Published online Jul 24, 2024. doi: 10.5306/wjco.v15.i7.953
Revised: May 8, 2024
Accepted: June 7, 2024
Published online: July 24, 2024
Processing time: 117 Days and 14.7 Hours
Primary malignant melanoma of the cervix (PMMC) is an extremely rare disease that originates from primary cervical malignant melanoma and frequently re
Here, we report a case of amelanotic PMMC, with a history of breast cancer and thyroid carcinoma. The patient was finally diagnosed by immunohistochemical staining and staged as IB2 based on the International Federation of Gynecology and Obstetrics with reference to National Comprehensive Cancer Network guide
The differential diagnosis process reenforced the notion that immunohistochemical staining is the most reliable approach for amelanotic PMMC diagnosis. Due to the lack of established therapeutic guidelines, empirical information from limited available studies does not provide the rationale for treatment-decision making. By integrating 'omics' technologies and patient-derived xenografts or mini-patient-derived xenograft models this will help to identify selective thera
Core Tip: We report a case of unsuspected amelanotic primary malignant melanoma of the cervix (PMMC) with a history of breast cancer. The patient underwent radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy, and then received radiotherapy combined with immunotherapy. She has remained free of disease for more than 1 year. The successful management of this patient underscores the critical role of routine immunohistochemical staining during cervical cancer diagnosis to exclude unsuspected PMMC, and adjuvant immunotherapy may be an option for PMMC.
- Citation: Duan JL, Yang J, Zhang YL, Huang WT. Amelanotic primary cervical malignant melanoma: A case report and review of literature. World J Clin Oncol 2024; 15(7): 953-960
- URL: https://www.wjgnet.com/2218-4333/full/v15/i7/953.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i7.953
Primary malignant melanoma of the cervix (PMMC) is extremely rare. Due to a lack of melanocytes in the cervix, PMMC represents a challenge in clinical diagnosis. Currently, there is no consensus or guidelines for the treatment and management of PMMC. In most cases, treatment follows the surgical criteria for cervical squamous cell carcinoma. PMMC can be managed postoperatively or preoperatively.
A 56-year-old woman presented to our hospital in December 2022 with one-day postmenopausal bleeding.
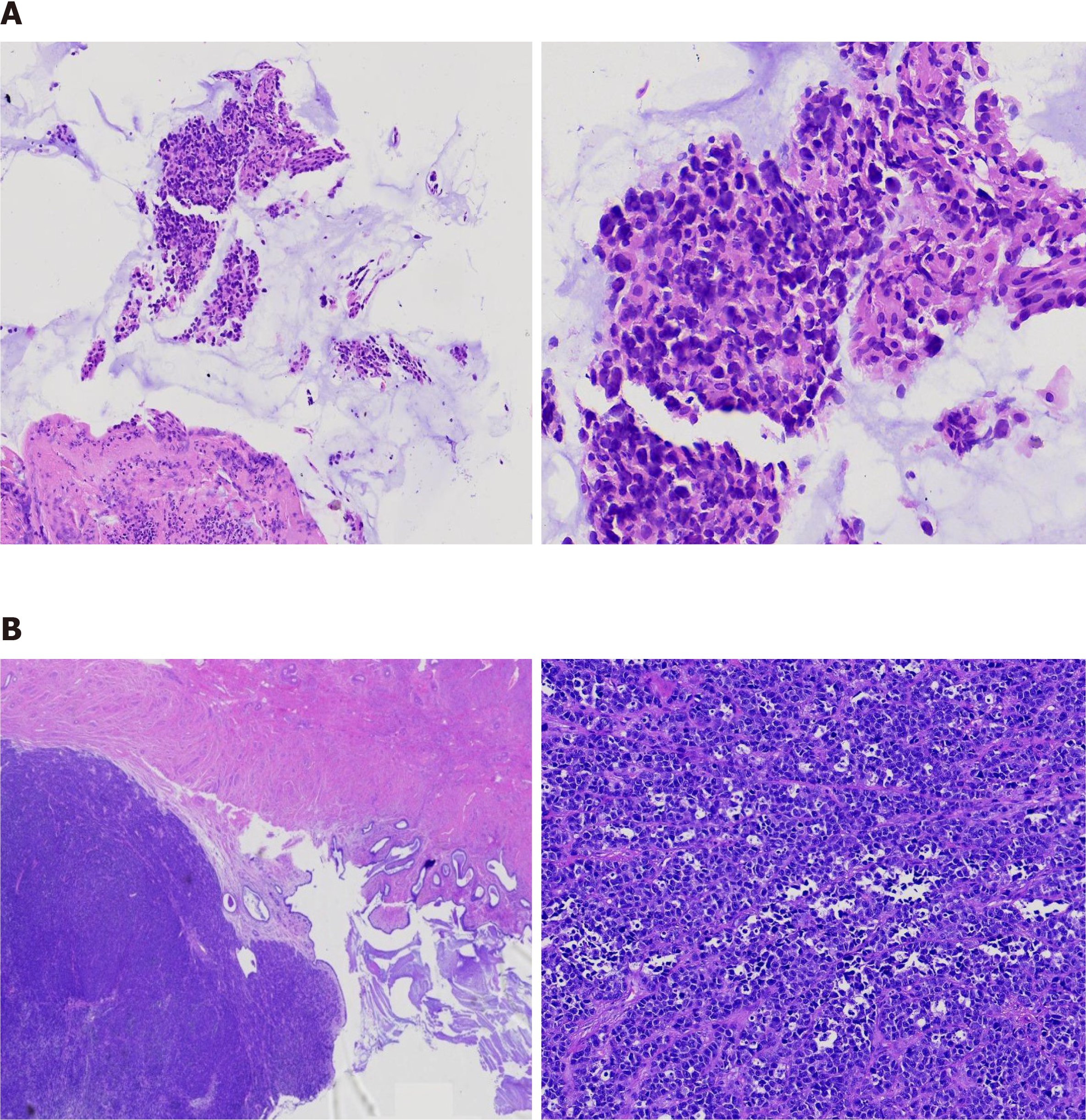
The surgery was planned by a multidisciplinary team and she underwent radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy, and one lesion (2.8 cm × 1.5 cm × 1.3 cm) was observed in the lower end of the cervix and its section appeared white in color. Biopsies were further evaluated by pathological examination. The tumor had invaded into 1/2 layer of the cervical muscle wall, and the depth of tumor invasion in the cervix was approximately 6 mm. The endometrium, bilateral adnexa, lymph nodes, and vaginal stump were free of tumors.
Eighteen years ago, she was diagnosed with breast duct carcinoma, and she underwent radical left unilateral mastectomy and then right unilateral mastectomy in 2014. She additionally underwent thyroidectomy two years later due to thyroid carcinoma.
Eighteen years ago, she was diagnosed with breast duct carcinoma, and underwent radical left unilateral mastectomy and then right unilateral mastectomy in 2014. She additionally underwent thyroidectomy two years later due to thyroid carcinoma.
Slight bulging of the anterior vaginal wall and posterior vaginal fornix was observed.
Human papillomavirus screening was negative. Quantitative DNA ploidy analysis identified at least 3 heterotypic cells and the DNA index value was over 2.5. Unexpectedly, a routine serum chemistry panel and plasma tumor biomarker examination, including squamous cell carcinoma antigen, were all within normal limits.
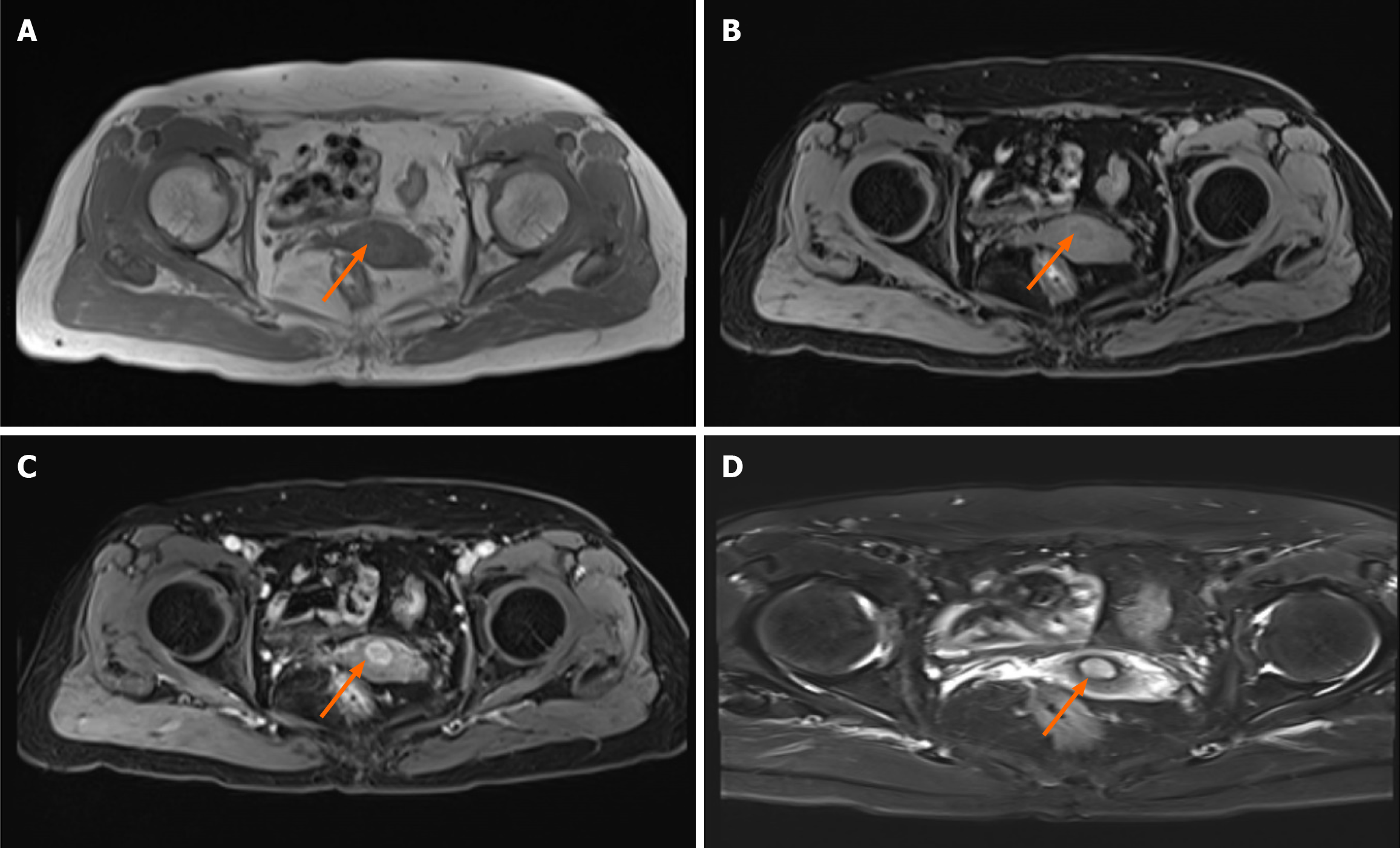
Ultrasound findings revealed a hypoechoic area measuring approximately 14 mm × 15 mm × 12 mm with clear boun
The surgery was planned by a multidisciplinary team and the patient underwent radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy.
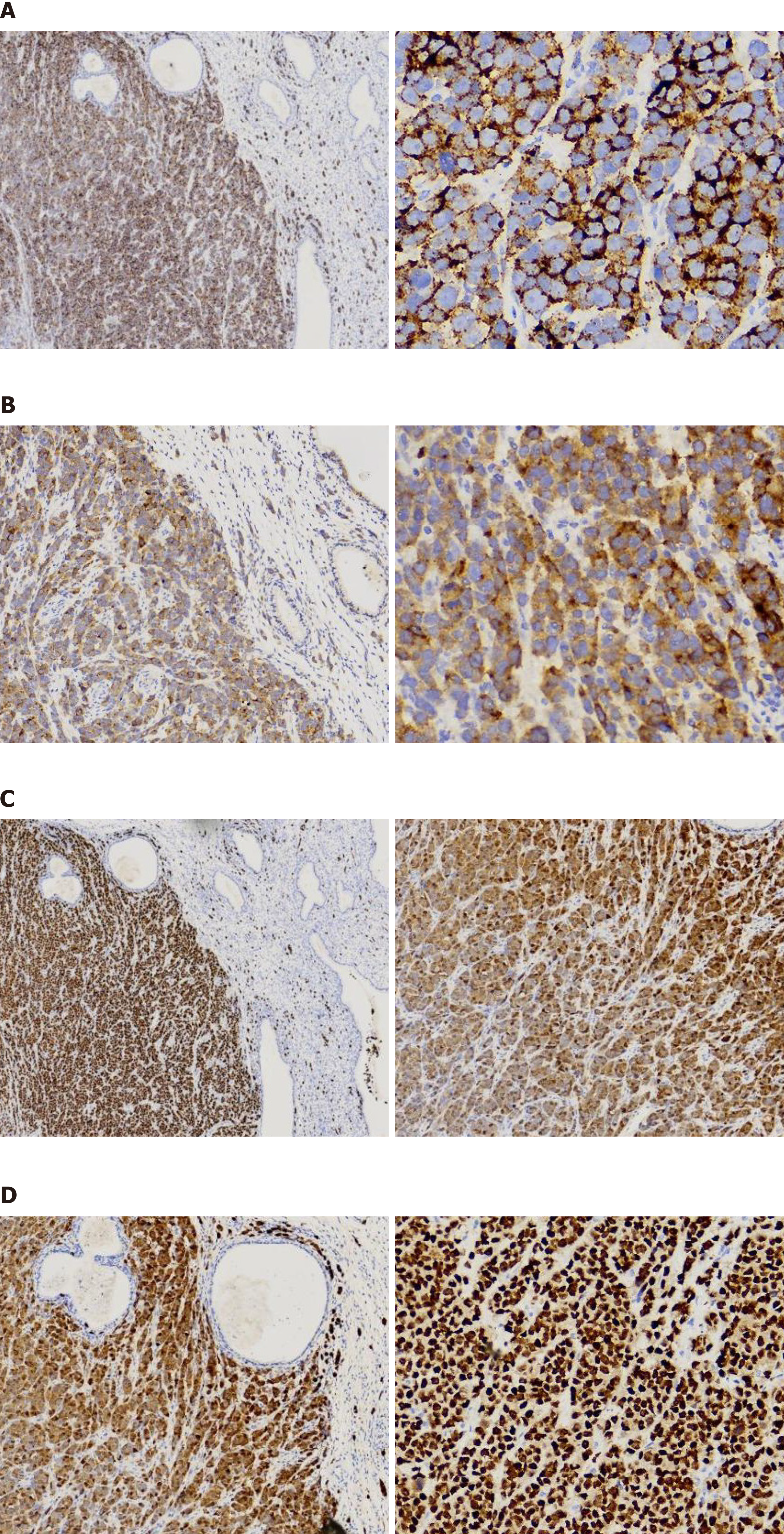
Considering the negative expression of pan-Keratin (AE1/AE3) and GATA3, as well the histological findings, this generally rules out the possibility that the tumor originated from primary breast cancer. Negative p40 and p16 reactivity in resected tumors on immunohistochemical (IHC) staining excluded the possibility of primary cervical cancer. Stepwise serial diagnostic IHC staining of biomarkers related to common cancers was performed. Surprisingly, the cells were strongly positive for MelanA, S-100, SOX-10 and HMB45, the biomarker for cervical melanoma (Figure 3). Due to the lack of melanin observed in the lesion, primary cervical malignant melanoma was not considered initially. The patient was finally diagnosed with primary cervical malignant melanoma.
Multiplex gene-panel testing indicated a genetic mutation of BRCA2 (exon11). She then received combination therapy consisting of the anti-PD1 antibody tislelizumab (200 mg, d1, q3w) and radiofrequency hyperthermia for 1.5 years.
The patient has undergone monthly follow-up visits. To date, she remains free of disease, without evidence of disease recurrence or metastasis for 1 year.
Primary cervical malignant melanoma represents an exceedingly uncommon tumor that can occur in the uterine cervix[1-4]. Since it was initially described as macroscopically "black cancer" of the cervix in 1889, only 149 cases have been reported to date[5]. The presence of melanin is one of the four criteria for the diagnosis of cervical melanoma[6]. Less than 20% of cases are, however, amelanotic and 3.5% cells in the cervical melanoma are melanin-containing cells compared with normal cervical epithelia[7,8]. Therefore, routine inclusion of IHC staining of combined S100 sensitivity, HBM45 specificity and MelanA staining is of great significance in facilitating the differential diagnosis of cervical malignancies without delays in situations where there is a lack of pigment. This is probably why IHC staining is more specific than Masson–Fontana staining[1,3]. Given that primary malignant melanoma frequently undergoes distant metastasis, excluding its origin from a primary cutaneous melanoma is a top priority for cervical melanoma diagnosis[9-12]. Both scanning and later positron emission tomography/computed tomography ruled out the presence of melanoma in other anatomic structures due to a distinct signal pattern from the paramagnetic properties of melanin[11,13]. This case was staged as IB2, without lymph node and distant metastasis, and she underwent regional lymph node dissection, although dissection of clinically negative regional lymph nodes is still controversial[14-18], indicating that a future study involving a larger sample size is necessary to determine the value of lymph node dissection in patients with PMMC.
Although melanoma is considered radio-resistant, the combination of ionizing radiation with hyperthermia provokes a systemic immune response and potentiates the efficacy of immunotherapy[19,20]. This case therefore received radiotherapy combined with immunotherapy, and the long-term effect is yet to be evaluated although she has been free of disease for 1 year. Notably, the combination of chemotherapy with either immunotherapy or radiotherapy has de
The differential diagnosis process reenforced the notion that immunohistochemical staining is the most reliable approach for amelanotic PMMC diagnosis. Due to the lack of established therapeutic guidelines, empirical information from limited previous studies does not provide the rationale for treatment-decision making. By integrating 'omics' technologies and PDXs or mini-PDX models this will help to identify selective therapeutic window(s) and screen the correct therapeutics for targeted therapies, immune checkpoint blockade or combination therapy strategies effectively and precisely that will ultimately improve patient survival.
| 1. | Pusceddu S, Bajetta E, Carcangiu ML, Formisano B, Ducceschi M, Buzzoni R. A literature overview of primary cervical malignant melanoma: an exceedingly rare cancer. Crit Rev Oncol Hematol. 2012;81:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Shi YF, Chen YQ, Chen HF, Hu X. An atypical primary malignant melanoma arising from the cervical nerve root: A case report and review of literture. World J Clin Cases. 2022;10:381-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 3. | Singh N, Tripathi R, Mala YM. Primary malignant melanoma of uterine cervix with probable origin from benign cervical melanosis. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Yin C, Yang A, Zhang Y, Tao L, Zou H, Ren Y, Liang W, Jiang J, Zhao J, Zhang W, Li F, Jia W. Primary Cervical Malignant Melanoma: 2 Cases and a Literature Review. Int J Gynecol Pathol. 2019;38:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Min A, Fu A, Huang M, Wang H, Chen H. Primary Malignant Melanoma of the Cervix: An Integrated Analysis of Case Reports and Series. Front Oncol. 2022;12:913964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 6. | Norris HJ, Taylor HB. Melanomas of the vagina. Am J Clin Pathol. 1966;46:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | CID JM. Melanoid pigmentation of the endocervix: a neurogenic visceral argument. Ann Anat Pathol (Paris). 1959;4:617-628. [PubMed] |
| 8. | Nigogosyan G, Delapava S, Pickren JW. Melanoblasts in vaginal mucosa. Origin for primary malignant melanoma. Cancer. 1964;17:912-913. [PubMed] [DOI] [Full Text] |
| 9. | Goldman RL. Melanomas of vagina. N Engl J Med. 1970;282:1492. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of Psoriasiform Eruption During Nivolumab Therapy for Primary Oral Mucosal Melanoma. JAMA Dermatol. 2015;151:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Piura B. Management of primary melanoma of the female urogenital tract. Lancet Oncol. 2008;9:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Yuan-Mou Yang J, Krishna GS, Macleod C, Oosthuysen W. Primary gastric mucosal melanoma. N Z Med J. 2008;121:96-99. [PubMed] |
| 13. | Sugiyama VE, Chan JK, Kapp DS. Management of melanomas of the female genital tract. Curr Opin Oncol. 2008;20:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Furuya M, Shimizu M, Nishihara H, Ito T, Sakuragi N, Ishikura H, Yoshiki T. Clear cell variant of malignant melanoma of the uterine cervix: a case report and review of the literature. Gynecol Oncol. 2001;80:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Jones HW 3rd, Droegemueller W, Makowski EL. A primary melanocarcinoma of the cervix. Am J Obstet Gynecol. 1971;111:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Cantuaria G, Angioli R, Fernandez-Abril A, Penalver M. Primary malignant melanoma of the uterine cervix: case report and review of the literature. Prim Care Update Ob Gyns. 1998;5:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Kim MS, Choi CH, Kim TJ, Lee JW, Lee J, Bae DS, Kim BG. Primary malignant melanoma of the uterine cervix treated with pembrolizumab after radical surgery: a case report and literature review. Obstet Gynecol Sci. 2018;61:524-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Mousavi AS, Fakor F, Nazari Z, Ghaemmaghami F, Hashemi FA, Jamali M. Primary malignant melanoma of the uterine cervix: case report and review of the literature. J Low Genit Tract Dis. 2006;10:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Tagliaferri L, Lancellotta V, Fionda B, Mangoni M, Casà C, Di Stefani A, Pagliara MM, D'Aviero A, Schinzari G, Chiesa S, Mazzarella C, Manfrida S, Colloca GF, Marazzi F, Morganti AG, Blasi MA, Peris K, Tortora G, Valentini V. Immunotherapy and radiotherapy in melanoma: a multidisciplinary comprehensive review. Hum Vaccin Immunother. 2022;18:1903827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 20. | Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. 2022;7:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 408] [Reference Citation Analysis (0)] |
| 21. | Wydra D, Sawicki S, Ciach K, Emerich J. Malignant melanoma of the uterine cervix. Eur J Obstet Gynecol Reprod Biol. 2006;124:257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 419] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 23. | Antonarakis ES, Abida W. Combining Poly(ADP)-Ribose Polymerase Inhibitors With Abiraterone in Castration-Resistant Prostate Cancer: Is Biomarker Testing Necessary? J Clin Oncol. 2023;41:3291-3294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3368] [Cited by in RCA: 3949] [Article Influence: 188.0] [Reference Citation Analysis (1)] |
| 25. | Konstantinopoulos PA, Cannistra SA. Comparing poly (ADP-ribose) polymerase inhibitors with standard chemotherapy in BRCA-mutated, recurrent ovarian cancer: lessons learned from a negative trial. J Clin Oncol. 2012;30:347-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Walsh C, Cass I. Poly(ADP-Ribose) Polymerase Inhibitors and Myeloid Neoplasm Risk-Clues to a Mechanistic Connection? JAMA Oncol. 2021;7:1763-1765. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, Balbin OA, Barbe S, Cai H, Casey F, Chatterjee S, Chiang DY, Chuai S, Cogan SM, Collins SD, Dammassa E, Ebel N, Embry M, Green J, Kauffmann A, Kowal C, Leary RJ, Lehar J, Liang Y, Loo A, Lorenzana E, Robert McDonald E 3rd, McLaughlin ME, Merkin J, Meyer R, Naylor TL, Patawaran M, Reddy A, Röelli C, Ruddy DA, Salangsang F, Santacroce F, Singh AP, Tang Y, Tinetto W, Tobler S, Velazquez R, Venkatesan K, Von Arx F, Wang HQ, Wang Z, Wiesmann M, Wyss D, Xu F, Bitter H, Atadja P, Lees E, Hofmann F, Li E, Keen N, Cozens R, Jensen MR, Pryer NK, Williams JA, Sellers WR. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21:1318-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 1027] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 28. | Garman B, Anastopoulos IN, Krepler C, Brafford P, Sproesser K, Jiang Y, Wubbenhorst B, Amaravadi R, Bennett J, Beqiri M, Elder D, Flaherty KT, Frederick DT, Gangadhar TC, Guarino M, Hoon D, Karakousis G, Liu Q, Mitra N, Petrelli NJ, Schuchter L, Shannan B, Shields CL, Wargo J, Wenz B, Wilson MA, Xiao M, Xu W, Xu X, Yin X, Zhang NR, Davies MA, Herlyn M, Nathanson KL. Genetic and Genomic Characterization of 462 Melanoma Patient-Derived Xenografts, Tumor Biopsies, and Cell Lines. Cell Rep. 2017;21:1936-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Gris-Oliver A, Palafox M, Monserrat L, Brasó-Maristany F, Òdena A, Sánchez-Guixé M, Ibrahim YH, Villacampa G, Grueso J, Parés M, Guzmán M, Rodríguez O, Bruna A, Hirst CS, Barnicle A, de Bruin EC, Reddy A, Schiavon G, Arribas J, Mills GB, Caldas C, Dienstmann R, Prat A, Nuciforo P, Razavi P, Scaltriti M, Turner NC, Saura C, Davies BR, Oliveira M, Serra V. Genetic Alterations in the PI3K/AKT Pathway and Baseline AKT Activity Define AKT Inhibitor Sensitivity in Breast Cancer Patient-derived Xenografts. Clin Cancer Res. 2020;26:3720-3731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Shattuck-Brandt RL, Chen SC, Murray E, Johnson CA, Crandall H, O'Neal JF, Al-Rohil RN, Nebhan CA, Bharti V, Dahlman KB, Ayers GD, Yan C, Kelley MC, Kauffmann RM, Hooks M, Grau A, Johnson DB, Vilgelm AE, Richmond A. Metastatic Melanoma Patient-Derived Xenografts Respond to MDM2 Inhibition as a Single Agent or in Combination with BRAF/MEK Inhibition. Clin Cancer Res. 2020;26:3803-3818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Woo XY, Giordano J, Srivastava A, Zhao ZM, Lloyd MW, de Bruijn R, Suh YS, Patidar R, Chen L, Scherer S, Bailey MH, Yang CH, Cortes-Sanchez E, Xi Y, Wang J, Wickramasinghe J, Kossenkov AV, Rebecca VW, Sun H, Mashl RJ, Davies SR, Jeon R, Frech C, Randjelovic J, Rosains J, Galimi F, Bertotti A, Lafferty A, O'Farrell AC, Modave E, Lambrechts D, Ter Brugge P, Serra V, Marangoni E, El Botty R, Kim H, Kim JI, Yang HK, Lee C, Dean DA 2nd, Davis-Dusenbery B, Evrard YA, Doroshow JH, Welm AL, Welm BE, Lewis MT, Fang B, Roth JA, Meric-Bernstam F, Herlyn M, Davies MA, Ding L, Li S, Govindan R, Isella C, Moscow JA, Trusolino L, Byrne AT, Jonkers J, Bult CJ, Medico E, Chuang JH; PDXNET Consortium; EurOPDX Consortium. Conservation of copy number profiles during engraftment and passaging of patient-derived cancer xenografts. Nat Genet. 2021;53:86-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 32. | Basak A, Lotfipour F. Modulating furin activity with designed mini-PDX peptides: synthesis and in vitro kinetic evaluation. FEBS Lett. 2005;579:4813-4821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Long Z, Lu Y, Li M, Ji C, Chen G, Li J, Xiang L, Yu H, Wang Q, Wang Z. Predicting chemosensitivity based on mini patient-derived xenografts in osteosarcoma patients: A retrospective study. J Cancer Res Ther. 2023;19:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Zhang F, Wang W, Long Y, Liu H, Cheng J, Guo L, Li R, Meng C, Yu S, Zhao Q, Lu S, Wang L, Wang H, Wen D. Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun (Lond). 2018;38:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Zhao P, Chen H, Wen D, Mou S, Zhang F, Zheng S. Personalized treatment based on mini patient-derived xenografts and WES/RNA sequencing in a patient with metastatic duodenal adenocarcinoma. Cancer Commun (Lond). 2018;38:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Zhu X, Xu X, Zhang B, Dong Y, Gong S, Gong T, Zhang F, Jin C. Individualized therapy based on the combination of mini-PDX and NGS for a patient with metastatic AFP-producing and HER-2 amplified gastric cancer. Oncol Lett. 2022;24:411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Kai M, Tamura K, Ohno M, Ohkura Y. Simple determination of forphenicinol in human plasma and erythrocytes by HPLC with native fluorescence detection. Biomed Chromatogr. 1986;1:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/