Published online Dec 24, 2024. doi: 10.5306/wjco.v15.i12.1520
Revised: September 19, 2024
Accepted: October 15, 2024
Published online: December 24, 2024
Processing time: 90 Days and 17.4 Hours
In the ongoing quest for new treatments in medicine, traditional Chinese medicine offers unique insights and potential. Recently, studies on the ability of Calculus bovis to inhibit M2-type tumour-associated macrophage polarisation by modu
Core Tip: Calculus bovis (C. bovis) has shown remarkable potential in liver cancer treatment research. It was found to modulate the Wnt/β-catenin signalling pathway and inhibit the polarisation of tumour-associated macrophages, thereby inhibiting liver cancer progression. These findings not only reveal the immunoregulatory mechanism of C. bovis but also provide a new strategy and theoretical basis for the treatment of liver cancer. However, since the specific anticancer components of C. bovis are not known, future studies should focus on the inhibition of the liver cancer pathway mediated by specific components of C. bovis to facilitate clinical advancements in liver cancer treatment.
- Citation: Du H, Chen HB, Zhao Y. Exploring a new chapter in traditional Chinese medicine: The potential of Calculus bovis in liver cancer treatment. World J Clin Oncol 2024; 15(12): 1520-1527
- URL: https://www.wjgnet.com/2218-4333/full/v15/i12/1520.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i12.1520
The latest figures for 2020 indicate that liver cancer was the sixth most common cancer and the third leading cause of cancer deaths worldwide in 2020. There are approximately 906000 new cases per year, with an incidence rate of 9.5 per 100000, or 4.7 per cent, and 830000 deaths, with a mortality rate of 8.7 per 100000, or 8.3 per cent. Its incidence is growing globally, and it is estimated that by 2025, more than 1 million people will develop liver cancer each year. Hepatocellular carcinoma is the most common type of liver cancer, accounting for approximately 90 per cent of all cases[1]. Liver cancer is projected to become the third leading cause of malignancy-related deaths by 2030[2]. Hepatitis B virus (HBV) infection is the most important risk factor for the development of primary liver cancer, accounting for approximately 50 per cent of all cases. Although the risk of liver cancer due to hepatitis C virus (HCV) infection has been greatly reduced in patients with hepatitis C as a result of a sustained viral response with antiviral medications, patients with cirrhosis are considered to have an increased risk of developing primary liver cancer even after HCV clearance[3]. Alcohol consumption[4], aflatoxin exposure[5], and genetic predispositions[6] are also crucial contributors to liver cancer. Nonalcoholic steatohepatitis, which is linked to metabolic syndrome and diabetes mellitus, is emerging as the most rapidly increasing source of liver cancer in Western countries[7]. In addition, aristolochic acid and tobacco have also been reported as cocausal factors in liver cancer[8,9].
The main treatments for primary liver cancer currently include surgery, radiotherapy, hepatic artery chemoembolisation (TACE), systemic therapy, and traditional Chinese medicine (TCM).
Surgical treatment, including hepatectomy and liver transplantation, has been the mainstay of primary liver cancer treatment and achieves the best results, with a 5-year survival rate of approximately 70-80 per cent[10,11]. The decision to resect or transplant must consider the patient's liver function, degree of portal hypertension, fitness status and tumour characteristics. The treatment of choice for liver cancer patients without cirrhosis is hepatectomy. However, the re
Extracorporeal radiation therapy achieves a radiological response to different tumour sizes and stages of liver cancer and attenuates extrahepatic metastases[15]. A comprehensive analysis examined the prognosis of 102 patients with unresectable primary liver cancer and Child-Pugh A liver function who received more than 6 fractions of 24-54 Gy photonic stereotactic body radiotherapy, 54% of whom achieved objective remission with a median overall survival of 17 months[16].
TACE therapy has a positive impact on the survival of patients with intermediate-stage liver cancer and has been recognized as the standard of care for this patient population worldwide[17]. The American Association for the Study of Liver Diseases guidelines recommend TACE for the treatment of patients with intermediate-stage liver cancer, a decision supported by level 2 evidence[18].
The majority of liver cancer patients are diagnosed at an advanced stage, which often results in the loss of the opportunity for radical surgical treatment and leads to a poor clinical prognosis. It is estimated that approximately 50%-60% of patients with liver cancer will use systemic therapy during their lifetime[19], especially in the advanced stages of the disease, and the median survival time of symptomatic advanced liver cancer patients receiving systemic therapy is approximately 1.0-1.5 years[20]. Receptor tyrosine kinase inhibitors have been the first-line treatment of choice for patients with advanced liver cancer for nearly a decade or more[21,22]. At present, on the basis of positive phase III data, there are three regimens in the guidelines (regorafenib, ramucirumab, and cabozantinib) approved for second-line tre
PROTACs can induce the degradation of target proteins through the ubiquitin-proteasome system, offering a highly effective approach for eliminating proteins that were previously considered 'undruggable'[29]. Currently, most PROTACs used for the treatment of liver cancer, such as BETd-260 (targeting the BET protein)[30], ARV-771 (a BRD degrader)[31], JB170 (an Aurora A degrader)[32], CP-10 (targeting CDK4)[33], and BSJ-03-123 (targeting CDK6)[33], are still in the preclinical study stage, and their clinical efficacy still needs to be further verified. Recently, two PROTAC probes developed by Arvinas LLC, ARV-110 and ARV-471 (with undisclosed structures), have been in phase І clinical trials (NCT03888612 and NCT04072952 at clinicaltrials.gov) for prostate and breast cancer, respectively[34]. However, further clinical studies on liver cancer are still needed (Table 1).
| Name | Target | Stage | Potential pros | Potential cons |
| PROTACs | ||||
| JB170 | AURKA | Preclinical | Versatile drug design potential; high target specificity; reduced risk of drug resistance | Higher complexity in design and development cost off-target effects; limited clinical data available |
| BETd-260 | BET | Preclinical | ||
| ARV-771 | BRD2/3/4 | Preclinical | ||
| CP-10 | CDK6 | Preclinical | ||
| BSJ-03-123 | CDK6 | Preclinical | ||
| ADCs | ||||
| MGC018 | CD276 | Preclinical | High target specificity; reduced systemic toxicity | Limited scope of targetable antigens off-target effects; challenges in stability and drug release |
| ABBV-400 | MET | Preclinical | ||
| hYP7-DC | GPC3 | Preclinical | ||
| CLDN6-DM1 | CLDN6 | Preclinical | ||
| mRNA vaccines | ||||
| PGV002 | Neoantigen | Phase I | Personalized treatment; potential; potential for broader; immune response; lower drug toxic | Potential for immune-related; side effects; Limited clinical data in; cancer treatment |
| PCV | Neoantigen | Phase I | ||
| ABOR2014 | Neoantigen | Phase I |
ADCs represent a novel class of agents specifically designed to deliver potent cytotoxic substances directly into tumours with high specificity. They are composed of three main components: Monoclonal antibodies, linkers, and cytotoxic drugs[35,36]. Preclinical studies have demonstrated the therapeutic potential of ADCs in the treatment of liver cancer[37,38]. GPC3 is highly expressed in liver cancer and is considered a potential therapeutic target. Studies have shown that GPC3-specific ADCs exhibit potent tumour killing activity in GPC3-positive liver cancer cell lines (Hep3B and A431-GPC3 cells) and their xenograft models[38]. GPC3-targeted chimeric antigen receptor (CAR)-T-cell therapy has a high safety profile in patients with liver cancer. The combination of GPC3-CAR-T cells and sorafenib was shown to increase tumour cell apoptosis in mouse experiments, demonstrating the clinical potential of GPC3-targeted CAR-T-cell therapy for liver cancer[39]. Moreover, GPC3 is a potential diagnostic and therapeutic target for liver cancer. An et al[40] successfully developed sdAb-derived GPC3-targeted immuno-PET imaging strategies and demonstrated their excellent diagnostic accuracy in preclinical liver cancer models[40]. Furthermore, dysregulation of CLDN6 promotes the pheno
mRNA vaccines have emerged as a new trend in the treatment of liver cancer[42,43]. Typically, mRNA vaccines deliver engineered mRNAs into tumour cells, inducing the production of tumour-specific antigens or neoantigens. This process activates the immune system, enabling it to recognize and eliminate cancer cells that present these antigens, thereby enhancing the antitumour immune response. By expressing multiple neoantigens, mRNA-based cancer vaccines have the potential to address the high degree of heterogeneity among tumour cells[42]. In 2017, a study reported the first clinical use of an mRNA neoantigen vaccine that demonstrated a significant reduction in the risk of metastasis and prolonged progression-free survival (PFS) in patients with melanoma[44]. Subsequent studies have further demonstrated the therapeutic value of mRNA vaccines in many types of cancer, including lung, colorectal and pancreatic cancers[45-47]. mRNA-based cancer vaccines have therapeutic potential for treating malignant tumours, including those in liver cancer. Deng et al[48] optimized and synthesized stable mRNA encoding the costimulator Oxford 40 ligand (OX40L). In vivo studies in mice revealed that LNPs with OX40L mRNA significantly reduced the rate of tumour growth and increased the survival rate of H22 tumour-bearing mice[48]. A phase I trial (NCT05738447) conducted in 2023 aimed to apply mRNA immunotherapy technology in patients with HBV-related refractory liver cancer. Another ongoing trial is a phase I trial (NCT05738447) that aims to apply mRNA immunotherapy technology in patients with HBV-related refractory liver cancer[49] (Table 1).
Calculus bovis (C. bovis), a valuable herb used in TCM, has long been recognized in folklore for its unique therapeutic effects. However, with advancements in modern science and technology-particularly the rapid progress in molecular biology and bioinformatics-we are now able to analyse the scientific mechanisms underlying its effects more deeply. This study reveals the immunomodulatory effects of C. bovis in the treatment of liver cancer, particularly its impact on immune cells within the tumour microenvironment. Through detailed compositional analyses and network pharmacological predictions, this research represents a significant breakthrough in the modernization of TCM.
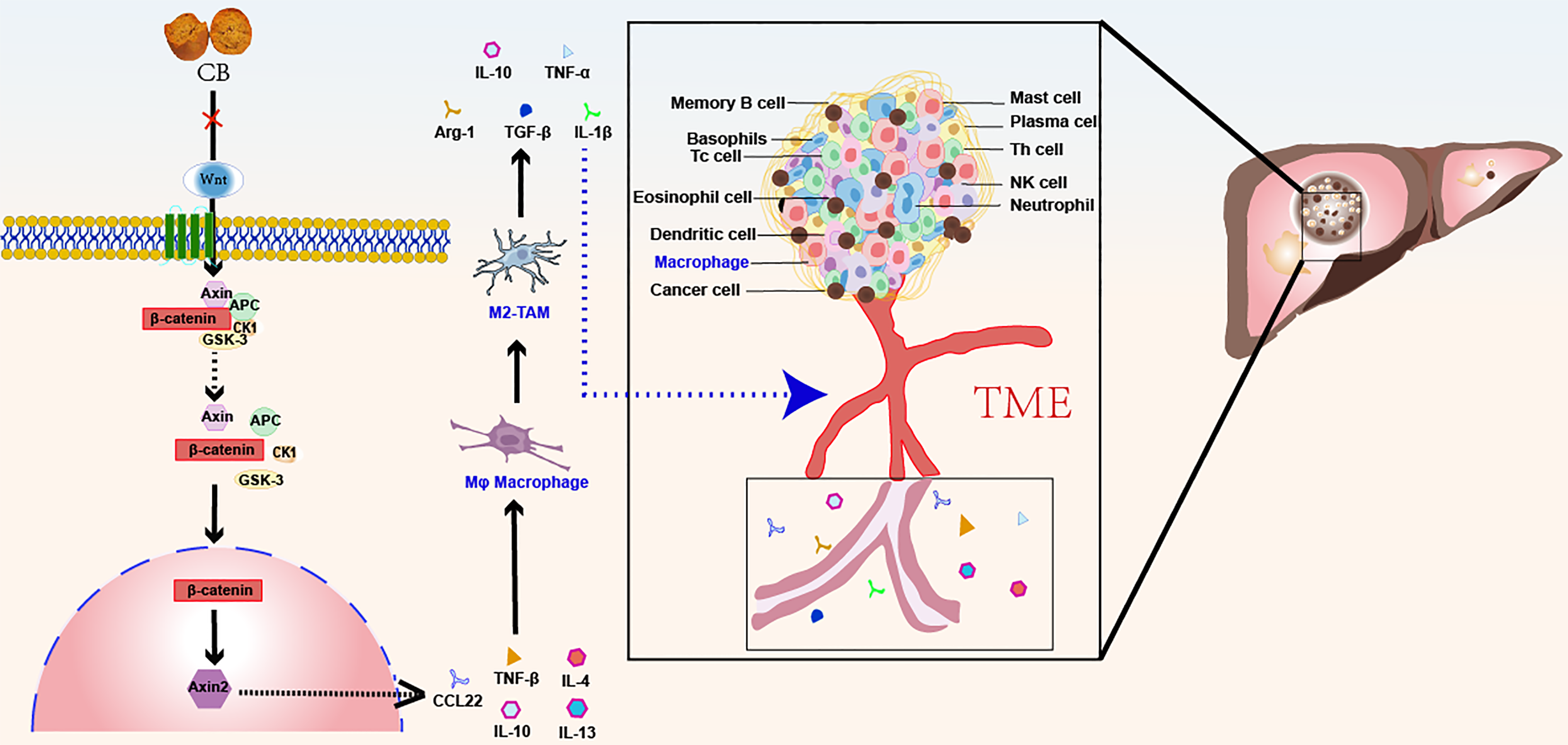
The tumour microenvironment is a critical site for tumorigenesis, progression, and metastasis, where tumour-associated macrophages (TAMs), a key population of immune cells, directly influence tumour progression through their polarisation status. M2-type TAMs have emerged as significant targets for tumour therapy because of their roles in promoting tumour growth, angiogenesis, and immunosuppression. In this study, through advanced techniques such as transcriptome sequencing, we demonstrated that C. bovis inhibits the progression of liver cancer by regulating the polarization of M2-type TAMs. This finding not only enhances our understanding of the mechanisms underlying tumour-immune microenvironment regulation but also provides an important theoretical foundation for the development of novel anticancer drugs.
More strikingly, this study revealed the crucial role of the Wnt/β-catenin signalling pathway in the regulation of M2-type TAM polarisation by C. bovis. As a signalling pathway that plays a vital role in cell proliferation, differentiation, and migration, the aberrant activation of Wnt/β-catenin signalling is closely associated with the development of various tumours. By inhibiting the activation of this pathway, C. bovis effectively blocks the polarization process of M2-type TAMs, opening new avenues for the treatment of liver cancer. The elucidation of this mechanism not only provides a scientific explanation for the anticancer effects of C. bovis but also offers guidance for future drug development targeting this pathway (Figure 1)[50].
This study provides new vigour and hope for the study of primary liver cancer; however, this study did not fully con
Second, this study proposes that C. bovis inhibits the growth of liver cancer through the Wnt/β-catenin pathway, but further experimental validation is needed to assess its future clinical translational potential. In addition to its complex compositional factors, Wnt/β-catenin signalling pathway immunotherapy-related drugs are currently used for targeted inhibition of liver cancer, and the process of clinical treatment has achieved positive therapeutic effects[55]. Therefore, future research should focus on the inhibition of liver cancer pathways mediated by specific components to promote the development of clinical treatments for liver cancer, and future studies should focus on the structure-function rela
The findings concerning the use of C. bovis in the treatment of liver cancer represent a microcosm of the modernisation of TCM. TCM contains a wealth of bioactive components and unique pharmacological mechanisms. As long as we use modern scientific and technological methods for in-depth exploration and elucidation, we can discover more drugs or drug-like compounds with potential clinical application value. In the future, with the continuous expansion of research and advancements in technology, we believe that TCM will play an increasingly important role in the treatment of cancer and the entire field of medicine.
Future research on liver cancer should consider the following: (1) The combination of an ICI with a TKI or VEGF inhibitor almost doubles the response rate and survival benefit for tumours compared with a single agent[23]. It is unknown whether combination regimens with other drugs are equally effective; (2) While therapeutic approaches related to vaccines have not yet led to notable clinical outcomes, there is an increasing focus on cell-based strategies such as CAR T-cell therapy, which is now being studied in early-stage liver cancer[60]; and (3) Researchers are using naked antibodies and antibody-drug couplings to find novel antibody targets against epitopes unique to liver cancer[61].
The treatment of liver cancer remains a global challenge, with its incidence continuously increasing worldwide. The need for neoadjuvant and adjuvant therapies for liver cancer has not yet been met. Exploring the pathogenesis of liver cancer and discovering new biomarkers will aid in the development of emerging combination therapies. TCM, an established medical system, has been recognized by the World Health Organization. To date, increasing evidence has confirmed that Chinese herbs such as Pien Tze Huang, Bupleurum, Astragalus, and Poria have anti-liver cancer effects[62]. Therefore, advancements in the understanding of the mechanisms involved in TCM and liver cancer are expected to lead to the development of new therapeutic drugs. However, most current research has focused on the effects of complex TCM components on liver cancer signalling pathways, and it is still unclear which compounds play a role in regulating these pathways, thus further clarification from future studies is necessary. Research on C. bovis in the treatment of liver cancer not only reveals its unique anticancer mechanisms but also provides valuable experience and insights for the modernisation of TCM. Future clinical or animal studies should be dedicated to investigating the specific components that regulate liver cancer signalling pathways to facilitate the development of new drugs and provide more effective treatments for clinical use.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68777] [Article Influence: 13755.4] [Reference Citation Analysis (202)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5376] [Article Influence: 448.0] [Reference Citation Analysis (0)] |
| 3. | Shi JF, Cao M, Wang Y, Bai FZ, Lei L, Peng J, Feletto E, Canfell K, Qu C, Chen W. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer. 2021;148:1051-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 4. | Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 897] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 5. | Groopman JD, Smith JW, Rivera-Andrade A, Alvarez CS, Kroker-Lobos MF, Egner PA, Gharzouzi E, Dean M, McGlynn KA, Ramírez-Zea M. Aflatoxin and the Etiology of Liver Cancer and Its Implications for Guatemala. World Mycotoxin J. 2021;14:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Qiu Z, Li H, Zhang Z, Zhu Z, He S, Wang X, Wang P, Qin J, Zhuang L, Wang W, Xie F, Gu Y, Zou K, Li C, Li C, Wang C, Cen J, Chen X, Shu Y, Zhang Z, Sun L, Min L, Fu Y, Huang X, Lv H, Zhou H, Ji Y, Zhang Z, Meng Z, Shi X, Zhang H, Li Y, Hui L. A Pharmacogenomic Landscape in Human Liver Cancers. Cancer Cell. 2019;36:179-193.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Kim H, Lee DS, An TH, Park HJ, Kim WK, Bae KH, Oh KJ. Metabolic Spectrum of Liver Failure in Type 2 Diabetes and Obesity: From NAFLD to NASH to HCC. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Li Y, Zhu S, Xue M, Jing Y, Liu X, Cai D, Zhao Y, Bian Y, Zhang Z, Zhang L. Aristolochic acid I promotes the invasion and migration of hepatocellular carcinoma cells by activating the C3a/C3aR complement system. Toxicol Lett. 2023;378:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Premkumar M, Anand AC. Tobacco, Cigarettes, and the Liver: The Smoking Gun. J Clin Exp Hepatol. 2021;11:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Newman JL, Stiers DL, Anderson WH, Schmid HH. Phase behavior of synthetic N-acylethanolamine phospholipids. Chem Phys Lipids. 1986;42:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Halbreich U, Goldstein S, Cooper T, Mathé AA. Different pattern of association of beta-endorphin and cortisol responses to dextroamphetamine in postmenopausal women and young men. Psychiatry Res. 1985;16:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 13. | Belghiti J. Resection and liver transplantation for HCC. J Gastroenterol. 2009;44 Suppl 19:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Franssen B, Jibara G, Tabrizian P, Schwartz ME, Roayaie S. Actual 10-year survival following hepatectomy for hepatocellular carcinoma. HPB (Oxford). 2014;16:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Chen CP. Role of External Beam Radiotherapy in Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:701-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 612] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 17. | Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E; ESMO Guidelines Committee. Corrigendum to "Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up": [Annals of Oncology 29 suppl. 4 (2018) v238-iv255]. Ann Oncol. 2022;33:666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 18. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3152] [Article Influence: 394.0] [Reference Citation Analysis (3)] |
| 19. | Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 258] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 20. | Galle PR, Dufour JF, Peck-Radosavljevic M, Trojan J, Vogel A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021;17:1237-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Hsu CH, Shen YC, Shao YY, Hsu C, Cheng AL. Sorafenib in advanced hepatocellular carcinoma: current status and future perspectives. J Hepatocell Carcinoma. 2014;1:85-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Park MK, Lee YB, Moon H, Choi NR, Kim MA, Jang H, Nam JY, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH. Effectiveness of Lenvatinib Versus Sorafenib for Unresectable Hepatocellular Carcinoma in Patients with Hepatic Decompensation. Dig Dis Sci. 2022;67:4939-4949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 926] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 24. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 1054] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 25. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1984] [Article Influence: 248.0] [Reference Citation Analysis (0)] |
| 26. | Yang X, Yang C, Zhang S, Geng H, Zhu AX, Bernards R, Qin W, Fan J, Wang C, Gao Q. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell. 2024;42:180-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 261] [Reference Citation Analysis (0)] |
| 27. | Yang C, Guo Y, Qian R, Huang Y, Zhang L, Wang J, Huang X, Liu Z, Qin W, Wang C, Chen H, Ma X, Zhang D. Mapping the landscape of synthetic lethal interactions in liver cancer. Theranostics. 2021;11:9038-9053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 502] [Reference Citation Analysis (0)] |
| 29. | Salvadori S, Minozzi L, Tomatis R. Opioid peptides. Structure-activity relationships in [OGly4] dermorphin tetrapeptides. X. Farmaco Sci. 1986;41:103-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 30. | Yoshikawa H, Yamada M, Yoshida Y. Freeze-fracture study of Blastocystis hominis. J Protozool. 1988;35:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Deng Y, Yu C, Chen L, Zhang X, Lei Q, Liu Q, Cai G, Liu F. ARV-771 Acts as an Inducer of Cell Cycle Arrest and Apoptosis to Suppress Hepatocellular Carcinoma Progression. Front Pharmacol. 2022;13:858901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Adhikari B, Bozilovic J, Diebold M, Schwarz JD, Hofstetter J, Schröder M, Wanior M, Narain A, Vogt M, Dudvarski Stankovic N, Baluapuri A, Schönemann L, Eing L, Bhandare P, Kuster B, Schlosser A, Heinzlmeir S, Sotriffer C, Knapp S, Wolf E. PROTAC-mediated degradation reveals a non-catalytic function of AURORA-A kinase. Nat Chem Biol. 2020;16:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 33. | Coggon D, Pannett B, Winter PD, Acheson ED, Bonsall J. Mortality of workers exposed to 2 methyl-4 chlorophenoxyacetic acid. Scand J Work Environ Health. 1986;12:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Li X, Song Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol. 2020;13:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 372] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 35. | Shiota K. Induction of neural tube defects and skeletal malformations in mice following brief hyperthermia in utero. Biol Neonate. 1988;53:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Tolcher AW. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann Oncol. 2016;27:2168-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Kong FE, Li GM, Tang YQ, Xi SY, Loong JHC, Li MM, Li HL, Cheng W, Zhu WJ, Mo JQ, Gong YF, Tang H, Zhao Y, Zhang Y, Ma S, Guan XY, Ma NF, Xie MB, Liu M. Targeting tumor lineage plasticity in hepatocellular carcinoma using an anti-CLDN6 antibody-drug conjugate. Sci Transl Med. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Scanu P, Hurault de Ligny B, Ryckelynck JP. Reversible acute renal insufficiency with combination of enalapril and diuretics in a patient with a single renal-artery stenosis. Nephron. 1987;45:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Wu X, Luo H, Shi B, Di S, Sun R, Su J, Liu Y, Li H, Jiang H, Li Z. Combined Antitumor Effects of Sorafenib and GPC3-CAR T Cells in Mouse Models of Hepatocellular Carcinoma. Mol Ther. 2019;27:1483-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 40. | An S, Zhang D, Zhang Y, Wang C, Shi L, Wei W, Huang G, Liu J. GPC3-targeted immunoPET imaging of hepatocellular carcinomas. Eur J Nucl Med Mol Imaging. 2022;49:2682-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, Moroose R, Santin AD, Kalinsky K, Picozzi V, O'Shaughnessy J, Gray JE, Komiya T, Lang JM, Chang JC, Starodub A, Goldenberg DM, Sharkey RM, Maliakal P, Hong Q, Wegener WA, Goswami T, Ocean AJ. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32:746-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 42. | He Q, Gao H, Tan D, Zhang H, Wang JZ. mRNA cancer vaccines: Advances, trends and challenges. Acta Pharm Sin B. 2022;12:2969-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 43. | Lorentzen CL, Haanen JB, Met Ö, Svane IM. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23:e450-e458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 348] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 44. | Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Brück AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Höller C, Utikal J, Huber C, Loquai C, Türeci Ö. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1814] [Article Influence: 201.6] [Reference Citation Analysis (0)] |
| 45. | Awad MM, Govindan R, Balogh KN, Spigel DR, Garon EB, Bushway ME, Poran A, Sheen JH, Kohler V, Esaulova E, Srouji J, Ramesh S, Vyasamneni R, Karki B, Sciuto TE, Sethi H, Dong JZ, Moles MA, Manson K, Rooney MS, Khondker ZS, DeMario M, Gaynor RB, Srinivasan L. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell. 2022;40:1010-1026.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 46. | Palmer CD, Rappaport AR, Davis MJ, Hart MG, Scallan CD, Hong SJ, Gitlin L, Kraemer LD, Kounlavouth S, Yang A, Smith L, Schenk D, Skoberne M, Taquechel K, Marrali M, Jaroslavsky JR, Nganje CN, Maloney E, Zhou R, Navarro-Gomez D, Greene AC, Grotenbreg G, Greer R, Blair W, Cao MD, Chan S, Bae K, Spira AI, Roychowdhury S, Carbone DP, Henick BS, Drake CG, Solomon BJ, Ahn DH, Mahipal A, Maron SB, Johnson B, Rousseau R, Yelensky R, Liao CY, Catenacci DVT, Allen A, Ferguson AR, Jooss K. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat Med. 2022;28:1619-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 47. | Wienbeck M, Blasberg M. Effects of an enkephalin analog on motility of the small and large intestine in the cat. Z Gastroenterol. 1986;24:179-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 964] [Cited by in RCA: 900] [Article Influence: 300.0] [Reference Citation Analysis (0)] |
| 48. | Deng Z, Yang H, Tian Y, Liu Z, Sun F, Yang P. An OX40L mRNA vaccine inhibits the growth of hepatocellular carcinoma. Front Oncol. 2022;12:975408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 49. | Tojjari A, Saeed A, Singh M, Cavalcante L, Sahin IH, Saeed A. A Comprehensive Review on Cancer Vaccines and Vaccine Strategies in Hepatocellular Carcinoma. Vaccines (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 50. | Huang Z, Meng FY, Lu LZ, Guo QQ, Lv CJ, Tan NH, Deng Z, Chen JY, Zhang ZS, Zou B, Long HP, Zhou Q, Tian S, Mei S, Tian XF. Calculus bovis inhibits M2 tumor-associated macrophage polarization via Wnt/β-catenin pathway modulation to suppress liver cancer. World J Gastroenterol. 2024;30:3511-3533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (6)] |
| 51. | Zhang Z, Zeng P, Gao W, Wu R, Deng T, Chen S, Tian X. Exploration of the Potential Mechanism of Calculus Bovis in Treatment of Primary Liver Cancer by Network Pharmacology. Comb Chem High Throughput Screen. 2021;24:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Luo ZY, Tian Q, Cheng NM, Liu WH, Yang Y, Chen W, Zhang XZ, Zheng XY, Chen MS, Zhuang QY, Zhao BX, Liu CS, Liu XL, Li Q, Wang YC. Pien Tze Huang Inhibits Migration and Invasion of Hepatocellular Carcinoma Cells by Repressing PDGFRB/YAP/CCN2 Axis Activity. Chin J Integr Med. 2024;30:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Sun L, Chen W, Zhao P, Zhao B, Lei G, Han L, Zhang Y. Anticancer Effects of Wild Baicalin on Hepatocellular Carcinoma: Downregulation of AKR1B10 and PI3K/AKT Signaling Pathways. Cancer Manag Res. 2024;16:477-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 54. | Wu Z, Wang J. Curcumol Enhances the Antitumor Effect of Lenvatinib on Hepatocellular Carcinoma Cells. Discov Med. 2024;36:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 55. | Daniele B, Di Maio M. Target therapy for hepatocellular carcinoma: is sorafenib for everybody? J Clin Gastroenterol. 2009;43:389-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Yu ZJ, Xu Y, Peng W, Liu YJ, Zhang JM, Li JS, Sun T, Wang P. Calculus bovis: A review of the traditional usages, origin, chemistry, pharmacological activities and toxicology. J Ethnopharmacol. 2020;254:112649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (2)] |
| 57. | Wan TC, Cheng FY, Liu YT, Lin LC, Sakata R. Study on bioactive compounds of in vitro cultured Calculus Suis and natural Calculus Bovis. Anim Sci J. 2009;80:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Shimada K, Azuma Y, Kawase M, Takahashi T, Schaffer SW, Takahashi K. Taurine as a marker for the identification of natural Calculus Bovis and its substitutes. Adv Exp Med Biol. 2013;776:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Wang J, Lu YY, Xie MX, He K, Shang BY, Wang T, Wang HQ. [Research status of Bovis Calculus and relevant Chinese patent medicines based on bibliometric analysis]. Zhongguo Zhong Yao Za Zhi. 2023;48:2092-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 1070] [Article Influence: 152.9] [Reference Citation Analysis (0)] |
| 61. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4480] [Article Influence: 896.0] [Reference Citation Analysis (4)] |
| 62. | Ting CT, Li WC, Chen CY, Tsai TH. Preventive and therapeutic role of traditional Chinese herbal medicine in hepatocellular carcinoma. J Chin Med Assoc. 2015;78:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/