Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.99527
Revised: November 10, 2024
Accepted: December 30, 2024
Published online: March 24, 2025
Processing time: 180 Days and 21.2 Hours
Colorectal cancer (CRC) is a leading cause of cancer-related mortality worldwide. In cases of metastatic CRC (mCRC) that are resistant to conventional chemo
We present the case of a 69-year-old woman diagnosed with mCRC with an NRAS p.G12V mutation and microsatellite stability, identified through tumor sequencing, along with HER2 overexpression detected by immunohistochemistry. She exhibited an excellent response to disitamab vedotin-containing therapy. To our knowledge, this is the first reported case of mCRC with HER2 overexpression and an NRAS p.G12V mutation achieving a remarkable clinical response to anti-HER2 therapy.
Disitamab vedotin demonstrates promising anti-tumor effects in HER2-overexpressing mCRC, offering patients an additional treatment option.
Core Tip: Colorectal cancer (CRC) is a leading cause of cancer-related mortality. In cases of metastatic CRC (mCRC) that is resistant to conventional chemotherapy, human epidermal growth factor receptor 2 (HER2)-targeted therapies have shown promise. We describe a 69-year-old woman with HER2-overexpressing mCRC, featuring an NRAS p.G12V mutation and microsatellite stability, who achieved an excellent response to disitamab vedotin treatment. This case is notable as it is the first recorded observation of a notable clinical response to anti-HER2 therapy in an mCRC patient with HER2 overexpression and an NRAS p.G12V mutation.
- Citation: Yan HC, Liu Y, Feng Y, Li JM, Sheng LM, Chen X, Xie YP, Li N. Efficacy of disitamab vedotin-containing therapy in metastatic colorectal cancer: A case report. World J Clin Oncol 2025; 16(3): 99527
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/99527.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.99527
Colorectal cancer (CRC) is a malignant tumor originating from the epithelial lining of the colon or rectum. It is the third most frequently diagnosed cancer and the third leading cause of cancer-related fatalities worldwide. Approximately 20% of patients with CRC present with metastatic disease at diagnosis, and up to 40% of those initially diagnosed with localized disease progress to recurrent disease. For patients with metastatic CRC (mCRC) that is resistant to che
The human epidermal growth factor receptor 2 (HER2) serves as a significant oncogenic driver in both breast and gastric cancers and is increasingly recognized as a therapeutic target in mCRC. It is estimated that 3%-5% of patients with mCRC exhibit HER2-positive tumors[3-5]. Notably, this figure doubles to approximately 10% in cases where the tumors are RAS wild-type[4]. The prevalence of HER2 overexpression or amplification is affected by the site of the primary tumor location, showing a higher incidence in rectal cancers compared to colon cancers, and in left-sided tumors compared to right-sided ones[6,7]. HER2 overexpressed or amplified mCRC has exhibited positive responses to therapies specifically targeting HER2[8-10].
Disitamab vedotin is an innovative anti-HER2 antibody-drug conjugate, which comprises the novel anti-HER2 monoclonal antibody trastuzumab, linked to the cytotoxic agent monomethyl auristatin E via a cleavable linker[11]. This drug is specifically approved for the treatment of patients with HER2+ [immunohistochemistry (IHC) 2+/3+] locally advanced or metastatic gastric carcinoma/gastric and gastroesophageal junction carcinoma, as well as urothelial carcinoma, who have received at least two prior systemic chemotherapy regimens[12]. Here, we present a HER2-overexpressing (IHC3+) mCRC case responding significantly to disitamab vedotin-containing therapy.
A 69-year-old female patient was admitted to the hospital 20 days after onset of left lower abdominal pain on March 17, 2023.
The patient reported no history of present illness.
The patient reported no history of past illness.
The patient reported no history of personal and family history.
Physical examination was not performed.
Laboratory examinations were not performed.
The patient underwent colonoscopy and biopsy, which indicated sigmoid colon adenocarcinoma. Assessment and examination were performed after admission. No evidence of distant disease was found in a staging body computed tomography scan, but extensive locoregional infiltration was detected.
Based on biopsy and imaging findings, the patient was diagnosed with sigmoid colon adenocarcinoma (cT2-T3N2M0).
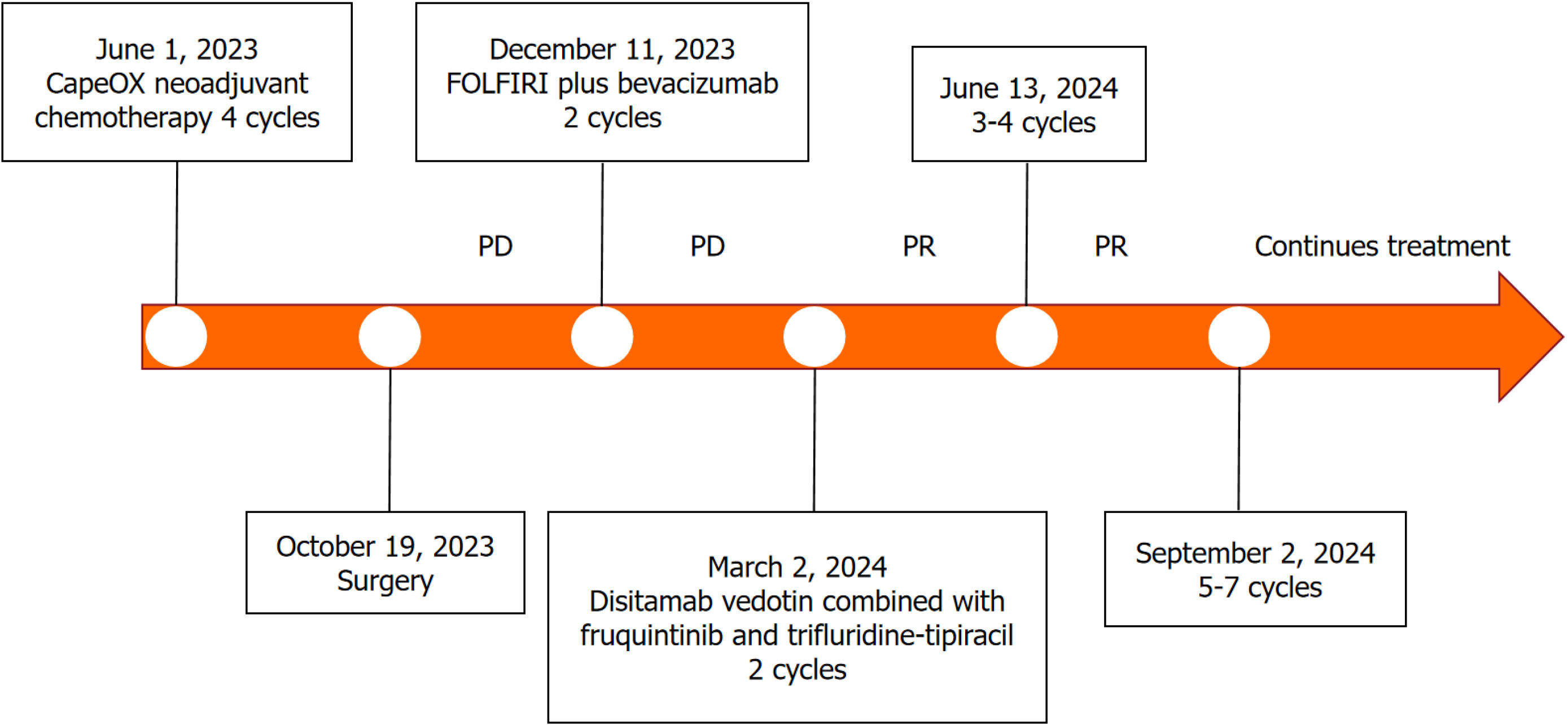
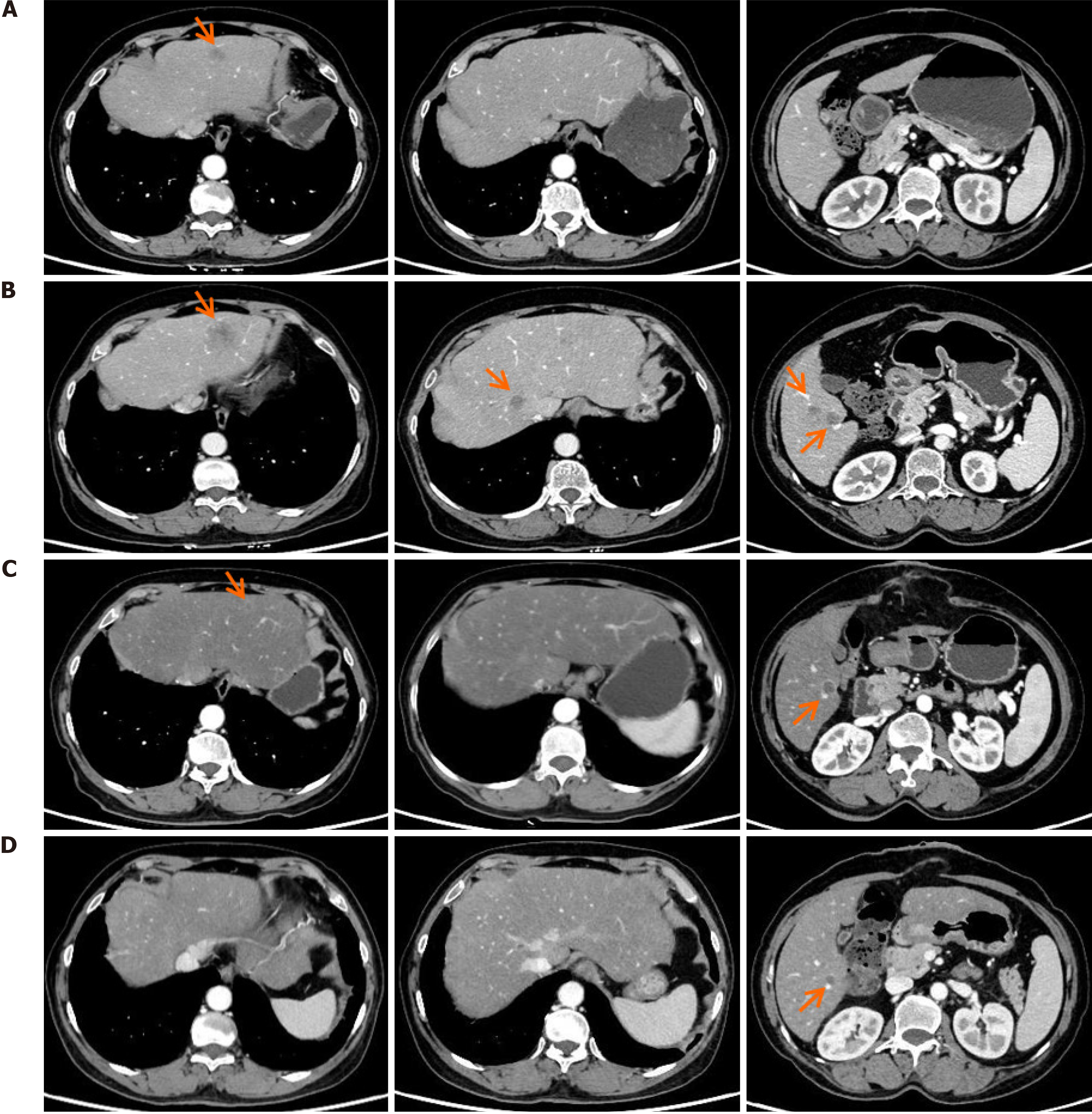
The treatment process is summarized in Figure 1. Given the difficulty of performing curative surgery, the patient underwent four cycles of neoadjuvant chemotherapy using the CapeOX regimen, which included intravenous infusion of oxaliplatin (130 mg/m2) for 3 hours and oral administration of capecitabine (1000 mg/m2) twice daily on days 1-14 in 21-day cycles. Subsequently, she underwent curative surgery, with final pathology revealing stage IIIC (ypT3N2bM0, American Joint Committee on Cancer 8th edition). Postoperative pathological analysis indicated moderately differentiated adenocarcinoma with proficient mismatch repair and HER2 overexpression (IHC3+). Unfortunately, one month after surgery, an abdominal computed tomography scan in November 2023 revealed multiple liver metastases (Figure 2A).
Tumor samples and peripheral blood plasma of the patient were sent for next generation sequencing and the results revealed NRAS p.G12V mutation, microsatellite stable status, and a tumor mutational burden of 7.9 mut/MB. Sub
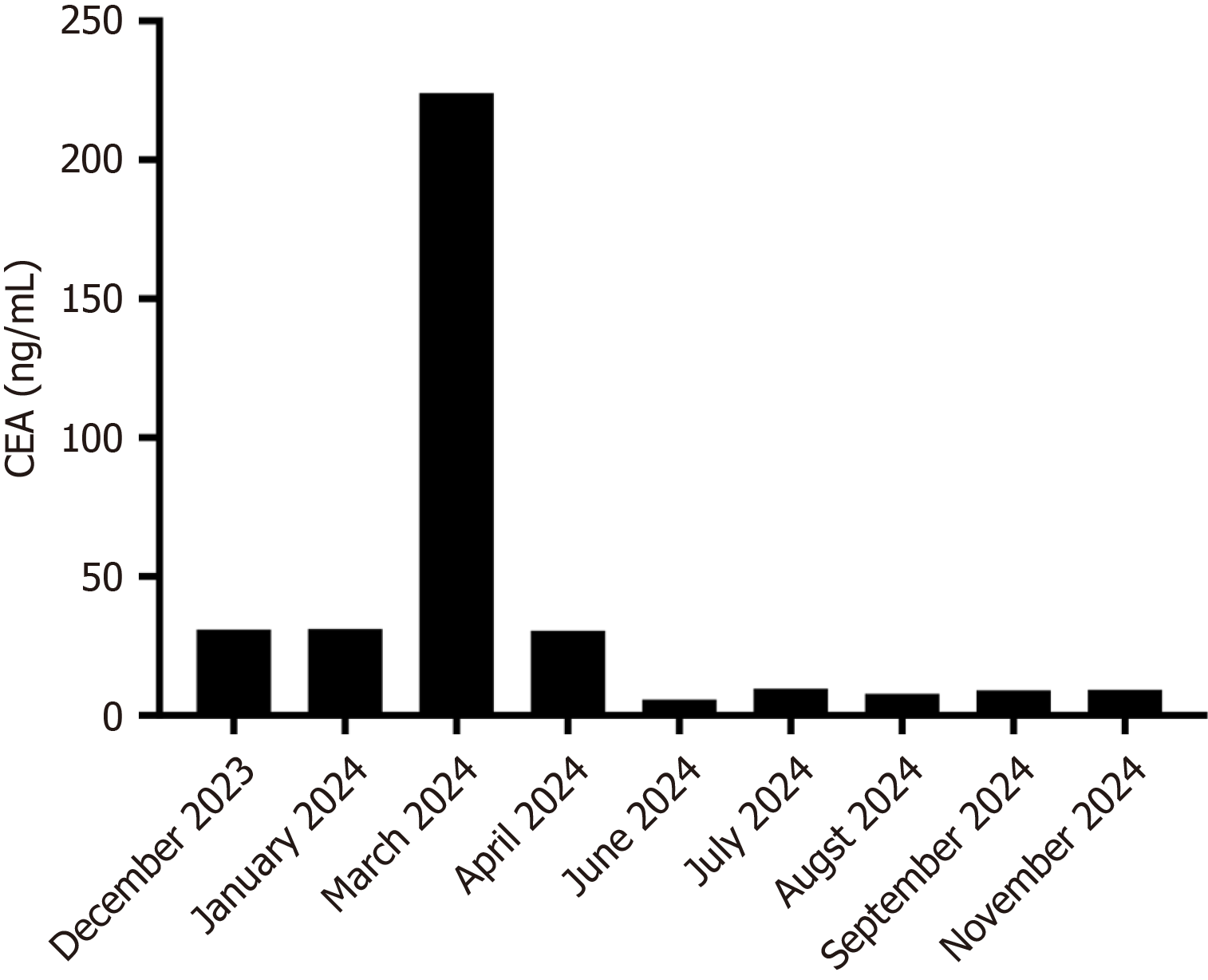
Changes in carcinoembryonic antigen (CEA) levels are shown in Figure 2. In December 2023, upon the initial diagnosis of liver metastasis, the patient’s CEA level was 30.9 ng/mL. After four cycles of FOLFIRI plus bevacizumab regimen, the CEA level increased to 224.0 ng/mL by March 2024. However, following six cycles of disitamab vedotin combined with fruquintinib and trifluridine-tipiracil, a significant decline in the CEA (from 224.0 ng/mL to 9.29 ng/mL) was observed on September 2024 (Figure 3). For the liver metastasis foci, a partial response was achieved based on the Modified Response Evaluation Criteria in Solid Tumors.
To date, the patient has completed 6 cycles of treatment, with a progression-free survival (PFS) of 7 months, and continues receiving treatment. There were no significant adverse events during treatment.
The overall 5-year survival rate for mCRC patients is 15%. In recent years, and despite some advances, the overall efficacy of chemotherapy for mCRC has reached a plateau. In a placebo-controlled phase 3 trial including 800 patients with refractory mCRC, the median PFS for cases treated with trifluridine-tipiracil monotherapy was 2.0 months, and the ORR was 1.6%[2]. Of note, the efficacy of the anti-angiogenic drug fruquintinib is also limited. In a placebo-controlled clinical trial involving 416 patients with refractory mCRC, the ORR and median PFS for patients treated with fruquintinib were 4.7% and 3.71 months, respectively[3].
HER2 amplification occurs in approximately 10% of RAS wild-type mCRC[4,5] and is associated with resistance to epidermal growth factor receptor-based treatment[13,14]. As per HER2-positive metastatic colorectal cancer diagnostic criteria, HER2 overexpression is defined as a 3+ HER2 score in > 50% of CRC cells as determined by IHC or a 3+ HER2 score in 10%-50% of CRC cells by IHC with positive amplification confirmed by fluorescent in situ hybridization in ≥ 50% of CRC cells[15]. The HER2 oncogene, when amplified or overexpressed, triggers excessive ligand-independent sti
As in the above clinical trial, most clinical studies of HER2-positive mCRC to date included patients with RAS wild-type tumors, with only a few investigations addressing populations with dual HER2-positive and RAS-mutant status[9,10]. RAS mutation is considered to be associated with resistance to anti-HER2 therapy[9]. Accordingly, in HER2-am
Disitamab vedotin demonstrates promising anti-tumor effects in HER2-overexpressing mCRC, offering patients addi
| 1. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2200] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 2. | Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 1060] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 3. | Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 4. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1064] [Article Influence: 212.8] [Reference Citation Analysis (16)] |
| 5. | Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 766] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 6. | Gong J, Cho M, Sy M, Salgia R, Fakih M. Molecular profiling of metastatic colorectal tumors using next-generation sequencing: a single-institution experience. Oncotarget. 2017;8:42198-42213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, Gatalica Z, Philip PA, Shields AF, Lenz HJ, Marshall JL. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8:86356-86368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Tosi F, Sartore-Bianchi A, Lonardi S, Amatu A, Leone F, Ghezzi S, Martino C, Bencardino K, Bonazzina E, Bergamo F, Fenocchio E, Martinelli E, Troiani T, Siravegna G, Mauri G, Torri V, Marrapese G, Valtorta E, Cassingena A, Cappello G, Bonoldi E, Vanzulli A, Regge D, Ciardiello F, Zagonel V, Bardelli A, Trusolino L, Marsoni S, Siena S. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2020;19:256-262.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, Yuki S, Masuishi T, Nishina T, Ebi H, Sawada K, Taniguchi H, Fuse N, Nomura S, Fukui M, Matsuda S, Sakamoto Y, Uchigata H, Kitajima K, Kuramoto N, Asakawa T, Olsen S, Odegaard JI, Sato A, Fujii S, Ohtsu A, Yoshino T. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med. 2021;27:1899-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 10. | Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 11. | Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022;29:1335-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 12. | Deeks ED. Disitamab Vedotin: First Approval. Drugs. 2021;81:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM, Oumie A, Spink KG, Brown SR, Jones M, Kerr D, Handley K, Gray R, Seymour M, Quirke P. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238:562-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 14. | Raghav K, Loree JM, Morris JS, Overman MJ, Yu R, Meric-Bernstam F, Menter D, Korphaisarn K, Kee B, Muranyi A, Singh S, Routbort M, Chen K, Shaw KRM, Katkhuda R, Shanmugam K, Maru D, Fakih M, Kopetz S. Validation of HER2 Amplification as a Predictive Biomarker for Anti-Epidermal Growth Factor Receptor Antibody Therapy in Metastatic Colorectal Cancer. JCO Precis Oncol. 2019;3:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, Rugge M, Grigioni W, Bencardino K, Lonardi S, Zagonel V, Leone F, Noe J, Ciardiello F, Pinto C, Labianca R, Mosconi S, Graiff C, Aprile G, Frau B, Garufi C, Loupakis F, Racca P, Tonini G, Lauricella C, Veronese S, Truini M, Siena S, Marsoni S, Gambacorta M. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015;28:1481-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001;12 Suppl 1:S9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Ahn ER, Rothe M, Mangat PK, Garrett-Mayer E, Ali-Ahmad HM, Chan J, Maitland ML, Patel SR, Reese Z, Balmanoukian AS, Drescher CW, Li R, Tsimberidou AM, Leath CA 3rd, O'Lone R, Grantham GN, Halabi S, Schilsky RL. Pertuzumab Plus Trastuzumab in Patients With Endometrial Cancer With ERBB2/3 Amplification, Overexpression, or Mutation: Results From the TAPUR Study. JCO Precis Oncol. 2023;7:e2200609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Fakih M, Elez E, Rodriguez J, Ciardiello F, Komatsu Y, Esaki T, Chung K, Wainberg Z, Sartore-Bianchi A, Saxena K, Yamamoto E, Bako E, Okuda Y, Shahidi J, Grothey A, Yoshino T; DESTINY-CRC01 investigators. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 19. | Sartore-Bianchi A, Lonardi S, Martino C, Fenocchio E, Tosi F, Ghezzi S, Leone F, Bergamo F, Zagonel V, Ciardiello F, Ardizzoni A, Amatu A, Bencardino K, Valtorta E, Grassi E, Torri V, Bonoldi E, Sapino A, Vanzulli A, Regge D, Cappello G, Bardelli A, Trusolino L, Marsoni S, Siena S. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open. 2020;5:e000911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Strickler J, Zemla T, Ou F, Cercek A, Wu C, Sanchez F, Hubbard J, Jaszewski B, Bandel L, Schweitzer B, Niedzwiecki D, Kemeny N, Boland P, Ng K, Bekaii-saab T. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): Initial results from the MOUNTAINEER trial. Ann Oncol. 2019;30:v200. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |