Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101766
Revised: November 6, 2024
Accepted: December 9, 2024
Published online: March 24, 2025
Processing time: 117 Days and 18.5 Hours
Most non-small cell lung cancer patients have epidermal growth factor receptor (EGFR) activating mutations, such as exon 19 deletion and exon 21 replacement mutations. Osimertinib is a third-generation EGFR-tyrosine kinase inhibitors ap
We report a case of a 56-year-old woman who was diagnosed with lung adenocarcinoma with lung hilum, mediastinal lymph nodes and brain metastases (T4N3
Retreatment with furmonertinib under prednisone could be considered as an effective therapeutic option after risk-benefit assessment for EGFR-mutant lung adenocarcinoma patients.
Core Tip: We report a case of a 56-year-old woman who was diagnosed with lung adenocarcinoma with lung hilum, media
- Citation: Wei FF, Zhang J, Jia Z, Yao ZC, Chen CQ. Furmonertinib re-challenge for epidermal growth factor receptor-mutant lung adenocarcinoma after osimertinib-induced interstitial lung disease: A case report. World J Clin Oncol 2025; 16(3): 101766
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101766.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101766
Lung cancer is the most common cause of cancer-related deaths worldwide, and 85% are non-small cell lung cancer (NSCLC). Most NSCLC patients have epidermal growth factor receptor (EGFR) activation mutations, such as exon 19 deletion and exon 21 replacement mutations. The development of tyrosine kinase inhibitors (TKIs) targeting EGFR activating mutations has improved the prognosis of many lung cancer patients[1]. Osimertinib is a third-generation EGFR-TKI approved for the treatment of lung cancer patients carrying EGFR activating mutations. The common adverse reactions include diarrhea, rash, paronychia, oral mucositis, etc. Osimertinib-induced interstitial lung disease (ILD) is a rare and potentially fatal pulmonary toxicity of drugs disease[2]. Herein we report a lung adenocarcinoma patient who developed ILD following treatment with osimertinib. After active symptomatic and hormone treatment, her lung injury alleviated. Then the patient was retreated with furmonertinib combined with prednisone and did not experience ILD again. It is suggested that retreatment with furmonertinib under steroid coverage could be considered as an effective treatment option after careful risk-benefit assessment for patients with EGFR-mutant lung adenocarcinoma. It is also necessary to closely monitor adverse reactions and efficacy in order to adjust the treatment plan in a timely manner.
A 56-years-old woman complained of dizziness and discomfort without obvious inducement in December 2022, ac
The patient suffered from repeated dizziness and headache for over a month without obvious cause.
The patient had had grade II hypertension before admission to our hospital. She denied a history of diabetes, coronary heart disease, hepatitis, tuberculosis or other infectious diseases. She had no history of major surgery, trauma, or blood transfusion. She also denied any history of medication, or food allergies.
The patient had no history of exposure to toxic substances or radioactive substances. She had no smoking or alcohol addiction either. She denied any family history of lung cancer or malignant tumors.
Her physical examination revealed clear consciousness, normal calculation, memory, orientation, judgment, and logical thinking abilities. Her performance status score was 1, Glasgow Coma Scale score was 15 (E4V5M6). The head size is normal without any deformities. There is no enlargement of the thyroid gland. Bilateral percussion shows clear sounds, with coarse breathing sounds in both lungs and slight moist rales in both lower lungs. The heart rate is 88 beats per minute and the rhythm is consistent. There is no tenderness or rebound pain in the entire abdomen, no percussion pain in the liver and kidney areas, and no edema in both lower limbs.
Tumor markers were as follows: (1) Alpha fetoprotein: 2.34 ng/mL (reference value: ≤ 7.0 ng/mL); (2) Carcinoembryonic antigen: 5.10 ng/mL (reference value: 0-5 ng/mL); (3) Carbohydrate antigen 125: 10.40 U/mL (reference value: 0-25 U/mL); (4) Carbohydrate antigen 19-9: 6.31 U/mL (reference value: ≤ 30 U/mL); (5) Carbohydrate antigen 15-3: 77.70 U/mL (reference value: ≤ 24 U/mL); (6) Carbohydrate antigen 72-4: 7.08 U/mL (reference value: ≤ 6.9 U/mL); (7) Squamous cell carcinoma antigen: 1.02 ng/mL (reference value: 0.5-3 ng/mL); (8) Ferritin: 251.00 ng/mL (reference value: 25-325 ng/mL); (8) Neuron-specific enolase: 18.40 ng/mL (reference value: 0-16.3 ng/mL); (9) Cytokeratin 19 fragment: 4.30 ng/mL (reference value: 0-3.3 ng/mL); and (10) B subunit of human chorionic gonadotropin: 1.0 IU/L (reference value: ≤ 2.0 IU/L).
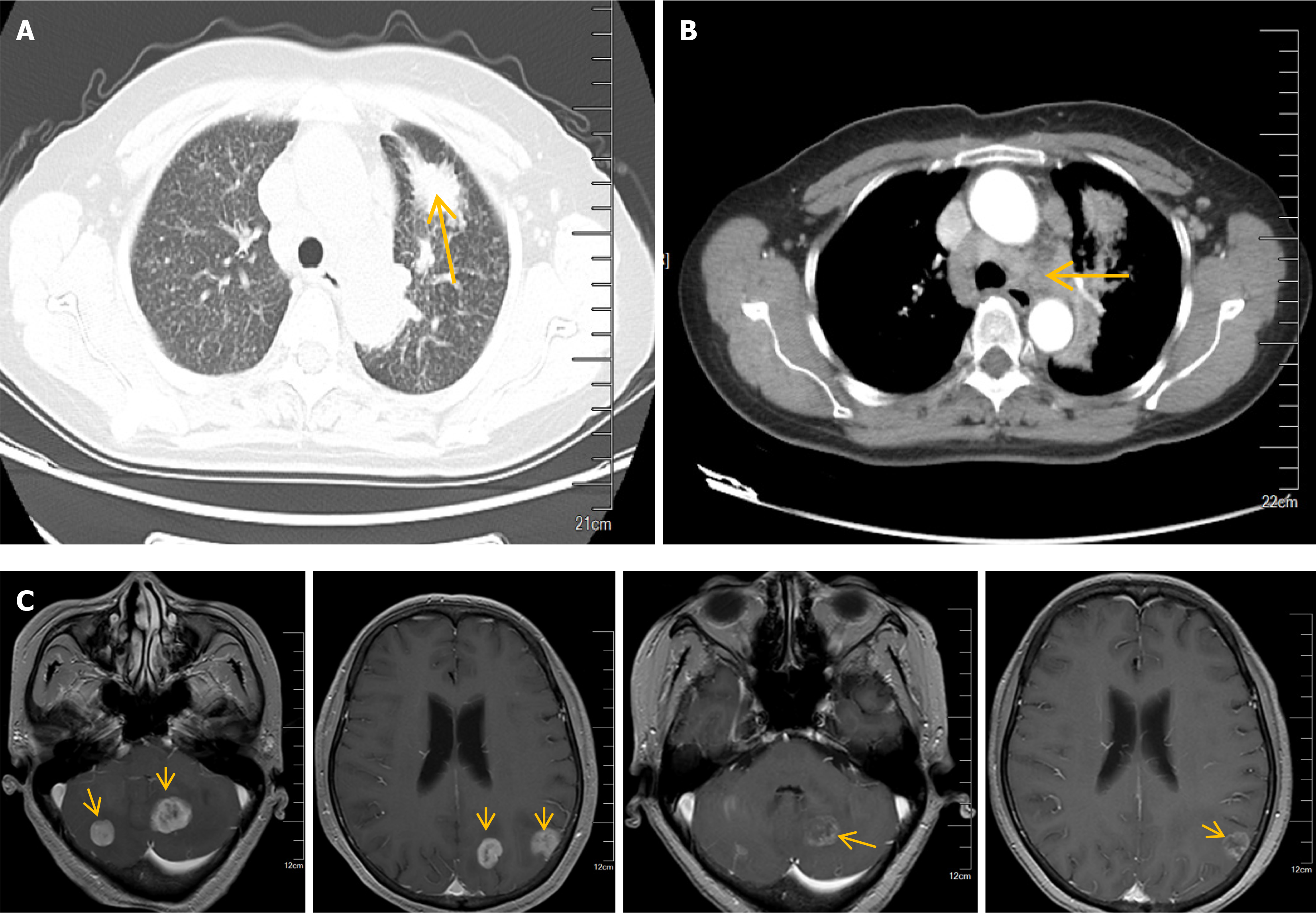
Chest CT indicated that: (1) A space occupying lesion in the anterior segment of the left upper lobe, suggesting central type lung cancer; (2) Diffuse miliary nodules in both lungs; (3) Enlarged lymph nodes in both pulmonary hilum and mediastinum, considering metastases; and (4) Magnetic resonance imaging of the brain revealed multiple abnormal signals in the left occipital lobe, bilateral parietal lobes, bilateral cerebellum, and cerebellar vermis, suggesting the possibility of metastatic tumors (Figure 1). Ultrasound examinations found no significant abnormalities in the urinary system, abdomen, cervical vascular system, or gynecological system.
Puncture biopsy was performed on the mass in the anterior segment of the left upper lobe of the lung, and immunohistochemical examinations showed the following results: Pan-cytokeratin (+), cytokeratin 7 (+), NapsinA (+), thyroid transcription factor 1 (+), cytokeratin 5/6 (-), P40 (-), CD56 (-), Ki-67 (+, 5%). The results suggested invasive adenocarcinoma of the lung.
The patient was diagnosed with lung adenocarcinoma with lung hilum, mediastinal lymph nodes and brain metastases (T4N3M1c stage IVB).
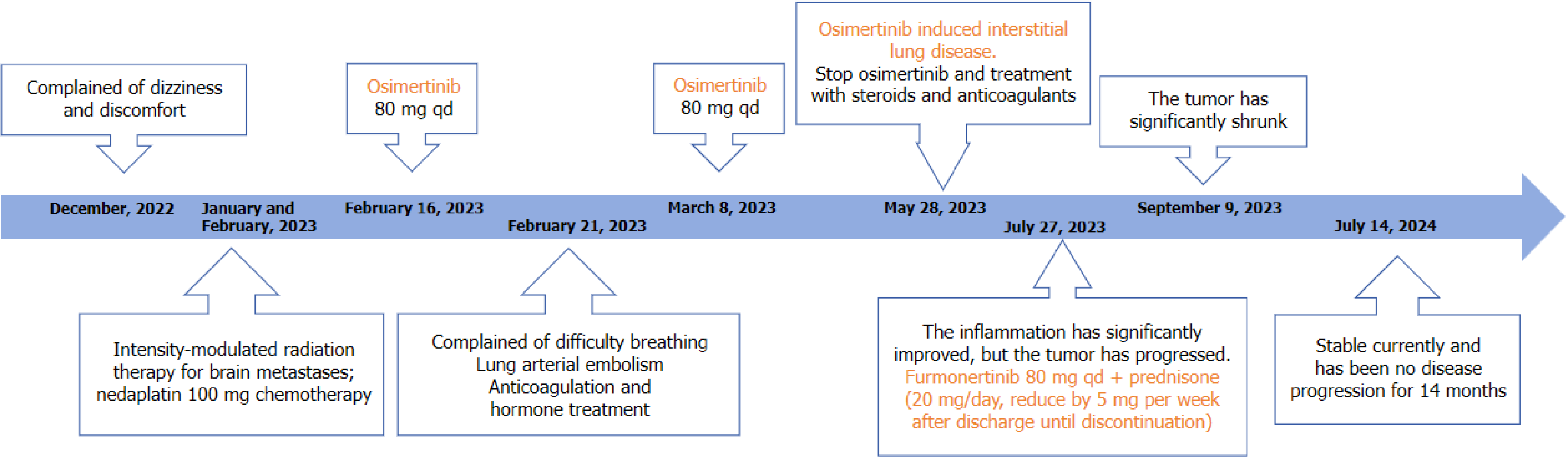
The patient underwent intensity-modulated radiation therapy for brain metastases from January 18 to February 15, 2023, with 95% gross tumor volume 5600 cGy/20 fractions/28 days and 95% planning target volume dose of 4000 cGy/20 fractions/28 days, respectively. On January 30, during the same period, nedaplatin 100 mg chemotherapy was administered. Genetic testing results indicated EGFR19 mutation, with an abundance of 62%. Starting from February 16, regular targeted therapy with 80 mg of osimertinib was administered once a day.
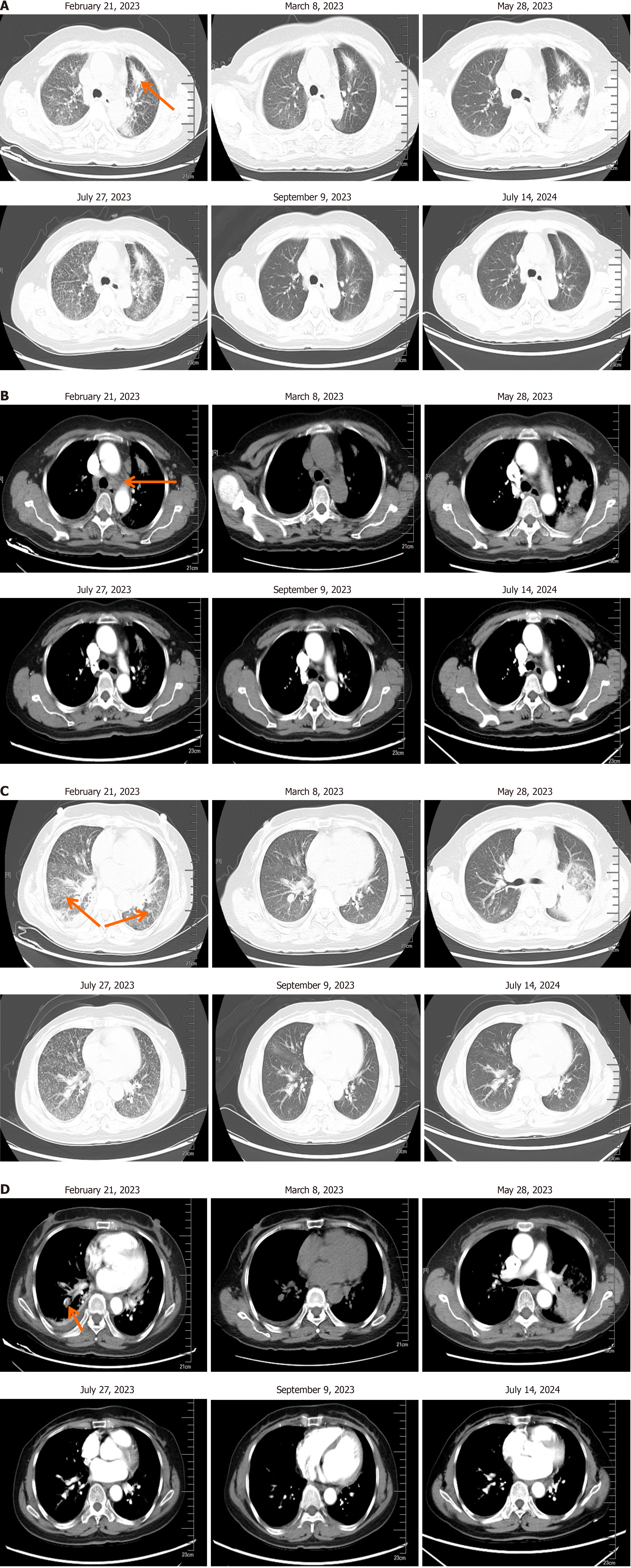
On February 21, 2023, the patient complained of difficulty breathing. Her lung CT showed that the mass in the upper lobe of the left lung was reduced compared to before, and there were pleural effusions on the right side. There were also arterial embolisms in the lateral segment of the lower lobe of the right lung. Blood tests including white blood cells, C-reactive protein, procalcitonin, aspergillus, and tests for detection of galactomannan (GM) showed no abnormalities. Anticoagulation and hormone symptomatic treatment were given. On March 8, the chest CT showed a decrease in bilateral lung patchy shadows compared to before, and the lesion in the lower lobe of the right lung was slightly reduced compared to before. The patient continued taking targeted treatment with osimertinib afterwards. On May 28, her chest CT showed multiple consolidations, nodules, and new patchy high-density shadows in the upper lobe of the left lung. Broncho-alveolar lavage fluid showed no gram-positive bacteria, gram negative bacteria, fungi, or acid-fast bacilli. Blood routine, C-reactive protein, and procalcitonin were normal. Respiratory syncytial virus, mycoplasma pneumoniae, cytomegalovirus, adenovirus infections, influenza A and B virus, parainfluenza virus, and aspergillus were all negative, as well as tests for detection of GM. The patient discontinued treatment with osimertinib and received symptomatic treatment with steroids and anticoagulants. On July 27, 2023, her chest CT showed a reduced range of patchy shadows in the upper lobe of the left lung near the hilum, and absorption and disappearance of patchy shadows in both lungs compared to before. The inflammation was significantly improved, but the tumor progressed. On July 27, the patient started taking 80 mg per day of furmonertinib combined with prednisone (20 mg/day, reducing by 5 mg per week after discharge until discontinuation) for retreatment. On September 9, her lung CT showed no inflammatory change in the lungs, and the tumor significantly shrank. Regular follow-up examinations were conducted to ensure stable conditions of lung tumors and brain metastases. The last follow-up was on July 14, 2024 (Figure 2). The treatment regimen is shown in Figure 3.
The patient received targeted treatment with 80 mg osimertinib per day after radiotherapy and chemotherapy. Due to intolerable side effects of osimertinib, the treatment was discontinued and the patient was retreated with 80 mg per day of furmonertinib combined with prednisone (20 mg/day, reducing by 5 mg per week after discharge for one week until discontinuation). After furmonertinib administration, no ILD occurred and the tumor significantly shrank. The patient’s condition has been stable currently and has had no disease progression for 14 months since.
ILD is a group of diffuse lung diseases that involve extensive changes in the lung interstitium, alveolar cavity, or bron
The clinical manifestations of ILD include cough, acute/subacute dyspnea, fever, chest pain, fatigue, etc[3]. The risk factors for ILD after using EGFR-TKIs mainly include: (1) Basic characteristics: Male, age ≥ 55 years, smoking, per
In this report, the patient underwent radiotherapy and chemotherapy treatment from January to February 2023. After genetic testing for EGFR19 mutation, targeted treatment with osimertinib was started on February 16. After taking osimertinib for 6 days, the patient complained of respiratory distress. CT scan of the lung showed right-sided pleural effusion and right lung hypoplasia, as well as arterial embolisms in the lateral segment of the lower lobe of the right lung. After 16 days of hormone and anticoagulant therapy, the inflammation improved upon follow-up. There are reports[8,9] on the re-challenge of osimertinib. For grade 1-2 osimertinib related ILD, re-challenge of osimertinib is relatively safe after clinical symptoms improve. For grade 3-4 patients, the risk of re-challenge is higher. However, there are also successful cases of re-challenge. On March 8, our patient underwent a follow-up chest CT, which showed a reduction in both lung patchy shadows, and a slight reduction in the lesion in the lower lobe of the right lung compared to before. The patient continued to take targeted treatment with osimertinib. About 3 months later, the patient developed lung lesions again, with multiple consolidations, nodules, and newly observed patchy high-density shadows in the upper lobe of the left lung. Osimertinib was discontinued. The risk factors for EGFR-TKIs related ILD in this case include age ≥ 55 years and a recent history of radiotherapy and chemotherapy (6 months ago). In addition, the infection indicators of the patient did not increase, and the broncho-alveolar lavage fluid showed no gram-positive bacteria, gram-negative bacteria, fungi, or acid-fast bacilli. Blood routine, C-reactive protein, and procalcitonin were normal. Respiratory syncytial virus, myco
The incidence of ILD caused by osimertinib is higher than that by the first-generation EGFR-TKIs, and there is a greater risk of ILD after reusing EGFR-TKIs. To reduce the incidence of ILD after reusing EGFR-TKIs, measures can be taken such as switching to other EGFR-TKIs, using EGFR-TKIs in combination with glucocorticoids, or adjusting the dosage of EGFR-TKIs[8,11]. Compared with the first/second-generation EGFR-TKIs, the use of third-generation EGFR-TKIs re
We report a lung adenocarcinoma patient who developed ILD after receiving treatment with osimertinib. After active symptomatic and hormone treatment, the lung injury was relieved. The patient was retreated with furmonertinib combined with prednisone and did not experience ILD again. Currently, there has been no disease progression for 14 months. It is suggested that retreatment with furmonertinib under steroid coverage could be considered as an effective treatment option after careful risk-benefit assessment for patients with EGFR-mutant lung adenocarcinoma.
| 1. | Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol. 2018;29:i20-i27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 2. | Chang HL, Chen YH, Taiwan HC, Yang CJ. EGFR Tyrosine Kinase Inhibitor-Associated Interstitial Lung Disease During the Coronavirus Disease 2019 Pandemic. J Thorac Oncol. 2020;15:e129-e131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet. 2022;400:769-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 4. | Kodama H, Wakuda K, Yabe M, Nishioka N, Miyawaki E, Miyawaki T, Mamesaya N, Kawamura T, Kobayashi H, Omori S, Ono A, Kenmotsu H, Naito T, Murakami H, Endo M, Takahashi T. Retrospective analysis of osimertinib re-challenge after osimertinib-induced interstitial lung disease in patients with EGFR-mutant non-small cell lung carcinoma. Invest New Drugs. 2021;39:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 5. | Shah RR. Tyrosine Kinase Inhibitor-Induced Interstitial Lung Disease: Clinical Features, Diagnostic Challenges, and Therapeutic Dilemmas. Drug Saf. 2016;39:1073-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Kikuchi R, Takoi H, Tsuji T, Nagatomo Y, Tanaka A, Kinoshita H, Ono M, Ishiwari M, Toriyama K, Kono Y, Togashi Y, Yamaguchi K, Yoshimura A, Abe S. Glasgow Prognostic Score predicts chemotherapy-triggered acute exacerbation-interstitial lung disease in patients with non-small cell lung cancer. Thorac Cancer. 2021;12:667-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Gu J, Bai F, Song L, Wang Y. [Osimertinib Re-challenge for EGFR-mutant NSCLC after Osimertinib-induced Interstitial Lung Disease: A Case Report]. Zhongguo Fei Ai Za Zhi. 2021;24:804-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Kiriu T, Tamura D, Tachihara M, Sekiya R, Hazama D, Katsurada M, Nakata K, Nagano T, Yamamoto M, Kamiryo H, Kobayashi K, Nishimura Y. Successful Osimertinib Rechallenge with Steroid Therapy after Osimertinib-induced Interstitial Lung Disease. Intern Med. 2018;57:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Mohammed T, Mangeshkar S, Rathmann J. Successful Rechallenge with Osimertinib after Very Acute Onset of Drug-Induced Pneumonitis. Case Rep Oncol. 2021;14:733-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Fintelmann FJ, Graur A, Oueidat K, Simon J, Barnes JMH, McDermott S, Genshaft SJ, Healey TT, Suh RD, Maxwell AWP, Abtin F. Ablation of Stage I-II Non-Small Cell Lung Cancer in Patients With Interstitial Lung Disease: A Multicenter Retrospective Study. Am J Roentgenol. 2024;222:e2330300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kashiwabara K, Semba H, Fujii S, Tsumura S. Outcome in advanced non-small cell lung cancer patients with successful rechallenge after recovery from epidermal growth factor receptor tyrosine kinase inhibitor-induced interstitial lung disease. Cancer Chemother Pharmacol. 2017;79:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Nagasaka M, Zhu VW, Lim SM, Greco M, Wu F, Ou SI. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors For Advanced EGFR+ NSCLC. J Thorac Oncol. 2021;16:740-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 13. | Jia K, Yang S, Chen B, Yu J, Wu Y, Li W, Zhou F, Wu F, Feng G, Ren S. Advanced lung adenocarcinoma patient with EGFR exon 20 insertion benefits from high-dose furmonertinib for nine months after progression from mobocertinib: a case report. Ann Transl Med. 2022;10:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, Liu C, Zhu S, Zhang X, Li Y, Liu J, Cao L, Cheng Y, Zhao H, Zhang S, Zang A, Cui J, Feng J, Yang N, Liu F, Jiang Y, Gu C; FURLONG investigators. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;10:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |