Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.100723
Revised: November 18, 2024
Accepted: December 13, 2024
Published online: March 24, 2025
Processing time: 149 Days and 21.2 Hours
Pancreatic cancer (PC) is one of the most aggressive malignancies characterized by rapid progression and poor prognosis. The involvement of cancer stem cells (CSCs) and Octamer transcription factor 4 (OCT4) in PC pathobiology is being increasingly recognized.
To investigate the role of OCT4 in pancreatic CSCs and its effect on PC cell proliferation, migration, drug sensitivity, and stemness maintenance.
We analyzed OCT4 and CD133 expression in PC tissues and cell lines. BxPC-3 cells were used to assess the effects of OCT4 modulation on cellular behavior. Proliferation, migration, and stemness of BxPC-3 cells were evaluated, and the PI3K/AKT/mTOR pathway was examined to gain mechanistic insights.
OCT4 and CD133 were significantly overexpressed in PC tissues. OCT4 mo
OCT4 plays a crucial role in PCSCs by influencing the aggressiveness and drug resistance of PC cells, thus presenting itself as a potential therapeutic target.
Core Tip: This study introduces a novel perspective on Octamer transcription factor 4 (OCT4)'s role in pancreatic cancer (PC) stem cells (PCSCs) and demonstrated that OCT4 overexpression enhances activity and drug resistance of PCSCs, with notable implications for PC treatment.
- Citation: Shi XY, Wang XL, Zhao J, Yang SH, Zhang CH. Role of octamer transcription factor 4 in proliferation, migration, drug sensitivity, and stemness maintenance of pancreatic cancer cells. World J Clin Oncol 2025; 16(3): 100723
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/100723.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.100723
Pancreatic cancer (PC) is one of the most fatal malignancies globally and is characterized by rapid progression and poor prognosis[1]. The insidious nature of PC often results in the absence of discernible early symptoms, leading to a dearth of effective biomarkers for early diagnosis[2]. Consequently, most patients are diagnosed at an advanced stage, missing the window for surgical intervention[3]. Furthermore, development of chemotherapeutic resistance significantly impedes the efficacy of treatment strategies. As a result, the 5-year survival rate for PC remains alarmingly low at less than 8%[4-6]. The identification of more precise diagnostic markers and therapeutic targets, along with the elucidation of the molecular framework of drug resistance, represents a formidable challenge in oncology. Addressing these issues is imperative for advancing our understanding of PC and developing more efficacious treatment modalities.
In recent years, accumulating evidence has underscored the role of cancer stem cells (CSCs) in the etiology of tumorigenesis, heterogeneity, metastasis, and recurrence, as well as in the development of drug resistance mechanisms[7-9]. Despite these insights, the precise mechanisms by which CSCs contribute to PC pathogenesis remain unclear. Therefore, elucidating the pathogenesis of CSCs in PC is crucial as it has the potential to inform the development of targeted therapeutics aimed at eradicating these cells. Such advancements may enable interventions at the initial stages of PC, thereby enhancing clinical outcomes. A deeper understanding of the role of CSCs in PC is critical. It may reshape our treatment approach through improved patient prognosis and the potential to mitigate the impact of this disease.
Octamer transcription factor 4 (OCT4) is a hallmark of embryonic stem cell (ESC) totipotency and serves as a pivotal regulator of preimplantation embryonic development. It is a critical transcription factor, governing the self-renewal of ESCs and the maintenance of their totipotency. Furthermore, OCT4 is essential for establishing and inducing multipotent stem cells, including induced pluripotent stem cells (iPSCs), in vitro[10]. Experimental evidence has demonstrated that inactivation of OCT4 within ESCs results in the loss of their totipotent state, thereby impeding normal embryonic progression. This finding underscores the indispensable role of OCT4 in establishing stemness in ESCs. CSCs share numerous biological characteristics with ESCs, including the expression of similar stemness markers[11]. Overexpression of OCT4 significantly influences the development, metastasis, and drug resistance of PC stem cells (PCSCs). However, the precise molecular mechanisms underlying these effects remain unknown.
In the present study, we used PC tissue microarray samples and established cultures of the human PC cell line, BxPC-3, to investigate the influence of alterations in OCT4 expression on the stemness characteristics of PCSCs. We also examined the contribution of these genetic factors to the proliferation, migration, and drug resistance of PCSCs. Our approach was designed to provide a comprehensive assessment of OCT4's role in modulating the stemness of PCSCs, as well as its impact on key cellular processes that are critical to the progression of malignancy and therapeutic response. In this study, we aimed to advance our understanding of the molecular mechanisms underlying the aggressive behavior of PC and identify potential therapeutic targets.
A PC tissue chip was purchased from Shanghai Outdo Biotech Co., LTD (order number: HPan-Ade180Sur-02), which contains patient clinical data and pathological samples. The survival time of 100 patients with PC (80 cases at the site of cancer/adjacent tissues, the other 20 cases were cancerous tissue), all of which were in situ carcinomas, was analyzed. The study included 63 men and 37 women, aged 34-85 years. The tumor sites included the head of the pancreas, tail of the pancreatic body, neck of the pancreas, and sulcus process of the head of the pancreas. The pathological types included duct adenocarcinoma (91 cases), adenocarcinoma (two cases), mucinous adenocarcinoma (two cases), and cystadenoma (one case). The clinical stages, according to the seventh edition of the AJCC, were stages 1A, 1B, 2A, 2B, and 4, and the TNM stages were mainly stages T2-T3. There were 60 cases of lymphatic metastasis, 40 cases without metastasis, and only two cases of distant metastasis. Survival time was 0-87 months. The surgeries were conducted between September 2004 and December 2008; the follow-up period was from December 2011 to a period of 3-7 years. Clinical data included: Smoking history, drinking history, diabetes history, hepatitis B test, family history and others.
A fully automatic immunohistochemical pretreatment system and fully automatic immunohistochemical staining system were provided by Dako. The tissue chip was placed in the oven, the temperature set to 63°C, and the wax was baked for 1 hour. Dewaxing was done in a fully automatic dyeing machine, according to the "PTLink concise operating procedures”. The slide was then placed into the antigen repair solution; after the repair was completed, it was placed in distilled water at room temperature for more than 10 minutes and cooled naturally. The chip was removed and rinsed with PBST buffer. The diluted working liquid was added and refrigerated at 4°C overnight. The slides were then removed from the refrigerator, rewarmed at room temperature for 45 minutes, and washed with PBST buffer. Slides were placed into the DAKO automatic immunohistochemical staining system instrument; blocking, secondary antibody binding, and DAB color development procedures were performed according to the "Autostainer Link 48 Usage Guide”. Hematoxylin staining was performed for 1 minute, after which the sections were immersed in 0.25% hydrochloric acid alcohol (400 mL 70% alcohol + 1 mL concentrated hydrochloric acid) for at least 2 seconds and rinsed with tap water for at least 2 minutes. The slides were then dried at room temperature and examined using a neutral resin seal.
OCT4 and CD133 showed cytoplasmic staining in PC and adjacent tissues, whereas a small number of nuclei were stained in the intercellular tissue, and the positive staining revealed brown-yellow particles. Five fields (× 100) were randomly selected for each section under a microscope, and the expression of OCT4 and CD133 proteins was analyzed using Image Pro Plus 6.0 image analysis software. If the average gray value was large (weak staining intensity and strong transmittance), the protein expression was low, and vice versa. All datas were t tested using SPSS statistical software. A P value < 0.05 was considered statistically significant.
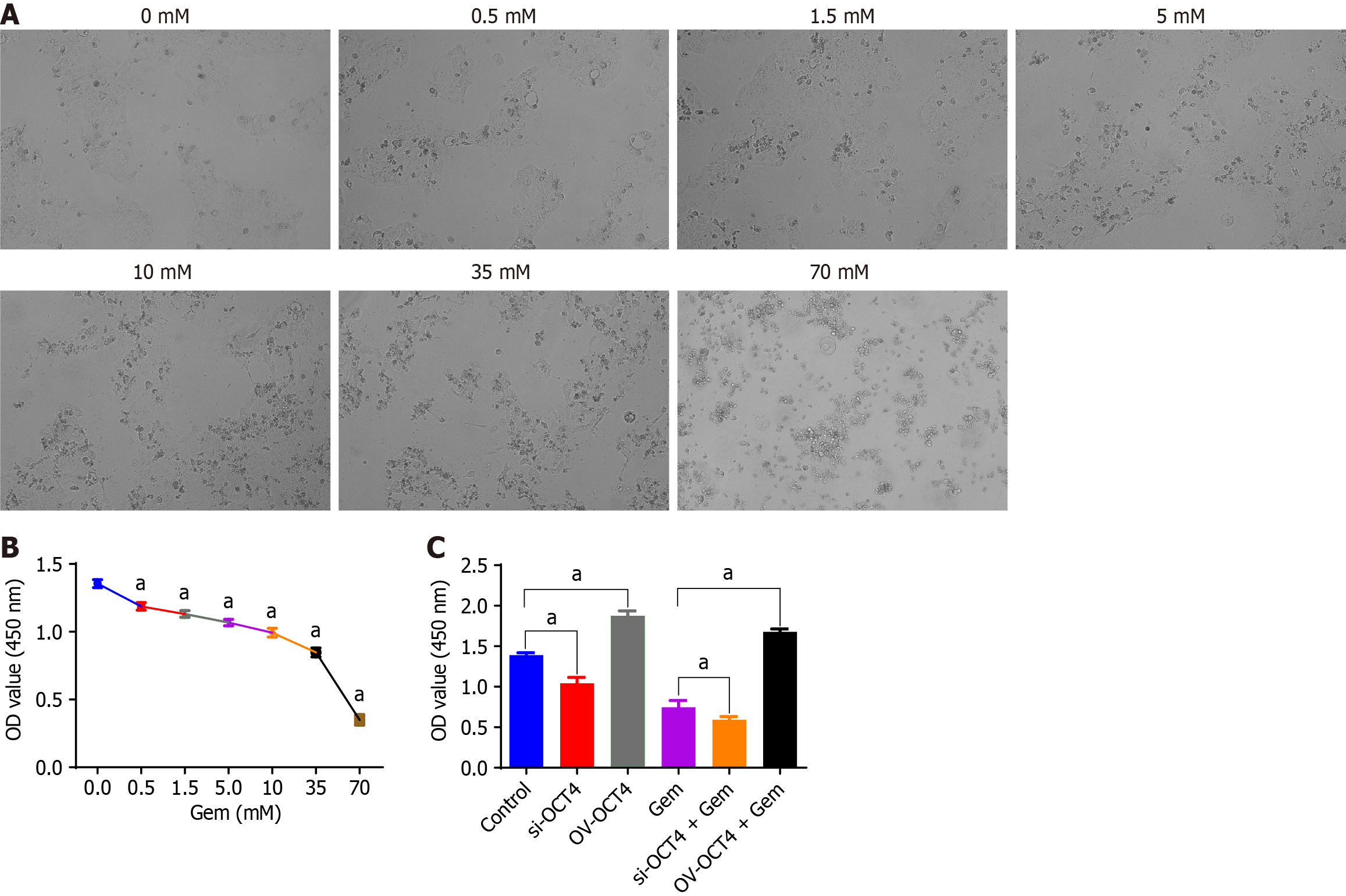
BxPC3 cells were purchased from Shanghai Biocon Biotech Co. and maintained in RPMI 1640 + 10%FBS + 1%PS (cyanostreptomycin) (Biosharp, United States). BxPC3 cells were cultured at 1 × 104 cells/well in 96-well plates with six wells per group. After an overnight culture, different concentrations of gemcitabine (0.5 mmol/L, 1.5 mmol/L, 5 mmol/L, 10, 35, and 70 mmol/L), dissolved in water, were added according to the manufacturer’s protocol. The cell plates were cultured at 37 °C in 5% CO2 incubator for 24 hours. Ten μL CCK8 solution was added to each well and incubated at 37 °C for 1 hour. The absorbance at 450 nm was measured using an enzyme-linked immunosorbent assay (ELISA) instrument and the data were recorded. The half of the determined inhibitory concentration (IC50) of gemcitabine is 50 mmol/L.
Small interfering RNAs (siRNAs) targeting OCT4 (siRNA-1, siRNA-2, siRNA-3, and siRNA-4) were synthesized by Sangon Biotech (Shanghai, China). Subsequently, 10 μL of siRNA was transfected into the cells using Lipofectamine 2000 (11668-019, Invitrogen, California, United States) After 48 hours, the cells were collected, and OCT4 expression was assessed. The siRNA sequences are listed in Table 1.
| Name | Sense (5′-3′) | Antisense (5′-3′) |
| OCT4 (human) siRNA-1# | GAGGCAACCUGGAGAAUUUTT | AAAUUCUCCAGGUUGCCUCTT |
| OCT4 (human) siRNA-2# | GCGACUAUGCACAACGAGATT | UCUCGUUGUGCAUAGUCGCTT |
| OCT4 (human) siRNA-3# | CAGAAGGGCAAGCGAUCAATT | UUGAUCGCUUGCCCUUCUGTT |
| OCT4 (human) siRNA-4# | GCAGAAAGAACUCGAGCAATT | UUGCUCGAGUUCUUUCUGCTT |
| Negative control | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
The overexpression plasmid (Gene ID: 5460) was synthesized by General Biosystems (Anhui) Co., Ltd. (Anhui, China).
Total RNA was isolated from the indicated cell lines using the CW Total RNA Kit (CoWin Biotech Co., China). The cells were cultured overnight and transfected using the RNA transfection system. Final concentration of inhibitor and siRNA in transfected cells was 100 nM, and the final concentration of control siRNA was 50 nM. For plasmid transfection, the 24-well plate was transfected with 0.5 μg/well and the 6-well plate was transfected with 2.5 μg/well. cDNA was synthesized using qPCR and amplified using real-time PCR. The results were automatically analyzed using a real-time fluorescence quantitative PCR instrument; the threshold and baseline were adjusted according to the negative control to determine the Ct value of each specimen and whether the Ct value was valid according to the melting curve. The results were derived and the expression difference of the target gene between the control group and each test group was analyzed using the 2-△△CT method. The measurement was made using the following formula: △Ct = Ct target gene -Ct internal reference; then, the average of △Ct in the control group was obtained and recorded as the △Ct control average. The △Ct value was obtained by the subtraction of △Ct control average from the △Ct in each group, that is, △Ct = △Ct sample -△Ct control average. The 2-△△CT value in each group was then calculated, which was the relative expression of genes in each group.
In this study, we categorized the cells into distinct experimental groups: Control, OCT4 overexpression (OV-OCT4), and OCT4 interference (si-OCT4). Each group was subjected to a 48-hour culture period. The cells were transfected into their respective groups for 48 hours, followed by the assessment of EDU incorporation (supplied by Biyuntian Biotechnology) and the tumor sphere formation. For EDU detection, BxPC3 cells were plated at a density of 1 × 105 cells per well in a 24-well plate. After transfection, in accordance with the experimental protocol, the cells were subjected to EDU detection following the manufacturer's instructions provided with the Biyuntian kit. Following enzymatic digestion, the cells were harvested by centrifugation, the serum-containing medium was aspirated, and the cells were rinsed twice with phosphate buffered saline (PBS) to remove any traces of serum. Subsequently, the cells were seeded at a density of 1 × 103 cells per well in a 96-well ultra-low attachment plate and incubated. The cells were then transfected according to the experimental protocol, and tumor spheres were photographed after a 5-day incubation period.
Apoptosis assay: The cells were transfected for 48 hours, and then apoptosis was examined using flow cytometry. Annexin V-FITC was tested through the FITC detection channel (Ex = 488 nm; Em = 530 nm), and PI was performed using the PI detection channel (Ex = 535 nm; Em = 615 nm).
Transwell assay: Migration assay: After the cells were digested, they were collected by centrifugation at 1500 rpm for 5 minutes. The cells were washed twice with PBS and resuspended in an appropriate volume of serum-free culture medium. The cell density was adjusted to approximately 2 × 105/mL. The Lipofectamine 2000 transfection reagent was used for cell transfection. A 24-well culture plate was prepared with 600 μL of complete medium containing 10% FBS in each well, and a Falcon 8 μm pore size PET membrane Transwell insert was placed in each well. The transfected cell-lipid mixture (200 μL) was added to the upper chamber of the Transwell, and the plate was incubated in a CO2 incubator. After 48 hours, the plate and the inserts were carefully removed. The supernatant was discarded and the cells on the upper surface were gently removed with a moist cotton swab. The cells were then fixed with 4% paraformaldehyde for 15 minutes and stained with a 0.1% crystal violet solution for 20 minutes. The number of cells in each field of view were counted under an inverted microscope (100 × magnification) and photographed for preservation.
Invasion assay: The assay method was the same as that of the migration assay, with the only difference being that the upper layer of the Transwell chamber PET film was uniformly covered with Matrigel matrix glue. The specific ex
The cells were harvested and subsequently subjected to a 48-hour culture period to ensure optimal growth conditions. The cells were then fixed using a suitable fixative solution for 1 hour to preserve the cellular structures. To facilitate the penetration of antibodies, the cells were permeabilized and sealed, allowing for enhanced accessibility of antigens within the cells. This process was completed in 1 hour. Subsequently, the cells were incubated with antibodies [phycoerythrin (PE) anti-human CD133 antibody (W6B3C1), E-AB-F1268D; Elabscience Bionovation Inc. Wuhan, China, and FITC Anti-Human CD44 Antibody (Hermes-1), E-AB-F1215C; Elabscience Bionovation Inc. Wuhan, China] for 1 hour to ensure adequate binding. To stain the cell nuclei, the cells were then treated with DAPI for 10 minutes, which binds to DNA and emits fluorescence upon excitation. The preparation was mounted on a microscope slide using an anti-fade mounting medium to prevent photobleaching and ensure the long-term preservation of fluorescence. After sealing, the slide was scanned using a fluorescence microscope to capture dual-stained immunofluorescence images, allowing for simultaneous visualization of both the antigen of interest and cell nuclei.
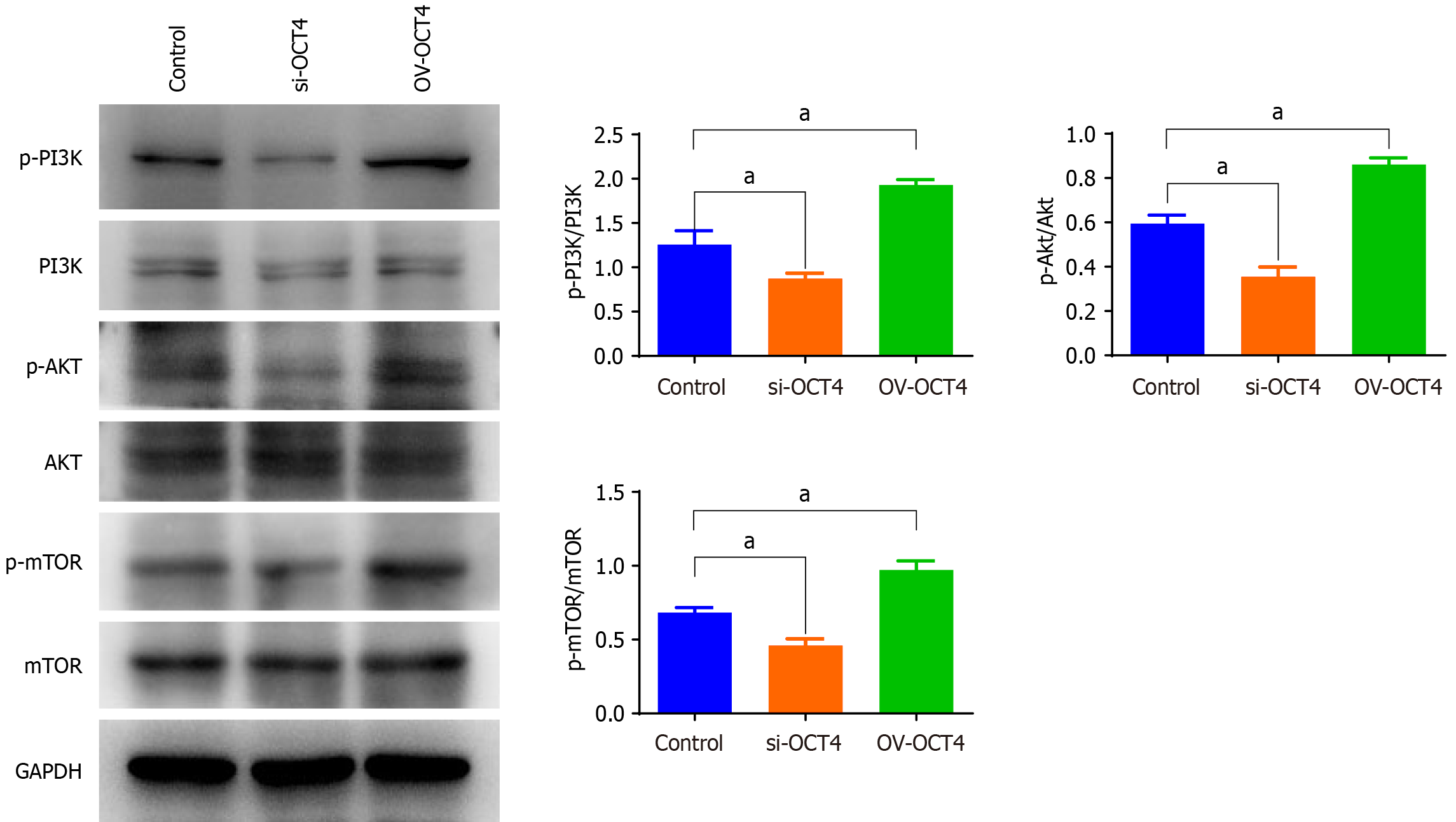
Western blot analysis was performed to investigate the expression profiles of the PI3K/AKT/mTOR signaling pathway. Cell samples from each experimental group were prepared and lysed using RIPA buffer to extract total cellular proteins. The resulting lysate was centrifuged to pellet the cellular debris, and the supernatant containing the lysed protein solution was carefully collected. The protein concentration in the lysates was quantified using a BCA protein assay kit to ensure accurate measurement and normalization of the protein content. The protein concentrations of the experimental samples were standardized to an optimal range to facilitate comparisons across samples. Subsequently, SDS-PAGE was performed. The gel was prepared using a resolving gel and stacking gel, both formulated with specific concentrations of acrylamide and bis-acrylamide, to resolve proteins within the molecular weight range of interest. The prepared samples were loaded onto a gel and subjected to electrophoresis under denaturing conditions, which allowed the separation of proteins based on their molecular weights. Following electrophoresis, the proteins were transferred onto a PVDF membrane, which was blocked to prevent nonspecific antibody binding. The membrane was incubated with primary antibodies specific to the PI3K/AKT/mTOR pathway, allowing selective binding of these antibodies to their respective antigens. After extensive washing to remove unbound primary antibodies, the membrane was incubated with hor
| Item No. | Supplier | Country | |
| AKT Monoclonal antibody | 60203-2-Ig | Proteintech | China |
| Phospho-AKT (Ser473) Polyclonal antibody | 28731-1-AP | Proteintech | China |
| PI3 Kinase p85 Alpha Monoclonal antibody | 60225-1-Ig | Proteintech | China |
| Phospho-PI3K p85 (Tyr458) [Tyr467]/p55 (Tyr199) Antibody | AF3242 | Affinity | China |
| mTOR Monoclonal antibody | 66888-1-Ig | Proteintech | China |
| Phospho-mTOR (Ser2448) Monoclonal antibody | 67778-1-Ig | Proteintech | China |
| HRP conjugated Goat Anti-Mouse IgG (H+L) | GB23301 | Servicebio | China |
| HRP conjugated Goat Anti-Rabbit IgG (H+L) | GB23303 | Servicebio | China |
In this study, we assessed the effects of altered OCT4 expression on the chemosensitivity of BxPC3 cells to gemcitabine. The cells were divided into several groups: Control, OV-OCT4, OCT4 knockdown (si-OCT4), gemcitabine-only (1/2 IC50), OCT4 overexpression plus gemcitabine (OV-OCT4 + gemcitabine at 1/2 IC50), and OCT4 knockdown plus gemcitabine (si-OCT4 + gemcitabine at 1/2 IC50).
BxPC3 cells were incubated overnight to allow attachment. Subsequently, transfection with the respective constructs was performed, followed by a 24-hour incubation period to facilitate changes in gene expression. The cells were treated with gemcitabine at a concentration equivalent to IC50. Following drug exposure, the cells were cultured for an additional 24 hours.
To evaluate cell viability, CCK-8 solution was added to each well and the cells were incubated for 1 hour. The absorbance, indicative of cellular metabolic activity, was measured at 450 nm using a spectrophotometric ELISA reader. The recorded absorbance values provided quantitative data regarding the response of the cells to gemcitabine in the context of OCT4 modulation.
This approach allowed us to systematically investigate the impact of OCT4 modulation influences the sensitivity of PC cells to standard chemotherapy, offering insights into potential therapeutic strategies.
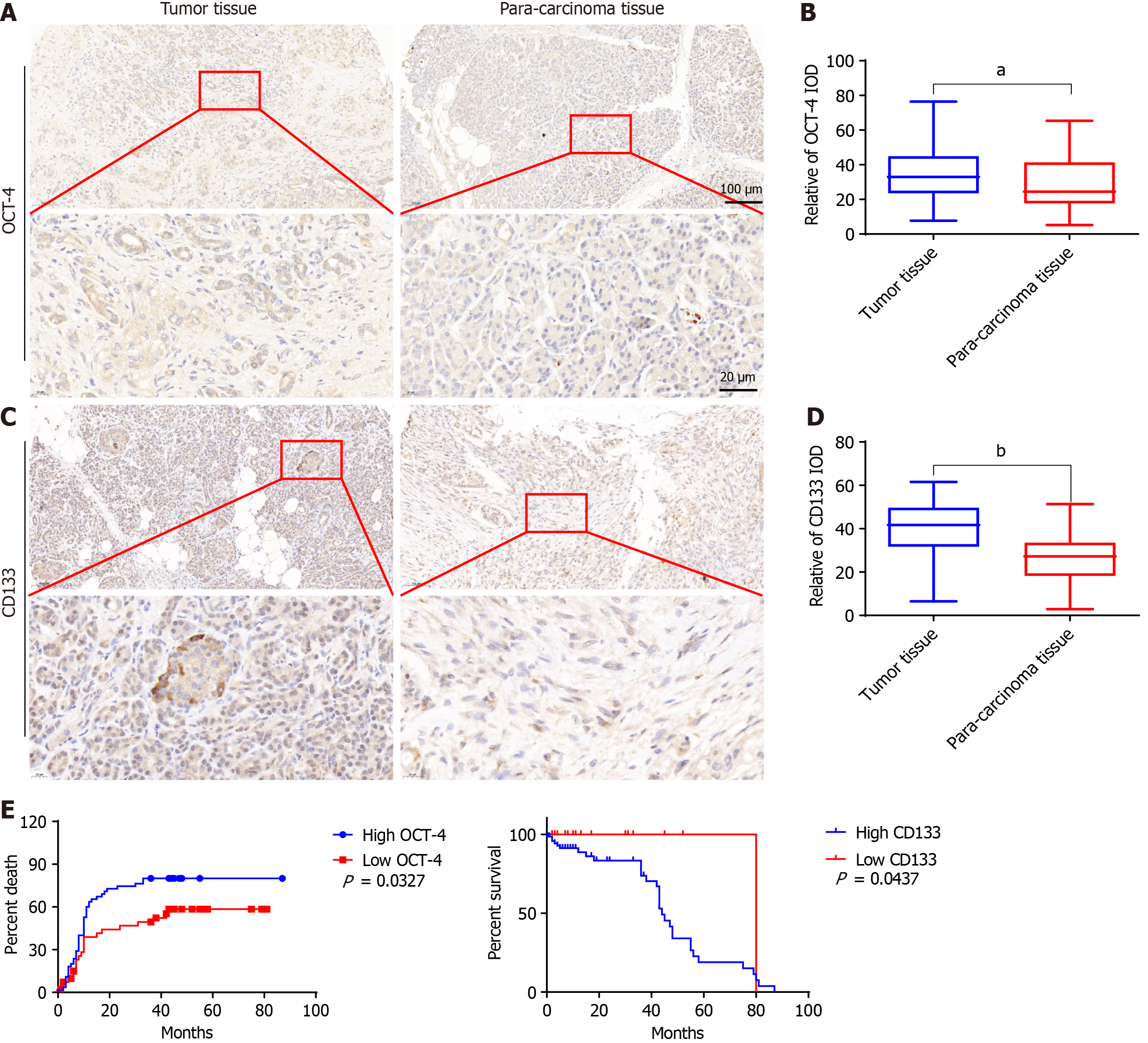
The expression of OCT4 in PC was significantly increased (P < 0.01). The expression of CD133 in PC was also significantly increased (P < 0.0001) (Figure 1). The influence of high and low OCT4 and CD133 expression on the survival rate of PC tissues is shown in Figure 1.
Among the 100 tissue samples, sex age, pathological grade, TNM, and clinical stage were distributed as follows: 37 samples were from women and 63 from men; 60 patients were < 65 years old and 60 were ≥ 65 years old; 69 patients had a pathological grade I-II and 31 patients had pathological grade III; and 79 patients had a TNM stage I-II and 21 patients had grade III. Clinical, physiological, and pathological characteristics 44 patients with stage 1A-1B, and 56 patients with stage 2A-2B were compared. Results showed that the changes in the expression of OCT4 was statistically significant (P < 0.05) (Table 3).
| Clinicopathological characteristic | n | CD133 high | CD133 low | P value | OCT4 high | OCT4 low | P value |
| Gender | |||||||
| Female | 37 | 26 | 11 | 0.0336 | 35 | 28 | 0.0244 |
| Male | 63 | 46 | 17 | 23 | 14 | ||
| Age | |||||||
| < 65 | 60 | 45 | 15 | 0.0206 | 36 | 24 | 0.0098 |
| ≥ 65 | 40 | 29 | 11 | 22 | 18 | ||
| Pathologic grading | |||||||
| I-II | 69 | 48 | 21 | 0.1138 | 38 | 31 | 0.4874 |
| II-IV | 31 | 26 | 5 | 20 | 11 | ||
| TNM | |||||||
| I-II | 79 | 61 | 18 | 0.0059 | 46 | 33 | 0.0115 |
| III | 21 | 13 | 8 | 12 | 9 | ||
| Pathologic staging | |||||||
| 1A-1B | 44 | 36 | 8 | 0.0435 | 25 | 19 | 0.3277 |
| 2A-2B | 56 | 39 | 17 | 33 | 23 | ||
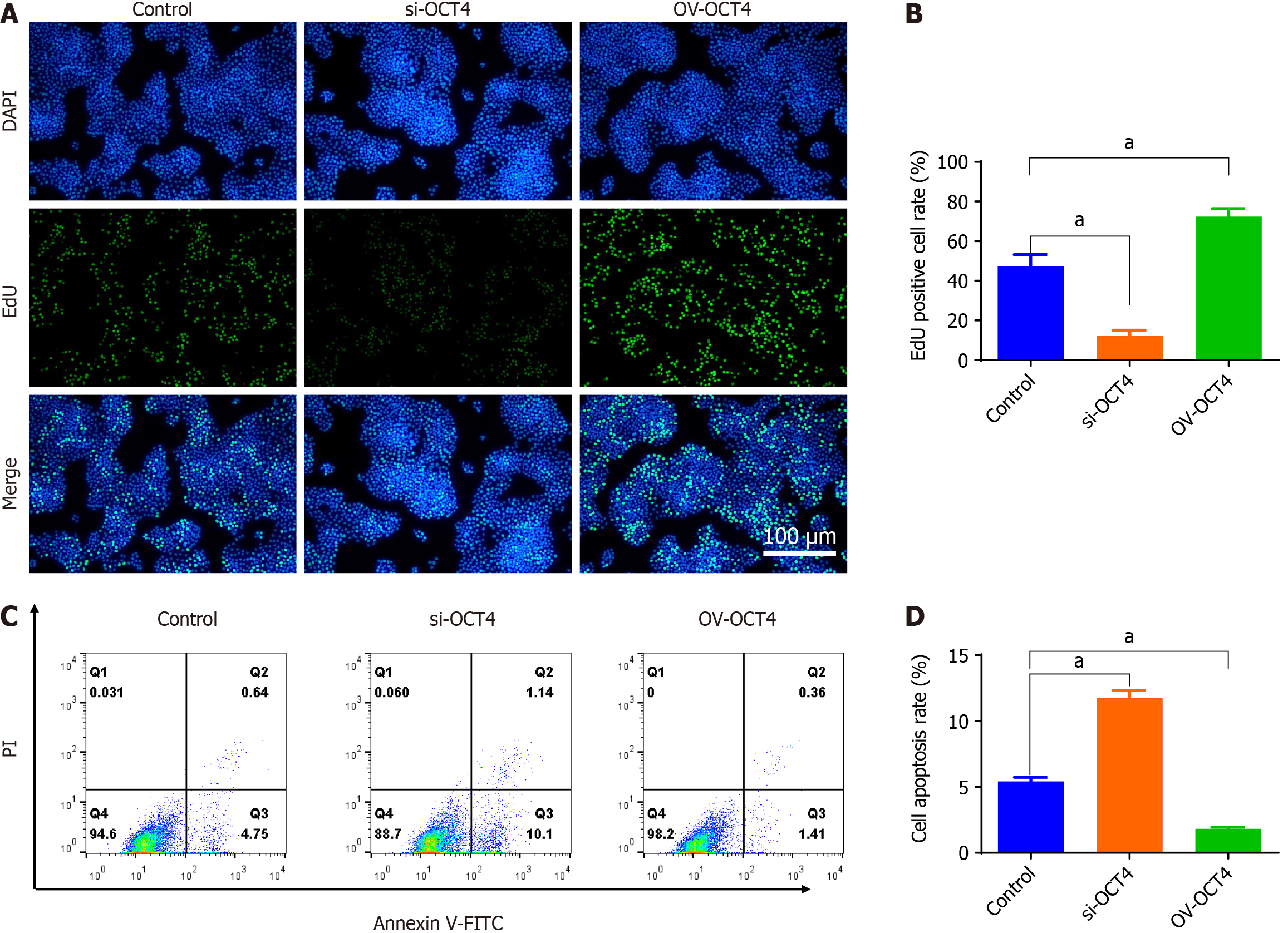
The siRNAs were synthesized and screened, siRNA fragments were transfected, and an si-OCT4 was constructed. The OV-OCT4 was transfected into BxPC3 cells to construct OV-OCT4 (Supplementary Figure 1). EdU incorporation assays revealed a significant decrease in cell proliferation in the si-OCT4 group compared to that in the controls, indicating the inhibitory effect of OCT4 knockdown. Conversely, the OV-OCT4 group showed a notable increase in EdU-positive cells, suggesting that OCT4 overexpression promotes cell proliferation (Figure 2A and B).
As shown in Figure 2C and D, using the normal expression of OCT4 group as the control, the apoptosis rate of si-OCT4 group (26.86 ± 0.65) was significantly higher than that of the control group (12.26 ± 0.48), and that of OV-OCT4 group (5.37 ± 0.53) was significantly lower than that of the control group (P < 0.05) (Figure 2).
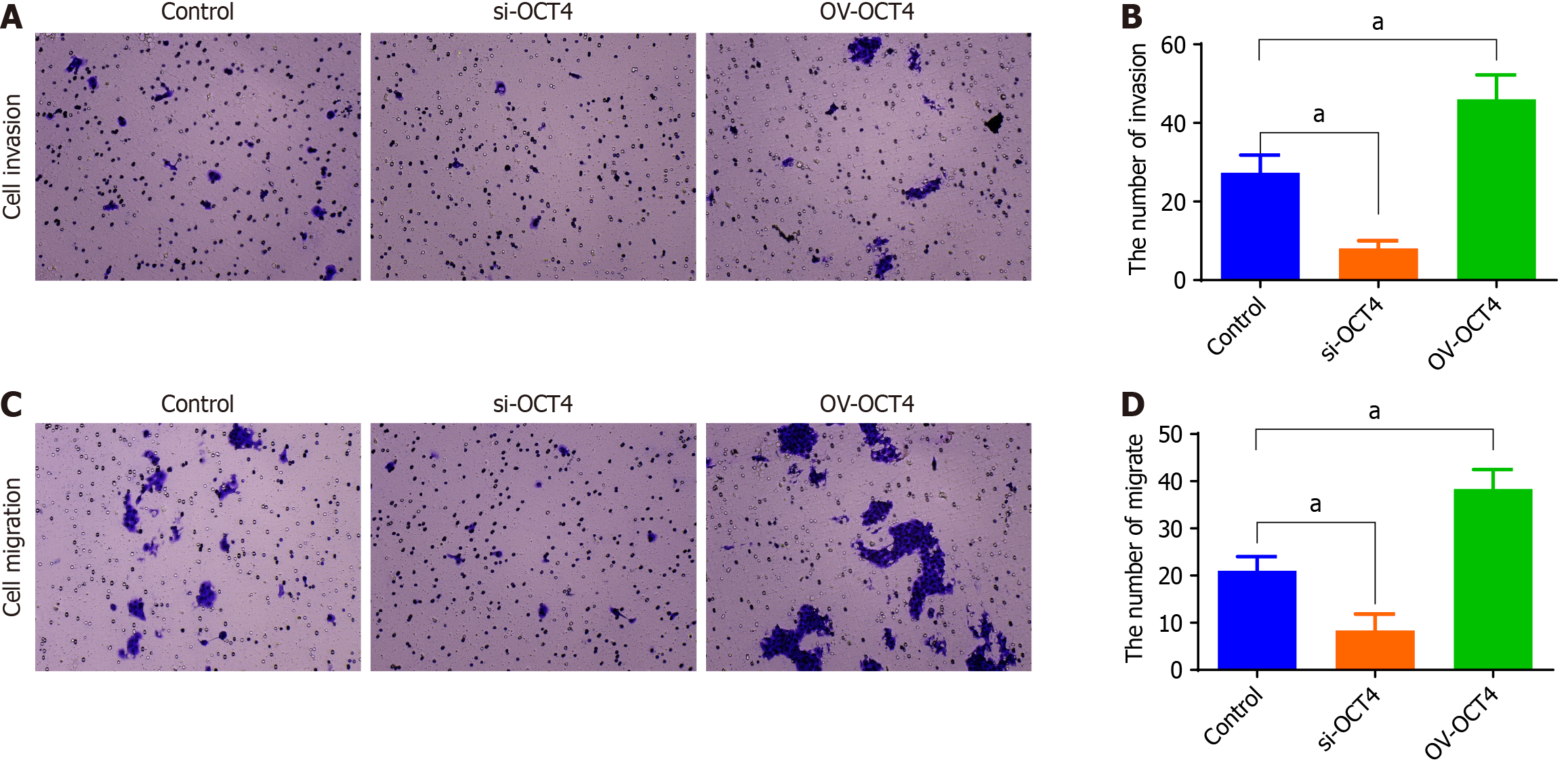
The cell migration and invasion experiment showed that compared with those of the control OCT4 group, the invasion and migration ability of si-OCT4 group was significantly lower, whereas the invasion and migration ability of OV-OCT4 group was significantly higher (P value) (Figure 3).
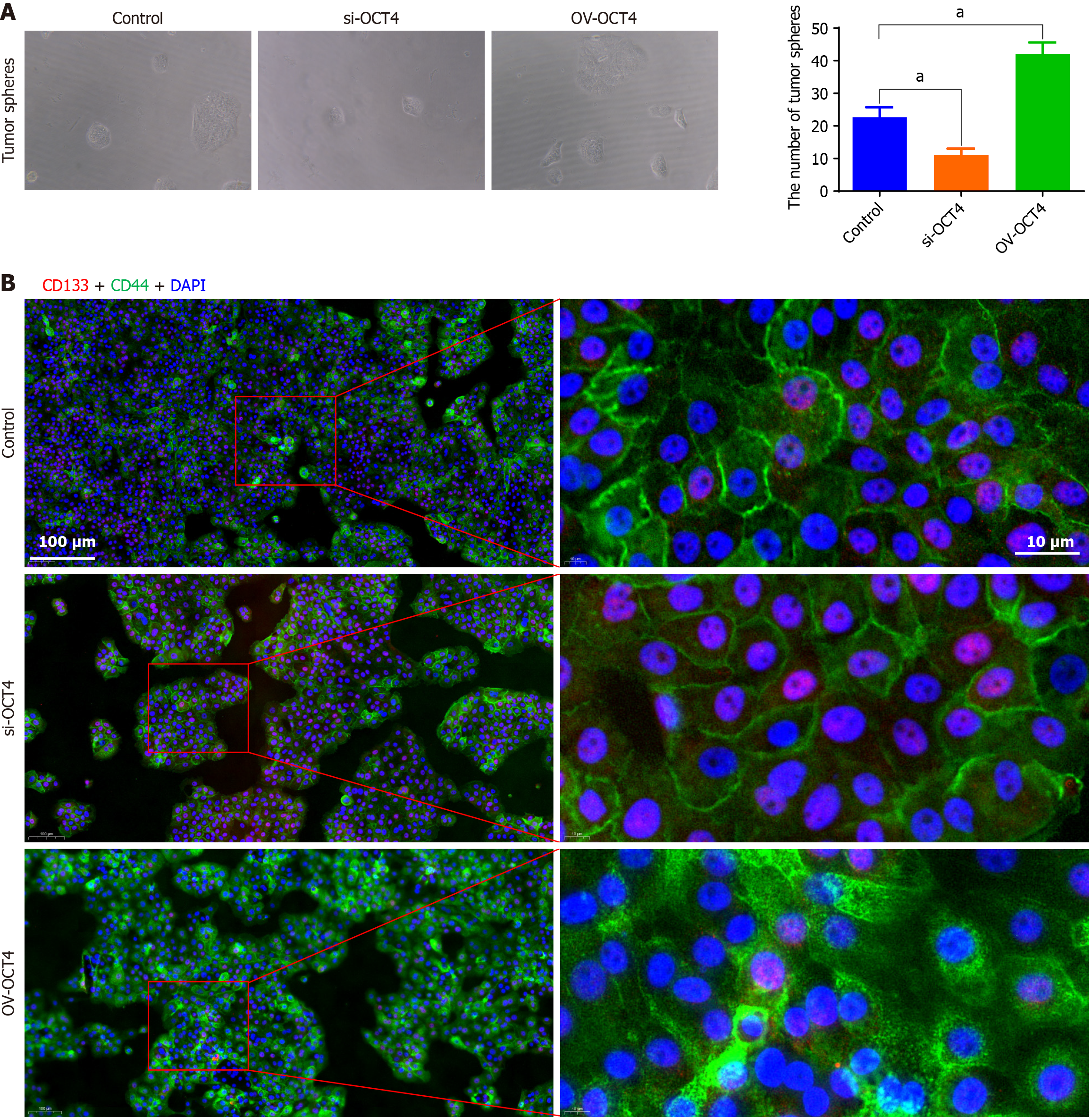
FITC-CD133 and PE-CD44 markers were used to detect the expression of CSCs surface antigens using flow cytometry. Compared with that of the control group, the number of stem cells in the si-OCT4 group was significantly less, whereas the number of stem cells in the OV-OCT4 group was significantly higher (Figure 4).
Using gemcitabine concentration of 0.5 mmol/L, 1.5 mmol/L, 5 mmol/L, 10 mmol/L, 100 mmol/L, and 800 mmol/L (Figure 5A), the proliferative capacity of BxPC3 cells decreased with increasing drug concentrations (Figure 5B).
Cell groups: Control group, OV-OCT4, si-OCT4, gemcitabine group (1/2 IC50), OV-OCT4 + gemcitabine group (1/2 IC50), si-OCT4) + gemcitabine group (1/2 IC50). The proliferative capacity of BxPC-3 cells in the OV-OCT4 group was significantly higher than that of the cells in the si-OCT4 group. After drug intervention, the proliferative ability of the OV-OCT4 group remained significantly higher than that of the si-OCT4 group (P < 0.05) (Figure 5C).
Taking the cell group with normal expression of OCT4 as the control, the phosphorylated p-PI3K/p-AKT/p-mTOR pathway protein expression in the si-OCT4 group was found to be significantly lower, whereas those in the OV-OCT4 group was significantly higher, as assessed using western blot analysis (Figure 6). However, no significant changes were detected in the levels of PI3K/AKT/mTOR pathway phosphorylated proteins upon OCT4 knockdown or overexpression.
OCT4 is a major regulator of cellular pluripotency and plays an important role in embryonic development. It is also known as POU5F1; OCT3, OTF3, and OTF4, which are transcription factors of the POU family that bind to DNA-containing octamer motifs (ATGCAAAT) and transcribe and activate the expression of their downstream target genes[12,13]. OCT4 is the core substance that maintains the characteristics of stem cells and can promote the proliferation of CSCs, reduce the sensitivity of cancer cells to chemotherapy drugs, and promote the malignant progression of cancer cells[14].
OCT4 is an ESC, which is capable of totipotency and an important regulatory factor in pre-implantation embryonic development[15,16]. It can promote the formation of intracastocyst cell mass, maintain the undifferentiated state of ESCs, and promote their proliferation[17-20]. Therefore, it is an important transcription factors involved in regulating the self-renewal of ESCs and maintaining their totipotency. It is also a key gene for establishing iPSCs in vitro[21-23]. Inactivation of OCT4 in ESC leads to the loss of totipotency of these cells, such that the embryo cannot develop normally. OCT4 is essential for establishing the stemness of ESCs. PCSCs and ESC have similar stem cell potential, and OCT4 maintains the characteristics of stem cells. Therefore, OCT4 plays an important role in the development of human embryos and maintains the stemness of CSCs. Reportedly, overexpression of OCT4 mediates drug sensitivity in various tumors; it can significantly promote resistance of melanoma cells to cisplatin and hypoxia[24,25]. Overexpression of OCT4 can also activate the expression of TCL1, AKT, and ABCG2, and upregulate drug resistance in liver cancer cells[26]. When the PI3K/AKT pathway is activated, the expression of OCT4 and Nanog is upregulated and the stemness of breast CSCs is increased[26].
In this study, we tested 100 PC tissue samples and found that the incidence of PC was associated with multiple factors. We analyzed the correlation between sex and age and found that the incidence of cancer in men was higher than that in women, and that the incidence of cancer was higher in patients aged < 65 years than in those aged > 65 years. We compared the expression changes of OCT4 and CD133 in different pathological grades, TNM stages, and clinical stages and found that the number of people with high expression was higher than that of those with low expression. The expression levels of OCT4 and CD133 in PC and adjacent tissues were detected using immunohistochemistry. The expression level of OCT4 were 28.87 ± 1.797 and 34.56 ± 1.351, respectively, and CD133 were 39.77 ± 1.313 and 26.07 ± 1.210, respectively. The mortality rate in patients with high OCT4 expression was significantly higher than that in patients with low expression. The survival rate of the patients with high CD133 expression was significantly lower than that of the patients with low CD133 expression. The results showed that OCT4 and CD133 were closely related to PC and positively correlated with the survival time of PC. These findings also indicated that increasing the expression levels of OCT4 and CD133 can effectively promote the proliferation and metastasis of PC cells and accelerate mortality in patients. This study provides a theoretical basis for the identification of clinical therapeutic targets for PC.
Reportedly, the proliferation ability of malignant tumors decreases with the increase in gemcitabine concentration. However, there are few reports on the effect of OCT4 gene expression on the proliferation, metastasis, apoptosis, and drug sensitivity of PC cells and the regulation of the transduction pathway of PCSCs by OCT4. This study examined the variation in the proliferation, metastasis, invasion, apoptosis, and drug sensitivity of PC cells when the expression level of OCT4 was changed. We found that OCT4 overexpression improved the proliferation, metastasis, and drug sensitivity of PC cells, reduced apoptosis, and increased the number of PC cells.
The stemness of PC cells was detected using flow cytometry, and the levels of CD133 and CD44, which are markers of PC cells, were significantly higher in the OV-OCT4 group than in the control group. This further demonstrated the positive effect of OCT4 on PC stemness. Increase in the phosphorylated proteins of the PI3K/AKT/mTOR pathway proved that overexpression of OCT4 could activate the PI3K/AKT/mTOR pathway, further indicating that OCT4 regulates PCSCs through this pathway.
In conclusion, our study provides compelling evidence that OCT4 significantly influences the biology of PCSCs by driving their proliferation, metastasis, and drug resistance. High expression levels of OCT4 were associated with enhanced PCSC activity and increased drug resistance, suggesting that OCT4 is a potential therapeutic target for PC intervention. Further research is warranted to explore the clinical applications of OCT4 as a prognostic marker and therapeutic agent.
We thank Dr. Guo Shao for guidance with the submission process and assistance with data analysis.
| 1. | Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 2. | Torphy RJ, Fujiwara Y, Schulick RD. Pancreatic cancer treatment: better, but a long way to go. Surg Today. 2020;50:1117-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 3. | Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 4. | Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2',2'-difluorodeoxycytidine (gemcitabine). Drug Resist Updat. 2002;5:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Alvarellos ML, Lamba J, Sangkuhl K, Thorn CF, Wang L, Klein DJ, Altman RB, Klein TE. PharmGKB summary: gemcitabine pathway. Pharmacogenet Genomics. 2014;24:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1075] [Article Influence: 134.4] [Reference Citation Analysis (3)] |
| 7. | Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339-9344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2176] [Cited by in RCA: 2213] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 8. | Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 809] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 9. | Adorno-Cruz V, Kibria G, Liu X, Doherty M, Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M, Alvarado A, Caplan AI, Rich J, Gerson SL, Lathia J, Liu H. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Zhao HX, Li Y, Jin HF, Xie L, Liu C, Jiang F, Luo YN, Yin GW, Li Y, Wang J, Li LS, Yao YQ, Wang XH. Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG. Differentiation. 2010;80:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 430] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731-24737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 853] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 13. | Rizzino A, Wuebben EL. Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis. Biochim Biophys Acta. 2016;1859:780-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (11)] |
| 14. | Mohiuddin IS, Wei SJ, Kang MH. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 15. | Singh N, George A, Sharma R, Singla SK, Palta P, Manik R, Chauhan MS, Singh D. Characterization of POU5F1 (OCT4) gene and its promoter in buffalo ESC-like cells identifies multiple transcription start sites and expression of four pseudogenes. Gene. 2012;491:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ebrahimi M, Forouzesh M, Raoufi S, Ramazii M, Ghaedrahmati F, Farzaneh M. Differentiation of human induced pluripotent stem cells into erythroid cells. Stem Cell Res Ther. 2020;11:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10552] [Article Influence: 376.9] [Reference Citation Analysis (0)] |
| 18. | Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D, Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 19. | Dekel C, Morey R, Hanna J, Laurent LC, Ben-Yosef D, Amir H. Stabilization of hESCs in two distinct substates along the continuum of pluripotency. iScience. 2022;25:105469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Varzideh F, Gambardella J, Kansakar U, Jankauskas SS, Santulli G. Molecular Mechanisms Underlying Pluripotency and Self-Renewal of Embryonic Stem Cells. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Wang G, Farzaneh M. Mini Review; Differentiation of Human Pluripotent Stem Cells into Oocytes. Curr Stem Cell Res Ther. 2020;15:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Zhao N, Sheng M, Wang X, Li Y, Farzaneh M. Differentiation of Human Induced Pluripotent Stem Cells into Male Germ Cells. Curr Stem Cell Res Ther. 2021;16:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | van den Berg DLC, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 462] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 24. | Cordaro FG, De Presbiteris AL, Camerlingo R, Mozzillo N, Pirozzi G, Cavalcanti E, Manca A, Palmieri G, Cossu A, Ciliberto G, Ascierto PA, Travali S, Patriarca EJ, Caputo E. Phenotype characterization of human melanoma cells resistant to dabrafenib. Oncol Rep. 2017;38:2741-2751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Kaufhold S, Garbán H, Bonavida B. Yin Yang 1 is associated with cancer stem cell transcription factors (SOX2, OCT4, BMI1) and clinical implication. J Exp Clin Cancer Res. 2016;35:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Kuo KK, Lee KT, Chen KK, Yang YH, Lin YC, Tsai MH, Wuputra K, Lee YL, Ku CC, Miyoshi H, Nakamura Y, Saito S, Wu CC, Chai CY, Eckner R, Steve Lin CL, Wang SS, Wu DC, Lin CS, Yokoyama KK. Positive Feedback Loop of OCT4 and c-JUN Expedites Cancer Stemness in Liver Cancer. Stem Cells. 2016;34:2613-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |