multicenter collaboration to establish standardized validation frameworks. Second, a fundamental tension exists between the "black-box" nature of DL and clinical in
Postoperative recurrence of HCC is a fatal threat to patient survival. Statistically, approximately 50%-70% of HCC patients develop recurrent metastasis within 5 years after radical resection[54]. Early recurrence (within 2 years after surgery) is mostly associated with pathological features such as microvascular invasion (MVI) and the immune microenvironment, whereas late recurrence is closely related to neoplastic or cirrhotic progression. The unpredictability of recurrence results in not only delayed treatment but also a significant decrease in patient survival; therefore, the development of accurate and dynamic recurrence risk assessment tools is urgently needed.
MVI is defined as microscopically observed nests of cancer cells in the portal vein, hepatic vein, or tumor-enveloped blood vessels within paraneoplastic liver tissue[54]. MVI is an important predictor of recurrence after HCC surgery, and accurate diagnosis of MVI is crucial. However, the traditional pathological diagnosis of MVI is complicated by multiple limitations[55-57] and the current approach for diagnosing MVI focuses on preoperative prediction, and a fast and accurate assessment method for postoperative histologic MVI is lacking. Therefore, developing a postoperative MVI assessment model based on DL is highly important. Zhang et al[58] developed a DL AI diagnostic model called MVI-AIDM, which was designed to quickly and accurately identify MVI on WSIs of HCC. The MVI-AIDM model achieves this identification by mimicking the diagnostic process of a pathologist in three steps: Localization of the tumour region, segmentation of the microvasculature, and classification of cells. The model achieved 94.25% accuracy in an independent external validation cohort, with a significantly greater positive detection rate for MVI than that of conventional mi
In addition to MVI, the pathological characteristics of immune cells in HCC are characteristically important in predicting tumour recurrence. Qu et al[62] aimed to develop a DL-based deep pathomics score (DPS) to predict tumour recurrence after liver transplantation (LT) in patients with HCC. The study included 380 HCC patients who underwent LT, identified six HCC histologic structures using ResNet-50, and calculated individual risk scores and DPSs using a modified DeepSurv network. The DPS performed well in predicting recurrence, with a C-index of 0.827 and 0.794 in the training and validation sets, respectively, and immune cells were the most significant histologic category for predicting recurrence after LT. Multivariate analysis demonstrated that the DPS was independently associated with recurrence-free survival (RFS). Patients in the high-risk group exhibited significantly shorter RFS and larger tumour diameters accompanied by poorly defined margins than those in low-risk patients. Cellular level analysis revealed that the greater the degree of natural killer (NK) cell infiltration within the tumour was, the lower the risk of recurrence. The DPS effectively predicted HCC recurrence after LT and identified relevant clinicopathological features, suggesting that immune cells in the tumour microenvironment (TME) of LT patients have important predictive significance. In addition to NK cells, studies have shown that macrophages or neutrophils may play a potentially important role in the progression of recurrence in HCC patients. The identification of prognostic pathological features via whole-slide imaging was used to construct a model with computable metrics that effectively differentiated patients' risk of recurrence; this model not only classified typical HCC tissues but also revealed immune infiltration within the tumour and surrounding structures and highlighted clinically important features for prognosis[63].
In summary, pathomics plays a critical role in predicting liver cancer recurrence, highlighting the transformative potential of DL frameworks in advancing precision medicine for liver cancer. By integrating MVI patterns, immune cell infiltration dynamics, and tumour cellular heterogeneity, the DL model overcomes the constraints of single-marker approaches, enabling holistic recurrence risk stratification. Nevertheless, clinical translation requires addressing key challenges, including data generalizability across diverse cohorts, validation of real-world clinical applicability, and elucidation of underlying biological mechanisms to ensure robust clinical utility. Future research should focus on interdisciplinary collaboration and multicentre validation to accelerate the translation of AI-driven pathology diagnostic tools to the clinic.
Liver cancer is one of the most lethal malignant tumours worldwide; accurate survival prediction tools for HCC and iCCA are urgently because of their high heterogeneity and substantial prognostic differences. While traditional pathology assessment relies on subjective interpretation, and quantification of complex biological features is challenging, pathomics introduces a transformative approach to prognostic evaluation and personalized treatment in liver cancer patients. Integration of AI with digital pathology enables comprehensive analysis of tissue morphology, cellular characteristics, and molecular signatures, advancing the field of precision oncology. In recent years, several studies have explored the potential value of pathomics in the prognostic prediction of HCC using DL frameworks and multiomics data.
The central challenge in liver cancer prognostication is the need to separate biologically and clinically relevant features from high-resolution WSIs. Zhou et al[64] pioneered a pathomics model leveraging HE-stained histopathological images from HCC patients, enabling the prediction of oncogene EZH2 expression levels. Their work further demonstrated that increased pathomics scores were independently correlated with poorer overall survival, highlighting the prognostic utility of this score in HCC management. This finding underscores that morphological patterns inherent in routine HE-stained tissue sections may represent latent molecular signatures, acting as histomorphometric proxies for critical oncogenic alterations such as EZH2 dysregulation or immune evasion mechanisms. Jia et al[65] investigated the tumour immune microenvironment in HCC by developing a ResNet 101V2-based DL model to quantify tumour-infiltrating lymphocytes (TILs) with high predictive accuracy (AUC > 0.95). Their findings identified the infiltration levels of TILs as an independent prognostic factor and culminated in the development of a prognostic nomogram integrating immune-specific features. Saillard et al[66] proposed a DL model (CHOWDER) that does not require manual annotation and significantly outperforms traditional clinical metrics in the prediction of survival after HCC (C-index: 0.75–0.78) and identified features such as the vascular luminal space and immune infiltration defects as morphological markers of poor prognosis.
The strength of pathomics lies not only in its predictive performance but also in its ability to reveal interpretable features of tumour biological behaviours. Ding et al[67] constructed an interpretable DL framework for iCCA and reported that the distribution of tertiary lymphoid structures, tumour-mesenchymal ratios, and nuclear morphology (e.g., distorted nuclear membranes, low texture contrast) are key morphological markers of prognosis and validated the associations of these markers with glycolysis, immune infiltration, and other pathways through multiomics. Xie et al[68] performed morphometric profiling of the spatial architecture of tumours and lymphocytes in iCCA, establishing an immunohistochemistry (IHC)-free prognostic framework (AUC = 0.68). Their analysis revealed that tumour architectural complexity and lymphocyte spatial topology are histospatial biomarkers that can be used to independently stratify survival outcomes in iCCA patients, decoupling prognosis from conventional IHC-dependent paradigms. Collectively, these studies establish pathomics as a transformative framework capable of converting qualitative histopathological descriptors—such as nuclear atypia or stromal architecture—into spatially resolved quantitative biomarkers and elucidating morphology-function interdependencies through multimodal integration of molecular and clinical data.
The clinical and research utility of the synergistic convergence of pathomics with genomic profiling and immune microenvironment characterization has significantly expanded. Shi et al[69] pioneered a weakly supervised DL framework that derives a tumour risk score (TRS) from HCC histopathology images. The TRS demonstrated robust correlations with key histopathological phenotypes, including hepatic sinusoid capillarization and nucleolar atypia. Furthermore, multiomics analyses revealed potential regulatory mechanisms linking increased TRSs to FAT3 mutations and immune evasion pathways. Similarly, the studies by Zhou et al[64] and Jia et al[65] correlated pathomics with immune checkpoint (PD-1/CTLA4) expression and key signalling pathways (e.g., mTORC1) from the perspectives of EZH2 expression and TIL heterogeneity, respectively, providing a paradigm for “image–gene–clinical” triple analysis.
Through DL and multiomics integration, pathomics has demonstrated three aspects of value in the prediction of HCC prognosis: First, mining key biological information such as EZH2 expression and immune infiltration from routine HE-stained sections to reduce the reliance on expensive molecular tests; second, quantifying the characteristics of the TME
Metastatic progression remains the primary contributor to cancer-related mortality, substantially diminishing patient survival rates across malignancies[70]. The liver is anatomically and immunologically permissive due to its dual blood supply, sinusoidal endothelial architecture, and tolerogenic microenvironment and serves as a predominant metastatic niche for diverse carcinomas, particularly colorectal, pancreatic, breast, and gastric cancers[71-73]. The unique dual vascular supply of the liver—receiving oxygenated blood via the hepatic artery and nutrient-rich, often tumour cell-laden blood from the portal vein—creates a permissive gateway for metastatic colonization[74,75]. This anatomical specialization is compounded by liver sinusoidal endothelial cells, which exhibit a fenestrated ("window-like") ultrastructure lacking a continuous basement membrane, enabling direct transmigration of tumour cells into the hepatic parenchyma[76]. Furthermore, the immune-tolerant niche of the liver facilitates metastatic immune evasion and outgrowth[75,77]. Notably, liver metastases are more common than primary liver tumours and tend to have a poorer prognosis than other metastatic sites, with approximately 30%–70% of cancer patients dying from liver metastases[73]. Unfortunately, existing treatments, including systemic chemotherapy, radiotherapy, immunotherapy and targeted therapy, have limited and poor results for metastases[74,78]. As the concept of precision medicine develops, individualized diagnosis and treatment of liver metastases face two core challenges: The difficulty of tracing the pathology due to the invisibility or substantial heterogeneity of the primary focus and the limitations of traditional histomorphometric assessment in predicting treatment response and prognosis. As an emerging field of cross-fertilization between digital pathology and AI, pa
Primary site identification constitutes the cornerstone of precision management for liver metastases. Chen et al[79] pioneered a hybrid approach that integrates handcrafted pathomics features (nuclear morphology, cytoplasmic texture) with DL-derived spatial patterns to classify the primary origins of metastases among four common sources (colorectal, breast, etc.), achieving moderate discriminative performance (AUC: 0.64-0.83). Although limited by cohort size, their work revealed morphologic continuity between primary tumours and metastases—specifically, moderately differentiated regions enriched with cohesive epithelial clusters emerged as topologic landmarks for origin tracing. Cutting-edge multimodal DL models have demonstrated remarkable potential in enhancing diagnostic accuracy. The HEPNET system developed by Albrecht et al[80] achieved an exceptional AUC of 0.997 in blinded testing for distinguishing iCCA from colorectal cancer liver metastases through analysis of HE-stained WSIs. This diagnostic performance surpasses even that of experienced pathologists. This technological breakthrough offers dual clinical advantages: It significantly reduces the reliance on costly IHC testing while minimizing diagnostic variability through standardized feature extraction. The capacity of this system to mitigate the observer-dependent interpretation biases inherent in traditional histopathological assessment is particularly noteworthy. Simultaneously, Jang et al[81] developed a pathomics-powered triple-classification framework that demonstrated exceptional discriminative ability (AUC > 0.995) in differentiating HCC, CCA, and colorectal cancer metastases. This achievement not only replicates previous successes in computational pathology but also crucially expands the application scope of AI to particularly challenging differential diagnostic scenarios in which conventional histopathological evaluation encounters inherent limitations.
After confirmation of primary tumour characteristics, histopathological growth pattern classification directly informs therapeutic decision-making. Höppener et al[82] developed a neural image compression algorithm that demonstrated exceptional discriminatory ability in automated differentiation between desmoplastic and non-desmoplastic colorectal liver metastases, achieving AUC values of 0.93–0.95. This automated system significantly outperformed manual as
The integration of spatial topological analysis has significantly expanded the prognostic evaluation framework. Qi et al[83] developed the CRLM-SPA framework, which transcends traditional morphological limitations by systematically quantifying 17 microenvironmental characteristics, including the tumour necrosis ratio and spatial distribution of lymphocyte infiltration. Through multicenter validation, their spatial organization feature (SOF) model amplified the 5-year survival stratification difference to 22.5%, demonstrating complementary prognostic value with the clinical risk score (CRS) [area under the receiver operating characteristic curve (AUROC) improvement, P = 0.004], indicating a paradigm shift from static morphological description to dynamic ecological analysis in pathomics. In preventive intervention research, Xiao et al[84] established a groundbreaking DL-based nomogram. Their study employed ResNet-50 networks to extract occult metastatic features from primary colorectal cancer lesions, constructing a predictive model (C-index, 0.81) integrated with pT/pN staging to identify populations at high risk for postoperative hepatic metastasis. Notably, the model's dynamic predictive ability for 1–3-year metastasis risk (AUC = 0.84) provides quantitative guidance for de
Pathomics has been used to establish comprehensive analytical frameworks spanning the entire diagnostic continuum of hepatic metastases—from primary lesion identification and molecular subtyping to metastatic risk stratification and TME characterization—through technological innovations incorporating CNNs and spatial topological quantification. However, three persistent challenges hinder clinical translation of these methods: (1) Intrinsic sample heterogeneity: Current architectures require massive annotated datasets, exemplified by the seven-class classification model of Kriegsman et al[85], which demands 204159 precisely labelled tissue regions for robust training; (2) Algorithm generalization limitations: Technical variances in pathological workflows constrain model adaptability, as highlighted by the report of Höppener et al[82] that their NIC algorithm results in staining protocol-dependent performance degradation; and (3) Translational implementation bottlenecks: Most systems lack interoperability with hospital infrastructure, parti
AI has revolutionized medical image analysis through advanced semantic segmentation techniques that precisely delineate anatomical structures and pathological anomalies, including pulmonary lobes, neoplastic lesions, and metastatic nodules. Clinically validated segmentation outputs are indispensable for surgical planning and therapeutic decision-making, enabling three-dimensional reconstruction of tumour morphology and the quantitative assessment of volumetric parameters, particularly in oncology applications where millimetre-level precision determines resection margins[86]. While whole-slide imaging technology has become increasingly prevalent in modern histopathology, the clinical implementation of this technology faces the dual challenges of massive data volumes (typically 1–3 GB per slide) and labour-intensive manual analysis protocols. The unique challenges of medical imaging require different approaches and solutions.
Recent advancements in DL have revolutionized histopathological image analysis. For nuclear segmentation, Lal et al[87] developed NucleiSegNet, which incorporates residual blocks and attention decoders to achieve efficient seg
To address data scarcity and annotation challenges, Jehanzaib et al[90] proposed the PathoSeg model coupled with PathopixGAN, which mitigates class imbalance through synthetic data generation. Their architecture combines a modified version of HRNet with CBAM attention mechanisms, which are supported by publicly available breast and prostate cancer datasets, facilitating cross-institutional validation. Hägele et al[91] pioneered a complementary label strategy to reduce reliance on pixel-level annotations, achieving a balanced accuracy of 0.91 in HCC segmentation and establishing new paradigms for weakly supervised learning. In classification tasks, Chen et al[92] demonstrated the efficacy of attention mechanisms using SENet models, achieving 95.27% accuracy in liver cancer differentiation grading, highlighting the universal value of DL in assisting with pathological diagnosis.
At the clinical integration level, Roy et al[93] developed HistoCAE with autoencoder reconstruction strategies for liver tumour segmentation, and their multiresolution MR-HistoCAE extension outperformed conventional segmentation networks in patch classification. Khened et al[94] created the DigiPathAI framework featuring a multimodel ensemble and uncertainty estimation, achieving state-of-the-art performance in breast, colon, and liver cancer WSI analysis. Their open-source pipeline enables complete workflow support from segmentation to tumour burden calculation, demon
The computational pathology field has three dominant innovation trajectories: (1) Architectural advancements incorporating attention mechanisms and residual optimization paradigms continue to push the boundaries of seg
The open-access availability of benchmark datasets (e.g., the KMC Hepatic Dataset) and diagnostic tools (e.g., DigiPathAI) will facilitate standardization in digital pathology. The strategic integration of pathological domain knowledge with DL architectures shows potential value for developing intelligent decision-support systems capable of clinical-grade diagnostic reasoning.
The molecular heterogeneity and complex microenvironmental features of HCC pose a serious challenge for precision diagnosis and treatment. In recent years, the in-depth integration of pathomics and transcriptomic data has provided a new dimension for analysing the biological nature of tumours. By integrating AI-driven digital pathology analysis with multiomics validation, researchers have gradually established interpretable associations from histomorphology to molecular phenotypes, opening innovative paths for prognostic stratification and treatment decisions.
In the field of molecular subtyping for liver cancer, the groundbreaking work by Calderaro et al[95] represents a pivotal milestone. Their team developed a DL framework that achieved precise reclassification of cHCC-CCA. This framework, in which a self-supervised pretrained ResNet50 feature extractor was synergistically integrated with an attention-based multiple instance learning framework, demonstrated exceptional diagnostic performance across the internal and external validation cohorts, achieving AUROC values of 0.99 and 0.94, respectively. Crucially, spatial transcriptomics validated the molecular underpinnings of model predictions: ICCA-dominant subtypes presented increased expression of biliary differentiation markers (e.g., KRT19, EPCAM), whereas HCC-dominant regions were enriched with hepatocytic markers
Notably, AI-driven pathomics transcends conventional morphological classification to enable therapeutic response prediction. The pioneering study by Zeng et al[96] provides compelling evidence for this paradigm shift. Through multiscale feature analysis of HCC histopathological images using DL architectures, such as clustering-constrained attention multiple instance learning, the team successfully constructed a predictive system for immune gene signature activation states, achieving validation AUCs of 0.81–0.92. Integrative digital pathology-transcriptomics analysis revealed that immune "hotspots" identified by the model correlated significantly with lymphocyte and plasma cell infiltration, establishing a visual decision-support framework for developing biomarkers targeting PD-1/PD-L1 inhibitors and other immunotherapies.
Collectively, these studies outline a technical blueprint for pathomics–transcriptomics integration. Calderaro et al[95] established a gold standard for morphology-molecular correlation through spatial multiomics validation, whereas the study by Zeng et al[96] pioneered a novel paradigm for therapy sensitivity prediction. These synergistic insights demonstrate that DL not only deciphers intrinsic tumour heterogeneity but also dynamically captures patterns in the spatiotemporal evolution of microenvironmental features and treatment responses. Future research should focus on incorporating multimodal data—including single-cell sequencing and dynamic radiomics—to develop interpretable cross-scale predictive models, ultimately enabling closed-loop clinical translation from histomorphological diagnosis to molecularly guided therapeutic intervention.
The field of liver cancer pathomics is currently at a critical juncture of technological innovation and clinical translation. AI-empowered digital pathology demonstrates transformative potential in enhancing diagnostic efficiency and optimizing personalized treatment strategies through predictive modeling and molecular subtype correlations[97,98]. In terms of technological breakthroughs, emerging technologies such as slide-free imaging systems and light sheet microscopy have begun to gain traction, signaling potential future technological pathways[99].
Despite landmark advancements, such as the United States Food and Drug Administration (FDA) approval of Paige Prostate[100] as the first AI-assisted diagnostic tool, persistent challenges hinder its widespread adoption. First, regulatory barriers remain significant: The approval process for AI medical devices is complex and time-consuming, particularly in pathology, where authorization lags far behind other fields like radiology. Laboratory-developed tests (LDTs) and in-house devices are facing increasingly stringent regulatory requirements (e.g., the FDA's proposed new regulations for LDTs and the European Union's In Vitro Diagnostic Medical Devices Regulation framework), which have raised the barriers and uncertainties in their development and clinical application. Additionally, disparities in regulatory frameworks across different countries and regions pose challenges to standardization and transnational deployment[101].
Second, data interoperability and integration with existing hospital information systems remain critical bottlenecks: Seamlessly embedding AI tools into clinical workflows, particularly achieving interoperability with LIS and PACS poses significant technical challenges. The lack of unified standards for pathological data formats, annotations, and model outputs further impedes data sharing and model generalizability. Meanwhile, the high infrastructure costs required for digital pathology itself and AI integration (including scanners, storage, and computational resources), along with the resulting infrastructure disparity—particularly in resource-constrained regions such as parts of Asia—severely limit its widespread adoption[102].
Third, the demand for rigorous prospective validation is critical: Although many models excel in retrospective studies, their clinical value and safety must be confirmed through large-scale, multicenter prospective trials in real-world settings to gain broad clinical acceptance[103-105]. Currently, most AI tools lack support from the highest levels of clinical evidence, and research dedicated to such validation remains limited. Validation must not only focus on technical accuracy but also demonstrate tangible clinical benefits[106]. Additionally, the sluggish standardization of HCC specific pathological data repositories, coupled with underdeveloped reimbursement frameworks and a lack of evidence-based clinical integration guidelines[107], collectively hinder the pace of clinical translation.
Current research should focus on discovering HCC -specific biomarkers and enhancing model interpretability through multi-omics data integration. To ultimately achieve clinical translation, it is imperative to establish an interdisciplinary collaboration framework to jointly develop standardized HCC pathomics data protocols, design cost-effective regionalized solutions, and formulate clinical practice guidelines based on real-world evidence, thereby advancing this technology from experimental research to precision diagnosis and treatment of HCC. Overcoming these interconnected challenges is pivotal to translating the potential of AI-driven pathomics into tangible improvements in patient care.
| 1. | Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1467] [Article Influence: 293.4] [Reference Citation Analysis (0)] |
| 2. | GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022-2050: a forecasting analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403:2204-2256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 569] [Article Influence: 284.5] [Reference Citation Analysis (0)] |
| 3. | Donne R, Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77:1773-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 435] [Article Influence: 145.0] [Reference Citation Analysis (1)] |
| 4. | Huang M, Ji Q, Huang H, Wang X, Wang L. Gut microbiota in hepatocellular carcinoma immunotherapy: immune microenvironment remodeling and gut microbiota modification. Gut Microbes. 2025;17:2486519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 5. | Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, Wei W, Lemmens VEPP, Soerjomataram I. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022;161:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 347] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 6. | Jiang Z, Zeng G, Dai H, Bian Y, Wang L, Cao W, Yang J. Global, regional and national burden of liver cancer 1990-2021: a systematic analysis of the global burden of disease study 2021. BMC Public Health. 2025;25:931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 7. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4448] [Article Influence: 889.6] [Reference Citation Analysis (4)] |
| 8. | Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 9. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12654] [Article Influence: 6327.0] [Reference Citation Analysis (6)] |
| 10. | Laws A, Punglia RS. Endocrine Therapy for Primary and Secondary Prevention After Diagnosis of High-Risk Breast Lesions or Preinvasive Breast Cancer. J Clin Oncol. 2023;41:3092-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (1)] |
| 11. | Jiang P, Sinha S, Aldape K, Hannenhalli S, Sahinalp C, Ruppin E. Big data in basic and translational cancer research. Nat Rev Cancer. 2022;22:625-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 12. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 21141] [Article Influence: 1921.9] [Reference Citation Analysis (2)] |
| 13. | Gupta R, Kurc T, Sharma A, Almeida JS, Saltz J. The Emergence of Pathomics. Curr Pathobiol Rep. 2019;7:73-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak JAWM; the CAMELYON16 Consortium, Hermsen M, Manson QF, Balkenhol M, Geessink O, Stathonikos N, van Dijk MC, Bult P, Beca F, Beck AH, Wang D, Khosla A, Gargeya R, Irshad H, Zhong A, Dou Q, Li Q, Chen H, Lin HJ, Heng PA, Haß C, Bruni E, Wong Q, Halici U, Öner MÜ, Cetin-Atalay R, Berseth M, Khvatkov V, Vylegzhanin A, Kraus O, Shaban M, Rajpoot N, Awan R, Sirinukunwattana K, Qaiser T, Tsang YW, Tellez D, Annuscheit J, Hufnagl P, Valkonen M, Kartasalo K, Latonen L, Ruusuvuori P, Liimatainen K, Albarqouni S, Mungal B, George A, Demirci S, Navab N, Watanabe S, Seno S, Takenaka Y, Matsuda H, Ahmady Phoulady H, Kovalev V, Kalinovsky A, Liauchuk V, Bueno G, Fernandez-Carrobles MM, Serrano I, Deniz O, Racoceanu D, Venâncio R. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA. 2017;318:2199-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1871] [Cited by in RCA: 1666] [Article Influence: 185.1] [Reference Citation Analysis (1)] |
| 15. | Naik N, Madani A, Esteva A, Keskar NS, Press MF, Ruderman D, Agus DB, Socher R. Deep learning-enabled breast cancer hormonal receptor status determination from base-level H&E stains. Nat Commun. 2020;11:5727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 16. | Kather JN, Heij LR, Grabsch HI, Loeffler C, Echle A, Muti HS, Krause J, Niehues JM, Sommer KAJ, Bankhead P, Kooreman LFS, Schulte JJ, Cipriani NA, Buelow RD, Boor P, Ortiz-Brüchle NN, Hanby AM, Speirs V, Kochanny S, Patnaik A, Srisuwananukorn A, Brenner H, Hoffmeister M, van den Brandt PA, Jäger D, Trautwein C, Pearson AT, Luedde T. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat Cancer. 2020;1:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 385] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 17. | Li F, Yang Y, Wei Y, He P, Chen J, Zheng Z, Bu H. Deep learning-based predictive biomarker of pathological complete response to neoadjuvant chemotherapy from histological images in breast cancer. J Transl Med. 2021;19:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Banna GL, Olivier T, Rundo F, Malapelle U, Fraggetta F, Libra M, Addeo A. The Promise of Digital Biopsy for the Prediction of Tumor Molecular Features and Clinical Outcomes Associated With Immunotherapy. Front Med (Lausanne). 2019;6:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Madabhushi A, Lee G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med Image Anal. 2016;33:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 576] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 20. | Ström P, Kartasalo K, Olsson H, Solorzano L, Delahunt B, Berney DM, Bostwick DG, Evans AJ, Grignon DJ, Humphrey PA, Iczkowski KA, Kench JG, Kristiansen G, van der Kwast TH, Leite KRM, McKenney JK, Oxley J, Pan CC, Samaratunga H, Srigley JR, Takahashi H, Tsuzuki T, Varma M, Zhou M, Lindberg J, Lindskog C, Ruusuvuori P, Wählby C, Grönberg H, Rantalainen M, Egevad L, Eklund M. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: a population-based, diagnostic study. Lancet Oncol. 2020;21:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 21. | Escobar Díaz Guerrero R, Carvalho L, Bocklitz T, Popp J, Oliveira JL. Software tools and platforms in Digital Pathology: a review for clinicians and computer scientists. J Pathol Inform. 2022;13:100103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5567] [Cited by in RCA: 5912] [Article Influence: 656.9] [Reference Citation Analysis (0)] |
| 23. | Jiang W, Mei WJ, Xu SY, Ling YH, Li WR, Kuang JB, Li HS, Hui H, Li JB, Cai MY, Pan ZZ, Zhang HZ, Li L, Ding PR. Clinical actionability of triaging DNA mismatch repair deficient colorectal cancer from biopsy samples using deep learning. EBioMedicine. 2022;81:104120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 24. | Chen S, Zhang M, Wang J, Xu M, Hu W, Wee L, Dekker A, Sheng W, Zhang Z. Automatic Tumor Grading on Colorectal Cancer Whole-Slide Images: Semi-Quantitative Gland Formation Percentage and New Indicator Exploration. Front Oncol. 2022;12:833978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Kather JN, Weis CA, Bianconi F, Melchers SM, Schad LR, Gaiser T, Marx A, Zöllner FG. Multi-class texture analysis in colorectal cancer histology. Sci Rep. 2016;6:27988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 26. | Bilgin CC, Rittscher J, Filkins R, Can A. Digitally adjusting chromogenic dye proportions in brightfield microscopy images. J Microsc. 2012;245:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Luo X, Zang X, Yang L, Huang J, Liang F, Rodriguez-Canales J, Wistuba II, Gazdar A, Xie Y, Xiao G. Comprehensive Computational Pathological Image Analysis Predicts Lung Cancer Prognosis. J Thorac Oncol. 2017;12:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Mousavi HS, Monga V, Rao G, Rao AU. Automated discrimination of lower and higher grade gliomas based on histopathological image analysis. J Pathol Inform. 2015;6:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Yu KH, Zhang C, Berry GJ, Altman RB, Ré C, Rubin DL, Snyder M. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun. 2016;7:12474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 610] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 30. | Stahlschmidt SR, Ulfenborg B, Synnergren J. Multimodal deep learning for biomedical data fusion: a review. Brief Bioinform. 2022;23:bbab569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 31. | Manivannan S, Li W, Zhang J, Trucco E, McKenna SJ. Structure Prediction for Gland Segmentation With Hand-Crafted and Deep Convolutional Features. IEEE Trans Med Imaging. 2018;37:210-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Nanni L, Brahnam S, Ghidoni S, Lumini A. Bioimage Classification with Handcrafted and Learned Features. IEEE/ACM Trans Comput Biol Bioinform. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Eckhardt CM, Madjarova SJ, Williams RJ, Ollivier M, Karlsson J, Pareek A, Nwachukwu BU. Unsupervised machine learning methods and emerging applications in healthcare. Knee Surg Sports Traumatol Arthrosc. 2023;31:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 34. | Wu IC, Chen YC, Karmakar R, Mukundan A, Gabriel G, Wang CC, Wang HC. Advancements in Hyperspectral Imaging and Computer-Aided Diagnostic Methods for the Enhanced Detection and Diagnosis of Head and Neck Cancer. Biomedicines. 2024;12:2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Chen Y, Wang A, Liu Z, Yue J, Zhang E, Li F, Zhang N. MoSViT: a lightweight vision transformer framework for efficient disease detection via precision attention mechanism. Front Artif Intell. 2025;8:1498025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Somers DC, Sheremata SL. Attention maps in the brain. Wiley Interdiscip Rev Cogn Sci. 2013;4:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Rong H, Liu G, Wang Y, Hu J, Sun Z, Gao N, Kee CS, Du B, Wei R. Using 3D Convolutional Neural Network and Corvis ST Corneal Dynamic Video for Detecting Forme Fruste Keratoconus. J Refract Surg. 2025;41:e356-e364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Li T, Qi W, Mao X, Jia G, Zhang W, Li X, Pan H, Wang D. Prediction of Lumbar Disc Degeneration Based on Interpretable Machine Learning Models: Retrospective Cohort Study. Spine J. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 40. | Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71:616-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 415] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 41. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4363] [Article Influence: 545.4] [Reference Citation Analysis (6)] |
| 42. | Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: An update. J Hepatol. 2021;74:1212-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 43. | Holczbauer Á, Factor VM, Andersen JB, Marquardt JU, Kleiner DE, Raggi C, Kitade M, Seo D, Akita H, Durkin ME, Thorgeirsson SS. Modeling pathogenesis of primary liver cancer in lineage-specific mouse cell types. Gastroenterology. 2013;145:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Zarella MD, Rivera Alvarez K. High-throughput whole-slide scanning to enable large-scale data repository building. J Pathol. 2022;257:383-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Calderaro J, Kather JN. Artificial intelligence-based pathology for gastrointestinal and hepatobiliary cancers. Gut. 2021;70:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 46. | Cheng N, Ren Y, Zhou J, Zhang Y, Wang D, Zhang X, Chen B, Liu F, Lv J, Cao Q, Chen S, Du H, Hui D, Weng Z, Liang Q, Su B, Tang L, Han L, Chen J, Shao C. Deep Learning-Based Classification of Hepatocellular Nodular Lesions on Whole-Slide Histopathologic Images. Gastroenterology. 2022;162:1948-1961.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 47. | Aatresh AA, Alabhya K, Lal S, Kini J, Saxena PUP. LiverNet: efficient and robust deep learning model for automatic diagnosis of sub-types of liver hepatocellular carcinoma cancer from H&E stained liver histopathology images. Int J Comput Assist Radiol Surg. 2021;16:1549-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Sun C, Xu A, Liu D, Xiong Z, Zhao F, Ding W. Deep Learning-Based Classification of Liver Cancer Histopathology Images Using Only Global Labels. IEEE J Biomed Health Inform. 2020;24:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Liao H, Long Y, Han R, Wang W, Xu L, Liao M, Zhang Z, Wu Z, Shang X, Li X, Peng J, Yuan K, Zeng Y. Deep learning-based classification and mutation prediction from histopathological images of hepatocellular carcinoma. Clin Transl Med. 2020;10:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 50. | Beaufrère A, Ouzir N, Zafar PE, Laurent-Bellue A, Albuquerque M, Lubuela G, Grégory J, Guettier C, Mondet K, Pesquet JC, Paradis V. Primary liver cancer classification from routine tumour biopsy using weakly supervised deep learning. JHEP Rep. 2024;6:101008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 51. | Dong X, Li M, Zhou P, Deng X, Li S, Zhao X, Wu Y, Qin J, Guo W. Fusing pre-trained convolutional neural networks features for multi-differentiated subtypes of liver cancer on histopathological images. BMC Med Inform Decis Mak. 2022;22:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 52. | Kiani A, Uyumazturk B, Rajpurkar P, Wang A, Gao R, Jones E, Yu Y, Langlotz CP, Ball RL, Montine TJ, Martin BA, Berry GJ, Ozawa MG, Hazard FK, Brown RA, Chen SB, Wood M, Allard LS, Ylagan L, Ng AY, Shen J. Impact of a deep learning assistant on the histopathologic classification of liver cancer. NPJ Digit Med. 2020;3:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 53. | Liu B, Li J, Yang X, Chen F, Zhang Y, Li H. Diagnosis of primary clear cell carcinoma of the liver based on Faster region-based convolutional neural network. Chin Med J (Engl). 2023;136:2706-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 54. | Sheng X, Ji Y, Ren GP, Lu CL, Yun JP, Chen LH, Meng B, Qu LJ, Duan GJ, Sun Q, Ye XQ, Li SS, Yang J, Liao B, Wang ZB, Zhou JH, Sun Y, Qiu XS, Wang L, Li ZS, Chen J, Xia CY, He S, Li CY, Xu EW, Geng JS, Pan C, Kuang D, Qin R, Guan HW, Wang ZD, Li LX, Zhang X, Wang H, Zhao Q, Wei B, Zhang WJ, Ling SP, Du X, Cong WM; Liver Cancer Pathology Group of China (LCPGC). A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14:1034-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 55. | Jiang H, Wei J, Fu F, Wei H, Qin Y, Duan T, Chen W, Xie K, Lee JM, Bashir MR, Wang M, Song B, Tian J. Predicting microvascular invasion in hepatocellular carcinoma: A dual-institution study on gadoxetate disodium-enhanced MRI. Liver Int. 2022;42:1158-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 56. | Zhang X, Ruan S, Xiao W, Shao J, Tian W, Liu W, Zhang Z, Wan D, Huang J, Huang Q, Yang Y, Yang H, Ding Y, Liang W, Bai X, Liang T. Contrast-enhanced CT radiomics for preoperative evaluation of microvascular invasion in hepatocellular carcinoma: A two-center study. Clin Transl Med. 2020;10:e111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 57. | Huo TI, Liu PH, Hsu CY. Detecting microvascular invasion in HCC with contrast-enhanced MRI: Is it a good idea? J Hepatol. 2018;68:862-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Zhang X, Yu X, Liang W, Zhang Z, Zhang S, Xu L, Zhang H, Feng Z, Song M, Zhang J, Feng S. Deep learning-based accurate diagnosis and quantitative evaluation of microvascular invasion in hepatocellular carcinoma on whole-slide histopathology images. Cancer Med. 2024;13:e7104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Laurent-Bellue A, Sadraoui A, Claude L, Calderaro J, Posseme K, Vibert E, Cherqui D, Rosmorduc O, Lewin M, Pesquet JC, Guettier C. Deep Learning Classification and Quantification of Pejorative and Nonpejorative Architectures in Resected Hepatocellular Carcinoma from Digital Histopathologic Images. Am J Pathol. 2024;194:1684-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Chen Q, Xiao H, Gu Y, Weng Z, Wei L, Li B, Liao B, Li J, Lin J, Hei M, Peng S, Wang W, Kuang M, Chen S. Deep learning for evaluation of microvascular invasion in hepatocellular carcinoma from tumor areas of histology images. Hepatol Int. 2022;16:590-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Feng S, Yu X, Liang W, Li X, Zhong W, Hu W, Zhang H, Feng Z, Song M, Zhang J, Zhang X. Development of a Deep Learning Model to Assist With Diagnosis of Hepatocellular Carcinoma. Front Oncol. 2021;11:762733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 62. | Qu WF, Tian MX, Lu HW, Zhou YF, Liu WR, Tang Z, Yao Z, Huang R, Zhu GQ, Jiang XF, Tao CY, Fang Y, Gao J, Wu XL, Chen JF, Zhao QF, Yang R, Chu TH, Zhou J, Fan J, Yu JH, Shi YH. Development of a deep pathomics score for predicting hepatocellular carcinoma recurrence after liver transplantation. Hepatol Int. 2023;17:927-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 63. | Qu WF, Tian MX, Qiu JT, Guo YC, Tao CY, Liu WR, Tang Z, Qian K, Wang ZX, Li XY, Hu WA, Zhou J, Fan J, Zou H, Hou YY, Shi YH. Exploring pathological signatures for predicting the recurrence of early-stage hepatocellular carcinoma based on deep learning. Front Oncol. 2022;12:968202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 64. | Zhou X, Man M, Cui M, Zhou X, Hu Y, Liu Q, Deng Y. Relationship between EZH2 expression and prognosis of patients with hepatocellular carcinoma using a pathomics predictive model. Heliyon. 2024;10:e38562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 65. | Jia W, Shi W, Yao Q, Mao Z, Chen C, Fan A, Wang Y, Zhao Z, Li J, Song W. Identifying immune infiltration by deep learning to assess the prognosis of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2023;149:12621-12635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Saillard C, Schmauch B, Laifa O, Moarii M, Toldo S, Zaslavskiy M, Pronier E, Laurent A, Amaddeo G, Regnault H, Sommacale D, Ziol M, Pawlotsky JM, Mulé S, Luciani A, Wainrib G, Clozel T, Courtiol P, Calderaro J. Predicting Survival After Hepatocellular Carcinoma Resection Using Deep Learning on Histological Slides. Hepatology. 2020;72:2000-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 67. | Ding GY, Tan WM, Lin YP, Ling Y, Huang W, Zhang S, Shi JY, Luo RK, Ji Y, Wang XY, Zhou J, Fan J, Cai MY, Yan B, Gao Q. Mining the interpretable prognostic features from pathological image of intrahepatic cholangiocarcinoma using multi-modal deep learning. BMC Med. 2024;22:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 68. | Xie J, Pu X, He J, Qiu Y, Lu C, Gao W, Wang X, Lu H, Shi J, Xu Y, Madabhushi A, Fan X, Chen J, Xu J. Survival prediction on intrahepatic cholangiocarcinoma with histomorphological analysis on the whole slide images. Comput Biol Med. 2022;146:105520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Shi JY, Wang X, Ding GY, Dong Z, Han J, Guan Z, Ma LJ, Zheng Y, Zhang L, Yu GZ, Wang XY, Ding ZB, Ke AW, Yang H, Wang L, Ai L, Cao Y, Zhou J, Fan J, Liu X, Gao Q. Exploring prognostic indicators in the pathological images of hepatocellular carcinoma based on deep learning. Gut. 2021;70:951-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 70. | Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020;20:483-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 71. | Brodt P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin Cancer Res. 2016;22:5971-5982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 72. | Williamson T, Sultanpuram N, Sendi H. The role of liver microenvironment in hepatic metastasis. Clin Transl Med. 2019;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Mielgo A, Schmid MC. Liver Tropism in Cancer: The Hepatic Metastatic Niche. Cold Spring Harb Perspect Med. 2020;10:a037259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 74. | Milette S, Sicklick JK, Lowy AM, Brodt P. Molecular Pathways: Targeting the Microenvironment of Liver Metastases. Clin Cancer Res. 2017;23:6390-6399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 75. | Zheng M, Tian Z. Liver-Mediated Adaptive Immune Tolerance. Front Immunol. 2019;10:2525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 76. | Clark AM, Ma B, Taylor DL, Griffith L, Wells A. Liver metastases: Microenvironments and ex-vivo models. Exp Biol Med (Maywood). 2016;241:1639-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 77. | Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol. 2011;11:879-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, Chopra Z, El Naqa I, Zhou J, Bian Y, Jiang L, Tezel A, Skvarce J, Achar RK, Sitto M, Rosen BS, Su F, Narayanan SP, Cao X, Wei S, Szeliga W, Vatan L, Mayo C, Morgan MA, Schonewolf CA, Cuneo K, Kryczek I, Ma VT, Lao CD, Lawrence TS, Ramnath N, Wen F, Chinnaiyan AM, Cieslik M, Alva A, Zou W. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 753] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 79. | Chen C, Lu C, Viswanathan V, Maveal B, Maheshwari B, Willis J, Madabhushi A. Identifying primary tumor site of origin for liver metastases via a combination of handcrafted and deep learning features. J Pathol Clin Res. 2024;10:e344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Albrecht T, Rossberg A, Albrecht JD, Nicolay JP, Straub BK, Gerber TS, Albrecht M, Brinkmann F, Charbel A, Schwab C, Schreck J, Brobeil A, Flechtenmacher C, von Winterfeld M, Köhler BC, Springfeld C, Mehrabi A, Singer S, Vogel MN, Neumann O, Stenzinger A, Schirmacher P, Weis CA, Roessler S, Kather JN, Goeppert B. Deep Learning-Enabled Diagnosis of Liver Adenocarcinoma. Gastroenterology. 2023;165:1262-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 81. | Jang HJ, Go JH, Kim Y, Lee SH. Deep Learning for the Pathologic Diagnosis of Hepatocellular Carcinoma, Cholangiocarcinoma, and Metastatic Colorectal Cancer. Cancers (Basel). 2023;15:5389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 82. | Höppener DJ, Aswolinskiy W, Qian Z, Tellez D, Nierop PMH, Starmans M, Nagtegaal ID, Doukas M, de Wilt JHW, Grünhagen DJ, van der Laak JAWM, Vermeulen P, Ciompi F, Verhoef C. Classifying histopathological growth patterns for resected colorectal liver metastasis with a deep learning analysis. BJS Open. 2024;8:zrae127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 83. | Qi L, Liang JY, Li ZW, Xi SY, Lai YN, Gao F, Zhang XR, Wang DS, Hu MT, Cao Y, Xu LJ, Chan RCK, Xing BC, Wang X, Li YH. Deep learning-derived spatial organization features on histology images predicts prognosis in colorectal liver metastasis patients after hepatectomy. iScience. 2023;26:107702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 84. | Xiao C, Zhou M, Yang X, Wang H, Tang Z, Zhou Z, Tian Z, Liu Q, Li X, Jiang W, Luo J. Accurate Prediction of Metachronous Liver Metastasis in Stage I-III Colorectal Cancer Patients Using Deep Learning With Digital Pathological Images. Front Oncol. 2022;12:844067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 85. | Kriegsmann M, Kriegsmann K, Steinbuss G, Zgorzelski C, Albrecht T, Heinrich S, Farkas S, Roth W, Dang H, Hausen A, Gaida MM. Implementation of deep learning in liver pathology optimizes diagnosis of benign lesions and adenocarcinoma metastasis. Clin Transl Med. 2023;13:e1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Khan RF, Lee BD, Lee MS. Transformers in medical image segmentation: a narrative review. Quant Imaging Med Surg. 2023;13:8747-8767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 87. | Lal S, Das D, Alabhya K, Kanfade A, Kumar A, Kini J. NucleiSegNet: Robust deep learning architecture for the nuclei segmentation of liver cancer histopathology images. Comput Biol Med. 2021;128:104075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 88. | Rong R, Sheng H, Jin KW, Wu F, Luo D, Wen Z, Tang C, Yang DM, Jia L, Amgad M, Cooper LAD, Xie Y, Zhan X, Wang S, Xiao G. A Deep Learning Approach for Histology-Based Nucleus Segmentation and Tumor Microenvironment Characterization. Mod Pathol. 2023;36:100196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 89. | Gu Z, Wang S, Rong R, Zhao Z, Wu F, Zhou Q, Wen Z, Chi Z, Fang Y, Peng Y, Jia L, Chen M, Yang DM, Hoshida Y, Xie Y, Xiao G. Cell Segmentation With Globally Optimized Boundaries (CSGO): A Deep Learning Pipeline for Whole-Cell Segmentation in Hematoxylin-and-Eosin-Stained Tissues. Lab Invest. 2025;105:102184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Jehanzaib M, Almalioglu Y, Ozyoruk KB, Williamson DFK, Abdullah T, Basak K, Demir D, Keles GE, Zafar K, Turan M. A robust image segmentation and synthesis pipeline for histopathology. Med Image Anal. 2025;99:103344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 91. | Hägele M, Eschrich J, Ruff L, Alber M, Schallenberg S, Guillot A, Roderburg C, Tacke F, Klauschen F. Leveraging weak complementary labels enhances semantic segmentation of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Sci Rep. 2024;14:24988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 92. | Chen C, Chen C, Ma M, Ma X, Lv X, Dong X, Yan Z, Zhu M, Chen J. Classification of multi-differentiated liver cancer pathological images based on deep learning attention mechanism. BMC Med Inform Decis Mak. 2022;22:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Roy M, Kong J, Kashyap S, Pastore VP, Wang F, Wong KCL, Mukherjee V. Convolutional autoencoder based model HistoCAE for segmentation of viable tumor regions in liver whole-slide images. Sci Rep. 2021;11:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Khened M, Kori A, Rajkumar H, Krishnamurthi G, Srinivasan B. A generalized deep learning framework for whole-slide image segmentation and analysis. Sci Rep. 2021;11:11579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 95. | Calderaro J, Ghaffari Laleh N, Zeng Q, Maille P, Favre L, Pujals A, Klein C, Bazille C, Heij LR, Uguen A, Luedde T, Di Tommaso L, Beaufrère A, Chatain A, Gastineau D, Nguyen CT, Nguyen-Canh H, Thi KN, Gnemmi V, Graham RP, Charlotte F, Wendum D, Vij M, Allende DS, Aucejo F, Diaz A, Rivière B, Herrero A, Evert K, Calvisi DF, Augustin J, Leow WQ, Leung HHW, Boleslawski E, Rela M, François A, Cha AW, Forner A, Reig M, Allaire M, Scatton O, Chatelain D, Boulagnon-Rombi C, Sturm N, Menahem B, Frouin E, Tougeron D, Tournigand C, Kempf E, Kim H, Ningarhari M, Michalak-Provost S, Gopal P, Brustia R, Vibert E, Schulze K, Rüther DF, Weidemann SA, Rhaiem R, Pawlotsky JM, Zhang X, Luciani A, Mulé S, Laurent A, Amaddeo G, Regnault H, De Martin E, Sempoux C, Navale P, Westerhoff M, Lo RC, Bednarsch J, Gouw A, Guettier C, Lequoy M, Harada K, Sripongpun P, Wetwittayaklang P, Loménie N, Tantipisit J, Kaewdech A, Shen J, Paradis V, Caruso S, Kather JN. Deep learning-based phenotyping reclassifies combined hepatocellular-cholangiocarcinoma. Nat Commun. 2023;14:8290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 96. | Zeng Q, Klein C, Caruso S, Maille P, Laleh NG, Sommacale D, Laurent A, Amaddeo G, Gentien D, Rapinat A, Regnault H, Charpy C, Nguyen CT, Tournigand C, Brustia R, Pawlotsky JM, Kather JN, Maiuri MC, Loménie N, Calderaro J. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022;77:116-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 97. | Wang X, Zhao J, Marostica E, Yuan W, Jin J, Zhang J, Li R, Tang H, Wang K, Li Y, Wang F, Peng Y, Zhu J, Zhang J, Jackson CR, Zhang J, Dillon D, Lin NU, Sholl L, Denize T, Meredith D, Ligon KL, Signoretti S, Ogino S, Golden JA, Nasrallah MP, Han X, Yang S, Yu KH. A pathology foundation model for cancer diagnosis and prognosis prediction. Nature. 2024;634:970-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 192] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 98. | Chen RJ, Ding T, Lu MY, Williamson DFK, Jaume G, Song AH, Chen B, Zhang A, Shao D, Shaban M, Williams M, Oldenburg L, Weishaupt LL, Wang JJ, Vaidya A, Le LP, Gerber G, Sahai S, Williams W, Mahmood F. Towards a general-purpose foundation model for computational pathology. Nat Med. 2024;30:850-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 486] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 99. | Orringer DA, Camelo-Piragua S. Fast and slide-free imaging. Nat Biomed Eng. 2017;1:926-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Eloy C, Marques A, Pinto J, Pinheiro J, Campelos S, Curado M, Vale J, Polónia A. Artificial intelligence-assisted cancer diagnosis improves the efficiency of pathologists in prostatic biopsies. Virchows Arch. 2023;482:595-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 101. | Pinto DG, Bychkov A, Tsuyama N, Fukuoka J, Eloy C. Real-World Implementation of Digital Pathology: Results From an Intercontinental Survey. Lab Invest. 2023;103:100261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Zehra T, Parwani A, Abdul-Ghafar J, Ahmad Z. A suggested way forward for adoption of AI-Enabled digital pathology in low resource organizations in the developing world. Diagn Pathol. 2023;18:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 103. | Hosseini MS, Bejnordi BE, Trinh VQ, Chan L, Hasan D, Li X, Yang S, Kim T, Zhang H, Wu T, Chinniah K, Maghsoudlou S, Zhang R, Zhu J, Khaki S, Buin A, Chaji F, Salehi A, Nguyen BN, Samaras D, Plataniotis KN. Computational pathology: A survey review and the way forward. J Pathol Inform. 2024;15:100357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 104. | Chauhan C, Gullapalli RR. Ethics of AI in Pathology: Current Paradigms and Emerging Issues. Am J Pathol. 2021;191:1673-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 105. | Zarella MD, McClintock DS, Batra H, Gullapalli RR, Valante M, Tan VO, Dayal S, Oh KS, Lara H, Garcia CA, Abels E. Artificial intelligence and digital pathology: clinical promise and deployment considerations. J Med Imaging (Bellingham). 2023;10:051802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 106. | Ghassemi M, Oakden-Rayner L, Beam AL. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit Health. 2021;3:e745-e750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 526] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 107. | Zhang DY, Venkat A, Khasawneh H, Sali R, Zhang V, Pei Z. Implementation of Digital Pathology and Artificial Intelligence in Routine Pathology Practice. Lab Invest. 2024;104:102111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/