Published online Jul 24, 2024. doi: 10.5306/wjco.v15.i7.818
Revised: June 20, 2024
Accepted: June 27, 2024
Published online: July 24, 2024
Processing time: 198 Days and 16.8 Hours
This review delved into the intricate relationship between circadian clocks and physiological processes, emphasizing their critical role in maintaining homeo
Core Tip: This review explored the pivotal role of circadian clocks in physiological processes and adverse health outcomes, including metabolic syndrome and cancer, linked to their disruption. Orchestrated by interlocked CLOCK genes, the circadian system regulates vital functions, from sleep-wake cycles to immune response. Disruptions, often from night shift work, correlate with increased risks, notably in endocrine tumors. Molecular insights highlight potential therapeutic vistas, emphasizing the need for integrating circadian considerations in personalized healthcare. Chronotherapy emerges as a promising approach for endocrine tumor treatment, urging further research into precise mechanisms and public health interventions to mitigate lifestyle-related circadian disruptions.
- Citation: Savvidis C, Kallistrou E, Kouroglou E, Dionysopoulou S, Gavriiloglou G, Ragia D, Tsiama V, Proikaki S, Belis K, Ilias I. Circadian rhythm disruption and endocrine-related tumors. World J Clin Oncol 2024; 15(7): 818-834
- URL: https://www.wjgnet.com/2218-4333/full/v15/i7/818.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i7.818
Circadian clocks are intrinsic oscillators in cells and organisms, coordinating multiple biological processes within an approximate 24-hour cycle. The circadian timekeeping system comprises a network of clock genes (CGs) interlocked in a complex of autoregulatory transcriptional-translational feedback loops that generate and synchronize circadian rhythms, primarily influenced by light-dark cycles[1]. This self-sustained internal system orchestrates fundamental physiological processes, including the sleep-wake cycle, energy metabolism, immune function, cognitive and physical performance, as well as cell proliferation and tumorigenesis[2,3]. Additionally, it contributes to the adaptation of organisms to environmental changes, thereby maintaining systematic and tissue homeostasis[3,4].
In mammals, the circadian timing system consists of a central oscillator located in the hypothalamic suprachiasmatic nucleus (SCN) and various peripheral tissue clocks. The core pacemaker of these intricate rhythms is the SCN, which is attuned to geophysical time and environmental changes[5]. This synchronization is mediated by light transmitted to the SCN, primarily via retinal ganglion cells, and subsequently temporal information is conveyed to peripheral tissue clocks, thereby aligning internal timing with the light-dark cycle[6].
However, it has been proposed that other environmental cues, with feeding times being the most crucial, can also synchronize peripheral oscillators[7]. Circadian homeostasis among central and peripheral clocks is well documented as integral to optimal health status and survival. In contrast, there is growing evidence of the detrimental effects of circadian rhythm disruption due to environmental or behavioral factors, which are often concomitants of modern society. These factors include night shift work, irregular sleep patterns or meal timing, and exposure to artificial light during the night, all of which can perturb the harmonious coordination among clocks, leading to aberrant epigenetic modifications and adverse health outcomes[8,9].
It is worth noting that circadian system dysregulation has been linked to a wide range of adverse health issues, in
The aim of this narrative review was to summarize our understanding of the critical interplay between circadian disruption and its clinical implications in a wide array of related diseases. Additionally, we discussed the latest evidence that connects molecular mechanisms of circadian disruption to tumorigenesis. We aimed in this way to highlight the importance of integrating biological timing into the clinical setting and the need to consider circadian-based approaches in personalized health care. Please note that in this review in murine (mouse and rat) gene nomenclature, gene symbols are typically italicized with only the initial letter capitalized, followed by lowercase letters (e.g., Gfap). Corresponding protein symbols maintain the same designation as the gene symbol but are not italicized with only the initial letter capitalized, followed by lowercase letters (e.g., Gfap). In human gene nomenclature, gene symbols are italicized and presented in all uppercase letters (e.g., TP53). Protein symbols follow the same naming convention as the gene symbols, except they are not italicized and remain in all uppercase letters, reflecting their human origin (e.g., TP53). mRNAs and cDNAs adopt the same formatting conventions as their respective gene symbols.
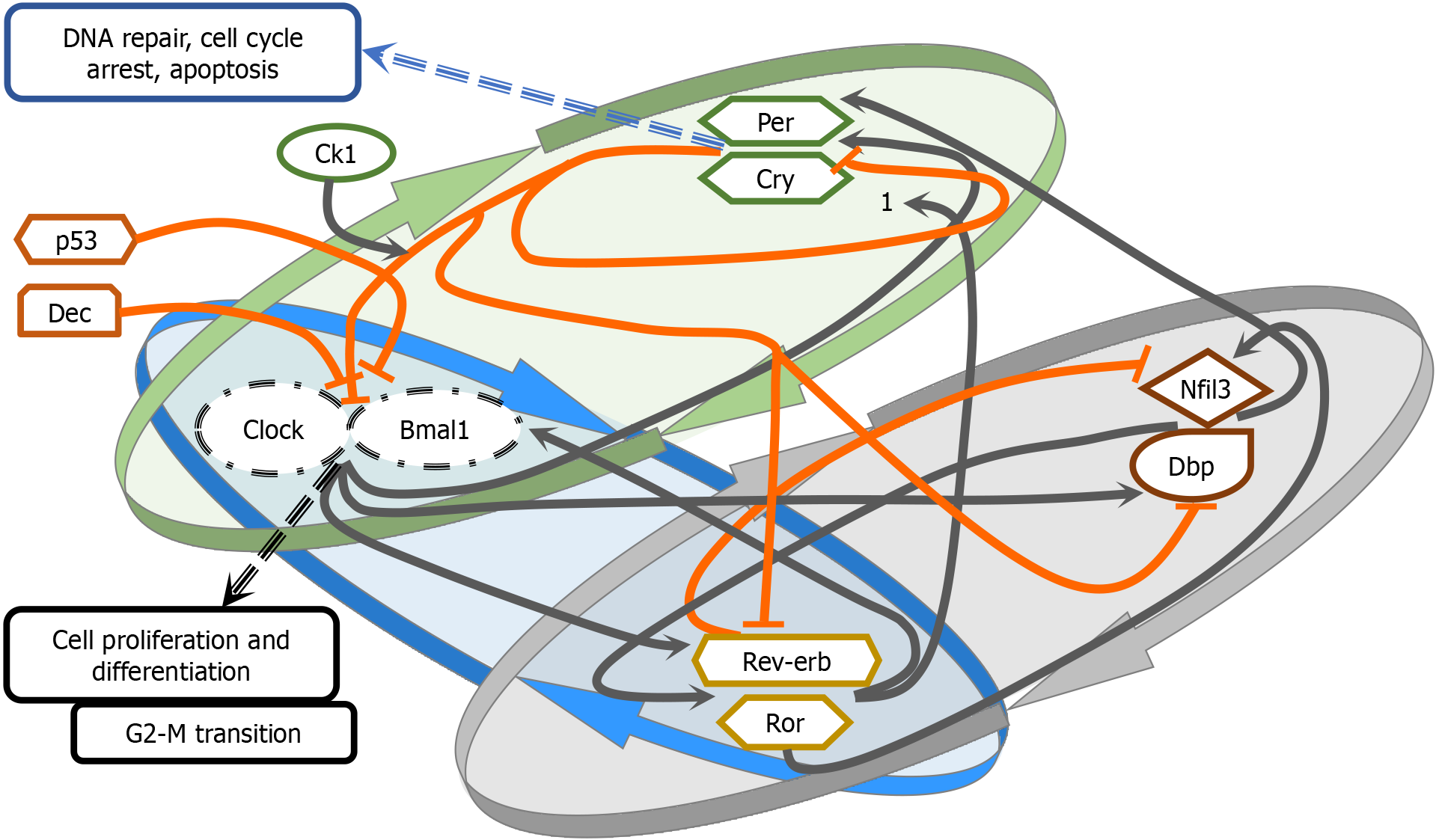
The CGs constitute a group of genes identified in mammals that collectively bear the responsibility of maintaining independent circadian rhythms in every individual cell[12]. To achieve this, CGs form three major transcriptional-translational feedback loops. The first identified CG, Period (Per), was discovered in Drosophila. In humans, PER encodes the PER protein, and its mRNA has been demonstrated to oscillate periodically[13,14]. The circadian locomotor output cycles kaput (Clock) gene, discovered in mice, encodes a regulatory transcriptional protein with a DNA-binding domain, a PAS dimerization domain, and a Q-rich transactivation domain[15]. While CLOCK mRNA does not exhibit periodic osci
There are three transcriptional feedback loops governing CGs. The primary loop involves the transcription of CLOCK and BMAL1 genes in the cytoplasm of mammalian cells. These proteins form a complex, CLOCK-BMAL1, which translocates to the nucleus and binds to enhancer box sequences (E-box), thereby regulating transcription[17]. The period (PER1, PER2, PER3) and cryptochrome (CRY1, CRY2) CGs, when activated by CLOCK-BMAL1, form a PER-CRY protein dimer that is translocated from the cytoplasm to the nucleus. This complex interacts with CLOCK-BMAL1, thereby halting its transcription in a negative feedback loop[21]. Once the protein levels of PER and CRY have decreased, the repression ceases, allowing CLOCK-BMAL1 to initiate gene transcription and commence a new oscillatory cycle[22].
The second loop involves the nuclear receptors REV-ERBa, REV-ERBβ (REV-ERBs), and the retinoic acid orphan receptor ROR (RORα, RORβ, RORγ), which bind to BMAL1 binding sites[23]. Specifically, REV-ERBs nuclear receptors repress the transcription of BMAL1, in contrast to the RORs, which positively regulate its expression by binding to retinoic acid receptor-related orphan receptor element (RORE) on the BMAL1 gene promoter[23].
The third loop involves CLOCK-BMAL1 and CRY1, which regulate D-box binding protein (DBP) and interleukin-3 regulated protein (NFIL3). These proteins bind to D-box elements, including RORα and RORβ[24]. NFIL3 can also bind to E-box and repress the transcription of PER2[24]. DBP can bind to the CRY1 promoter, influencing the timing of trans
Regulators of the core circadian CGs include certain genes forming minor transcriptional feedback loops in addition to the major transcriptional feedback loops. F-box protein 3 and 21 have been identified as degraders of CRY1[26,27]. Neuronal PAS family member 2 (NPAS2) shares homology with the CLOCK gene and is the largest member of the family[28]. Differentially expressed in chondrocyte (DEC1) and DEC2 inhibit CLOCK-BMAL1 transactivation by either binding with BMAL1 or E-boxes[29]. Other factors that may intervene in circadian clock rhythms include cAMP signaling, intracellular calcium flux, and membrane depolarization[30,31]. These factors are crucial for the SCN, potentially forming positive feedback loops on the central pacemaker of the circadian clock.
Post-translational regulation of the clock proteins includes phosphorylation, O-GlcNAcylation, and acetylation of the CLOCK-BMAL1 complex. These processes lead to either ubiquitination and degradation of core clock proteins or inhibition of their destruction, thereby promoting stabilization and a prolonged half-life. This delicate balance is crucial for maintaining the period of the molecular clock[32,33]. Molecules responsible for the amplitude of the clock period primarily include casein kinase II, PI3-kinase, and c-Jun N-terminal kinases[34].
Disruption of circadian rhythms has been increasingly linked to metabolic disorders. Recent guidelines for the mana
In line with epidemiological studies, there is ample evidence that circadian gene variations are associated with metabolic disorders. A CLOCK gene single nucleotide polymorphism has been associated with a lower risk of T2DM[41], while polymorphisms in CLOCK and ARNTL in patients with T2DM have been linked to myocardial infarction[42]. In recent years, research has focused on interventions that could improve metabolic profiles by influencing circadian rhythms. Time-restricted eating between 8 am and 2 pm appears to decrease morning fasting glucose and mean daily glucose levels and affect the expression of circadian CGs, such as BMAL-1, CRY1, CRY2[43]. Furthermore, according to a recent cross-sectional study, chronotype-aligned exercise has better outcomes in HbA1c, fasting glucose, and lipidemic profile of individuals with T2DM compared to exercise disregarding chronotype[44]. In addition, a three-meal diet with a rich carbohydrate breakfast appears to optimize HbA1c, reduce body weight and insulin requirements, and concomitantly upregulate the expression of CGs in patients with T2DM compared to a six-meal isocaloric diet[45]. Moreover, a 12-wk warm light exposure can alter the expression of REV-ERBα in shift workers[46], suggesting that light therapy could be used to rectify circadian disturbances. Based on current data, future research could uncover therapeutic methods for metabolic disorders based on circadian rhythms.
Several studies support the notion that disruption of the circadian clock can lead to oncogenesis through various path
The circadian system and the cell cycle are tightly interconnected. CGs control the transcription of key cell cycle regulators, gating the initiation of different phases (G1, S, G2, M)[51]. Transcriptional regulation can be facilitated either through E-boxes in gene promoters (Cyclins A, B1, D1, p53, c-Myc, and Wee1)[52,53] or through RORE regions on gene promoters that are targets of REV-ERBα[54]. For example, C-MYC expression (G0/G1 checkpoint and oncogene) is negatively regulated by BMAL1- CLOCK/NPAS through its E-box[52,55]. Bmal1-Clock controls both cyclin B1, an enhancer of G2/M transition, and Wee1, an inhibitor[56]. This relationship is bidirectional, as both Per2 and Rev-Erb transcription is regulated by genes like p53 and other tumor suppressor or cyclin kinase genes[57,58]. Therefore, CG disruption could contribute to tumorigenesis by interfering with different phases of the cell cycle[49,51].
A normal part of the cell cycle is the process of ensuring that any damage in the DNA are identified and repaired. Cryptochrome genes seem to be stimulated in states of genotoxic stress, and Cry2-deficient cells have been found to sustain damaged DNA[59]. CRYs and PER1 interact with the ATR/CHK1,2 signaling pathway involved in repairing DNA strands destroyed by ionizing radiation[60-62]. An attribute of cancer cells is their infinite replication potential. Telomerase activity, crucial for maintaining telomere length and preventing reproductive senescence[50], seems to be associated with CLOCK-BMAL expression[63]. Moreover, the circadian clock controls genes affecting cellular duplication (Ki-67)[64,65], the MDM-2 oncogene[52,66], cell apoptosis through Bax and Bcl-2[67], invasion and metastasis (MMP9)[68,69], and angiogenesis (VEGF)[64,70,71].
Dysregulation of the circadian clock can cause disruption of cellular metabolism. Chronic jet lag may be associated with the appearance of hepatocellular carcinoma via nonalcoholic fatty liver disease[72]. Increased glycolytic metabolism (Warburg effect) is crucial for cancer cell nutrition. For example, PI3-kinase/PdK1/AKT and hypoxia-induced factor 1 (HIF-1) pathways, which increase cell glycolysis, are influenced by circadian clock components[73,74].
Furthermore, the circadian clock controls the immune system and maintains homeostasis during inflammation. Disruption of the circadian clock can lead to immune dysfunction and immunosuppression through interference with antigen presentation, cell trafficking, and the function of T and B lymphocytes, natural killer cells, and monocytes[75,76]. Disruption of the circadian clock is also associated with chronic inflammation via cytokines, which have both paracrine and endocrine activity and affect cells locally and in distant tissues[47,48,50,77]. The TME, consisting of many different cells[78], dysfunctional vessel networks, cytokines, and deleterious intercellular signaling, is affected by CGs. This can be through the regulation of lymphocyte TME infiltration, impaired vigilance toward cancer cells, as well as the interaction between CGs and HIFs[71,79]. These processes can contribute to angiogenesis, metastasis, and immune system evasion[80,81].
Animal studies have shown that mutated CGs may act as tumor suppressors or oncogenes in various tissues[82-85]. These mutations might cause different disorders in different tissues. Genetic and genomic studies have shown that variations in the transcription of CGs are more common than mutations. Interestingly, it could be cancer cells themselves that disrupt the circadian clock, enhancing tumorigenesis[50].
Can shift work be solely responsible for the detrimental effects of circadian disruption? Studies have shown that night shift workers have an increased incidence of breast, prostate, and colorectal cancer[86-88]. Environmental factors such as light exposure and food consumption are indeed known circadian modulators. However, the effect of nocturnal activity, be it work or social life, is multifactorial, often mixed with other habits that increase cancer risk, such as alcohol or tobacco use, junk food consumption, and reduced exercise[89-93]. Some are skeptical of the association between cancer and the circadian clock, given certain reports of clockless animals that appear to be resistant to certain cancers[94]. Nevertheless, generalizations should be avoided; research should hone on the specific effects of distinct CGs on distinct forms of cancer and vice versa[94]. More studies that illustrate in detail the connections between the circadian clock and cancer and enable the design of new treatments are necessary.
An interesting but complex issue to address is the effect of circadian disruption in patients with metabolic disorders on the development and progression of cancer. In patients with diabetes, circadian clocks and sirtuins (SIRTs) influence oxidative stress, inflammation, and aging, contributing to organ-specific damages such as those seen in diabetic lung disease[95]. An imbalance of the circadian clock systems can lead to immune dysregulation and chronic inflammation that is linked to diabetes and obesity but additionally promotes cancer cell proliferation and invasion as well as evasion of immune surveillance[96]. Alterations in microbiota that are known to have circadian rhythmicity have been linked to metabolic disorders and could promote carcinogenesis especially in the colon[97]. Further exploration of the interconnections between metabolically-induced circadian disruption and carcinogenesis is beyond the objective of this review albeit being an exciting field.
Breast cancer (BC) is the most common cancer in females. According to a systematic review[98], in which the authors examined the relationship between BC and night shifts among nurses, most studies suggested a relationship between increased BC risk and cumulative years working in night shifts, particularly with three or more nights per month for at least 15 years. Furthermore, long-duration shift work is associated with estrogen and progesterone receptor-positive tumors, especially among young women with intensive shifts (12-h shifts). Night work schedules may shorten telomere length, a potential contributing factor to BC risk, and are associated with irregular menstrual cycles, which are considered a risk factor for BC.
The early onset and high mortality of BC are linked to a heterogeneous TME, consisting of various cell types such as cancer-associated fibroblasts, immune cells, and endothelial cells. Cancer-associated fibroblasts, in particular, contribute to tumor progression, metastasis, and drug resistance. Circadian CGs can modulate the TME, and their disruption can impact the biology of breast tissue, causing metabolic disturbances and immune dysfunction and potentially promoting tumor growth[99]. Additionally, in BC, there is lower expression of PER genes compared to normal breast epithelial cells. Loss-of-function mutations in PER2 have been linked to higher cancer incidence, especially radiation-induced cancer. Loss of PER2 inhibits programmed cell death, affecting p53 and cell cycle regulation[100]. Single nucleotide polymor
The role of protein arginine methyltransferase 6 (PRMT6) and poly ADP-ribose polymerase 1 (PARP1) in BC pro
Ovarian cancer is a common and deadly gynecologic malignancy, and research has shown that disturbances in circadian rhythms, such as shift work or irregular sleep patterns, might entail an increased risk[107]. Ovaries express circadian genes, controlling hormones during reproductive cycles, and lower expression of PER1, PER2, CRY2, and CLOCK in ovarian cancer compared to normal ovaries has been demonstrated[108]. Low expression of both BMAL1 and CRY1 was connected with a reduced overall survival rate. In contrast, CRY1, PER3, and BMAL1 showed higher and antiphasic (opposing) expression in ovarian malignancy.
CLOCK gene expression was linked to cisplatin resistance in ovarian cancer cells, and particularly upregulation of CLOCK reduced sensitivity to cisplatin. CLOCK knockdown increased the inhibitory effects of cisplatin on cell proliferation and induction of apoptosis[109]. Furthermore, Piwil2 and Pasd1, which are cancer/testis antigens, can interact with CGs like Bmal1 or Clock and interfere with circadian rhythms both in normal testis and cancer cells. Moreover, they negatively regulate the transcriptional activation of clock-controlled genes[110].
Prostate cancer (PCa) is a leading cause of cancer-related deaths in males. Studies that included immigrant populations suggested that environmental, lifestyle, and circadian rhythm disruptions may contribute to PCa formation. Both exogenous and endogenous factors might contribute to this process. Aging is closely connected to the circadian clock and changes in the SCN[111]. Sleep patterns, sleep quality, and duration are also factors that may affect PCa risk. Intermittent fasting and its effect on circadian rhythm regulation might offer protection against carcinogenesis, including PCa[112]. The circadian genes NPAS2, PER1, PER2, and PER3 play roles in controlling DNA damage, cell growth, and cell cycle regulation. Alterations in these genes can influence PCa risk and growth[113-118].
Chronic inflammation is proposed to be implicated in prostate carcinogenesis, and circadian rhythm disruption may contribute to the proinflammatory environment in PCa. The downregulation of PER2 is associated with the induction of epithelial–mesenchymal transition, which plays a role in cancer dissemination[119]. Increased melatonin is associated with protection against PCa progression[120], while inverse relationship of cortisol with the melatonin/cortisol ratio is linked to PCa emergence and stage[121]. The role of osteoblastic protein kinase D1 (PKD1) in promoting dormancy in PCa cells has been highlighted[122-124]. The results indicated that osteoblastic PKD1 induced dormancy in co-cultured PCa cells by activating cAMP responsive element binding protein 1 and increasing growth arrest specific 6 secretion. This osteoblastic PKD1-induced dormancy also enhanced the expression of core circadian clock molecules in PCa cells, which was linked to recurrence-free survival in metastatic PCa patients[122].
The rapid increase in the detection rate of thyroid cancer (TC) recently has been largely attributed to screening[125]. TC accounts for 3.4% of all cancers diagnosed annually worldwide[126], and recent data suggest that TC is increasing globally faster than other malignant lesions[127,128]. Several risk factors for TC have been identified by epidemiological research, namely female sex, advanced age[129], ionizing radiation[130,131], non-Hispanic white race[132], alterations in thyroid stimulating hormone (TSH) levels[133,134], obesity[135,136], and last but not least iodine deficiency[137]. However, even though the above-mentioned risk factors have been studied thoroughly, they cannot fully explain the variation in TC risk.
Growing evidence suggests that the disruption of circadian rhythm is a potential factor in thyroid tumorigenesis[138]. An epidemiological study in post-menopausal non-obese women proposed an association between insomnia and a higher incidence of TC[139], supporting the role of circadian dysregulation in TC development caused by sleep disorders. Furthermore, night shift work has been an established circadian disruptor and related to altered TSH plasma levels[140]. Artificial light at night suppresses melatonin, a fundamental regulator of circadian rhythms, causing circadian disruption[141]. A study in the United States[142] evaluated the hypothesis of a positive association between higher exposure to light at night and the risk of TC, especially papillary thyroid carcinoma. The same study suggested potential sex differences with a stronger association observed in females and a higher risk of anaplastic thyroid carcinoma for higher exposure to artificial light at night.
Based on current evidence, the SCN exerts control over the endocrine system. In humans, thyrotropin-releasing hormone and TSH show a nocturnal peak around 2 am to 4 am, while free thyroxine 4 has a less prominent circadian profile. The abovementioned hypothalamic nucleus directs neuronal outputs into the paraventricular hypothalamic nucleus and may be responsible for the circadian pattern of thyrotropin-releasing hormone[143].
At the genomic level, alterations in the characteristics of CGs were observed recently[144] in thyroid follicular malignancies. The cells were in vitro synchronized primary thyrocytes and cells recuperated by tissue biopsies. BMAL1 gene expression was 13-fold upregulated, while CRY2 expression was about 2-fold downregulated in papillary thyroid carcinoma nodules compared with benign thyroid nodules. In follicular thyroid carcinoma, BMAL1 showed 2-fold upregulated levels, with CRY2 downregulated equally by 2-fold. It is worth mentioning that upregulated levels of tissue inhibitor of metalloproteinase 1 were found, in contrast to downregulated levels of growth arrest and DNA damage inducible gene 153 transcripts in papillary thyroid carcinomas, a finding that was in agreement with previously published studies[145,146].
The transformation of normal thyroid tissue to nodular thyroid tissue with benign characteristics does not alter the circadian oscillator function[144]. However, malignant transformation of thyrocytes might implicate a change in circadian oscillator properties, namely the alteration of PER2 profile, especially in papillary thyroid carcinoma. PER2 can act as a tumor suppressor and has been found to play a key role by regulating responsive DNA damage pathways[52]. A recent cohort study evaluated the circadian rhythm genes involved in anaplastic thyroid carcinoma, a rare and extremely malignant type of endocrine cancer[147]. This work demonstrated that the NPAS2 gene promoted malignant phenotypes of anaplastic thyroid carcinoma by modulating the cell cycle as well as focal adhesion signals. This finding could be useful in the development of new therapeutic agents for this challenging type of endocrine cancer.
Dysregulation of various CGs including CRYs, PER1-2-3, REV-ERBs, and ROR α-β-γ have been associated with a higher risk of TC development[101,148]. In addition, increased levels of the circadian clock factor DEC1 have been found to promote the induction of several cell cycle-related genes, potentially leading to the development of TC[149].
As far as parathyroid tumors are concerned, available data is scarce. In parathyroid adenomas, mRNA of NFIL3, a gene responsible for repression of PER1 and PER2 expression, was downregulated. Moreover, NFIL3 and CRY2 mRNA levels were following the same pattern of downregulation. It is interesting though that no statistical difference was found between parathyroid adenomas and parathyroid hyperplasia concerning the core CGs[107]. Nevertheless, the me
Adrenal masses are encountered in 2% of the general population, and less than one-third of them are hormone-producing adenomas[150,151]. Adrenal carcinomas are scarce, with an incidence of 0.5-2.0 cases per million population per year[152,153]. Adrenal tumorigenesis is currently perceived as a gradual process of transformation from normal tissue to adeno
Cortisol-secreting adenomas (CSAs) were found to have reduced expression of PER1, CRY1, and REV-ERB mRNAs compared with adjacent normal adrenal tissue in humans[155]. The rhythmicity of glucocorticoid secretion is multi
Studies in mice show that the loss of core CGs like PER1 leads to deranged rhythmicity of glucocorticoid production[158]. Lack of Per1 or Per2 in mice causes higher circulating levels of glucocorticoids[158], whereas the lack of both Per2/Cry1 leads to the complete loss of the circadian rhythm of both adrenocorticotropic hormone and corticosterone, as well as other CGs[159]. Conversely, adrenal-specific knockout (KO) of Bmal1 did not have an effect on basal secretion of glucocorticoids in mice, but an increased glucocorticoid response to acute stress was observed[160]. Therefore, BMAL1 expressed in the adrenal glands has a local regulatory role.
The effect of circadian disruption on cortisol secretion is another interesting domain. Indicatively, shift work causes a partial adaptation in cortisol secretion in humans, as demonstrated by Koshy et al[161], who studied police officers after seven night shifts[161]. This was accompanied by a loss of morning/evening difference in CLOCK gene expression in both the oral mucosa and peripheral blood cells. Undoubtedly, more evidence is required, taking into consideration that work-related stress might be an additional driver of glucocorticoid production, independent of circadian rhythm dis
In human tissues from aldosterone-producing adenomas (APAs), all CGs were found to be upregulated compared to normal tissue but not with statistical significance[155]. Interestingly, PER1 and BMAL1 were more downregulated in APAs compared with CSAs[155]. Aldosterone stimulates Per gene transcription in mice[162]. CRY1 was found to be upregulated in human tissues of APAs, while CRY2 was downregulated[163]. Moreover, treatment with angiotensin II caused a significant upregulation of CRY1 and downregulation of CRY2. These studies imply that high levels of circulating aldosterone or angiotensin II are affecting the expression of CGs with consequent circadian disruption but cannot prove the opposite effect.
Further studies have revealed increased aldosterone levels in male mice, with global Per1 KO and kidney-specific Per1 KO on a normal salt diet[164-166]. This was accompanied by altered Na+ handling and non-dipping hypertension after high-salt and mineralocorticoid treatment. Per1 KO also caused an increase in Cry2 expression in the adrenals[165]. Adrenal-specific Bmal1 ablation in mice also led to changes in diurnal aldosterone levels and Na+ handling by the kidney[167]. These results would imply that Per1 and Bmal1 regulate the expression of aldosterone, but it is unknown if these sex-specific findings could translate similarly into human tissues.
In the murine adrenal medulla, Bmal, Per1, and Per3 seem to have a distinct circadian rhythm, while other CGs show weak expression[159]. There is yet no evidence of dysregulated CGs in human pheochromocytoma (PCC) tissues. However, research on PCCs and genes implicated in their development has revealed an interesting association between the hypoxia signaling pathway and the circadian clock; BMAL1, CLOCK, and PERIOD are heterodimerizing transcription factors that belong to the same family (bHLH-PAS) as HIF1α, 1β, 2α[168]. It is well known that mutations in CLUSTER 1 and 2 genes in PCC cells lead to stabilized and therefore increased hypoxia-inducible factors. As a result, there is increased hypoxia signaling in the environment of PCC cells and consequent overexpression of genes that control apoptosis, cell growth and proliferation, as well as VEGF, which leads to angiogenesis[169-171].
As members of the same family, BMAL1 might form a heterodimer with CLOCK or HIF-1β and HIF-1β a heterodimer with HIF-α or CLOCK, and they can bind to DNA regions that modulate gene expression of either the circadian or the hypoxia pathway. This leads to a bidirectional interaction. Animal studies have demonstrated how VEGF expression can be controlled by Bmal1 and Per2, causing angiogenesis driven by the circadian pathway[71,79]. Rutter et al[172] described an association between the redox state within the cell and the binding of CLOCK2-BMAL1 on E-box sites, indicating that circadian clock disruption can occur in states of oxidative stress[172].
One known clock-controlled gene in PCC cells is Atf5, which was found to be negatively regulated by Clock-Bmal1 in PC12 cells[173]. In the absence of Bmal, this gene is overexpressed[174], having a negative effect on neuronal differentiation in PC cells. On this occasion, cell differentiation is disrupted by a clock gene.
Recent studies on human adrenal tissue proclaim that there are different patterns of clock gene disruption in benign compared to malignant adrenal tumors. CLOCK, CRY1, and PER1 gene expression were amplified in adrenocortical carcinomas (ACCs) compared to CSAs. On the contrary, BMAL1, RORa, and REV-ERB expression were decreased[155]. Differences have been observed between ACC cells and normal tissue: Upregulation of CRY1 and PER1 and downregulation of BMAL1, RORa, and REV-ERB genes in ACC cells were noted[155]. The exact effects of clock gene disruption on tumorigenesis are not yet known.
Pituitary adenomas consist of typically monoclonal cell populations that can be either sporadic or familial. Four to five percent of pituitary adenomas are associated with clinical syndromes like multiple endocrine neoplasia 1 (Carney complex) and familial isolated pituitary adenoma, whose diverse genetic profiles are still under investigation[175]. A recent work has revealed the role of PER2, a key clock gene, in the pathogenesis of pituitary adenomas[176]. In human tissues obtained from prolactinomas and growth hormone-producing adenomas, the expression of several CGs was dysregulated compared to controls, with PER2 being consistently overexpressed. This overexpression of PER2 was confirmed in TSH-producing and adrenocorticotropic hormone-producing adenomas as well as non-functioning pituitary tumors.
Jet-lagged mice that had been inoculated with GH3 cells exhibited a faster rate of tumor growth that had a time-specific pattern[176]. The timing of higher cell proliferation was different compared to mice with intact circadian rhythm and correlated with elevated Per2 expression. In other groups of mice, one with estrogen-induced prolactinomas and one with xenograft GH3 tumors, KO of Per2 led to slower progression of the tumors as indicated by decreased Ki67 or smaller tumor mass and volume[176]. Apparently, Per2 plays an important role in cell cycle regulation, as its loss halts cells in the G2/M phase and negatively affects the number of pituitary cells in mitosis and positively those in apoptosis. More specifically, Per2 seems to act by upregulating key cell cycle genes (Ccnb2, Cdc20, Espl1) through HIF-1α[176].
Furthermore, a REV-ERBα antagonist administered to mice with GH3 tumors was found to counteract tumor growth by inhibiting Per2 expression. Interestingly, this agent was injected at a specific time of day that correlated with peak Per2 levels[176]. Patterns of PER2 expression in pituitary adenoma cells that might reflect times of higher cell proliferation could be useful guides for the timing of pharmacotherapeutic interventions in the future.
The expression of CGs has also been studied recently in gastric neuroendocrine tumors. In human gastric neuroendocrine tumor cells, CLOCK and BMAL1 were significantly upregulated, and REV-ERB was downregulated compared to normal tissue. On the contrary, the expression of PER2 and CRY1 was not altered significantly[177].
During the last decades, an increasing number of studies have investigated the interaction between circadian clocks and cancer therapy as well as the clinical utility of chronotherapy, which consists of three approaches[178,179].
The first approach is to promote and preserve an ideal circadian rhythm by entraining the circadian clock using daytime exposure to LED light[180], administering melatonin (apart from its antiproliferative, antioxidant, and immunological effects)[181], following intermittent fasting[182], doing exercise at different times of the day[183], or administering glucocorticoids[184] (the latter can alter the expression of CGs and display time-dependent pharmacokinetics)[185].
The second approach is to optimize chemotherapy and/or radiotherapy administration to maximize therapeutic results and reduce adverse effects by selecting a specific dosing time during the day[11,186,187]. This is based on the time-dependent expression of CGs, which affects the sensitivity of tumors to anticancer drugs[188] as well as the im
The third approach is to use small molecules that alter a circadian clock gene and can improve efficacy in combination with other anticancer treatments[178,179]. Almost all tumors are characterized by a disruption in the expression of CGs[190], and a number of small molecule modulators have been studied in several types of cancer. Additionally, chemo
Some small molecules have been shown to be effective against certain types of cancer. SR9009 and SR9011 are agonists of nuclear receptors Rev-Erbα/β, which reduce Bmal1 transcription[179], and appear to be specifically lethal to glioma cells without affecting normal cells. KL001, a stabilizer of Cry1/2, reduces glioma stem cell proliferation[191]. These molecules could potentially be useful in endocrine cancers, considering the significant upregulation of BMAL1 and downregulation of CRY2 in follicular and papillary TC[144], the synergistic interaction between Bmal1 and Hif-1a at the genomic level in PCC[73], as well as the significant increase of Bmal1 and reduced expression of REV-Erbs in gastric neuroendocrine cells compared to adjoining enterochromaffin-like tissue[177].
Another small molecule, LYC-55716, a RORγ agonist involved in the regulation of circadian rhythms and the immune system, has been successfully tested in a phase 1 open-label multicenter study in advanced tumors[192]. In addition, SR8278, a REV-ERB antagonist leading to the downregulation of pituitary expression of Per-2, reduces pituitary tumorigenesis[176]. Nevertheless, CGs display differential expression in different types of cancer[190]. Therefore, further clinical studies are required to elucidate the possible effects of chronomodulating small molecules in accordance with the diverse expression of CGs in cancerous tissues, including endocrine cancer. On the other hand, core clock proteins could be used as tumor biomarkers[193] or diagnostic preoperative markers[144].
Circadian CGs, particularly the PER gene family, play a crucial role in cancer progression and patient prognosis[194]. Mutations in these genes can affect cell function, metabolism, immunity, and response to treatment, contributing to varying cancer outcomes[194,195]. Studies have shown that the expression levels of circadian genes are significantly altered in cancers such as colorectal carcinoma, HER2-positive advanced gastric cancer, BC, pancreatic cancer, and obesity-related cancers[196-200]. These alterations could serve as biomarkers for cancer prognosis.
In colorectal cancer, the variable expression of circadian genes correlates with tumor progression and patient survival[197,201,202]. In pancreatic cancer, targeting circadian genes may optimize treatment and improve prognosis[199]. In BC, disruptions in circadian rhythms and alterations in circadian gene expression have been linked to different BC subtypes, tumor progression, and response to chemotherapy and radiotherapy[196,203-205]. Notably, lower expression of PER genes has been correlated with more aggressive tumor phenotypes and poorer survival rates in some cases[194,202], whereas in adrenocortical carcinoma PER1 expression is enhanced[155] and in pituitary adenomas there is marked overexpression of Per2[176]. It is evident that different types of tumors display distinct alterations in CGs that could be associated with prognosis and survival rates. Therefore, there is no single pattern that fits all. Further studies assessing the expression of circadian genes, including the PER family, can provide valuable prognostic information and guide personalized treatment strategies.
Disruptions in circadian rhythms, often a result of modern lifestyle factors such as shift work, irregular sleep patterns, and exposure to artificial light, lead to aberrant epigenetic modifications and adverse health outcomes. These disruptions have been linked to a range of diseases, including metabolic syndrome, cardiovascular diseases, and various types of cancer, emphasizing the critical role of biological clock homeostasis in human health. Circadian misalignments may lead to reductions in muscle insulin sensitivity, elevated fasting glucose levels, and an increased risk of T2DM among shift workers. Moreover, circadian gene variations have been associated with different metabolic disorders, highlighting the potential for interventions that could influence circadian rhythms to improve metabolic profiles.
The connection between circadian disruption and cancer involves multiple pathways, including interference with gene expression, DNA repair, and immune response. CGs control key cell cycle regulators and discuss the bidirectional relationship between the circadian system and the TME, which affects tumor growth, survival, and metastasis. An association between night shift work and increased BC risk, particularly with long-duration shift work, has been observed. Table 1 provides a comprehensive overview of how circadian disruption and CGs influence various endocrine tumors along with the related outcomes and interventions, highlighting the complex interplay between circadian rhythms and the pathology of endocrine tumors.
| Endocrine tumor | Circadian gene influence | Associated findings/interventions |
| Breast cancer | PER genes, PRMT6, PARP1 | Night shifts linked to increased risk, PER genes involved in tumor progression, PRMT6 and PARP1 implicated in cancer progression |
| Ovarian cancer | PER1, PER2, CRY2, CLOCK | Shift work/irregular patterns linked to risk, gene expression alterations, CLOCK gene linked to cisplatin resistance |
| Prostate cancer | NPAS2, PER1-3 | Environmental/lifestyle factors contribute, aging and sleep patterns, PER2 linked to cancer dissemination, intermittent fasting as a protective factor |
| Thyroid cancer | Various clock genes | Disruption as a potential contributing factor, alterations in gene expressions in follicular malignancies, NPAS2 gene promotes malignant phenotypes |
| Parathyroid tumors | NFIL3, CRY2 | Downregulation observed in adenomas, further research needed |
| Adrenal tumors | PER1, BMAL1, CRY1 | Altered expression in cortisol-secreting and aldosterone-producing adenomas, adrenal-specific gene influences, implications in glucocorticoid secretion and regulation |
| Pituitary tumors | PER2 | Overexpression linked to tumor growth, potential timing for pharmacotherapeutic interventions |
The evidence presented supports the potential for circadian rhythm-focused therapies, including chronotherapy, to treat endocrine tumors. Understanding the rhythmic patterns of drug efficacy and toxicity could optimize treatment schedules and minimize side effects. We call for further research to elucidate the precise mechanisms through which circadian genes influence tumor biology. Investigating specific pathways and molecular mechanisms involved can help identify new therapeutic targets and improve prognostic tools for various cancers. Given the associations between lifestyle factors like shift work and increased cancer risk, there is a significant opportunity for public health interventions. The importance of circadian rhythms in human health and disease is critical, and the need to consider the circadian dimension in understanding disease mechanisms and developing more effective, time-based therapeutic interventions should be emphasized.
| 1. | Pilorz V, Helfrich-Förster C, Oster H. The role of the circadian clock system in physiology. Pflugers Arch. 2018;470:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Zhou L, Zhang Z, Nice E, Huang C, Zhang W, Tang Y. Circadian rhythms and cancers: the intrinsic links and therapeutic potentials. J Hematol Oncol. 2022;15:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 3. | Ayyar VS, Sukumaran S. Circadian rhythms: influence on physiology, pharmacology, and therapeutic interventions. J Pharmacokinet Pharmacodyn. 2021;48:321-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Qu M. Molecular crosstalk between circadian clock and cancer and therapeutic implications. Front Nutr. 2023;10:1143001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Oosterman JE, Wopereis S, Kalsbeek A. The Circadian Clock, Shift Work, and Tissue-Specific Insulin Resistance. Endocrinology. 2020;161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Koronowski KB, Sassone-Corsi P. Communicating clocks shape circadian homeostasis. Science. 2021;371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 7. | Lane JM, Qian J, Mignot E, Redline S, Scheer FAJL, Saxena R. Genetics of circadian rhythms and sleep in human health and disease. Nat Rev Genet. 2023;24:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 8. | Giudice A, Crispo A, Grimaldi M, Polo A, Bimonte S, Capunzo M, Amore A, D'Arena G, Cerino P, Budillon A, Botti G, Costantini S, Montella M. The Effect of Light Exposure at Night (LAN) on Carcinogenesis via Decreased Nocturnal Melatonin Synthesis. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Meyer N, Harvey AG, Lockley SW, Dijk DJ. Circadian rhythms and disorders of the timing of sleep. Lancet. 2022;400:1061-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 200] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 10. | Rijo-Ferreira F, Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Med. 2019;11:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 367] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 11. | Lee Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp Mol Med. 2021;53:1529-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 975] [Cited by in RCA: 1044] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 13. | Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 522] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 617] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Vitaterna MH, Shimomura K, Jiang P. Genetics of Circadian Rhythms. Neurol Clin. 2019;37:487-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1575] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 18. | Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci U S A. 1998;95:6097-6102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 309] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1302] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 20. | Lee H, Chen R, Lee Y, Yoo S, Lee C. Essential roles of CKIdelta and CKIepsilon in the mammalian circadian clock. Proc Natl Acad Sci U S A. 2009;106:21359-21364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Michael AK, Fribourgh JL, Chelliah Y, Sandate CR, Hura GL, Schneidman-Duhovny D, Tripathi SM, Takahashi JS, Partch CL. Formation of a repressive complex in the mammalian circadian clock is mediated by the secondary pocket of CRY1. Proc Natl Acad Sci U S A. 2017;114:1560-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1898] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 23. | Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 847] [Article Influence: 38.5] [Reference Citation Analysis (6)] |
| 24. | Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 657] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Ukai-Tadenuma M, Yamada RG, Xu H, Ripperger JA, Liu AC, Ueda HR. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell. 2011;144:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 431] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 27. | Dardente H, Mendoza J, Fustin JM, Challet E, Hazlerigg DG. Implication of the F-Box Protein FBXL21 in circadian pacemaker function in mammals. PLoS One. 2008;3:e3530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 29. | Li Y, Song X, Ma Y, Liu J, Yang D, Yan B. DNA binding, but not interaction with Bmal1, is responsible for DEC1-mediated transcription regulation of the circadian gene mPer1. Biochem J. 2004;382:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682-7686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | de Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9:3725-3729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Spengler ML, Kuropatwinski KK, Schumer M, Antoch MP. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle. 2009;8:4138-4146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Yoshitane H, Takao T, Satomi Y, Du NH, Okano T, Fukada Y. Roles of CLOCK phosphorylation in suppression of E-box-dependent transcription. Mol Cell Biol. 2009;29:3675-3686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (14)] |
| 34. | Yagita K, Yamanaka I, Koinuma S, Shigeyoshi Y, Uchiyama Y. Mini screening of kinase inhibitors affecting period-length of mammalian cellular circadian clock. Acta Histochem Cytochem. 2009;42:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, Isaacs SD, Izuora KE, Low Wang CC, Twining CL, Umpierrez GE, Valencia WM. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm - 2023 Update. Endocr Pract. 2023;29:305-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 235] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 36. | Wefers J, van Moorsel D, Hansen J, Connell NJ, Havekes B, Hoeks J, van Marken Lichtenbelt WD, Duez H, Phielix E, Kalsbeek A, Boekschoten MV, Hooiveld GJ, Hesselink MKC, Kersten S, Staels B, Scheer FAJL, Schrauwen P. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci U S A. 2018;115:7789-7794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 37. | Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, Shi O, Deng J, Bi H, Lu Z. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 38. | Sun M, Feng W, Wang F, Li P, Li Z, Li M, Tse G, Vlaanderen J, Vermeulen R, Tse LA. Meta-analysis on shift work and risks of specific obesity types. Obes Rev. 2018;19:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 39. | Khosravipour M, Khanlari P, Khazaie S, Khosravipour H, Khazaie H. A systematic review and meta-analysis of the association between shift work and metabolic syndrome: The roles of sleep, gender, and type of shift work. Sleep Med Rev. 2021;57:101427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 40. | Dutheil F, Baker JS, Mermillod M, De Cesare M, Vidal A, Moustafa F, Pereira B, Navel V. Shift work, and particularly permanent night shifts, promote dyslipidaemia: A systematic review and meta-analysis. Atherosclerosis. 2020;313:156-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Corella D, Asensio EM, Coltell O, Sorlí JV, Estruch R, Martínez-González MÁ, Salas-Salvadó J, Castañer O, Arós F, Lapetra J, Serra-Majem L, Gómez-Gracia E, Ortega-Azorín C, Fiol M, Espino JD, Díaz-López A, Fitó M, Ros E, Ordovás JM. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc Diabetol. 2016;15:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Škrlec I, Milić J, Heffer M, Wagner J, Peterlin B. Circadian clock genes and circadian phenotypes in patients with myocardial infarction. Adv Med Sci. 2019;64:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (1)] |
| 44. | Menek MY, Budak M. Effect of exercises according to the circadian rhythm in type 2 diabetes: Parallel-group, single-blind, crossover study. Nutr Metab Cardiovasc Dis. 2022;32:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Jakubowicz D, Landau Z, Tsameret S, Wainstein J, Raz I, Ahren B, Chapnik N, Barnea M, Ganz T, Menaged M, Mor N, Bar-Dayan Y, Froy O. Reduction in Glycated Hemoglobin and Daily Insulin Dose Alongside Circadian Clock Upregulation in Patients With Type 2 Diabetes Consuming a Three-Meal Diet: A Randomized Clinical Trial. Diabetes Care. 2019;42:2171-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Rizza S, Luzi A, Mavilio M, Ballanti M, Massimi A, Porzio O, Magrini A, Hannemann J, Menghini R, Cridland J, Staels B, Grant PJ, Boger RH, Marx N, Federici M. Impact of light therapy on rotating night shift workers: the EuRhythDia study. Acta Diabetol. 2022;59:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Hojo H, Enya S, Arai M, Suzuki Y, Nojiri T, Kangawa K, Koyama S, Kawaoka S. Remote reprogramming of hepatic circadian transcriptome by breast cancer. Oncotarget. 2017;8:34128-34140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | de Assis LVM, Moraes MN, Magalhães-Marques KK, Kinker GS, da Silveira Cruz-Machado S, Castrucci AML. Non-Metastatic Cutaneous Melanoma Induces Chronodisruption in Central and Peripheral Circadian Clocks. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Masri S, Kinouchi K, Sassone-Corsi P. Circadian clocks, epigenetics, and cancer. Curr Opin Oncol. 2015;27:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 50. | Sulli G, Lam MTY, Panda S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer. 2019;5:475-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 51. | Soták M, Sumová A, Pácha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med. 2014;46:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 52. | Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 988] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 53. | Tan XM, Ye H, Yang K, Chen D, Wang QQ, Tang H, Zhao NB. Circadian variations of clock gene Per2 and cell cycle genes in different stages of carcinogenesis in golden hamster buccal mucosa. Sci Rep. 2015;5:9997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1726] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 55. | Dang CV. MYC on the path to cancer. Cell. 2012;149:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1984] [Cited by in RCA: 2720] [Article Influence: 194.3] [Reference Citation Analysis (0)] |
| 56. | Farshadi E, Yan J, Leclere P, Goldbeter A, Chaves I, van der Horst GTJ. The positive circadian regulators CLOCK and BMAL1 control G2/M cell cycle transition through Cyclin B1. Cell Cycle. 2019;18:16-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 57. | Miki T, Matsumoto T, Zhao Z, Lee CC. p53 regulates Period2 expression and the circadian clock. Nat Commun. 2013;4:2444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 58. | Zhao X, Hirota T, Han X, Cho H, Chong LW, Lamia K, Liu S, Atkins AR, Banayo E, Liddle C, Yu RT, Yates JR 3rd, Kay SA, Downes M, Evans RM. Circadian Amplitude Regulation via FBXW7-Targeted REV-ERBα Degradation. Cell. 2016;165:1644-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 59. | Papp SJ, Huber AL, Jordan SD, Kriebs A, Nguyen M, Moresco JJ, Yates JR, Lamia KA. DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010;584:2618-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 61. | Kang TH, Leem SH. Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res. 2014;42:4427-4434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 62. | Unsal-Kaçmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol. 2005;25:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 63. | Chen WD, Wen MS, Shie SS, Lo YL, Wo HT, Wang CC, Hsieh IC, Lee TH, Wang CY. The circadian rhythm controls telomeres and telomerase activity. Biochem Biophys Res Commun. 2014;451:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Ye H, Yang K, Tan XM, Fu XJ, Li HX. Daily rhythm variations of the clock gene PER1 and cancer-related genes during various stages of carcinogenesis in a golden hamster model of buccal mucosa carcinoma. Onco Targets Ther. 2015;8:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am J Pathol. 1999;154:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Hua H, Wang Y, Wan C, Liu Y, Zhu B, Yang C, Wang X, Wang Z, Cornelissen-Guillaume G, Halberg F. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006;97:589-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 67. | Granda TG, Liu XH, Smaaland R, Cermakian N, Filipski E, Sassone-Corsi P, Lévi F. Circadian regulation of cell cycle and apoptosis proteins in mouse bone marrow and tumor. FASEB J. 2005;19:304-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Anea CB, Ali MI, Osmond JM, Sullivan JC, Stepp DW, Merloiu AM, Rudic RD. Matrix metalloproteinase 2 and 9 dysfunction underlie vascular stiffness in circadian clock mutant mice. Arterioscler Thromb Vasc Biol. 2010;30:2535-2543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Zieker D, Jenne I, Koenigsrainer I, Zdichavsky M, Nieselt K, Buck K, Zieker J, Beckert S, Glatzle J, Spanagel R, Koenigsrainer A, Northoff H, Loeffler M. Circadian expression of clock- and tumor suppressor genes in human oral mucosa. Cell Physiol Biochem. 2010;26:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Koyanagi S, Kuramoto Y, Nakagawa H, Aramaki H, Ohdo S, Soeda S, Shimeno H. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. 2003;63:7277-7283. [PubMed] |
| 71. | Jensen LD, Cao Z, Nakamura M, Yang Y, Bräutigam L, Andersson P, Zhang Y, Wahlberg E, Länne T, Hosaka K, Cao Y. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep. 2012;2:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 72. | Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, Katchy CA, Lee C, Moore DD, Fu L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 2016;30:909-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (1)] |
| 73. | Wu Y, Tang D, Liu N, Xiong W, Huang H, Li Y, Ma Z, Zhao H, Chen P, Qi X, Zhang EE. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab. 2017;25:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 74. | Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, Bass J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017;25:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 318] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 75. | Cao Q, Zhao X, Bai J, Gery S, Sun H, Lin DC, Chen Q, Chen Z, Mack L, Yang H, Deng R, Shi X, Chong LW, Cho H, Xie J, Li QZ, Müschen M, Atkins AR, Liddle C, Yu RT, Alkan S, Said JW, Zheng Y, Downes M, Evans RM, Koeffler HP. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci U S A. 2017;114:12548-12553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 76. | Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, Mazzoccoli G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int. 2013;30:870-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 77. | Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, Jacks T, Sassone-Corsi P. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell. 2016;165:896-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 78. | Park H, Saravanakumar G, Kim J, Lim J, Kim WJ. Tumor Microenvironment Sensitive Nanocarriers for Bioimaging and Therapeutics. Adv Healthc Mater. 2021;10:e2000834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 79. | Jensen LD, Cao Y. Clock controls angiogenesis. Cell Cycle. 2013;12:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018;18:423-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 396] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 81. | Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 542] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 82. | Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 83. | Janich P, Toufighi K, Solanas G, Luis NM, Minkwitz S, Serrano L, Lehner B, Benitah SA. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013;13:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 84. | Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, Tothova Z, Tejero H, Heckl D, Järås M, Chen MC, Li H, Tamayo A, Cowley GS, Rozenblatt-Rosen O, Al-Shahrour F, Regev A, Ebert BL. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016;165:303-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 85. | Matsu-Ura T, Dovzhenok A, Aihara E, Rood J, Le H, Ren Y, Rosselot AE, Zhang T, Lee C, Obrietan K, Montrose MH, Lim S, Moore SR, Hong CI. Intercellular Coupling of the Cell Cycle and Circadian Clock in Adult Stem Cell Culture. Mol Cell. 2016;64:900-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 86. | Salamanca-Fernández E, Rodríguez-Barranco M, Guevara M, Ardanaz E, Olry de Labry Lima A, Sánchez MJ. Night-shift work and breast and prostate cancer risk: updating the evidence from epidemiological studies. An Sist Sanit Navar. 2018;41:211-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 87. | Papantoniou K, Castaño-Vinyals G, Espinosa A, Turner MC, Alonso-Aguado MH, Martin V, Aragonés N, Pérez-Gómez B, Pozo BM, Gómez-Acebo I, Ardanaz E, Altzibar JM, Peiro R, Tardon A, Lorca JA, Chirlaque MD, García-Palomo A, Jimenez-Moleon JJ, Dierssen T, Ederra M, Amiano P, Pollan M, Moreno V, Kogevinas M. Shift work and colorectal cancer risk in the MCC-Spain case-control study. Scand J Work Environ Health. 2017;43:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Papantoniou K, Devore EE, Massa J, Strohmaier S, Vetter C, Yang L, Shi Y, Giovannucci E, Speizer F, Schernhammer ES. Rotating night shift work and colorectal cancer risk in the nurses' health studies. Int J Cancer. 2018;143:2709-2717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 89. | Leung L, Grundy A, Siemiatycki J, Arseneau J, Gilbert L, Gotlieb WH, Provencher DM, Aronson KJ, Koushik A. Shift Work Patterns, Chronotype, and Epithelial Ovarian Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2019;28:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Bhatti P, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Nightshift work and risk of ovarian cancer. Occup Environ Med. 2013;70:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Carter BD, Diver WR, Hildebrand JS, Patel AV, Gapstur SM. Circadian disruption and fatal ovarian cancer. Am J Prev Med. 2014;46:S34-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Wendeu-Foyet MG, Bayon V, Cénée S, Trétarre B, Rébillard X, Cancel-Tassin G, Cussenot O, Lamy PJ, Faraut B, Ben Khedher S, Léger D, Menegaux F. Night work and prostate cancer risk: results from the EPICAP Study. Occup Environ Med. 2018;75:573-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Cordina-Duverger E, Menegaux F, Popa A, Rabstein S, Harth V, Pesch B, Brüning T, Fritschi L, Glass DC, Heyworth JS, Erren TC, Castaño-Vinyals G, Papantoniou K, Espinosa A, Kogevinas M, Grundy A, Spinelli JJ, Aronson KJ, Guénel P. Night shift work and breast cancer: a pooled analysis of population-based case-control studies with complete work history. Eur J Epidemiol. 2018;33:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 94. | Sancar A, Van Gelder RN. Clocks, cancer, and chronochemotherapy. Science. 2021;371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 95. | Zhou S, Dai YM, Zeng XF, Chen HZ. Circadian Clock and Sirtuins in Diabetic Lung: A Mechanistic Perspective. Front Endocrinol (Lausanne). 2020;11:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Shivshankar P, Fekry B, Eckel-Mahan K, Wetsel RA. Circadian Clock and Complement Immune System-Complementary Control of Physiology and Pathology? Front Cell Infect Microbiol. 2020;10:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Bishehsari F, Voigt RM, Keshavarzian A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol. 2020;16:731-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 98. | Fagundo-Rivera J, Gómez-Salgado J, García-Iglesias JJ, Gómez-Salgado C, Camacho-Martín S, Ruiz-Frutos C. Relationship between Night Shifts and Risk of Breast Cancer among Nurses: A Systematic Review. Medicina (Kaunas). 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 99. | Malla RR, Padmaraju V, Amajala KC, Chalikonda G, Nagaraju GP. Association between the Circadian Clock and the Tumor Microenvironment in Breast Cancer. Crit Rev Oncog. 2021;26:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Magni M, Buscemi G, Zannini L. Cell cycle and apoptosis regulator 2 at the interface between DNA damage response and cell physiology. Mutat Res Rev Mutat Res. 2018;776:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Morales-Santana S, Morell S, Leon J, Carazo-Gallego A, Jimenez-Lopez JC, Morell M. An Overview of the Polymorphisms of Circadian Genes Associated With Endocrine Cancer. Front Endocrinol (Lausanne). 2019;10:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 102. | Rizk SM, Shahin NN, Shaker OG. Association between SIRT1 Gene Polymorphisms and Breast Cancer in Egyptians. PLoS One. 2016;11:e0151901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 103. | Ball LJ, Palesh O, Kriegsfeld LJ. The Pathophysiologic Role of Disrupted Circadian and Neuroendocrine Rhythms in Breast Carcinogenesis. Endocr Rev. 2016;37:450-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 104. | Yang T, Huang W, Ma T, Yin X, Zhang J, Huo M, Hu T, Gao T, Liu W, Zhang D, Yu H, Teng X, Zhang M, Qin H, Yang Y, Yuan B, Wang Y. The PRMT6/PARP1/CRL4B Complex Regulates the Circadian Clock and Promotes Breast Tumorigenesis. Adv Sci (Weinh). 2023;10:e2202737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | El-Andaloussi N, Valovka T, Toueille M, Steinacher R, Focke F, Gehrig P, Covic M, Hassa PO, Schär P, Hübscher U, Hottiger MO. Arginine methylation regulates DNA polymerase beta. Mol Cell. 2006;22:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 106. | Gupta S, Kadumuri RV, Singh AK, Chavali S, Dhayalan A. Structure, Activity and Function of the Protein Arginine Methyltransferase 6. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Angelousi A, Kassi E, Ansari-Nasiri N, Randeva H, Kaltsas G, Chrousos G. Clock genes and cancer development in particular in endocrine tissues. Endocr Relat Cancer. 2019;26:R305-R317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | Tokunaga H, Takebayashi Y, Utsunomiya H, Akahira J, Higashimoto M, Mashiko M, Ito K, Niikura H, Takenoshita S, Yaegashi N. Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87:1060-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Xu H, Wang Z, Mo G, Chen H. Association between circadian gene CLOCK and cisplatin resistance in ovarian cancer cells: A preliminary study. Oncol Lett. 2018;15:8945-8950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Lu Y, Zheng X, Hu W, Bian S, Zhang Z, Tao D, Liu Y, Ma Y. Cancer/testis antigen PIWIL2 suppresses circadian rhythms by regulating the stability and activity of BMAL1 and CLOCK. Oncotarget. 2017;8:54913-54924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 111. | Zhu WZ, He QY, Feng DC, Wei Q, Yang L. Circadian rhythm in prostate cancer: time to take notice of the clock. Asian J Androl. 2023;25:184-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 112. | Kinouchi K, Magnan C, Ceglia N, Liu Y, Cervantes M, Pastore N, Huynh T, Ballabio A, Baldi P, Masri S, Sassone-Corsi P. Fasting Imparts a Switch to Alternative Daily Pathways in Liver and Muscle. Cell Rep. 2018;25:3299-3314.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 113. | Chu LW, Zhu Y, Yu K, Zheng T, Yu H, Zhang Y, Sesterhenn I, Chokkalingam AP, Danforth KN, Shen MC, Stanczyk FZ, Gao YT, Hsing AW. Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Zhu Y, Stevens RG, Hoffman AE, Fitzgerald LM, Kwon EM, Ostrander EA, Davis S, Zheng T, Stanford JL. Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res. 2009;69:9315-9322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 115. | Markt SC, Valdimarsdottir UA, Shui IM, Sigurdardottir LG, Rider JR, Tamimi RM, Batista JL, Haneuse S, Flynn-Evans E, Lockley SW, Czeisler CA, Stampfer MJ, Launer L, Harris T, Smith AV, Gudnason V, Lindstrom S, Kraft P, Mucci LA. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control. 2015;26:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 116. | Lin DW, FitzGerald LM, Fu R, Kwon EM, Zheng SL, Kolb S, Wiklund F, Stattin P, Isaacs WB, Xu J, Ostrander EA, Feng Z, Grönberg H, Stanford JL. Genetic variants in the LEPR, CRY1, RNASEL, IL4, and ARVCF genes are prognostic markers of prostate cancer-specific mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1928-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 117. | Mocellin S, Tropea S, Benna C, Rossi CR. Circadian pathway genetic variation and cancer risk: evidence from genome-wide association studies. BMC Med. 2018;16:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 118. | Yu CC, Chen LC, Chiou CY, Chang YJ, Lin VC, Huang CY, Lin IL, Chang TY, Lu TL, Lee CH, Huang SP, Bao BY. Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. 2019;19:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 119. | Hadadi E, Acloque H. Role of circadian rhythm disorders on EMT and tumour-immune interactions in endocrine-related cancers. Endocr Relat Cancer. 2021;28:R67-R80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 120. | Bartsch C, Bartsch H, Schmidt A, Ilg S, Bichler KH, Flüchter SH. Melatonin and 6-sulfatoxymelatonin circadian rhythms in serum and urine of primary prostate cancer patients: evidence for reduced pineal activity and relevance of urinary determinations. Clin Chim Acta. 1992;209:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Tai SY, Huang SP, Bao BY, Wu MT. Urinary melatonin-sulfate/cortisol ratio and the presence of prostate cancer: A case-control study. Sci Rep. 2016;6:29606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 122. | Li G, Fan M, Zheng Z, Zhang Y, Zhang Z, Huang Z, Luo W, Zhao W, Lai X, Chen H, Zeng F, Deng F. Osteoblastic protein kinase D1 contributes to the prostate cancer cells dormancy via GAS6-circadian clock signaling. Biochim Biophys Acta Mol Cell Res. 2022;1869:119296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 123. | Chen J, Deng F, Singh SV, Wang QJ. Protein kinase D3 (PKD3) contributes to prostate cancer cell growth and survival through a PKCepsilon/PKD3 pathway downstream of Akt and ERK 1/2. Cancer Res. 2008;68:3844-3853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 124. | Zou Z, Zeng F, Xu W, Wang C, Ke Z, Wang QJ, Deng F. PKD2 and PKD3 promote prostate cancer cell invasion by modulating NF-κB- and HDAC1-mediated expression and activation of uPA. J Cell Sci. 2012;125:4800-4811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 125. | Grodski S, Brown T, Sidhu S, Gill A, Robinson B, Learoyd D, Sywak M, Reeve T, Delbridge L. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144:1038-43; discussion 1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 126. | Chmielik E, Rusinek D, Oczko-Wojciechowska M, Jarzab M, Krajewska J, Czarniecka A, Jarzab B. Heterogeneity of Thyroid Cancer. Pathobiology. 2018;85:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 127. | Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. 2017;317:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1635] [Article Influence: 181.7] [Reference Citation Analysis (0)] |
| 128. | Haymart MR, Banerjee M, Reyes-Gastelum D, Caoili E, Norton EC. Thyroid Ultrasound and the Increase in Diagnosis of Low-Risk Thyroid Cancer. J Clin Endocrinol Metab. 2019;104:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 129. | Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C, Vigneri R. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med. 1992;93:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 283] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 130. | Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2366] [Cited by in RCA: 2226] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 131. | Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761-13766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1152] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 132. | Yu GP, Li JC, Branovan D, McCormick S, Schantz SP. Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid. 2010;20:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 133. | Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 134. | Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, Pinchera A, Vitti P. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 135. | Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de González A. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 136. | Zhao ZG, Guo XG, Ba CX, Wang W, Yang YY, Wang J, Cao HY. Overweight, obesity and thyroid cancer risk: a meta-analysis of cohort studies. J Int Med Res. 2012;40:2041-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 137. | Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 529] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 138. | Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol. 2019;15:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 139. | Luo J, Sands M, Wactawski-Wende J, Song Y, Margolis KL. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women's Health Initiative. Am J Epidemiol. 2013;177:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Moon SH, Lee BJ, Kim SJ, Kim HC. Relationship between thyroid stimulating hormone and night shift work. Ann Occup Environ Med. 2016;28:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 141. | Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog. 2007;13:303-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 142. | Zhang D, Jones RR, James P, Kitahara CM, Xiao Q. Associations between artificial light at night and risk for thyroid cancer: A large US cohort study. Cancer. 2021;127:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 143. | Kalsbeek A, Fliers E. Daily regulation of hormone profiles. Handb Exp Pharmacol. 2013;185-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 144. | Mannic T, Meyer P, Triponez F, Pusztaszeri M, Le Martelot G, Mariani O, Schmitter D, Sage D, Philippe J, Dibner C. Circadian clock characteristics are altered in human thyroid malignant nodules. J Clin Endocrinol Metab. 2013;98:4446-4456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 145. | Wasenius VM, Hemmer S, Kettunen E, Knuutila S, Franssila K, Joensuu H. Hepatocyte growth factor receptor, matrix metalloproteinase-11, tissue inhibitor of metalloproteinase-1, and fibronectin are up-regulated in papillary thyroid carcinoma: a cDNA and tissue microarray study. Clin Cancer Res. 2003;9:68-75. [PubMed] |
| 146. | Hawthorn L, Stein L, Varma R, Wiseman S, Loree T, Tan D. TIMP1 and SERPIN-A overexpression and TFF3 and CRABP1 underexpression as biomarkers for papillary thyroid carcinoma. Head Neck. 2004;26:1069-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 147. | Xu T, Jin T, Lu X, Pan Z, Tan Z, Zheng C, Liu Y, Hu X, Ba L, Ren H, Chen J, Zhu C, Ge M, Huang P. A signature of circadian rhythm genes in driving anaplastic thyroid carcinoma malignant progression. Cell Signal. 2022;95:110332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 148. | Kettner NM, Katchy CA, Fu L. Circadian gene variants in cancer. Ann Med. 2014;46:208-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 149. | Gallo C, Fragliasso V, Donati B, Torricelli F, Tameni A, Piana S, Ciarrocchi A. The bHLH transcription factor DEC1 promotes thyroid cancer aggressiveness by the interplay with NOTCH1. Cell Death Dis. 2018;9:871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 150. | Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, Pelsma I, Marina L, Lorenz K, Bancos I, Arlt W, Dekkers OM. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2023;189:G1-G42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 384] [Reference Citation Analysis (0)] |
| 151. | Arlt W, Biehl M, Taylor AE, Hahner S, Libé R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96:3775-3784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 152. | Wooten MD, King DK. Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer. 1993;72:3145-3155. [PubMed] [DOI] [Full Text] |
| 153. | Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 154. | Gicquel C, Baudin E, Lebouc Y, Schlumberger M. Adrenocortical carcinoma. Ann Oncol. 1997;8:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Angelousi A, Nasiri-Ansari N, Karapanagioti A, Kyriakopoulos G, Aggeli C, Zografos G, Choreftaki T, Parianos C, Kounadi T, Alexandraki K, Randeva HS, Kaltsas G, Papavassiliou AG, Kassi E. Expression of clock-related genes in benign and malignant adrenal tumors. Endocrine. 2020;68:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 156. | Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr Rev. 2017;38:3-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 381] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 157. | Minnetti M, Hasenmajer V, Pofi R, Venneri MA, Alexandraki KI, Isidori AM. Fixing the broken clock in adrenal disorders: focus on glucocorticoids and chronotherapy. J Endocrinol. 2020;246:R13-R31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 158. | Dallmann R, Touma C, Palme R, Albrecht U, Steinlechner S. Impaired daily glucocorticoid rhythm in Per1 ( Brd ) mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 159. | Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 403] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 160. | Engeland WC, Massman L, Miller L, Leng S, Pignatti E, Pantano L, Carlone DL, Kofuji P, Breault DT. Sex Differences in Adrenal Bmal1 Deletion-Induced Augmentation of Glucocorticoid Responses to Stress and ACTH in Mice. Endocrinology. 2019;160:2215-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 161. | Koshy A, Cuesta M, Boudreau P, Cermakian N, Boivin DB. Disruption of central and peripheral circadian clocks in police officers working at night. FASEB J. 2019;33:6789-6800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 162. | Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol. 2003;285:F664-F673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 163. | Tetti M, Castellano I, Venziano F, Magnino C, Veglio F, Mulatero P, Monticone S. Role of Cryptochrome-1 and Cryptochrome-2 in Aldosterone-Producing Adenomas and Adrenocortical Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 164. | Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxf). 2017;220:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 165. | Douma LG, Costello HM, Crislip GR, Cheng KY, Lynch IJ, Juffre A, Barral D, Masten S, Roig E, Beguiristain K, Li W, Bratanatawira P, Wingo CS, Gumz ML. Kidney-specific KO of the circadian clock protein PER1 alters renal Na(+) handling, aldosterone levels, and kidney/adrenal gene expression. Am J Physiol Renal Physiol. 2022;322:F449-F459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 166. | Douma LG, Crislip GR, Cheng KY, Barral D, Masten S, Holzworth M, Roig E, Glasford K, Beguiristain K, Li W, Bratanatawira P, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Knockout of the circadian clock protein PER1 results in sex-dependent alterations of ET-1 production in mice in response to a high-salt diet plus mineralocorticoid treatment. Can J Physiol Pharmacol. 2020;98:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 167. | Costello HM, Mckee A, Bratanatawira P, Crislip GR, Juffre A, Cheng K, Wingo CS, Gumz ML. A Role for the Adrenal Clock in Aldosterone Regulation and Renal Excretory Function. FASEB J. 2022;36. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 168. | Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. bHLH-PAS proteins in cancer. Nat Rev Cancer. 2013;13:827-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 169. | Kaelin WG Jr. Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 170. | Favier J, Gimenez-Roqueplo AP. Pheochromocytomas: the (pseudo)-hypoxia hypothesis. Best Pract Res Clin Endocrinol Metab. 2010;24:957-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 171. | Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF, Rötig A, Jeunemaitre X. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab. 2002;87:4771-4774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 172. | Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 742] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 173. | Lemos DR, Goodspeed L, Tonelli L, Antoch MP, Ojeda SR, Urbanski HF. Evidence for circadian regulation of activating transcription factor 5 but not tyrosine hydroxylase by the chromaffin cell clock. Endocrinology. 2007;148:5811-5821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 174. | Greene LA, Lee HY, Angelastro JM. The transcription factor ATF5: role in neurodevelopment and neural tumors. J Neurochem. 2009;108:11-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 175. | Daly AF, Tichomirowa MA, Beckers A. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 176. | Guo L, Cen H, Weng J, He Y, Guo X, He D, Liu K, Duan S, Yang J, Zhang X, Qin Z, Wan Y, Chen Z, Wu B. PER2 integrates circadian disruption and pituitary tumorigenesis. Theranostics. 2023;13:2657-2672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 177. | Schmoldt A, Benthe HF, Haberland G. Digitoxin metabolism by rat liver microsomes. Biochem Pharmacol. 1975;24:1639-1641. [PubMed] [DOI] [Full Text] |
| 178. | Sulli G, Manoogian ENC, Taub PR, Panda S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol Sci. 2018;39:812-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 179. | Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, Plikus MV, Verma IM, Panda S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018;553:351-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 180. | Dauchy RT, Wren-Dail MA, Dupepe LM, Hill SM, Xiang S, Anbalagan M, Belancio VP, Dauchy EM, Blask DE. Effect of Daytime Blue-enriched LED Light on the Nighttime Circadian Melatonin Inhibition of Hepatoma 7288CTC Warburg Effect and Progression. Comp Med. 2018;68:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 181. | Bhattacharya S, Patel KK, Dehari D, Agrawal AK, Singh S. Melatonin and its ubiquitous anticancer effects. Mol Cell Biochem. 2019;462:133-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 182. | Zhao X, Yang J, Huang R, Guo M, Zhou Y, Xu L. The role and its mechanism of intermittent fasting in tumors: friend or foe? Cancer Biol Med. 2021;18:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 183. | Weitzer J, Castaño-Vinyals G, Aragonés N, Gómez-Acebo I, Guevara M, Amiano P, Martín V, Molina-Barceló A, Alguacil J, Moreno V, Suarez-Calleja C, Jiménez-Moleón JJ, Marcos-Gragera R, Papantoniou K, Pérez-Gómez B, Llorca J, Ascunce N, Gil L, Gracia-Lavedan E, Casabonne D, Lope V, Pollán M, Kogevinas M. Effect of time of day of recreational and household physical activity on prostate and breast cancer risk (MCC-Spain study). Int J Cancer. 2021;148:1360-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 184. | Damato AR, Herzog ED. Circadian clock synchrony and chronotherapy opportunities in cancer treatment. Semin Cell Dev Biol. 2022;126:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 185. | Li J, Chen R, Yao QY, Liu SJ, Tian XY, Hao CY, Lu W, Zhou TY. Time-dependent pharmacokinetics of dexamethasone and its efficacy in human breast cancer xenograft mice: a semi-mechanism-based pharmacokinetic/pharmacodynamic model. Acta Pharmacol Sin. 2018;39:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 186. | Dakup PP, Porter KI, Little AA, Gajula RP, Zhang H, Skornyakov E, Kemp MG, Van Dongen HPA, Gaddameedhi S. The circadian clock regulates cisplatin-induced toxicity and tumor regression in melanoma mouse and human models. Oncotarget. 2018;9:14524-14538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 187. | Lee Y, Fong SY, Shon J, Zhang SL, Brooks R, Lahens NF, Chen D, Dang CV, Field JM, Sehgal A. Time-of-day specificity of anticancer drugs may be mediated by circadian regulation of the cell cycle. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 188. | Slat EA, Sponagel J, Marpegan L, Simon T, Kfoury N, Kim A, Binz A, Herzog ED, Rubin JB. Cell-intrinsic, Bmal1-dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma. J Biol Rhythms. 2017;32:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 189. | Harper E, Talbot CJ. Is it Time to Change Radiotherapy: The Dawning of Chronoradiotherapy? Clin Oncol (R Coll Radiol). 2019;31:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 190. | Ye Y, Xiang Y, Ozguc FM, Kim Y, Liu CJ, Park PK, Hu Q, Diao L, Lou Y, Lin C, Guo AY, Zhou B, Wang L, Chen Z, Takahashi JS, Mills GB, Yoo SH, Han L. The Genomic Landscape and Pharmacogenomic Interactions of Clock Genes in Cancer Chronotherapy. Cell Syst. 2018;6:314-328.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 191. | Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z, Prager BC, Wang X, Kim LJY, Morton AR, Dixit D, Zhou W, Huang H, Li B, Zhu Z, Bao S, Mack SC, Chavez L, Kay SA, Rich JN. Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock. Cancer Discov. 2019;9:1556-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 192. | Mahalingam D, Wang JS, Hamilton EP, Sarantopoulos J, Nemunaitis J, Weems G, Carter L, Hu X, Schreeder M, Wilkins HJ. Phase 1 Open-Label, Multicenter Study of First-in-Class RORγ Agonist LYC-55716 (Cintirorgon): Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin Cancer Res. 2019;25:3508-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 193. | Aroca-Siendones MI, Moreno-SanJuan S, Puentes-Pardo JD, Verbeni M, Arnedo J, Escudero-Feliu J, García-Costela M, García-Robles A, Carazo Á, León J. Core Circadian Clock Proteins as Biomarkers of Progression in Colorectal Cancer. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 194. | Chen K, Wang Y, Li D, Wu R, Wang J, Wei W, Zhu W, Xie W, Feng D, He Y. Biological clock regulation by the PER gene family: a new perspective on tumor development. Front Cell Dev Biol. 2024;12:1332506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 195. | Li HX. The role of circadian clock genes in tumors. Onco Targets Ther. 2019;12:3645-3660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 196. | Cadenas C, van de Sandt L, Edlund K, Lohr M, Hellwig B, Marchan R, Schmidt M, Rahnenführer J, Oster H, Hengstler JG. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle. 2014;13:3282-3291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 197. | Zhu H, Chen J, Wen Z, Li J, Yu Q, Liao W, Luo X. The role of circadian clock genes in colorectal carcinoma: Novel insights into regulatory mechanism and implications in clinical therapy. Life Sci. 2023;333:122145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 198. | Wang S, Khan S, Nabi G, Li HY. Circadian rhythm as a key player in cancer progression as well as a therapeutic target in HER2-positive advanced gastric cancer treatment. Front Oncol. 2023;13:1240676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 199. | Pourali G, Ahmadzade AM, Arastonejad M, Pourali R, Kazemi D, Ghasemirad H, Khazaei M, Fiuji H, Nassiri M, Hassanian SM, Ferns GA, Avan A. The circadian clock as a potential biomarker and therapeutic target in pancreatic cancer. Mol Cell Biochem. 2024;479:1243-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 200. | Miro C, Docimo A, Barrea L, Verde L, Cernea S, Sojat AS, Marina LV, Docimo G, Colao A, Dentice M, Muscogiuri G. "Time" for obesity-related cancer: The role of the circadian rhythm in cancer pathogenesis and treatment. Semin Cancer Biol. 2023;91:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 201. | Fonnes S, Donatsky AM, Gögenur I. Expression of core clock genes in colorectal tumour cells compared with normal mucosa: a systematic review of clinical trials. Colorectal Dis. 2015;17:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 202. | Karantanos T, Theodoropoulos G, Pektasides D, Gazouli M. Clock genes: their role in colorectal cancer. World J Gastroenterol. 2014;20:1986-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 203. | Rida P, Syed MI, Aneja R. Time will tell: Circadian clock dysregulation in triple negative breast cancer. Front Biosci (Schol Ed). 2019;11:178-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 204. | Tsuchiya Y, Umemura Y, Yagita K. Circadian clock and cancer: From a viewpoint of cellular differentiation. Int J Urol. 2020;27:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 205. | Reszka E, Przybek M. Circadian Genes in Breast Cancer. Adv Clin Chem. 2016;75:53-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/