Published online May 24, 2025. doi: 10.5306/wjco.v16.i5.104577
Revised: February 27, 2025
Accepted: March 10, 2025
Published online: May 24, 2025
Processing time: 146 Days and 16.7 Hours
Gastric neuroendocrine tumors (G-NETs) are rare tumors originating from enterochromaffin-like cells, with an incidence of 0.4 per 100000 annually. There are three main types: (1) Type I, linked to chronic atrophic gastritis and hypergastrinemia, makes up 75%–80% of G-NETs; (2) Type II, associated with Zollinger-Ellison syndrome (ZES) and multiple endocrine neoplasia, comprises 5%; and (3) Type III, sporadic tumors with a higher metastatic potential, accounting for 15%–25%. Diagnosis involves endoscopy, biopsy, and histological examination. Additional methods include serum gastrin testing, immunohistochemistry, and imaging techniques such as computer tomography or magnetic resonance imaging for detecting metastasis. Type I treatment usually involves endoscopic resection (ER), with surgical resection for recurrence. Somatostatin analogs (SSAs) can reduce tumor size, and the prognosis is generally excellent. Type II treatment centers on surgical removal of the gastrinoma, with ER for smaller lesions and SSAs for symptom management. Type III requires surgical resection (partial or total gastrectomy) with lymph node dissection, and possibly chemotherapy. This type has a worse prognosis due to its aggressive nature. Emerging treatments like Peptide Receptor Radionuclide Therapy are promising for advanced cases, and ongoing research into immunotherapies is expanding future treatment options. Regular endoscopic follow-up is crucial to monitor for recurrence or metastasis across all types. Our literature review explores the current perspectives on G-NETs and highlights the importance of further research to improve diagnostic precision and treatment, particularly for those associated with less favorable cases.
To improve diagnostic precision and treatment, particularly for those associated with less favorable cases.
A systematic search was conducted in PubMed, Scopus, and Web of Science until September 2024. Two independent reviewers screened titles, abstracts, and full texts for eligibility based on G-NET treatment in adults. Eligible studies included cohort studies, clinical trials, case series, and case reports, while in vitro, pediatric, and non-English studies were excluded. Relevant data were extracted independently, and disagreements were resolved through discussion. Study quality was assessed using appropriate tools.
G-NETs are rare, classified into three types: (1) Type I; (2) Type II; and (3) Type III. Type I G-NETs, often associated with chronic atrophic gastritis, are typically slow-growing and low-grade, with favorable outcomes following surgical resection. Type II G-NETs arise in hypergastrinemia conditions like multiple endocrine neoplasia and ZES, showing moderate malignancy risk. Type III G-NETs, the most aggressive and least common, present with distant metastases and poor prognosis. Diagnosis relies on endoscopy, imaging, and biomarkers like chromogranin A. Treatment varies by type, ranging from ER to aggressive surgery and chemotherapy for advanced cases. Regular follow-up is essential to monitor recurrence, particularly for type III G-NETs.
G-NETs require tailored diagnosis and treatment based on type and stage. Types I and II generally have better prognosis, while types III and IV are linked to poorer outcomes due to invasion and metastasis. Treatment strategies vary from ER for type I to extensive surgery for type III. Emerging therapies, like somatostatin analogs and peptide-receptor radionuclide therapies, show promise in advanced cases. Further research is essential to improve early diagnosis and treatment, particularly for high-risk lesions.
Core Tip: Gastric neuroendocrine tumors are classified into three types, with type I and type II having a better prognosis, while type III presents a more aggressive course. Early diagnosis and treatment, including endoscopic resection and emerging therapies like Peptide Receptor Radionuclide Therapy, are key to improving outcomes, particularly for advanced cases. Further research is needed to enhance diagnostic accuracy and treatment options.
- Citation: Christodoulidis G, Kouliou MN, Ragias D, Chatziisaak D, Agko ES, Schizas D, Zacharoulis D. Last decade of advances in gastric neuroendocrine tumors: Innovations, challenges, and future directions. World J Clin Oncol 2025; 16(5): 104577
- URL: https://www.wjgnet.com/2218-4333/full/v16/i5/104577.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i5.104577
Neuroendocrine tumors (NETs) are a rare type of neoplasm presenting with an incidence of 8.19/100000 and 6.98/100000 in the United States[1,2]. About 70% of all NETs occur in the gastrointestinal tract, including pancreatic NETs, followed by NETs primarily sited in the lungs, and only 5% of them are found in other organs[3]. Gastric NETs (G-NETs) are a distinct subgroup within the broader category of gastroenteropancreatic NETs (GEP-NETs). GEP-NETs represent the largest group of NETs, encompassing all NETs located in the stomach, small intestine, pancreas, and colon[4]. G-NETs account for 1.9%-2.2% of all NETs and 5%-15% of all gastroenteropancreatic NETs[5]. Recently, the frequency of their diagnosis has been reported to be increasing, due to the steadily increasing number of upper gastrointestinal endoscopies being performed today and the technical expertise of endoscopists[6].
NETs are heterogeneous malignancies, which arise mainly from cells constituting the neuroendocrine system, histamine-secreting enterochromaffin-like (ECL) cells[7,8]. The ECL cell serves as histamine’s producing, storage, and secretion site[9]. The latest World Health Organization (WHO) guidelines classify G-NETs into 4 different categories, based on the cells of origin. Histamine-producing ECL cell NET, somatostatin-producing D-cell NET, gastrin-producing G-cell NET, and serotonin-producing ECL cell NET[3]. The great majority of G-NETs consist of such ECL cells and are situated in the corpus-fundus (oxyntic) mucosa[10].
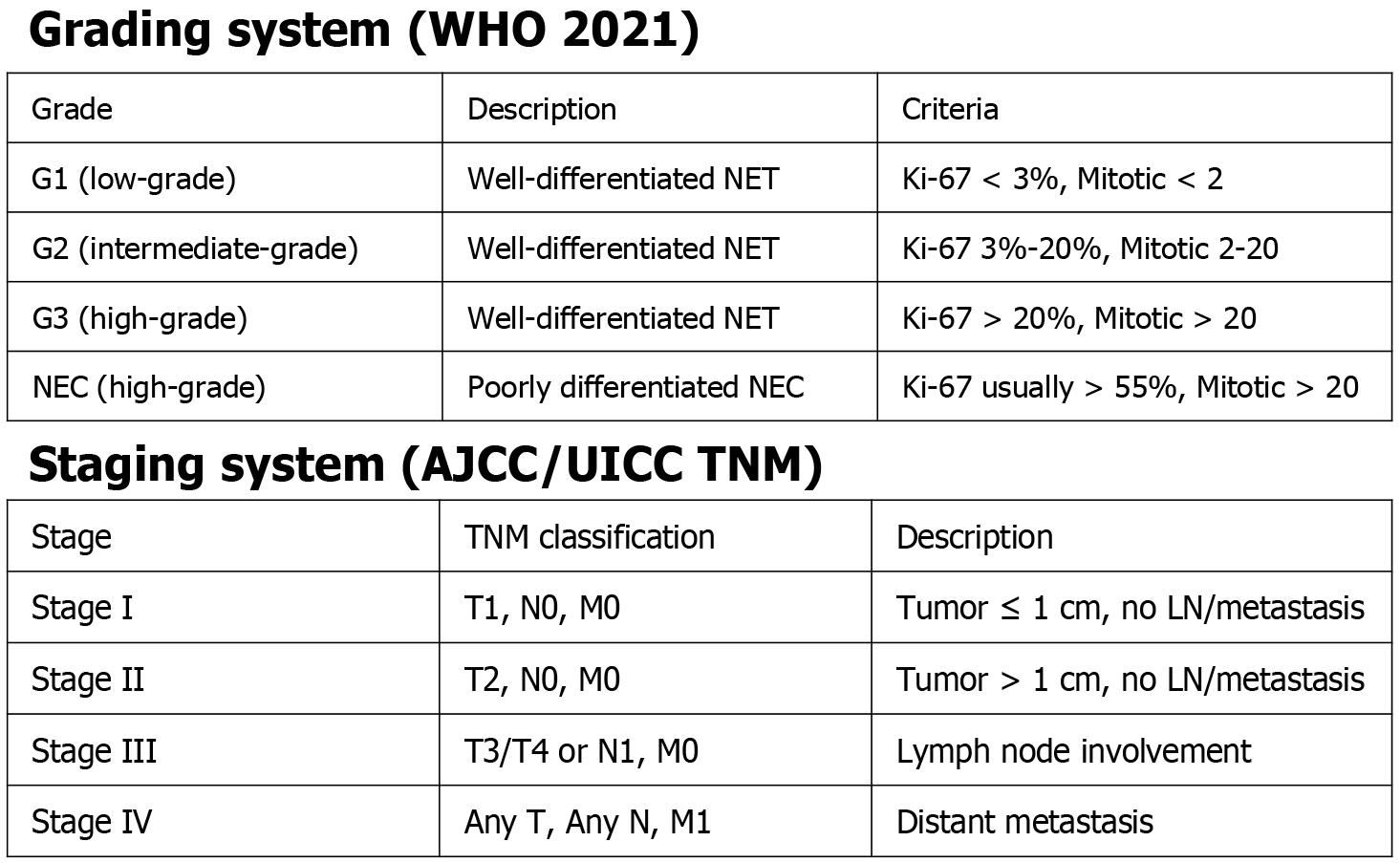
G-NETs are also histologically distinguished from other neuroendocrine neoplasms according to their level of differentiation and proliferation rate[11]. They are one of the two main subgroups of gastric neuroendocrine neoplasms, alongside gastric neuroendocrine carcinomas (NECs), while mixed neuroendocrine-non-neuroendocrine neoplasms are much less commonly traced. According to histologic-tumor proliferation, assessed by mitotic rate and Ki-67 index, WHO in 2010 classified well-differentiated GNETs as low grade (G1) or intermediate grade (G2), whereas high grade (G3) referred to poorly differentiated NECs[5,12,13]. In 2019 though, WHO introduced a novel classification, reclassifying well-differentiated GNETs as low grade (G1), intermediate grade (G2), and high grade (G3). Tumor grading in this classification was determined by the Ki-67 index, with G1 being ≤ 3%, G2 ranging from 3% to 20%, and G3 exceeding 20%[14-16]. NETs are histologically graded as the most well-differentiated subtype of any grade, while NECs and MinNENs represent highly malignant forms[10].
Based on their clinicopathological features and whether they originate from ECL cells, G-NETs are classified into types I to IV (Table 1). Types I and II are typically ECL cell-derived, type III can originate from either ECL or non-ECL cells, and type IV has a non-ECL cell origin[17,18]. Poorly differentiated gastric NEC, as well as mixed adeno-NEC, are often regarded as subtypes of type IV G-NETs[19,20]. Among these types, the most frequently identified NETs are type I, type II, and type III[21].
| Type I | Type II | Type III | Type IV | |
| Prevalence (%) | 70-80 | 5-7 | 10-20 | Rare |
| Predominance | Females | Same frequency male:female | Males | Not determined (possibly male) |
| Background | Chronic autoimmune gastritis | Zollinger-Ellison Syndrome (gastrinomas) | Normal gastric mucosa | Hypergastrinemia (1/3 of cases). Chronic atrophic gastritis (82% of cases) |
| Number of lesions | Multiple | Multiple | Single | Single |
| Size of lesions | 1-2 cm | < 10 mm | > 2 cm | > 4 cm (usually) |
| Site of lesions | Gastric body/fundus | Gastric body/fundus | Gastric body/fundus | Any part of the stomach |
| Underlying mucosa | Atrophic | Hypertrophic | Normal | Mainly atrophic (less frequently hypertrophic) |
| Cell of origin | ECL | ECL | ECL | Non-ECL |
| Serum gastrin levels | Elevated | Elevated | Normal | Elevated or normal |
| Gastric pH | High (> 7) | Low (< 2) | Normal | Approaching neutral to slightly alkaline |
| Aggressiveness | Low | Greater | High | High |
| Invasion | Rare | More common | Common | Common |
| Prognosis | Excellent | Very good | Poor | Poor |
G-NETs are furtherly staged using the Tumor (T), Node (N), and Metastasis (M) staging system established by the Union for International Cancer Control and the American Joint Committee on Cancer (AJCC)[22]. Both grading and staging of G-NETs are presented in Figure 1.
G-NETs are mainly non-functional, and, as a consequence, remain asymptomatic, resulting in delayed diagnosis[5,23]. Incidental symptoms such as non-specific abdominal pain, anemia, or upper gastrointestinal bleeding can therefore set the clinical suspicion[24]. After successful diagnosis, imaging, and staging, treatment follows. Therapeutic options consist of surveillance, endoscopic therapy or resection, surgical resection, and systemic therapies. Management of G-NETs is based on a variety of tumor-related factors such as tumor growth, the number of lesions, the extent of invasion, the differentiation of the tumor, and the presence or absence of metastases. Thus, as these factors get more seriously involved, the complexity of treatment options increases[5,23]. In that context, new treatment approaches have been suggested for each type[23].
In this literature review, we aim to provide an overview of all the new perspectives, diagnostic, and therapeutic on well-differentiated G-NETs and highlight emerging trends and future research directions to enhance effectiveness in the management of these lesions. Poorly differentiated NECs do not cover the purposes of this review and therefore will not be thoroughly described.
Articles from 3 databases (PubMed, Scopus, and Web of Science) were screened for further evaluation. Two independent reviewers (Kouliou MN and Ragias D) reviewed the titles and abstracts of potentially relevant articles for eligibility. The search was conducted by the end of September 2024. The search terms used were: (1) Gastric; (2) Stomach; (3) NET; (4) Neuroendocrine; (5) Tumor; (6) Neoplasm; and (7) Treatment, with Boolean operators 'AND' and 'OR' applied using all possible combinations.
The eligibility for inclusion of the screened articles was based on the treatment of adult patients with G-NETs. Review articles and original articles including cohort studies, randomized and non-randomized clinical trials, case series, and case reports were included and assessed for their eligibility by screening of the full text. In vitro studies, studies including pediatric population, and studies on animal models were excluded from our selection. Articles written in any language other than English were also excluded. Relevant data were extracted after reading the full text of the eligible publications by 2 independent reviewers (Kouliou MN and Ragias D). Disagreement on the inclusion or exclusion of specific articles was discussed and settled in meetings of all authors. Finally, the quality of the included studies was assessed with the use of available tools suitable for each type of study.
Epidemiology: Type I G-NETs represent the most prevalent NET subtype, accounting for 70%-80% of all the G-NETs consisting mainly of multiple small tumors 1-2 cm located in the gastric body and fundus[6,14,21,25,26]. A predominance of females is noted, comprising approximately 70% of all cases[21,27].
Pathophysiology/risk factors: Type I G-NETs develop mainly in the context of chronic autoimmune gastritis (AIG)[28,29]. AIG is distinguished by the production of autoantibodies directed against gastric parietal cells, targeting the H+/K+ ATPase, mainly in the cells of the gastric body and fundus[30]. The inflammation that occurs is followed by the destruction of parietal cells and subsequent reduction of their number, leading to reduced gastric acid secretion (hypochlorhydria). In an attempt to compensate for hypochlorhydria, and by the resulting disruption of the feedback inhibition, exerted normally via the somatostatin-producing D cells, the body induces hypergastrinemia, due to the persistent secretion of gastrin from the gastric G cells, which promotes ECL cell hyperplasia and potentially leads to emerging of type I G-NETs, based on cohort studies, in approximately 0.4%-0.7% of all patients suffering AIG yearly[5,30,31]. In later stages of the disease, the presence of antibodies against intrinsic factor, leads to its decreased production and vitamin B12 malabsorption. Long-standing vitamin B12 deficiency triggers pernicious anemia, a type of macrocytic anemia[32,33]. Recent research suggests that hypergastrinemia induced by the long-term use of proton pump inhibitors (PPIs) may contribute to the development of type I G-NETs[34,35]. Helicobacter pylori (H. Pylori) is commonly co-existent with AIG or as its causative factor and it has been suggested that it may play a significant role in type I G-NETs oncogenesis. However, this has not been sufficiently substantiated, and further research on this matter is necessary[36].
Diagnosis: Patients suffering G-NETs type I, are mostly incidentally traced in the context of esophagogastroduodenoscopy (EGD) conducted for screening purposes. The co-existing pernicious anemia or AIG and their clinical consequences are those that typically necessitate the screening examinations, while type I G-NETs exhibit predominantly an asymptomatic clinical presentation[37,38]. Immunohistochemically, positivity for markers such as chromogranin-A (CgA), synaptophysin, vesicular monoamine transporter 2 (VMAT2), and somatostatin receptor 2A (SSTR2A) indicates the presence of NET cells[21]. In histology, positive markers such as CgA, neuron-specific enolase (NSE), and VMAT2 (characteristic of histamine-producing cells) can also be detected[8].
During EGD, type I G-NETs appear as multiple small(< 10mm) subepithelial reddish polyps or nodules, smooth and rounded with or without central depression, contrasting with the smooth and reddish normal mucosa as well as the pale, yellowish, and transparent blood vessels of the antral mucosa. These lesions are located predominantly in the gastric body and fundus[6,38]. The definite diagnosis is only set after the conduction of both EGD and biopsy sampling, indicative of atrophic mucosa with intestinal and pseudopyloric metaplasia, absence of parietal cells, and neuroendocrine cell hyperplasia[6]. Furthermore, gastric acidity measurement demonstrates an elevated pH (pH ≥ 7)[21,26,39]. Once the initial diagnosis is settled, each lesion exceeding 1 cm, must be further evaluated with endoscopic ultrasound (EUS). EUS is used to highlight the hypoechoic or isoechoic lesions surrounded by well-defined margins within the lamina propria (second echo layer) or the submucosa (third echo layer), as well as to assess any possible involvement of regional lymph nodes (LNs) and determine the depth of invasion, a determining factor on whether an endoscopic resection (ER) is a viable option or not[21,26,39].
To determine tumor staging, further evaluation through computer tomography (CT), magnetic resonance imaging(MRI), octreoscan (SSTR scintigraphy), or positron emission tomography (PET), can provide insights into the tumor’s local infiltration and remote metastasis[40]. The utility of the latter remains under question. Contrast-enhanced abdominal CT can detect possible liver metastasis and is recommended for tumors larger than 2 cm[41,42]. MRI with the use of Gd-DOTA as a contrast agent provides higher detection capability than CT, guiding the handling of some patients[40]. Octreoscan is capable of detecting liver, bone, or LN metastases, however with poorer qualitative results in terms of cost-effectiveness and identification success rate than those achieved with 68Ga-labeled PET tracers. Additional imaging utilizing 68Ga-labeled PET tracers (68Ga-DOTATOC, 68Ga-DOTANOC, and 68Ga-DOTATATE), remains under investigation, though showing encouraging initial outcomes in comparison to the previously mentioned octreoscan[41,42].
Treatment choices: Type I G-NETs treatment should be personalized, taking into consideration factors such as tumor size, number of lesions, and overall patient condition[8]. Endoscopic management is promising, with optimal prognosis, and therefore suggested as first-line treatment, as long as the tumor consists of small, well-differentiated lesions[6,43,44]. Endoscopic management can vary from simple endoscopic polypectomy to endoscopic mucosal resection or endoscopic submucosal dissection[8]. Tumors less than 1 cm in size are typically managed through routine endoscopic monitoring or endoscopic removal. For lesions measuring between 1 cm and 2 cm, when six or fewer lesions are present, either ER or surveillance via EGD is advised[43]. In cases where there are six or fewer tumors larger than 2 cm, management options include ER when feasible or surgical resection as necessary[43].
Surgical treatment should be reserved for cases where ER is considered unfeasible or adverse prognostic factors are identified[6,44]. Frequent recurrences and multiple large lesions are cited as the primary unfavorable factors, however, precise definitions of these remain under debate. For patients presenting with more than six lesions exceeding 2 cm simultaneously, surgery is recommended as the sole treatment option[45]. Additionally, for lesions larger than 1 cm, extensive (involving the muscularis propria on EUS), multifocal (> 5), and recurrent on a site where ER was conducted, wedge resection of the stomach or even gastric antrectomy could turn out as useful methods in minimizing gastrin secretion[8]. Another definitive indication for surgical treatment is deep tissue invasion by tumors, LN metastasis, or in general factors that render ER ineffective. In contrast, necrosis, vascular invasion, or elevated Ki-67 alone are insufficient factors and can not urge surgery[6,45].
For patients not capable of undergoing surgical resection, clinical handling of type I G-NETs through the administration of somatostatin analogs (octreotide) could be an option. Current literature reveals, though, that the decrease in serum gastrin induced by these drugs lasts less than a year long, following their discontinuation. Due to this and similar considerations, therapy with octreotide should be considered as a last resort for patients unable to undergo surgery[6,44]. Another drug that can contribute to the management of such tumors is Netazepide, a gastrin/cholecystokinin 2 receptor antagonist. Netazepide acts mainly by blocking the gastrin-regulated signaling pathway, decreasing the secretion of gastrin, as well as serum plasma CgA levels, and shrinking the number and size of type I G-NETs[46,47]. Evidence from clinical trials highlights its utility in type I G-NETs by demonstrating its role in reducing the abundance of multiple gastrin-regulated genes, including ECL cell–specific markers such as CHGA and HDC, as well as pregnancy-associated plasma protein A2, a metalloproteinase implicated in insulin-like growth factor signaling and gastric tumor development, thereby contributing to tumor regression[46].
The 5-year survival rate for type I G-NETs following treatment, as demonstrated in recent patient series, is excellent, reaching rates as high as 100%, with no disease-associated mortality reported[8,18].
Challenges in the surgical treatment: A significant controversy has arisen regarding the ideal type of surgery. Antrectomy (removal of the antrum) targets the removal of G-cells (gastrin-producing cells), though, can be complicated, either by incomplete removal of these cells or by the independent function and secretion of ECL cells. Since that's the case, the focus is directed towards alternative surgical methods such as subtotal or total gastrectomy with or without lymphadenectomy. By subtotal gastrectomy, adequate removal of G-cells is achieved. Total gastrectomy is rarely the treatment of choice and is generally reserved only for cases with significant disease in the gastric fundus[6,44]. Regarding lymphadenectomy, it should be undertaken when there is any indication of extra-gastric disease or the presence of adverse prognostic factors. The extent of the procedure is not clearly defined by the available literature, and the decision between D1, D1+, or D2 lymphadenectomy should be made on a case-by-case basis, given the lack of consensus in the literature. Minimally invasive techniques are suitable when appropriate[46].
Follow-up: According to former National Comprehensive Cancer Network (NCCN) guidelines, the ideal approach for the follow-up of patients with small (< 20 mm) type I tumors was the annual conduction of the EGD after the first three postoperative years. On the other hand, for tumors exceeding 20 mm a re-evaluation of the patient course was proposed every 3 months to 12 months postoperatively for the first 3 year period, and every 6 months to 12 months thereafter recent guidelines proposed by the European Neuroendocrine Tumor Society (ENETS) recommend yearly follow-up with EGD for patients with type I G-NEΤs, after the complete ER of the lesion. Also, according to these guidelines, lesions not requiring resection should be primarily reevaluated with EGD in 6-12 months and then every 1-2 years. However, the risk of gastric adenocarcinoma development arising from chronic AIG in these patients underscores the significance of yearly follow-up with the use of EGD[21,27]. Seemingly, the NCCN guidelines suggest that the mostly benign nature of type I G-NETs and the low recurrence rate, promote a long-term but less frequent patient follow-up consisting of EGD, medical history taking, and clinical assessment[48].
Patients with type I G-NETs, as already stated, commonly present with atrophic gastritis, which increases the risk of deficiencies in essential nutrients, particularly vitamin B12 and iron, due to impaired gastric absorption. Regular monitoring is thus advised to evaluate their nutritional status. Supplementing with vitamin B12 and other necessary micronutrients helps prevent deficiency-related complications, allowing healthcare providers to manage these patients’ health more effectively[6,49].
Epidemiology: Type II G-NETs account for about 5%-7% of all the G-NETs[8,25], a percentage that ranks them as the rarest occurring G-NETs[26,43,44,50,51]. This type of G-NETs occurs with the same frequency in both male and female patients[43,51]. They exhibit a bimodal age distribution, impacting both younger adults with conditions such as MEN-1 and Zollinger-Ellison syndrome (ZES), as well as older adults[51].
Pathophysiology/risk factors: MEN-1 and ZES alone are considered the two main causes of type 2 G-NETs[8,44,50]. Sporadic ZES, though infrequent, rarely leads to type II G-NETs, while these tumors are more commonly associated with MEN-1 and the ZES that results from it. Specifically, MEN-1-ZES is identified as the cause of the tumors in 13%-43% of cases, while less than 1% can be attributed to sporadic ZES[8]. Pathophysiologically, the gastrinomas (gastrin-producing G-cell neoplasms) developed in the context of both conditions and located mostly in the pancreas or duodenum, are responsible for the excessive gastrin secretion, resulting in hypergastrinemia that drives the pathogenesis of type 2 G-NETs[5,43]. Hypergastrinemia in type 2 G-NETs activates the parietal cells, which produce hydrochloric acid (HCl), leading to a decrease in gastric pH and the development of hyperchlorhydria (gastric pH ≤ 2). In contrast, in type 1 G-NETs, although hypergastrinemia is also present, the pH remains high due to the destruction of parietal cells caused by chronic AIG, which are thus unable to produce HCl[8].
In patients, where MEN-1 is suspected, evaluation is necessary through serum sequencing for the MEN-1 gene located on chromosome 11q13[8,43]. MEN-1 syndrome, follows an autosomal dominant inheritance pattern, necessitating genetic evaluation. This need for genetic counseling is of great importance in patients who meet the clinical criteria for MEN-1, due to potential implications for other associated tumors, such as the aforementioned gastrinomas, and pancreatic NETs, including insulinomas, glucagonomas, vipomas, somatostatinomas[24,52]. MEN-1 gene normally encodes menin, a tumor suppressor protein[8,51]. MEN-1 gene mutations, traced in the context of MEN-1 syndrome, lead to its dysfunction. In particular, in about 9 out of 10 MEN-1-associated G-NETs, the biallelic inactivation of menin occurs following the mutation of one allele and loss of the wild-type remaining one[53]. This genetic alteration contributes to the transformation of G-NETs. Additionally, neuroendocrine microadenomatosis is a common feature seen in patients with MEN-1, further highlighting the complexity of this condition[51].
In comparison to type I G-NETs, type II ones tend to exhibit greater aggression and carry a higher, though still low, metastatic potential, estimated at around 10% to 30%[43,44,50,51]. A metastasis is to be expected mostly in tumors that are over 2 cm in length, invade the muscularis propria, and exhibit vascular invasion[51]. Furthermore, the risk of metastasis is estimated as higher, by the presence of positive diagnostic molecular markers such as elevated gastrin levels, elevated CgA levels, SSTR expression, Ki-67 > 2%, vascular endothelial growth factors overexpression, menin, and CDKN1B mutations[27]. The 5-year survival rate for Type II G-NETs following treatment is considered good, with percentages ranging from 70% to 90%[8].
Molecular heterogeneity of type II G-NETs and subsequent implications: Type II G-NETs are characterized by high molecular heterogeneity, regarding the many different pathways and alterations observed and the grade that they are involved in each tumor respectively[5]. The involvement of elevated gastrin levels in these tumors is one of the main characteristics, especially in the proliferation of the neuroendocrine tumor cells. It has been recently shown that more aggressive type II tumors may benefit even by a small increase in gastrin levels, suggesting that sensitivity to gastrin may vary even for lesions of the same type, as a result of alterations that can occur in that pathway[5].
MEN-1 also promotes tumorigenesis in type II G-NETs as a result of mutations of the MEN-1 gene, which lead to dysregulation of the protein it encodes, the menin protein. Subsequently, the large number of variants of this gene results in clinical heterogeneity not only of MEN-1 but of the associated lesions as well[4].
Despite MEN-1 being the most common example, regarding its direct relation to type II G-NETs, a significant part of genes and pathways has also been investigated for their involvement. Dysregulation of phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin and TP53, as well as mutations of PRKAR1A, and CDKN1B/p27 genes, have also been associated with the development of type II G-NETs. Mutations in the PRKAR1A gene found in these tumors have been demonstrated to disrupt the cyclic AMP (cAMP) pathway. The alterations caused by these mutations in the cAMP-dependent protein kinase type I-alpha regulatory subunit encoded by that gene are associated strongly with tumorigenesis in these lesions. Furthermore, CDKN1B/p27 mutations have been shown to affect cell cycle regulation[14].
Finally, epigenetic changes, especially DNA methylation, along with chromosomal and microsatellite instability, constitute a big chapter of such alterations. They also promote tumorigenesis and tumor cell proliferation and their relation to type II G-NETs has been extensively investigated for the association of the variants with the different clinical phenotypes they produce[4].
Apart from the identification of the different molecular alterations that contribute to the high heterogeneity of type II G-NETs, it is of high importance to connect these mutations with the subsequent changes in tumor cells’ phenotype and the possible implications this may have[14]. Regarding prognosis, lesions characterized by the presence of mutations of certain genes, such as TP53, are more aggressive and the prognosis is thus expected to be poorer. These features of the tumor cells also reflect on the treatment options. Recently, trials testing new agents that target the involved pathways are in progress. This opens the way for targeted and personalized medicine in the G-NETs family. However, in the context of the high molecular heterogeneity, further research is necessary for the identification of more features of the molecular profiles of these tumors for the development of new targeted and possibly more effective agents.
Diagnosis: Type II G-NETs can be asymptomatic on their own or cause non-specific symptoms, in the context of the co-existent peptic ulcers, developed due to the hypergastrinemia state[20]. This is also the reason why the diagnosis of such cases can delay up to 5 years, from the disease’s onset. Although this is the standard, there are also cases where the hypergastrinemia induced in the context of type 2 G-NETs can provoke the appearance of symptoms such as abdominal pain, due to the irritation of the so-developed gastric ulcers, or even secretory diarrhea[21,54].
Previously disease-free patients, once exposed to hypergastrinemia, undergo a transition of the normal gastric mucosa into a hypertrophic state, also characterized by ECL cell hyperplasia and dysplasia[8,43]. Morphologically there are two typical structures of type II G-NETs. An EGD can reveal either numerous, small(< 10 mm), polypoid lesions or yellowish smooth, hemispherical submucosal ones. When the second is the case, EGD with white light, manifests reddish hemispherical polyps, with or without a central depression, indicative of subepithelial tumor growth[20]. High-resolution magnifying endoscopy utilizing narrow-band imaging may uncover gastric pit-like structures on the tumor's surface; however, these features can be difficult to discern during conventional EGD since G-NETs are usually obscured by normal mucosa. In the region of the central depression, these pit-like structures are often lacking, with dilated blackish-brown subepithelial vessels displaying corkscrew-shaped capillaries instead[21,38]. Moreover, EGD may reveal associated peptic ulcer disease, with lesions predominantly located in the fundus but sometimes also present in the antrum[50].
Type 2 G-NETs are typically classified as well-differentiated NETs that are confined to the mucosa and/or submucosa[8]. Histopathological analysis shows a gastric oxyntic mucosa featuring an increased mass of oxyntic cells, which results from unchecked gastrin stimulation. Additionally, the analysis reveals nodules of neuroendocrine tumor nests that demonstrate negligible mitotic activity, no necrosis, and usually a low Ki-67 proliferation index (below 3%), indicating compatibility with a well-differentiated neuroendocrine tumor categorized as G1. Rarely, a G2 classification may be observed when the Ki-67 proliferation index is between 3% and 20%[51].
Furthermore, while the different histological types of ECL cell hyperplasia (including diffuse, linear, and micronodular) in the peritumoral gastric mucosa are found at similar frequencies in patients with both MEN-1 associated gastrinoma and sporadic gastrinoma, the presence of ECL cell dysplasia and ECL cell NETs has been observed solely in individuals with MEN-1[55].
Elevated gastric secretions, marked by hypergastrinemia, are characteristic of G-NETs type II, often presenting with high serum acidity resulting from hypochlorhydria[8].
In patients with either type I or type II G-NETs, serum gastrin levels frequently exceed 1000 pg/mL[43,51]. For type II G-NETs, particularly those associated with ZES, diagnosis not only requires elevated gastrin levels but also involves measuring serum CgA and assessing for possible MEN-1[56]. Alongside EGD and serum gastrin evaluation, genetic testing for MEN-1 and localization studies for gastrinomas are recommended. Screening for additional tumors associated with MEN-1, such as those affecting the parathyroid and pituitary glands, is also advised. Detection of gastrinomas can further be supported by sequential serum gastrin measurements following secretin injection; while secretin administration typically reduces serum gastrin in healthy individuals, an abrupt increase in levels indicates the presence of a gastrinoma[50].
Treatment choices: Once the diagnosis is confirmed, localizing the gastrinoma should become a priority to guide potential surgical intervention for its removal. Gastrinomas are commonly found within the 'gastrinoma triangle', defined by the junction of the cystic duct and the common hepatic duct, the transition from the second to third portions of the duodenum, and the pancreatic neck[15]. However, identifying the lesion can be challenging. Thus, imaging techniques such as CT, MRI, EUS, octreotide scintigraphy, selective angiography, PET, and intraoperative ultrasonography can be valuable in localizing the tumor[44].
The role of EUS in patients with type II G-NETs is to assess the depth of the tumor's invasion, specify its precise location within the layers of the gastric wall, and determine whether an ER is a viable option. EUS typically shows G-NETs as hypoechoic, intramural structures located within the second (deeper mucosal) or third (submucosal) echo layers of the gastric wall[21,43]. Other, less commonly used imaging techniques, such as CT and MRI, provide valuable information on local tumor spread, and distant metastasis, and help with tumor staging[20,57]. Abdominal CT is recommended for tumors larger than 2 cm, while MRI is generally reserved for select cases where it can offer specific insights[43,44,51]. Octreoscan has a high sensitivity in detecting liver, bone, and LN metastasis. PET-CT scanning, though generally reserved for specific, extensive cases of type II G-NETs, has shown a higher ability to identify lesions at relatively reasonable costs. In NETs, PET imaging with 68Ga-labeled tracers is effective in detecting metastatic disease, though its specific role in identifying primary G-NET tumors requires further research[21,41-43].
Type II G-NETs are associated with a greater likelihood of LN involvement and metastasis compared to type I G-NETs, warranting a more intensive treatment approach[43,49,56]. The ENETS guidelines specifically recommend surgical resection for all type II G-NETs due to their greater metastatic risk, whereas the NCCN guidelines generally align the management of type II G-NETs with type I, though with some variations given these differences[21,49,56,57]. Management typically focuses on localizing and resecting the gastrinoma when present, as it often accompanies type II G-NETs and can influence disease progression[8,44,51]. For localized G-NET lesions without invasion beyond the submucosa, ER is generally sufficient. However, for invasive or metastatic lesions, an oncologic resection is preferred to address these aggressive characteristics[50,56,57]. When no adverse prognostic factors are present, ER may be adequate for small, localized tumors. Larger or multiple lesions, however, often call for a combined approach using both EGD and surgery[43,44,56,57].
In cases where surgical resection is not feasible, acid hypersecretion can be managed to prevent peptic ulcer disease through the use of PPIs or somatostatin analogs (SSAs)[49]. Currently, high-dose PPIs are the preferred treatment strategy, as SSAs, though also used, have been associated with potential worsening of type II G-NETs and elevated serum gastrin levels in some studies[8,43,48,56].
Follow-up: For type II GNET-s, annual endoscopic surveillance is advocated by the ENETS guidelines for recurrence, particularly if ZES persists from an in situ gastrinoma[20]. In contrast, based on the NCCN guidelines, a patient’s follow-up consisting of EGD, medical history taking, and clinical assessment is the ideal approach for patients with type II tumors. After the first three postoperative years, annual conduction of the EGD is recommended. For tumors exceeding 20 mm a re-evaluation of the patient course is proposed every 3 months to 12 months postoperatively for the first 3 year period, and every 6 to 12 months thereafter[48].
Epidemiology: Type III G-NETs manifest intermittently. These lesions constitute approximately 10%-20% of all G-NETs, representing the second most common type. They are distinguished by their high potential for malignant transformation[21,50,51]. A predominance of males is noted. Type III G-NETs have a significantly poorer prognosis compared to the other G-NET types, with the average survival being estimated at about 28 months, with a mortality rate reaching as high as 30%[50,51,56]. Additionally, it is estimated that between 5% and 10% of these patients may also develop concurrent gastric adenocarcinoma[56].
Pathophysiology/risk factors: To date, no association has been suggested between the occurrence of type III G-NETs and any predisposing conditions such as ECL-cell hyperplasia, hypergastrinemia, chronic atrophic gastritis, MEN-1, or ZES[21,51]. The current WHO 2019 classification categorizes them based on tumor grading (G1, G2, and G3) using mitotic count and Ki-67 index, but molecular markers are not yet widely incorporated into routine clinical classification[3]. TP53 and RB1 mutations, as well as alterations in chromatin remodeling genes, could play a role in stratifying these tumors further. Additionally, markers like p53 and RB expression may help distinguish aggressive type III G-NETs from NECs, aiding in treatment decisions[3,50,57]. The tumor’s single lesion appears on an otherwise normal-appearing gastric mucosa[50,57].
Diagnosis: The diagnosis can be corroborated by measuring plasma CgA, histamine, serotonin, and gastrin levels, which should be within the normal range[50]. Additionally, for well-differentiated tumors, CgA and NSE can serve as useful biomarkers[57].
Type III G-NETs are highly aggressive and frequently present with distant metastases at the time of diagnosis. They commonly spread to regional LNs and the liver[21,51]. The propensity for metastasis is associated with specific factors, including tumor size, angioinvasion, and deep infiltration into the muscularis propria[51]. According to the AJCC (8th edition), LN metastasis (N1) is observed in about 71% of type III cases, while distant metastasis to the liver (M1) occurs in about 69% of patients[51]. Therefore most type III lesions fit stages IIa, IIb (T3 N0 M0), IIIa (T4 N0 M0), IIIb (T1 N1 M0), or even IV (any T, any N, M0)[51].
When symptomatic, type III G-NETs exhibit symptoms similar to those caused in the setting of gastric adenocarcinoma, such as anemia, loss of appetite, dyspepsia, gastrointestinal bleeding, and weight loss[21,50,57]. Given that type III G-NETs are sporadic and unassociated with other conditions, they typically become symptomatic only when they reach a size that is sufficient to cause pain, bleeding, or noticeable weight loss[50,57].
While uncommon, a "carcinoid syndrome" may occur in the context of type III G-NETs, especially when liver metastasis is present[20]. Symptoms are mostly flushing, rapid heartbeat, and diarrhea[20]. In very rare cases, an atypical carcinoid syndrome linked to increased histamine release from ECL cells might also occur in the other G-NETs types, types I and II. This atypical presentation can involve persistent flushing, itching, wheals, bronchospasms, headaches, and tearing, potentially triggered by tyramine-rich foods or spontaneously[55,56]. Elevated levels of the histamine metabolite methylimidazole acetic acid in the urine, may be indicative in cases with atypical carcinoid syndrome[56].
The role of EGD, as a diagnostic tool, is crucial for identifying G-NETs. When it comes to type III G-NETs, a solitary lesion larger than 2 cm is typically detected, arising within normal-appearing gastric mucosa. These tumors can often have an ulcerated appearance, leading to significant hemorrhage. Though frequently coinciding with H. pylori-related gastritis, they are not related to gastric atrophy or peptic ulcers[57]. It is important to note that both the size and quantity of the tumor should be recorded. Biopsies should be conducted on the lesions themselves, as well as on the healthy areas of gastric mucosa along the greater and lesser curves, to facilitate comparison of ECL cell density[50].
Histologically, these tumors consist of various sporadically developed endocrine cells and do not correlate with gastrin hypersecretion[57]. Immunohistochemical profiling shows that type III G-NETs react positively to CgA, NSE, synaptophysin, and S-100 proteins[50]. Many cases demonstrate intermediate cytological atypia, characterized by abundant amphophilic cytoplasm, enlarged nuclei with open chromatin, and prominent nucleoli. Additional features such as single-cell apoptosis or significant necrosis may be present, with a Ki-67 proliferation index frequently exceeding 2%. Tumors with a Ki-67 index greater than 20% are classified as grade 3 (G3), while those below this threshold are generally categorized as grade 2 (G2)[51]. Mitosis rate > 1 per HPF. Invasion beyond the submucosa is common, and concurrent gastric adenocarcinoma is observed in 5%-10% of cases[17,57].
EUS assesses the depth of tumor invasion and highlights its position within the layers of the gastric wall. G-NETs are commonly located in the second (deeper mucosal) or third (submucosal) echo layers, appearing as hypoechoic structures within the stomach wall[7,20].
Imaging modalities such as CT and MRI are valuable tools that provide additional information about local spread and distant metastasis directing tumor staging, particularly in patients with extensive disease and those with type III lesions, where staging is essential[20]. In particular, CT or MRI scans are advantageous when there are concerns regarding metastatic disease or LN involvement. Conversely, radiolabeled somatostatin analogs, which assist in determining the location and extent of tumors, are not commonly utilized[50]. In the context of metastasis detection, Octreoscan identifies liver, bone, and LN metastases[21]. Recent studies have highlighted the advantages of using fluorodeoxyglucose PET, particularly with 68Ga-labeled PET tracers (such as 68Ga-DOTATOC, 68Ga-DOTANOC, and 68Ga-DOTATATE), offering a higher rate of lesion identification and lower costs compared to traditional Octreoscan methods. However, the application of 68Ga-labeled PET in G-NET assessment is still being evaluated, and further research is necessary to fully understand its utility in this patient population[41,42].
Treatment choices: Management of type III G-NETs follows the same protocols as that of gastric adenocarcinomas. According to the ENETS guidelines, surgical resection, either partial or total gastrectomy, along with LN dissection, is recommended[21]. The NCCN guidelines also advocate for radical gastric resection combined with perigastric LN dissection for localized type III G-NETs[47]. These tumors are typically approached with more aggressive treatment strategies, which may include subtotal or total gastrectomy based on their location. Given the resectability, liver metastases should be surgically removed. If not, options such as arterial embolization or radio ablation can be pursued, with a success rate of approximately 50%. For type III lesions that exhibit extrahepatic metastasis or recurrent disease, systemic chemotherapy or molecular-targeted therapies may be utilized[51].
The NCCN guidelines indicate that endoscopic or wedge resection is an option for type III G-NETs measuring under 2 cm. Scherübl et al[58] have recommended that smaller, well-differentiated (G1) type III G-NETs can be managed using ER. Furthermore, Kwon et al[59] noted that if a type III tumor is confined to the submucosal layer, is less than 2 cm in size, and shows no signs of lymphovascular invasion, then endoscopic treatment should be considered as the first line of action. Management strategies for well-differentiated G2 type III G-NETs are often the same as those used for types I and II, though the sequence of treatments may vary. Patients with G3 type III tumors typically follow the same pattern of treatment as patients suffering from adenocarcinoma[56]. In contrast, patients with advanced G3 type III tumors typically receive chemotherapy exclusively, with specific regimens designed based on the tumor's morphological features, in line with the latest grading system[56].
SSAs may be utilized as agents that either slow tumor growth or manage symptoms evoked by carcinoid syndrome. These analogs can also help suppress gastrin secretion, mitigating the growth-promoting effects on ECL cells in G-NETs. Although these therapies tend to be well tolerated and can lead to reductions in tumor size, recurrence is common once treatment ceases, and no substantial enhancements in patient outcomes have been linked to the use of SSAs.
In advanced disease scenarios, chemotherapy can result in a response rate ranging from 20% to 40%, employing well-established drugs such as streptozotocin, 5-fluorouracil, cyclophosphamide, etoposide, and doxorubicin[50].
Follow-up: For patients with type III G-NETs, follow-up care is recommended every 3 months to 12 months post-resection, transitioning to every 6 months to 12 months afterward, in line with NCCN guidelines[48]. The monitoring approach for type III G-NETs aligns closely with that for gastric adenocarcinoma, which involves annual endoscopic examinations and thorough clinical assessments[56]. Additionally, the ENETS guidelines emphasize the importance of regular surveillance for individuals who have had gastrectomy for type III tumors to detect any potential recurrence early[21].
G-NETs are a lesser but not least part of the GEP-NETs. The diagnostic patterns include the use of a variety of methods, from endoscopic biopsy for the pathological identification of the lesion to PET/CT scan for the search for metastases[60]. However, the limitations in the use of each method pose a serious challenge in the diagnostic process. Despite the EUS being the first imaging choice after EGD for type I G-NETs, it is, as mentioned above, used for the staging of lesions exceeding 1 cm. Moreover, it has been suggested that, after ER, the method was of significantly lower accuracy regarding the staging of submucosal tumors compared to pathological staging[61]. In addition, CT and, especially, multi-phase CT imaging of G-NETs, is based on the arterial phase enhancement of these lesions[60]. A few years ago, this knowledge was further advanced in pancreatic NETs, by the demonstration that the arterial phase enhancement of the tumor in multi-phase contrast-enhanced CT was negatively associated with the tumor’s grade. It is, however, important to mention that the accuracy of the method varied between 69%-82%, its negative predictive value between 62%-77%, and its sensitivity between 45%-75%[62]. Therefore, more studies are needed to prove the significance of these findings in G-NETs and to further evolve the method to increase its accuracy.
Delayed detection due to the lack of early symptoms also poses serious challenges for the specification of the extent of the disease at the time of diagnosis. Depending on the characteristics of the tumor, size, expansion to adjunct structures and nodes, and metastases, as well as those of the tumor cells, like proliferation index and SSTRs expressed, management including either surgical, non-surgical, or a combination of them may be performed. Tumor resection, however, such as endoscopic submucosal excision for smaller and surgical resection for bigger lesions, remains a basic option.
In addition to the excision of the lesion, a number of alternative treatment modalities have been proposed, including the use of somatostatin analogs, chemotherapeutic agents, and radionucleotide agents. SSTRs found on the cell membrane of the NETs of the gastroenteropancreatic system play a key role in the diagnosis and treatment of the primary tumor, as well as of the metastases. In a study by Diamantopoulos et al[63], above-label doses of somatostatin analogs were shown to be more effective than the label doses in the treatment of GEP-NETs. This context reflects the need for identification of the limits of the administered doses regarding both efficacy in disease regression and safety for patients. Furthermore, the combination of somatostatin analogs with interferon has been extensively investigated for its potential as an antiproliferative treatment in GEP-NETs during the past decades. However, its use remains rare due to insufficient evidence[64]. Although chemotherapy regimens and the combination of them have been extensively studied in GEP-NETs, the comparison of their effectiveness with that of the most recently generated peptide-receptor radionucleotide treatment was inevitable. Another possible prognostic and therapeutic target investigated was immune checkpoint inhibitors. The rate of expression of programmed death -1/programmed death-ligand 1 in GEP-NETs was found to vary between 1%-8% and a relationship with disease progression (P < 0.01) was demonstrated. The efficacy of treatment with immune checkpoint inhibitors as monotherapy or in combinations though has proven to be insignificant[65].
Among the most used scintigraphic radiopharmaceutical agents used in PET/CT imaging for the detection of both primary and metastatic lesions is the 68Ga-DOTATATE. The 111In-DTPAOC and 177Lu-DOTATATE have also been studied for their diagnostic value[66-68]. Moreover, their use as peptide-receptor radionucleotide therapeutic agents has been thoroughly studied, and relevant data have been published regarding their effectiveness, more importantly in patients with advanced and unresectable diseases. Based on these data, in 2018, 177Lu-DOTATATE received Food and Drug Administration approval for the treatment of GEP-NETs[69]. Strosberg et al[70], in their randomized trial, addressed the side effects associated with 177Lu-DOTATATE therapy. Common adverse events included nausea, vomiting, fatigue, abdominal pain, and diarrhea[70]. While no cases of renal toxicity were reported in either the treatment or control group, patients receiving 177Lu-DOTATATE exhibited a higher incidence of hematological toxicity, as indicated by increased rates of neutropenia, thrombocytopenia, and lymphopenia[70]. These findings suggest a potential vulnerability to myelodysplastic syndromes in patients treated with the study demonstrated that 177Lu-DOTATATE significantly improved progression-free survival (PFS), reducing the risk of progression or death by 79% [hazard ratio (HR) = 0.21, P < 0.001], with a 20-month PFS of 65.2% vs 10.8% in controls[70]. Interim overall survival (OS) analysis suggested a survival benefit (48-month OS: 66% vs 54%)[69]. Quality of life improved, with delayed symptom progression (HR = 0.40, P = 0.003)[70]. Late toxicities included grade 3–4 thrombocytopenia (9%) and lymphopenia (2%), with 1.7% developing multidimensional scaling[70]. Despite that, the publication of new studies urges hopes for more favorable outcomes[68,71]. As such, evidence of the effectiveness of radioembolization with holmium-166 after treatment with 177Lu-DOTATATE for the treatment of liver metastases from primary NETs of various sites has been presented[72]. The limits of the administered dose have been studied due to the radiation of critical organs, kidneys, and bone marrow by this agent. Data from radiotherapy can assist in this estimation of the thresholds, but further evaluation and clarification are under investigation[73]. The way of administration has also been shown to be of importance with the intra-arterial administration offering increased uptake[74].
Prognostication has consistently represented a significant challenge, not only in the context of G-NETs but across the entire spectrum of medical specialties. A variety of scores have been suggested as tools for clinicians regarding the prognosis of different treatment approaches for GEP-NETs[75,76]. A few months ago, in April 2024, a nomogram regarding the OS in GEP-NETs was published, based on data from a large patient-based study including 42662 patients[77]. The commonly accepted tool for the staging and prognosis of GEP-NETs however remains the AJCC Cancer Staging System[78]. This process of decision-making represents a further crucial element in the management of GEP-NETs. Effective communication and collaboration between the patient and the physician are crucial for achieving the optimal outcome. In 2018, Wagner et al[79] provided a comprehensive guide to the reflective multicriteria decision analysis (MCDA) regarding patients in the United States, facilitating alignment between the patient and the physician. Subsequently, new evidence was generated from the application of the MCDA to a specific subset of United States patients, namely Spanish patients[80].
In the future, it will be crucial to focus on the development of new biomarkers for earlier diagnosis and more accurate prognosis of G-NETs. Additionally, optimizing multidisciplinary diagnosis and treatment approaches will be essential in improving patient outcomes. Research into standardizing treatment protocols and exploring innovative therapeutic agents should be prioritized to achieve more effective and personalized care for G-NET patients.
G-NETs pose a challenge for modern medicine. The variety of histological patterns and clinical features underscore the need for detailed diagnostic and treatment management specific to grade, type, and stage. High clinical suspicion should arise from the presence of known risk factors. The gastric chloride background of each individual could be considered highly important in differentiating between the distinct types I, II, and III. The expected outcomes and prognosis are strongly linked to each specific subtype, with types I and II being associated with a better prognosis, while types III and IV are linked to less favorable outcomes due to tissue and lymphovascular invasion, as well as metastasis. For type I G-NETs, ER of the lesions is considered the gold standard. For type II, either ER or surgery is preferred, based on oncologic counseling. Type III, if resectable, requires detailed and extensive surgery. Nowadays, innovative and emerging alternative therapies, such as somatostatin analogs and peptide-receptor radionuclide therapies, offer promising outcomes, especially in advanced cases. Our study emphasizes the need for further in-depth research to improve diagnostic accuracy in early stages, and treatment efficacy, resulting in more favorable outcomes, especially in patients with lesions linked to a poorer prognosis.
| 1. | Wu P, He D, Chang H, Zhang X. Epidemiologic trends of and factors associated with overall survival in patients with neuroendocrine tumors over the last two decades in the USA. Endocr Connect. 2023;12:e230331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 2. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2661] [Article Influence: 295.7] [Reference Citation Analysis (5)] |
| 3. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2734] [Article Influence: 455.7] [Reference Citation Analysis (3)] |
| 4. | Fernandes CJ, Leung G, Eads JR, Katona BW. Gastroenteropancreatic Neuroendocrine Tumors. Gastroenterol Clin North Am. 2022;51:625-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 5. | Sok C, Ajay PS, Tsagkalidis V, Kooby DA, Shah MM. Management of Gastric Neuroendocrine Tumors: A Review. Ann Surg Oncol. 2024;31:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Dias AR, Azevedo BC, Alban LBV, Yagi OK, Ramos MFKP, Jacob CE, Barchi LC, Cecconello I, Ribeiro U Jr, Zilberstein B. Gastric Neuroendocrine Tumor: Review and Update. Arq Bras Cir Dig. 2017;30:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Carrasco P, Zuazo-Gaztelu I, Casanovas O. Sprouting strategies and dead ends in anti-angiogenic targeting of NETs. J Mol Endocrinol. 2017;59:R77-R91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (20)] |
| 9. | Tsolakis AV, Grimelius L, Granerus G, Stridsberg M, Falkmer SE, Janson ET. Histidine decarboxylase and urinary methylimidazoleacetic acid in gastric neuroendocrine cells and tumours. World J Gastroenterol. 2015;21:13240-13249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Mastracci L, Rindi G, Grillo F, Solcia E, Campora M, Fassan M, Parente P, Vanoli A, La Rosa S. Neuroendocrine neoplasms of the esophagus and stomach. Pathologica. 2021;113:5-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Ichikawa Y, Kobayashi N, Takano S, Kato I, Endo K, Inoue T. Neuroendocrine tumor theranostics. Cancer Sci. 2022;113:1930-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | van Velthuysen ML, Groen EJ, van der Noort V, van de Pol A, Tesselaar ME, Korse CM. Grading of neuroendocrine neoplasms: mitoses and Ki-67 are both essential. Neuroendocrinology. 2014;100:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Zappi A, Persano I, Galvani L, Parlagreco E, Andrini E, Campana D, Brizzi MP, Lamberti G, La Salvia A. Chemotherapy in Well Differentiated Neuroendocrine Tumors (NET) G1, G2, and G3: A Narrative Review. J Clin Med. 2023;12:717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 14. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 617] [Article Influence: 154.3] [Reference Citation Analysis (2)] |
| 15. | Uri I, Avniel-Polak S, Gross DJ, Grozinsky-Glasberg S. Update in the Therapy of Advanced Neuroendocrine Tumors. Curr Treat Options Oncol. 2017;18:72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Childs A, Kirkwood A, Edeline J, Luong TV, Watkins J, Lamarca A, Alrifai D, Nsiah-Sarbeng P, Gillmore R, Mayer A, Thirlwell C, Sarker D, Valle JW, Meyer T. Ki-67 index and response to chemotherapy in patients with neuroendocrine tumours. Endocr Relat Cancer. 2016;23:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Li TT, Qiu F, Qian ZR, Wan J, Qi XK, Wu BY. Classification, clinicopathologic features and treatment of gastric neuroendocrine tumors. World J Gastroenterol. 2014;20:118-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Postlewait LM, Baptiste GG, Ethun CG, Le N, Cardona K, Russell MC, Willingham FF, Kooby DA, Staley CA, Maithel SK. A 15-year experience with gastric neuroendocrine tumors: Does type make a difference? J Surg Oncol. 2016;114:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Zhang P, Zhang Y, Zhang C, Shi Y, Liu J, Liu Q, Yu L, Wang M, Zou G, Lou J, Chen J, Tan H. [Subtype classification and clinicopathological characteristics of gastric neuroendocrine neoplasms: an analysis of 241 cases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:1241-1246. [PubMed] |
| 20. | Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 773] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 21. | Sato Y, Hashimoto S, Mizuno K, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22:6817-6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (5)] |
| 22. | Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, Giordano T, Halfdanarson TR, Halperin D, He J, Heaney A, Heslin MJ, Kandeel F, Kardan A, Khan SA, Kuvshinoff BW, Lieu C, Miller K, Pillarisetty VG, Reidy D, Salgado SA, Shaheen S, Soares HP, Soulen MC, Strosberg JR, Sussman CR, Trikalinos NA, Uboha NA, Vijayvergia N, Wong T, Lynn B, Hochstetler C. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:839-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 23. | Gonzalez RS. Diagnosis and Management of Gastrointestinal Neuroendocrine Neoplasms. Surg Pathol Clin. 2020;13:377-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Gluckman CR, Metz DC. Gastric Neuroendocrine Tumors (Carcinoids). Curr Gastroenterol Rep. 2019;21:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 25. | Manfredi S, Walter T, Baudin E, Coriat R, Ruszniewski P, Lecomte T, Laurenty AP, Goichot B, Rohmer V, Roquin G, Cojocarasu OZ, Lombard-Bohas C, Lepage C, Morcet J, Cadiot G. Management of gastric neuro-endocrine tumours in a large French national cohort (GTE). Endocrine. 2017;57:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Roberto GA, Rodrigues CMB, Peixoto RD, Younes RN. Gastric neuroendocrine tumor: A practical literature review. World J Gastrointest Oncol. 2020;12:850-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (8)] |
| 27. | Panzuto F, Ramage J, Pritchard DM, van Velthuysen MF, Schrader J, Begum N, Sundin A, Falconi M, O'Toole D. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1-G3. J Neuroendocrinol. 2023;35:e13306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 28. | Sheikh-Ahmad M, Saiegh L, Shalata A, Bejar J, Kreizman-Shefer H, Sirhan MF, Matter I, Swaid F, Laniado M, Mubariki N, Rainis T, Rosenblatt I, Yovanovich E, Agbarya A. Factors Predicting Type I Gastric Neuroendocrine Neoplasia Recurrence: A Single-Center Study. Biomedicines. 2023;11:828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Pachuashvili NV, Nagornaya DP, Tertychnyi AS. [Metachronous tumors of the stomach in a patient with autoimmune gastritis]. Arkh Patol. 2023;85:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Massironi S, Gallo C, Elvevi A, Stegagnini M, Coltro LA, Invernizzi P. Incidence and prevalence of gastric neuroendocrine tumors in patients with chronic atrophic autoimmune gastritis. World J Gastrointest Oncol. 2023;15:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Lahner E, Esposito G, Pilozzi E, Purchiaroni F, Corleto VD, Di Giulio E, Annibale B. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand J Gastroenterol. 2015;50:856-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Carabotti M, Annibale B, Lahner E. Common Pitfalls in the Management of Patients with Micronutrient Deficiency: Keep in Mind the Stomach. Nutrients. 2021;13:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Lenti MV, Miceli E, Cococcia S, Klersy C, Staiani M, Guglielmi F, Giuffrida P, Vanoli A, Luinetti O, De Grazia F, Di Stefano M, Corazza GR, Di Sabatino A. Determinants of diagnostic delay in autoimmune atrophic gastritis. Aliment Pharmacol Ther. 2019;50:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Nandy N, Hanson JA, Strickland RG, McCarthy DM. Solitary Gastric Carcinoid Tumor Associated with Long-Term Use of Omeprazole: A Case Report and Review of the Literature. Dig Dis Sci. 2016;61:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Cavalcoli F, Zilli A, Conte D, Ciafardini C, Massironi S. Gastric neuroendocrine neoplasms and proton pump inhibitors: fact or coincidence? Scand J Gastroenterol. 2015;50:1397-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 36. | Sánchez Caraballo A, Guzmán Y, Sánchez J, Munera M, Garcia E, Gonzalez-Devia D. Potential contribution of Helicobacter pylori proteins in the pathogenesis of type 1 gastric neuroendocrine tumor and urticaria. In silico approach. PLoS One. 2023;18:e0281485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Sato Y. Clinical features and management of type I gastric carcinoids. Clin J Gastroenterol. 2014;7:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Sato Y. Endoscopic diagnosis and management of type I neuroendocrine tumors. World J Gastrointest Endosc. 2015;7:346-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 39. | Chin JL, O'Toole D. Diagnosis and Management of Upper Gastrointestinal Neuroendocrine Tumors. Clin Endosc. 2017;50:520-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Ito T, Masui T, Komoto I, Doi R, Osamura RY, Sakurai A, Ikeda M, Takano K, Igarashi H, Shimatsu A, Nakamura K, Nakamoto Y, Hijioka S, Morita K, Ishikawa Y, Ohike N, Kasajima A, Kushima R, Kojima M, Sasano H, Hirano S, Mizuno N, Aoki T, Aoki T, Ohtsuka T, Okumura T, Kimura Y, Kudo A, Konishi T, Matsumoto I, Kobayashi N, Fujimori N, Honma Y, Morizane C, Uchino S, Horiuchi K, Yamasaki M, Matsubayashi J, Sato Y, Sekiguchi M, Abe S, Okusaka T, Kida M, Kimura W, Tanaka M, Majima Y, Jensen RT, Hirata K, Imamura M, Uemoto S. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol. 2021;56:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 41. | Kjaer A, Knigge U. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand J Gastroenterol. 2015;50:740-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 43. | Maciejewski K, Buchalska B, Solnik M, Fudalej M, Deptała A, Badowska-Kozakiewicz A. Gastroenteropancreatic neuroendocrine tumours–an overview. Med Stud. 2022;38:361-376. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Alshahrani NA, Alnemari RA, Aldafeery NN, Aldandan RG, Alfayi BA, Alharbi MH, Allihyani MA, Alsalhi AA, Atallah RO, Almazeedi AA, Alzaher SA, Alshahrani NA. Neuroendocrine tumors. IAJPS. 2019;6:1536-1540. |
| 45. | Laird AM, Libutti SK. Management of Other Gastric and Duodenal Neuroendocrine Tumors. Surg Oncol Clin N Am. 2020;29:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Lloyd KA, Parsons BN, Burkitt MD, Moore AR, Papoutsopoulou S, Boyce M, Duckworth CA, Exarchou K, Howes N, Rainbow L, Fang Y, Oxvig C, Dodd S, Varro A, Hall N, Pritchard DM. Netazepide Inhibits Expression of Pappalysin 2 in Type 1 Gastric Neuroendocrine Tumors. Cell Mol Gastroenterol Hepatol. 2020;10:113-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Boyce M, Moore AR, Sagatun L, Parsons BN, Varro A, Campbell F, Fossmark R, Waldum HL, Pritchard DM. Netazepide, a gastrin/cholecystokinin-2 receptor antagonist, can eradicate gastric neuroendocrine tumours in patients with autoimmune chronic atrophic gastritis. Br J Clin Pharmacol. 2017;83:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Barchi LC, Jacob CE, Bresciani CJ, Yagi OK, Mucerino DR, Lopasso FP, Mester M, Ribeiro-Júnior U, Dias AR, Ramos MF, Cecconello I, Zilberstein B. Minimally Invasive Surgery For Gastric Cancer: Time To Change The Paradigm. Arq Bras Cir Dig. 2016;29:117-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Kulke MH, Shah MH, Benson AB 3rd, Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF, Fanta P, Giordano T, Goldner WS, Halfdanarson TR, Heslin MJ, Kandeel F, Kunz PL, Kuvshinoff BW 2nd, Lieu C, Moley JF, Munene G, Pillarisetty VG, Saltz L, Sosa JA, Strosberg JR, Vauthey JN, Wolfgang C, Yao JC, Burns J, Freedman-Cass D; National comprehensive cancer network. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 50. | Corey B, Chen H. Neuroendocrine Tumors of the Stomach. Surg Clin North Am. 2017;97:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 51. | Algashaamy K, Garcia-Buitrago M. Multifocal G1-G2 gastric neuroendocrine tumors: Differentiating between Type I, II and III, a clinicopathologic review. World J Clin Cases. 2019;7:2413-2419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Brandi ML, Agarwal SK, Perrier ND, Lines KE, Valk GD, Thakker RV. Multiple Endocrine Neoplasia Type 1: Latest Insights. Endocr Rev. 2021;42:133-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 53. | Kim JY, Hong SM. Recent Updates on Neuroendocrine Tumors From the Gastrointestinal and Pancreatobiliary Tracts. Arch Pathol Lab Med. 2016;140:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 54. | Rossi RE, Elvevi A, Citterio D, Coppa J, Invernizzi P, Mazzaferro V, Massironi S. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J Gastroenterol. 2021;27:5890-5907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (11)] |
| 55. | La Rosa S, Vanoli A. Gastric neuroendocrine neoplasms and related precursor lesions. J Clin Pathol. 2014;67:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Grozinsky-Glasberg S, Alexandraki KI, Angelousi A, Chatzellis E, Sougioultzis S, Kaltsas G. Gastric Carcinoids. Endocrinol Metab Clin North Am. 2018;47:645-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39:1071-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Kwon YH, Jeon SW, Kim GH, Kim JI, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD, Moon JS. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. 2013;19:8703-8708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Corrias G, Monti S, Horvat N, Tang L, Basturk O, Saba L, Mannelli L. Imaging features of malignant abdominal neuroendocrine tumors with rare presentation. Clin Imaging. 2018;51:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 62. | Belousova E, Karmazanovsky G, Kriger A, Kalinin D, Mannelli L, Glotov A, Karelskaya N, Paklina O, Kaldarov A. Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours: correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clin Radiol. 2017;72:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Diamantopoulos LN, Laskaratos FM, Kalligeros M, Shah R, Navalkissoor S, Gnanasegaran G, Banks J, Smith J, Jacobs B, Galanopoulos M, Mandair D, Caplin M, Toumpanakis C. Antiproliferative Effect of Above-Label Doses of Somatostatin Analogs for the Management of Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology. 2021;111:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Al-Toubah T, Strosberg J. Somatostatin Analogs and Interferon in the Treatment of Neuroendocrine Tumors. In: Yalcin S, Öberg K, editor. Neuroendocrine Tumours. Berlin: Springer, 2024. [DOI] [Full Text] |
| 65. | Garcia-Alvarez A, Cubero JH, Capdevila J. What Is the Status of Immunotherapy in Neuroendocrine Neoplasms? Curr Oncol Rep. 2022;24:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 66. | Tierney JF, Kosche C, Schadde E, Ali A, Virmani S, Pappas SG, Poirier J, Keutgen XM. (68)Gallium-DOTATATE positron emission tomography-computed tomography (PET CT) changes management in a majority of patients with neuroendocrine tumors. Surgery. 2019;165:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Chen SH, Chang YC, Hwang TL, Chen JS, Chou WC, Hsieh CH, Yeh TS, Hsu JT, Yeh CN, Tseng JH, Chen TC, Yen TC. 68Ga-DOTATOC and 18F-FDG PET/CT for identifying the primary lesions of suspected and metastatic neuroendocrine tumors: A prospective study in Taiwan. J Formos Med Assoc. 2018;117:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | O'Dorisio TM, Anthony LB. A 25-Year Experience of Gastroenteropancreatic Neuroendocrine Tumors and Somatostatin (Congeners) Analogs: From Symptom Control to Antineoplastic Therapy. Front Horm Res. 2015;44:177-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Hope TA, Bodei L, Chan JA, El-Haddad G, Fidelman N, Kunz PL, Mailman J, Menda Y, Metz DC, Mittra ES, Pryma DA, Reidy-Lagunes DL, Singh S, Strosberg JR. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of (177)Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J Nucl Med. 2020;61:222-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 70. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2427] [Article Influence: 269.7] [Reference Citation Analysis (0)] |
| 71. | Aggarwal P, Kumar A, Sood A, Walia R, Bhadada SK, Mittal BR. 177 Lu-DOTATATE Salvage Therapy in Rapidly Progressing Neuroendocrine Tumor With Poor Performance Status. Clin Nucl Med. 2024;49:561-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 72. | Singh S, Halperin D, Myrehaug S, Herrmann K, Pavel M, Kunz PL, Chasen B, Tafuto S, Lastoria S, Capdevila J, García-Burillo A, Oh DY, Yoo C, Halfdanarson TR, Falk S, Folitar I, Zhang Y, Aimone P, de Herder WW, Ferone D; all the NETTER-2 Trial Investigators. [(177)Lu]Lu-DOTA-TATE plus long-acting octreotide versus highdose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): an open-label, randomised, phase 3 study. Lancet. 2024;403:2807-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 173] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 73. | Kovan B, Özkan ZG, Demir B, Tunçman D, Işik EG, Şimşek DH, Büyükkaya F, Türkmen C, Şanli Y. An Analysis for Therapeutic Doses of Patients with Neuroendocrine Tumor Treated with Lutetium 177 ((177)Lu)-DOTATATE. Cancer Biother Radiopharm. 2022;37:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Pool SE, Kam BL, Koning GA, Konijnenberg M, Ten Hagen TL, Breeman WA, Krenning EP, de Jong M, van Eijck CH. [(111)In-DTPA]octreotide tumor uptake in GEPNET liver metastases after intra-arterial administration: an overview of preclinical and clinical observations and implications for tumor radiation dose after peptide radionuclide therapy. Cancer Biother Radiopharm. 2014;29:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Das S, Du L, Schad A, Jain S, Jessop A, Shah C, Eisner D, Cardin D, Ciombor K, Goff L, Bradshaw M, Delbeke D, Sandler M, Berlin J. A clinical score for neuroendocrine tumor patients under consideration for Lu-177-DOTATATE therapy. Endocr Relat Cancer. 2021;28:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Cao LL, Lu J, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Chen QY, Lin M, Tu RH, Huang CM. A novel predictive model based on preoperative blood neutrophil-to-lymphocyte ratio for survival prognosis in patients with gastric neuroendocrine neoplasms. Oncotarget. 2016;7:42045-42058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Wu Z, Shang G, Zhang K, Wang W, Fan M, Lin R. A nomogram incorporating treatment data for predicting overall survival in gastroenteropancreatic neuroendocrine tumors: a population-based cohort study. Int J Surg. 2024;110:2178-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Chan K, Chauhan A, Shi C. AJCC Cancer Staging System Version 9: Practice-Informing Updates for Gastroenteropancreatic Neuroendocrine Tumors. Ann Surg Oncol. 2024;31:4834-4836. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Wagner M, Samaha D, Khoury H, O'Neil WM, Lavoie L, Bennetts L, Badgley D, Gabriel S, Berthon A, Dolan J, Kulke MH, Goetghebeur M. Development of a Framework Based on Reflective MCDA to Support Patient-Clinician Shared Decision-Making: The Case of the Management of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NET) in the United States. Adv Ther. 2018;35:81-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Wagner M, Samaha D, Cuervo J, Patel H, Martinez M, O'Neil WM, Jimenez-Fonseca P. Applying Reflective Multicriteria Decision Analysis (MCDA) to Patient-Clinician Shared Decision-Making on the Management of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NET) in the Spanish Context. Adv Ther. 2018;35:1215-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/