Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.112029
Revised: August 23, 2025
Accepted: October 27, 2025
Published online: November 24, 2025
Processing time: 128 Days and 16.3 Hours
Human epidermal growth factor receptor 2 (HER2)-positive gastric cancer (GC) represents a distinct molecular cancer subtype that is often associated with a poor prognosis. While perioperative chemotherapy regimens are currently the primary recommendation for locally advanced HER2-positive GC, combination therapies incorporating immune checkpoint inhibitors are under active investigation.
The present case describes a patient with locally advanced HER2-positive GC who underwent perioperative treatment with chemotherapy combined with trastu
Large-scale phase III clinical trials are needed to verify the efficacy of combined neoadjuvant treatment application for GC.
Core Tip: The efficacy and safety of immune checkpoint inhibitors (ICIs) combination therapy in the perioperative period of human epidermal growth factor receptor 2 (HER2)-positive gastric cancer (GC) have not been confirmed by large-scale clinical studies. This study introduces a case of neoadjuvant treatment for HER2-positive GC using standard chemotherapy combined with anti-HER2 therapy, and systematically summarizes the current related research on chemotherapy combined with targeted therapy and ICI-based combined therapy. The efficacy of ICI combined therapy during the perioperative period still requires further confirmation through a phase III clinical trial.
- Citation: Ma XT, Yao GY, Li JL, Wang XC, Ba Y. Perioperative immunotherapy combined with standard therapy for human epidermal growth factor receptor 2-positive locally advanced gastric cancer: A case report. World J Clin Oncol 2025; 16(11): 112029
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/112029.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.112029
Human epidermal growth factor receptor 2 (HER2) is a member of the epidermal growth factor receptor (EGFR) family and is encoded on human chromosome 17q21. It is a single-chain transmembrane glycoprotein with a relative molecular mass of 185 kDa and tyrosine protein kinase activity, classifying it as a type I transmembrane tyrosine kinase. Notably, HER2 is unique among EGFR family members as it lacks a high-affinity specific ligand. Consequently, HER2 requires homodimerization or heterodimerization with other family members and their ligands to activate downstream signaling pathways[1]. HER2 heterodimerization represents the most critical signal transduction pathway among these dimer formations[2]. HER2 is predominantly expressed during embryonic development under normal physiological conditions, with limited expression in only a few adult tissues. However, HER2 gene amplification or HER2 protein overexpression has been identified in various malignancies, including breast, ovarian, lung, gastric, colorectal, and prostate cancers.
The HER2 positivity rate in gastric cancer (GC) ranges from approximately 12% to 20%[3]. Patients with HER2-positive GC typically exhibit poor prognosis, with the highest positivity rates observed in intestinal-type and gastric/gastroesophageal junction (G/GEJ) adenocarcinoma, while diffuse-type and distal tumors demonstrate lower positivity rates[4]. Trastuzumab is a monoclonal antibody targeting the extracellular domain of the HER2 receptor. It binds to the IV region of the HER2 cell membrane, thereby inhibiting downstream PI3K/AKT and RAS/MEK signaling pathways and exerting anti-tumor effects[5,6]. Although the ToGA trial in 2010 marked the beginning of precision targeted therapy for GC, progress in HER2-positive GC research remained relatively limited over the following decade. However, recent break
The combination of ICIs with traditional therapies has been conclusively demonstrated to enhance overall treatment efficacy and prolong survival in individuals with HER2-positive advanced GC, establishing this approach as the standard of care for this patient population[7,8]. However, the perioperative treatment for advanced HER2-positive GC based on conventional chemotherapy regimens has yet to achieve significant advances. The present report describes a patient with HER2-positive locally advanced GC who experienced some benefits following neoadjuvant therapy with chemotherapy combined with trastuzumab.
Anemia lasting for over one year and fatigue persisting for eight months.
A 60-year-old male patient was diagnosed with anemia at local hospital in October 2023, with hemoglobin levels fluctuating around 100 g/L. The patient experienced intermittent constipation and melena despite long-term oral iron supplementation. In July 2024, the patient developed generalized fatigue and dizziness, particularly with postural changes, accompanied by decreased physical strength and a weight loss of approximately 3 kg over the course of one month, for which he did not seek medical attention. In October 2024, he visited local hospital for tumor marker eva
The patient has been diagnosed with type 2 diabetes mellitus for three years. He adhered to metformin therapy, achieving optimal glycemic control.
The patient’s mother, uncle, and cousin suffered from pancreatic cancer, intestinal cancer, and liver cancer, respectively. No other special personal histories or family histories of diseases were noted.
The patient's mental state, appetite, and sleep have been normal since the onset of the disease. Urination was also normal. The physical examination indicated mild pallor of the conjunctiva. The breathing sounds in both lungs were clear, the heart rhythm was regular, there were no abnormal heart murmurs, no positive signs were found in the abdomen, and there was no edema in both lower extremities.
The blood routine test indicated mild anemia, the stool routine test was positive for occult blood, and no significant abnormalities were found in the other laboratory tests.
An enhanced abdominal and pelvic computed tomography scan performed on October 21, 2024 at our hospital confirmed gastric wall thickening in the lesser curvature, consistent with clinical stage cT4aN2M0, with a small amount of pelvic fluid observed.
Gastric adenocarcinoma (cT4aN2M0, stage III).
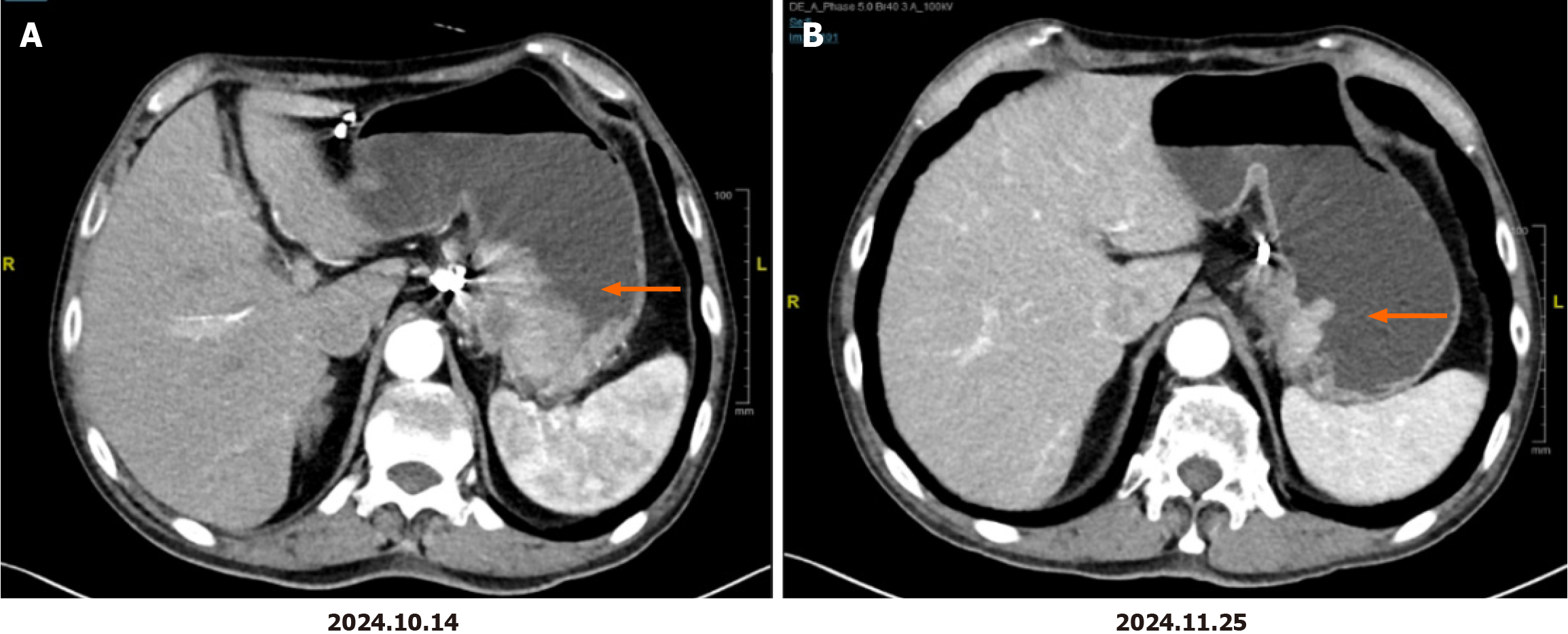
The patient received four cycles of oxaliplatin, S-1 (SOX) combined with trastuzumab treatment in our department starting on October 31, 2024. The specific SOX regimen was as follows: Oxaliplatin at a dose of 130 mg/m2 (2-hour intravenous infusion) on day 1 and S-1 taken orally at a dose of 120 mg/day for body surface areas of > 1.5 m2 on days 1-14 every three weeks. The initial trastuzumab dose was 8 mg/kg, and the subsequent dose was 6 mg/kg on day 1 and then every three weeks. The patient's medication dosage has not yet been reduced. The adverse events were grade 1 neurotoxicity and grade 1 nausea. No drug-related myelosuppression or cardiotoxicity occurred. Follow-up imaging after two cycles of treatment revealed a significant improvement in gastric wall thickening at the lesser curvature and a reduction of adjacent lymph node size, achieving a partial response (PR) that was maintained after four cycles (Figure 1). The patient reported marked improvement in fatigue symptoms. On February 6, 2025, the patient underwent robot-assisted radical distal gastrectomy at our hospital. Postoperative pathology results confirmed moderately differentiated adenocarcinoma (Lauren classification: Intestinal type) infiltrating the muscular layer and subserosa, with extensive foam cell aggregation, fibrous tissue hyperplasia, and inflammatory cell infiltration. The tumor occupied 20% of the tumor bed area, which was consistent with partial treatment response (College of American Pathologists grade 2), without serosal invasion, with negative surgical margins, no evidence of neural or vascular invasion, and lymph node metastasis (1/46). Final pathological staging was pT3N1, stage II.
The patient recovered well postoperatively and planned to return to our hospital for further adjuvant treatment.
HER2-positive GC represents a distinct molecular cancer subtype characterized by specific genetic alterations. As a proto-oncogene, HER2 plays a crucial role in promoting cell proliferation and inhibiting apoptosis. HER2 positivity in GC is associated with enhanced cancer cell proliferation, reflecting greater tumor aggressiveness and poorer prognosis. A comprehensive systematic analysis encompassing 42 studies demonstrated that 71% of these studies confirmed the association between HER2 gene amplification or HER2 protein overexpression and adverse clinical outcomes in GC patients[9]. The three-year survival rate for HER2-positive GC patients remains below 10%.
The use of ICIs for immunotherapy has emerged as a rapidly advancing therapeutic domain in recent years. Supported by the success of multiple phase III clinical trials, the combination of ICIs with chemotherapy has been incorporated into international guidelines as the first-line treatment for advanced GC. The KEYNOTE-811 study exemplifies this advan
Mechanistically, HER2-positive GC exhibits distinct tumor immune microenvironment characteristics, including increased levels of tumor-associated antigens, elevated numbers of tumor-infiltrating lymphocytes, higher tumor mutational burden, and enhanced PD-L1 expression. These features render HER2-positive GC more susceptible to ICI therapy. Trastuzumab exerts its anti-tumor effects via dual mechanisms of intracellular and extracellular pathways. In
Current guidelines primarily recommend perioperative chemotherapy regimens for locally advanced HER2-positive GC (Table 1)[15-18]. The present study performed a systematic search of the PubMed/MEDLINE, EMBASE, Cochrane Library, and Web of Science databases between 2014 and 2024 to capture contemporary evidence. Case reports, retros
| Clinical trial number | Regimen name | Experi-mental stage | Enrolled participants number | Treatment | Conclusions |
| NCT01472029 | HER-FLOT | II | 56 | DOF + trastuzumab | pCR: 21.4%; median DFS: 42.5 months; 2-year OS rate: 89.3%. Trastuzumab shows promising potential in the neoadjuvant treatment of gastric cancer |
| NCT01130337 | NEOHX | II | 36 | XELOX + trastuzumab | pCR rate: 9.6%; 18-month DFS rate: 71%; 5-year OS rate: 58%. The efficacy of XELOX combined with trastuzumab in the neoadjuvant treatment of gastric cancer has been preliminarily established |
| NCT02581462 | PETRARCA | II/III | 40 vs 41 | FLOT + trastuzumab + pertuzumab vs FLOT | pCR: 35% vs 12%, R0 resection rate: 93% vs 90%. Further evidence has confirmed the efficacy and safety of neoadjuvant targeted therapy |
| NCT02205047 | INNOVATION (EORTC-1203-GITCG) | II | 70 vs 67 vs 35 | Trastuzumab + | R0 resection rate: 85.9% vs 90.3% vs 83.9%; MPR rate: 26.4% vs 37.0% vs 23.3%; trastuzumab combined with chemotherapy in the perioperative setting demonstrates favorable pCR rates |
| NCT05034887 | EPOC2003 | II | 27 | Trastuzumab Deruxtecan | MPR rate: 14.8%; pCR rate: 3.7%. The short-term efficacy of T-DXd monotherapy in locally advanced HER2-positive G/GEJ adenocarcinoma has been unsatisfactory |
| II | 27 | Disitamab vedotin + toripalimab + tegafur (S-1) | Among them, 20 patients underwent surgery. R0 resection rate: 100%; MPR rate: 50%; pCR rate: 30%; median DFS or OS not reached. Provides a safe and effective neoadjuvant treatment option for patients with locally advanced HER2-positive G/GEJ adenocarcinoma | ||
| NCT06385873 | - | II | Estimated 32 | Disitamab vedotin + toripalimab + apatinib + tegafur (S-1) | Ongoing |
| NCT0466115 | - | II | 21 vs 21 | Atezolizumab + trastuzumab + XELOX vs trastuzumab + XELOX | pCR rate: 38.1% vs 14.3%; the addition of atezolizumab to the trastuzumab combined with XELOX regimen is effective in treating patients with HER2-positive, locally advanced, resectable G/GEJ adenocarcinoma |
| NCT04819971 | II | 25 | Tislelizumab + trastuzumab + DOS | Four patients achieved cCR, and 18 patients underwent surgery with an R0 resection rate of 100%; pCR + cCR rate: 54.5%; MPR rate: 72.2%; T-stage reduction rate: 88.9%; indicates the potent tumor-reducing and downstaging effects of preoperative neoadjuvant therapy |
However, large-scale studies investigating ICI application in the perioperative treatment of HER2-positive GC remain limited. Preliminary exploratory studies have provided initial insights. A retrospective analysis of eight patients with HER2-positive locally advanced GC treated with neoadjuvant therapy combining programmed cell death 1 (PD-1) and HER2 inhibitors with or without chemotherapy, demonstrated a PR rate of 85.7% (6/7 evaluable patients) and a disease control rate of 100%. In a phase II prospective study conducted by Peng et al[21]. 42 Asian patients were randomized to receive either atezolizumab combined with trastuzumab and XELOX or trastuzumab combined with XELOX alone. The pCR rates were 38.1% and 14.3%, respectively, with the significantly higher pCR rate in the combination therapy group providing preliminary evidence for the efficacy of PD-L1 inhibitor-based regimens. Subgroup analysis revealed that age of < 65 years, male gender, and intestinal-type histology were associated with higher pCR rates in the atezolizumab combination group. Immunohistochemical analysis of tumor specimens showed significantly higher expression levels of ERBB2, PANCK, and IRF8 in patients achieving pCR compared to those in non-responders (P < 0.05). Conversely, CD163 expression was markedly reduced in the pCR cohort. These findings suggest enhanced anti-tumor immunity and attenuated immunosuppressive activity within the tumor microenvironment of pCR patients. In addition, the number of CD56+ cells around HER2- and PANCK-positive cells in the tumor tissues of patients who achieved pCR was lower, suggesting that the natural killer cell activity in the bodies of such patients is higher, which may be one of the reasons for the better treatment effect observed in pCR patients. These results also indicate that atezolizumab may improve the treatment effect by enhancing the anti-tumor immune response and improving the tumor microenvironment. Further evidence was presented at the 2024 ESMO Congress, where a single-arm study enrolled 25 patients with resectable, histologically confirmed HER2-positive G/GEJ adenocarcinoma (cT2-4 Nx M0 or cTx N+ M0). Patients received one cycle of tislelizumab combined with trastuzumab as induction therapy, followed by three cycles of tislelizumab combined with trastuzumab and DOS (docetaxel, oxaliplatin, and S-1) regimen. Among the 18 patients who underwent surgery, four achieved clinical complete response (cCR) after neoadjuvant therapy and declined surgery. The combined pCR and cCR rate was 54.5%, with 72.2% of patients achieving a major pathological response. Tumor downstaging was observed in 88.9% of cases (16/18), underscoring the potent role of preoperative neoadjuvant therapy in reducing tumor burden[22]. Additionally, case reports have highlighted the potential of PD-1 inhibitors combined with trastuzumab and chemotherapy for conversional treatment. Ibrahim et al[23] reported four cases of HER2-positive advanced GC with retroperitoneal lymph node metastases at initial diagnosis, which were clinically identified as stage IV. Three patients received three cycles of SOX combined with trastuzumab and sintilimab, while the fourth received five cycles of FLOT combined with trastuzumab and sintilimab. All four patients achieved R0 resection, with two attaining pCR and the other two downstaged to ypT1N0M0 and ypT2N0M0. Postoperative pathology results confirmed the absence of neural or vascular invasion. These cases demonstrate that pCR may be achievable through chemotherapy combined with ICIs and trastuzumab even in unresectable advanced GC.
The tumor in the present case showed significant shrinkage after the patient received perioperative treatment combining chemotherapy and trastuzumab, but failed to achieve pCR, highlighting the limitations of the current treatment regimen. This might be due to the heterogeneity of HER2 expression within the tumor, which affects drug efficacy. Additionally, there are regulatory T cells, myeloid-derived suppressor cells, and other immunosuppressive cells in the tumor microenvironment. The tumor cells inhibit T cell activity by highly expressing immune checkpoint molecules, such as PD-L1, which may shape the inhibitory immune microenvironment factors. The ICI combination therapy may potentially improve the response rate. Current research on the combination of PD-1 inhibitors with standard perioperative treatment regimens remains exploratory and cannot yet serve as an evidence-based foundation. However, this treatment can be further considered as an effective neoadjuvant treatment option in the future based on the downstaging potential of ICI combination therapy.
The efficacy and safety of ICI combination therapy in the perioperative period of HER2-positive GC have not been confirmed by large-scale clinical studies. The present report introduces a case of neoadjuvant treatment for HER2-positive GC using standard chemotherapy combined with anti-HER2 therapy and systematically summarizes the current related research on chemotherapy combined with targeted therapy and ICI-based combined therapy. The efficacy of combined ICI therapy during the perioperative period requires a further confirmation through a phase III clinical trial.
| 1. | Milanezi F, Carvalho S, Schmitt FC. EGFR/HER2 in breast cancer: a biological approach for molecular diagnosis and therapy. Expert Rev Mol Diagn. 2008;8:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12 Suppl 1:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 456] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 4. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5525] [Article Influence: 345.3] [Reference Citation Analysis (3)] |
| 5. | Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol. 2011;8:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Chung HC, Bang YJ, S Fuchs C, Qin SK, Satoh T, Shitara K, Tabernero J, Van Cutsem E, Alsina M, Cao ZA, Lu J, Bhagia P, Shih CS, Janjigian YY. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol. 2021;17:491-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 8. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 2221] [Article Influence: 444.2] [Reference Citation Analysis (1)] |
| 9. | Jørgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer. 2012;3:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Gu CL, Zhu HX, Deng L, Meng XQ, Li K, Xu W, Zhao L, Liu YQ, Zhu ZP, Huang HM. Bispecific antibody simultaneously targeting PD1 and HER2 inhibits tumor growth via direct tumor cell killing in combination with PD1/PDL1 blockade and HER2 inhibition. Acta Pharmacol Sin. 2022;43:672-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Xia L, Wen L, Qin Y, Dobson HE, Zhang T, Comer FI, Hinrichs MJ, Oberst MD, Coats SR, Chang AE, Liu Y, Bao Y, Dai F, Wicha MS, Li Q. HER2-targeted antibody-drug conjugate induces host immunity against cancer stem cells. Cell Chem Biol. 2021;28:610-624.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Wu X, Xu L, Li X, Zhou Y, Han X, Zhang W, Wang W, Guo W, Liu W, Xu Q, Gu Y. A HER2-targeting antibody-MMAE conjugate RC48 sensitizes immunotherapy in HER2-positive colon cancer by triggering the cGAS-STING pathway. Cell Death Dis. 2023;14:550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 13. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10705] [Article Influence: 764.6] [Reference Citation Analysis (34)] |
| 14. | Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (1)] |
| 15. | Hofheinz R, Hegewisch-Becker S, Thuss-Patience PC, Kunzmann V, Fuchs M, Graeven U, Homann N, Heinemann V, Pohl M, Tannapfel A, Al-Batran S. HER-FLOT: Trastuzumab in combination with FLOT as perioperative treatment for patients with HER2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the AIO Gastric Cancer Study Group. J Clin Oncol. 2014;32:4073-4073. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Rivera F, Izquierdo-Manuel M, García-Alfonso P, Martínez de Castro E, Gallego J, Limón ML, Alsina M, López L, Galán M, Falcó E, Manzano JL, González E, Muñoz-Unceta N, López C, Aranda E, Fernández E, Jorge M, Jiménez-Fonseca P. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Hofheinz RD, Merx K, Haag GM, Springfeld C, Ettrich T, Borchert K, Kretzschmar A, Teschendorf C, Siegler G, Ebert MP, Goekkurt E, Mahlberg R, Homann N, Pink D, Bechstein W, Reichardt P, Flach H, Gaiser T, Battmann A, Oduncu FS, Loose M, Sookthai D, Pauligk C, Göetze TO, Al-Batran SE. FLOT Versus FLOT/Trastuzumab/Pertuzumab Perioperative Therapy of Human Epidermal Growth Factor Receptor 2-Positive Resectable Esophagogastric Adenocarcinoma: A Randomized Phase II Trial of the AIO EGA Study Group. J Clin Oncol. 2022;40:3750-3761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Wagner AD, Grabsch HI, Mauer M, Marreaud S, Caballero C, Thuss-Patience P, Mueller L, Elme A, Moehler MH, Martens U, Kang YK, Rha SY, Cats A, Tokunaga M, Lordick F. EORTC-1203-GITCG - the "INNOVATION"-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 19. | Takahari D, Kawazoe A, Machida N, Minashi K, Yamagata Y, Hara H, Omori T, Yamamoto M, Yasui H, Nakayama I, Yamada T, Kano M, Yoshikawa T, Wakabayashi M, Komura Y, Sato A, Kuwata T, Kojima M, Kinoshita T, Shitara K. Phase 2 study of trastuzumab deruxtecan as neoadjuvant treatment for HER2-positive gastric and gastroesophageal junction adenocarcinoma (EPOC2003). J Clin Oncol. 2024;42:309-309. [DOI] [Full Text] |
| 20. | Chai J, Wang L, Liu L, Liu B, Sun D. Efficacy and safety of disitamab vedotin (RC48) combined with camrelizumab and S-1 for neoadjuvant therapy of locally advanced gastric cancer with HER2 overexpression: Preliminary results of a prospective, single-arm, phase II study. J Clin Oncol. 2024;42:e16100-e16100. [DOI] [Full Text] |
| 21. | Peng Z, Zhang X, Liang H, Zheng Z, Wang Z, Liu H, Hu J, Sun Y, Zhang Y, Yan H, Tong L, Xu J, Xie J, Ji J, Shen L. Atezolizumab and trastuzumab plus chemotherapy in patients with HER2+ locally advanced resectable gastric cancer or adenocarcinoma of the gastroesophageal junction: A multicenter, randomized, open-label phase II study. J Clin Oncol. 2024;42:312-312. [DOI] [Full Text] |
| 22. | Wang F, Zhao C, Xia J, Liu Z, Meng X, Shan Z, Jiang J, Liu X, Li H, Sun J, Ding C, Zhang H, Liang W, Wang J, Ren C, Wang Z, Yang X, Li S. 1466P Efficacy and safety of perioperative chemotherapy combined with tislelizumab and trastuzumab for HER2-positive resectable gastric/gastroesophageal junction cancer (GC/EGJC): Data update from a phase II single-arm study. Ann Oncol. 2024;35:S908. [DOI] [Full Text] |
| 23. | Ibrahim S, Maimaitiaili A, Zhu G, Ye S. Efficacy of sintilimab combined with neoadjuvant chemotherapy and trastuzumab in conversional treatment of locally advanced HER2-positive gastric cancer: case analysis and literature review. J Cancer Res Clin Oncol. 2024;150:507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/