INTRODUCTION
The incidence of thyroid cancer has increased significantly over the last decades in many countries. The Globocan 2020 incidence rates of thyroid cancer were 10.1 per 100000 women and 3.1 per 100000 men, while the mortality rates were 0.5 per 100000 women and 0.3 per 100000 men[1]. The rise in thyroid cancer incidence is mostly attributed to papillary thyroid carcinoma early diagnosis. However, the incidence of stage IV thyroid cancer has increased as well[2]. Of note, papillary thyroid cancer detection has risen by 240% in the last three decades[3]. Of the major histologic types, about 79%-90% are papillary thyroid carcinomas, 4%-5% are follicular thyroid carcinomas, 2%-5% are Hürthle-cell carcinomas, 2%-4% are medullary thyroid carcinomas, and 1%-2% are anaplastic thyroid carcinomas[4,5]. In iodine-deficient countries the incidence of follicular thyroid cancer can be up to 27%-28%, while papillary thyroid cancer is less frequent (47%-53%)[6,7]. In the cases of delayed diagnosis approximately 10%-15% of differentiated thyroid cancer can progress to more aggressive histological types[8].
The diagnosis of thyroid cancer is based on the Bethesda classification system, which plays the central role in interpreting cytology results[9-11]. Fine-needle aspiration has proven to be an efficient, simple and safe method for triage of patients with thyroid nodules[12]. It became the standard diagnostic tool for thyroid nodules in the late 1980s and replaced large-needle biopsy due to its high diagnostic accuracy and low complication rate[13]. However, its use in clinical practice demonstrated several weak points that require strengthening.
The rate of non-informative fine needle aspiration (FNA) is 10%-20% and up to 50% in repeated FNA. However, the FNA diagnostic accuracy depends on the skills of a medical specialist and the pathologist, who interprets the results. As was to be shown, approximately 17%-20% of FNA are classified as insufficient samples[14-16]. Cytological categories Bethesda III and IV do not provide an accurate diagnosis, since they require additional testing or surgery. Moreover, thyroid surgery complication rate is relatively high, while most cases of thyroid follicular neoplasia are benign[17,18]. Additional diagnostic tests are needed to overcome challenges in evaluating thyroid nodules[19].
In the light of these difficulties, large-needle biopsy has re-emerged as a new diagnostic method in the form or core-needle biopsy (CNB). Advanced technical construction of automatic and semi-automatic guns has led to the possibility of safe and efficient obtainment of thyroid histological specimens before surgery. CNB is now a widely implemented procedure used in difficult and suspicious cases of thyroid neoplasia with uncertain malignant potential owing to the progress in thyroid ultrasonography (USG) imaging[20].
CURRENT POSITION PAPERS AND GUIDELINES
Several documents reinforced the use of CNB on a national level. In 2013 and 2017 the Korean Society of Thyroid Radiology published “Core needle biopsy of thyroid nodules: Consensus statement and recommendations”[21,22]. In 2015 the Korean Endocrine Pathology Thyroid CNB Study Group published a positional paper “Pathology Reporting of Thyroid Core Needle Biopsy” [23]. The National Cancer Institute, American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi proposed CNB for thyroid nodules with non-diagnostic FNA results and persistently non-diagnostic nodules[22,24,25].
PREPARATION BEFORE THE PROCEDURE
Core needle biopsy is performed under local anesthesia and there is no need for fasting before the procedure. The Korean Society of Thyroid Radiology recommends withdrawal from aspirin and clopidogrel for 7-10 d, warfarin for 3-5 d, and heparin for 4-6 h before the procedure. Initiation of aspirin and clopidogrel bisulfate after CNB is recommended on the next day after procedure followed by heparin administered 2 h later after CNB and warfarin at night[22]. There have been studies that ultrasound-guided FNA of thyroid nodules can be safely used in patients who receive antiplatelet agents and anticoagulants without increase in adverse events or decrease in diagnostic rate[26]. Similar reports have been published for patients undergoing CNB of other organs[27]. Nevertheless, thyroid gland is a highly vascularized organ and there should be further studies to assess antiplatelet agents and anticoagulants security profile before administering them to the patients undergoing this procedure. Therefore, it seems reasonable that the Korean Society of Thyroid Radiology recommendations should be implemented.
CNB TECHNIQUE
The device for CNB should meet the parameters for the depth of needle penetration, taking into account the size of the mass. The optimal goal is maximum capture of the thyroid mass with minimal damage to the surrounding tissues. As for the thyroid gland, it is optimal to use a needle length of 6-10 cm, with an excursion of 1.5-2.2 cm. An ultrasound probe is insulated with a sterile sleeve, and sterile gel or saline are used to improve conductivity.
The procedure is performed in aseptic conditions. The neck area is disinfected with an antiseptic solution and subcutaneous tissue is infiltrated with local anesthetics. Additionally, it is possible to use non-steroidal anti-inflammatory drugs to reduce pain.
The thyroid gland CNB is performed under ultrasound control with an 18-21G cutting biopsy needle. The size of the needle affects the volume of material obtained; the thinner the needle, the less tissue will be obtained, but it is also related with the lower risk of trauma to the organ and lower complication rate. Therefore, in the majority of cases it is optimal to use 18 G needles. The procedure is performed by trained surgeon and a sonographer using the “free hand” method.
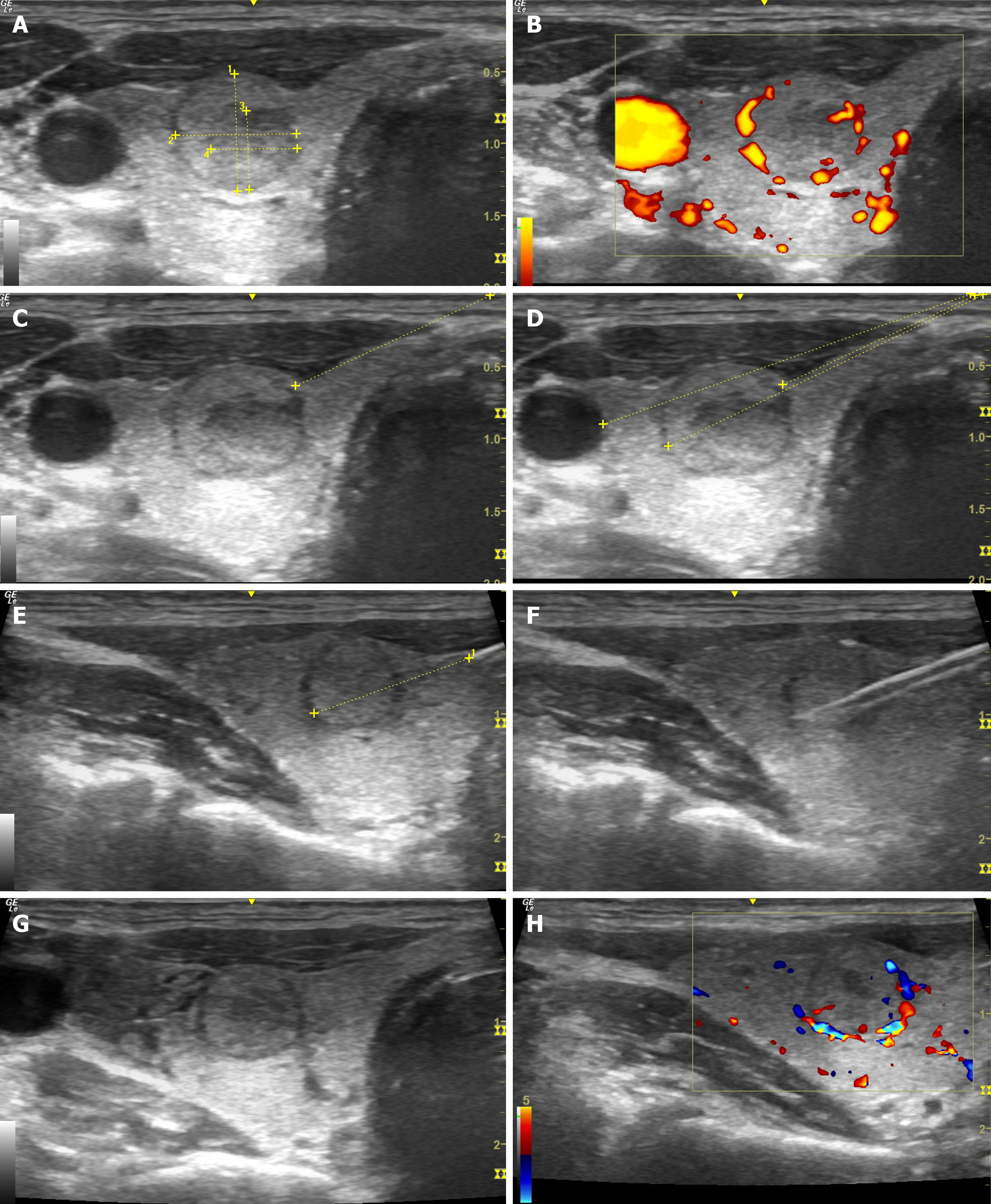
The needle is inserted using ultrasound navigation to the level of the thyroid gland. The needle tip is positioned so it remains on a pre-selected trajectory away from the major vessels (carotid artery and jugular vein) and trachea, and the biopsy needle firing distance is measured. After the procedure, finger pressure is applied to the biopsy area for 20-30 min. There are several ways to collect material: Biopsy of the mass formation itself, marginal biopsy of the mass with the adjacent zone of the capsule and healthy tissue, and a combination of these methods. At least two biopsies of the thyroid nodule are required (Figure 1). The obtained biological material is placed in containers with a 10% formaldehyde solution and they are marked in advance for a subsequent histological and, if necessary, immunohistochemical examination and/or molecular genetic testing.
Figure 1 Ultrasonography-guided core-needle biopsy of the thyroid gland.
A: Assessment of the size of the mass; B: Assessment in the Doppler mode; C: Marking the optimal trajectory for core-needle biopsy (CNB); D: Calculation of the distance till the mass and major vessels; E: Control of the needle along its length; F: Control during the biopsy; G: Assessment of the mass after CNB; H: Assessment of the node in Doppler mode after CNB.
ULTRASONOGRAPHY-GUIDANCE
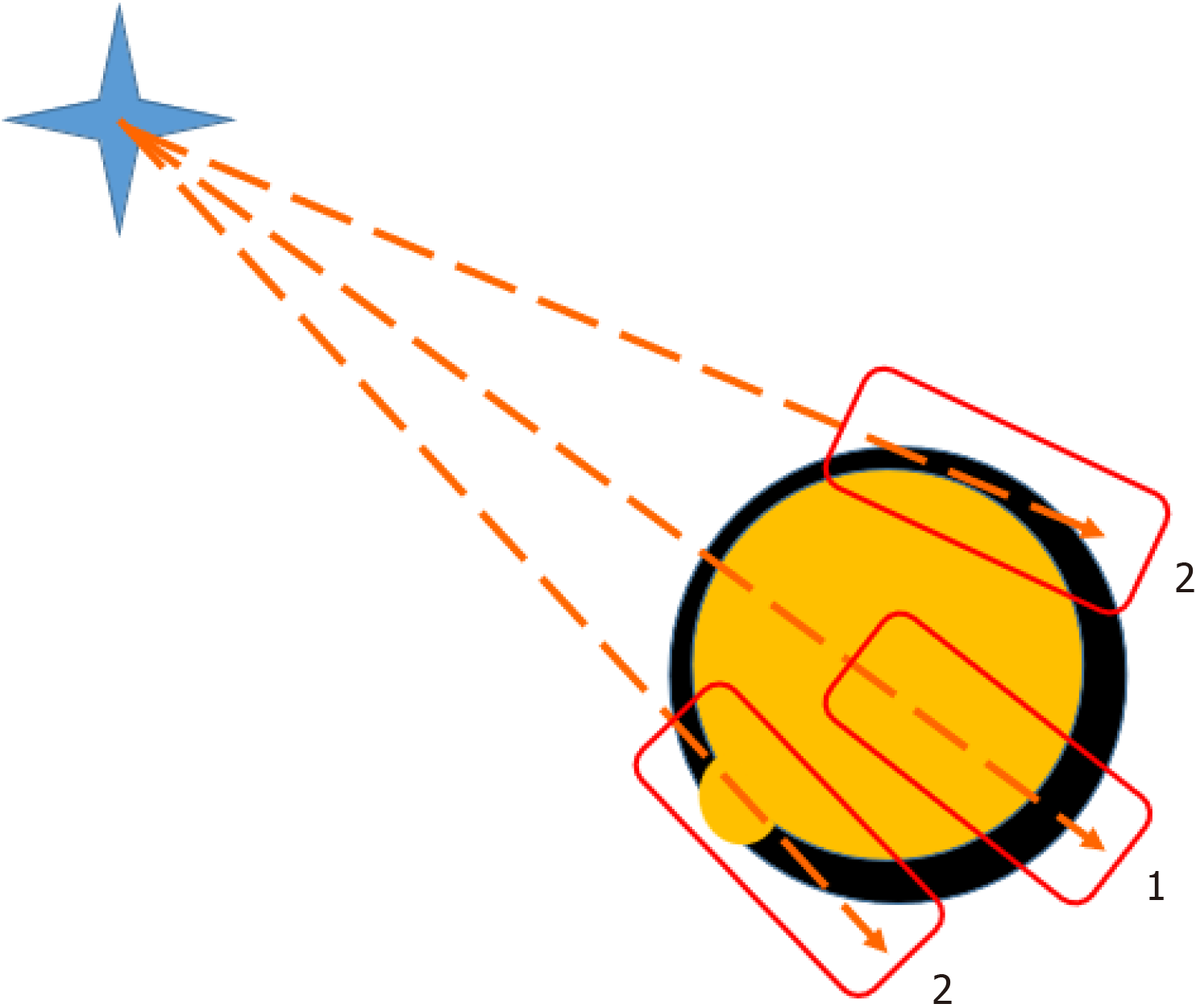
The thyroid gland CNB is performed under ultrasonography control with preliminary assessment of the mass blood supply, Thyroid Imaging Reporting and Data System (TI-RADS) score, and identification of potentially malignant areas. Optimal visualization is reached using a linear probe with a frequency of 7-12 MHz. The probe is aimed perpendicular to the skin surface in the projection of the mass. The needle is inserted strictly into the area of interest on the scanning plane. Ultrasound control is carried out parallel to the inserted needle along its length with mandatory visualization of the needle tip. It is necessary that the ultrasound scanning field includes the main vessels (carotid artery and jugular vein) and the trachea in order to avoid their injury. It is possible to assess the penetration distance before firing using the Vernier caliper. After the procedure, a re-evaluation of the mass is performed. The stages of ultrasonography-guidance are presented in Figure 2.
Figure 2
Core-needle biopsy of a thyroid node may be marginal 2 or through the node 1.
DIAGNOSTIC ACCURACY
A recent meta-analysis that included nine eligible studies, with 2240 patients and 2245 thyroid nodules involved, demonstrated that the pooled proportion for non-diagnostic results, inconclusive results and malignancy was 1.8% (95%CI: 0.4%-3.2%), 25.1% (95%CI: 15.4%–34.9%) and 18.9% (95%CI: 8.4%–29.5%), respectively. The sensitivity of CNB varied, ranging from 44.7% to 85.0%, while the specificity was 100% in all cases[28]. Second CNB in the case of non-diagnostic results provides a definitive diagnosis in almost all cases[29].
Furthermore, as CNB has advantages over FNA in amount of tissue obtained, it is possible to perform immunohistochemistry (IHC) or molecular testing using additional paraffin-embedded tissue sections. A combination of IHC markers consisting of galectin-3, HBME-1, cytokeratin 19 and CD56 is commonly used for the diagnosis of papillary carcinoma[22]. Molecular testing based on the FNA does not always provide adequate results. Rate of false-negative BRAF mutation testing using FNA increases in cases of old age, indeterminate FNA pathology results, and certain thyroid cancer subtypes[30]. The material extracted from CNB is usually sufficient to perform IHC and genetic tests[31,32].
CNB may have its limitation in small thyroid nodules. Some studies demonstrate that superiority of CNB to FNA is found in thyroid nodules larger than 2.0 cm and classified by American College of Radiology as TI-RADS or Korean-TIRADS category 4[33]. CNB is also superior to FNA in cases of possible lymph node metastases[34].
SAFETY AND COMPLICATIONS
An analysis of 6169 patients who underwent ultrasound-guided CNB demonstrated a complication rate of 0.81% (53 complications, of which only 4 were major). The complications included hematoma, pseudoaneurysm formation, dysphonia, carotid injury, tracheal puncture, dysphagia, oedema, vertebral puncture, and vasovagal reaction[35]. Most of the reported cases of bleeding could be treated with manual pressure[36]. Another study that included 4412 CNB demonstrated that minor complications occurred in 2.2% of CNB, and major in four procedures (0.09%)[29]. As it seems, in high volume centers the complication rate is around 1%-2% and major complications are exceedingly rare.
Treatment tactics for bleeding depends primarily on the blood volume loss and airway patency. In order to assess the degree of surrounding tissues compression, the most optimal method is USG. Decompression can be employed by performing USG-guided puncture or by installing drainage into the bleeding area. In most cases, bleeding stops with conservative therapy. In the case of dyspnea aggravation, uncontrolled hemorrhage or hemodynamic instability, emergency surgical intervention is indicated. Conservative treatment should include antibacterial therapy to prevent abscess formation, and anti-inflammatory therapy to reduce swelling. If a patient condition worsens, emergency intubation to protect airways is indicated and surgical treatment is performed, which most often includes stopping bleeding and hemithyroidectomy[37].
The literature presents isolated cases of transient laryngeal nerve paresis after CNB biopsy of the thyroid gland. In such cases, conservative treatment methods can be used: Drug therapy (neostigmine and other drugs that increase conductivity in the nervous system), exercises with a speech therapist, vocal cord augmentation using botulinum toxin injections and surgery (laryngoplasty, reinnervation)[38].
CONCLUSION
Modern oncology is largely based on histopathological examination of preoperative specimens. Nevertheless, thyroid surgery is carried out on the basis of fine-needle aspiration cytology. It has been presumed that CNB is an invasive procedure for an abundantly vascularized gland. However, latest advances in this filed demonstrate that CNB is a safe procedure that can decrease the number of unnecessary thyroid surgical operations and can provide additional information in undetermined cases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Russia
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade C
Scientific Significance: Grade B
P-Reviewer: Wu J, China S-Editor: Liu H L-Editor: A P-Editor: Zhao YQ