Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101251
Revised: December 1, 2024
Accepted: December 25, 2024
Published online: March 24, 2025
Processing time: 134 Days and 4.9 Hours
Esophageal squamous cell carcinoma (ESCC) is a common malignancy in China, often diagnosed at an advanced stage, with poor prognosis. Standard treatments such as definitive chemoradiotherapy offer limited survival benefits. Recent advances in immune checkpoint inhibitors combined with chemotherapy have shown promise, but their effectiveness and safety in conjunction with radio
To assess the safety and effectiveness of induction chemoimmunotherapy fo
This retrospective study included 80 patients with locally advanced unresectable ESCC who underwent induction chemoimmunotherapy followed by definitive radiotherapy, recruited from Zhejiang Cancer Hospital. All patients received 2-4 cycles of chemotherapy plus programmed cell death 1/programmed cell death ligand 1 inhibitor, were re-evaluated to be inoperable, then received definitive radiotherapy or CCRT. Primary endpoint was treatment safety and tolerance. SPSS 26.0 software was used for data analysis. Th Kaplan-Meier method was used for survival analysis.
Thirty-seven (46.3%) patients received CCRT and 43 (53.7%) received radiotherapy alone. The most common treatment-related adverse events included radiation esophagitis (32/80, 40.0%) and anemia (49/80, 61.3%), with 22 (27.5%) experiencing grade ≥ 3 adverse events. No treatment-related deaths occurred. After median follow-up of 16.5 months, the median progression-free survival (PFS) was 14.2 months, and median overall survival (OS) was 19.9 months. The 1-year and 2-year PFS and OS were 55.8% and 31.6%, and 67.5% and 44.1%, respectively. Patients with partial response had better outcomes than those with stable disease: 1-year PFS 69.4% vs 43.9% (P = 0.011) and OS 83.2% vs 48.8% (P = 0.007). Induction therapy effectiveness and immunotherapy maintenance were independent prognostic factors for OS.
Chemotherapy combined with programmed cell death 1/programmed cell death ligand 1 inhibitor followed by definitive radiotherapy or CCRT in patients with locally advanced ESCC was safe and effective.
Core Tip: This study highlighted the safety and effectiveness of combining induction chemotherapy with programmed cell death 1/programmed cell death ligand 1 inhibitors followed by definitive radiotherapy or chemoradiotherapy, for locally advanced unresectable esophageal squamous cell carcinoma. It identified induction therapy effectiveness and maintenance immunotherapy as key prognostic factors, emphasizing the need for further validation through prospective trials.
- Citation: Wei ZJ, Wang L, Wang RQ, Wang Y, Chen H, Ma HL, Xu YJ. Safety and effectiveness of induction chemoimmunotherapy followed by definitive radiotherapy or concurrent chemoradiotherapy in esophageal squamous cell carcinoma. World J Clin Oncol 2025; 16(3): 101251
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101251.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101251
Esophageal carcinoma (EC) is one of the prevalent malignant tumors of the digestive system, with approximately 90% of cases in China being attributed to the histological subtype, esophageal squamous cell carcinoma (ESCC)[1]. Owing to the often latent nature of early-stage EC, patients are frequently diagnosed at an advanced, less treatable phase. The 5-year survival rate for patients with locally advanced EC, deemed inoperable or opting against surgical intervention, is 20%-30%, accompanied by a daunting local recurrence rate ranging from 40% to 60%[2,3]. Consequently, there is a pressing demand for innovative treatment modalities.
In recent times, immune checkpoint inhibitors (ICIs) have become prominent in tumor therapy, marking substantial progress in the management of advanced EC. Consequently, the combination of chemotherapy with immunotherapy has become the new standard of care for advanced EC[4-6]. A series of small-sample phase 2 trials are presently investigating the therapeutic potential of neoadjuvant chemotherapy coupled with immunotherapy for locally advanced operable EC. The notable efficacy observed in treating advanced EC has prompted the exploration of conversion therapy - utilizing induction chemotherapy in conjunction with immunotherapy - to render locally advanced unresectable EC operable. While this approach has demonstrated a degree of efficacy, a significant portion of unresectable tumors undergoing chemoimmunotherapy remain nonresectable, necessitating the eventual consideration of radiotherapy or chemoradiotherapy[7,8]. Preclinical investigations have illuminated the synergistic potential of combining radiotherapy with immunotherapy, showcasing benefits such as the enhancement of the immune microenvironment surrounding tumor cells, increased contact inhibition of tumor cells, and improved distant effects[9,10]. Despite these promising findings, the safety profile and overall benefits of this treatment approach have yet to be comprehensively documented.
We undertook a retrospective analysis of clinical data from 80 patients diagnosed with locally advanced ESCC admitted to Zhejiang Cancer Hospital from July 2019 to October 2021. These patients underwent chemoimmunotherapy prior to radiotherapy. Our objective was to assess the safety and efficacy of this treatment strategy, with the ultimate aim of offering a novel and effective treatment method for patients with locally advanced ESCC. Inclusion criteria were as follows: (1) ESCC diagnosed by histological or cytological pathology; (2) Tumor node metastasis stage II-IVA according to the 8th edition of the American Joint Committee on Cancer staging system; (3) Initially evaluated as inoperable, and then re-evaluated as inoperable after 2-4 cycles of chemotherapy combined with immunotherapy or the patient refused surgery, and received definitive radiotherapy; (4) Eastern Cooperative Oncology Group performance status score 0-1; and (5) Age 18-75 years. Exclusion criteria included: (1) Previous history of other malignant tumors; (2) Total irradiation dose < 45.0 Gy; and (3) The presence of esophageal fistula before treatment initiation; and coexistence of serious diseases, such as uncontrolled diabetes, active infections and severe cardiovascular disease or major organ dysfunction, such as cardiac function class III/IV (according to the New York Society of Cardiology classification), pulmonary function tests showing severe ventilatory dysfunction, and glomerular filtration rate < 15 mL/min/1.73 m².
Radiotherapy was performed using an intensity-modulated technique. Computed tomography (CT) simulation localization was performed with the patient in the supine position. Radiotherapy target areas were treated with involved-field and elective nodal irradiation (ENI), and the gross target volume (GTV) of the primary lesion included esophageal tumors detected by esophagography, enhanced CT, endoscopy, or positron emission tomography (PET)/CT. GTV of regional lymph nodes referred to visible metastatic lymph nodes, with a short diameter ≥ 10 mm or ≥ 5 mm in the paraesophageal and tracheo-esophageal grooves, as shown by CT, magnetic resonance imaging, or PET/CT. On PET/CT imaging, lymph nodes demonstrated high standardized uptake value (excluding inflammatory lymph nodes). Although not meeting the aforementioned standardized uptake value threshold, these lymph nodes exhibited obvious necrosis, and ring-like enhancement, with enhancement intensity similar to the primary lesion, or eccentric calcification. The involved field irradiation includes clinical target volume-primary (CTVp) and clinical target volume-node (CTVn). CTVp was defined as 5 mm of GTVp in the anterior-posterior and left-right directions, and 30 mm of GTVp in each of the up-and-down directions. CTVn was defined as the GTVn expanded by 5 mm in all directions. ENI in addition to targeting the primary tumor and clinically or radiologically involved lymph nodes, prophylactically irradiates specific lymph node regions that are not overtly involved but are at high risk for potential metastasis. The planned target volume (PTV) was generated by 5 mm externally in all directions based on CTV.
The evaluation of AEs was conducted in accordance with the World Health Organization grading standards for AEs associated with chemotherapy. Acute radiation reactions were assessed using the standards of the United States Radiation Therapy Oncology Collaborative Group. Therapeutic effectiveness was classified as complete response, partial response (PR), stable disease (SD), and progressive disease by the World Health Organization standards for evaluating the therapeutic effectiveness of solid tumors. Overall survival (OS) was defined as the time from the start of treatment until the patient’s death from any cause. Progression-free survival (PFS) was defined as the time from the start of treatment to disease progression or death from any cause.
Follow-up was once in the first month after completion of radiotherapy, every 3 months on an outpatient basis for the first 2 years, every 6 months on an outpatient basis for 3-5 years, and once a year on an outpatient basis thereafter. Follow-up included blood tests (such as blood count, liver function and tumor markers), chest/abdominal CT, and/or PET/CT. Locoregional recurrence was defined as persistence or recurrence within the esophagus or regional lymph nodes, and distant recurrence included distant organ metastases and non-regional lymph node metastases (supraclavicular or para-abdominal aortic lymph nodes).
SPSS 26.0 statistical software was used for data processing, and the measurement data were expressed as M (range) and N (score). The Kaplan-Meier method was used for survival analysis, the log-rank method was used for univariate analysis, the Cox regression model was used for multivariate analysis, and GraphPad Prism 9.0 was used to draw the survival curve graphs. P < 0.05 was considered a significant difference.
A total of 80 eligible patients were included in this study: 75 male and five female. The age range was 44-75 years with a median age of 63 years. Induction chemotherapy regimens included a paclitaxel-based combination of platinum in 78 cases (97.5%) and a fluorouracil-based combination of platinum in two (2.5%). The induction immunotherapy regimen included programmed cell death 1 inhibitors (camrelizumab, pembrolizumab, sintilimab, tislelizumab, nivolumab, or tislelizumab) and programmed cell death ligand 1 inhibitor (durvalumab). These chemotherapy and immunotherapy regimens were chosen because they are recommended as first choice or standard of care in National Comprehensive Cancer Network guidelines and European Society for Medical Oncology guidelines. In China, the standard dose of radiotherapy for esophageal cancer is 50 Gy. Therefore, patients were divided into two groups: 15 (18.8%) received ≤ 50 Gy and 65 cases (81.2%) > 50 Gy. Thirty-seven (46.3%) patients received concurrent chemoradiotherapy (CCRT) and 43 (53.7%) radiotherapy alone. Concurrent chemotherapy regimens included paclitaxel-based combined with platinum in 28 patients (75.7%), single-agent albumin paclitaxel in two (5.4%), and single-agent tegafur in seven (18.9%). Clinical characteristics are shown in Table 1.
| Characteristics | Data |
| Sex | |
| Male | 75 (93.8) |
| Female | 5 (6.2) |
| Age, year, median (range) | 63 (44-75) |
| Length of primary focus, cm, median (range) | 7 (2-17) |
| Tumor location | |
| Cervical segment | 1 (1.2) |
| Upper thoracic segment | 13 (16.3) |
| Middle thoracic segment | 36 (45.0) |
| Inferior thoracic segment | 30 (37.5) |
| Clinical T stage | |
| T1 | 2 (2.5) |
| T2 | 16 (20.0) |
| T3 | 54 (67.5) |
| T4 | 8 (10.0) |
| Clinical N stage | |
| N0 | 3 (3.7) |
| N1 | 37 (46.3) |
| N2 | 21 (26.3) |
| N3 | 19 (23.7) |
| Clinical M stage | |
| M0 | 69 (86.2) |
| M1 | 11 (13.8) |
| Radiotherapy regimens | |
| Chemoradiotherapy | 37(46.3) |
| Radiotherapy alone | 43 (53.7) |
| Radiation dose | |
| ≤ 50 Gy | 15 (18.8) |
| > 50 Gy | 65 (81.2) |
| Induction chemotherapy regimen | |
| Paclitaxel-based combination of platinum | 78 (97.5) |
| Fluorouracil-based combination of platinum | 2 (2.5) |
| Induction immunotherapy regimen | |
| Camrelizumab | 45 (56.2) |
| Pembrolizumab | 14 (17.5) |
| Sintilimab | 10 (12.5) |
| Tislelizumab | 5 (6.2) |
| Nivolumab | 1 (1.3) |
| Toripalimab | 1 (1.3) |
| Durvalumab | 4 (5.0) |
| Concurrent chemotherapy regimen | |
| Paclitaxel-based combination of platinum | 27 (88.8) |
| Single-agent albumin paclitaxel | 2 (2.5) |
| Single-agent tegafur | 7 (8.7) |
| Reasons for no surgery | |
| Unresectable or medically inoperable | 57 (71.3) |
| Refusal of surgery | 23 (28.7) |
AEs were mainly anemia and radiation esophagitis. A total of 61 patients (76.3%) developed varying degrees of myelosuppression, including anemia in 49 (61.3%), leukocyte decline in 42 (52.5%), platelet decline in 30 (37.5%), and neutrophil decline in nine (11.3%). Nonhematological AEs occurred in 49 (61.3%) patients, including 32 (40.0%) with radiation esophagitis, 25 (31.3%) with hypothyroidism, 22 (27.5%) with radiation pneumonitis, six (7.5%) with radiation dermatitis, three (3.8%) with radiation mucositis, and 11 (13.8%) with nausea and vomiting. The incidence of grade 3 or higher AEs was 27.5%, mainly including radiation esophagitis in 12 (15.0%) patients and radiation dermatitis in three (3.8%). No patients died from treatment-related AEs.
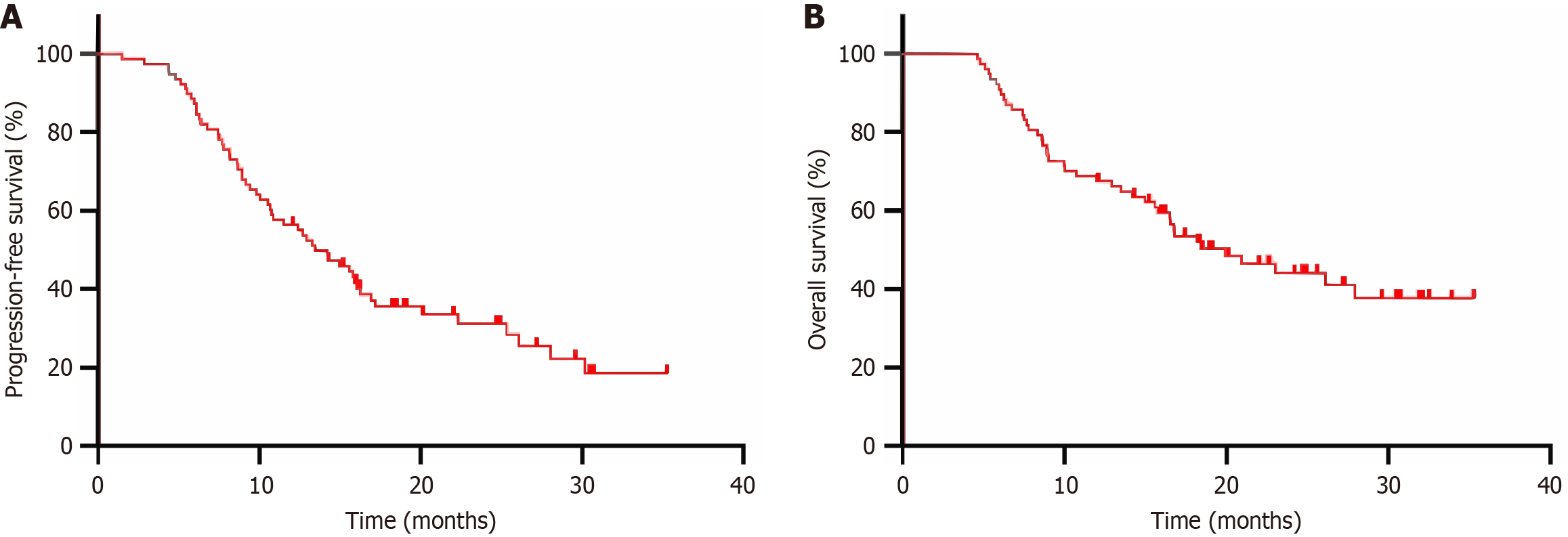
As of December 17, 2022, the median follow-up time was 16.5 (4.6-35.3) months. A total of 80 eligible patients were included in this study with three lost to follow-up, and the follow-up rate was 96.3%. Among the 77 patients followed up, 54 (71.1%) had progressive disease, 42 died, and 35 survived, with a median PFS and median OS of 14.2 months [95% confidence interval (CI): 10.3-18.1 months] and 19.9 months (95%CI: 13.8-26.0 months), and 1-year and 2-year PFS and OS rates of 55.8% and 31.6%, and 67.5% and 44.1%, respectively (Figure 1).
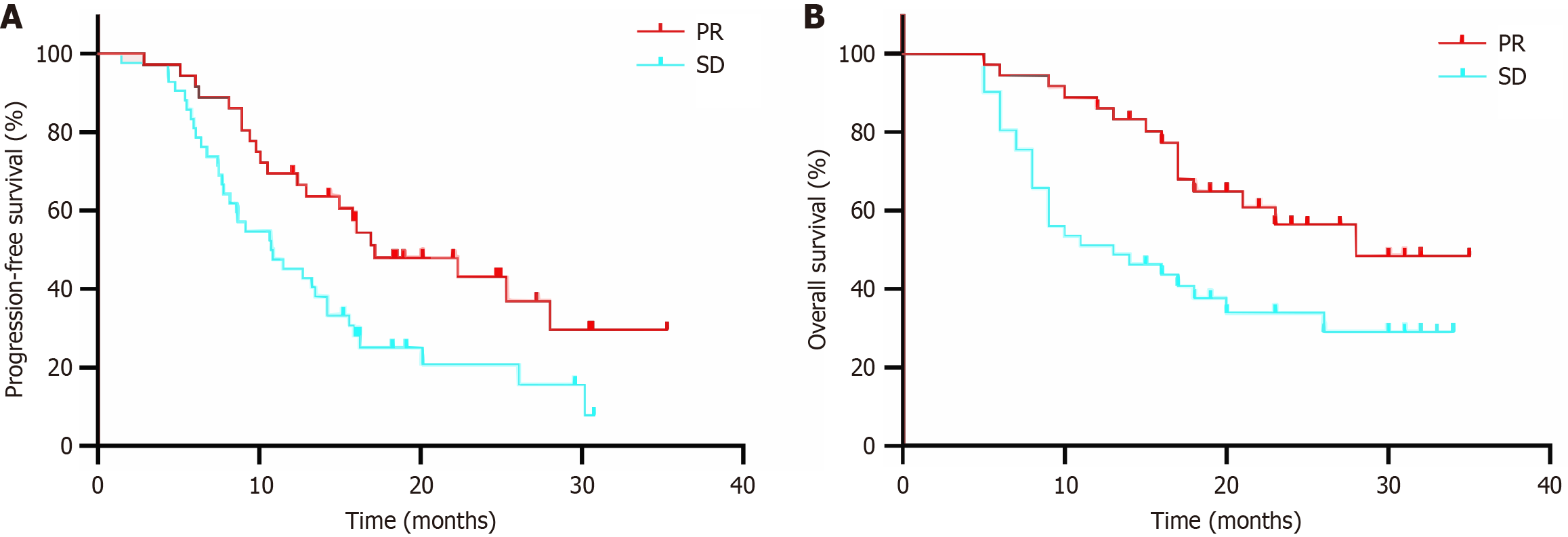
The results of univariate analysis showed that the effectiveness of induction therapy was a relevant factor affecting PFS, and the effectiveness of induction therapy, PTV dose, and maintenance immunotherapy were relevant factors affecting OS (all P < 0.05, Table 2). The results of multifactorial analysis showed that the therapeutic effectiveness of induction therapy and maintenance immunotherapy were independent prognostic factors affecting OS (both P < 0.05, Table 3). The median PFS in the subgroup of therapeutic effectiveness for PR and SD after induction therapy was 17.2 months (95%CI: 9.5-24.8 months) and 10.8 months (95%CI: 7.2-4.3 months), respectively, with a 1-year PFS of 69.4% vs 43.9% (P = 0.011), and 1-year OS of 83.2% vs 48.8% (P = 0.007) (Figure 2).
| Characteristics | Data, n (%) | PFS, χ2 value | PFS, P value | OS, χ2 value | OS, P value |
| Sex | 0.296 | 0.604 | 0.198 | 0.657 | |
| Male | 72 (93.5) | ||||
| Female | 5 (6.5) | ||||
| Age, year | 1.354 | 0.245 | 1.784 | 0.182 | |
| < 65 | 43 (55.8) | ||||
| ≥ 65 | 34 (44.2) | ||||
| Tumor length | 0.138 | 0.710 | 0.023 | 0.879 | |
| < 7 cm | 37 (48.1) | ||||
| ≥ 7 cm | 40 (51.9) | ||||
| Tumor location | 2.235 | 0.525 | 4.433 | 0.218 | |
| Cervical segment | 1 (1.2) | ||||
| Upper thoracic segment | 13 (16.3) | ||||
| Middle thoracic segment | 36 (45.0) | ||||
| Inferior thoracic segment | 30 (37.5) | ||||
| Clinical T stage | 0.795 | 0.372 | 0.069 | 0.793 | |
| T1-2 | 17 (22.1) | ||||
| T3-4 | 60 (77.9) | ||||
| Clinical N stage | 1.826 | 0.177 | 0.001 | 0.976 | |
| N0-1 | 39 (50.6) | ||||
| N2-3 | 38 (49.4) | ||||
| Clinical M stage | 0.004 | 0.948 | 0.003 | 0.995 | |
| M0 | 66 (85.7) | ||||
| M1 | 11 (14.3) | ||||
| Induction immunotherapy regimen | 5.558 | 0.475 | 4.851 | 0.563 | |
| Camrelizumab | 45 (56.2) | ||||
| Pembrolizumab | 14 (17.5) | ||||
| Sintilimab | 10 (12.5) | ||||
| Tislelizumab | 5 (6.2) | ||||
| Nivolumab | 1 (1.3) | ||||
| Toripalimab | 1 (1.3) | ||||
| Durvalumab | 4 (5.0) | ||||
| Therapeutic effectiveness of induction therapy | 6.509 | 0.011 | 7.341 | 0.007 | |
| Partial response | 36 (45.0) | ||||
| Stable disease | 41 (52.5) | ||||
| Radiotherapy modalities | 0.894 | 0.344 | 1.949 | 0.163 | |
| Radiotherapy | 36 (46.8) | ||||
| CCRT | 41 (53.2) | ||||
| PTV dose | 1.733 | 0.188 | 4.223 | 0.040 | |
| ≤ 50 Gy | 13 (16.8) | ||||
| > 50 Gy | 64 (83.1) | ||||
| Whether maintenance of immunotherapy | 1.811 | 0.178 | 6.349 | 0.012 | |
| Yes | 23 (29.9) | ||||
| No | 54 (70.1) |
| Characteristics | HR | 95%CI | P value |
| Therapeutic effectiveness of induction therapy | |||
| Partial response | 1.000 | ||
| Stable disease | 2.113 | 1.113-4.013 | 0.022 |
| PTV dose | |||
| ≤ 50 Gy | 1.000 | ||
| > 50 Gy | 0.620 | 0.300-1.284 | 0.198 |
| Maintenance of immunotherapy | |||
| Yes | 1.000 | ||
| No | 0.402 | 0.185-0.957 | 0.039 |
The current gold standard for the treatment of locally advanced unresectable EC is definitive CRT, according to National Comprehensive Cancer Network guidelines. However, despite this approach, the median survival time remains short; typically < 10 months[11]. Unfortunately, optimizing factors such as the chemotherapy regimen, irradiation target volumes and radiation dose have not yielded significant improvements in prognosis. In the landscape of advanced EC, the combination of ICIs with chemotherapy has emerged as the standard of care. Several randomized controlled clinical trials have underscored the outstanding safety profile of immunotherapy while simultaneously demonstrating a profound enhancement in patient prognosis[12,13]. Notably, the synergistic effect of immunotherapy and chemotherapy has demonstrated initial benefits in locally advanced EC. A study involving 155 patients revealed that neoadjuvant immunotherapy combined with chemotherapy successfully converted a substantial percentage of initially unresectable cases to resectable, with a conversion rate of 74.8%[14]. However, small-scale research on immunotherapy sequential radiotherapy has pointed to a tendency toward a higher incidence of harmful AEs, such as checkpoint inhibitor pneumonitis[15]. Thus, there is much interest in researching the safety and efficacy of immunotherapy in combination with chemotherapy for locally advanced EC. This study retrospectively analyzed the long-term survival outcomes and AEs in patients with locally advanced ESCC using this treatment method.
Our study demonstrated that the treatment-associated AEs were consistent with those observed in radical CCRT alone and combined with immunotherapy. In the study of radical CCRT for locally advanced EC[2], AEs of grade 3 and above occurred in both groups with different chemotherapy regimens and reached 50%, and the incidence of hematological and nonhematological AEs after radiotherapy was lower in this study. These findings could be attributed to the following factors. Pharmacological interventions were generally timely in the pre-myelosuppressive stage of the patients in this study. The risk of radiation injury has been gradually reduced with the continuous improvement of radiotherapy technology in recent years. In comparison to the EC-CRT-001 study exploring toripalimab combined with definitive CRT[16], our study exhibited a higher incidence of grade 3 and higher radiation esophagitis (15.0% vs 10.0%). This dis
The benefit of definitive CRT in conjunction with immunotherapy for patients with locally advanced ESCC has been confirmed by several trials. Zhang et al[15] initiated a study of camrelizumab combined with concurrent radiotherapy for patients with locally advanced ESCC, which showed that the objective response rate of the 20 patients reached 65%. The feasibility of definitive CRT combined with camrelizumab for first-line treatment of ESCC was confirmed. A further single-arm phase 2 trial, designated EC-CRT-001, demonstrated that 26 patients (62%) achieved complete response with a median duration of response of 12.1 months. It also showed that toripalimab combined with radical concurrent ra
In terms of survival, the 1-year and 2-year PFS and OS were 55.8% and 31.6%, and 67.5% and 44.1%, respectively, which were not significantly different from those observed in several radical CCRT studies, including the Radiation Therapy Oncology Collaborative Group 0436 study[17-20]. In our study, the 1-year PFS was similar to that in the EC-CRT-001 study (54.5% vs 55.8%)[16], while the 1-year OS was slightly lower (78.4% vs 67.5%). Several factors could have contributed to these observations. The concurrent combination of radiotherapy with immunotherapy may exert a more potent inhibitory effect on tumor cells, and the influx of lymphocytes induced by ICIs may be susceptible to radiotherapy-induced cell death, thereby impacting therapeutic efficacy. Simultaneously, radiotherapy may hinder the activity of effector T cells through the cGAS-STING pathway, leading to lymphopenia, which, in turn, can affect the overall treatment response[21,22]. These complex interactions highlight the intricate interplay between immunotherapy and radiotherapy, emphasizing the need for further investigation and understanding of the underlying mechanisms.
The subsequent stratified analysis of diverse clinical factors in our study revealed that patients who achieved PR during induction therapy exhibited a more favorable prognosis, with significantly improved median PFS and median OS compared to those with SD (17.16 vs 10.75 months and 27.95 vs 13.48 months). This favorable outcome may be attributed to the potential recovery of residual tumor vascular function following a reduction in tumor burden. This recovery may contribute to an improved hypoxic microenvironment, sensitizing the tumors to subsequent radiotherapy. Our study affirmed that PTV > 50 Gy significantly correlated with enhanced patient survival, consistent with previous studies[19]. Our study also highlighted that immune maintenance therapy after definitive radiotherapy substantially prolonged OS in patients with locally advanced ESCC. This suggests that immune maintenance therapy could confer a similar survival benefit in patients with locally advanced EC as demonstrated in advanced cases. These findings underscore the potential significance of tailored therapeutic strategies based on individual patient characteristics and treatment responses.
This study had several limitations. Firstly, due to its retrospective nature and single-arm design, there was inherent bias resulting in a low level of evidence-based support. Secondly, the sample size was small, necessitating further expansion to enhance validation. Thirdly, the follow-up period was limited, precluding the assessment of long-term survival benefits and late toxicity.
Our study was an initial investigation to establish the efficacy and safety of chemotherapy combined with immunotherapy followed by radiotherapy as the primary intervention for patients diagnosed with locally advanced ESCC. Chemotherapy combined with immunotherapy followed by radiotherapy had a favorable safety profile in the mana
| 1. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2470] [Article Influence: 617.5] [Reference Citation Analysis (2)] |
| 2. | Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, Tang H, Fan M, Li L, Lin Q, Xia Y, Li Y, Li J, Jia H, Lu S, Zhang Z, Zhao K. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial. J Clin Oncol. 2019;37:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Buckstein M, Liu J. Cervical Esophageal Cancers: Challenges and Opportunities. Curr Oncol Rep. 2019;21:46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, Park SR, Ping L, Jiang Y, Zhang J, Wu X, Yao Y, Shen L, Kojima T, Gotovkin E, Ishihara R, Wyrwicz L, Van Cutsem E, Jimenez-Fonseca P, Lin CY, Wang L, Shi J, Li L, Yoon HH. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023;24:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 5. | Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 774] [Article Influence: 193.5] [Reference Citation Analysis (2)] |
| 6. | Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K; KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 1057] [Article Influence: 211.4] [Reference Citation Analysis (0)] |
| 7. | Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, Wu Y, Feng X, Qi W, Chen K, Xiang J, Li J, Lerut T, Li H. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. 2021;144:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 8. | van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, Meijer SL, Schokker S, Dings MPG, Bergman JJGHM, Haj Mohammad N, Ruurda JP, van Hillegersberg R, Mook S, Nieuwdorp M, de Gruijl TD, Soeratram TTD, Ylstra B, van Grieken NCT, Bijlsma MF, Hulshof MCCM, van Laarhoven HWM. Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasibility Trial (PERFECT). Clin Cancer Res. 2021;27:3351-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 9. | Lee SJ, Yang H, Kim WR, Lee YS, Lee WS, Kong SJ, Lee HJ, Kim JH, Cheon J, Kang B, Chon HJ, Kim C. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J Immunother Cancer. 2021;9:e002195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Liu ZG, Yuan H, Deng W, Li J, Huang Y, Kim BYS, Story MD, Jiang W. The Reciprocity between Radiotherapy and Cancer Immunotherapy. Clin Cancer Res. 2019;25:1709-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, Willett C, Stiles B, Sharma P, Tang L, Wijnhoven BPL, Hofstetter WL. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:2677-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 12. | Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH; ESCORT-1st Investigators. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021;326:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 547] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 13. | Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wang J, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Liu Y, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Zhang Y, Zhang Y, Chen X, Dai G, He J, Li J, Li G, Liu Y, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Liu Z, Tang P, Wang T, Wang W, Zhang J, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022;40:277-288.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 330] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 14. | Huang S, Wu H, Cheng C, Zhou M, Xu E, Lin W, Wang G, Tang J, Ben X, Zhang D, Xie L, Zhou H, Chen G, Zhuang W, Tang Y, Xu F, Du Z, Xie Z, Wang F, He Z, Zhang H, Sun X, Li Z, Sun T, Liu J, Yang S, Xie S, Fu J, Qiao G. Conversion Surgery Following Immunochemotherapy in Initially Unresectable Locally Advanced Esophageal Squamous Cell Carcinoma-A Real-World Multicenter Study (RICE-Retro). Front Immunol. 2022;13:935374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Zhang W, Yan C, Zhang T, Chen X, Dong J, Zhao J, Han D, Wang J, Zhao G, Cao F, Zhou D, Jiang H, Tang P, Zhao L, Yuan Z, Wang Q, Wang P, Pang Q. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study. Oncoimmunology. 2021;10:1971418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 16. | Zhu Y, Wen J, Li Q, Chen B, Zhao L, Liu S, Yang Y, Wang S, Lv Y, Li J, Zhang L, Hu Y, Liu M, Xi M. Toripalimab combined with definitive chemoradiotherapy in locally advanced oesophageal squamous cell carcinoma (EC-CRT-001): a single-arm, phase 2 trial. Lancet Oncol. 2023;24:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 17. | Suntharalingam M, Winter K, Ilson D, Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar H, Horiba N, Dubey A, Greenberger JS, Raben A, Giguere J, Roof K, Videtic G, Pollock J, Safran H, Crane CH. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2017;3:1520-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, Etienne PL, Boige V, Martel-Lafay I, Michel P, Llacer-Moscardo C, François E, Créhange G, Abdelghani MB, Juzyna B, Bedenne L, Adenis A; Fédération Francophone de Cancérologie Digestive and UNICANCER-GI Group. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Hulshof MCCM, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, Nuyttens JJME, van der Sangen MJC, Jeene PM, Reinders JG, van Berge Henegouwen MI, Thano A, van Hooft JE, van Laarhoven HWM, van der Gaast A. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (ARTDECO Study). J Clin Oncol. 2021;39:2816-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 20. | Ai D, Ye J, Wei S, Li Y, Luo H, Cao J, Zhu Z, Zhao W, Lin Q, Yang H, Zheng X, Zhou J, Huang G, Li L, Li J, Zhang Z, Zhou G, Gu D, Du M, Mo M, Jia H, Zhang Z, Zhao K. Comparison of 3 Paclitaxel-Based Chemoradiotherapy Regimens for Patients With Locally Advanced Esophageal Squamous Cell Cancer: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e220120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 1019] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 22. | Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 1400] [Article Influence: 155.6] [Reference Citation Analysis (0)] |