Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1293
Revised: August 9, 2024
Accepted: August 23, 2024
Published online: October 24, 2024
Processing time: 107 Days and 21.5 Hours
In China banxia xiexin decoction (BXD) has been used in treating gastric cancer (GC) for thousands of years and BXD has a good role in reversing GC histopa
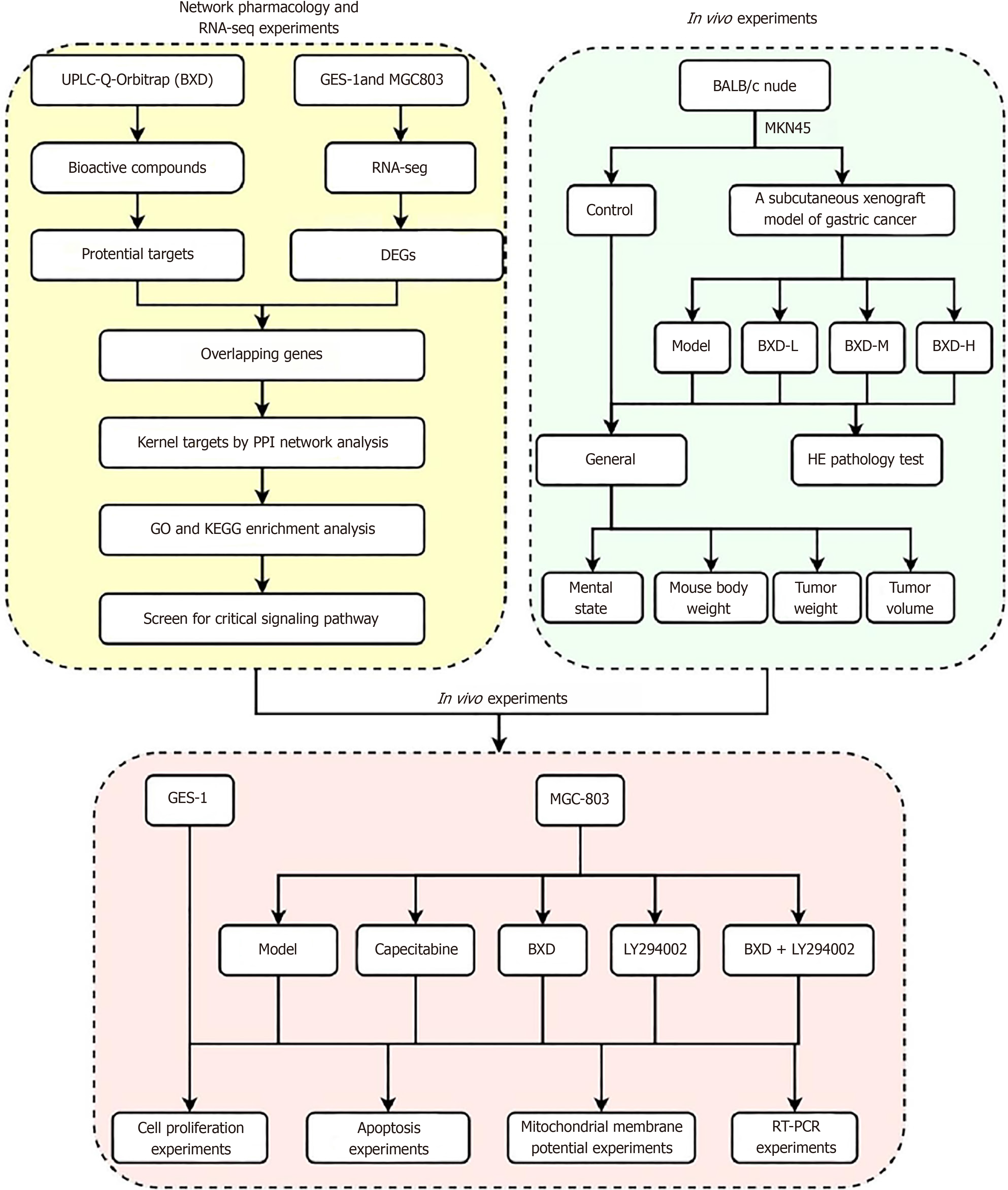
To investigate the mechanism of action of BXD against GC based on transcriptomics, network pharmacology, in vivo and in vitro experiments.
The transplanted tumor model was prepared, and the nude mouse were pathologically examined after administration, and hematoxylin-eosin staining was performed. The active ingredients of BXD were quality controlled and identified using ultra-performance liquid chromatography tandem quadrupole electrostatic field orbitrap mass spectrometry (UPLC-Q-Orbitrap MS/MS), and traditional Chinese medicines systems pharmacology platform, drug bank and the Swiss target prediction platform to predict the relevant targets, the differentially exp
All dosing groups inhibited the growth of transplanted tumors in laboratory-bred strain nude, with the capecitabine group and the BXD medium-dose group being the best. A total of 29 compounds and 859 potential targets in BXD were identified by UPLC-Q-Orbitrap MS/MS and network pharmacology, RNA-seq sequencing found 4767 GC DEGs, which were combined with network pharmacology and analyzed 246 potential therapeutic targets were obtained and pathway results showed that BXD may against GC through the Phosphoinositide 3-kinase (PI3K)/protein kinase B (AKt) signaling pathway. In vitro cellular experiments confirmed that BXD-containing serum and LY294002 could inhibit the proliferation of GC cells, promote apoptosis, and inhibit the migration of GC cells by decreasing the expression of EGFR, PIK3CA, IL6, BCL2 and AKT1 in the PI3K-Akt pathway in MGC-803 expression.
BXD has the effect of inhibiting tumor growth rate and delaying the development of GC. Its mechanism of action may be related to the regulation of PI3K-Akt signaling pathway.
Core Tip: Based on the clinical efficacy of traditional Chinese medicine banxia xiexin decoction (BXD) in the prevention and treatment of gastric cancer, this paper explored the drug composition and drug authenticity of BXD based on Ultra-performance liquid chromatography tandem quadrupole electrostatic field orbitrap mass spectrometry technology, predicted the molecular mechanism of BXD in the treatment of gastric cancer based on network pharmacology and transcriptomics sequencing, and verified its molecular mechanism by combining in vivo and in vitro experiments.
- Citation: Zu GX, Sun KY, Liu XJ, Tang JQ, Huang HL, Han T. Banxia xiexin decoction prevents the development of gastric cancer. World J Clin Oncol 2024; 15(10): 1293-1308
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1293.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1293
Gastric cancer (GC), as a global disease, has the fifth highest incidence rate and the fourth highest mortality rate[1]. According to the World Health Organization data in 2020, China has the highest incidence and mortality rate of GC patients, accounting for 44.0% and 48.6% of the global incidence and mortality respectively[2]. According to the national comprehensive cancer network[3] a treatment strategy that proposes multidisciplinary participation and coexistence of multiple therapeutic modalities for GC treatment, the most common ones are surgical resection, chemotherapy and adjuvant therapy[4]. However, the metastatic rate of postoperative tumors is still high due to the invasion and migration of GC cells to tumor lymph nodes and distal organs[5]. Therefore, it is particularly important to seek an efficient and safe strategy for GC prevention and treatment.
In recent years, under the guidance of Chinese medicine theory, the traditional Chinese medicine formula banxia xiexin decoction (BXD), with the effects of cold and heat leveling, dispersing knots and removing plaques, has remarkable efficacy in the prevention and treatment of gastrointestinal plaques, and its efficacy in the clinical application of GC and precancer is remarkable, which not only reduces the pain of the patients, the medical costs and the It not only reduces patients’ pain, medical cost and economic burden, but also prolongs patients’ life cycle and improves their quality of life[6-9]. Therefore, it is necessary to conduct detailed, in-depth and systematic research on it by using modern scientific research means to further popularize its application.
Previous studies have found that phosphatidyl inositide 3-kinase (PI3K) is an intracellular phosphatidylinositol kinase, which belongs to an important component of inositol and phosphatidylinositol, and has the function of activating protein kinase B (protein kinase B, Akt)[8]. The PI3K/Akt signaling pathway plays a key role in GC tumorigenesis and prognosis, and is involved in cell proliferation, differentiation, apoptosis and other physiological activities[10]. Clinical studies have shown that LY294002, as a general inhibitor of the PI3K signaling pathway, not only enhances the chemo sensitizing effect of 5-fluorouracil, but also enhances the inhibitory effect of 5-fluorouracil on human GC in vitro[10].
PI3K-Akt signaling pathway is a classical cancer pathway, and its abnormal expression in cancer is closely related to the occurrence and development of cancer, but BXD, as a traditional Chinese medicine, has a multi-component, multi-target, and multi-pathway therapeutic mechanism. Clinical evidence shows that BXD has good efficacy on the prevention and postoperative treatment of GC, but the understanding of the mechanism is still shallow, and network pharmacology, as an emerging mechanism prediction technology, combined with bioinformatics can elucidate the interactions between drugs and disease targets through a complex network of interactions[11]. Therefore, in this experiment, MKN45 cells were used to prepare a nude mouse xenograft model, and the effects of different doses of BXD on subcutaneous xenograft were studied. Then, we used ultra-performance liquid chromatography tandem quadrupole electrostatic field orbitrap mass spectrometry (UPLC-Q-Orbitrap MS/MS) technology and network pharmacology to analyze the active ingredients and targets of BXD, and obtained the differentially expressed genes of GC using RNA-seq sequencing. Then, explored the effects of BXD on the proliferation of the GC cell line MGC-803 cells based on the overlapping targets enriched to the PI3K/Akt signaling pathway. MGC-803 cell proliferation, apoptosis, migration and mitochondrial membrane potential. Figure 1 shows the general idea and flow chart of this experiment.
Methanol (chromatographic purity, Thermo Fisher Scientific), formic acid (chromatographic purity, Shanghai Aladdin Biochemical Technology Co., Ltd.), polypropylene centrifuge tubes (1.5 mL, 2.0 mL, Wuhan Xavier Biotechnology Co., Ltd.), direct-Q5 pure/ultra pure water system (Merck Millipore, Germany), D3024R microfuge freezer centrifuge (Beijing Dahlong Xingchuang Experimental Instrument Co. Ltd.), MX-F vortex oscillator (Wuhan Xavier Biotechnology Co., Ltd.), JP-040S ultrasonic cleaner (Shenzhen Jiemeng Cleaning Equipment Co., Ltd.), Eppendorf pipettes (2.0-20.0 µL, 20.0-200 µL, 200~1000 µL), Ulti Mate 3000 RS chromatography (Thermo Fisher Scientific Ltd.), QUALIFICATIONS (Thermo Fisher Scientific), Q exactive high resolution mass spectrometer (Thermo Fisher Scientific), Steady pure rapid RNA extraction kit (Acres Bio, AG21023), Annexin V-AbFluor™ 488/PI dual staining apoptosis detection kit (Abbkine,KTA0002), the Cell Counting Kit-8 (CCK-8) Kit (Biosharp Biotechnology Co., Ltd., C0038), mitochondrial membrane potential detection kit (Biosharp Biotechnology Co., Ltd., C2006), Prime Script™ RT reagent Kit (Takara, RR037A), fluorescence quantitative polymerase chain reaction (PCR) Kit (biosharp, BL698A), capecitabine bin tablets (Qilu Pharmaceutical Co., 0G0823DE3).
BXD: Banxia (Hubei province, 20090302), Huangqin (Hebei province, 20080201), Huanglian (Sichuan province, 20071202), Renshen (Jilin province, 20050504), Gancao (Inner Mongolia, 20092401), Ganjiang (Sichuan province, 20082002), Dazao (Xinjiang province, 20062303). The above herbs were purchased from Shandong Jichengtang Herbal Medicine Company. and all the herbs were accompanied by quality inspection reports, which were in compliance with the requirements of the pharmacopoeia of China. The above seven Chinese medicinal herbs were soaked for 30 min and then boiled over high heat, the dregs were removed, and then concentrated to a raw volume of 2.46 g/mL. The BXD solution was placed in a refrigerator at 4 °C for use in UPLC-Q-Orbitrap MS/MS analysis and in vivo experiments.
Forty specific pathogen-free (SPF)-grade male Wistar rats, 7-8 weeks old, were purchased from Beijing Viton Lever Animal Laboratory Technology Co. Ltd (Beijing 2016-0006), and 60 SPF-grade male laboratory-bred strain nude rats, 4-5 weeks old, were purchased from background Viton Lever Animal Laboratory Technology Co. Ltd (Beijing 2021-0006) in the Shandong province in the Animal Experiment Center of Shandong University of Traditional Chinese Medicine after 7 days of adaptive feeding. This experiment was reviewed by the Animal Welfare and Ethics Committee of Shandong University of Traditional Chinese Medicine (SDUTCM20230828016). Human Gastric Epithelial Cells (GES)-1 and GC cells MGC-803, both purchased from the Cell Resource Center of Peking Union Medical College, and human GC cells MKN45, purchased from Wuhan Sevier Company, were cultured in the Animal Experiment Center of Shandong University of Traditional Chinese Medicine.
Sample processing: 100 µL of sample was taken, 300 µL of methanol was added, vortexed for 10 min, centrifuged for 10 min at 4 °C with a force of 20000 g, and the supernatant was taken for analysis.
Determination of BXD components by UPLC-Q-Orbitrap MS/MS: Compositional chromatographic separation of BXD was carried out using an UltiMate 3000 RS chromatograph, and then the components of BXD were analyzed using a Q Exactive high-resolution mass spectrometer. The samples were analyzed on an AQ-C18 column (150 mm × 2.1 mm, 1.8 µm) at 35 °C. The samples were analyzed by ESI. For the qualitative analysis, an electrospray ionization source (ESI) was used as the ion source, and the detection was carried out in Full mass/dd-MS2, with a resolution of 70000 (full mass) and 17500 (dd-MS2), and the scanning range (scan range) was from 100.0 to 1500.0 m/z under the positive-negative switching scanning mode. For quantitative analysis, single ion monitoring mode was selected for the analysis. The data collected by the high-resolution liquid-liquid acquisition were searched and compared with the database (mz Cloud) by Compound Discoverer 3.3 (Thermo Fisher) after the initial organization of the data.
Cell culture: MKN45 cells were cultured in Roswell Park Memorial Institute 1640 medium containing 10% fetal bovine serum, 1% dual antibody, and incubated in a 37 °C, 5% carbon dioxide constant temperature incubator, and when the cells in the culture flasks reached 80%, the cells were digested and passaged with 0.25% trypsin, and the logarithmic growth phase MKN45 cells were taken to prepare cell suspensions for the establishment of an animal model.
GC transplant tumor model construction: Well-grown MKN45 GC cells were taken, cells were digested with trypsin and counted, cell concentration was adjusted to 6 × 107 cells/mL, and phosphate buffered saline (PBS) was used to dilute the cells into tumor cell suspension, 200 μL of the above cell suspension was taken and injected into nude mouse subcutaneously in the right axilla using a syringe. Observe the growth of mouse after inoculation of cells, and after the tumor grows to a certain volume, measure the long diameter (A) and short diameter (B) of the tumor of mouse every week, and estimate the volume size of the tumor according to the formula V = AB2/2.
Experimental grouping and drug administration: One week after the inoculation of MKN45 cells, the subcutaneous tumor volume in the axilla of mouse was calculated by using vernier calipers, and the mouse were randomly divided into control, capecitabine, BXD low-dose, BXD medium-dose, and BXD high-dose groups when the volume of the tumors was more than 100 mm3, with three mouse in each group, and the BXD low-, medium-, and high-dose groups were ad
Experimental sampling: At the end of drug administration, blood was collected from the eyeballs of tumor-bearing mouse, and the supernatant was centrifuged at 3500 rpm for 15 min after standing for 2 hours. The supernatant was transferred to a new 1.5 mL Eppendorf tube and stored in the refrigerator at -80 °C for spare use. After the mouse were executed, the tumor tissues of each group of tumor-bearing mouse were peeled off, and the ulcerated part of the surface of the tumor tissues was separated, and the tumors were cleaned up and photographed at the place of reorganization of the light, so as to compare the size of the tumors. After photographing, the tumor was divided into two parts, one part was fixed with 4% paraformaldehyde and stored in a refrigerator at 4 °C; the other part was placed in a freezing tube and stored in a refrigerator at -80 °C after quick-freezing with liquid nitrogen.
Hematoxylin-eosin staining: Tumors were taken and fixed in 4% paraformaldehyde to make paraffin sections. The paraffin sections were baked at 60 °C for 2 hours; the sections were placed in xylene for 15 min, 3 times. 100%, 95%, 85% and 75% ethanol were placed in each stage for 5 min; the sections were then rinsed in distilled water for 5 min; he
BXD chemical composition and target screening and identification: The mass spectrometry data were processed and analyzed according to the UPLC-Q- Orbitrap MS/MS results, and the three dimensional results were obtained from Pub chem database, and BXD potential targets were evaluated and predicted by traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP)[12], Swiss target prediction, encyclopedia of traditional Chinese medicine, and drug bank databases[13].
RNA-seq sequencing screened differentially expressed genes for GC: The experiment was divided into MGC-803 group and GES-1 group, according to the guideline. Three biological replicates were used in each group, and the measurement of the RNA concentration was carried out using a Qubit according to the manufacturer’s recommendations, dilution buffer to dilute the RNA samples to the appropriate concentration, and then the quality of the extracted RNA samples was checked using a Q-sep quality checker. Samples with RNA quality number values > 8 were selected for subsequent library construction experiments to generate sequencing libraries, and index codes were added to the attribute sequences of each sample. Differentially expressed genes were identified using data encryption standard eq software. the threshold of differentially expressed genes was set to fold-change (FC) log2 |FC|≥ 1.0, P < 0.05.
Protein-protein interactions analysis: Search tool for the retrieval of interaction gene/proteins (STRING) 11.0 database (https://string-db.org) was used to analyze the intersecting targets, saved as a tab separated values file, and imported into Cyto scape software for visualization, and mapped the protein-protein interactions (PPIs). Network Aalyzer was used to analyze the degree of network topology, the higher the degree, the larger the corresponding node, indicating that the target protein is more important, and the core target of BXD anti-GC was obtained.
Gene ontology and Kyoto encyclopedia of genes and genomes function analysis: The Metascape (http://metascape.org/gp/index.html) database was used to fully annotate the intersecting targets functionally and genetically, and the data results were saved for visualization and analysis.
GC cell culture, passaging: GC cells MGC-803 cells were cultured in 1640 medium with 10% fetal bovine serum and 1% penicillin-streptomycin in a cellular thermostat incubator, and when the cells in the culture flasks reached 80%, the cells were digested and passaged with 0.25% trypsin.
Effect of different concentrations of BXD-containing serum on the viability of MGC-803 cells: Human GC cells MGC-803 in logarithmic growth phase were taken, and 2 × 104 /wells of GC cells were inoculated into 96-well culture plates, with 5 replicate wells in each group, and the cells were completely adhered to the wall under the microscope, and then added with BXD-containing serum (10%, 20%, and 40%) after treatment for 24 hours and 48 hours, respectively, and then added with 10 μL of CCK-8 solution per well, and then cultured at 37 °C for 2 hours. The optical density value of the cells was determined by an enzyme labeler at 450 nm wavelength to determine the optical density value and calculate the cell proliferation capacity.
Experimental grouping and administration of drugs: The experiment was divided into MGC-803 group, BXD group, capecitabine group, LY294002 group and combined BXD + LY294002 group, to which 20% blank serum, 20% BXD-containing serum, 20% capecitabine-containing serum, 15 μM LY294002, 20% BXD-containing serum + 15 uM LY294002 were added respectively.
CCK-8 detects proliferation level of GC cells: MGC-803 cells in logarithmic growth phase were taken and inoculated into 96-well plates at 5 × 103/well, after the cells were attached to the wall, they were cultured for 24 hours and 48 hours according to the experimental grouping of 2.4.3 and given the corresponding drugs, respectively, and 10 μL of CCK-8 was added to each well, and the cells were cultured for 2 hours. Optical density value was measured by an enzyme counter at 450 nm and the cell proliferation capacity was calculated.
Annexin V-Ab Fluor™ 488/PI double staining for apoptosis detection: MGC-803 cells were inoculated on cell crawls and observed under the microscope to be completely adherent to the wall, and then cultured for 24 hours after administration of the drugs according to the experimental grouping and washed twice with PBS, and then 100 µL of 1 × Annexin V Binding Buffer, 5 µL of L-Annexin V-AbFluor™ 488, and 2 µL of PI were added to each well and incubated at room temperature and protected from light for 15 min, and then washed twice with 1 × Annexin V Binding Buffer and ob
Scratch assay to detect cell migration: MGC-803 cells in logarithmic growth phase were selected and inoculated in 6-well culture plates, 2 × 105 cells were inoculated in each well, and cell scratches were created with a 200 μL lance tip, after the scratches were completed, the floating cells were washed with PBS, and photos were taken to record the positions of the scratches, and the cells were continued to be cultured in a constant temperature incubator after the addition of the relative medications according to the experimental grouping under a microscope every 24 hours and the cell migration was observed and recorded.
Mitochondrial membrane potential assay kit with JC-1 staining to detect mitochondrial membrane potential changes: GES-1, and MGC-803 cells in logarithmic growth phase were selected and inoculated in 6-well culture plates, 2 × 105 cells were inoculated in each well, after the cells were wall-approximated, 10%, 20% and 40% of BXD-containing serum were added and cultured for 24 hours. After being washed with PBS, 1 mL of JC-1 staining workup solution was added and incubated for 20 min at 37 °C. The cells were washed with JC-1 staining buffer for two times and then incubated in the mitochondrial membrane potential changes were observed under fluorescence microscope.
Quantitative reverse transcription polymerase chain reaction to detect key mRNA expression on the PI3K/Akt sig
| Primer name | Upstream primer sequence (5’-3’) | Downstream primer sequences (5’-3’) |
| AKT1 | GCTCAGCCCACCCTTCAAG | GCTGTCATCTTGGTCAGGTGGT |
| PIK3CA | TAGTGTCCGGGAAAATGGCT | GGCATGCTCTTCGATCACAG |
| EGFR | GCCAAGGCACGAGTAACAAGC | GGGCAATGAGGACATAACCAGC |
| BCL2 | AGACGGAAGAGAAATTCACTGG | GCCATAATCTCATCAGGGG |
| IL6 | GTAGTGAGGTTCAAG | GCTGTCATCTTGGTCAGGTGGT |
| GAPDH | GAAGGTCGGAGTCAACGGAT | CCTGGAAGATGGTGATGGG |
Statistical product and service solutions 22.0 statistical software was used to analyze the experimental data, GraphPad Prism 8.0 software was used to graph the data, all the measurements were expressed as mean ± SD, one-way analysis of variance was used for multiple group comparisons, and two-by-two comparisons were made by t-test.
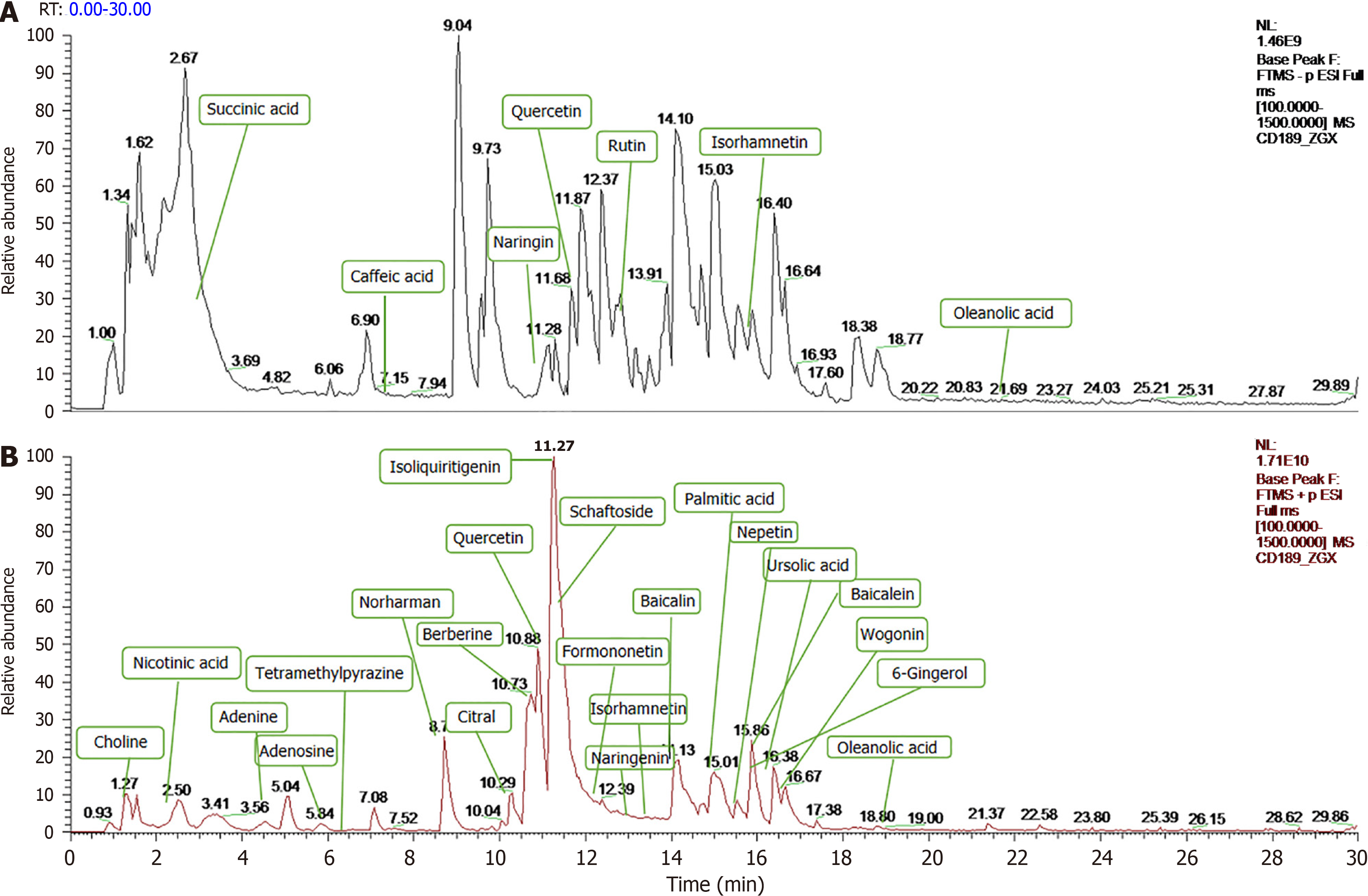
Under the optimized analytical conditions, the total ion flow diagrams of BXD in positive and negative ion modes are shown in Figure 2. A total of 832 compounds were matched by BXD medicinal solution in mzCloud, and 477 compounds with total score greater than 60 in the mzCloud best match. Through the comparative analysis of MS and MS/MS mass spectral information and structural identification by combining with databases (chemsrc, Pubchem, TCMSP) and relevant literature reports, a total of 29 constituents of BXD were obtained, as shown in Figure 1.
Comparison of growth status, body weight, tumor volume, tumor weight and tumor inhibition rate of nude mouse: The nude mouse in the model group were listless, sluggish, and had dry and fluffy hair, and the drug administration groups showed different degrees of relief after drug intervention. Compared with the control group, the body weight of the model group was significantly decreased (P < 0.01), and the body weight of nude mouse in each administration group increased compared with the model group, and the difference was statistically significant (P < 0.01). Different concentrations of BXD and capecitabine had inhibitory effects on GC transplantation tumors in mouse, among which the tumor volume inhibition rate of capecitabine was 62.41%, 55.75% in the low-dose BXD group, 66.99% in the medium-dose BXD group, and 58.48% in the high-dose BXD group, and the statistical difference between the administration groups and the model group was (P < 0.01) (Table 2). The above experimental results showed that BXD could inhibit the growth of GC in nude mouse.
| Group | Number | Weight/g | Tumor volume/mm3 | Tumor weight/g | Anti-tumor rate (%) |
| Control | 3 | 18.83 ± 0.15 | |||
| Model | 3 | 15.133 ± 0.40b | 1641.48 ± 499.84 | 1.25 ± 0.13 | |
| Capecitabine | 3 | 17.633 ± 0.06b | 616.98 ± 156.00a | 0.56 ± 0.15b | 62.41 |
| BXD-L | 3 | 16.833 ± 0.06b | 726.37 ± 168.48a | 0.77 ± 0.08b | 55.75 |
| BXD-M | 3 | 17.673 ± 0.15b | 541.85 ± 95.72a | 0.51 ± 0.09b | 66.99 |
| BXD-H | 3 | 17 ± 0.1b | 682.00 ± 281.61a | 0.55 ± 0.13b | 58.48 |
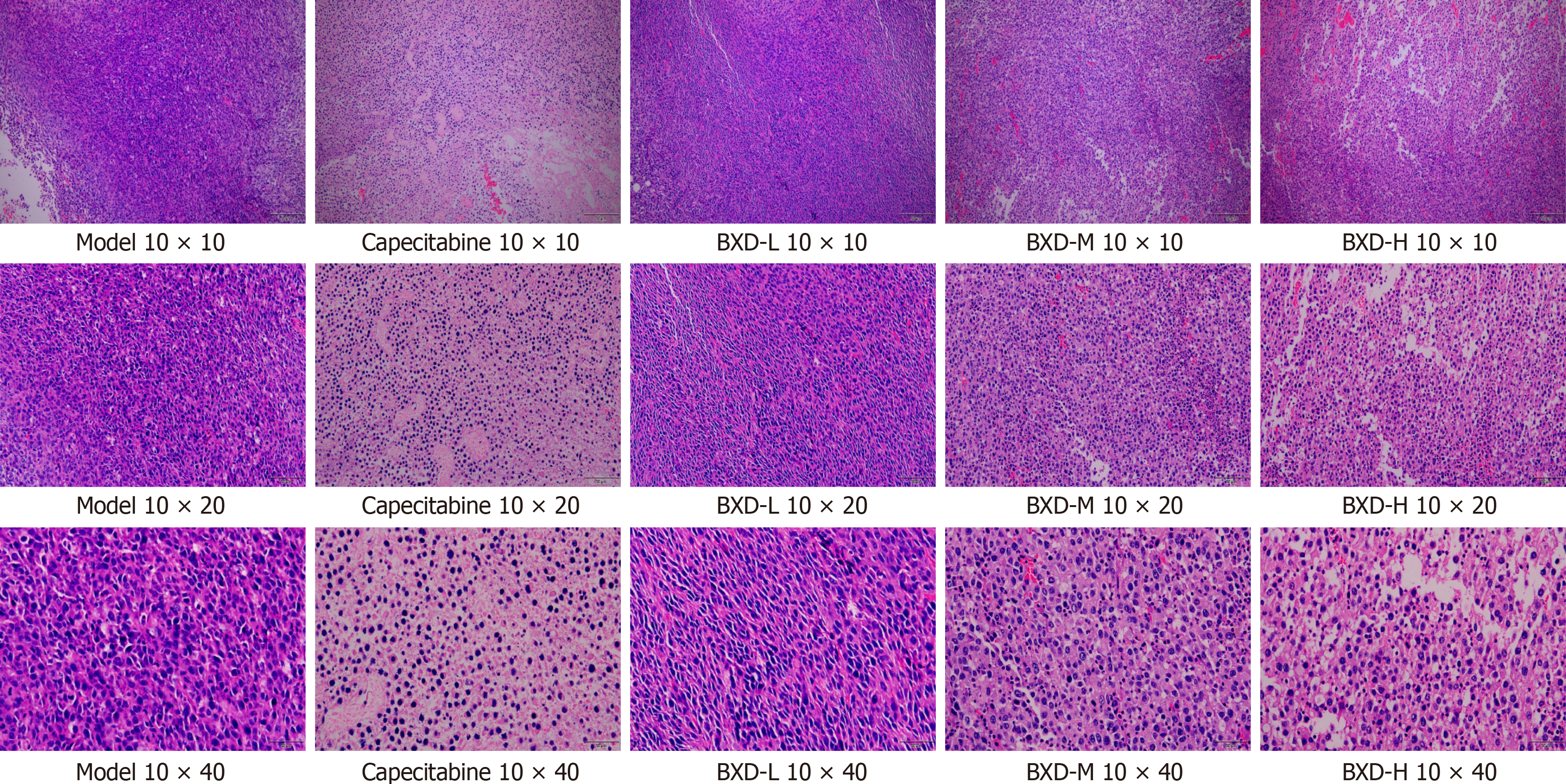
Pathological observations of BXD on mouse tumors: Hematoxylin-eosin staining of tumor tissue sections, Figure 3 shows that in the model group, the number of cells is large and tightly arranged, the volume is large, the nucleus is large and deeply stained, the tumor cell isoform is obvious, and the interstitial blood vessels are rich in capecitabine group and BXD medium-dose group can be seen in the reduced number of tumor cells, loosely arranged, the size of the cells is obviously reduced, the nucleus is solidified, and apoptotic and necrotic cells can be seen in the tissue in scattered distribution, and the gap between the cells is appeared. The number of tumor cells in capecitabine group and BXD medium-dose group was reduced, the number of tumor cells was laxly arranged, the cell volume was obviously reduced, the apoptotic and necrotic cells were scattered in the tissue, and there was a gap between the cells, the number of mesenchymal stromal cells was reduced, and there was an increase in the collagen fibers.
Screening of BXD active ingredients and collection of potential targets: The components in the combined UPLC-Q-Orbitrap MS/MS analysis results were utilized to extract the target genes of each chemical component in TCMSP, drug bank, and Swiss target prediction platforms, and a total of 859 potential targets of BXD were obtained after merging and removing duplicates. Compared with the GES-1 control group, there were 4767 differential genes in the model group, including 2657 up-regulated genes and 2107 down-regulated genes.
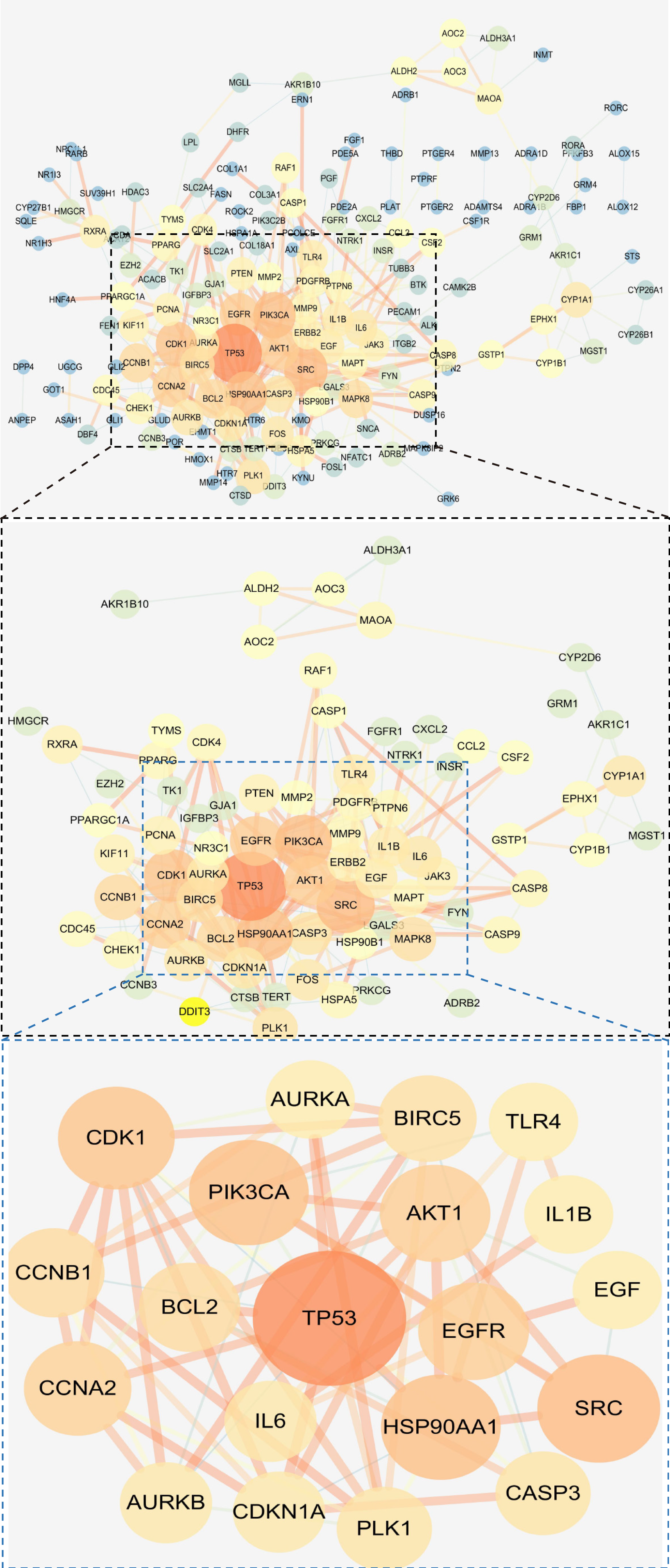
Construction of PPI network and screening of core targets: A total of 246 coincident targets were imported into the STRING online database to obtain PPI network diagrams, with an average node degree of 3.91 and an average near-centrality of 0.405. The key genes were screened with 1 times and 2 times the values of “nodal degree” and “near centrality”, respectively, and the screening threshold was ≥ 7.82 and near-centrality ≥ 0.81, and 20 potential core targets were screened, which were TP53, SRC, HSP90AA1, PIK3CA, CDK1, AKT1, EGFR, CCNA2, BCL2, CCNB1, BIRC5, PLK1, CASP3, IL6, AURKB, CDKN1A, AURKA, IL1B, TLR4, and EGF, suggesting that these gene targets may be key targets for BXD in the treatment of GC, see Figure 4 for details.
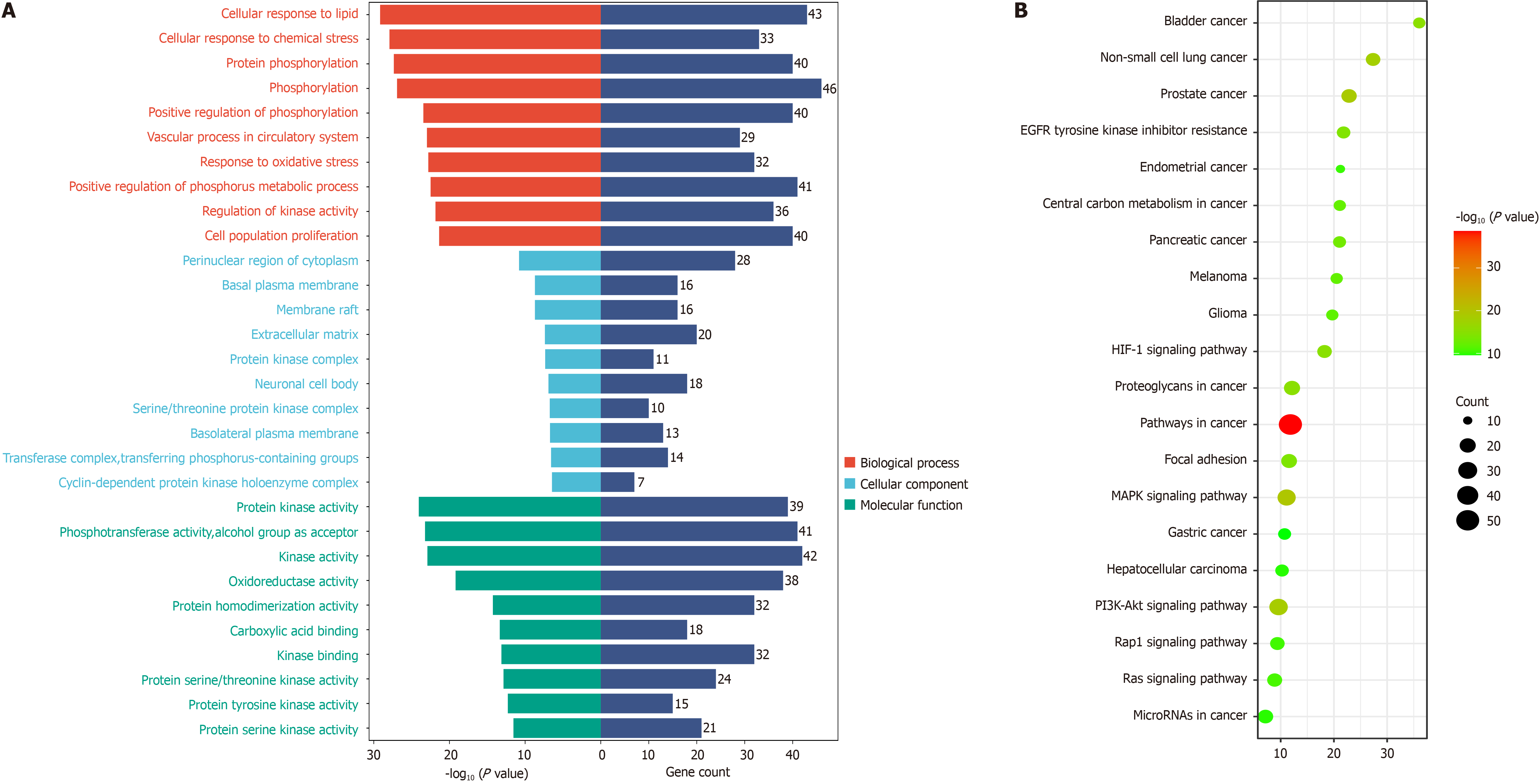
Gene ontology enrichment and Kyoto encyclopedia of genes and genomes signaling pathway enrichment analysis: A total of 2068 gene ontology results were obtained by using Meta scape database to set P < 0.05 and enrichment > 1.5, including 129 cellular component (CC) entries, 1692 biological process (BP) entries, and 247 molecular function (MF) related entries. According to the enrichment, the top 10 BP, CC, and MF were taken for visualization, as shown in Figure 5A. The results showed that BXD positively regulated protein kinase activity, phosphotransferase activity, pho
Effect of different concentrations of BXD-containing serum on the survival of MGC-803 cells: CCK-8 assay was performed after BXD-containing serum intervened in MGC-803 cells, and the results showed that both 20% and 40% of BXD-containing serum significantly inhibited the proliferative effect of GC cells in a dose-dependent manner. The IC50 of BXD-containing serum at 24 hours and 48 hours was calculated to be 20%, as shown in Table 3. Subsequent experiments were conducted with 20% BXD-containing serum.
| Group | 24 hours OD | Proliferation rate (%) | 48 hours OD | Proliferation rate (%) |
| Control | 1.88 ± 0.02 | 2.73 ± 0.04 | ||
| 10% BXD | 1.85 ± 0.01 | 98 | 2.36 ± 0.06b | 86.09 |
| 20% BXD | 0.91 ± 0.07b | 45.78 | 1.53 ± 0.02b | 54.62 |
| 40% BXD | 0.48 ± 0.01b | 21.18 | 0.54 ± 0.02b | 17.09 |
| Capecitabine | 1.00 ± 0.06b | 50.48 | 1.65 ± 0.19b | 59.11 |
| LY294002 | 1.00 ± 0.01b | 50.25 | 1.00 ± 0.07b | 34.7 |
| BXD + LY294002 | 0.64 ± 0.04b | 30.07 | 0.77 ± 0.05b | 26.05 |
CCK-8 detection of GC cell proliferation level: Compared with the model group, the capecitabine group, PI3K inhibitor group and the combination group significantly inhibited the proliferative activity of GC cells at different incubation times (P < 0.01), and the combination group of BXD + LY294002 had the best inhibitory capacity of GC cells in terms of proliferation inhibition, as shown in Table 3, which showed that the LY294002 and the BXD-containing serum complemented each other in inhibiting the proliferation of GC cells and played a promoting role.
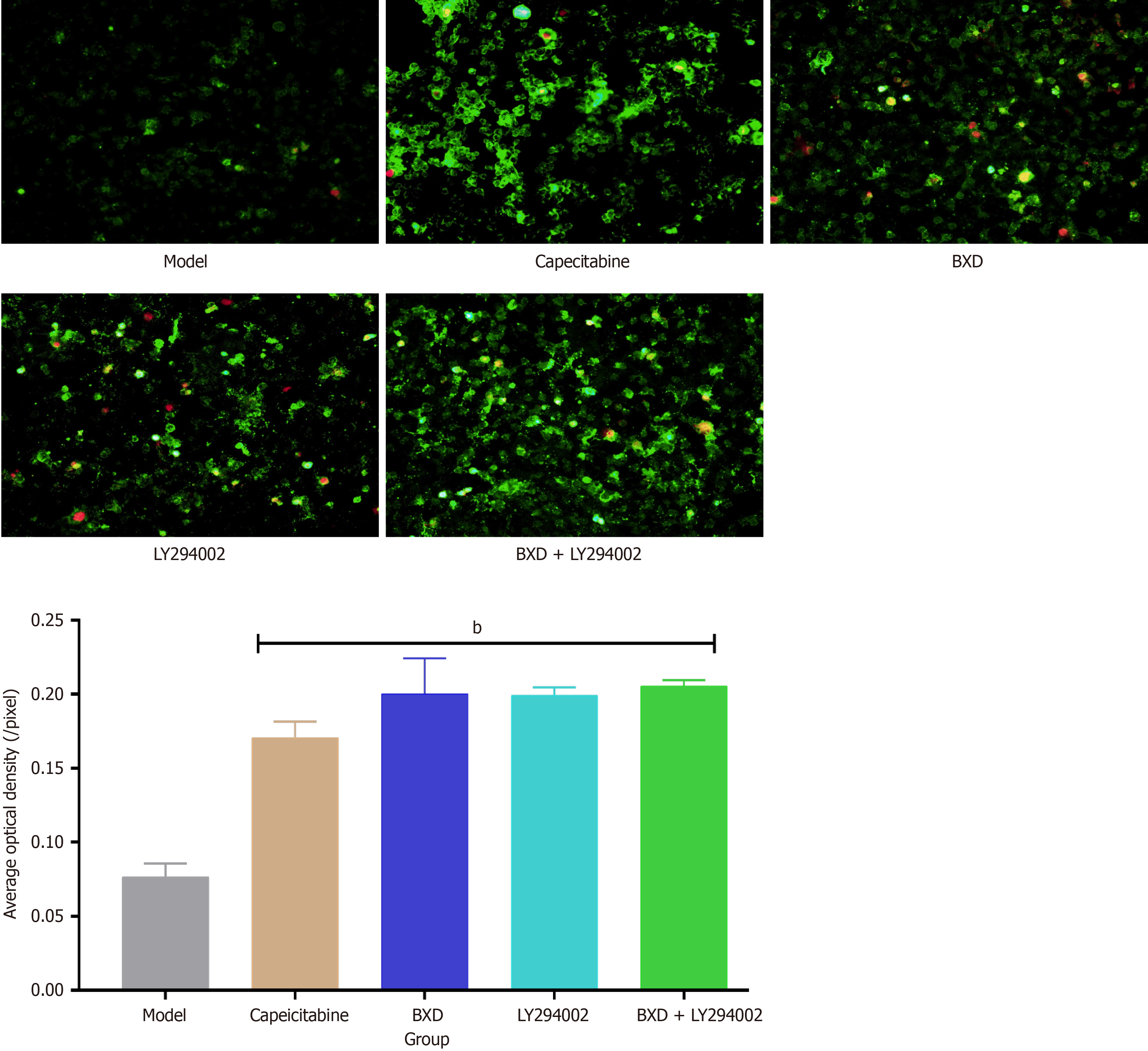
Annexin V-Ab Fluor™ 488/PI double staining for apoptosis detection: The pathogenesis of cancer may be related to apoptotic processes including phosphatidylserine externalization and DNA breakage, and targeting apoptosis in drug development may be the ultimate process in treating cancer. The results showed that the average optical density of cells in the model group was 0.076 ± 0.009 (/pixel), the average optical density of cells in the Capecitabine group was 0.171 ± 0.011 (/pixel), the average optical density of BXD was 0.200 ± 0.024 (/pixel), and the average optical density of cells in the LY294002 group was 0.199 ± 0.005 (/pixel), BXD + the mean optical density of cells in the LY294002 group was 0.205 ± 0.004 (/pixel) and all of them promoted apoptosis of MGC-803 cells as shown in Figure 6.
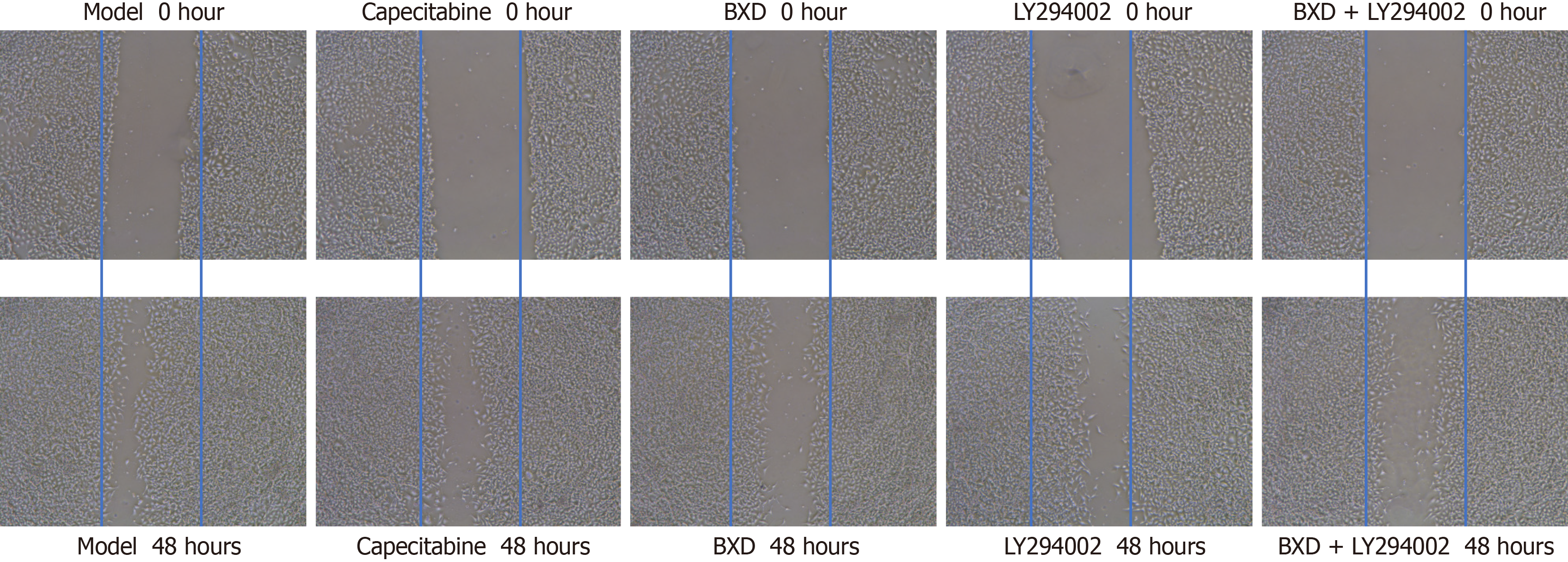
Scratch assay to detect cell migration: The results of cell migration assay showed that the migration rate of cells in the 48 hours model group was (81.25 ± 3.58%), and compared with the model group, the different administration groups could inhibit the migration of GC cells, and the best effect was found in the combination group of BXD + LY294002, see Figure 7.
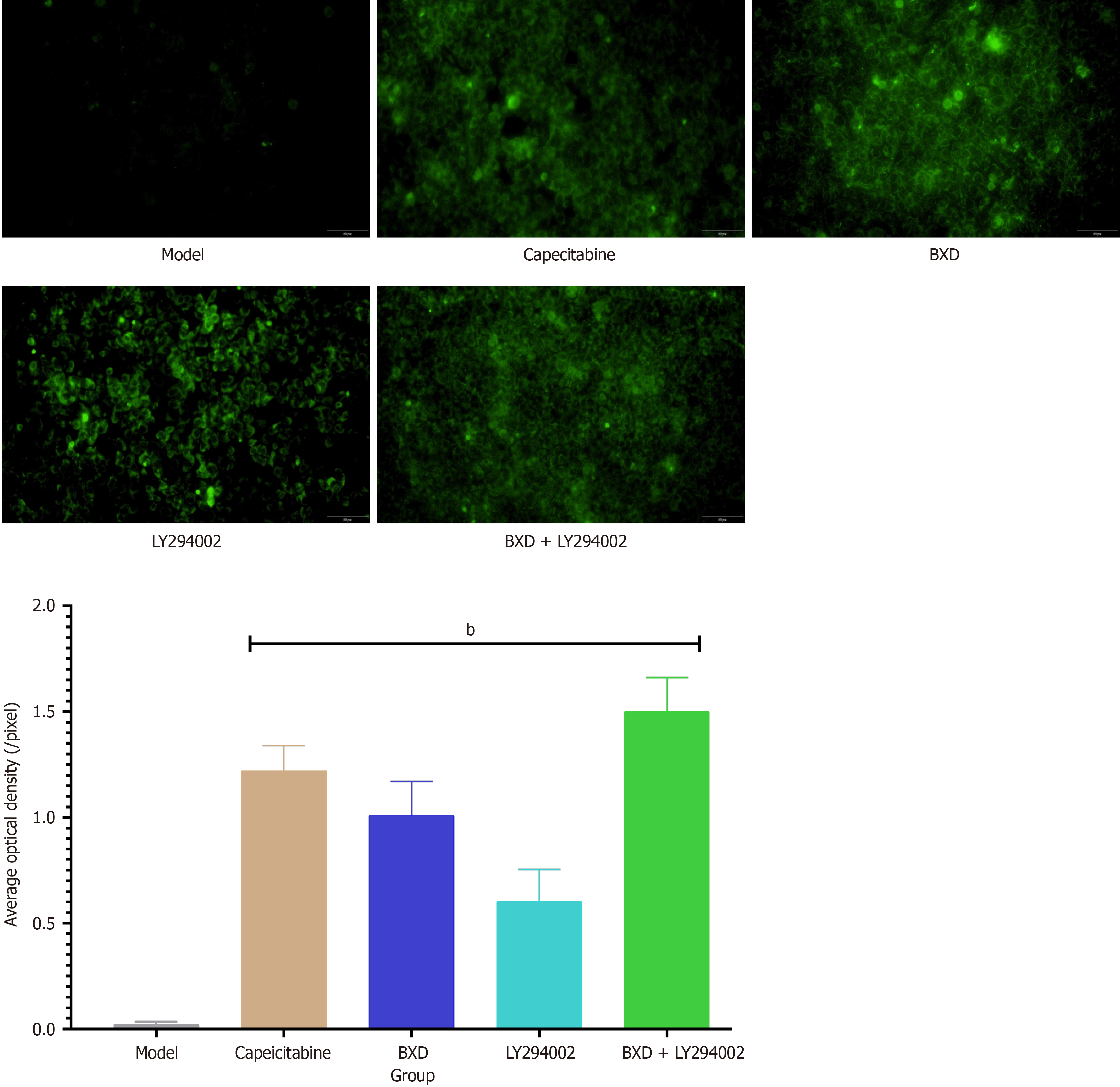
JC-1 staining to detect mitochondrial membrane potential changes: The results showed that the mitochondrial mem
Effect of BXD on mRNA expression on PI3K-Akt signaling pathway: The effects of mRNA expression levels of AKT1, PIK3CA, EGFR, IL6, BCL2 were analyzed by qRT-PCR using GAPDH as an internal reference in the results of transcriptome sequencing and are shown in Table 4. Relative to the model group, the BXD group, the capecitabine group, the LY294002 group, and the combination group decreased AKT1 to different degrees, PIK3CA, EGFR, IL6, BCL2 and AKT1 expression, and the combination of BXD and LY294002 may contribute to the prevention and treatment of GC.
| Group | EGFR | PIK3CA | AKT1 | IL6 | BCL2 |
| Model | 1.00 ± 0.1 | 0.80 ± 0.25 | 1.01 ± 0.11 | 1.56 ± 0.06 | 2.23 ± 0.52 |
| Capecitabine | 0.97 ± 0.4 | 0.34 ± 0.17a | 0.07 ± 0.04b | 1.01 ± 0.14b | 0.52 ± 0.13b |
| BXD | 0.79 ± 0.39a | 0.41 ± 0.18 | 0.04 ± 0.01b | 1.52 ± 0.25 | 1.09 ± 0.56a |
| LY294002 | 0.25 ± 0.14b | 0.07 ± 0.02b | 0.09 ± 0.05b | 1.59 ± 0.17 | 0.85 ± 0.47a |
| BXD + LY294002 | 0.01 ± 0.01b | 0.38 ± 0.21 | 0.06 ± 0.01b | 0.74 ± 0.07b | 0.40 ± 0.11b |
GC is a highly migratory and heterogeneous tumor involving the activation of multiple signaling pathways, which has a high lethality rate and its death rate ranks the first among the mortality rates of malignant tumors in China. Its pa
BXD is a traditional Chinese compound formula in which semenxia, dry ginger, scutellaria baicalensis, and rhizoma coptidis calmly regulate cold and heat so as to balance yin and yang of the internal organs, and the pungent, bitter, and descending make the elevation and elevation of the qi smooth, and the glycyrrhiza, jujubae uralensis, and ginseng support the righteousness of qi, and drag the toxicity out of the body. It has been clinically proved that the addition and subtraction of BXD can delay the development of GC and improve the adverse digestive reaction of postoperative chemotherapy for GC. Liao et al[14] observed that BXD addition and subtraction had better curative effect on GC of damp-heat type in spleen and stomach. Moreover, the use of BXD plus and minus can alleviate the symptoms of diarrhea, hidden pain in the abdomen, acid swallowing, nausea, low food intake, loss of appetite, abdominal distension and so on, which can improve the quality of life of GC patients after chemotherapy, so BXD can be used in the clinical treatment of GC[15] it is evident that BXD has a solid foundation in the clinical treatment of GC, but the exact mechanism behind it is not clear. Therefore, the research group predicted the possible mechanism of action of BXD in treating GC through an integrated approach of network pharmacology and transcriptomic sequencing, and explored the pathway of action of BXD in treating GC through in vitro experiments and analyzing the corresponding changes in biochemical indexes.
In this study, the therapeutic effects of BXD were found using transcriptomics and network pharmacology analyses that BXD may act through AKT1, PIK3CA, IL6, BCL2 and EGFR-mediated PI3K-Akt signaling pathway, and the results of in vitro experiments showed that BXD dose-dependently inhibited the proliferative activity of MGC-803 cells, and furthermore, BXD and the PI3K inhibitor LY294002 had the ability to inhibit the proliferation and migration level of GC cells, promote apoptosis and mitochondrial membrane potential expression level of GC cells, and at the same time reduce the expression level of key mRNAs on the PI3K-Akt signaling pathway in different degrees, which plays a role in delaying the development of GC. The PI3K-Akt signaling pathway acts as an inhibitor of apoptosis and is activated to join in the phosphorylation of NF-κB P65, nuclear translocation, contributing to the exacerbation of the inflammatory response. AKT, the central link in the PI3K/Akt signaling pathway, is an important signaling marker and effector mo
EGFR is a multifunctional transmembrane glycoprotein with tyrosine protein kinase activity, which affects cell growth, differentiation, proliferation and apoptosis levels, in which it is found that EGFR is highly expressed in malignant tumors such as GC, lung cancer, colon cancer, breast cancer and prostate cancer, which may be an important cause of tumor cell mutation and spread. It may be an important cause of tumor cell mutation and proliferation. In addition, studies have confirmed that the expression content of EGFR in GC tissues is higher than that in paraneoplastic tissues, which is positively correlated with the degree of tumor differentiation, depth of infiltration, lymph node metastasis, and clinical staging[20]. Thus, EGFR can be used as a target for targeted therapy in the process of GC occurrence and development. PIK3CA gene is located in region 3 of locus 26 on the long arm of chromosome 3, with a total length of 34 kb, and encodes the catalytic subunit of p110α of type IA PI3K, i.e., the PI3K p110α protein. PIK3CA is closely related to the development of various malignant tumors, such as lung cancer, brain cancer, breast cancer, colon cancer[21], GC[22], etc. It was found that the positive expression rate of PIK3CA in GC and para cancerous tissues was significantly higher than that in normal gastric mucous membrane tissues and was positively correlated with the expression of p-Akt, which further indicated that the expression level of PIK3CA had an effect on the activity of the PI3K/Akt signaling pathway. Previous studies have shown that PIK3CA encodes the catalytic subunit of PI3K, p110α, which is an important component of the PI3K/Akt signaling pathway[23]. Thus, it is inferred that the up-regulation of PIK3CA expression in gastric tumor tissues leads to the enhancement of the catalytic activity of PI3K p110α, which leads to the phosphorylation of Akt, thus activating the PI3K/Akt signaling pathway and contributing to the pathogenesis of GC[24,25], and this led to the activation of PI3K/Akt signaling pathway, promoting the development of GC.
In conclusion, BXD may inhibit GC cell proliferation and migration, induce apoptosis and mitochondrial membrane potential expression by inhibiting the PI3K/Akt signaling pathway, which provides in vitro experimental evidence for the treatment of GC and lays the foundation for further understanding of the mechanism of BXD in GC, and provides a scientific basis for constructing GC animal models for in vivo experiments, and for further exploring the inhibition of the malignant biological behavior of GC by BXD. And provide scientific basis for constructing GC animal models for in vivo experiments to further investigate the molecular mechanism of action of BXD in inhibiting the malignant biological behavior of GC.
The authors thank Experimental Center, Shandong University of Traditional Chinese Medicine in cellular, animal feeding, and molecular imaging techniques to assist with tissue processing and staining.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68351] [Article Influence: 13670.2] [Reference Citation Analysis (201)] |
| 2. | Wang Z, Han W, Xue F, Zhao Y, Wu P, Chen Y, Yang C, Gu W, Jiang J. Nationwide gastric cancer prevention in China, 2021-2035: a decision analysis on effect, affordability and cost-effectiveness optimisation. Gut. 2022;71:2391-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 3. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 1155] [Article Influence: 288.8] [Reference Citation Analysis (0)] |
| 4. | Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother. 2022;146:112542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Deng YQ, Gao M, Lu D, Liu QP, Zhang RJ, Ye J, Zhao J, Feng ZH, Li QZ, Zhang H. Compound-composed Chinese medicine of Huachansu triggers apoptosis of gastric cancer cells through increase of reactive oxygen species levels and suppression of proteasome activities. Phytomedicine. 2024;123:155169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 6. | Dai X, Yu Y, Zou C, Pan B, Wang H, Wang S, Wang X, Wang C, Liu D, Liu Y. Traditional Banxia Xiexin decoction inhibits invasion, metastasis, and epithelial mesenchymal transition in gastric cancer by reducing lncRNA TUC338 expression. Heliyon. 2023;9:e21064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Feng X, Xue F, He G, Ni Q, Huang S. Banxia xiexin decoction affects drug sensitivity in gastric cancer cells by regulating MGMT expression via IL-6/JAK/STAT3-mediated PD-L1 activity. Int J Mol Med. 2021;48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Li Y, Li L, Wang X, Huang H, Han T. Determining the Mechanism of Banxia Xiexin Decoction for Gastric Cancer Treatment through Network Analysis and Experimental Validation. ACS Omega. 2024;9:10119-10131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Sun X, Xue D, Zhang K, Jiang F, Li D. Acrid-release and bitter-downbearing therapy and banxia xiexin decoction regulate Wnt/β-catenin pathway, inhibit proliferation and invasion, and induce apoptosis in gastric cancer cells. Am J Transl Res. 2021;13:6211-6220. [PubMed] |
| 10. | Ren J, Hu Z, Niu G, Xia J, Wang X, Hong R, Gu J, Wang D, Ke C. Annexin A1 induces oxaliplatin resistance of gastric cancer through autophagy by targeting PI3K/AKT/mTOR. FASEB J. 2023;37:e22790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 11. | Zhang R, Zhu X, Bai H, Ning K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front Pharmacol. 2019;10:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 827] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 12. | Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 3410] [Article Influence: 284.2] [Reference Citation Analysis (0)] |
| 13. | Gfeller D, Michielin O, Zoete V. Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013;29:3073-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 14. | Liao Y, Gui Y, Li Q, An J, Wang D. The signaling pathways and targets of natural products from traditional Chinese medicine treating gastric cancer provide new candidate therapeutic strategies. Biochim Biophys Acta Rev Cancer. 2023;1878:188998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 15. | Zhu YJ, Wu XY, Wang W, Chang XS, Zhan DD, Diao DC, Xiao J, Li Y, Ma D, Hu M, Li JC, Wan J, Wu GN, Ke CF, Sun KY, Huang ZL, Cao TY, Zhai XH, Chen YD, Peng JJ, Mao JJ, Zhang HB. Acupuncture for Quality of Life in Gastric Cancer Patients Undergoing Adjuvant Chemotherapy. J Pain Symptom Manage. 2022;63:210-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Ding MR, Qu YJ, Hu B, An HM. Signal pathways in the treatment of Alzheimer's disease with traditional Chinese medicine. Biomed Pharmacother. 2022;152:113208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 17. | Gu C, Zhang Q, Li Y, Li R, Feng J, Chen W, Ahmed W, Soufiany I, Huang S, Long J, Chen L. The PI3K/AKT Pathway-The Potential Key Mechanisms of Traditional Chinese Medicine for Stroke. Front Med (Lausanne). 2022;9:900809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Hinz N, Jücker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal. 2019;17:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 19. | Toulany M, Maier J, Iida M, Rebholz S, Holler M, Grottke A, Jüker M, Wheeler DL, Rothbauer U, Rodemann HP. Akt1 and Akt3 but not Akt2 through interaction with DNA-PKcs stimulate proliferation and post-irradiation cell survival of K-RAS-mutated cancer cells. Cell Death Discov. 2017;3:17072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Feng X, Xue F, He G, Huang S, Ni Q. Banxia Xiexin Decoction Inhibits the Expression of PD-L1 Through Multi-Target and Multi-Pathway Regulation of Major Oncogenes in Gastric Cancer. Onco Targets Ther. 2021;14:3297-3307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol. 2012;18:3745-3751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, So S, Chen X, Yuen ST, Leung SY. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Ye B, Jiang LL, Xu HT, Zhou DW, Li ZS. Expression of PI3K/AKT pathway in gastric cancer and its blockade suppresses tumor growth and metastasis. Int J Immunopathol Pharmacol. 2012;25:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 24. | Feng Y, Ren Y, Zhang X, Yang S, Jiao Q, Li Q, Jiang W. Metabolites of traditional Chinese medicine targeting PI3K/AKT signaling pathway for hypoglycemic effect in type 2 diabetes. Front Pharmacol. 2024;15:1373711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Chu F, Lin J, Li Y, Johnson N, Zhang J, Gai C, Su Z, Cheng H, Wang L, Ding X. Erianin, the main active ingredient of Dendrobium chrysotoxum Lindl, inhibits precancerous lesions of gastric cancer (PLGC) through suppression of the HRAS-PI3K-AKT signaling pathway as revealed by network pharmacology and in vitro experimental verification. J Ethnopharmacol. 2021;279:114399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/