Published online Apr 24, 2024. doi: 10.5306/wjco.v15.i4.496
Peer-review started: December 15, 2023
First decision: January 15, 2024
Revised: January 29, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: April 24, 2024
Processing time: 128 Days and 10.1 Hours
Endometriosis is an estrogen-dependent inflammatory disease, defined by the presence of functional endometrial tissue outside of the uterine cavity. This disease is one of the main gynecological diseases, affecting around 10%-15% women and girls of reproductive age, being a common gynecologic disorder. Although endometriosis is a benign disease, it shares several characteristics with invasive cancer. Studies support that it has been linked with an increased chance of developing endometrial ovarian cancer, representing an earlier stage of neoplastic processes. This is particularly true for women with clear cell carcinoma, low-grade serous carcinoma and endometrioid. However, the carcinogenic pathways between both pathologies remain poorly understood. Current studies suggest a connection between endometriosis and endometriosis-associated ovarian cancers (EAOCs) via pathways associated with oxidative stress, inflammation, and hyperestrogenism. This article aims to review current data on the molecular events linked to the development of EAOCs from endometriosis, specifically focusing on the complex relationship between the immune response to endometriosis and cancer, including the molecular mechanisms and their ramifications. Examining recent developments in immunotherapy and their potential to boost the effectiveness of future treatments.
Core Tip: Current investigations imply a relationship between endometriosis and endometriosis-associated ovarian cancers (EAOCs) through pathways involving oxidative stress, inflammation, and hyperestrogenism. This article endeavors to examine the current data on the molecular events associated with the development of EAOCs from endometriosis, with a particular emphasis on the intricate relationship between the immune response to endometriosis and cancer, including the molecular mechanisms and their implications.
- Citation: Calmon MS, Lemos FFB, Silva Luz M, Rocha Pinheiro SL, de Oliveira Silva LG, Correa Santos GL, Rocha GR, Freire de Melo F. Immune pathway through endometriosis to ovarian cancer. World J Clin Oncol 2024; 15(4): 496-522
- URL: https://www.wjgnet.com/2218-4333/full/v15/i4/496.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i4.496
Endometriosis, an estrogen-dependent inflammatory disease, is defined by the presence of functional endometrial tissue (stromal cells and gland) outside of the uterine cavity. It involves ectopic implantation of endometrial cells, marked by heightened proliferation, infiltration, and migration. This condition is one of the main gynecological diseases, affecting around 10%-15% women and girls of reproductive age. It can reach 50% of women facing infertility and often correlates with dysmenorrhea and pelvic pain[1,2]. Notably, more than 52% of women diagnosed with endometriosis are between 18-29 years[3].
Regrettably, there is a substantial delay of almost 6 years between the onset of symptoms and diagnosis in primary care, which has a detrimental impact on the quality of life of many women and the subsequent treatment of a large number of patients[4]. Currently, there is no definitive cure for endometriosis, and available treatments primarily focus on symptom management, lacking measures to prevent recurrence of the disease. Risk factors for endometriosis include early menarche, nulliparity, dysfunctional uterine bleeding, aberrant estrogen levels, and low body mass index[5-7].
The underlying mechanisms of this disease have yet to be determined, despite numerous theories attempting to clarify their nature. The most widely accepted theory posits that retrograde menstruation allows endometriotic cells to evade the apoptotic pathway, leading to a disruption of the immune balance in the surrounding endometrioid tissue and triggering an immunological cascade that produces a mixture of pro- and anti-inflammatory factors[8]. However, the precise nature of these alterations remains unclear and is the subject of ongoing research.
Although endometriosis is a benign disease, more and more studies support that it has been linked with an increased chance of developing ovarian cancer, representing an earlier stage of neoplastic processes[9-11]. The first histological correlation between endometriosis tissue and ovarian cancer specimens was first presented by Sampson (1925), proposing that endometrial ovarian cancer may develop from endometriotic tissue, creating some criteria for diagnosis: (1) Evidence of coexisting tumor and endometriosis in the same ovarian location; (2) exclusion of a second malignancy elsewhere; and (3) histological pattern that resembles endometrial origin[12]. Later on, in 1953, Scott added a fourth criteria: Histological demonstration of benign lesions of endometriosis adjacent to malignant tissue[13].
Ovarian cancer ranks as the fifth leading cause of cancer-related death among women, surpassing other female reproductive system cancers. Ovarian epithelial tumors are divided into two categories: Type I tumors, which include clear cell carcinoma, low-grade serous carcinoma, endometrioid, and mucinous carcinoma; and Type II tumors, represented by high-grade serous carcinomas[14]. Endometrioid and clear cell tumors are linked to endometriosis and are classified as endometriosis-associated ovarian cancers (EAOCs), exhibiting a crescent correlation as they progress from endometriotic cyst epithelium through various stages of tumor development[15]. Both endometriosis and cancer share certain characteristics, such as the ability to evade apoptosis, form new blood vessels, and metastasize to distant sites, as well as the capability to create a supportive microenvironment that promotes growth and immune system mobilization[11].
Our review focuses on the intricate relationships between the immune response to endometriosis and cancer, particularly on the molecular mechanisms and their consequences. We designed this article by following a chain of thought, starting with the characteristics of the diseases, the immune alterations found in both pathologies, followed by the correlations between endometriosis and ovarian cancer, and how the immune response of endometriosis can lead to the onset of ovarian cancer, or at least favor its development. We explore how the microenvironment and imbalances due to endometriosis can trigger the development of ovarian cancer. Furthermore, we provide an overview of recent advancements in immunotherapy and their potential to enhance the efficacy of future treatments of these pathologies until the new findings for treatment and the current hurdle this field faces.
Despite being a benign disorder, numerous epidemiological studies consistently identify endometriosis as a risk factor for ovarian cancer, which is the most lethal among gynecological malignancies and rank as the third most prevalent[16-18]. Several mechanistic theories are linked to endometriosis, such as the reflux of endometrial tissue through the fallopian tubes during menstruation, coelomic metaplasia, embryonic cell rests, and lymphatic and vascular dissemination[12,19,20]. However, the etiology of endometriosis is generally considered multifactorial due to factors like genetics, hormones, and immunity. Remarkably, endometriosis can also develop in postmenopausal women, and although rare, it carries the risk of malignant transformation[21-24]. Hence, the observation of the occurrence and malignant transformation of endometriotic lesions in a hypoestrogenic environment with the absence of menstrual cycles emphasize the need for a more comprehensive understanding of the underlying mechanisms in the disease’s pathogenesis, that goes beyond classical theories centered on estrogen and retrograde menstrual flow[25,26].
The invasive potential of endometriosis and the persistent maintenance of ectopic tissue are characteristics that resemble cancer[27]. Notably, a significant association has been established between a history of endometriosis and an increased risk of developing specific subtypes of epithelial ovarian carcinoma, namely, endometrioid carcinoma, clear cell carcinoma, and low-grade serous tumors[19].
It is estimated that endometriosis increases the risk by approximately 3-fold for endometrioid and clear cell carcinomas[28,29]. Through histopathological analysis, it has been observed that the carcinogenesis of these cancer subtypes can originate from cysts and other endometriotic lesions that progress to a phase of endometriosis with higher oncogenic potential, known as atypical endometriosis[30-32]. In this condition, two main histological findings are noteworthy and may be present simultaneously or independently: Cellular atypia (cytologic atypia) and architectural atypia (hyperplasia)[33]. Furthermore, a subsequent prospective histological study found that endometriosis displaying architectural atypia, and consequently exhibiting higher proliferative activity, is most strongly linked to endometriosis-associated ovarian cancer[34].
From a molecular perspective, atypical endometriosis and clear cell carcinoma share mutations in hepatocyte nuclear factor-1β and the AT-rich interactive domain-containing protein 1A gene (ARID1A), which encodes the tumor suppressor protein BAF250. Therefore, absence of the BAF250a protein can be a useful early biomarker indicating malignant transformation of endometriosis[35,36]. In contrast, endometrioid adenocarcinoma primarily exhibits mutations in CTNNB1 (catenin beta 1), phosphatase and tensin homolog (PTEN), and ARID1A[35].
The association between endometriosis and low-grade serous tumors is relatively recent. Previously, epidemiological studies were primarily conducted alongside the high-grade serous tumor subtype, which does not exhibit an association with endometriosis[19]. The two histological subtypes may differ in terms of etiology, with low-grade typically originating from a malignant transformation process of borderline serous tumor, while high-grade tumors often arising from intraepithelial tubal carcinoma, with rarely observed progression from low-grade to high-grade serous tumors[37,38].
Genetically, TP53 mutations are more restricted to high-grade serous carcinomas[39], whereas low-grade serous carcinomas typically exhibit mutations in Kirsten Rat Sarcoma (KRAS) and B-type Raf kinase (BRAF), along with overexpression of hormone receptors (estrogen and/or progesterone)[38]. It’s worth noting that KRAS mutations are generally associated with a worse prognosis compared to exclusive BRAF mutations[40]. This underscores the need for additional genetic research in this field.
Endometriosis has garnered significant attention in the field of reproductive health due to its elusive etiology. While several theories have been proposed to explain its origin, the most widely accepted among them is Sampson's theory of retrograde menstruation[41]. According to this theory, during menstruation, endometrial cells retrograde into the fallopian tubes and subsequently into the peritoneal cavity[42,43]. In healthy females, these displaced cells typically undergo programmed cell death and are efficiently cleared by phagocytes and natural killer (NK) cells—a phenomenon known as immune surveillance[44,45]. However, in the context of endometriosis, compromised cell-mediated immune responses can disrupt this natural clearance process. Consequently, eutopic endometrial cells can adhere to the peritoneal wall, where they proliferate and eventually form endometriotic lesions[45,46].
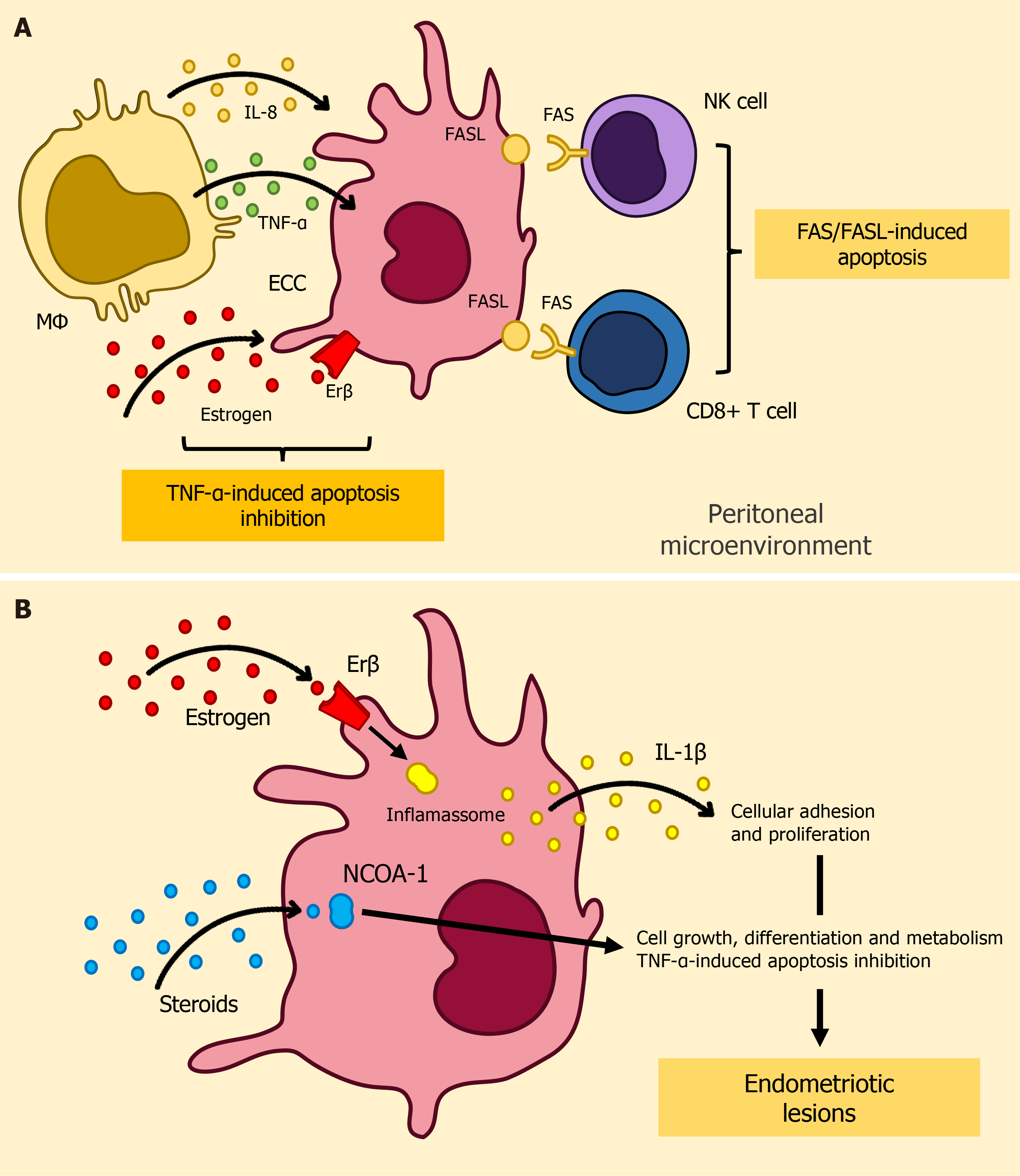
The precise mechanisms responsible for the evasion of immunosurveillance by ectopic endometrial cells (EECs) remain poorly understood. Several hypotheses have been proposed to elucidate this phenomenon, including the possible dysregulation of programmed cell death pathways[47]. The FAS (CD95) and FAS ligand (FASL) extrinsic apoptosis signaling system, which is well-documented for its significant role in immune modulation[48-50], appears to hold a pivotal position in the context of endometriosis[51,52]. In response to the peritoneal microenvironment, specially elevated interleukin(IL)-8 levels, a conspicuous increase in the expression of FASL is detected within EECs[53-55]. This heightened FASL expression seems to initiate apoptotic processes through the FAS-mediated pathway in immune cells expressing FAS, including T cells and NK cells[55].
In a parallel manner, alterations in tumor necrosis factor (TNF)-α-mediated cell death signaling contribute to the advancement of endometriosis. In retrograde menstruation, the entry of menstrual tissues into the peritoneal cavity stimulates macrophages to release cytotoxic cytokines, including TNF-α, thereby initiating apoptosis signaling in the extrauterine endometrial fragments that need to be eliminated[56]. However, in individuals with endometriosis, estrogen-dependent molecular alterations in retrograde menstrual tissues enable them to evade TNF-α-mediated apoptosis[57]. In their pioneering research, Han et al[58] presented compelling evidence showcasing elevated expression levels of Estrogen Receptor β (ERβ) within endometriotic tissues. Erβ exerts regulatory influence over cellular apoptotic processes by impeding apoptosis initiated by TNF-α. Furthermore, ERβ engages in interactions with cytoplasmic inflammasome constituents, consequently stimulating heightened production of interleukin-1β (IL-1β)[58]. This augmentation, in turn, amplifies cellular adhesion and proliferation characteristics.
Concurrently, within endometriotic lesions, there is a notable increase in the abundance of the nuclear receptor co
Within the framework of endometriosis, perturbation of the intrinsic apoptotic pathway carries substantial ramifications. The B-cell lymphoma/Leukemia-2 gene (Bcl-2) represents a novel class of proto-oncogenes characterized by their ability to inhibit apoptosis independently of cell proliferation stimulation[63,64]. In the context of endometriosis, an upregulation of Bcl-2 protein expression was observed in the proliferative eutopic endometrial tissue of affected patients[65,66]. Conversely, Bax expression was notably absent in the proliferative endometrial phase, but displayed increased expression during the secretory phase in both patients and control subjects[66]. Accordingly, research findings demonstrate that the utilization of Gonadotropin Releasing Hormones analogs elicits an upregulation in the expression of the proapoptotic protein Bax[67], a putative antagonist protein, concomitant with a downregulation in the expression of the antiapoptotic protein Bcl-2 which can suggest potential targets for therapeutic interventions in this condition.
Dysregulation of the mitogen-activated protein kinase (MAPK) signaling pathway also seems to exert a significant influence on the advancement of the disease[68]. In tandem with the conveyance of antiapoptotic signals to endometriotic tissues, this intricate cascade promotes the recruitment of immune cells, intensifies the inflammatory response, and augments the expression of growth factors[69,70]. This coordinated synchronization of cellular processes seems to promote the initiation and progression of endometriotic lesions, while concurrently fostering a microenvironment conducive to the development of endometriosis.
Increasing evidence suggests that the peritoneal fluid features macrophages as the most predominant immune cells, within physiological parameters, and that they may be related to the pathogenesis of endometriosis[71]. The ectopic growth of endometriotic tissue within the peritoneal cavity leads to the onset of an inflammatory response, which, in turn, results in increased recruitment of these cells, a phenomenon mediated by colony stimulating factor-1 (CSF-1), monocyte chemoattractant protein-1 (MCP-1/CCL2), interleukin (IL)-8, and RANTES (CCL5)[72-74].
Upon recruitment, macrophages undergo activation, thus adopting specific functional profiles (M1/M2) that can either intensify inflammatory processes or contribute to tissue repair and immune regulation. In this sense, the polarization of macrophages into the M2 phenotype seems to be beneficial to the angiogenesis of endometriotic lesions, through secretion of vascular endothelial growth factor (VEGF)[75]. Additionally, the secretion of factors such as IL-10 and transforming growth factor-beta (TGF-β) by M2-polarized macrophages contributes to the growth of endometriotic lesions, since it impairs the cytotoxicity of NK cells[76-78]. Interestingly, there is also growing interest in the role of IL-17A in endometriosis, as it appears to be associated with macrophage recruitment, triggering M2 phenotype polarization, angiogenesis and maintenance of the inflammatory cascade[79,80].
In addition to the intricate interplay involving macrophages and the aforementioned mechanisms, it is essential to address the role of fibrosis in this context. In this sense, TGF-β secreted by M2 macrophages induces fibrosis, as it promotes the differentiation of fibroblasts into myofibroblasts and stimulate the synthesis of collagen and fibronectin[81-83]. On the other hand, Barcz et al[84] suggests that increased levels of VEGF are negatively associated to endometriosis-related pelvic fibrotic adhesions.
Macrophages also play a role in neurogenesis and, consequently, in the onset of endometriosis-associated pain[85]. Overall, nerve fibers originating from endometriotic lesions have the capacity to secrete chemokines such as CCL2 and CSF-1, which, as previously discussed, are pivotal in the recruitment of macrophages[81,86]. As a result, macrophages release neurotrophic factors, including brain-derived neurotrophic factor and neurotrophin-3, thus contributing to the heightened sensitivity and pain experienced by individuals with endometriosis[86].
Furthermore, iron accumulation into the peritoneal cavity, stemming from retrograde menstruation, holds a particular interplay with macrophages and plays a role in the pathogenesis of endometriosis[87]. Macrophages in the pelvic cavity carry out erythrocyte phagocytosis and iron metabolism, thereby resulting in elevated iron concentrations within the peritoneal fluid[88]. As a result, iron overload can trigger oxidative stress and contribute to chronic inflammation, thus leading to increased proliferative capacity of endometriotic lesions[89].
Finally, it is crucial to acknowledge that the phenotypic distinction between M1 and M2 macrophages is currently viewed as oversimplified[87]. Indeed, emerging evidence underscores the plasticity of macrophages, suggesting the potential for a hybrid M1/M2 profile or a dynamic switch between these phenotypes[87,88]. This adaptability appears to be influenced by the specific microenvironment to which these cells are exposed[88]. Such complexity in the interplay between macrophages and the pathogenesis of endometriosis emphasizes the need for further studies to thoroughly elucidate these intricate mechanisms.
Natural Killer cells play a crucial role in the immune system's surveillance and defense against endometriosis. These immune cells are capable of identifying and eliminating abnormal endometrial cells, thereby contributing to the body's efforts to combat this condition[90,91]. Additionally, NK cells help regulate inflammation, modulate angiogenesis, and assist in maintaining immune tolerance within the endometrial environment[92,93].
In individuals with endometriosis, the peripheral circulation is characterized by a predominance of CD16+/CD56dim NK cells, which are well-known for their heightened cytotoxic capabilities. In contrast, the endometrium and peritoneal fluid (PF) predominantly harbor CD16-/CD56bright NK cells, renowned for their robust production of cytokines[94]. Accordingly, among females diagnosed with endometriosis, there is a noteworthy decrease in cytotoxicity observed in NK cells present within the peritoneum and PF[95]. Reduced cytotoxicity in the context of endometriosis may result from a complex interplay of cytokines within the intricate microenvironment of endometriotic lesions.
For instance, Yang et al[76] recently proposed that the interaction between macrophages and endometrial stromal cells (ESCs) could downregulate NK cell cytotoxicity. This downregulation might occur through the induction of cytokine secretion, including IL-10 and TGF-β, by the interacting cells. Such an interaction could potentially facilitate immune evasion by ectopic fragments and contribute to the development of endometriosis. Building on this idea, Kang et al[96] demonstrated that an increased level of IL-6 in the PF of patients with endometriosis might also suppress NK cell activity via regulation of SHP-2 expression. Likewise, elevated IL-15 levels were demonstrated to foster the proliferation and invasive behavior of ESCs while concurrently suppressing the cytotoxic capabilities of NK cells in individuals with endometriosis[97]. These cytokines, each wielding a distinct mechanism, collectively contribute to the precise regulation of NK cell responses in various immunological contexts.
The NK cell detection system employs a set of receptors on the surface of NK cells, including activating receptors like NKG2D and CD16 (FcgRIIIa), to regulate NK cell activities. This system is crucial for the immune system's ability to identify and eliminate abnormal cells—such as ECCs. In comparison to healthy women, the PF of individuals with endometriosis exhibited decreased levels of various markers associated with NK cell cytotoxicity. These markers include the natural receptors NKp46, NKp44, and NKG2D, as well as CD16 and CD107a, which are indicative of NK cell activation, and CD69[98].
Conversely, González-Foruria et al[99] demonstrated that there is a notable rise in soluble NKG2D ligands in the PF of endometriosis patients, indicating reduced expression of these ligands on the surface of ectopic endometrial cells[99]. These soluble NKG2D ligands serve as decoy receptors, contributing to increased evasion from NK cell recognition[100]. Notably, several studies have also reported elevated levels of Inhibitory Receptor Tyrosine-based Inhibition Motif-Killer Immunoglobulin-like Receptors, including KIR2DL1, Natural Killer Cell Inhibitory Receptor NKB1, EB6, soluble intracellular adhesion molecule-1, and Human Leukocyte Antigen class I in the PF of endometriosis patients[101-104].
Thus, the immune dysregulation associated with endometriosis involves intricate interactions between NK cells, various immune cells, and cytokines, ultimately impacting NK cell function and contributing to the development and persistence of this condition.
In the context of endometriosis, there is a notable reduction in the Th1/Th2 cell ratio within the peritoneal fluid (PF) when compared to women with a healthy condition[105]. This shift is accompanied by increased concentrations of IFN-γ and IL-10, resulting in elevated IL-4/IFN-γ, IL-4/IL-2, IL-10/IFN-γ, and IL-10/IL-2 ratios within endometriotic lesions[106]. Furthermore, individuals with endometriosis show a substantial reduction in the T-bet/GATA-3 protein ratio compared to their healthy counterparts[107].
To our current understanding, T-bet regulates the expression of the Th1-specific cytokine IFN-γ while inhibiting the production of the Th2-specific cytokine IL-4[108-110]. Conversely, GATA-3, a transcription factor specific to Th2 differentiation, orchestrates the differentiation of Th2 cells and promotes the production of Th2 cytokines, including IL-4, IL-6, and IL-10[111-113]. Within endometriotic lesions, there is a significant upregulation of GATA-3 protein mRNA levels influenced by estrogen, a hormone central to GATA-3 regulation[107]. Consequently, the interplay between GATA-3 and estrogen signaling governs the production of Th2-type cytokines in affected endometrial cells[114]. This dynamic contributes to the elevated levels of Th2-type cytokines in endometriotic lesions—despite the increase in IFN-γ concentrations—ultimately promoting the progression of endometriosis.
Recent research conducted by Xia et al[115] underscores the diagnostic significance of serum cytokine concentrations in the context of endometriosis-associated pelvic pain (EAPP). Specifically, the study identifies IFN-γ and IL-2 as independent protective factors against EAPP, while recognizing IL-4 and IL-10 as independent risk factors for the condition[115]. Notably, IL-4, a hallmark cytokine associated with the Th2 immune response, is shown to elevate localized estrogen levels, thereby facilitating the estrogen-dependent progression of endometriosis[116]. Furthermore, this cytokine enhances the proliferation of endometriotic stromal cells through the activation of pathways such as p38 MAPK, stress-activated protein kinase/c-Jun kinase, and p42/44 MAPK, thereby leading to the advancement of the disease[117].
Apart from Th2 cells, T-helper-17 (Th17) cells and Regulatory T (Treg) cells may also be involved in endometriosis[118]. Khan et al[118] recently demonstrated that CD4+IL-17A+ Th17 cell percentage was consistently reduced in both peripheral blood and PF of individuals with early and advanced endometriosis. In contrast, Gogacz and colleagues reported an elevated proportion of Th17 cells in PF when compared to peripheral blood in individuals with endometriosis[119]. Their findings further indicated that the percentage of Th17 cells in PF was associated with the severity of endometriosis[119].
Zhang et al[120] pioneered the empirical validation of elevated IL-17 levels in the PF of individuals with endometriosis. Their research provided substantial evidence of statistically significant increases in IL-17 concentrations in individuals with minimal/mild endometriosis compared to those with moderate/severe disease and healthy individuals[120]. Subsequent to their groundbreaking work, multiple other authors have also confirmed elevated IL-17 levels in the PF of women diagnosed with this condition[121].
Interleukin-17A exhibits the capability to induce the secretion of IL-8 and the upregulation of cyclooxygenase-2 (COX-2) expression, thereby instigating inflammatory reactions and fostering the proliferation of stromal cells associated with endometriosis[122]. In a similar vein, the research conducted by Ahn et al[79] has provided evidence that IL-17A also contributes to the pathogenesis of endometriosis by triggering the expression of angiogenic factors such as VEGF and IL-8, as well as proinflammatory cytokines including IL-6 and IL-1β, along with chemotactic cytokines such as granulocyte colony-stimulating factor, C-X-C motif chemokine ligand 12 (CXCL12), C-X-C motif chemokine ligand 1 (CXCL1), and C-X3-C motif chemokine ligand 1[79].
Subsequently, it was also observed that the presence of IL-10+Th17 cells significantly rises in the PF of females suffering from endometriosis[123]. Additionally, there is an upregulation of IL-27, IL-6, and TGF-β in this context. In comparison to peripheral CD4+ T cells, endometrial CD4+ T cells exhibit a pronounced expression of IL-27 receptors, particularly in the ectopic endometrium. Apparently, in later stages endometriosis, IL-27 seems to plays a role in suppressing the development of Th17 cells while stimulating the production of IL-10 within these cells through the c-Maf/RORC/Blimp-1 complex—thereby contributing to the establishment of an immune tolerance pattern[123]. Consequently, these Th17 cells, which produce IL-10, enhance the growth, adhesion, invasion, and deep infiltration of endometrial stromal cells, thereby hastening the progression of endometriosis[123,124].
In contrast to the CD4+IL-17A+Th17 cell subset, there is a substantial increase in the proportions of CD25+FOXP3+ Treg cells within the CD4+ T-cell population among patients with advanced endometriosis, as opposed to those with early-stage endometriosis or control subjects (P < 0.05 in both instances)[118]. The induction of Treg cells, characterized by the expression of the transcription factor FOXP3, may be facilitated by specific cytokines, notably TGF-β and IL-10[125]. Consistent with these findings, heightened levels of TGF-β and IL-10 have been consistently documented in the PF of individuals afflicted with endometriosis[90,126]. Notwithstanding this, endometriosis is correlated with elevated PF concentrations of numerous cytokines, encompassing various chemotactic and activatory factors, such as RANTES and MCP-1, known as robust chemoattractants for Treg cells[127,128]. Hence, the heightened prevalence of the CD4+ T cell phenotype may arise from either local stimulation or represent a secondary occurrence associated with their chemotactic response due to the sustained presence of a local inflammatory response[118].
It is hypothesized that the abundance of Treg cells within the peritoneal cavity hinders the recognition and selective targeting of ectopic endometrial tissues—thereby contributing to the persistence of ectopic lesions.
Notably, the role of B cells in endometriosis is an area of ongoing research, and the exact mechanisms underlying their dysregulation in the disease are not fully understood. One intriguing finding is the decreased B-cell leukemia lymphoma (Bcl)-6 and increased B lymphocyte inducer of maturation program (Blimp)-1—transcription factors that regulate B-cell function—in the peritoneal cavity of patients with endometriosis[129].
Blimp-1 serves as a pivotal regulator of plasma cell differentiation[130,131]. The pronounced elevation of Blimp-1 in individuals suffering from endometriosis implies a heightened commitment to the differentiation of B cells into plasma cells. This observation raises the intriguing possibility of an intensified antibody response occurring within the peritoneal cavities of these patients, potentially bearing significance for their immune function. Conversely, Bcl-6 functions as an antagonist to Blimp-1, primarily inhibiting the process of plasma cell differentiation[132]. The diminished levels of Bcl-6 in endometriosis patients suggest a compromised ability to regulate the differentiation of B cells into plasma cells. This imbalance may contribute to an exaggerated antibody response or potentially exert influence over other facets of immune function.
The PF of individuals with endometriosis also seems to express high levels of the B lymphocyte stimulator (BLys)[133]—a protein that plays a critical role in the development of B cells and their differentiation into plasma cells[134]. Increased BLys levels in endometriotic lesions, in turn, suggest that the local microenvironment within the peritoneal cavity of endometriosis patients may be conducive to enhanced B-cell activation and maturation.
Accordingly, the presence of autoantibody responses targeting endometrial antigens represents a prevalent characteristic in endometriosis. In 1980, Startseva[135] was the first to report an elevated responsiveness of B cells in individuals with endometriosis. Since then, antibody responses directed against a range of both serum and tissue antigens, including alpha(2)-Heremans Schmidt glycoprotein (alpha(2)-HSG), transferrin, and carbonic anhydrase, have been discerned in this condition[136]. Nevertheless, additional research is required to gain a more comprehensive understanding of the connection between autoantibodies and the disease's onset and progression.
The development of endometriotic lesions relies on estradiol—an estrogenic steroid hormone[137]. The heightened activity within the 17β-estradiol axis serves as a pivotal trigger for the activation of macrophages intricately associated with endometriosis pathogenesis[138-141]. In response to the escalated signaling of estradiol, the ectopic endometrial tissue, a hallmark feature of endometriosis, undergoes a noteworthy upregulation in the expression of ERβ. Indeed, higher ERβ levels, as opposed to ERα, have been observed in endometriotic tissues when compared to normal endometrial tissues[142]. An elevated ERβ-to-ERα ratio within endometriotic stromal cells is linked to the downregulation of progesterone receptors and an upsurge in cyclo-oxygenase-2 Levels, thereby playing a role in the development of progesterone resistance and inflammation[143,144].
Moreover, elevated prostaglandin levels hinder the immune system, enabling ectopic endometrial cells to evade immune surveillance and form endometriotic lesions. Additionally, ERβ engages in interactions with cytoplasmic inflammasome components and TNF-α-mediated programmed cell death pathways, resulting in increased production of IL-1β and enhanced cellular adhesion and proliferation[58]. This intricate modulation ultimately creates a cellular environment favoring enhanced cell survival and the sustained orchestration of the inflammatory response, both of which are pivotal factors in the perpetuation of endometriosis.
The correlation between chronic inflammation and development of tumors is not a unique feature of ovarian cancer. It has been described for many years, as various risk factors of cancer development are linked to inflammatory processes, such as viral infections, smoking and UV exposure[145]. The process of ovarian carcinogenesis is attributed to multiple factors, and while inflammation does not account for all of them, it serves as a pivotal element in the development of this particular disease[146]. Firstly, despite ovulation being a physiological process, multiple factors that alter the ovulation cycle, such as contraceptive pills, parity and age of menarche and menopause are related to a reduced risk of ovarian cancer development[147]. Fathalla proposed, in 1971, the theory of incessant ovulation, suggesting that the repetitive damage and subsequent repair of the ovarian epithelium may elevate the risk of neoplastic development and be the reason for the above-mentioned risk factors[147].
Presently, it is well-established that ovulation is closely connected to the inflammatory cascade, as the ovarian population of immune cells play critical roles in various processes within the menstrual cycle. As an example, ovarian macrophages contribute to tissue repair and proliferation through the secretion of several growth factors, TGF-β and IL-10, as well as apoptosis via the secretion of Reactive Oxygen Species (ROS), and IL-1β during physiological destruction, resulting in the necessary rupture of the follicle wall for ovum liberation and remodeling processes in the ovarian epithelium associated with the menstrual cycle phases[148].
The result of this is a chronic and periodic exposition of ovarian epithelial cells to a complex and dysregulated interplay of molecular events, involving both inflammation and tissue proliferation stimuli. These events encompass the nuclear factor-kappa B (NF-κB) activation, which has been reported to be an important element in tumorigenesis and further fueling the inflammatory milieu, as it enhances cytokine and growth factors production, induces cell proliferation and impedes cell apoptosis[149-151]. It is also important to note that the high levels of ROS may induce DNA damage that can facilitate the development of mutations that could induce the ovarian carcinogenesis process[152]. Ultimately, the complicated network of interacting events offers insights into its plausible involvement in instigating the mechanisms underpinning ovarian carcinogenesis.
Furthermore, various inflammatory conditions, including infections and reproductive system disorders, have been identified as risk factors for the development of ovarian cancer. Lin and colleagues, in 2011, found that women with Pelvic Inflammatory Disease exhibited an adjusted Hazard Ratio for ovarian tumor development almost twice as high as non-affected women[145]. Additionally, in 2012, a combination of results from 13 case-control studies demonstrated a significant association between the presence of clear-cell, low-grade serous, and endometrioid invasive ovarian cancers and a history of endometriosis among patients[19].
A determining factor in how ovarian cancer will progress, is the individual aspects of the tumor microenvironment. Increased number of Tumor infiltrating lymphocytes (TILs) with active CD3+ T cells have been associated with increased survival rate in patients with ovarian cancer[153,154]. On the other hand, the anti-tumor action of these cells can be rendered less effective by Tumor-infiltrating immune cells with immunosuppressive activity, such as M2 macrophages, regulatory T cells and Myeloid-derived Suppressive Cells, whose increased presence have been consistently related with poor prognosis of the disease[155,156].
Previous studies have evaluated the specific action of live Treg cells in this scenario. Initially, production of CCL22 chemokine by cancerous cells and macrophages attracts CCR4 expressing Treg cells to tumor site. After reaching the tumor microenvironment, these cells highly express several immunosuppressive cytokines, such as IL-10, IL-35, and TGF-β[157,158] and immune checkpoint inhibitors (CPI), such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), that binds to CD80/CD86 in Antigen presenting cells (APC), decreasing co-stimulation of T cells and inhibiting their activity[157]. In summary, the interplay among all of these factors underscores a multifaceted tumor microenvironment that hinders immune surveillance and the fight against malignant cells.
Another important molecule for the immunomodulator properties of ovarian cancer microenvironment is T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), associated with higher IL-10 production and inhibition of T cell action in multiple tumors[158]. Currently, it is being tested in preclinical settings as a possible target for monoclonal antibodies in cancer treatment in association with PD-1/PD-L1 inhibitors[159,160].
It is important to note that proinflammatory cytokines, such as IL-6 and TNF-α, can also exhibit high expression levels within the context of ovarian cancer[161]. The correlation between IL-6 and the tumor's progression is well-established, and it is believed to contribute to various functions, primarily through the JAK/STAT pathway, including angiogenesis, cell proliferation, differentiation, and resistance to chemotherapy[162]. This could explain why the overexpression of the IL-6 receptor (IL-6R) in ovarian tissue has been linked to a poor prognosis for the disease[163]. Regarding M2 macrophages, in addition to playing an immunosuppressive role, they can induce angiogenesis and fibroblast proliferation by secreting various growth factors that promote the replication of cancer cells[164]. This is particularly significant in the clinical context of ovarian cancer, as vasculogenesis driven by VEGF secretion is the primary target of bevacizumab, one of the immunotherapy drugs currently used in the treatment of this condition[165].
Another type of CPI is PD-1, whose ligands PD-L1 and PD-L2 (both members of B7 superfamily) can be expressed by both immune and cancerous cells in the tumor microenvironment. When the PD-1 receptor of T cells binds to its ligands, it causes a reduction in proliferation, cell activity, IFN-γ and IL-2 production, and may even induce apoptosis of these cells[157,166,167]. Given the pressing need for additional therapeutic targets to enhance outcomes in combined ovarian cancer treatment, current research focuses on investigating the potential roles of other B7 family members, specifically B7-H3 (CD276) and B7-H4 (B7x) in TME immune suppression, as their expression relate to poor survival rates and treatment resistance[168-170].
Research suggests that B7-H3 and B7-H4 exhibit distinct expression patterns within the ovarian cancer microenvironment, as B7-H3 has been shown to be present both in stromal and tumor cells in Epithelial Ovarian Cancer TME, whereas B7-H4 seems to be primarily restricted to tumor cells within the ovarian cancer[171]. Furthermore, the expression of B7-H4 by Tumor-Associated Macrophages is induced by IL-6 and IL-10 and B7-H4 expressing TAMs in the ovarian cancer microenvironment exhibit an enhanced suppressive effect on T cell responses[172]. B7-H4 is believed to trigger cell cycle arrest in T lymphocytes, leading to the inhibition of cell division and proliferation.
Additionally, it has been linked to reduced cytokine production and a decrease in the cytotoxic capabilities of these immune cells[173]. On the other hand, B7-H3 has a complex and conflicting role in the immune response, as it may function as both a co-stimulatory and immunoregulatory molecule. This dual role could explain why a study discovered a positive correlation between B7-H3 expression, CD8+ cell infiltration in the TME, and improved prognosis for patients with pancreatic cancer[174]. However, in most neoplasms, including ovarian cancer, high B7-H3 expression doesn't appear to be associated with a favorable prognosis. Instead, it is correlated with increased therapy resistance and enhanced proliferation of cancerous cells, both in vitro and in vivo[170].
B7-H3 may be expressed in Antigen-Presenting Cells, and the knockout of its expression has been shown to induce enhanced cytolytic activity in tumor antigen-specific CD8+ cells among Tumor-Infiltrating Lymphocytes of mice, resulting from a higher production of IFN-γ and Granzyme B enzymes[175]. It is important to note, however, that the precise mechanisms and functions of B7-H3 and B7-H4 in immune cell activity are still areas of active research. Despite this, these proteins offer potential avenues for future tailored immunotherapies that can improve outcomes in ovarian cancer treatment.
The presence of endometriosis leads to an increased risk of malignant tumors, and it is a well-documented finding that both pathologies appear together[176]. Suggesting some kind of transformation from endometriosis constituents into tumor cells[177]. Current molecular studies aim to establish links between endometriosis and EAOCs through pathways related to oxidative stress, inflammation, and hyperestrogenism[178]. Several researches have indicated that atypical endometriosis precedes clear cell or endometrioid ovarian cancers[31,32,179-181], suggesting a precancerous behavior[182]. A study conducted by Kato et al[183] concluded that certain epithelial cells in ovarian endometriosis, including atypical endometriosis and endometriosis - with many inflammatory and regenerative changes - have already acquired a clear cell phenotype.
Thinking about the pathway through endometriosis to derived ovarian cancer, the first thing to point out is the common component of both pathologies, the inflammatory pattern and immune system mobilization[11]. Those alterations can lead to a disruption and tumor formation. Endometriosis patients may have an inflammation profile similar to those with EAOC, even present in patients with benign lesions[184], suggesting that tumor-like immune signatures may develop even earlier than imagined. Deep infiltrating endometriosis with infrequent occurrences exhibits a tumor-like behavior, and may even resemble a metastatic disease[185,186].
Suryawanshi et al[184], in 2014, demonstrated a correlation between upregulation of the complement pathway and the KRAS and PTEN-regulated pathways, those two frequently related in oncogenesis and maintenance of the cancer phenotype in vitro. Also, this study showed that 33% of the patients with endometriosis revealed a tumor-like inflammation. Complement was previously linked with the support of tumor growth, being engaged in both chronic and acute inflammation[187,188].
It is crucial to comprehend the onset of the lesion and its connection to the inflammatory component. The development of endometriosis initiates with the implantation of ectopic tissue, which leads to bleeding. This is followed by inflammation, which triggers fibrin deposition and adhesion formation, eventually leading to scarring and distortion of the affected surfaces[189]. It has been reported that eutopic endometrium has a significant decrease in apoptosis compared to women without endometriosis[190]. The inflammatory process of endometriosis is strongly correlated with the peritoneal space experiencing high levels of oxidative stress, this leads to the proliferation of endometriosis as well as increased angiogenesis[191].
Increased proliferation of endometrial tissue and the occurrence of retrograde menstruation result in elevated levels of hemoglobin, heme, and iron[192,193]. Intense hemolysis observed in endometriosis results in high levels of free heme and iron; these molecules have a prominent effect as proinflammatory factors[193]. Excessive exposure to iron in the context of endometriosis sustains a state of chronic inflammation, modulates several mechanisms for the progression of endometrial lesions, and generates intracellular reactive oxygen species, as well as activating neutrophil responses[194,195].
These substances modify crucial structures, enhance adhesion of refluxed endometrial cells, result in cell damage and DNA methylation, and consequently lead to the development of fibrosis and progression of endometriosis[189,193,196]. Subsequent transcription activation occurs (NF-kB, AP-1, and SP-1), along with oxidative burst, production of ROS, and IL-8[197-199]. Iron overload worsens the activation of peritoneal macrophages. And help to maintain a state of chronic inflammation. The inflammatory is further accentuated by the increased expression and activity of COX- 2, interleukins, and oxidative stress that act through the MAPK pathways[190].
The peritoneal fluid in cases of endometriosis typically has elevated levels of activated cytokines and macrophages[200,201]. Macrophage activation is implicated in the pathogenesis of endometriosis and its association with ovarian cancer. Primarily due to the trophic factors secreted by macrophages that promote the growth of neoplastic lesions while at the same time increasing the conditions of oxidative stress due to the production of lipid peroxides[202,203]. Macrophage proliferation can alter the immune response at the site of inflammation, M2 phenotype molecules attract additional proinflammatory mediators to the lesion site, amplifying the inflammatory microenvironment[204].
Tumor-associated macrophages (TAMS) are a key component of the tumor stroma, essential for angiogenesis and matrix remodeling[189], they spontaneously release large amounts of IL-10 to TGF47, and some chemokines induce IL-10 in macrophages and the monocyte chemotactic protein-1 polarizes immunity in the Th2 direction[205,206].
Other than that, macrophages secrete many products such as TGF-beta, VEGF, IL-1, Prostaglandin E2 (PGE2) and macrophage migration inhibitory factor (MIF)[35]. Importantly, MIF sustains macrophage viability, which sustains inflammation through TAMS activation, and leads to tumor progression and the development of metastases[207]. Also, MIF upregulates COX-2 synthesis and PGE2 secretion in ectopic endometrial cells[208].
In a synergistic manner, IL-1 is associated with the induction of COX-2 and IL-8 expression, which facilitate migration, proliferation, and angiogenesis in endometriotic tissue[209]. And as a cytokine that promotes tumor growth, IL-1 triggers an ongoing chemical dialogue between the progressing tumor and its supportive stroma[210]. In the ovary, COX-2 is involved in the early events of neoplastic transformation; it is rarely found in normal ovarian epithelium, but is present in endometriosis and in ovarian cysts considered to be premalignant[211].
In addition, other cytokines and chemokines are present in increased concentrations, on both ovarian CA and endometriosis. These include TNF-α, IL-1β, IL-6, IL-8 and RANTES. The proinflammatory cytokines TNF-α and IL-1β are elevated in the peritoneal fluid of women with endometriosis[201]. IL-1β induces expression of RANTES more in endometriotic stromal cells than in normal endometrial stromal cells, functioning as a chemoattractant for monocytes, memory T cells, and eosinophils[193,201]. TNF also can be found in malignant and/or stromal cells in human ovarian and the expression of TNF-α is increased on clear cell ovarian carcinoma, when compared with normal ovarian tissue[205,211]. Elevated TNF network gene expression resulted in increased signaling related to angiogenesis, cell adhesion, cell cycle and inflammation[211]. IL-8 increases ERα activity to induce ovarian cancer cell proliferation, it acts as an autocrine growth factor to promote proliferation of endometrial stromal cells in normal endometrioma and endometriotic cells.
Endometrial fragments are able to adhere to surfaces due to the presence of molecules that regulate cell-matrix and cell-cell interactions expressed by endometrial cells. These molecules include cadherins, integrins, proteoglycans such as the immunoglobulin superfamily, and CD44[212]. Cell-cell and cell-matrix adhesion molecules are engaged both in cancer tumors and endometriosis[213]. Endometriosis is reported to have an overall highly variable and aberrant integrin expression as compared with eutopic endometrium[190,214-216]. Endometrioid tissue may share molecular mechanisms of invasion and metastasis with carcinoma cells that are related to the level of E-cadherin expression[213,216].
The failure to remove fragments of menstrual effluent from the abdominal cavity induces excessive local inflammation[217,218]. The chronic aberrant expression of proinflammatory cytokines alters regulatory signaling pathways, which may facilitate cancer growth, invasion and metastasis through DNA damage and inhibition of DNA repair via reactive oxygen, autocrine/paracrine growth, survival factors for malignant cells, induction of vascular permeability and extravasation of fibrin/fibronectin[205]. And, result in accumulation of genetic mutations in endometriotic cells, through the changing of physiological homeostasis and progressive transcriptional changes can drive sustained proliferation and increase the rate of DNA repair[189].
Another factor that has an imperative role on disruption of homeostasis, and inflammation, is the hormonal component. As previously mentioned, hormonal management, upregulation of estrogen and intolerance to progesterone, plays a fundamental role in the maintenance and development of endometriosis, as well as on the tumor development. This said, an important alteration is the estrogen role on endometriosis, and its intense presence on peritoneum of afflicted women, and the consequently exacerbation of the immune inflammatory pathway.
Endometriotic stromal cells contain numerous specific epigenetic defects that favor overproduction of E2 and overexpression of the steroid receptor ER-beta that mediates an intense and E2-induced inflammatory process involving overproduction of cytokines and prostaglandins[219-221]. Being a major regulator of all key pathological processes in endometriosis and enhances lesion survival and inflammation leading to pain[220]. And excess E2 can result in cellular proliferation through the stimulation of cytokine production, specifically IL-8 and RANTES[222]. In addition, E2 stimulates the production of PGE2, the micro-environment in endometriotic tissue is marked by proliferative pressure with an enhanced level of reparative activity and thus, a higher chance for DNA damage and mutations[178]. Thus, PGE2 is a central mediator of the inflammatory response on endometriosis, but it has also been shown to regulate vital processes related to tumor growth, including angiogenesis, proliferation and inhibition of apoptosis[223].
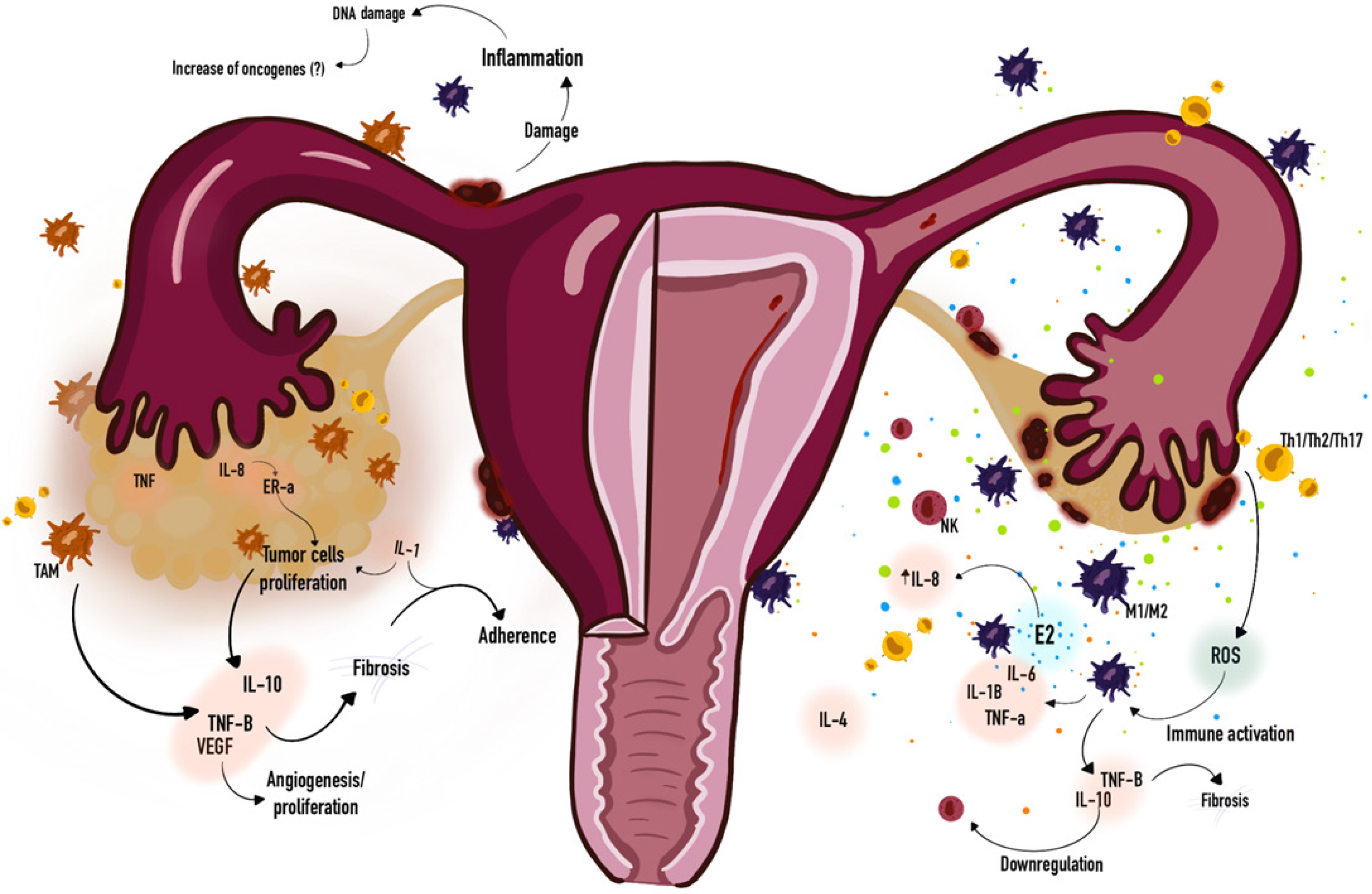
Moreover, massive concentrations of estrogen in the ovary may also stimulate the inflammatory process via ER-beta in endometriotic stromal cells, which may contribute to the carcinogenic process in neighboring epithelial cells[58,224]. Is speculated that intense inflammation, progesterone resistance, and high levels of E2 (unopposed by progesterone action) in the stromal component led to a high proliferative activity and enrichment of driver mutations (e.g., PIK3CA, KRAS, ARID1A) in attached endometriotic epithelial cells[68]. And it is associated with pro-inflammatory cytokines, which leads to (VEGF) expression, cell cycle activation, and activation of the anti-apoptotic gene Bcl-2[225,226]. Figure 2 depicts a simplified schematic illustrating of general immune response on endometriosis and ovarian cancer, and their similarities.
The dysregulation of apoptotic pathways and subsequent resistance to apoptosis contribute to the failure of immune clearance[45,227]. Accumulation of mutations in tumor suppressor genes and oncogenes is a crucial step during tumor development[228]. It is known that hormonal dysregulation in endometriotic implants, along with Inflammatory responses, may drive carcinogenesis[71]. Mutations secondary to endometriosis, is a fair finding. A study conducted by Koppolu et al[185], in 2021, showed that all the patients with endometriosis recruited on the study had no history or features of neoplastic disease, however the results revealed mutations in known cancer driver genes, especially in ectopic lesions. Some cancer-related mutations are found in endometriosis without cancer, in particular recurrent KRAS mutations[229]. Otherwise, some studies have shown confirmatory evidence that mutations found in endometriosis-associated cancers are found in adjacent endometriosis[36,230-232], and has been reported to exhibit a high percentage of PIK3CA and KRAS activating mutations and ARID1A and PTEN inactivating mutations[232,233]. A study conducted in 2023, with mice, was capable of successfully developed carcinoma by inducing the knockout (KO) of ARID1A and PTEN in the epithelium of endometriotic cysts, which were formed by the transplantation of small uterine pieces onto the peritoneum or ovarian surface, having a EAOC developed as early as 4 wk after the KO[232]. Helping to consolidate and bring up more data surrounding the intrinsic correlation between the dysfunctions caused by endometriosis and the onset of ovarian cancer.
Unlike the traditional strategies of killing tumor cells, immunotherapy is a treatment approach that utilizes cells, viruses, peptides, small molecules, or antibodies to activate or modulate the immune system to attack cancer cells[234]. This treatment has brought about a significant transformation in the approach of various solid tumors, including malignant melanoma, non-small-cell lung cancer, and renal cell carcinoma. It has become the leading choice for managing recurrent or metastatic solid tumors, surpassing conventional chemotherapy and targeted therapy[235].
Over the past few decades, immunotherapy has surfaced as a hopeful treatment alternative for gynecologic malig
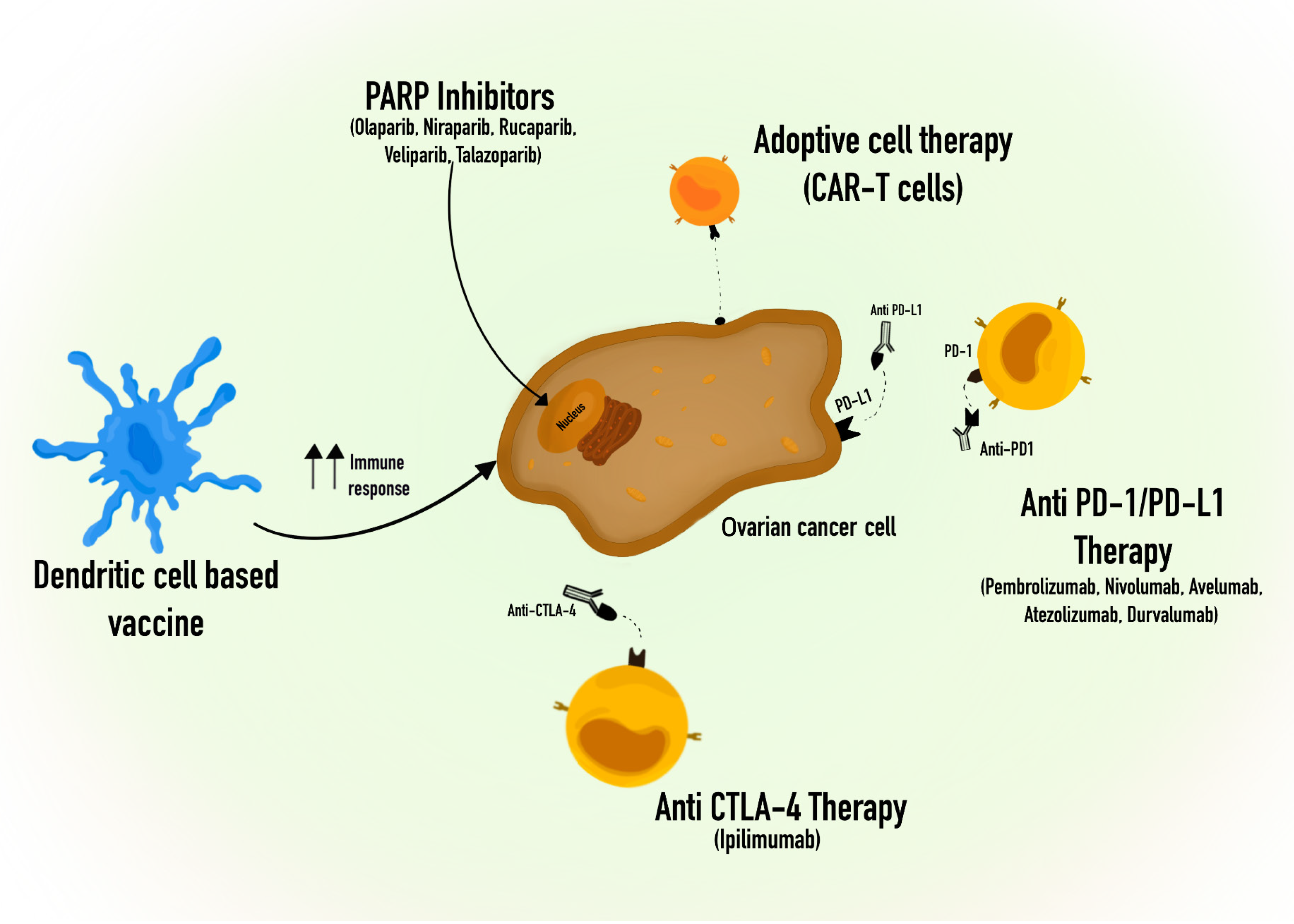
Effective immunotherapy for ovarian cancer hinges on activating antigen-presenting cells, reducing the immunosuppressive microenvironment, and enhancing the performance of effector T cells. The T cell-mediated immune response is controlled through both inhibitory and activating signals, and immune checkpoint receptors play a crucial role in restraining T cell activation to prevent excessive stimulation. Nonetheless, numerous types of tumors exhibit immune checkpoint expression, which results in immune evasion. Consequently, inhibitors targeting immune checkpoints play a significant role in immunotherapy[238]. Up until now, the most promising immune checkpoint inhibitors for solid tumors have been antibodies that hinder CTLA4, PD-1 and PD-L1, which are presented in some drugs approved by The Food and Drug Administration, such as CTLA4 antibodies (Ipilimumab), PD-1 antibodies (Pembrolizumab and Nivolumab), and PD-L1 antibodies (Avelumab, Atezolizumab and Durvalumab)[239,240]. However, the clinical application of checkpoint inhibitors in ovarian cancer has yielded limited success, with single-agent response rates in clinical trials typically hovering around 6%-15%[241-243]. As single agents, the results in ongoing clinical trials showed modest effects of immune checkpoint inhibitors (ICI) in ovarian cancer, limiting its approval for use in patients with ovarian cancer[244].
Another potential target of immune checkpoint blockade is B7-H3, which is an immunosuppressive molecule present on tumor cells but absent in host cells, and researchers have explored the therapeutic impacts of blocking B7-H3 and PD-1 in the context of cancer[238]. The results indicate that, in ID8 tumor-bearing mice with ovarian cancer, it is B7-H3 inhibition, not PD-1 blockade, that prolongs the median survival time[245].
Immune checkpoint inhibitors monotherapy has limited anti-tumor effects in ovarian cancer, and the efficacy of ICI depends on the condition of TILs and the expression of specific molecules. Hence, ideal candidates for this strategy should be well-chosen. To surpass these barriers, combining therapies are being tested to improve the anti-tumor activity in ovarian cancer[246].
The poly (ADP-ribose) polymerase (PARP) is a well-acknowledged detector of DNA damage, renowned for its invol
Adoptive cell therapy (ACT) primarily relates to the utilization of chimeric antigen receptor (CAR)-modified T cells, T-cell receptor (TCR)-engineered T cells, natural tumor-infiltrating lymphocytes (TILs), CAR-NK cells, and CAR-macrophages. ACT has brought about a significant breakthrough in treating blood-related tumors. However, when it comes to solid tumors like ovarian cancer, ACT appears to be inadequate in inducing substantial anti-tumor responses[255]. Up until now, there has not been a notable therapeutic effectiveness. The primary challenges lie in the weak binding affinity and inconsistent presence of targetable surface antigens, as well as obstacles related to the infiltration and viability of CAR-T cells[256]. Therefore, additional clinical data is necessary to verify their effectiveness in individuals with ovarian cancer.
Vaccination plays a crucial role in immunotherapy, offering advantages to individuals affected by diverse forms of cancer, and there is extensive research into therapeutic vaccines in the context of ovarian cancer. The investigation into the potential of single application of a cancer vaccine for ovarian cancer is ongoing, and this includes the examination of various types of vaccines such as peptide vaccines, whole tumor cell vaccines, cancer stem cell vaccines, APC vaccines, DNA/RNA vaccines, bacterial vaccines, and more[255]. Many of these vaccines enhance the body's immune response against ovarian cancer, but clinical evidence has only demonstrated modest effectiveness in the majority of patients[257-259]. It is feasible to assess the therapeutic potential in a broader group of patients. The use of dendritic cell-based vaccines is another treatment approach, which is also under investigation in patients with ovarian cancer, being shown that it can produce improved outcomes[236,246]. However, the evaluation of the clinical use of vaccines in cancer patients has certain limitations, such as the heterogeneity of antigen expression within a tumor[260,261].
The immune system has a significant role in endometriosis. Therefore, immune therapy holds promise as a potential treatment approach for this condition. The reduced macrophagic phagocytosis observed in the serum and peritoneal fluid of individuals with endometriosis is a significant contributor to the disruption of immune balance[262]. In this sense, the expression of CD47 is used by macrophages to distinguish “self” or “non-self” cells. The CD47 site inhibitor disrupts this signal, enabling macrophages to carry out regular phagocytosis[263]. Clinical observations have revealed a substantial elevation in CD47 Levels within the ectopic endometrial tissue of individuals with endometriosis[264]. Immunotherapy targeting the CD47-SIRPa signaling pathway appears to show promise in the management of endometriosis[265].
Furthermore, it has been reported that exosomes originating from endometriosis can induce a shift in macrophage phenotype toward M2 polarization, leading to a reduction in macrophage phagocytic activity both in laboratory settings (in vitro) and within the body (in vivo)[262]. As a result, employing anti-exocrine therapy for individuals with endometriosis appears to have a notable effect on attracting macrophages to ectopic lesions[266]. This therapy can decrease the presence of M2-type macrophages, leading to an overall enhancement in macrophage-mediated phagocytosis of ectopic endometrial cells[98].
Moreover, encouraging NK cell cytotoxic activity is a potential novel therapeutic approach for managing endometriosis[267]. The treatment is based on NK inhibitory receptors that dampen their response to ectopic or malignant cells. The PD1, one such receptor that interacts with the PDL1 Ligand has already shown promise in cancer immunotherapy[268]. This approach aims to potentially enhance the rescue of endometrial cells, counteract the suppression of NK cells' regulatory function, and enable the elimination of misplaced endometrial cells. The proposed immunotherapy strategy suggests utilizing existing medications, commonly employed for conditions like cancer, to aid in identifying and prompting the removal of endometrial cells through apoptosis[269].
Besides this, vitamin D has been noted for its immunomodulatory properties in the medical management of endometriosis[270]. In a recent animal model study focused on endometriosis, it was observed that the use of synthetic vitamin D derivatives significantly curbed the progression of both endometriosis and peritonitis[271]. Considering the role of inflammatory cytokines in the development of endometriosis, recent studies have suggested that the impact of 1,25(OH)2D3 on the cytokine production within human endometrial stromal cells could be a contributing factor to the therapeutic benefits of this compound in treating endometriosis. Additionally, another investigation has shown that 1,25(OH)2D suppresses the immune response of Th1 cells while promoting the response of Th2 cells. It achieves this by inhibiting the release of IL-12, IL-2, and TNF from T cells, macrophages, and DCs, respectively[272].
The direct involvement of tumor-associated macrophages (TAMs) in the oncogenesis and prognosis of ovarian cancer based on the imbalance in polarization between M1 (pro-inflammatory) and M2 (anti-inflammatory) macrophages has been previously established[273]. Thus, it was discovered that the presence of a majority of M2 (anti-inflammatory) macrophages in the ovarian environment is a determining factor for a worse cancer prognosis, through the interaction of specific receptors of these cells with the immune system and also through the stimulation of various cytokines, generating an environment more conducive to the establishment of ovarian cancer of all types, including EAOC[274]. In this way, investigation of new ways of interfering in this process using the specific receptors expressed on M2 macrophages and the cytokines produced can be a new approach in order to achieve better therapeutic results and greater survival[275].
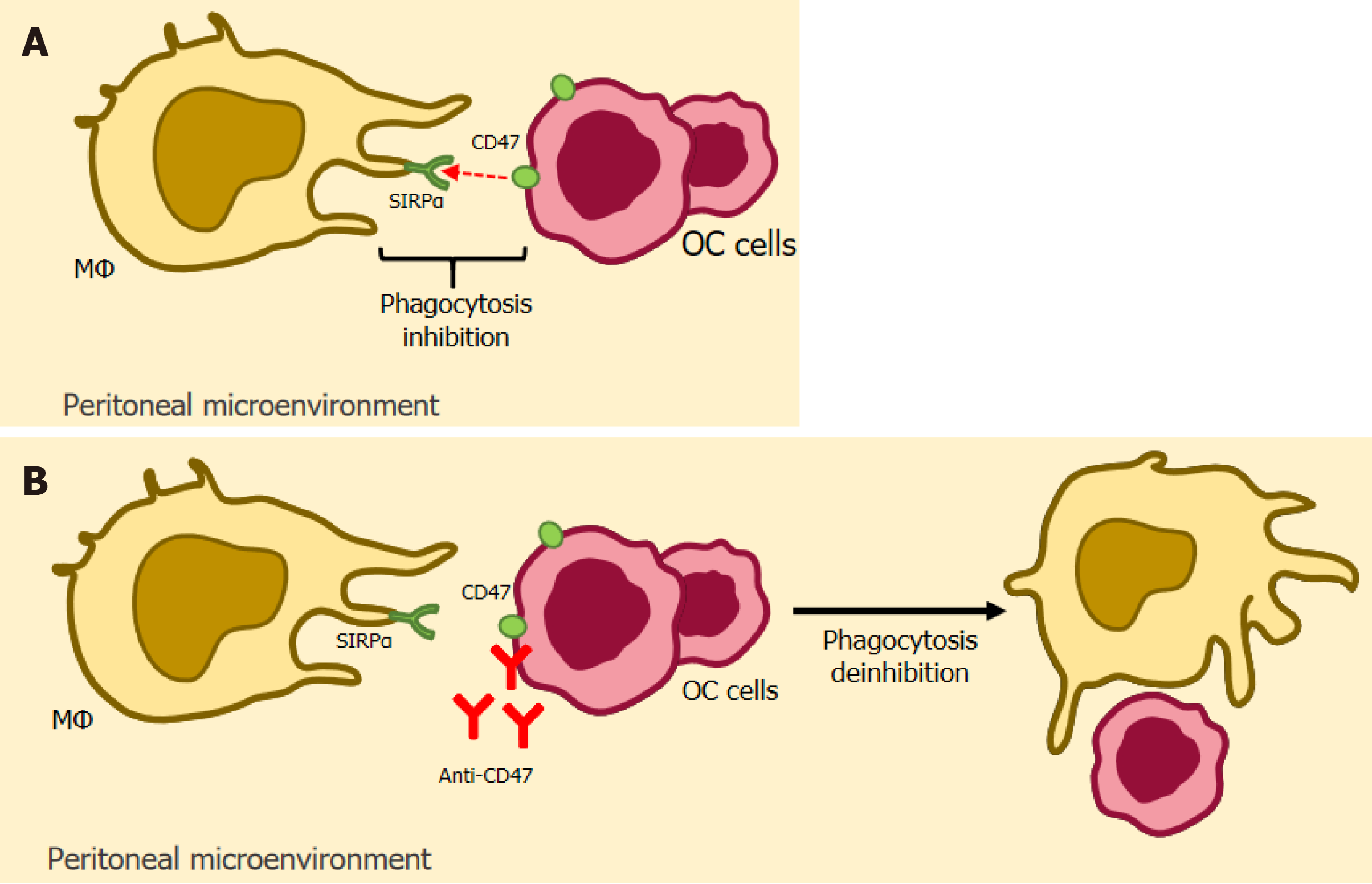
CD47- SIRPα: CD47 is a glycoprotein that is very present in the tumor environment and exerts its inhibitory activity by binding to its counter-receptor, the signal regulatory protein-α (SIRPα), expressed in macrophages[276,277]. Liu et al[277] conclude that the impact of this inhibitory process is a reduction in phagocytosis by these macrophages (known as the "don't eat me signal"), which culminates in the progression of the tumor microenvironment, thus serving as one of the strategies for inhibiting immune activity by tumor cells[277].
Based on this principle, therapies based on blocking CD47 (anti-CD47) and SIRPα have emerged, which aim to interfere with the inhibitory process[278]. Son et al[278] demonstrated that these two therapies are promising in the treatment of solid cancers, such as ovarian cancer, and have shown good progress in terms of improved anti-tumor activity of macrophages based on the established blockade of inhibitory receptors.
Kaur et al[279] reported a series of preclinical studies in mice and humanized clinical studies of anti-CD47 therapy, bringing a positive result, since both the preclinical study and the humanized clinical studies showed promising data in relation to limiting tumor cell growth.
Sikic et al[280] also demonstrated a promising result when they carried out a phase I study in humans using the anti-CD47 antibody Hu5F9-G4, which showed safety, tolerability and also tumor regression in some patients undergoing therapy. It is also important to note that Tian et al[281] were able to demonstrate that anti-CD47 therapy has a promising future in improving the activity of innate immunity and the oncolytic process.
In addition, Li et al[264] also reported a study showing high CD47 expression in patients with endometriosis, in which CD47 blocking treatment resulted in an increase in the phagocytic process by macrophages and also in an increase in apoptosis of endometrial stromal cells, representing a protective effect against the development of EAOC. Figure 4 depicts a simplified schematic illustrating of CD47- SIRP-based immunotherapy.
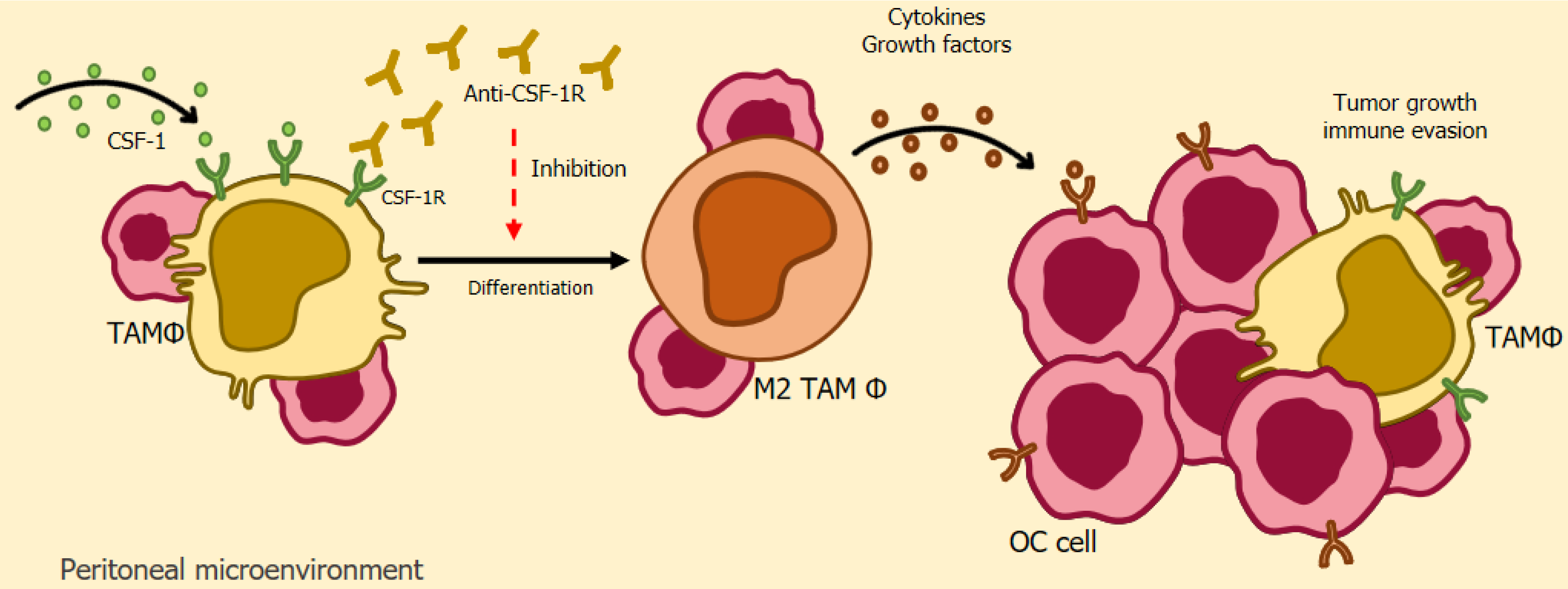
CSF-1/CSF-1R: The colony-stimulating factor-1 receptor (CSF-1R) is a receptor that exists in several human cells during homeostasis, but is overexpressed in tumor-associated macrophages in several types of cancer, including ovarian cancer[282]. The specific ligand of this glycoprotein is CSF-1, which is found in high levels in tumor cells and the binding of these two receptors culminates in an oncogenic role through the release of growth factors and substances that stimulate the cellular differentiation of macrophages into M2[282]. In this way, the relationship between both glycoproteins has become the object of study for the development of new therapeutic techniques, based on the inhibition of these receptors, with the aim of reducing TAMs and tumor growth[283].
Based on this premise, Ries et al[284] used a monoclonal antibody directed at blocking CSF-1R in cancer patients, with the aim of manipulating the activity of TAMs, and the result was promising, since a reduction in tumor-associated macrophages was achieved in patients as well as an increase in the levels of TCD8/CD4 cells in animal models.
Lu et al[285] conducted a study in murine models of ovarian cancer and associated a CSF-1R inhibitor with docetaxel and concluded that treatment with the inhibitor alone resulted in increased cell apoptosis of tumor-associated macrophages due to the repolarization effect, transforming them into M1. Combined treatment resulted in a significant reduction in tumor growth, a reduction in TAMs and increased levels of TCD8+ cells[285].
Finally, Xiaocui et al[286] identified a significant presence of the glycoprotein CSF-1 in patients with endometriosis, which resulted in an elevated appearance of macrophages with an anti-inflammatory effect and, consequently, a depletion of the activity of the immune system in this environment, leaving it vulnerable to the appearance of possible ovarian cancer. This study also found that the use of an anti-CSF-1 antibody reduced these negative effects, demonstrating the promising activity of this immunotherapeutic field[286]. Figure 5 depicts a simplified schematic illustrating of CSF-1/CSF-1R-based immunotherapy.
CCL2/C-C motif chemokine receptor 2: The CCL2/C-C motif chemokine receptor 2 (CCR2) axis is involved in the recruitment of monocytes from the bloodstream to the tumor environment, since CCL2 is a chemokine that attracts CCR2+ monocytes, which will become M2 macrophages when they arrive in the tumor environment[287].
This axis has also become a therapeutic target, as demonstrated by Miyamoto et al[288] who based their study on blocking the CCL2/CCR2 interaction. The study achieved a positive result in mice by reducing M2 macrophages and increasing TCD8+ cells and IFNγ by inhibiting the CCL2/CCR2 relationship[288].
In addition, it is also necessary to highlight the relationship between CCL2 and endometriosis, as reported by Hogg et al[289], who reported that high levels of CCL2 are present in endometriosis, thus resulting in greater recruitment of macrophages that may impact on the inflammatory environment and contribute to the process of malignization of the lesion, generating ovarian cancer.
CXCR4-CXCL12: C-X-C receptor 4 (CXCR4) is a chemokine that functions through its binding to the CXCL12, derived from stromal cells, and the activation of this axis is closely linked to the initiation and progression of ovarian tumors from the invasion of ovarian cancer cells[290,291].
With this in mind, Xue et al[292] used an antagonist of the CXCR4 receptor in order to reduce the impacts derived from its activity and achieved promising results, which are the blocking of the activation of the NF-κB signaling pathway and, consequently, a reduction in tumor growth and a reduction in the possibility of metastasis.
Pharmacological modulation of TAMs: The polarization of macrophages and their influence on the oncogenesis and progression of ovarian cancer has also extended the discussion into the field of pharmacological modulation, with the clear aim of finding a way to revert M2 macrophages into M1, promoting anti-tumour activity and, consequently, regression of the already established tumour[293,294].
In their study, Bolli et al[295] used imidazoquinoline (IMDQ), an agonist of the Toll 7/8 receptor, which was coupled to an antibody and infused intravenously into patients with ovarian cancer with the aim of blocking the macrophage mannose receptor (MMR) and causing a repolarization of macrophages from M2 to M1. The results of the study were promising and showed a reduction in tumor growth, associated with an increase in the pro-inflammatory process derived from the change in the types of macrophages employed in the environment[295].
On the other hand, the study by Xiao et al[296] addressed the development of a nanodrug specific for tumor-associated M2 macrophages, from siRNA IKKβ (an activator of NF-kβ and STAT6, participants in the polarization process), with the clear objective of inhibiting STAT6, thus resulting in a safe conversion of macrophages and the reduction of tumor growth with the activation of the antitumor axis in an in vivo experiment.
Finally, Hsieh et al[297] presented a study based on the use of vorinostat in mice with EAOC, in which the result was a reduction in the tumor from the inhibition of the polarization of M2 macrophages.
COX-2 is an enzyme directly linked to inflammatory processes from arachidonic acid and the consequent production of prostaglandins[298]. COX-2 has been described as an overexpressed marker in ovarian cancer, playing a direct role in the poor prognosis of patients with this type of cancer, since it participates in the migration and invasion of tumor cells[299]. In addition, Zhang et al[300] and Lai et al[301] bring up the direct involvement of COX-2 in endometriosis, since, as found in the study, cyclooxygenase-2 promotes increased cell proliferation, reduced apoptosis and increased angiogenesis, factors that are also linked to oncogenesis.
Thus, studies such as that by Li et al[302] show the importance of investigating the impact of anti-inflammatory drugs on ovarian cancer, since, as reported in this analysis, a positive effect was found when associating a COX-1 inhibitor with Cisplatin or Taxol in order to reduce angiogenesis in ovarian cancer in murine models.
Therefore, the direct involvement of COX-1 and COX-2 in the oncogenesis and progression of ovarian cancer, as well as in the progression of endometriosis, has already been established. Therefore, new clinical studies with a large number of patients using COX inhibitors are still needed in order to establish an even more efficient treatment for ovarian cancer and endometriosis.
Endometriosis and ovarian cancer are still underreported diseases. This means that much involved in their patho
Future studies should aim to better elucidate the roles of oxidative stress, inflammation, and estrogen in EAOC development. Existing evidence suggests a shared microenvironment between endometriosis and EAOC in terms of cytokines and mediators, but further research is necessary to confirm a direct link between the two. Additionally, further research should focus on discovering biomarkers capable of identifying endometriosis cases with oncogenic potential, with the aim of detecting premalignant lesions, and consequently develop better interventions in order to decrease the incidence of EAOCs.
Research efforts should prioritize establishing model systems for endometriosis. These investigations could yield valuable knowledge about risk factors, molecular traits specific to subtypes, novel therapeutic testing, and the factors that contribute to the development of EAOCs. Additionally, it is crucial to emphasize the necessity of current interventions that lower the risk of ovarian cancer, including endometrioid carcinoma and clear cell carcinoma.
Moreover, non-invasive methods to help diagnose the disease should be a priority, as well as clarification of the genetics and genomics that control disease development, environmental contributions, and the involvement of the immune system. Other than that, well-designed clinical trials are essential to determine which therapies are safe and effective, and which markers and targets on the immune system may be useful in treatment and management. And confirmation of the veracity of these biomarkers can help in the development of true research, with larger populations, to understand endometriosis in particular.
| 1. | Moradi Y, Shams-Beyranvand M, Khateri S, Gharahjeh S, Tehrani S, Varse F, Tiyuri A, Najmi Z. A systematic review on the prevalence of endometriosis in women. Indian J Med Res. 2021;154:446-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 97] [Reference Citation Analysis (0)] |
| 2. | Barreta A, Sarian L, Ferracini AC, Eloy L, Brito ABC, de Angelo Andrade L, Derchain S. Endometriosis-Associated Ovarian Cancer: Population Characteristics and Prognosis. Int J Gynecol Cancer. 2018;28:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Fuldeore MJ, Soliman AM. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol Obstet Invest. 2017;82:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT; World Endometriosis Research Foundation Global Study of Women's Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366-373.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1100] [Cited by in RCA: 1042] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 5. | Dawson A, Fernandez ML, Anglesio M, Yong PJ, Carey MS. Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience. 2018;12:803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | McLeod BS, Retzloff MG. Epidemiology of endometriosis: an assessment of risk factors. Clin Obstet Gynecol. 2010;53:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Moga MA, Bălan A, Dimienescu OG, Burtea V, Dragomir RM, Anastasiu CV. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer-An Overview. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Throwba H PK, Unnikrishnan L, Pangath M, Vasudevan K, Jayaraman S, Li M, Iyaswamy A, Palaniyandi K, Gnanasampanthapandian D. The epigenetic correlation among ovarian cancer, endometriosis and PCOS: A review. Crit Rev Oncol Hematol. 2022;180:103852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 9. | Whelan E, Kalliala I, Semertzidou A, Raglan O, Bowden S, Kechagias K, Markozannes G, Cividini S, McNeish I, Marchesi J, MacIntyre D, Bennett P, Tsilidis K, Kyrgiou M. Risk Factors for Ovarian Cancer: An Umbrella Review of the Literature. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The relation between endometriosis and ovarian cancer - a review. Acta Obstet Gynecol Scand. 2014;93:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Králíčková M, Losan P, Vetvicka V. Endometriosis and cancer. Womens Health (Lond). 2014;10:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Archives of Surgery. 1925;10:1-72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 518] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283-289. |
| 14. | Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1399] [Cited by in RCA: 1307] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 15. | Maeda D, Shih IeM. Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms. Adv Anat Pathol. 2013;20:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Borgfeldt C, Andolf E. Cancer risk after hospital discharge diagnosis of benign ovarian cysts and endometriosis. Acta Obstet Gynecol Scand. 2004;83:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Melin A, Sparén P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006;21:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 584] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 19. | Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A; Ovarian Cancer Association Consortium. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 701] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 20. | Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet. 2002;76:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Haas D, Chvatal R, Reichert B, Renner S, Shebl O, Binder H, Wurm P, Oppelt P. Endometriosis: a premenopausal disease? Age pattern in 42,079 patients with endometriosis. Arch Gynecol Obstet. 2012;286:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Gemmell LC, Webster KE, Kirtley S, Vincent K, Zondervan KT, Becker CM. The management of menopause in women with a history of endometriosis: a systematic review. Hum Reprod Update. 2017;23:481-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Giannella L, Marconi C, Di Giuseppe J, Delli Carpini G, Fichera M, Grelloni C, Giuliani L, Montanari M, Insinga S, Ciavattini A. Malignant Transformation of Postmenopausal Endometriosis: A Systematic Review of the Literature. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Lee HJ, Lee B, Choi H, Kim T, Kim Y, Kim YB. Impact of Hormone Replacement Therapy on Risk of Ovarian Cancer in Postmenopausal Women with De Novo Endometriosis or a History of Endometriosis. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 25. | Nap AW, Groothuis PG, Demir AY, Evers JL, Dunselman GA. Pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Bulun SE. Endometriosis. N Engl J Med. 2009;360:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1369] [Cited by in RCA: 1417] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 27. | Gomes VA, Bonocher CM, Rosa-E-Silva JC, de Paz CCP, Ferriani RA, Meola J. The Apoptotic, Angiogenic and Cell Proliferation Genes CD63, S100A6 e GNB2L1 are Altered in Patients with Endometriosis. Rev Bras Ginecol Obstet. 2018;40:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Gourley C. Link between endometriosis and ovarian-cancer subtypes. Lancet Oncol. 2012;13:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Risk of epithelial ovarian cancer in relation to benign ovarian conditions and ovarian surgery. Cancer Causes Control. 2008;19:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Ogawa S, Kaku T, Amada S, Kobayashi H, Hirakawa T, Ariyoshi K, Kamura T, Nakano H. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol. 2000;77:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249-255. [RCA] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 234] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol. 2012;124:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Ñiguez Sevilla I, Machado Linde F, Marín Sánchez MDP, Arense JJ, Torroba A, Nieto Díaz A, Sánchez Ferrer ML. Prognostic importance of atypical endometriosis with architectural hyperplasia vs cytologic atypia in endometriosis-associated ovarian cancer. J Gynecol Oncol. 2019;30:e63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Mikhaleva LM, Davydov AI, Patsap OI, Mikhaylenko EV, Nikolenko VN, Neganova ME, Klochkov SG, Somasundaram SG, Kirkland CE, Aliev G. Malignant Transformation and Associated Biomarkers of Ovarian Endometriosis: A Narrative Review. Adv Ther. 2020;37:2580-2603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih IeM, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1422] [Cited by in RCA: 1332] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 37. | Gilks CB. Molecular abnormalities in ovarian cancer subtypes other than high-grade serous carcinoma. J Oncol. 2010;2010:740968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Singer G, Shih IeM, Truskinovsky A, Umudum H, Kurman RJ. Mutational analysis of K-ras segregates ovarian serous carcinomas into two types: invasive MPSC (low-grade tumor) and conventional serous carcinoma (high-grade tumor). Int J Gynecol Pathol. 2003;22:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Silwal-Pandit L, Langerød A, Børresen-Dale AL. TP53 Mutations in Breast and Ovarian Cancer. Cold Spring Harb Perspect Med. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 40. | Cheasley D, Nigam A, Zethoven M, Hunter S, Etemadmoghadam D, Semple T, Allan P, Carey MS, Fernandez ML, Dawson A, Köbel M, Huntsman DG, Le Page C, Mes-Masson AM, Provencher D, Hacker N, Gao Y, Bowtell D, deFazio A, Gorringe KL, Campbell IG. Genomic analysis of low-grade serous ovarian carcinoma to identify key drivers and therapeutic vulnerabilities. J Pathol. 2021;253:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 41. | Lamceva J, Uljanovs R, Strumfa I. The Main Theories on the Pathogenesis of Endometriosis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 167] [Reference Citation Analysis (0)] |
| 42. | Yovich JL, Rowlands PK, Lingham S, Sillender M, Srinivasan S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod Biomed Online. 2020;40:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 44. | Abramiuk M, Grywalska E, Małkowska P, Sierawska O, Hrynkiewicz R, Niedźwiedzka-Rystwej P. The Role of the Immune System in the Development of Endometriosis. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 45. | Riccio LDGC, Santulli P, Marcellin L, Abrão MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 46. | Paul Dmowski W, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:245-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Carbone G, Nelson K, Baumgartner C, Bode AM, Takahashi A, Chefetz I. Endometriosis: Cell Death and Cell Signaling Machinery. Endocrinology. 2023;164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 48. | Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1447] [Cited by in RCA: 1456] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 49. | Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 411] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 50. | O'Connell J, Bennett MW, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: cancer as a site of immune privilege. Immunol Today. 1999;20:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Sbracia M, Valeri C, Antonini G, Biagiotti G, Pacchiarotti A. Fas and Fas-Ligand in Eutopic and Ectopic Endometrium of Women With Endometriosis: The Possible Immune Privilege of Ectopic Endometrium. Reprod Sci. 2016;23:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Sbracia M, McKinnon B, Scarpellini F, Marconi D, Rossi G, Simmilion C, Mueller MD, Barnea ER, Mueller M. PreImplantation Factor in endometriosis: A potential role in inducing immune privilege for ectopic endometrium. PLoS One. 2017;12:e0184399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Selam B, Kayisli UA, Garcia-Velasco JA, Akbas GE, Arici A. Regulation of fas ligand expression by IL-8 in human endometrium. J Clin Endocrinol Metab. 2002;87:3921-3927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Selam B, Kayisli UA, Akbas GE, Basar M, Arici A. Regulation of FAS ligand expression by chemokine ligand 2 in human endometrial cells. Biol Reprod. 2006;75:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Selam B, Kayisli UA, Garcia-Velasco JA, Arici A. Extracellular matrix-dependent regulation of Fas ligand expression in human endometrial stromal cells. Biol Reprod. 2002;66:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Leavy O. Reproductive immunology: Evading immunosurveillance in endometriosis. Nat Rev Immunol. 2015;15:729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Cho Y, Chun H, Kim S, Koh D, Park J, Park D. Analysis of Eradication Rate of Helicobacter pylori According to Treatment Duration by Using 13C-Urea Breath Test Comparison of OAC 7, 10 or 14 days regimen. Korean J Gastrointest Endosc. 2001;23:207-212. |
| 58. | Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O'Malley BW. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell. 2015;163:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 59. | Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O'Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Meng Z, Wang X, Zhang D, Lan Z, Cai X, Bian C, Zhang J. Steroid receptor coactivator-1: The central intermediator linking multiple signals and functions in the brain and spinal cord. Genes Dis. 2022;9:1281-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 61. | Walsh CA, Qin L, Tien JC, Young LS, Xu J. The function of steroid receptor coactivator-1 in normal tissues and cancer. Int J Biol Sci. 2012;8:470-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, Meijer OC. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci U S A. 2009;106:8038-8042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun. 2018;500:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 64. | Siddiqui NR, Garvey WT, Khaled MA. H. pylori-Induced Higher C-Reactive Protein in Obese African Americans. Artery Res. 2009;3:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Goumenou A, Panayiotides I, Matalliotakis I, Vlachonikolis I, Tzardi M, Koumantakis E. Bcl-2 and Bax expression in human endometriotic and adenomyotic tissues. Eur J Obstet Gynecol Reprod Biol. 2001;99:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000;74:760-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Bilotas M, Barañao RI, Buquet R, Sueldo C, Tesone M, Meresman G. Effect of GnRH analogues on apoptosis and expression of Bcl-2, Bax, Fas and FasL proteins in endometrial epithelial cell cultures from patients with endometriosis and controls. Hum Reprod. 2007;22:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Bora G, Yaba A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J Obstet Gynaecol Res. 2021;47:1610-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 69. | Wu XG, Chen JJ, Zhou HL, Wu Y, Lin F, Shi J, Wu HZ, Xiao HQ, Wang W. Identification and Validation of the Signatures of Infiltrating Immune Cells in the Eutopic Endometrium Endometria of Women With Endometriosis. Front Immunol. 2021;12:671201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 70. | Giacomini E, Minetto S, Li Piani L, Pagliardini L, Somigliana E, Viganò P. Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 71. | Kubicka U, Olszewski WL, Tarnowski W, Bielecki K, Ziółkowska A, Wierzbicki Z. Normal human immune peritoneal cells: subpopulations and functional characteristics. Scand J Immunol. 1996;44:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 73. | Cao X, Yang D, Song M, Murphy A, Parthasarathy S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: implications for endometriosis. Fertil Steril. 2004;82 Suppl 3:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Budrys NM, Nair HB, Liu YG, Kirma NB, Binkley PA, Kumar S, Schenken RS, Tekmal RR. Increased expression of macrophage colony-stimulating factor and its receptor in patients with endometriosis. Fertil Steril. 2012;97:1129-35.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Müller KH, Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 349] [Article Influence: 11.6] [Reference Citation Analysis (8)] |
| 76. | Yang HL, Zhou WJ, Chang KK, Mei J, Huang LQ, Wang MY, Meng Y, Ha SY, Li DJ, Li MQ. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction. 2017;154:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 77. | Suen JL, Chang Y, Chiu PR, Hsieh TH, Hsi E, Chen YC, Chen YF, Tsai EM. Serum level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am J Pathol. 2014;184:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 79. | Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J Immunol. 2015;195:2591-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 80. | Miller JE, Ahn SH, Marks RM, Monsanto SP, Fazleabas AT, Koti M, Tayade C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front Immunol. 2020;11:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 81. | Chen S, Liu Y, Zhong Z, Wei C, Zhu X. Peritoneal immune microenvironment of endometriosis: Role and therapeutic perspectives. Front Immunol. 2023;14:1134663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 82. | Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 961] [Article Influence: 64.1] [Reference Citation Analysis (6)] |
| 83. | Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337-4345. [PubMed] |
| 84. | Barcz E, Milewski Ł, Dziunycz P, Kamiński P, Płoski R, Malejczyk J. Peritoneal cytokines and adhesion formation in endometriosis: an inverse association with vascular endothelial growth factor concentration. Fertil Steril. 2012;97:1380-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Wu J, Xie H, Yao S, Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. 2017;14:53. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 86. | Greaves E, Temp J, Esnal-Zufiurre A, Mechsner S, Horne AW, Saunders PT. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am J Pathol. 2015;185:2286-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 87. | Guo M, Bafligil C, Tapmeier T, Hubbard C, Manek S, Shang C, Martinez FO, Schmidt N, Obendorf M, Hess-Stumpp H, Zollner TM, Kennedy S, Becker CM, Zondervan KT, Cribbs AP, Oppermann U. Mass cytometry analysis reveals a distinct immune environment in peritoneal fluid in endometriosis: a characterisation study. BMC Med. 2020;18:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 88. | Malyshev I, Malyshev Y. Current Concept and Update of the Macrophage Plasticity Concept: Intracellular Mechanisms of Reprogramming and M3 Macrophage "Switch" Phenotype. Biomed Res Int. 2015;2015:341308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 89. | Donnez J, Binda MM, Donnez O, Dolmans MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. 2016;106:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 90. | Sikora J, Mielczarek-Palacz A, Kondera-Anasz Z. Role of natural killer cell activity in the pathogenesis of endometriosis. Curr Med Chem. 2011;18:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Thiruchelvam U, Wingfield M, O'Farrelly C. Natural Killer Cells: Key Players in Endometriosis. Am J Reprod Immunol. 2015;74:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 92. | Fraser R, Zenclussen AC. Killer Timing: The Temporal Uterine Natural Killer Cell Differentiation Pathway and Implications for Female Reproductive Health. Front Endocrinol (Lausanne). 2022;13:904744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Rätsep MT, Felker AM, Kay VR, Tolusso L, Hofmann AP, Croy BA. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction. 2015;149:R91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 94. | Fukui A, Mai C, Saeki S, Yamamoto M, Takeyama R, Kato T, Ukita Y, Wakimoto Y, Yamaya A, Shibahara H. Pelvic endometriosis and natural killer cell immunity. Am J Reprod Immunol. 2021;85:e13342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Jeung I, Cheon K, Kim MR. Decreased Cytotoxicity of Peripheral and Peritoneal Natural Killer Cell in Endometriosis. Biomed Res Int. 2016;2016:2916070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 96. | Kang YJ, Jeung IC, Park A, Park YJ, Jung H, Kim TD, Lee HG, Choi I, Yoon SR. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum Reprod. 2014;29:2176-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 97. | Yu JJ, Sun HT, Zhang ZF, Shi RX, Liu LB, Shang WQ, Wei CY, Chang KK, Shao J, Wang MY, Li MQ. IL15 promotes growth and invasion of endometrial stromal cells and inhibits killing activity of NK cells in endometriosis. Reproduction. 2016;152:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 98. | Jeung IC, Chung YJ, Chae B, Kang SY, Song JY, Jo HH, Lew YO, Kim JH, Kim MR. Effect of helixor A on natural killer cell activity in endometriosis. Int J Med Sci. 2015;12:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | González-Foruria I, Santulli P, Chouzenoux S, Carmona F, Batteux F, Chapron C. Soluble ligands for the NKG2D receptor are released during endometriosis and correlate with disease severity. PLoS One. 2015;10:e0119961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Xu H. Expressions of natural cytotoxicity receptor, NKG2D and NKG2D ligands in endometriosis. J Reprod Immunol. 2019;136:102615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Matsuoka S, Maeda N, Izumiya C, Yamashita C, Nishimori Y, Fukaya T. Expression of inhibitory-motif killer immunoglobulin-like receptor, KIR2DL1, is increased in natural killer cells from women with pelvic endometriosis. Am J Reprod Immunol. 2005;53:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 102. | Wu MY, Yang JH, Chao KH, Hwang JL, Yang YS, Ho HN. Increase in the expression of killer cell inhibitory receptors on peritoneal natural killer cells in women with endometriosis. Fertil Steril. 2000;74:1187-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Kuessel L, Wenzl R, Proestling K, Balendran S, Pateisky P, Yotova 1st, Yerlikaya G, Streubel B, Husslein H. Soluble VCAM-1/soluble ICAM-1 ratio is a promising biomarker for diagnosing endometriosis. Hum Reprod. 2017;32:770-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 104. | Baka S, Frangou-Plemenou M, Panagiotopoulou E, Makrakis E, Kaltsakas G, Hassiakos D, Kondi-Pafiti A. The expression of human leukocyte antigens class I and II in women with endometriosis or adenomyosis. Gynecol Endocrinol. 2011;27:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Chang LY, Shan J, Hou XX, Li DJ, Wang XQ. Synergy between Th1 and Th2 responses during endometriosis: A review of current understanding. J Reprod Immunol. 2023;158:103975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 106. | Podgaec S, Abrao MS, Dias JA Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 107. | Chen P, Zhang Z, Chen Q, Ren F, Li T, Zhang C, Wang D. Expression of Th1 and Th2 cytokine-associated transcription factors, T-bet and GATA-3, in the eutopic endometrium of women with endometriosis. Acta Histochem. 2012;114:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2754] [Article Influence: 105.9] [Reference Citation Analysis (5)] |
| 109. | Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, Lord GM, Jenner RG. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 110. | Hertweck A, Vila de Mucha M, Barber PR, Dagil R, Porter H, Ramos A, Lord GM, Jenner RG. The TH1 cell lineage-determining transcription factor T-bet suppresses TH2 gene expression by redistributing GATA3 away from TH2 genes. Nucleic Acids Res. 2022;50:4557-4573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Chakir H, Wang H, Lefebvre DE, Webb J, Scott FW. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: predominant role of GATA-3. J Immunol Methods. 2003;278:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 112. | Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 318] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 113. | Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O'Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 313] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 114. | Chen P, Wang DB, Liang YM. Evaluation of estrogen in endometriosis patients: Regulation of GATA-3 in endometrial cells and effects on Th2 cytokines. J Obstet Gynaecol Res. 2016;42:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Xia T, Zeng K, Peng Q, Wu X, Lei X. Clinical significance of serum Th1/Th2 cytokines in patients with endometriosis. Women Health. 2023;63:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 116. | Urata Y, Osuga Y, Akiyama I, Nagai M, Izumi G, Takamura M, Hasegawa A, Harada M, Hirata T, Hirota Y, Yoshino O, Koga K, Kozuma S. Interleukin-4 and prostaglandin E2 synergistically up-regulate 3β-hydroxysteroid dehydrogenase type 2 in endometrioma stromal cells. J Clin Endocrinol Metab. 2013;98:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | OuYang Z, Hirota Y, Osuga Y, Hamasaki K, Hasegawa A, Tajima T, Hirata T, Koga K, Yoshino O, Harada M, Takemura Y, Nose E, Yano T, Taketani Y. Interleukin-4 stimulates proliferation of endometriotic stromal cells. Am J Pathol. 2008;173:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Khan KN, Yamamoto K, Fujishita A, Muto H, Koshiba A, Kuroboshi H, Saito S, Teramukai S, Nakashima M, Kitawaki J. Differential Levels of Regulatory T Cells and T-Helper-17 Cells in Women With Early and Advanced Endometriosis. J Clin Endocrinol Metab. 2019;104:4715-4729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 119. | Gogacz M, Winkler I, Bojarska-Junak A, Tabarkiewicz J, Semczuk A, Rechberger T, Adamiak A. Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J Reprod Immunol. 2016;117:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 120. | Zhang X, Xu H, Lin J, Qian Y, Deng L. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG. 2005;112:1153-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 121. | Shi JL, Zheng ZM, Chen M, Shen HH, Li MQ, Shao J. IL-17: an important pathogenic factor in endometriosis. Int J Med Sci. 2022;19:769-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 122. | Hirata T, Osuga Y, Hamasaki K, Yoshino O, Ito M, Hasegawa A, Takemura Y, Hirota Y, Nose E, Morimoto C, Harada M, Koga K, Tajima T, Saito S, Yano T, Taketani Y. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology. 2008;149:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 123. | Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, Wei CY, Zhou WJ, Zhu XY, Shao J, Li DJ, Li MQ. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORγt/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017;8:e2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 124. | Qiu XM, Lai ZZ, Ha SY, Yang HL, Liu LB, Wang Y, Shi JW, Ruan LY, Ye JF, Wu JN, Fu Q, Yi XF, Chang KK, Li MQ. IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-γ and IL-10 in endometriosis. Reproduction. 2020;159:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 125. | Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 313] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 126. | Nanda A, K T, Banerjee P, Dutta M, Wangdi T, Sharma P, Chaudhury K, Jana SK. Cytokines, Angiogenesis, and Extracellular Matrix Degradation are Augmented by Oxidative Stress in Endometriosis. Ann Lab Med. 2020;40:390-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 127. | Heidari S, Kolahdouz-Mohammadi R, Khodaverdi S, Tajik N, Delbandi A-A. Expression levels of MCP-1, HGF, and IGF-1 in endometriotic patients compared with non-endometriotic controls. BMC Women’s Health. 2021;21:422. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 128. | Gueuvoghlanian-Silva BY, Bellelis P, Barbeiro DF, Hernandes C, Podgaec S. Treg and NK cells related cytokines are associated with deep rectosigmoid endometriosis and clinical symptoms related to the disease. J Reprod Immunol. 2018;126:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 129. | Yeol SG, Won YS, Kim YI, Lee JW, Choi YJ, Park DC. Decreased Bcl-6 and increased Blimp-1 in the peritoneal cavity of patients with endometriosis. Clin Exp Obstet Gynecol. 2015;42:156-160. |
| 130. | Turner CA Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 622] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 131. | Nutt SL, Fairfax KA, Kallies A. BLIMP1 guides the fate of effector B and T cells. Nat Rev Immunol. 2007;7:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 132. | Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, Staudt LM. BCL-6 expression during B-cell activation. Blood. 1996;87:5257-5268. |
| 133. | Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, Rojas J, Herrera R, Grigoriadis D, White E, Conlon PJ, Maki RA, Zlotnik A. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci USA. 2007;104:12451-12456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 134. | Do RK, Chen-Kiang S. Mechanism of BLyS action in B cell immunity. Cytokine Growth Factor Rev. 2002;13:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Startseva NV. [Clinical immunological aspects of genital endometriosis]. Akush Ginekol (Mosk). 1980;3:23-26. [PubMed] |
| 136. | Lang GA, Yeaman GR. Autoantibodies in endometriosis sera recognize a Thomsen-Friedenreich-like carbohydrate antigen. J Autoimmun. 2001;16:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 137. | Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397:839-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 791] [Article Influence: 158.2] [Reference Citation Analysis (0)] |
| 138. | Hong M, Zhu Q. Macrophages are activated by 17b-estradiol: possible permission role in endometriosis. Exp Toxicol Pathol. 2004;55:385-391. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 139. | Delvoux B, Groothuis P, D'Hooghe T, Kyama C, Dunselman G, Romano A. Increased production of 17beta-estradiol in endometriosis lesions is the result of impaired metabolism. J Clin Endocrinol Metab. 2009;94:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 140. | Khan KN, Kitajima M, Inoue T, Fujishita A, Nakashima M, Masuzaki H. 17β-estradiol and lipopolysaccharide additively promote pelvic inflammation and growth of endometriosis. Reprod Sci. 2015;22:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 141. | Hayashi T, Yamada K, Esaki T, Muto E, Chaudhuri G, Iguchi A. Physiological concentrations of 17beta-estradiol inhibit the synthesis of nitric oxide synthase in macrophages via a receptor-mediated system. J Cardiovasc Pharmacol. 1998;31:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 142. | Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 143. | Han SJ, Lee JE, Cho YJ, Park MJ, O'Malley BW. Genomic Function of Estrogen Receptor β in Endometriosis. Endocrinology. 2019;160:2495-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 144. | Chantalat E, Valera MC, Vaysse C, Noirrit E, Rusidze M, Weyl A, Vergriete K, Buscail E, Lluel P, Fontaine C, Arnal JF, Lenfant F. Estrogen Receptors and Endometriosis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 145. | Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 146. | Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin WZ, Wu SC, Lai YL. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;12:900-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 147. | Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 746] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 148. | Yang Z, Kong B, Mosser DM, Zhang X. TLRs, macrophages, and NK cells: our understandings of their functions in uterus and ovary. Int Immunopharmacol. 2011;11:1442-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 149. | Verzella D, Pescatore A, Capece D, Vecchiotti D, Ursini MV, Franzoso G, Alesse E, Zazzeroni F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 2020;11:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 150. | Macciò A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58:133-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 151. | Tago K, Funakoshi-Tago M, Ohta S, Kawata H, Saitoh H, Horie H, Aoki-Ohmura C, Yamauchi J, Tanaka A, Matsugi J, Yanagisawa K. Oncogenic Ras mutant causes the hyperactivation of NF-κB via acceleration of its transcriptional activation. Mol Oncol. 2019;13:2493-2510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 152. | Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1147] [Cited by in RCA: 1488] [Article Influence: 212.6] [Reference Citation Analysis (0)] |
| 153. | Tomsová M, Melichar B, Sedláková I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 154. | Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2457] [Cited by in RCA: 2691] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 155. | Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, Li J, Li F, Tan HB. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 1039] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 156. | Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, Lu J, Liu J. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 157. | Cassar E, Kartikasari AER, Plebanski M. Regulatory T Cells in Ovarian Carcinogenesis and Future Therapeutic Opportunities. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 158. | Bu M, Shen Y, Seeger WL, An S, Qi R, Sanderson JA, Cai Y. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8(+) T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour Biol. 2016;37:3949-3956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 159. | Yiong CS, Lin TP, Lim VY, Toh TB, Yang VS. Biomarkers for immune checkpoint inhibition in sarcomas - are we close to clinical implementation? Biomark Res. 2023;11:75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 160. | Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 722] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 161. | Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, Coward JI, Schioppa T, Robinson SC, Gallagher WM, Galletta L; Australian Ovarian Cancer Study Group, Salako MA, Smyth JF, Hagemann T, Brennan DJ, Bowtell DD, Balkwill FR. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 162. | Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 552] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 163. | Isobe A, Sawada K, Kinose Y, Ohyagi-Hara C, Nakatsuka E, Makino H, Ogura T, Mizuno T, Suzuki N, Morii E, Nakamura K, Sawada I, Toda A, Hashimoto K, Mabuchi S, Ohta T, Morishige K, Kurachi H, Kimura T. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PLoS One. 2015;10:e0118080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 164. | Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1138] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 165. | Mao CL, Seow KM, Chen KH. The Utilization of Bevacizumab in Patients with Advanced Ovarian Cancer: A Systematic Review of the Mechanisms and Effects. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 166. | Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 167. | Dumitru A, Dobrica EC, Croitoru A, Cretoiu SM, Gaspar BS. Focus on PD-1/PD-L1 as a Therapeutic Target in Ovarian Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 168. | Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, Spriggs DR, Dupont J, Allison JP. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 169. | Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900-8905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 170. | Zhou L, Zhao Y. B7-H3 Induces Ovarian Cancer Drugs Resistance Through An PI3K/AKT/BCL-2 Signaling Pathway. Cancer Manag Res. 2019;11:10205-10214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 171. | MacGregor HL, Sayad A, Elia A, Wang BX, Katz SR, Shaw PA, Clarke BA, Crome SQ, Robert-Tissot C, Bernardini MQ, Nguyen LT, Ohashi PS. High expression of B7-H3 on stromal cells defines tumor and stromal compartments in epithelial ovarian cancer and is associated with limited immune activation. J Immunother Cancer. 2019;7:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 172. | Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 577] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 173. | Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 546] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 174. | Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, Kleeff J, Friess H. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 175. | Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, Park HJ, Jeong M, Chang SH, Kim BS, Xiong W, Zang W, Guo L, Liu Y, Dong ZJ, Overwijk WW, Hwu P, Yi Q, Kwak L, Yang Z, Mak TW, Li W, Radvanyi LG, Ni L, Liu D, Dong C. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 176. | Vercellini P, Parazzini F, Bolis G, Carinelli S, Dindelli M, Vendola N, Luchini L, Crosignani PG. Endometriosis and ovarian cancer. Am J Obstet Gynecol. 1993;169:181-182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 177. | Giudice LC, Kao LC. Endometriosis. The Lancet. 2004;364:1789-1799. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2295] [Cited by in RCA: 2492] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 178. | Worley MJ, Welch WR, Berkowitz RS, Ng SW. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. 2013;14:5367-5379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 179. | Moll UM, Chumas JC, Chalas E, Mann WJ. Ovarian carcinoma arising in atypical endometriosis. Obstet Gynecol. 1990;75:537-539. |
| 180. | Chalas E, Chumas J, Barbieri R, Mann WJ. Nucleolar organizer regions in endometriosis, atypical endometriosis, and clear cell and endometrioid carcinomas. Gynecol Oncol. 1991;40:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 181. | Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol. 1996;15:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 182. | Brinton LA, Sakoda LC, Sherman ME, Frederiksen K, Kjaer SK, Graubard BI, Olsen JH, Mellemkjaer L. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiol Biomarkers Prev. 2005;14:2929-2935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 183. | Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2006;19:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 184. | Suryawanshi S, Huang X, Elishaev E, Budiu RA, Zhang L, Kim S, Donnellan N, Mantia-Smaldone G, Ma T, Tseng G, Lee T, Mansuria S, Edwards RP, Vlad AM. Complement pathway is frequently altered in endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res. 2014;20:6163-6174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 185. | Koppolu A, Maksym RB, Paskal W, Machnicki M, Rak B, Pępek M, Garbicz F, Pełka K, Kuśmierczyk Z, Jacko J, Rydzanicz M, Banach-Orłowska M, Stokłosa T, Płoski R, Malejczyk J, Włodarski PK. Epithelial Cells of Deep Infiltrating Endometriosis Harbor Mutations in Cancer Driver Genes. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 186. | Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M, McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C, Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL, Vogelstein B, Yong PJ, Huntsman DG, Shih IM. Cancer-Associated Mutations in Endometriosis without Cancer. N Engl J Med. 2017;376:1835-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 455] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 187. | Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the complement cascade. Mol Cancer Res. 2010;8:1453-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 188. | Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2932] [Cited by in RCA: 2853] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 189. | Wei JJ, William J, Bulun S. Endometriosis and ovarian cancer: a review of clinical, pathologic, and molecular aspects. Int J Gynecol Pathol. 2011;30:553-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 190. | Laganà AS, Garzon S, Götte M, Viganò P, Franchi M, Ghezzi F, Martin DC. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 191. | Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 192. | Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 193. | Kobayashi H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sakata M, Sado T, Oi H. The role of iron in the pathogenesis of endometriosis. Gynecol Endocrinol. 2009;25:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 194. | Defrère S, Lousse JC, González-Ramos R, Colette S, Donnez J, Van Langendonckt A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008;14:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 195. | Van Langendonckt A, Casanas-Roux F, Dolmans MM, Donnez J. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil Steril. 2002;77:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 196. | Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S, Sasano H, Yaegashi N. Loss of estrogen receptor beta isoform expression and its correlation with aberrant DNA methylation of the 5'-untranslated region in human epithelial ovarian carcinoma. Cancer Sci. 2008;99:2365-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 197. | Giudice LC, Tazuke SI, Swiersz L. Status of current research on endometriosis. J Reprod Med. 1998;43:252-262. [PubMed] |
| 198. | Ferrándiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 199. | Kato J, Kobune M, Ohkubo S, Fujikawa K, Tanaka M, Takimoto R, Takada K, Takahari D, Kawano Y, Kohgo Y, Niitsu Y. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 200. | Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65:925-930. [PubMed] |
| 201. | McKinnon BD, Bertschi D, Bersinger NA, Mueller MD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. 2015;26:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 202. | Vitale SG, Capriglione S, Peterlunger I, La Rosa VL, Vitagliano A, Noventa M, Valenti G, Sapia F, Angioli R, Lopez S, Sarpietro G, Rossetti D, Zito G. The Role of Oxidative Stress and Membrane Transport Systems during Endometriosis: A Fresh Look at a Busy Corner. Oxid Med Cell Longev. 2018;2018:7924021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 203. | Capobianco A, Monno A, Cottone L, Venneri MA, Biziato D, Di Puppo F, Ferrari S, De Palma M, Manfredi AA, Rovere-Querini P. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol. 2011;179:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 204. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2596] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 205. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5849] [Article Influence: 234.0] [Reference Citation Analysis (1)] |
| 206. | Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 652] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 207. | Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 487] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 208. | Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150:3128-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 209. | Bergqvist A, Bruse C, Carlberg M, Carlström K. Interleukin 1beta, interleukin-6, and tumor necrosis factor-alpha in endometriotic tissue and in endometrium. Fertil Steril. 2001;75:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 210. | Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1330] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 211. | Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson JL, Wilbanks GD, Burke F, Balkwill FR. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006;5:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 212. | Rutherford EJ, Hill ADK, Hopkins AM. Adhesion in Physiological, Benign and Malignant Proliferative States of the Endometrium: Microenvironment and the Clinical Big Picture. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 213. | Starzinski-Powitz A, Handrow-Metzmacher H, Kotzian S. The putative role of cell adhesion molecules in endometriosis: can we learn from tumour metastasis? Mol Med Today. 1999;5:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 214. | Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 398] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 215. | Regidor PA, Vogel C, Regidor M, Schindler AE, Winterhager E. Expression pattern of integrin adhesion molecules in endometriosis and human endometrium. Hum Reprod Update. 1998;4:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 216. | Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 217. | Martínez-Román S, Balasch J, Creus M, Fábregues F, Carmona F, Vilella R, Vanrell JA. Transferrin receptor (CD71) expression in peritoneal macrophages from fertile and infertile women with and without endometriosis. Am J Reprod Immunol. 1997;38:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 218. | Raiter-Tenenbaum A, Barañao RI, Etchepareborda JJ, Meresman GF, Rumi LS. Functional and phenotypic alterations in peritoneal macrophages from patients with early and advanced endometriosis. Arch Gynecol Obstet. 1998;261:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 219. | Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 220. | Dyson MT, Kakinuma T, Pavone ME, Monsivais D, Navarro A, Malpani SS, Ono M, Bulun SE. Aberrant expression and localization of deoxyribonucleic acid methyltransferase 3B in endometriotic stromal cells. Fertil Steril. 2015;104:953-963.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 221. | Dyson MT, Roqueiro D, Monsivais D, Ercan CM, Pavone ME, Brooks DC, Kakinuma T, Ono M, Jafari N, Dai Y, Bulun SE. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10:e1004158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 222. | Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169:1545-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 223. | Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 224. | Monsivais D, Dyson MT, Yin P, Coon JS, Navarro A, Feng G, Malpani SS, Ono M, Ercan CM, Wei JJ, Pavone ME, Su E, Bulun SE. ERβ- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Mol Endocrinol. 2014;28:1304-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 225. | Laganà AS, Vitale SG, Salmeri FM, Triolo O, Ban Frangež H, Vrtačnik-Bokal E, Stojanovska L, Apostolopoulos V, Granese R, Sofo V. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 2017;103:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 226. | Agic A, Djalali S, Diedrich K, Hornung D. Apoptosis in endometriosis. Gynecol Obstet Invest. 2009;68:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 227. | Santulli P, Marcellin L, Tosti C, Chouzenoux S, Cerles O, Borghese B, Batteux F, Chapron C. MAP kinases and the inflammatory signaling cascade as targets for the treatment of endometriosis? Expert Opin Ther Targets. 2015;19:1465-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 228. | Krawczyk N, Banys-Paluchowski M, Schmidt D, Ulrich U, Fehm T. Endometriosis-associated Malignancy. Geburtshilfe Frauenheilkd. 2016;76:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 229. | Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, Yamawaki K, Adachi S, Takahashi T, Kase H, Tanaka K, Yamamoto T, Motoyama T, Inoue I, Enomoto T. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018;24:1777-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 301] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 230. | Stamp JP, Gilks CB, Wesseling M, Eshragh S, Ceballos K, Anglesio MS, Kwon JS, Tone A, Huntsman DG, Carey MS. BAF250a Expression in Atypical Endometriosis and Endometriosis-Associated Ovarian Cancer. Int J Gynecol Cancer. 2016;26:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 231. | Anglesio MS, Bashashati A, Wang YK, Senz J, Ha G, Yang W, Aniba MR, Prentice LM, Farahani H, Li Chang H, Karnezis AN, Marra MA, Yong PJ, Hirst M, Gilks B, Shah SP, Huntsman DG. Multifocal endometriotic lesions associated with cancer are clonal and carry a high mutation burden. J Pathol. 2015;236:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 232. | Chene G, Ouellet V, Rahimi K, Barres V, Provencher D, Mes-Masson AM. The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int J Gynaecol Obstet. 2015;130:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 233. | Matsumoto T, Yamazaki M, Takahashi H, Kajita S, Suzuki E, Tsuruta T, Saegusa M. Distinct β-catenin and PIK3CA mutation profiles in endometriosis-associated ovarian endometrioid and clear cell carcinomas. Am J Clin Pathol. 2015;144:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 234. | Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1656] [Cited by in RCA: 1864] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 235. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 10124] [Article Influence: 723.1] [Reference Citation Analysis (5)] |
| 236. | Siminiak N, Czepczyński R, Zaborowski MP, Iżycki D. Immunotherapy in Ovarian Cancer. Arch Immunol Ther Exp (Warsz). 2022;70:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 237. | An Y, Yang Q. Tumor-associated macrophage-targeted therapeutics in ovarian cancer. Int J Cancer. 2021;149:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 238. | Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G, Jin WL. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front Immunol. 2020;11:577869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 239. | Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 2170] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 240. | Wang W, Liu JR, Zou W. Immunotherapy in Ovarian Cancer. Surg Oncol Clin N Am. 2019;28:447-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 241. | Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2015;33:4015-4022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 938] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 242. | Hinchcliff E, Hong D, Le H, Chisholm G, Iyer R, Naing A, Hwu P, Jazaeri A. Characteristics and outcomes of patients with recurrent ovarian cancer undergoing early phase immune checkpoint inhibitor clinical trials. Gynecol Oncol. 2018;151:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 243. | Demircan NC, Boussios S, Tasci T, Öztürk MA. Current and future immunotherapy approaches in ovarian cancer. Ann Transl Med. 2020;8:1714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 244. | Yoon H, Kim A, Jang H. Immunotherapeutic Approaches in Ovarian Cancer. Curr Issues Mol Biol. 2023;45:1233-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 245. | Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, Li P, Lyu N, Sun T, Xie S, Guo L, Ni L, Jin L, Dong C. Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol. 2020;17:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 246. | Morand S, Devanaboyina M, Staats H, Stanbery L, Nemunaitis J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 247. | LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20:e15-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 248. | Kurnit KC, Avila M, Hinchcliff EM, Coleman RL, Westin SN. PARP inhibition in the ovarian cancer patient: Current approvals and future directions. Pharmacol Ther. 2020;213:107588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 249. | Mirza MR, Coleman RL, González-Martín A, Moore KN, Colombo N, Ray-Coquard I, Pignata S. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31:1148-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 250. | Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, Christensen RD, Berek JS, Dørum A, Tinker AV, du Bois A, González-Martín A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA; ENGOT-OV16/NOVA Investigators. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375:2154-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1936] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 251. | Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G, Leary A, Holloway RW, Gancedo MA, Fong PC, Goh JC, O'Malley DM, Armstrong DK, Garcia-Donas J, Swisher EM, Floquet A, Konecny GE, McNeish IA, Scott CL, Cameron T, Maloney L, Isaacson J, Goble S, Grace C, Harding TC, Raponi M, Sun J, Lin KK, Giordano H, Ledermann JA; ARIEL3 investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1371] [Article Influence: 152.3] [Reference Citation Analysis (0)] |
| 252. | Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, González-Martín A, Aghajanian C, Bradley W, Mathews C, Liu J, Lowe ES, Bloomfield R, DiSilvestro P. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018;379:2495-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 2095] [Article Influence: 261.9] [Reference Citation Analysis (0)] |
| 253. | Ledermann JA, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp AR, Scambia G, Leary A, Holloway RW, Gancedo MA, Fong PC, Goh JC, O'Malley DM, Armstrong DK, Banerjee S, García-Donas J, Swisher EM, Cameron T, Maloney L, Goble S, Coleman RL. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:710-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 254. | Frenel JS, Kim JW, Aryal N, Asher R, Berton D, Vidal L, Pautier P, Ledermann JA, Penson RT, Oza AM, Korach J, Huzarski T, Pignata S, Colombo N, Park-Simon TW, Tamura K, Sonke GS, Freimund AE, Lee CK, Pujade-Lauraine E. Efficacy of subsequent chemotherapy for patients with BRCA1/2-mutated recurrent epithelial ovarian cancer progressing on olaparib vs placebo maintenance: post-hoc analyses of the SOLO2/ENGOT Ov-21 trial. Ann Oncol. 2022;33:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 255. | Hu X, Bian C, Zhao X, Yi T. Efficacy evaluation of multi-immunotherapy in ovarian cancer: From bench to bed. Front Immunol. 2022;13:1034903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 256. | Moreno V, Hernandez T, de Miguel M, Doger B, Calvo E. Adoptive cell therapy for solid tumors: Chimeric antigen receptor T cells and beyond. Curr Opin Pharmacol. 2021;59:70-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 257. | Adams SF, Grimm AJ, Chiang CL, Mookerjee A, Flies D, Jean S, McCann GA, Michaux J, Pak H, Huber F, Neal C, Dangaj D, Bassani-Sternberg M, Rusakiewicz S, Facciabene A, Coukos G, Gimotty PA, Kandalaft LE. Rapid tumor vaccine using Toll-like receptor-activated ovarian cancer ascites monocytes. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 258. | Kalli KR, Block MS, Kasi PM, Erskine CL, Hobday TJ, Dietz A, Padley D, Gustafson MP, Shreeder B, Puglisi-Knutson D, Visscher DW, Mangskau TK, Wilson G, Knutson KL. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin Cancer Res. 2018;24:3014-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 259. | Morisaki T, Hikichi T, Onishi H, Morisaki T, Kubo M, Hirano T, Yoshimura S, Kiyotani K, Nakamura Y. Intranodal Administration of Neoantigen Peptide-loaded Dendritic Cell Vaccine Elicits Epitope-specific T Cell Responses and Clinical Effects in a Patient with Chemorefractory Ovarian Cancer with Malignant Ascites. Immunol Invest. 2021;50:562-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 260. | Friese C, Harbst K, Borch TH, Westergaard MCW, Pedersen M, Kverneland A, Jönsson G, Donia M, Svane IM, Met Ö. CTLA-4 blockade boosts the expansion of tumor-reactive CD8(+) tumor-infiltrating lymphocytes in ovarian cancer. Sci Rep. 2020;10:3914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 261. | Levinson K, Dorigo O, Rubin K, Moore K. Immunotherapy in Gynecologic Cancers: What We Know Now and Where We Are Headed. Am Soc Clin Oncol Educ Book. 2019;39:e126-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 262. | Li W, Lin A, Qi L, Lv X, Yan S, Xue J, Mu N. Immunotherapy: A promising novel endometriosis therapy. Front Immunol. 2023;14:1128301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 263. | Jiang Z, Sun H, Yu J, Tian W, Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol. 2021;14:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 264. | Li J, Yan S, Li Q, Huang Y, Ji M, Jiao X, Yuan M, Wang G. Macrophage-associated immune checkpoint CD47 blocking ameliorates endometriosis. Mol Hum Reprod. 2022;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 265. | Sun H, Li D, Yuan M, Li Q, Zhen Q, Li N, Wang G. Macrophages alternatively activated by endometriosis-exosomes contribute to the development of lesions in mice. Mol Hum Reprod. 2019;25:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 266. | Freger S, Leonardi M, Foster WG. Exosomes and their cargo are important regulators of cell function in endometriosis. Reprod Biomed Online. 2021;43:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 267. | Itoh H, Sashihara T, Hosono A, Kaminogawa S, Uchida M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology. 2011;63:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 268. | Maksym RB, Hoffmann-Młodzianowska M, Skibińska M, Rabijewski M, Mackiewicz A, Kieda C. Immunology and Immunotherapy of Endometriosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 269. | Ścieżyńska A, Komorowski M, Soszyńska M, Malejczyk J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 270. | Sayegh L, Fuleihan Gel-H, Nassar AH. Vitamin D in endometriosis: a causative or confounding factor? Metabolism. 2014;63:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 271. | Bertone-Johnson ER, Chocano-Bedoya PO, Zagarins SE, Micka AE, Ronnenberg AG. Dietary vitamin D intake, 25-hydroxyvitamin D3 Levels and premenstrual syndrome in a college-aged population. J Steroid Biochem Mol Biol. 2010;121:434-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 272. | D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 510] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 273. | Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, Ma X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol. 2017;147:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 274. | Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, Choinzonov E, Kzhyshkowska J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front Oncol. 2020;10:566511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 296] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 275. | Schweer D, McAtee A, Neupane K, Richards C, Ueland F, Kolesar J. Tumor-Associated Macrophages and Ovarian Cancer: Implications for Therapy. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 276. | Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 439] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 277. | Liu R, Wei H, Gao P, Yu H, Wang K, Fu Z, Ju B, Zhao M, Dong S, Li Z, He Y, Huang Y, Yao Z. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget. 2017;8:39021-39032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 278. | Son J, Hsieh RC, Lin HY, Krause KJ, Yuan Y, Biter AB, Welsh J, Curran MA, Hong DS. Inhibition of the CD47-SIRPα axis for cancer therapy: A systematic review and meta-analysis of emerging clinical data. Front Immunol. 2022;13:1027235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 279. | Kaur S, Cicalese KV, Bannerjee R, Roberts DD. Preclinical and Clinical Development of Therapeutic Antibodies Targeting Functions of CD47 in the Tumor Microenvironment. Antib Ther. 2020;3:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 280. | Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, Colevas AD, O'Rourke T, Narayanan S, Papadopoulos K, Fisher GA, Villalobos V, Prohaska SS, Howard M, Beeram M, Chao MP, Agoram B, Chen JY, Huang J, Axt M, Liu J, Volkmer JP, Majeti R, Weissman IL, Takimoto CH, Supan D, Wakelee HA, Aoki R, Pegram MD, Padda SK. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients With Advanced Cancers. J Clin Oncol. 2019;37:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 486] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 281. | Tian L, Xu B, Teng KY, Song M, Zhu Z, Chen Y, Wang J, Zhang J, Feng M, Kaur B, Rodriguez L, Caligiuri MA, Yu J. Targeting Fc Receptor-Mediated Effects and the "Don't Eat Me" Signal with an Oncolytic Virus Expressing an Anti-CD47 Antibody to Treat Metastatic Ovarian Cancer. Clin Cancer Res. 2022;28:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 282. | Achkova D, Maher J. Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem Soc Trans. 2016;44:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 283. | Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 588] [Cited by in RCA: 816] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 284. | Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord JP, Levitsky H, Blay JY, Rüttinger D. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 1094] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 285. | Lu X, Meng T. Depletion of tumor-associated macrophages enhances the anti-tumor effect of docetaxel in a murine epithelial ovarian cancer. Immunobiology. 2019;224:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 286. | Xiaocui L, Wei H, Yunlang C, Zhenzhen Z, Min A. CSF-1-induced DC-SIGN(+) macrophages are present in the ovarian endometriosis. Reprod Biol Endocrinol. 2022;20:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 287. | Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 288. | Miyamoto T, Murakami R, Hamanishi J, Tanigaki K, Hosoe Y, Mise N, Takamatsu S, Mise Y, Ukita M, Taki M, Yamanoi K, Horikawa N, Abiko K, Yamaguchi K, Baba T, Matsumura N, Mandai M. B7-H3 Suppresses Antitumor Immunity via the CCL2-CCR2-M2 Macrophage Axis and Contributes to Ovarian Cancer Progression. Cancer Immunol Res. 2022;10:56-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 289. | Hogg C, Horne AW, Greaves E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front Endocrinol (Lausanne). 2020;11:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 290. | Mao TL, Fan KF, Liu CL. Targeting the CXCR4/CXCL12 axis in treating epithelial ovarian cancer. Gene Ther. 2017;24:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 291. | Luo N, Chen DD, Liu L, Li L, Cheng ZP. CXCL12 promotes human ovarian cancer cell invasion through suppressing ARHGAP10 expression. Biochem Biophys Res Commun. 2019;518:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 292. | Xue J, Li R, Gao D, Chen F, Xie H. CXCL12/CXCR4 Axis-Targeted Dual-Functional Nano-Drug Delivery System Against Ovarian Cancer. Int J Nanomedicine. 2020;15:5701-5718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 293. | Zheng X, Turkowski K, Mora J, Brüne B, Seeger W, Weigert A, Savai R. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. 2017;8:48436-48452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 227] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 294. | Wang N, Wang S, Wang X, Zheng Y, Yang B, Zhang J, Pan B, Gao J, Wang Z. Research trends in pharmacological modulation of tumor-associated macrophages. Clin Transl Med. 2021;11:e288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 295. | Bolli E, Scherger M, Arnouk SM, Pombo Antunes AR, Straßburger D, Urschbach M, Stickdorn J, De Vlaminck K, Movahedi K, Räder HJ, Hernot S, Besenius P, Van Ginderachter JA, Nuhn L. Targeted Repolarization of Tumor-Associated Macrophages via Imidazoquinoline-Linked Nanobodies. Adv Sci (Weinh). 2021;8:2004574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 296. | Xiao H, Guo Y, Li B, Li X, Wang Y, Han S, Cheng D, Shuai X. M2-Like Tumor-Associated Macrophage-Targeted Codelivery of STAT6 Inhibitor and IKKβ siRNA Induces M2-to-M1 Repolarization for Cancer Immunotherapy with Low Immune Side Effects. ACS Cent Sci. 2020;6:1208-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 297. | Hsieh TH, Hsu CY, Wu CW, Wang SH, Yeh CH, Cheng KH, Tsai EM. Vorinostat decrease M2 macrophage polarization through ARID1A(6488delG)/HDAC6/IL-10 signaling pathway in endometriosis-associated ovarian carcinoma. Biomed Pharmacother. 2023;161:114500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 298. | Pannunzio A, Coluccia M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals (Basel). 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 299. | Sun H, Zhang X, Sun D, Jia X, Xu L, Qiao Y, Jin Y. COX-2 expression in ovarian cancer: an updated meta-analysis. Oncotarget. 2017;8:88152-88162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 300. | Zhang X, Yan K, Deng L, Liang J, Liang H, Feng D, Ling B. Cyclooxygenase 2 Promotes Proliferation and Invasion in Ovarian Cancer Cells via the PGE2/NF-κB Pathway. Cell Transplant. 2019;28:1S-13S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 301. | Lai ZZ, Yang HL, Ha SY, Chang KK, Mei J, Zhou WJ, Qiu XM, Wang XQ, Zhu R, Li DJ, Li MQ. Cyclooxygenase-2 in Endometriosis. Int J Biol Sci. 2019;15:2783-2797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 302. | Li W, Wan L, Zhai LY, Wang J. Effects of SC-560 in combination with cisplatin or taxol on angiogenesis in human ovarian cancer xenografts. Int J Mol Sci. 2014;15:19265-19280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Silva P, United States S-Editor: Liu JH L-Editor: A P-Editor: Zhao S