Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1359
Revised: September 14, 2024
Accepted: September 19, 2024
Published online: October 24, 2024
Processing time: 155 Days and 12.2 Hours
Herlyn-Werner-Wunderlich (HWW) syndrome is a rare Müllerian duct anomaly, characterized by a combination of urogenital abnormalities. The occurrence of primary cervico-vaginal carcinomas in patients with HWW syndrome is excep
We report a rare case of primary clear cell carcinoma of the vagina complicated in a 40-year-old woman with HWW syndrome. The patient presented with irregular vaginal bleeding for 4 years. On gynecological examination, an oblique vaginal septum was suspected. Surgical resection of the vaginal septum revealed a com
Cervico-vaginal adenocarcinomas in patients with HWW syndrome are occult, and require early surgical intervention and regular imaging surveillance.
Core Tip: Cervico-vaginal adenocarcinomas in patients with Herlyn-Werner-Wunderlich (HWW) syndrome are extremely rare. The present case and a literature review of this rare condition, indicate that patients with HWW syndrome may have a higher risk of developing cervico-vaginal adenocarcinomas, compared to the general female population. These cancers typically occur on the obstructed side, making them occult and difficult to detect. Therefore, it is crucial for patients with HWW syndrome to undergo septum resection upon diagnosis or, at a minimum, have regular imaging evaluations of the cervix and vagina, to facilitate early detection and management.
- Citation: Lei XG, Zhang H. Vaginal clear cell adenocarcinoma in Herlyn-Werner-Wunderlich syndrome: A case report. World J Clin Oncol 2024; 15(10): 1359-1365
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1359.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1359
Herlyn-Werner-Wunderlich (HWW) syndrome is a complex genitourinary disorder characterized by a constellation of anatomical anomalies, primarily reported as uterus didelphys, oblique vaginal septum with obstructed hemivagina, and ipsilateral renal agenesis[1-3]. This syndrome constitutes a minor proportion of Müllerian duct anomalies and is ex
The 40-year-old multiparous female patient had a 4-year history of irregular vaginal bleeding.
The patient presented to our gynecological department, complaining of irregular vaginal bleeding for 4+ years. Prior to her visit, she had received various medical treatments at different hospitals with minimal improvement.
The patient was diagnosed with uterus didelphys during a cesarean section performed 16 years ago. She denied a history of prenatal diethylstilbestrol (DES) exposure. The patient had no significant history of past illness.
The patient reported an allergy to cefmetazole and had no significant family medical history.
During gynecological examination, an oblique vaginal septum was suspected. The cervix was only partially visualized.
Serum tumor markers, including alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen (CA) 125, CA19-9 and human chorionic gonadotropin were all within the normal range. The results of a complete blood count were normal. Cervical cytology was performed and was found to be normal. Human papillomavirus 16 was positive.
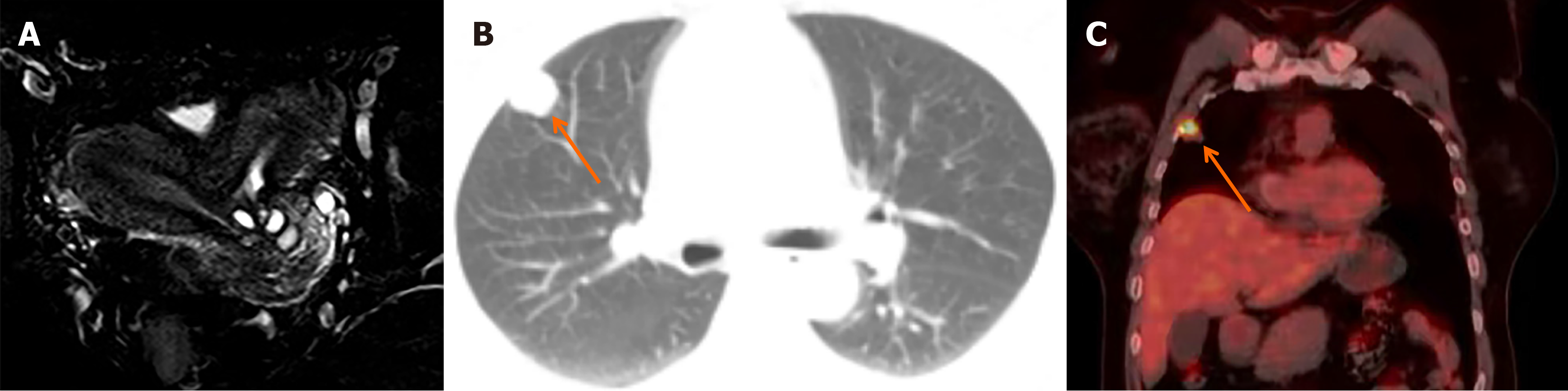
Transvaginal ultrasound revealed uterus didelphys, both uteri anteverted with normal endometrium lining. After surgical excision of the oblique vaginal septum, contrast-enhanced pelvic magnetic resonance imaging (MRI) was performed. The MRI scan showed no obvious lesions in the vagina or paravaginal tissue. The cervix was duplicated and multiple Nabothian cysts. The uterus was didelphic, displaying normal signal intensity and enhancement (Figure 1A). The ovaries, urethra, bladder, and rectum displayed normal signal characteristics. There was no evidence of enlarged pelvic or inguinal lymph nodes. The left kidney was absent.
Clear cell carcinoma of the vagina, stage I; HWW syndrome.
Surgical resection of the vaginal septum was initially performed. During the procedure, a septum was identified along with a 1.0 cm communicating fistula within the septum. Following septal resection, the left cervix and vagina were revealed, and a 2 cm × 1 cm lesion was discovered on the left fornix, left wall of the upper vagina, and the left side of the septum. An excisional biopsy of the lesion was conducted. One month later, after pathological confirmation of vaginal clear cell carcinoma, the patient underwent a radical hysterectomy, vaginectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection. A cycle of systemic chemotherapy, comprising 240 mg of paclitaxel and 100 mg of cisplatin, was administered. Subsequently, the patient declined further treatment due to significant side effects and discomfort.
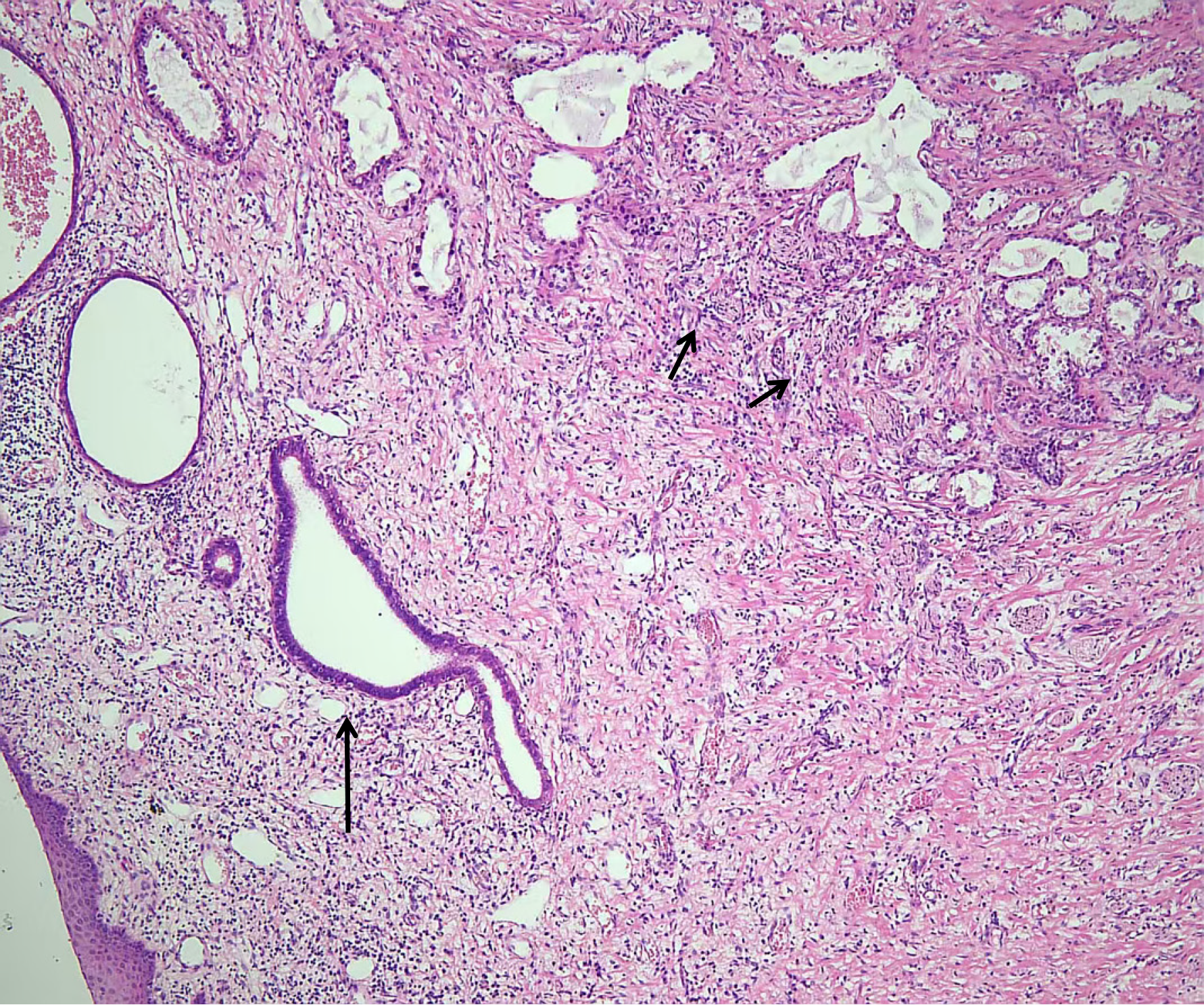
Clear cell adenocarcinoma of the upper vagina was identified, originating from the malignant transformation of vaginal adenosis (Figure 2). The lesion was confined within the vaginal wall and extended to the fornix and the surgical margin. It did not involve the paravaginal or paracervical tissues on either side, the lateral pelvic walls, or the adnexa bilaterally. Examined lymph nodes showed reactive hyperplasia without any evidence of metastatic cancer. Immunohistochemical results were as follows: Napsin A+++, CD15++, P16+++, P53-, WT-1-, PAX-8+++, ER-, PR-, CEA- and Ki67 with a positivity rate of 45%.
After discharge, the patient continued follow-up at an external hospital. One year post-operation, a follow-up computed tomography (CT) scan revealed a 0.8 cm nodule in the anterior segment of the upper lobe of the right lung. Three years later, the nodule had grown to 1.8 cm (Figure 1B) and was accompanied by signs of pleural indentation. Positron emission tomography/CT showed high fluorodeoxyglucose uptake in the nodule, indicating malignancy (Figure 1C). A thoracoscopic wedge resection of the right upper lobe was performed, and the postoperative pathology confirmed metastatic clear cell carcinoma. The patient has been under follow-up for 5 years and currently has no evidence of recurrence or metastasis.
This report describes vaginal clear cell adenocarcinoma in a patient with HWW syndrome. Given the related origins of cervical and vaginal adenocarcinomas, we summarized 13 cases of primary cervical or vaginal carcinoma in HWW syndrome reported in the English literature to explore this rare entity (Table 1)[5-12].
| Ref. | Age (years) | Gravida/para | DES exposure | Symptoms | Site | Stage | Side (obstructed or non-obstructed) | Pathology | Adenosis | Treatment | Outcome | ||
| Follow-up time (years) | Outcome | ||||||||||||
| 1 | GRANT et al[5] | 35 | G0P0 | / | Irregular vaginal bleeding | Cervix | II | Non-obstructed side | AC | / | Hysterectomy | / | NED |
| 2 | Cordoba et al[6] | 37 | G7P2 | / | Menorrhagia and irregular vaginal bleeding | Cervix | IIIa | Obstructed side | AC | / | Aortic lymphadenectomy + concomitant chemoradiotherapy | 3 | NED |
| 3 | Kaba et al[7] | 49 | P2 | / | Menorrhagia and intermenstrual bleeding | Cervix | Ib1 | Obstructed side | EAC | / | Radical hysterectomy + bilateral salpingo-oophorectomy + pelvic-paraaortic lymphadenectomy + omentectomy; radical parametrectomy and proximal vaginectomy | 1.3 | NED |
| 4 | Watanabe et al[8] | 33 | G2P2 | No | Irregular vaginal bleeding | Cervix | IVa | Obstructed side | EAC | / | Chemotherapy+ external radiation; anterior pelvic exenteration + total vaginectomy | / | / |
| 5 | Kusunoki et al[9] | 65 | G3P2 | No | Postmenopausal bleeding | Cervix | I | Obstructed side | CCA | / | Hysterectomy + tumor resection + concurrent Chemoradiation | 1 | NED |
| 6 | Zong et al[10] | 20 | / | / | / | Cervix | I | / | CCA | / | Radical hysterectomy + bilateral salpingo-oophorectomy + pelvic lymphadenectomy + total vaginectomy | 3.0 | NED |
| 7 | Zong et al[10] | 27 | / | / | / | Cervix | IIb | / | / | / | Concurrent chemoradiation, hysterectomy + bilateral salpingo-oophorectomy + total vaginectomy, chemotherapy | 2 | Local recurrence and kidney failure 1 year later; DOD |
| 8 | Zong et al[10] | 31 | / | / | / | Cervix | IIa | / | CCA | / | Chemotherapy,hysterectomy + bilateral salpingo-oophorectomy + pelvic-paraaortic lymphadenectomy, chemotherapy, concurrent Chemoradiation | 1.5 | Local recurrence and distant metastases 4 months later; DOD |
| 9 | Zong et al[10] | 38 | / | / | / | Cervix | IIa | / | / | / | Radical hysterectomy + bilateral salpingo-oophorectomy + pelvic-paraaortic lymphadenectomy, radiotherapy | 2 | Distant metastases after 1 year; DOD |
| 10 | Zeeshan-ud-din et al[11] | 27 | / | No | Irregular vaginal bleeding | Vagina | III | Obstructed side | CCA | / | Wertheim's hysterectomy + partial vaginectomy + pelvic lymphadenectomy | / | / |
| 11 | Watanabe et al[8] | 53 | G2P2 | No | Vaginal pain and irregular vaginal bleeding | Vagina | I | Obstructed side | CCA | / | Radical hysterectomy | / | / |
| 12 | Uehara et al[12] | 54 | G4P3 | No | Vaginal bleeding | Vagina | I | Obstructed side | CCA | Yes | Anterior pelvic exenteration | 3.6 | NED |
| 13 | Present case | 40 | G1P1 | No | Irregular vaginal bleeding | Vagina | I | Obstructed side | CCA | Yes | Hysterectomy + tumor resection + chemotherapy (1 cycle) | 5 | Lung metastasis 1 year post-operative, had it resected; NED till now |
The average age of the patients was 37 years (range, 20-69 years). Among the 9 patients with available data on parity, 8 had delivered. Based on the patients’ age and delivery history, as well as intra-operative findings in some cases[7-9,11,12], these patients were most likely suffering from communicant HWW syndrome. Non-communicant HWW syndrome usually has earlier onset and more acute and severe symptoms, such as acute abdominal pain. Therefore, these patients can usually receive timely treatment and relief of obstruction[1,2]. In contrast, communicant HWW syndrome has more chronic and tolerable symptoms, such as irregular vaginal bleeding[1,2], which may lead to the disease being neglected until complications arise, such as endometriosis and cervico-vaginal carcinoma, as observed in these 13 patients.
The primary symptom was vaginal bleeding, either irregular or post-menopausal bleeding; additionally, 2 patients had menorrhagia and 1 patient had vaginal pain. Irregular vaginal bleeding is a common yet nonspecific symptom for both cervico-vaginal carcinoma and HWW syndrome. Consequently, it might lead to a delayed diagnosis of carcinoma com
Of these 13 patients, 9 had cervical carcinoma, and 4 had vaginal carcinoma, with staging from I to IV. Histopathologically, all 13 patients had adenocarcinoma, including 7 with clear cell adenocarcinoma, 4 with adenocarcinoma and 2 with endometroid adenocarcinoma. This is different to patients without Müllerian duct anomalies. For the general population, adenocarcinoma only accounts for 8%-10% of primary vaginal malignancies[13] and 10-25% of all cervical carcinomas[14]. This aligns with previous research by Zong et al[10], suggesting that congenital Müllerian duct anomalies may be linked to an increased risk of cervico-vaginal adenocarcinoma. One possible explanation for this correlation is that Müllerian duct anomalies are associated with vaginal or cervical adenosis, which have persisted for a long time and are subject to genetic and hormonal changes[9,10]. Both our case and the case reported by Uehara et al[12] had vaginal adenosis. Furthermore, a study of 27 patients with HWW syndrome found vaginal adenosis in 8 of the resected septa (29.6%)[15]. Although prenatal DES exposure is strongly associated with an increased risk of vaginal adenosis and vaginal clear cell adenocarcinoma[16,17], none of the 6 patients with available data on DES exposure reported being exposed to DES[8,9,11,12].
Of the 9 patients for whom data on tumor location were available, eight tumors were found on the obstructed side[6-9,11-13], while only one was found on the non-obstructed side[5]. Cervico-vaginal carcinomas on the obstructed side in patients with HWW syndrome are occult, particularly in those not previously diagnosed with the syndrome. Conse
Once diagnosed, the treatment for cervico-vaginal adenocarcinomas in patients with HWW syndrome generally follows similar protocols to those for patients without congenital anomalies. However, anatomical abnormalities as
The impact of congenital anomalies on the prognosis of cervico-vaginal adenocarcinoma remains unclear due to the limited number of reported cases. Among the 10 patients with follow-up data, monitored over a period of 1-5 years, three died due to the disease, six showed no evidence of disease recurrence[5-8,10], and the present patient underwent suc
Our case highlights the risk of cervico-vaginal adenocarcinomas in patients with HWW syndrome and the challenges in detecting and treating such malignancies. Although these occurrences are exceedingly rare, they tend to manifest on the obstructed side, which is often occult and difficult to detect. This can significantly hinder timely diagnosis and treatment. Therefore, it is crucial for patients with HWW syndrome to have their septum resected upon diagnosis, including those with communicating fistulas. For patients whose obstructions are not surgically corrected, regular imaging evaluations of the cervix and vagina, particularly using MRI, should be implemented to facilitate early detection and management. Tailored management with routine imaging and early surgical intervention may improve outcomes in these rare but complex cases, and should be incorporated into clinical practice when managing HWW syndrome.
| 1. | Zhang H, Zheng Y, Ning G, Fu C, Bao L. Preoperative MRI presentations of Herlyn-Werner-Wunderlich syndrome. Congenit Anom (Kyoto). 2022;62:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Zhu L, Chen N, Tong JL, Wang W, Zhang L, Lang JH. New classification of Herlyn-Werner-Wunderlich syndrome. Chin Med J (Engl). 2015;128:222-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Fedele L, Motta F, Frontino G, Restelli E, Bianchi S. Double uterus with obstructed hemivagina and ipsilateral renal agenesis: pelvic anatomic variants in 87 cases. Hum Reprod. 2013;28:1580-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Acién P, Acién M. The presentation and management of complex female genital malformations. Hum Reprod Update. 2016;22:48-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Grant RN, Pierce VK. Adenocarcinoma occurring in one cervix of a uterus didelphys associated with solitary kidney. Am J Obstet Gynecol. 1952;63:212-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Cordoba A, Escande A, Comte P, Fumagalli I, Bresson L, Mubiayi N, Lartigau E. Locally advanced adenocarcinoma of the cervix on uterus didelphys: a case report. J Contemp Brachytherapy. 2017;9:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Kaba M, Gungor T, Baser E, Ozdal B, Sirvan L. Cervical cancer in a patient with uterus didelphys and obstructive hemivagina, ipsilateral renal anomaly (OHVIRA) syndrome. Arch Gynecol Obstet. 2013;288:229-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Watanabe Y, Etoh T, Nakai H. Adenocarcinoma of the lower female genital tract in patients with Herlyn-Werner-Wunderlich syndrome. Am J Obstet Gynecol. 2012;207:e5-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Kusunoki S, Huang KG, Magno A. Laparoscopic en bloc resection of a para-cervical cancer with OHVIRA syndrome. Taiwan J Obstet Gynecol. 2018;57:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Zong L, Wang W, He Y, Cheng N, Xiang Y. Carcinoma of the lower female genital tract in patients with genitourinary malformations: a clinicopathologic analysis of 36 cases. J Cancer. 2019;10:3054-3061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zeeshan-ud-din, Ahsan A. Vaginal clear cell adenocarcinoma with associated Müllerian duct anomalies, renal agenesis and situs inversus: report of a case with no known in-utero exposure with diethyl stilboestrol. J Pak Med Assoc. 2009;59:568-570. [PubMed] |
| 12. | Uehara T, Onda T, Sasajima Y, Sawada M, Kasamatsu T. A case of vaginal clear cell adenocarcinoma complicated with congenital anomalies of the genitourinary tract and metanephric remnant without prenatal diethylstilbestrol exposure. J Obstet Gynaecol Res. 2010;36:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Adams TS, Rogers LJ, Cuello MA. Cancer of the vagina: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Ohya A, Asaka S, Fujinaga Y, Kadoya M. Uterine cervical adenocarcinoma associated with lobular endocervical glandular hyperplasia: Radiologic-pathologic correlation. J Obstet Gynaecol Res. 2018;44:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Smith NA, Laufer MR. Obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome: management and follow-up. Fertil Steril. 2007;87:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1923] [Cited by in RCA: 1438] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation. 2012;84:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Lei C, Huang M, Li N, An J, Xiong S, Li X, Wu L. IMRT and HDR-ICBT for Locally Advanced Clear Cell Adenocarcinoma of the Cervix in Uterus Didelphys Associated With Unilateral Renal Agenesis. Front Oncol. 2020;10:1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/