Published online Feb 24, 2024. doi: 10.5306/wjco.v15.i2.290
Peer-review started: December 13, 2023
First decision: December 22, 2023
Revised: December 25, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: February 24, 2024
Processing time: 68 Days and 21.3 Hours
Sessile serrated lesions (SSLs) are considered precancerous colorectal lesions that should be detected and removed to prevent colorectal cancer. Previous studies in Vietnam mainly investigated the adenoma pathway, with limited data on the serrated pathway.
To evaluate the prevalence, risk factors, and BRAF mutations of SSLs in the Vietnamese population.
This is a cross-sectional study conducted on patients with lower gastrointestinal symptoms who underwent colonoscopy at a tertiary hospital in Vietnam. SSLs were diagnosed on histopathology according to the 2019 World Health Organi
There were 2489 patients, with a mean age of 52.1 ± 13.1 and a female-to-male ratio of 1:1.1. The prevalence of SSLs was 4.2% [95% confidence interval (CI): 3.5-5.1]. In the multivariate analysis, factors significantly associated with SSLs were age ≥ 40 [odds ratio (OR): 3.303; 95%CI: 1.607-6.790], male sex (OR: 2.032; 95%CI: 1.204-3.429), diabetes mellitus (OR: 2.721; 95%CI: 1.551-4.772), and hypertension (OR: 1.650, 95%CI: 1.045-2.605). The rate of BRAF mutations in SSLs was 35.5%.
The prevalence of SSLs was 4.2%. BRAF mutations were present in one-third of SSLs. Significant risk factors for SSLs included age ≥ 40, male sex, diabetes mellitus, and hypertension.
Core Tip: This was a cross-sectional study conducted on patients with lower gastrointestinal symptoms who underwent colonoscopy at a tertiary hospital in Vietnam. Sessile serrated lesions (SSLs) were diagnosed on histopathology according to the 2019 World Health Organization classification. BRAF mutation analysis was performed using the Sanger DNA sequencing method. There were 2489 patients, with a mean age of 52.1 years. The prevalence of SSLs was 4.2%. In the multivariate analysis, factors significantly associated with SSLs were age ≥ 40, male sex, diabetes mellitus, and hypertension. The rate of BRAF mutations in SSLs was 35.5%.
- Citation: Vu NTH, Le HM, Vo DTN, Vu HA, Le NQ, Ho DDQ, Quach DT. Prevalence, risk factors, and BRAF mutation of colorectal sessile serrated lesions among Vietnamese patients. World J Clin Oncol 2024; 15(2): 290-301
- URL: https://www.wjgnet.com/2218-4333/full/v15/i2/290.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i2.290
Colorectal cancer (CRC) remains the third most prevalent cancer and the second cause of cancer-related mortality worldwide[1]. More than 182000 new cases were diagnosed in Vietnam, and 122000 deaths were recorded in 2020[1]. For a long time, it was considered that colorectal adenomas were the only precursor lesions for CRC. However, recent studies have indicated that despite a high adenoma detection rate, postcolonoscopy CRC still occurs, with serrated lesions being the most likely cause[2]. There is mounting evidence that 15%-30% of all CRCs develop from serrated lesions via the serrated neoplasia pathway[3]. Furthermore, proximal serrated lesions pose a higher risk of metachronous neoplasia than tubular adenomas, emphasizing the significance of screening and surveillance[4]. According to the World Health Organization (WHO) classification 2019, serrated lesions were divided into hyperplastic polyps, sessile serrated lesions (SSLs), SSLs with dysplasia, traditional serrated adenomas, and unclassified serrated adenomas[5]. SSLs are the most common and significant precancerous serrated lesions due to their rapid growth and high potential for malignant transformation[6]. Nevertheless, SSLs are challenging to detect by colonoscopy due to their typically subtle appearance as flat or sessile lesions and having an overlying mucous cap[7]. Moreover, they are often incompletely resected because of the indistinct borders, thus related to interval and synchronous CRC[8]. The histopathological identification of SSLs also tends to be difficult for both expert and non-expert pathologists[9]. Consequently, the reported prevalence of SSLs varies widely across studies, leading to an underestimation of their prevalence and subsequent biased analysis[10,11].
Studies analyzing risk factors for SSLs have been limited and controversial, primarily due to obstacles posed by their evolving histological definition and the relatively low prevalence of these polyps. In addition, most published data have included all histopathological types of serrated lesions with different malignant progression risks[12]. As a result, there were inconsistent findings, and the risk factors for SSLs could not be accurately evaluated. Moreover, existing studies have shown that serrated lesions and adenomas share common risk factors[12,13]. Nonetheless, several factors correlated with serrated lesions differently than conventional adenomas[13,14].
In contrast to the conventional pathway, the serrated pathway is characterized by BRAF mutations and a CpG island methylator phenotype with or without microsatellite instability (MSI)[15]. Most BRAF mutations in CRC and other tumors are a thymidine-to-adenine transversion at nucleotide 1796, converting a valine at amino acid 600 to glutamic acid (V600E)[16]. It has been demonstrated that proximal serrated lesions with BRAF mutations have a considerably high risk for malignant progression[17]. Hence, detecting BRAF mutations as an adjunct diagnostic tool in clinical pathology may reliably identify proximal serrated lesions with cancer risk, which require more aggressive therapy and attentive clinical surveillance. Additionally, BRAF mutations may serve as a potential therapeutic target for serrated colorectal neoplasia[18].
In Vietnam, previous research on colorectal polyps has focused heavily on the adenoma pathway, with limited data on the serrated pathway. Despite the importance of SSLs, there remains a paucity of evidence on their prevalence, risk factors, and molecular characteristics. Thus, this study aimed to evaluate the prevalence, risk factors, and BRAF mutations of SSLs in Vietnamese patients. These findings may provide valuable insights into the prevention, early detection, enhancement of treatment, and surveillance approaches for SSLs in the Vietnamese population.
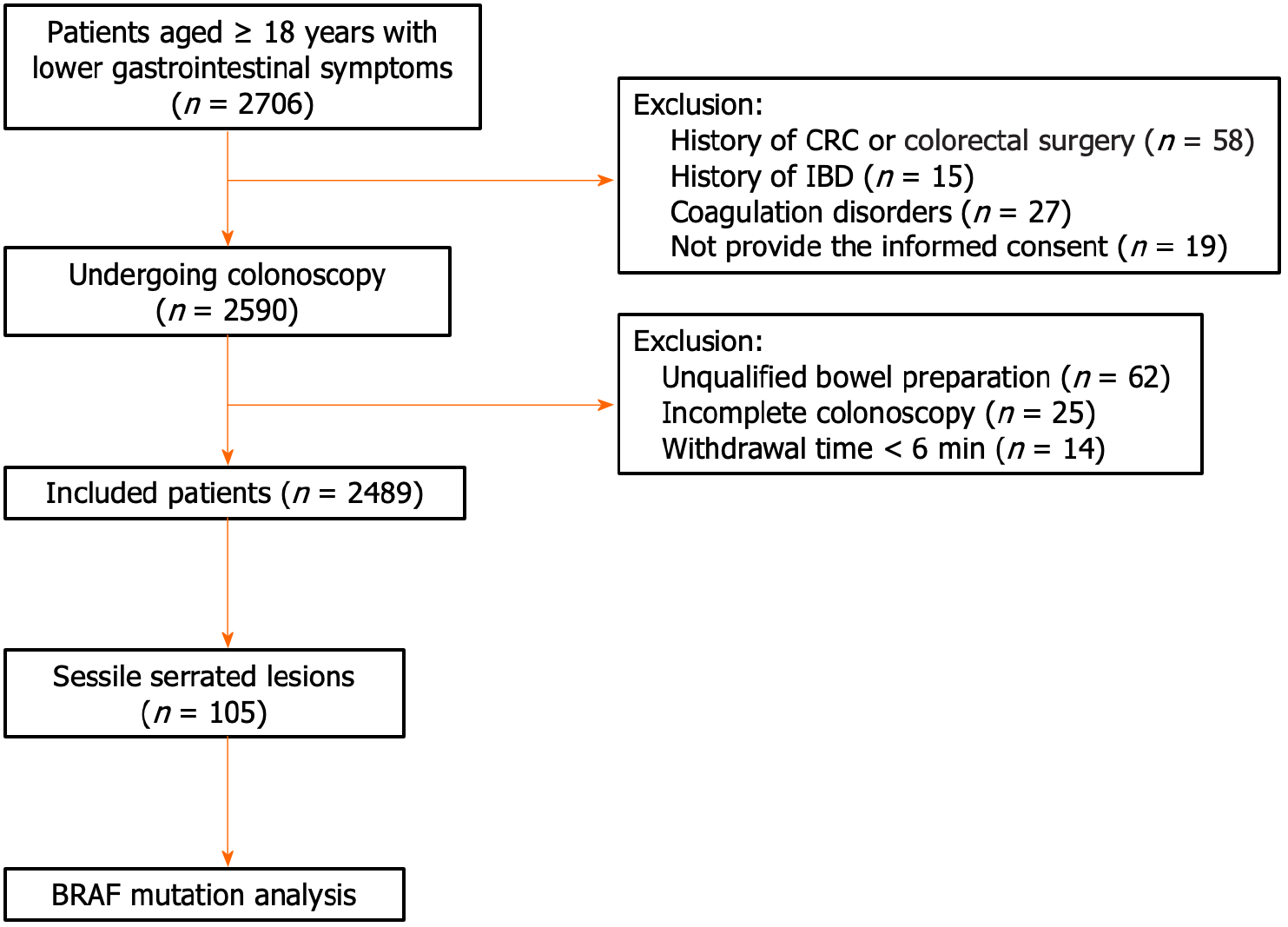
From March 2022 to July 2023, a cross-sectional study was carried out on outpatients over 18 who presented with lower gastrointestinal symptoms and underwent colonoscopy at the University Medical Center at Hochiminh City, Vietnam. Only the first colonoscopy was included if patients received multiple colonoscopies during the study period to ensure a per-patient analysis. Exclusion criteria comprised a history of CRC or colorectal surgery, inflammatory bowel disease, inherited cancer syndromes, coagulation disorders, unqualified bowel preparation according to the Boston Bowel Preparation Scale (BBPS) with a total score of < 6 and each region score of < 2, incomplete colonoscopy, withdrawal time of less than 6 minutes, and not willing to participate in the research.
This study's sample size was determined by applying the following formula:
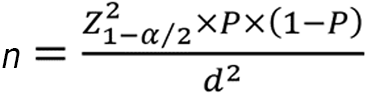
The P values were chosen based on the prevalence of SSLs and BRAF mutations in previous Asian studies, with 2.6% and 82%, respectively[19,20]. The estimated loss of samples would be 10% during the study. Thus, the minimum sample size was 2411 patients.
The demographic, clinical, endoscopic, pathologic, and BRAF mutation data were recorded and evaluated. Smoking conditions were classified as "never," "former," and "current" users. Obesity was defined as having a body mass index equal to or greater than 25.0 kg/m2. Nonalcohol consumption was identified as either never drinking or drinking alcohol once per month or less. Hypertension was diagnosed when systolic blood pressure was ≥ 140 mmHg and/or their diastolic blood pressure was ≥ 90 mmHg or when they were on antihypertensive medication. Patients were classified as having diabetes mellitus (DM) if they fulfilled any of the following criteria: (1) A random venous plasma glucose concentration ≥ 11.1 mmol/L; (2) a fasting plasma glucose concentration ≥ 7.0 mmol/L; (3) HbA1C ≥ 6.5%; and (4) on medication for hyperglycemia with a history of DM. The definition of a family history of CRC was the presence of at least one affected first-degree relative.
All eligible patients signed a written informed consent form. The study protocol was approved by the Board of Ethics in Biomedical Research of the University of Medicine and Pharmacy at Ho Chi Minh City (ID number: 615/HDDD-DHYD, signed on November 19, 2021).
Precolonoscopy bowel preparations were obtained with three liters of polyethylene glycol-based (Fortrans®, Beaufour Ipsen Industrie, France). The colonoscopies were carried out by experienced endoscopists using the Olympus Evis Exera III High Definition CV-190 (Olympus Co., Ltd, Tokyo, Japan). All participating endoscopists had performed at least 3000 colonoscopic procedures over the last five years and had an adenoma detection rate of over 25%, resulting in high-performance colonoscopies. Moreover, all endoscopists had attended the web-based educational program (CATCH project) to detect flat and depressed colorectal lesions before the commencement of the study[21]. In addition, they also participated in a local training session to standardize the examination process and the interpretation of colon lesions suspected as SSLs.
The assessment of bowel preparation quality was conducted utilizing the BBPS. The colon was divided into three sections, including the right colon, transverse colon, and left colon, and each section was scored from 0 to 3. Qualified bowel preparation was defined as a total score of ≥ 6 and each region score of ≥ 2[22]. Confirmation of cecal intubation was achieved by identifying the appendiceal orifice, cecal valve, or intubation of the ileum. Stopwatches were used to record withdrawal times, which were mandated at least six minutes after excluding the time required for polypectomies.
The Paris classification was utilized to classify the macroscopic type, including three categories: type 0-I: polypoid (0-Is: sessile, 0-Ip: pedunculated), type 0-II: nonpolypoid (0-IIa: slightly elevated, 0-IIb: flat, 0-IIc: slightly depressed), and type 0-III: excavated[23]. The Japan NBI Expert Team (JNET) classification was used to categorize the colorectal lesions by magnifying narrow-band imaging endoscopic findings[24]. Type 1 is identified by an invisible vascular pattern and a surface pattern with dark and white spots, resembling the surrounding normal mucosa. Type 2A is defined by its regular surface pattern and vessel pattern, including a regular caliber or distribution. Type 2B is characterized by vessels with variable caliber, irregular distribution, and an irregular or obscure surface pattern. Type 3 is recognized by loose vessel regions, thick vessel disruptions and/or a surface pattern of amorphous areas. The proximal colon included the cecum, ascending colon, hepatic flexure, and transverse colon. The distal colon included the splenic flexure, descending colon, sigmoid colon, and rectum. The size of the lesions was measured by comparing them with open (with a width of 7 mm) or closed (equivalent to 3 mm) biopsy forceps or polypectomy snares of known diameters. Multiple polyps were identified as having at least two lesions. The most advanced or largest lesion was considered for the primary diagnosis in subjects with multiple lesions. All detected polyps were removed and obtained for histopathological evaluation.
All resected lesions were collected in distinct jars and preserved in 10% formalin. Sections were cut at 4 µm thickness and stained with hematoxylin-eosin. Serrated lesions were classified in accordance with the updated WHO 2019 criteria[5]. The presence of a single unequivocally distorted crypt was regarded as a diagnostic criterion for SSL. An obviously distorted serrated crypt had at least one of the following histologic characteristics: (1) Horizontally growing crypt along the muscularis mucosa (L-shaped or inverted T-shaped crypt); (2) crypt base dilation, comprising the basal one-third of the crypt; (3) serrations that extend into the crypt base; and (4) asymmetrical expansion of the crypts. SSLs with dysplasia were subclassified into SSLs with dysplasia. The dysplastic component is identified as clearly separated from the SSL with architectural modifications, including the presence of villous architecture, elongated crypts, crowded crypts with complicated branching, cribriforming, and excessive or diminished luminal serration compared with the background SSL[5]. Cells may exhibit intestinal dysplasia similar to that observed in conventional adenomas; serrated dysplasia with round atypical nuclei, large nucleoli, multiple mitoses, and eosinophilic cytoplasm; or (less frequently) subtle cytological atypia, including hypermucinous alterations. Stratification of dysplasia into low-grade and high-grade is not applicable in this study. All histopathological diagnoses were made by two experienced gastrointestinal pathologists. Any disa
BRAF mutation analysis was conducted on all specimens diagnosed with SSLs using Sanger sequencing at the Center for Molecular Biomedicine, University of Medicine and Pharmacy at Ho Chi Minh City. The ReliaPrep FFPE gDNA Miniprep System Kit (Promega, Madison, WI, USA) was used to extract genomic DNA from formalin-fixed paraffin-embedded tissues according to the manufacturer's protocol. Exon 15 of BRAF was amplified utilising TaKaRa Taq HotStart Polymerase (Takara Bio, Shiga, Japan) with a primer pair (forward: 5’-ACTCTTCATAATGCTTGCTC-3’ and reverse: 5’-CCACAAAATGGATCCAGACA-3’). Following purification with ExoSAP-IT reagent (Thermo Fisher, Scientific, Waltham, MA, United States), the products of the polymerase chain reaction were sequenced in both the sense and antisense directions using an ABI 3500 Genetic Analyser (Applied Biosystems, Foster City, CA, United States) and the BigDye Terminator v3.1 Kit. SeqScape Software version 2.6 (Thermo Fisher, Scientific, Waltham, MA, USA) was utilised to analyse mutations.
All statistical analyses were performed using SPSS software version 23 (SPSS Inc., Chicago, IL). The Kolmogorov-Smirnov test was used to determine the normality of continuous variables. The t-test was utilized to compare variables that followed a normal distribution and were presented as the mean and standard deviation (SD). Those with a nonnormal distribution were reported as the median (upper and lower quartiles) and compared using the Mann–Whitney U test. The categorical variables were compared using Pearson's chi-squared test and presented as numbers and percentages. The prevalence was estimated by dividing the proportion of colonoscopies containing at least one SSL by the total number of performed colonoscopies. The prevalence and risk factors for SSLs were determined on a per-patient basis. In addition, the data were analyzed per lesion to determine the characteristics of SSLs and BRAF mutations. The SSL patient group was compared to the control group, with no polyps detected. Univariate analyses were conducted in order to evaluate the factors correlated with SSLs. Variables with a P value of 0.2 or less were included in a multivariate logistic regression model. All P values of less than 0.05 were considered statistically significant.
There were 2706 patients with lower gastrointestinal symptoms who were referred for colonoscopy. Among them, 2590 participants met the inclusion criteria and underwent colonoscopy. We excluded 101 cases due to incomplete colonoscopy, unqualified bowel preparation, and withdrawal time of less than 6 min. As a result, a total of 2489 patients were included in the analysis (Figure 1).
The main indications for colonoscopy were abdominal pain (62.4%), diarrhea (51%), constipation (29.2%), and hematochezia (13.8%). The mean age of the participants was 52.1 ± 13.1 (range: 19–87 years). The female-to-male ratio was 1:1.1.
There were 1009 participants with at least one colorectal polyp (40.5%). A total of 121 specimens from 105 patients were histopathologically confirmed to have SSLs, with a prevalence of 4.2% [95% confidence interval (CI): 3.5-5.1]. The demographic and clinical features of the SSL patients are presented in Table 1. The mean age of the SSL patients was 57.6 ± 12 years, ranging from 21 to 84 years, and 91.4% were over 40 years. Male patients accounted for a larger proportion, with 61% of all cases. There was a remarkable trend in the prevalence of SSLs with increasing age. The SSL prevalence in patients under 40 was only 8.6%; meanwhile, this prevalence increased to 33.3% and 44.8% in those aged 50 to 59 years and those aged ≥ 60, respectively.
| Characteristics | Total (n = 1585) | No polyp (n = 1480) | SSLs (n = 105) | P value1 |
| Sex | ||||
| Male | 657 (41.5) | 593 (40.1) | 64 (61) | < 0.001 |
| Female | 928 (58.5) | 887 (59.9) | 41 (39) | |
| Age | ||||
| < 40 | 402 (25.4) | 393 (26.6) | 9 (8.6) | |
| 40-49 | 378 (23.8) | 364 (24.6) | 14 (13.3) | 0.232 |
| 50-59 | 437 (27.6) | 402 (27.2) | 35 (33.3) | < 0.001 |
| 60-69 | 283 (17.9) | 253 (17.1) | 30 (28.6) | < 0.001 |
| ≥ 70 | 85 (5.4) | 68 (4.6) | 17 (16.2) | < 0.001 |
| Obesity | ||||
| Yes | 296 (18.7) | 271 (18.3) | 25 (23.8) | 0.162 |
| No | 1289 (81.3) | 1209 (81.7) | 80 (76.2) | |
| Smoking | ||||
| Yes/ever | 300 (18.9) | 262 (17.7) | 38 (36.2) | < 0.001 |
| No | 1285 (81.1) | 1218 (82.3) | 67 (63.8) | |
| Alcohol consumption | ||||
| Yes | 189 (11.9) | 169 (11.4) | 20 (19) | 0.020 |
| No | 1396 (88.1) | 1311 (88.6) | 85 (81) | |
| Hypertension | ||||
| Yes | 307 (19.4) | 272 (18.4) | 35 (33.3) | < 0.001 |
| No | 1278 (80.6) | 1208 (81.6) | 70 (66.7) | |
| Diabetes mellitus | ||||
| Yes | 113 (7.1) | 93 (6.3) | 20 (19) | < 0.001 |
| No | 1472 (92.9) | 1387 (93.7) | 85 (81) | |
| Family history of CRC | ||||
| Yes | 100 (6.8) | 100 (6.8) | 7 (6.7) | 0.972 |
| No | 1478 (93.2) | 1380 (93.2) | 98 (93.3) |
In the per-patient univariate analysis, age ≥ 40, male sex, current or ex-smokers, DM, and hypertension were significantly related to SSLs (Table 1). Table 2 displays the results of the multivariate logistic regression models. The factors significantly associated with SSLs in the multivariate model were age ≥ 40 (odds ratio [OR]: 3.303; 95%CI: 1.607-6.790; P = 0.001), male sex (OR: 2.032; 95%CI: 1.204-3.429; P = 0.008), DM (OR: 2.721; 95%CI: 1.551-4.772; P < 0.001), and hypertension (OR: 1.650; 95%CI: 1.045-2.605; P = 0.031).
| Risk factors | Univariable | Multivariable | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Male | 2.335 | 1.556-3.503 | < 0.001 | 2.032 | 1.204-3.429 | 0.008 |
| Age ≥ 40 | 3.856 | 1.929-7.711 | < 0.001 | 3.303 | 1.607-6.790 | 0.001 |
| Obesity | 1.394 | 0.873-2.226 | 0.162 | 1.105 | 0.679-1.799 | 0.688 |
| Smoking | 2.637 | 1.733-4.012 | < 0.001 | 1.519 | 0.896-2.577 | 0.121 |
| Alcohol consumption | 1.825 | 1.093-3.048 | 0.020 | 1.310 | 0.738-2.326 | 0.356 |
| Hypertension | 2.221 | 1.450-3.402 | < 0.001 | 1.650 | 1.045-2.605 | 0.031 |
| Diabetes mellitus | 3.509 | 2.065-5.964 | < 0.001 | 2.721 | 1.551-4.772 | < 0.001 |
A total of 121 Lesions from 105 patients were diagnosed with SSLs. There were 90 patients (85.7%) who had one SSL, and 15 patients had two SSLs (14.3%). The mean size of the SSLs was 8.8 ± 4.8 mm. Endoscopic features of SSLs are described in Table 3.
| Characteristics | SSLs, n = 121 |
| Location | |
| Proximal | 85 (70.2) |
| Distal | 36 (29.8) |
| Size (mm) | |
| ≤ 5 | 40 (33.1) |
| 6-10 | 55 (45.5) |
| 11-20 | 24 (19.8) |
| > 20 | 2 (1.7) |
| Shape (Paris classification) | |
| 0-Is | 84 (69.4) |
| 0-Ip | 12 (9.9) |
| 0-IIa | 23 (19) |
| 0-IIb | 2 (1.7) |
| JNET type | |
| JNET 1 | 64 (52.9) |
| JNET 2A | 56 (46.3) |
| JNET 2B | 1 (0.8) |
BRAF mutations were observed in 43 (35.5%) out of 121 Lesions from patients diagnosed with SSLs. The majority were V600E, amounting to 95.3%, while the others were D594N and K601E. Risk factors correlated with BRAF mutations are described in Table 4. Male patients comprised 55.1% of the SSL group without BRAF mutations and 62.8% of those with BRAF mutations; this difference was not statistically significant (P = 0.410). In univariate analysis, age less than 60 years old and SSLs without dysplasia were correlated with BRAF mutations (P < 0.05). However, multivariate analysis indicated no risk factors with statistically significant differences.
| Factors | SSLs without BRAF mutation (n = 80), n (%) | SSLs with BRAF mutation (n = 41), n (%) | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |||
| Age < 60 | 15 (34.9) | 42 (53.8) | 2.178 (1.009-4.699) | 0.045 | 2.118 (0.935-4.797) | 0.072 |
| Proximal localization | 51 (65.4) | 34 (79.1) | 2 (0.383-4.775) | 0.115 | 1.967 (0.777-4.979) | 0.153 |
| Size ≥ 10 mm | 40 (51.3) | 15 (34.9) | 0.509 (0.236-1.097) | 0.083 | 0.875 (0.368-2.082) | 0.762 |
| Flat morphology | 12 (15.4) | 13 (30.2) | 2.383 (0.973-5.836) | 0.054 | 1.766 (0.646-4.826) | 0.268 |
| No dysplasia | 53 (67.9) | 19 (44.2) | 2.627 (1.219-5.663) | 0.013 | 2.020 (0.880-4.633) | 0.097 |
To the best of our knowledge, this is the first study evaluating the prevalence, related factors, and BRAF mutations for SSLs among Vietnamese individuals. The prevalence of SSLs has shown considerable heterogeneity across various investigations, with reported rates ranging from 1% to 14%[25-27]. According to a recent systematic analysis, SSL prevalence varied by region, with rates of 2.6% in Asia, 3.9% in Europe, 5.1% in the United States, and 10.5% in Australia[19]. The differences in patient demographics, ethnicity, colonoscopy qualities, pathology criteria, and interobserver variability among endoscopists and pathologists may explain these discrepancies[19,28,29]. Our findings indicated that the prevalence rate of SSLs was 4.2%, which was higher than that in most previous studies conducted in Asia. This discrepancy can be partly explained by several reasons. First, the research subjects in most previous studies in Asia screened asymptomatic participants, while our study was on patients with lower gastrointestinal symptoms. Second, pathological diagnostic criteria for SSLs in other Asian studies applied the old WHO classification, which might lower the prevalence of SSLs. The old WHO classification for the diagnosis of SSLs necessitated the presence of two to three distorted serrated crypts[30]. In contrast, the recent WHO criteria 2019 have been revised to require just one distorted serrated crypt for SSL diagnosis[5]. Third, in some studies, only one pathologist was responsible for interpreting the results. In our study, all lesions were assessed by two experienced gastrointestinal pathologists using the WHO criteria 2019 to minimize interobserver variation and increase diagnostic accuracy. Fourth, the participating endoscopists in our study also received training in recognizing SSLs prior to the commencement of the investigation. It is anticipated that the elements mentioned above might contribute to our investigation's enhanced precision of SSL prevalence.
Our data revealed that age ≥ 40, male sex, DM, and hypertension were independent risk factors for SSLs. Prior studies also concluded that older age was a risk factor for SSLs. However, the age cutoff in these studies was higher than in our analysis, with cutoffs over 50 or 60 years old[13,25,31]. These observed disparities could be attributed to our study participants having a lower mean age and gastrointestinal symptoms. As early-onset CRC (i.e., < 50 years of age) is prevalent in Vietnam, colonoscopy is generally considered in Vietnamese patients with lower gastrointestinal symptoms at a lower threshold of age, as recommended by the Asia-Pacific consensus recommendations for CRC screening[32]. One previous study in Vietnamese reported a significantly high proportion of early-onset CRC (i.e., < 50 years of age) of 28%, of which 11% of patients were under 40[33]. Nonetheless, it remains controversial that several studies have found no association between age and SSLs[26,34,35]. Meester et al[19] conducted a systematic review and reported that SSL prevalence increased with age. Nevertheless, the relationship was smaller than that for adenomas and did not reach statistical significance. Therefore, additional research is needed to ascertain the connection between age and the formation of SSLs, especially in the Vietnamese population.
There have been conflicting results about the relationship between gender and SSLs. Some research revealed no differences based on sex[12,27,35], whereas others showed an association between SSLs and female sex[36]. In our study, male sex increased the odds of SSLs. In a recent meta-analysis, males also had a higher rate of serrated lesions than females, and estrogen or hormone replacement therapy may reduce the risk of CRC and serrated lesions[37]. One clinical trial found a reduction of 40% in the risk of CRC in postmenopausal women receiving hormone replacement therapy, which may contribute to a decreased risk of serrated lesions[38].
The association between DM and the development of CRC and conventional adenomas has been established in previous research[39,40]. In addition, there is a correlation between the usage of diabetes drugs and CRC. Metformin use has been shown in recent meta-analyses to significantly reduce the incidence of CRC, and improve CRC outcomes[41,42]. Conversely, hyperinsulinemia is hypothesized to stimulate the development and proliferation of cells[43]. The relationship between insulin therapy and cancer growth is biologically connected via hyperinsulinemia. One meta-analysis of observational studies suggested that insulin therapy might increase the risk of CRC[44]. However, additional prospective cohort studies with extended follow-up periods are necessary to validate this correlation. Regarding the relationship between DM and SSLs, limited data have addressed this matter. Lui et al[13] provided evidence that DM increased the risk of developing SSLs in the screening population aged 50-70. Burnett-Hartman et al[45] included symptomatic patients, and DM was considered a risk factor for SSLs. These findings were consistent with our results. Nevertheless, Macarie et al[46] demonstrated that DM was unrelated to SSLs, although hypertension and obesity were statistically associated with SSL development. Hence, the association between DM and SSLs remains unclear. Further investigations should be carried out to clarify this issue.
Limited studies have evaluated the relationship between hypertension and SSL patients, and the results were inconsistent. Our results indicated that hypertension was statistically associated with SSLs. This finding was also reported by a case-control study conducted in Romania[46]. One study in Korea also showed that SSLs with dysplasia/carcinoma were significantly associated with metabolic diseases, including hypertension, type 2 DM, and dyslipidemia[47]. In contrast, two studies in the Chinese population found no association between hypertension and SSLs[13]. Therefore, this is a crucial issue that needs to be further investigated.
The most prominent critical feature in the initial phase of the serrated neoplastic pathway is a mutation in the BRAF proto-oncogene, which activates the mitogen-activated protein kinase (MAPK) cascade. This BRAF mutation leads to uncontrolled cell proliferation, similar to the KRAS mutation in adenomas[17]. The incidence of BRAF mutations has been reported in 50%-72% of microvesicular hyperplastic polyps, 70%-80% of SSLs, and only 1% of tubular adenomas[48]. However, there have been differences in BRAF mutation rates in Eastern and Western countries. Based on data from Western countries, the prevalence of BRAF mutations in SSLs ranges from 63% to 100%[17,49-53], which is higher than that in East Asian nations, with reported rates ranging from 14% to 86%[54-58]. In our study, the percentage of BRAF mutations was 35.5%. These dissimilarities indicated that BRAF mutations in SSLs may depend on ethnicity, lifestyle, and diet. Moreover, BRAF mutations may not be a significant molecular feature of the serrated neoplasia pathway in a subset of Eastern populations, as in Western populations. The low frequency of BRAF mutations in SSLs in Asia may also partially explain the low prevalence of SSLs compared to Western countries. Furthermore, our results reported no statistically significant association with age, sex, endoscopic, or pathologic factors. More future studies will be needed to better investigate the molecular characteristics of SSLs in the Vietnamese population.
This study has several limitations. First, this is a single-center study. Second, some potential risk factors, such as dietary habits, dyslipidemia, and medication histories, have not been investigated. Third, the study participants were symptomatic patients undergoing colonoscopy. These limitations could limit the generalizability of the study findings. Fourth, the available data about diabetes duration and therapy used in the patients with DM in our study are insufficient and constrained. Therefore, we could not analyze these data in our manuscript.
In conclusion, we reported for the first time a prevalence of SSLs of 4.2% in Vietnamese patients with lower gas
Sessile serrated lesions (SSLs) are precancerous colorectal lesions that should be identified and removed to prevent colorectal cancer. However, previous research in Vietnam mainly focused on the adenoma pathway, with limited information on the serrated pathway.
The reported prevalence of SSLs varies widely across studies, leading to underestimating their prevalence and biased analysis. Moreover, studies analyzing risk factors for SSLs have been limited and controversial. Furthermore, BRAF mutations may be a potential therapeutic target in serrated colorectal neoplasia.
Our study aimed to evaluate the prevalence, risk factors, and BRAF mutations of SSLs in Vietnamese patients. These findings may provide valuable insights into the prevention, early detection, treatment improvement, and surveillance approaches for SSLs in the Vietnamese population.
This is a cross-sectional study carried out on patients with lower gastrointestinal symptoms who underwent colonoscopy at the University Medical Center at Ho Chi Minh City, Vietnam. SSLs were diagnosed on histopathology according to the updated 2019 WHO classification. BRAF mutation analysis was performed using the Sanger DNA sequencing method. Univariate and multivariate logistic regression analyses were used to determine SSL-associated factors.
There were 2489 patients were included in the analysis, with a mean age of 52.1 ± 13.1 and a female-to-male ratio of 1:1.1. A total of 121 specimens from 105 patients were histopathologically confirmed to have SSLs, with a prevalence of 4.2% [95% confidence interval (CI): 3.5-5.1]. The factors significantly associated with SSLs in the multivariate model were age ≥ 40 [odds ratio (OR): 3.303; 95%CI: 1.607-6.790; P = 0.001], male sex (OR: 2.032; 95%CI: 1.204-3.429; P = 0.008), diabetes mellitus (OR: 2.721; 95%CI: 1.551-4.772; P < 0.001), and hypertension (OR: 1.650; 95%CI: 1.045-2.605; P = 0.031). The rate of BRAF mutations in SSLs was 35.5%.
We reported for the first time a prevalence of SSLs in Vietnamese patients with lower gastrointestinal symptoms. Our data revealed that age ≥ 40, male sex, diabetes mellitus, and hypertension were independent risk factors for SSLs. Additional investigations are needed to ascertain the connection between these risk factors and SSLs, especially in the Vietnamese population.
It is crucial to conduct multicenter, prospective, and follow-up studies to determine the prevalence, risk factors, and molecular characteristics of SSLs, especially in the Vietnamese population.
The authors would like to thank Drs. Truc Le Thanh Tran, Mai Ngoc Luu, Quang Dinh Le, Doan Thi Nha Nguyen, and Vy Ly Thao Tran at the University of Medicine and Pharmacy at Ho Chi Minh City and the University Medical Center at Ho Chi Minh City for their support.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68525] [Article Influence: 13705.0] [Reference Citation Analysis (201)] |
| 2. | van Toledo DEFWM, IJspeert JEG, Bossuyt PMM, Bleijenberg AGC, van Leerdam ME, van der Vlugt M, Lansdorp-Vogelaar I, Spaander MCW, Dekker E. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022;7:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, Ket SN, Leedham SJ, Phull PS, Rutter MD, Shepherd NA, Tomlinson I, Rees CJ. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 4. | Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2740] [Article Influence: 456.7] [Reference Citation Analysis (3)] |
| 6. | Sacco M, De Palma FDE, Guadagno E, Giglio MC, Peltrini R, Marra E, Manfreda A, Amendola A, Cassese G, Dinuzzi VP, Pegoraro F, Tropeano FP, Luglio G, De Palma GD. Serrated lesions of the colon and rectum: Emergent epidemiological data and molecular pathways. Open Med (Wars). 2020;15:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Hazewinkel Y, López-Cerón M, East JE, Rastogi A, Pellisé M, Nakajima T, van Eeden S, Tytgat KM, Fockens P, Dekker E. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Rashtak S, Rego R, Sweetser SR, Sinicrope FA. Sessile Serrated Polyps and Colon Cancer Prevention. Cancer Prev Res (Phila). 2017;10:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Ensari A, Bilezikçi B, Carneiro F, Doğusoy GB, Driessen A, Dursun A, Flejou JF, Geboes K, de Hertogh G, Jouret-Mourin A, Langner C, Nagtegaal ID, Offerhaus J, Orlowska J, Ristimäki A, Sanz-Ortega J, Savaş B, Sotiropoulou M, Villanacci V, Kurşun N, Bosman F. Serrated polyps of the colon: how reproducible is their classification? Virchows Arch. 2012;461:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Payne SR, Church TR, Wandell M, Rösch T, Osborn N, Snover D, Day RW, Ransohoff DF, Rex DK. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol. 2014;12:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 11. | Abdeljawad K, Vemulapalli KC, Kahi CJ, Cummings OW, Snover DC, Rex DK. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc. 2015;81:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Burnett-Hartman AN, Passarelli MN, Adams SV, Upton MP, Zhu LC, Potter JD, Newcomb PA. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol. 2013;177:625-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Lui RN, Kyaw MH, Lam TYT, Ching JYL, Chan VCW, Wong MCS, Sung JJY. Prevalence and risk factors for sessile serrated lesions in an average risk colorectal cancer screening population. J Gastroenterol Hepatol. 2021;36:1656-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Davenport JR, Su T, Zhao Z, Coleman HG, Smalley WE, Ness RM, Zheng W, Shrubsole MJ. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polyps. Gut. 2018;67:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | O'Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, Amorosino M, Farraye FA. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 386] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 16. | Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7459] [Cited by in RCA: 7752] [Article Influence: 323.0] [Reference Citation Analysis (0)] |
| 17. | Mesteri I, Bayer G, Meyer J, Capper D, Schoppmann SF, von Deimling A, Birner P. Improved molecular classification of serrated lesions of the colon by immunohistochemical detection of BRAF V600E. Mod Pathol. 2014;27:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Rad R, Cadiñanos J, Rad L, Varela I, Strong A, Kriegl L, Constantino-Casas F, Eser S, Hieber M, Seidler B, Price S, Fraga MF, Calvanese V, Hoffman G, Ponstingl H, Schneider G, Yusa K, Grove C, Schmid RM, Wang W, Vassiliou G, Kirchner T, McDermott U, Liu P, Saur D, Bradley A. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell. 2013;24:15-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 19. | Meester RGS, van Herk MMAGC, Lansdorp-Vogelaar I, Ladabaum U. Prevalence and Clinical Features of Sessile Serrated Polyps: A Systematic Review. Gastroenterology. 2020;159:105-118.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Cho H, Hashimoto T, Yoshida H, Taniguchi H, Ogawa R, Mori T, Hiraoka N, Saito Y, Sekine S. Reappraisal of the genetic heterogeneity of sessile serrated adenoma/polyp. Histopathology. 2018;73:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Iwatate M, Hirata D, Francisco CPD, Co JT, Byeon JS, Joshi N, Banerjee R, Quach DT, Aye TT, Chiu HM, Lau LHS, Ng SC, Ang TL, Khomvilai S, Li XB, Ho SH, Sano W, Hattori S, Fujita M, Murakami Y, Shimatani M, Kodama Y, Sano Y; CATCH project team. Efficacy of international web-based educational intervention in the detection of high-risk flat and depressed colorectal lesions higher (CATCH project) with a video: Randomized trial. Dig Endosc. 2022;34:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 22. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 987] [Article Influence: 58.1] [Reference Citation Analysis (1)] |
| 23. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 675] [Article Influence: 32.1] [Reference Citation Analysis (2)] |
| 24. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 25. | IJspeert JE, de Wit K, van der Vlugt M, Bastiaansen BA, Fockens P, Dekker E. Prevalence, distribution and risk of sessile serrated adenomas/polyps at a center with a high adenoma detection rate and experienced pathologists. Endoscopy. 2016;48:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (3)] |
| 26. | Hazewinkel Y, de Wijkerslooth TR, Stoop EM, Bossuyt PM, Biermann K, van de Vijver MJ, Fockens P, van Leerdam ME, Kuipers EJ, Dekker E. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S, O'Brien MJ, Farraye FA. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 269] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 28. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L, Whitehall V, Leggett B. Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol. 2014;38:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Gourevitch RA, Rose S, Crockett SD, Morris M, Carrell DS, Greer JB, Pai RK, Schoen RE, Mehrotra A. Variation in Pathologist Classification of Colorectal Adenomas and Serrated Polyps. Am J Gastroenterol. 2018;113:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Bosman FT; World Health Organization; International Agency for Research on Cancer. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer, 2010. |
| 31. | Sekiguchi M, Kakugawa Y, Matsumoto M, Nakamura K, Mizuguchi Y, Takamaru H, Yamada M, Sakamoto T, Saito Y, Matsuda T. Prevalence of serrated lesions, risk factors, and their association with synchronous advanced colorectal neoplasia in asymptomatic screened individuals. J Gastroenterol Hepatol. 2020;35:1938-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Sung JJY, Chiu HM, Lieberman D, Kuipers EJ, Rutter MD, Macrae F, Yeoh KG, Ang TL, Chong VH, John S, Li J, Wu K, Ng SSM, Makharia GK, Abdullah M, Kobayashi N, Sekiguchi M, Byeon JS, Kim HS, Parry S, Cabral-Prodigalidad PAI, Wu DC, Khomvilai S, Lui RN, Wong S, Lin YM, Dekker E. Third Asia-Pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance. Gut. 2022;71:2152-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 33. | Quach DT, Nguyen OT. Clinical, endoscopic and pathogical characteristics of early-onset colorectal cancer in Vietnamese. Asian Pac J Cancer Prev. 2012;13:1767-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Cao HL, Chen X, Du SC, Song WJ, Wang WQ, Xu MQ, Wang SN, Piao MY, Cao XC, Wang BM. Detection Rate, Distribution, Clinical and Pathological Features of Colorectal Serrated Polyps. Chin Med J (Engl). 2016;129:2427-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Zhang R, Ni Y, Guo CL, Lui RN, Wu WK, Sung JJ, Wong VW, Wong SH. Risk factors for sessile serrated lesions among Chinese patients undergoing colonoscopy. J Gastroenterol Hepatol. 2023;38:1468-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 37. | Huang J, Chan PSF, Pang TWY, Choi P, Chen X, Lok V, Zheng ZJ, Wong MCS. Rate of detection of serrated lesions at colonoscopy in an average-risk population: a meta-analysis of 129,001 individuals. Endosc Int Open. 2021;9:E472-E481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Slattery ML, Potter JD, Curtin K, Edwards S, Ma KN, Anderson K, Schaffer D, Samowitz WS. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. 2001;61:126-130. [PubMed] |
| 39. | Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106:1911-21; quiz 1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 40. | Eddi R, Karki A, Shah A, DeBari VA, DePasquale JR. Association of type 2 diabetes and colon adenomas. J Gastrointest Cancer. 2012;43:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Ng CW, Jiang AA, Toh EMS, Ng CH, Ong ZH, Peng S, Tham HY, Sundar R, Chong CS, Khoo CM. Metformin and colorectal cancer: a systematic review, meta-analysis and meta-regression. Int J Colorectal Dis. 2020;35:1501-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Kobiela J, Dobrzycka M, Jędrusik P, Kobiela P, Spychalski P, Śledziński Z, Zdrojewski T. Metformin and Colorectal Cancer - A Systematic Review. Exp Clin Endocrinol Diabetes. 2019;127:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG; Diabetes and Cancer Research Consortium. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 44. | Bu WJ, Song L, Zhao DY, Guo B, Liu J. Insulin therapy and the risk of colorectal cancer in patients with type 2 diabetes: a meta-analysis of observational studies. Br J Clin Pharmacol. 2014;78:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Anderson JC, Rangasamy P, Rustagi T, Myers M, Sanders M, Vaziri H, Wu G, Birk JW, Protiva P. Risk factors for sessile serrated adenomas. J Clin Gastroenterol. 2011;45:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Macarie M, Bataga S, Mocan S, Pantea M, Opaschi R, Voidazan S, Macarie I. Correlation of Metabolic Risk Factors with Sessile Serrated Lesions. J Gastrointestin Liver Dis. 2020;29:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Jung P, Kim HW, Park SB, Kang DH, Choi CW, Kim SJ, Nam HS, Ryu DG, Shin DH, Na JY, Yun MS. Clinical and endoscopic characteristics of sessile serrated lesions with dysplasia/carcinoma. Korean J Intern Med. 2023;38:349-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 48. | Fernando WC, Miranda MS, Worthley DL, Togashi K, Watters DJ, Leggett BA, Spring KJ. The CIMP Phenotype in BRAF Mutant Serrated Polyps from a Prospective Colonoscopy Patient Cohort. Gastroenterol Res Pract. 2014;2014:374926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Liu C, Bettington ML, Walker NI, Dwine J, Hartel GF, Leggett BA, Whitehall VLJ. CpG Island Methylation in Sessile Serrated Adenomas Increases With Age, Indicating Lower Risk of Malignancy in Young Patients. Gastroenterology. 2018;155:1362-1365.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Kim YH, Kakar S, Cun L, Deng G, Kim YS. Distinct CpG island methylation profiles and BRAF mutation status in serrated and adenomatous colorectal polyps. Int J Cancer. 2008;123:2587-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 51. | Rau TT, Agaimy A, Gehoff A, Geppert C, Jung K, Knobloch K, Langner C, Lugli A, Groenbus-Lurkin I, Nagtegaal ID, Rüschoff J, Saegert X, Sarbia M, Schneider-Stock R, Vieth M, Zwarthoff EC, Hartmann A. Defined morphological criteria allow reliable diagnosis of colorectal serrated polyps and predict polyp genetics. Virchows Arch. 2014;464:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Rau TT, Atreya R, Aust D, Baretton G, Eck M, Erlenbach-Wünsch K, Hartmann A, Lugli A, Stöhr R, Vieth M, Wirsing AM, Zlobec I, Katzenberger T. Inflammatory response in serrated precursor lesions of the colon classified according to WHO entities, clinical parameters and phenotype-genotype correlation. J Pathol Clin Res. 2016;2:113-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Sandmeier D, Benhattar J, Martin P, Bouzourene H. Serrated polyps of the large intestine: a molecular study comparing sessile serrated adenomas and hyperplastic polyps. Histopathology. 2009;55:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Fujita K, Yamamoto H, Matsumoto T, Hirahashi M, Gushima M, Kishimoto J, Nishiyama K, Taguchi T, Yao T, Oda Y. Sessile serrated adenoma with early neoplastic progression: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 55. | Kim KM, Lee EJ, Ha S, Kang SY, Jang KT, Park CK, Kim JY, Kim YH, Chang DK, Odze RD. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol. 2011;35:1274-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Maeda T, Suzuki K, Togashi K, Nokubi M, Saito M, Tsujinaka S, Kamiyama H, Konishi F. Sessile serrated adenoma shares similar genetic and epigenetic features with microsatellite unstable colon cancer in a location-dependent manner. Exp Ther Med. 2011;2:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Qiu Y, Fu X, Zhang W, Xu Y, Xiao L, Chen X, Shi L, Zhou X, Xia G, Peng Y, Deng M. Prevalence and molecular characterisation of the sessile serrated adenoma in a subset of the Chinese population. J Clin Pathol. 2014;67:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Tanaka Y, Yamano HO, Yamamoto E, Matushita HO, Aoki H, Yoshikawa K, Takagi R, Harada E, Nakaoka M, Yoshida Y, Eizuka M, Sugai T, Suzuki H, Nakase H. Endoscopic and molecular characterization of colorectal sessile serrated adenoma/polyps with cytologic dysplasia. Gastrointest Endosc. 2017;86:1131-1138.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Viet Nam
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cigrovski Berkovic M, Croatia S-Editor: Gong ZM L-Editor: A P-Editor: Zhang XD