Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1351
Revised: August 18, 2024
Accepted: August 29, 2024
Published online: October 24, 2024
Processing time: 166 Days and 17.2 Hours
In patients with human epidermal growth factor receptor 2 (HER2)-overexpressing gastric cancer (GC), the combination of HER2 targeting and a standard first-line chemotherapy regimen has been demonstrated to significantly improve their prognosis. However, in a proportion of patients, cancer progresses within a short period of time, and there is currently no standard treatment after disease progression.
This study presents a case of a 51-year-old male with advanced GC who un
Despite the unfavorable prognosis associated with advanced GC, the imple
Core Tip: Apatinib exhibits synergistic effect with pan-HER inhibitor and reverses acquired resistance in human epidermal growth factor receptor 2 (HER2)-positive gastric cancer (GC) via stem cell factor/c-kit signaling and its downstream pathways. The patient treated with HER2-targeted therapy (disitamab vedotin, RC48) and small molecule antiangiogenesis targeted therapy with apatinib experiencing excellent survival, which provide related data for posterior line treatment of advanced GC.
- Citation: Li XQ, Yang J, Liu B, Han SM. Disitamab vedotin combined with apatinib in gastric cancer: A case report and review of literature. World J Clin Oncol 2024; 15(10): 1351-1358
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1351.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1351
Gastric cancer (GC) is the fifth most commonly diagnosed malignant tumor worldwide and the fourth leading cause of cancer-related mortality[1]. The most prominent therapeutic target in advanced patients is human epidermal growth factor receptor 2 (HER2)[2]. Approximately 12% of patients with GC exhibit HER2 overexpression in China[3]. Prior research has demonstrated that trastuzumab in conjunction with chemotherapy markedly improves overall survival (OS) rates in comparison with chemotherapy alone. This finding has established trastuzumab as the primary treatment for HER2-positive GC and has initiated a new era of targeted therapy for GC[4]. Moreover, immune checkpoint inhibitors have been employed in the treatment of advanced GC, with notable efficacy. The most recent data indicate that, in comparison with a placebo, the combination of pembrolizumab with first-line trastuzumab and chemotherapy results in a significantly improved progression-free survival (PFS) rate in patients with metastatic HER2-positive gastroesophageal cancer[5]. However, patients with advanced disease tend to experience progression and metastasis, which portends a poor prognosis. Apatinib, a small-molecule antiangiogenic targeted drug, has been demonstrated to significantly prolong survival following the failure of standard chemotherapy for advanced GC. In addition, apatinib has been shown to exhibit a synergistic effect when combined with a pan-HER inhibitor, effectively reversing acquired resistance in HER2-positive GC through the reversal of stem cell factor/c-kit signaling and its downstream pathways[6]. In this case report, we present a case of a patient with advanced GC with multiple organ metastasis who received second-line treatment comprising HER2-targeted therapy (disitamab vedotin, RC48) in combination with the small-molecule antiangiogenic targeted drug apatinib. The patient survived for a relatively long period of time, with a total survival of 25.8 months.
The patient had hidden abdominal pain for 2 months.
A 51-year-old Chinese man was admitted to the Shandong Cancer Hospital due to hidden abdominal pain.
There was no past medical history.
There was no other relevant personal or family history.
Abdominal examination revealed tenderness in the upper abdomen.
Increased tumor makers of carbohydrate antigen 19-9 (CA19-9) and carbohydrate antigen 125 (CA125), decreased hemoglobin and serum albumin.
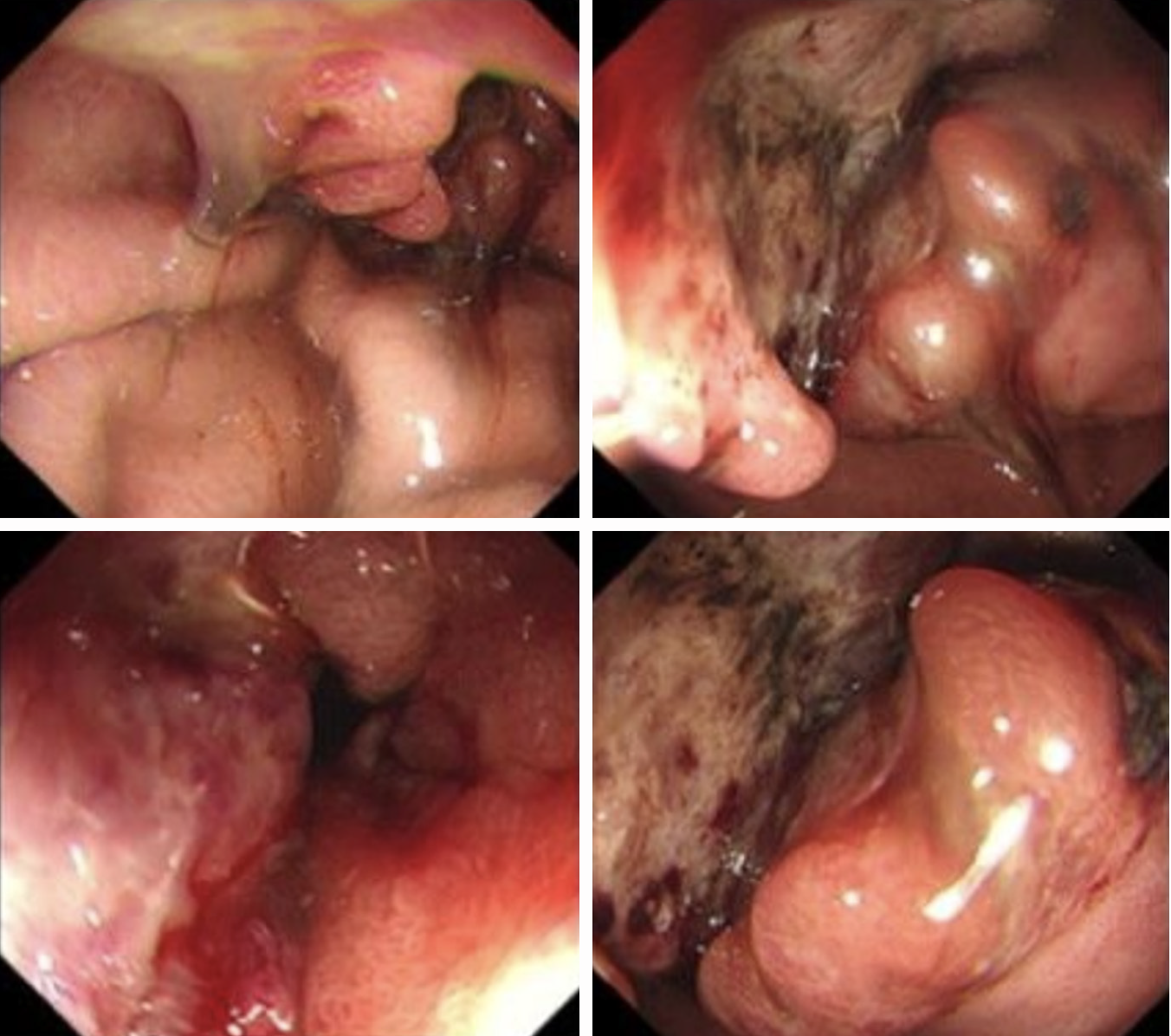
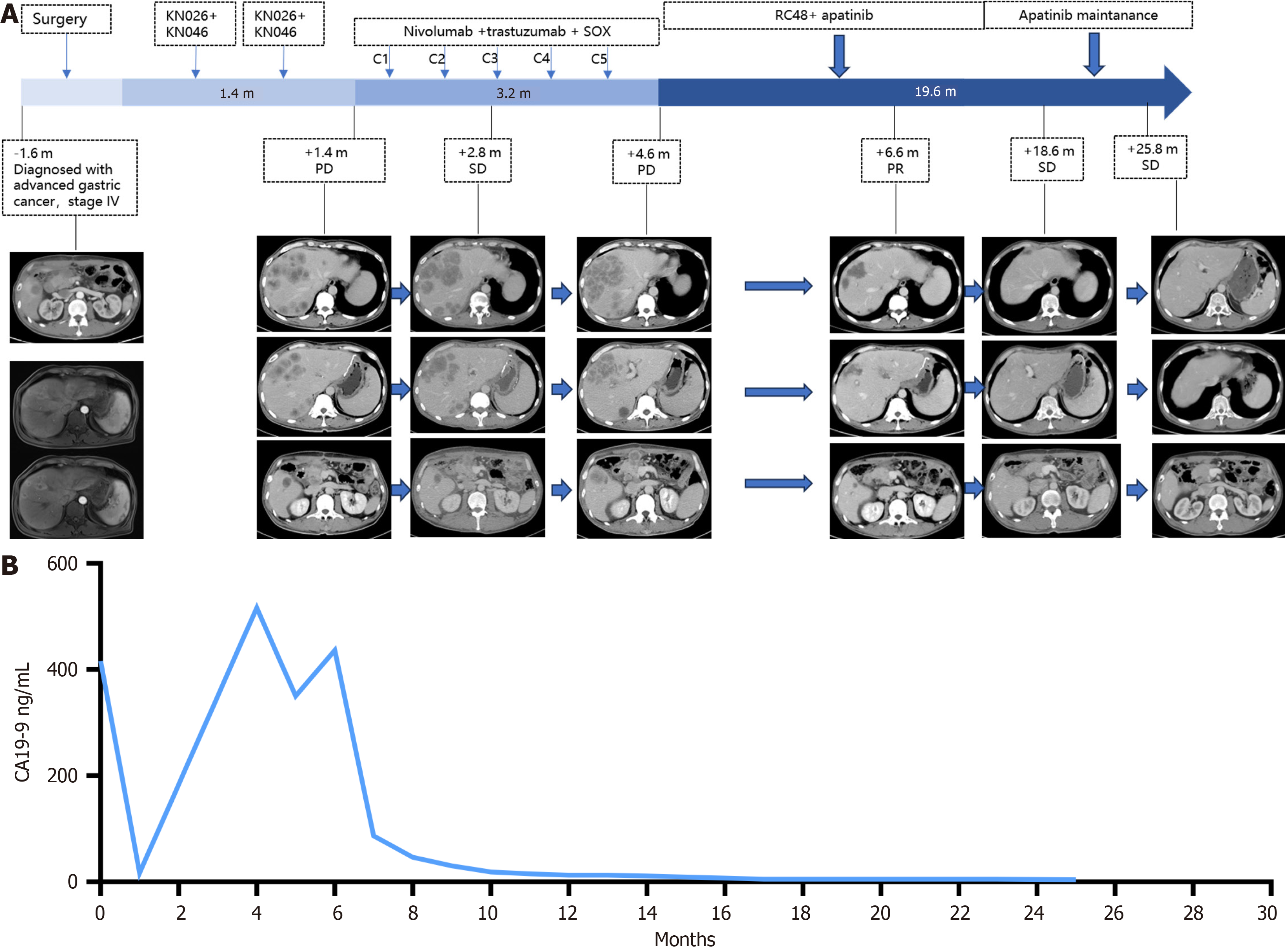
A gastroscopic examination revealed the presence of ulcerative lesions in the pyloric antrum (Figure 1). Computed tomography (CT) imaging (Figure 2) revealed a mass within the gastric antrum, accompanied by the presence of multiple lymph node and liver metastases.
The patient could receive first-line treatment or enroll in clinical studies.
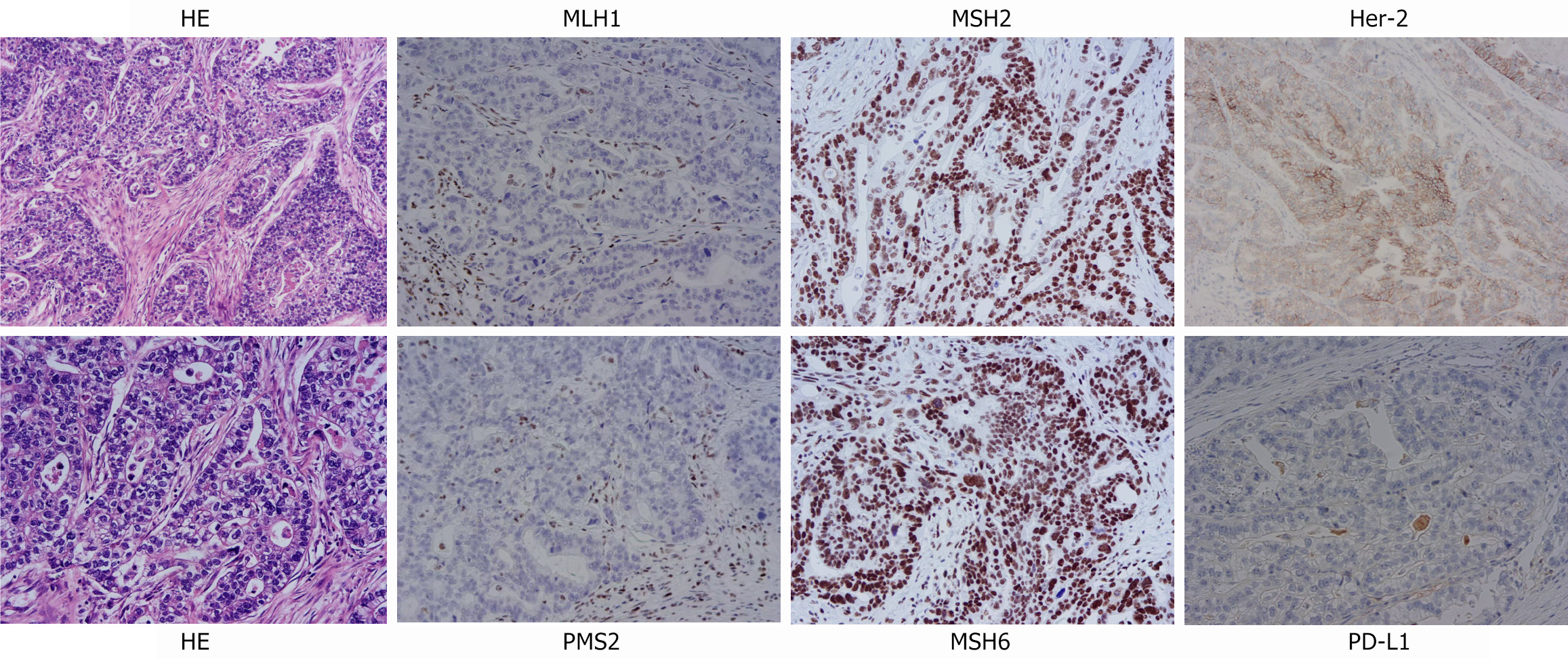
A diagnosis of GC was established through gastroscopic biopsy. A follow-up chest CT scan conducted on December 20, 2021, revealed disease progression in the liver, neck of the pancreas, and celiac lymph nodes. Immunohistochemical staining revealed the presence of HER2 (2+), the absence of MLH1, the presence of MSH2 and MSH6, the absence of PMS2, and a Ki67 Labeling index ranging from 30% to 40%. Additionally, PD-L1 expression was less than 5%. These findings are illustrated in Figure 3. The gene test results revealed ERBB2 amplification, a KRAS p.G13D mutation, and an ERBB2 p.R678Q mutation, with a PD-L1 expression level of less than 5%. The laboratory tumor markers demonstrated the following values: Carcinoembryonic antigen (CEA) at 2.33 ng/mL, CA19-9 at 516 U/mL, and CA125 at 118 U/mL. On the basis of the aforementioned pathological examination, CT scan, and laboratory results, the diagnosis was advanced GC, pT4N1M1.
Radical surgery and liver metastasis resection were performed on November 23, 2021. Postoperative pathology revealed poorly differentiated adenocarcinoma with full layer invasion, nerve invasion, a vascular cancer thrombus, and a negative incisal margin. Two of the 36 lymph nodes presented evidence of metastasis, and the pathology of the resected liver also revealed poorly differentiated adenocarcinoma. A multidisciplinary consultation recommended either first-line treatment or enrollment in clinical studies. For patients with advanced HER2-positive GC, dual-antibody treatment in conjunction with conventional chemotherapy has demonstrated favorable survival outcomes, with a median PFS of 8.6 months and a median OS of 19.3 months[7]. Following the consultation, the patient and his family expressed a strong willingness to participate in a clinical trial. Following comprehensive communication and the provision of informed consent, the patient commenced participation in a clinical trial (NCT 04521179; registration date: August 20, 2022). The patient was treated with KN026 (a bispecific antibody targeting HER2, 1890 mg on day 1) and KN046 (a bispecific antibody targeting PD-L1/CTLA-4, 325 mg on day 1). Following three cycles of treatment, there was evidence of the progression of metastases that involved multiple organs, including the liver, celiac lymph nodes, and newly enlarged superior phrenic lymph nodes. The laboratory tumor marker levels were markedly elevated (CEA 2 ng/mL; CA19-9 516 U/mL; and CA125 118 U/mL).
In light of the absence of chemotherapy in the first-line treatment regimen, a second-line regimen comprising five cycles of nivolumab (160 mg on day 0) in combination with trastuzumab (6 mg/kg on day 0) and SOX (oxaliplatin 130 mg/m2 on day 1 and tegafur 60 mg bid from day 1 to day 14) was selected. A CT scan conducted on May 13, 2022, demonstrated increases in the sizes of the liver, pancreas, and celiac lymph nodes. Additionally, the serum tumor marker levels (CEA 2.97 ng/mL; CA19-9 436 U/mL; and CA125 203 U/mL) were notably elevated.
Up to this point, the patient had been treated with targeted combined immunotherapy and chemotherapy via two lines of treatment. In patients with HER2-positive advanced GC, apatinib has demonstrated significant clinical benefits in prolonging both PFS and OS. The guidelines recommend its use in third-line and subsequent treatment settings. Furthermore, RC48, which was approved by the United States Food and Drug Administration in June 2022, is considered a third-line treatment for patients with advanced or metastatic GC with high HER2-positive expression. On June 16, 2022, the targeted drug for subsequent treatment was changed to RC48 (2.5 mg/kg Q2W) combined with apatinib (250 mg orally once per day) in accordance with the aforementioned approval.
A subsequent CT scan conducted on July 28, 2022, indicated a regression of the disease, with the involvement of multiple organs. The efficacy evaluation was classified as partial remission. The concentration of CA199 decreased from 436 U/mL to within the normal range, and the concentration of CA125 decreased from 203 U/mL to within the normal range. During the course of treatment, the patient exhibited only grade 2 myelosuppression and grade 2 neurotoxicity, two common toxic side effects observed in the majority of patients treated with apatinib. At the time of this report, the patient remained in remission until the last follow-up on August 10, 2024. The PFS after third-line therapy was 25.8 months.
The present report details the case of a patient with HER2-positive metastatic GC who experienced excellent survival benefits (PFS of 25.8 months at the time of this writing) from third-line RC48 combined with apatinib. This new combination therapy provides a novel regimen option for the second-line treatment of advanced GC.
In 2011, the analysis of the ToGA study paved the way for the development of precision anti-HER2 targeted therapy for GC. The data from the ToGA study demonstrated that the median OS was 13.8 months in patients with HER2-positive advanced GC who received trastuzumab in combination with chemotherapy, whereas it was 11.1 months in those who received chemotherapy alone. In light of the findings from numerous large-scale clinical trials, trastuzumab has been incorporated into the standard chemotherapy regimen for patients with advanced or metastatic GC, resulting in notable benefits. Nevertheless, over half of the patients experience recurrence within six months. Additional studies have sought to incorporate pembrolizumab into the initial treatment regimen for patients with HER2-positive advanced GC, resulting in a superior response rate and prolonged survival[8,9]. A recently conducted large-scale randomized phase III clinical trial, designated KEYNOTE811, demonstrated that the administration of pembrolizumab in conjunction with first-line trastuzumab and chemotherapy for metastatic HER2-positive GC had the capacity to markedly increase PFS, particularly in patients exhibiting tumors with a PD-L1-positive score of 1 or greater. In this study, the patient was successfully enrolled in a phase II clinical trial of KN026 combined with KN046 and received targeted therapy and immunotherapy. Unfortunately, the disease progressed after only three cycles. In the clinical setting, transient progressive disease was observed, followed by a partial response that was described as pseudoprogression. Previous studies have demonstrated that the incidence of pseudoprogression ranges from 0% to 15%, with some authors additionally reporting disease stabilization following a first progression[10]. The second-line treatment regimen with nivolumab combined with trastuzumab and chemotherapy, which also included targeted therapy and immunotherapy, yielded a PFS of 3.5 months. This result highlights the necessity for further investigation into the phenomenon of pseudoprogression following first-line therapy. However, studies on pseudoprogression in GC are scarce.
Previous studies have demonstrated that HER2 positivity may be lost following neoadjuvant therapy with or without trastuzumab[11,12]. In GC, multiple investigators have reported the loss of HER2 positivity in 29%-61% of biopsied tissues and 21%-42% of surgical samples obtained from patients following trastuzumab-based chemotherapy[13-17]. In this case, HER2 positivity may have been altered following the administration of KN026 as a first-line treatment and trastuzumab-based chemotherapy as a second-line treatment. Unfortunately, the patient was not rebiopsied, and no tissue samples were available for testing. Research has indicated that histological tumor cells associated with chemoresistance may be important factors in the loss of HER2 positivity after trastuzumab-based chemotherapy[18]. This may be the reason for the patient's progression after five cycles of second-line therapy.
A previous study has indicated that acquired resistance is a contributing factor to disease progression following trastuzumab treatment[19]. Various mechanisms of acquired trastuzumab resistance, including the activation of bypass pathways (insulin-like growth factor 1 receptor, HER3, etc.) and the upregulation of downstream signaling pathways[20-22], have been identified in preclinical studies. Additionally, macrophage-mediated angiogenesis has been identified as a mechanism promoting trastuzumab resistance. The efficacy of antiangiogenic drugs in reversing trastuzumab resistance was demonstrated in a previous study[23]. Apatinib is a small-molecule tyrosine kinase inhibitor that exhibits high selectivity and potent inhibitory activity against VEGFR-2[24]. In multiple randomized controlled trials, apatinib demonstrated significant antitumor efficacy in Chinese patients with advanced gastric or gastroesophageal junction adenocarcinoma as a third-line or later treatment[25-27]. A previous study has demonstrated that apatinib reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters[28]. Apatinib may also reverse HER2 resistance and be beneficial for patients with breast cancer[29]. RC48 is a novel HER2-targeted antibody-drug conjugate comprising trastuzumab coupled with monomethyl auristatin E via a cleavable linker. Compared with trastuzumab, hertuzumab has a greater affinity for HER2. A phase I study of RC48 combination immunotherapy indicated that patients with low HER2 expression and G/GEJ cancer also achieved an ORR of 46%, which is a promising outcome when compared with that of the currently available treatment regimen for this population[30].
In light of the aforementioned research and guidelines, apatinib in conjunction with anti-HER2-targeted therapy (RC48) was employed as a third-line treatment in this patient, yielding a favorable clinical outcome. This case study suggests that HER2-targeted therapy in combination with apatinib may increase survival rates in patients with HER2-positive GC when it is used as a subsequent line of therapy. This may represent a novel chemotherapy-free approach for patients. Furthermore, apatinib may reverse HER2 resistance in GC, as evidenced by the long-term survival observed. In conclusion, we present the successful management of a patient with advanced GC who was treated with RC48 and apatinib. This combination of dual-targeted therapy may provide additional survival benefits for patients with advanced or metastatic HER2-positive GC. However, the reliability of this approach remains to be verified, and the underlying mechanism still needs to be validated by further experimental research and clinical studies.
Despite the unfavorable prognosis associated with advanced GC, the imple
We are very grateful for this patient’s willingness to share this case.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68318] [Article Influence: 13663.6] [Reference Citation Analysis (201)] |
| 2. | Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 756] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 3. | Kim WH, Gomez-Izquierdo L, Vilardell F, Chu KM, Soucy G, Dos Santos LV, Monges G, Viale G, Brito MJ, Osborne S, Noé J, Du X. HER2 Status in Gastric and Gastroesophageal Junction Cancer: Results of the Large, Multinational HER-EAGLE Study. Appl Immunohistochem Mol Morphol. 2018;26:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5520] [Article Influence: 345.0] [Reference Citation Analysis (3)] |
| 5. | Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yanez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin SK, Van Cutsem E, Tabernero J, Li K, Shih CS, Bhagia P, Rha SY; KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402:2197-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 282] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 6. | Su B, Huang T, Jin Y, Yin H, Qiu H, Yuan X. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer. 2021;24:352-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Rha SY, Lee C, Kim HS, Kang B, Jung M, Bae WK, Koo D, Shin S, Jeung H, Zang DY, Chung HC. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC). J Clin Oncol. 2020;38:3081. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ, Momtaz P, Shcherba M, Ku GY, Zervoudakis A, Won ES, Kelsen DP, Ilson DH, Nagy RJ, Lanman RB, Ptashkin RN, Donoghue MTA, Capanu M, Taylor BS, Solit DB, Schultz N, Hechtman JF. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 9. | Chung HC, Bang YJ, S Fuchs C, Qin SK, Satoh T, Shitara K, Tabernero J, Van Cutsem E, Alsina M, Cao ZA, Lu J, Bhagia P, Shih CS, Janjigian YY. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol. 2021;17:491-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 10. | Frelaut M, du Rusquec P, de Moura A, Le Tourneau C, Borcoman E. Pseudoprogression and Hyperprogression as New Forms of Response to Immunotherapy. BioDrugs. 2020;34:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, Esteva FJ, Buzdar AU, Chen H, Eksambi S, Hortobagyi GN, Baselga J, Gonzalez-Angulo AM. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381-7388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Guarneri V, Dieci MV, Barbieri E, Piacentini F, Omarini C, Ficarra G, Bettelli S, Conte PF. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24:2990-2994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Seo S, Ryu MH, Park YS, Ahn JY, Park Y, Park SR, Ryoo BY, Lee GH, Jung HY, Kang YK. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019;22:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 14. | Pietrantonio F, Caporale M, Morano F, Scartozzi M, Gloghini A, De Vita F, Giommoni E, Fornaro L, Aprile G, Melisi D, Berenato R, Mennitto A, Volpi CC, Laterza MM, Pusceddu V, Antonuzzo L, Vasile E, Ongaro E, Simionato F, de Braud F, Torri V, Di Bartolomeo M. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer. 2016;139:2859-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 15. | Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, Narita Y, Satake H, Matsuda Y, Sonoda H, Shimokawa M, Maehara Y; Kyushu Study Group of Clinical Cancer (KSCC). Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer. 2018;105:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Watson S, Validire P, Cervera P, Zorkani N, Scriva A, Lemay F, Tournigand C, Perniceni T, Garcia ML, Bennamoun M, Paye F, Louvet C. Combined HER2 analysis of biopsies and surgical specimens to optimize detection of trastuzumab-eligible patients in eso-gastric adenocarcinoma: a GERCOR study. Ann Oncol. 2013;24:3035-3039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Hedner C, Borg D, Nodin B, Karnevi E, Jirström K, Eberhard J. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in oesophageal and gastric adenocarcinomas preneoadjuvant and postneoadjuvant treatment. J Clin Pathol. 2018;71:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Kijima T, Arigami T, Uenosono Y, Hiraki T, Yanagita S, Matsushita D, Okubo K, Shimonosono M, Ishigami S, Maemura K, Tanimoto A, Natsugoe S. Comparison of HER2 Status Before and After Trastuzumab-based Chemotherapy in Patients With Advanced Gastric Cancer. Anticancer Res. 2020;40:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Piro G, Carbone C, Cataldo I, Di Nicolantonio F, Giacopuzzi S, Aprile G, Simionato F, Boschi F, Zanotto M, Mina MM, Santoro R, Merz V, Sbarbati A, de Manzoni G, Scarpa A, Tortora G, Melisi D. An FGFR3 Autocrine Loop Sustains Acquired Resistance to Trastuzumab in Gastric Cancer Patients. Clin Cancer Res. 2016;22:6164-6175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Gambardella V, Fleitas T, Tarazona N, Cejalvo JM, Gimeno-Valiente F, Martinez-Ciarpaglini C, Huerta M, Roselló S, Castillo J, Roda D, Cervantes A. Towards precision oncology for HER2 blockade in gastroesophageal adenocarcinoma. Ann Oncol. 2019;30:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1213] [Cited by in RCA: 1266] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 22. | Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res. 2009;15:7479-7491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 376] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 23. | Hu X, Ma Z, Xu B, Li S, Yao Z, Liang B, Wang J, Liao W, Lin L, Wang C, Zheng S, Wu Q, Huang Q, Yu L, Wang F, Shi M. Glutamine metabolic microenvironment drives M2 macrophage polarization to mediate trastuzumab resistance in HER2-positive gastric cancer. Cancer Commun (Lond). 2023;43:909-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 24. | Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 25. | Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 26. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 764] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 27. | Li J, Qin S, Wen L, Wang J, Deng W, Guo W, Jia T, Jiang D, Zhang G, He Y, Ba Y, Zhong H, Wang L, Lin X, Yang J, Zhao J, Bai Y, Wu X, Gao F, Sun G, Wu Y, Ye F, Wang Q, Xie Z, Yi T, Huang Y, Yu G, Lu L, Yuan Y, Li W, Liu L, Sun Y, Sun Y, Yin L, Hou Z. Safety and efficacy of apatinib in patients with advanced gastric or gastroesophageal junction adenocarcinoma after the failure of two or more lines of chemotherapy (AHEAD): a prospective, single-arm, multicenter, phase IV study. BMC Med. 2023;21:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, Ma XX, To KK, Ambudkar SV, Chen ZS, Fu LW. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981-7991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 29. | He L, Shen X, Liu Y, Gao L, Wu J, Yu C, Li G, Wang X, Shao X. The reversal of anti-HER2 resistance in advanced HER2-positive breast cancer using apatinib: two cases reports and literature review. Transl Cancer Res. 2022;11:4206-4217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Gong J, Wang A, Wei J, Peng Z, Wang X, Zhou J, Qi C, Liu D, Li J, Lu M, Lu Z, Cao Y, Yuan J, Zhang R, Fang J, Zhang X, Shen L. Disitamab vedotin (RC48) plus toripalimab for HER2-expressing advanced gastric or gastroesophageal junction and other solid tumours: a multicentre, open label, dose escalation and expansion phase 1 trial. EClinicalMedicine. 2024;68:102415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/