Published online Jul 24, 2023. doi: 10.5306/wjco.v14.i7.265
Peer-review started: December 28, 2022
First decision: April 13, 2023
Revised: May 15, 2023
Accepted: June 27, 2023
Article in press: June 27, 2023
Published online: July 24, 2023
Processing time: 202 Days and 15.5 Hours
Literature focused on cancer screening and management is lacking in the trans
To action to increase contributions to the scientific literature that drives the creation of cancer screening and management protocols for transgender and gender nonconforming (TGNC) patients.
We performed a systematic search of PubMed on January 5th, 2022, with the following terms: “TGNC”, OR “transgender”, OR “gender non-conforming”, OR “gender nonbinary” AND “cancer screening”, AND “breast cancer”, AND “cervical cancer”, AND “uterine cancer”, AND “ovarian cancer”, AND “prostate cancer”, AND “testicular cancer”, AND “surveillance”, AND “follow-up”, AND “management”. 70 unique publications were used. The findings are discussed under “Screening” and “Management” categories.
Screening: Current cancer screening recommendations default to cis-gender protocols. However, long-term gender-affirming hormone therapy and loss to follow-up from the gender-specific specialties contribute to a higher risk for cancer development and possible delayed detection. The only known screening guidelines made specifically for this population are from the American College of Radiology for breast cancer. Management: Prior to undergoing Gender Affirmation Surgery (GAS), discussion should address cancer screening and management in the organs remaining in situ. Cancer treatment in this population requires consideration for chemotherapy, radiation, surgery and/or reconstruction. Modification of hormone therapy is decided on a case-by-case basis. The use of prophylactic vs aesthetic techniques in surgery is still debated.
When assessing transgender individuals for GAS, a discussion on the future oncologic risk of the sex-specific organs remaining in situ is essential. Cancer management in this population requires a multidisciplinary approach while the care should be highly individualized with considerations to social, medical, surgical and gender affirming surgery related specifications. Special considerations have to be made during planning for GAS as surgery will alter the anatomy and may render the organ difficult to sample for screening purposes. A discussion with the patient regarding the oncologic risk of remaining organs is imperative prior to GAS. Other special considerations to screening such as the conscious or unconscious will to unassociated with their remaining organs is also a key point to address. We currently lack high quality studies pertinent to the cancer topic in the gender affirmation literature. Further research is required to ensure more comprehensive and individualized care for this population.
Core Tip: Currently, a comprehensive guideline for cancer screening in the transgender and gender diverse (TGGD) population is lacking. Caring for the TGGD population undergoing Gender Affirmation Surgery is highly individualized and requires consideration of factors such as age at which individuals commenced hormonal therapy and the stage of transition. Once diagnosed with cancer, TGGD patients should receive care at institutions capable of providing a multi-disciplinary approach. This collective approach will ensure record upkeep and help delay any unnecessary delays in care.
- Citation: Panichella JC, Araya S, Nannapaneni S, Robinson SG, You S, Gubara SM, Gebreyesus MT, Webster T, Patel SA, Hamidian Jahromi A. Cancer screening and management in the transgender population: Review of literature and special considerations for gender affirmation surgery. World J Clin Oncol 2023; 14(7): 265-284
- URL: https://www.wjgnet.com/2218-4333/full/v14/i7/265.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i7.265
The transgender and gender diverse (TGGD) population in the United States is estimated to be around 1.4 million, constituting 0.6% of the United States adult population[1]. There exists no census data to back this estimate and may be higher in the younger population. It is well known that cancer screening has led to a decrease in cancer mortality. Many organizations including American Cancer Society (ACS), United States Preventive Services Task Force (USPSTF) have clear recommendations for the early detection of cancer in cis-gender individuals. However, the TGGD population currently has no cancer screening recommendations specific to the TGGD population. The World Professional Association for Transgender Health, a non-profit, interdisciplinary professional and educational organization devoted to transgender health, states that due to a lack of prospective studies, there is not enough evidence for the recommendation of the appropriate type and frequency of screening in this population[2].
In addition to screening, no studies have commented on gender affirming surgery (GAS) and its impact on the screening, management, and surveillance of cancer in the TGGD population. Special considerations must be made during planning for GAS as surgery will alter the anatomy and may render the organ difficult to sample for screening purposes i.e., prostate evaluation following the penile inversion vaginoplasty in the transgender woman. A discussion with the patient regarding the oncologic risk of remaining organs is imperative prior to GAS.
Of note, in this article, the distinction between sex and gender is made based on the former referring objectively to biology and the latter subjectively being psychosocially constructed. Overall, this article aims to review the current guidelines and practice patterns with regard to cancer screening and management in each sex-specific organ for the TGGD population.
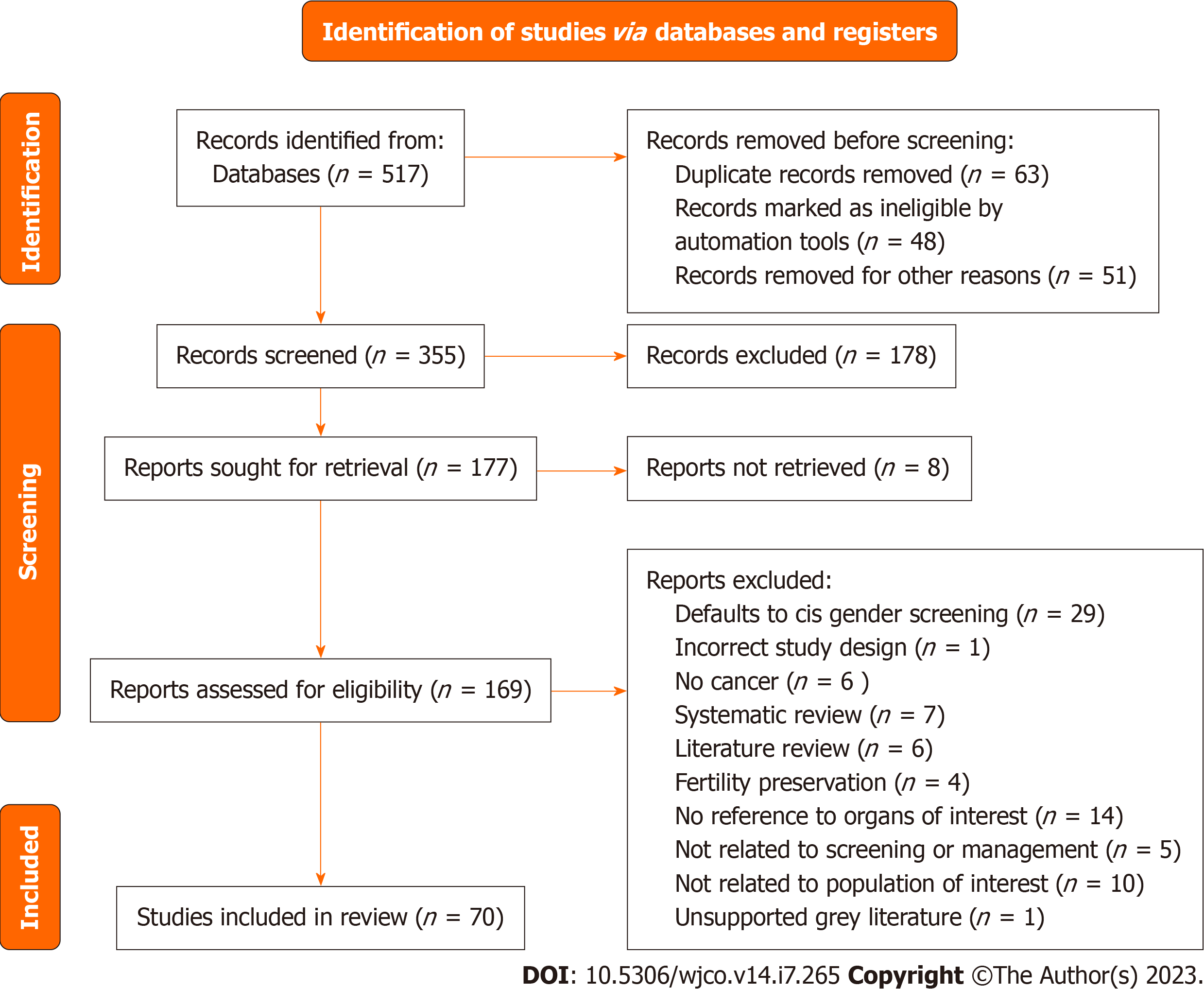
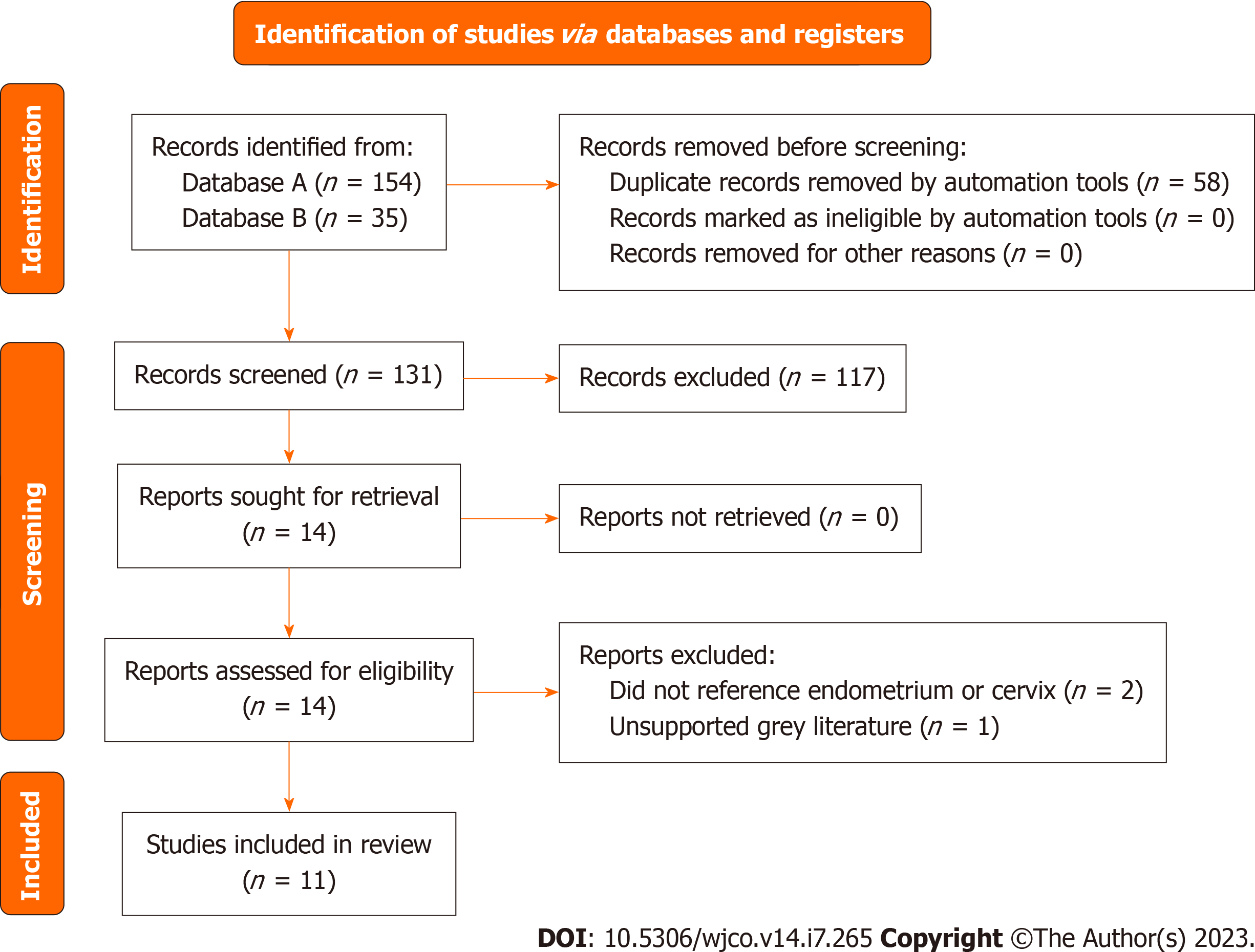
A systematic search of PubMed on January 5th, 2022, with the following terms: “TGNC”, OR “transgender”, OR “gender non-conforming”, OR “gender nonbinary” AND “cancer screening”, AND “breast cancer”, AND “cervical cancer”, AND “uterine cancer”, AND “ovarian cancer”, AND “prostate cancer”, AND “testicular cancer”, AND “surveillance”, AND “follow-up”, AND “management”. After eliminating review articles, duplicates, abstracts, articles not relevant to the section topic or opinion pieces a total of 70 studies with original data were obtained (Figure 1). Articles relevant to the section topic, including the search terms were included in this systematic review. Search parameters were performed according to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Two independent reviewers Araya S and Nannapaneni S carried out independent abstract revisions on January 11th, 2022, using systematic review software “Rayyan”[3] registered in Cambridge Massachusetts.
The PubMed Database was queried from April 1968 to January 2022 using the search text of “(gender nonbinary) OR (transgender and gender non conforming) OR (transsexual) AND (breast cancer)”. This search produced 190 unique articles. Of these articles, 60 were assessed for eligibility and sub-classified based on the primary content of the paper as either screening or management relating to the breast. The term “transsexual” is outdated. However, as our search would span to the remote past, we used this term to be able to identify older publications.
In addition to the physical limitations that GAS can impose on cancer screening, it is equally important to acknowledge the psychological health of each individual patient and the impact of gender dysphoria on their attitude towards the cancer screening process. The lack of protocols and education surrounding TGGD patients provided to healthcare workers has led to an environment where both providers and patients are uncomfortable with the quality healthcare currently being provided[4-10]. Finally, GAS adds to the technical complexity of oncologic screening protocols.
In different retrospective population studies, authors reported that while 92% of studied transgender men have retained their cervixes, they were 60% less likely to undergo cervical cancer screening, 70% less likely to have breast cancer screening, and 50% less likely to have colorectal cancer screening compared to cis-gender patients[9,11,12]. Of note, it is uncommon to remove the prostate during vaginoplasty in transgender women and these patients are also significantly less likely to receive prostate cancer screenings compared to their cis-gender counterparts[13].
While some of these discrepancies can be attributed to differences in demographics as TGGD patients tend to be of a lower socioeconomic status, there are also hurdles these patients face within the healthcare system - including history of prior trauma, provider knowledge deficits, fear of mistreatment or mis-gendering, and lack of appropriate restrooms, gender affirming spaces or educational material[4-9]. There are also disparities of gender affirmation care, gender friendly facilities and services between different parts of the country.
As an example, the ACS recommendation for mammograms for women would miss screening of trans men or nonbinary people for whom the “chest” screening is relevant. Additionally, the lack of gender friendly language may create an additional barrier to care. Some TGGD individuals may want to mentally detach themselves from gender attributed organs i.e., prostate in transgender women or breast in transgender men and attributed screening i.e., a mammogram in the case of a transgender man as this may exacerbate their gender dysphoria. The mention of organs such as “breast” instead of “chest” or “vagina” instead of “current canal” can further promote gender dysphoria in TGGD individuals, and as a result, they are less likely to receive such life-saving screening[4].
Seventy percent of TGGD patients have reported some form of distrust with the healthcare system, and 33% of patients in this population have had negative experiences with healthcare providers that have ranged from incompetent providers and being refused care to harassment and assault[8,9,12]. During the time of the coronavirus disease 2019 pandemic, there has been an increase in anxiety, depression, and suicidal ideation among TGGD patients so providers should be mindful of the mental stress that these patients undergo in addition to the fear and mistrust they have experienced within the healthcare system[14]. Not only do providers need to be explicit in their welcoming of TGGD patients, but they need to invoke flexible methods of meeting the patients’ needs, such as patient-collected HPV swabs, interviewing the patient prior to disrobing, creating a gender friendly environment i.e., introducing themselves with their pronouns and the use of gender-inclusive language[4]. Providers also need to remain up to date on TGGD cancer screening recommendations as a study of gynecological providers found that only 35% felt comfortable providing gynecologic care to this community and even fewer (29%) felt equipped to do so[10]. The utilization of health navigators offers an additional form of support and knowledge for both patients and providers in accomplishing the best care of the patient[15].
Breast cancer is the most common form of cancer in cis-gender women and the second most common cause of cancer mortality in cis-gender women in the United States[16]. However, the reported lifetime risk for TGGD individuals is not reported due to insufficient data and research. Every year, more case studies are reported of TGGD individuals developing breast cancer. Studies have shown increased rates of breast cancer in TGGD women compared with cis-gender males, as well as a decreased risk of breast cancer in TGGD men compared to cis-gender females. For transgender men who have undergone chest surgery to remove the breasts, the decreased risk of breast cancer is an expected finding and consistent with risk reducing mastectomy in the cis-gender female population[17]. However, there is a lack of data and recommendations on breast cancer screening and management of TGGD patients. This is compounded by the inherent risk of discrimination and poor access/barriers to healthcare in the TGGD population, leading to a high rate of disease progression before diagnosis[18,19]. This systematic review aims to elucidate the screening and management of breast cancer in TGGD individuals with a goal of improved care and treatment.
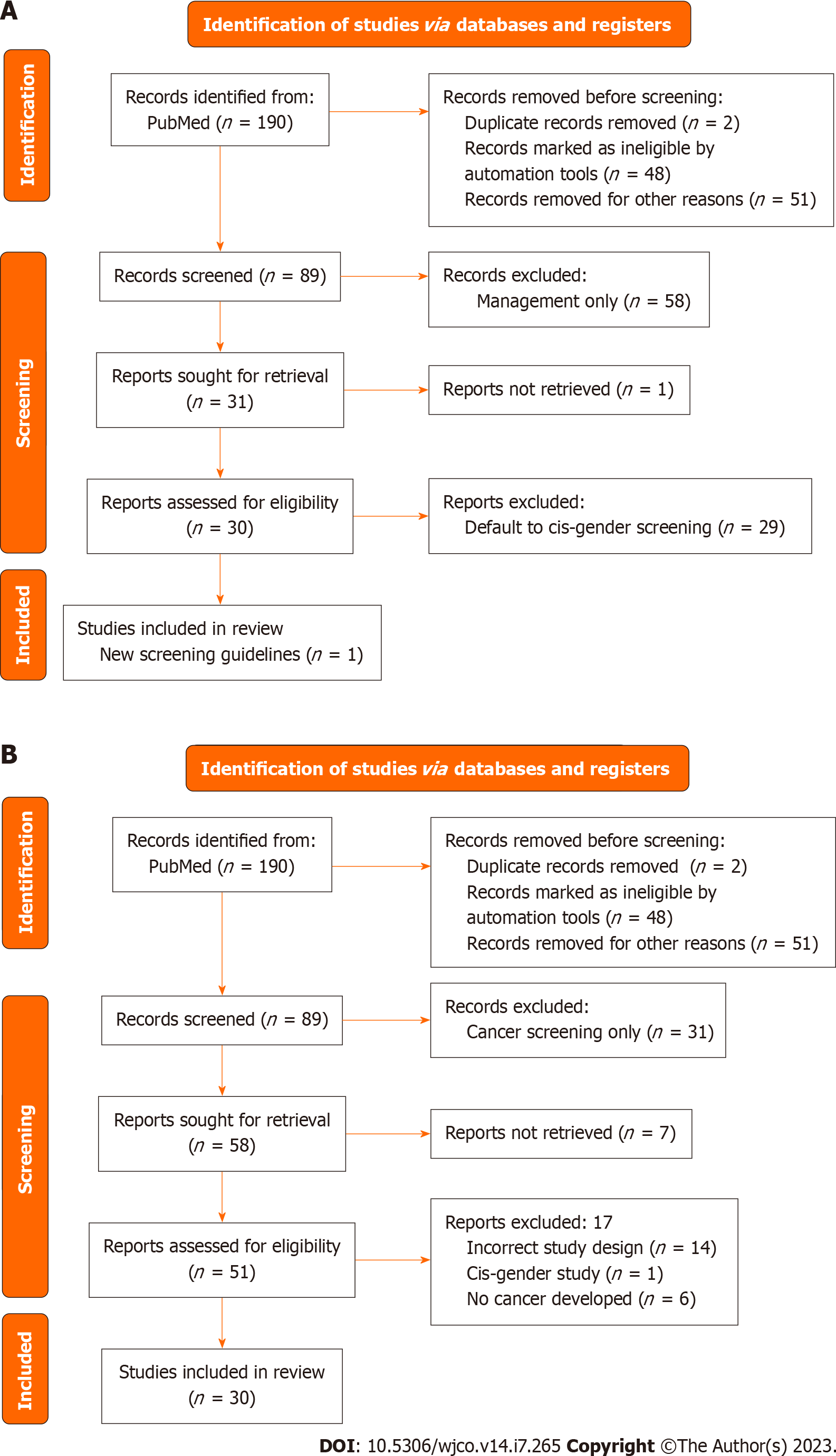
Of the 89 records screened, 30 records were sought for retrieval pertaining to screening for breast cancer in TGGD patients. The majority of these articles (n = 29) deferred management to cis-gender guidelines for TGGD patients or called for more studies on TGGD-specific screening recommendations (Figure 2A). Nevertheless, our review identified and included one article that was a comprehensively covered, evidence-based, breast cancer screening guideline for TGGD individuals provided by the American College of Radiology Appropriateness Criteria in 2021 (Figure 2A)[20]. These guidelines cover eight different variants of screening based on classification of gender affirming surgery, age, duration of exogenous hormone use, and risk category. Recommendations are graded for each variant by appropriateness categories including “Usually appropriate”, “May be appropriate”, and “Usually not appropriate”. Each modality is also considered in relation to the amount of radiation involved. Screening modalities include digital breast tomosynthesis (DBT) screening, mammography screening, magnetic resonance imaging (MRI) breast with and without IV contrast, and ultrasound of breast. Overall, the higher the age, longer the length of use of hormones, and higher the risk category, the more appropriate the use of DBT and MRI becomes.
Transgender women can undergo a variety of breast augmentation surgery procedures to create a feminine appearing chest. Included in this population are non binary individuals who may also undergo breast augmentation procedures. Breasts can be created through a variety of methods, including hormone therapy, fat grafting, saline implants or silicone implants, or autologous reconstruction. Chest masculinization, colloquially referred to as “top surgery”, can be performed to create a more masculine appearing chest. Breast tissue is either reduced or completely removed via liposuction, mastopexy, or mastectomy to create a flat chest, while the nipples can be completely removed and/or resized and repositioned. The authors believe and practice with the gender spectrum concept and as such acknowledge the desired chest to be a spectrum.
Breast cancer in the cis-gender individuals is managed surgically with breast conserving surgery (lumpectomy and radiation), and/or mastectomy. Treatment may also include adjuvant or neoadjuvant chemotherapy and/or radiation pending nodal status along with hormonal therapy with anti-estrogen agents pending hormone receptor status. Currently, breast cancer in the TGGD individual is managed similarly. However, in TGGD patients, the timing of cancer presentation in relation to gender affirming surgery, as well as timing in relation to the use of hormone therapy are additional variables that will affect management.
Of the 89 records screened, 58 of them were sought for retrieval related specifically to management of breast cancer in TGGD patients. Of that 58, there were 30 case reports of breast cancer in TGGD patients (Figure 2B).
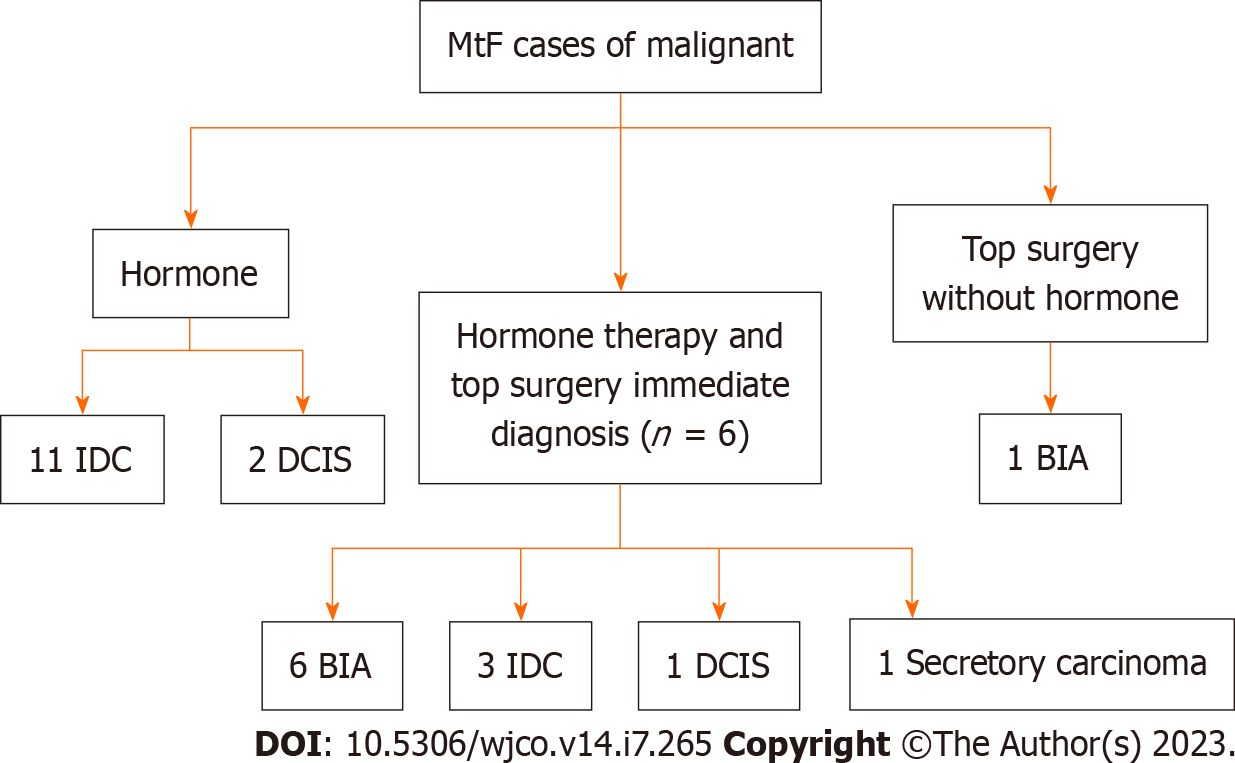
There was a total of 25 male to female (MtF) gender affirming surgery cases among 18 case studies. Each group was further categorized according to hormonal status, gender affirming surgery, and the timing of detection (immediate or delayed) (Figure 3). Immediate detection describes patients whose breast cancer was discovered at the time of gender-affirming breast augmentation. Delayed detection describes cases of breast cancer that were detected after breast augmentation. Patients that did not undergo surgery or hormone therapy were excluded as we were largely interested in understanding how these factors influenced breast cancer detection and management. Patients who were not diagnosed with breast cancer were excluded (Figure 2B).
Eleven papers identified 13 patients who were on estrogen hormone therapy regimens before gender affirming breast surgery. Of the 13 patient studies, two were diagnosed with ductal carcinoma in situ (DCIS) and 11 patients were diagnosed with Infiltrating Ductal Carcinoma (IDC). The average age of cancer diagnosis was 53.8 years old. The average time on hormone therapy prior to the surgery was 16.5 years. All patients mentioned were diagnosed with cancer.
Of the two patients who were diagnosed with DCIS, both had cancer that are Estrogen receptor (ER) positive. One patient demonstrated Progesterone receptor (PR) positive DCIS and hormone treatment was discontinued in the other patient who was PR negative. Additionally, the latter patient was further treated with lumpectomy and radiation with sentinel lymph node sampling, adjuvant chemotherapy, and aromatase inhibitor without any reported recurrence[21]. The patient with ER+/PR+ cancer reported family history of ovarian cancer and a mutation in Chek2 p.I157T, which confers a 1.4 increased risk of breast cancer development. Despite the higher risks, hormone therapy was not discontinued, and the patient was treated with breast conservation surgery and radiation without anti-estrogen therapy according to the patient's wishes. No follow-up recurrence was reported[22]. The difference in the treatment can be attributed to patient desires. Despite being aware of the higher risk, the patient opted to continue hormonal therapy and forgo anti-estrogen therapy.
In the group of 11 patients who were diagnosed with IDC, there was a variety of hormonal receptor status, treatments, and outcomes. Six patients had ER+/PR+ cancers. Of these six, two were positive for BRCA2 mutations. Both patients elected to discontinue hormone therapy[23,24]. The first patient declined tamoxifen and was just surgically treated with a simple unilateral mastectomy of the right side with sentinel lymph node biopsy. Local recurrence occurred 30 mo later and treatment with radiation therapy and adjuvant chemotherapy with aromatase inhibitors (epirubicin plus cyclophosphamide w/paclitaxel)[23]. The other patient was treated with bilateral mastectomy and sentinel lymph node dissection, neoadjuvant tamoxifen and adjuvant radiation (patient declined chemotherapy). No recurrence was reported[24]. This brings up the discussion on what treatment options should be for patients who are positive for BRCA2 mutations. Additionally, it is difficult to know whether ER positivity in these two patients is due to hormone therapy or the mutation itself[24]. This may require more research to determine the effect of BRCA2 mutations on ER+ cancer in the presence of gender affirming hormone (GAH) therapy.
The other four ER+ IDC patients were treated with tamoxifen. One of these patients did not stop hormone therapy and had good outcomes from treatment while two patients who did stop hormone therapy treatment did die from complications of metastatic breast cancer, 22 mo and 6.5 years after their diagnoses[25,26]. This further highlights the necessity to determine what the real impact of GAH therapy in on cancer. Further research is required to mitigate risk of gender-affirming care hormone therapy continuation.
Three patients were diagnosed with triple negative IDC, each of whom were taking hormone therapy for more than 10 years. One patient was only treated with tamoxifen after local wide excision and axillary clearance and did not discontinue their hormone therapy, Premarin. This patient reached remission and remained cancer free after 1 year of follow up[25]. The other two patients did discontinue hormone therapy treatment. Both of these patients were non-surgically treated with neoadjuvant chemotherapy and adjuvant radiation[27,28]. The first patient had no family history of breast cancer and genetic testing found no clinically significant mutations that would increase her risk. However, it should be noted that this patient had significant comorbidities including HIV that was well managed with medication, and depression that was managed through counseling. Additionally, though the patient was ER (-), her healthcare team decided to discontinue use of estrogen therapy to prevent the development of an ER+ tumor subset/second tumor. In addition to management of her breast cancer the patient attended counseling for management of psychological distress attributed to the cessation of estrogen[27].
A second patient with comorbid severe depression on antipsychotic medications and possible secondary hyperprolactinemia attributed to the medications was managed with cessation of GAHs. The patient’s cancer progressed and ultimately expired by intentional drug overdose[28]. Concerns of a patient’s mental status due to aggravation of gender dysphoria and loss of feminine characteristics when halting hormone therapy and even creation of suicidal ideation vs the risk/benefit of hormone therapy on prolactin and cancer incidence is a debated issue in the current literature[28]. Authors describe prolactin screening for patients on long term estrogen given the possible tumor promoter actions in breast and prostate cancer[28].
Patients taking GAHs: This group received treatment with hormone therapy and underwent gender affirming breast augmentation surgery with implants prior to cancer diagnosis. There were 11 patients[6], who were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), three who were diagnosed with IDC, one who was diagnosed with DCIS, and one patient who presented with a triple negative secretory carcinoma caused by a ETV6-NTRK3 gene fusion mutation though no treatment was discussed[29]. The average age of cancer diagnosis was 45.3 years old. The average time on hormone therapy was 14.2 years.
The finding of six TGGD patients diagnosed with BIA-ALCL has implications on health care. For surgical treatment, all were treated with implant removal, capsulectomy, and tumor resection as per treatment in cis-gender women with ALCL[30-32]. This treatment has been shown to improve disease-free survival[32]. Complete surgical resection with en-bloc removal of the disease, implant, and capsule provides the best survival outcomes. However, for patients with extensive disease and regional lymph node involvement, adjuvant chemotherapy and/or radiation may be recommended[30].
According to the most recent National Comprehensive Cancer Network (NCCN) guidelines in the United States, adjuvant radiation therapy is indicated for patients with local residual disease with or without regional lymph node involvement or unresectable disease with chest wall invasion. Systemic chemotherapy is indicated for patients with Stage II–IV disease[32]. All six patients received textured implants, a possible risk factor for the formation of BIA-ALCL[33].
Three patients were diagnosed with IDC. One of the patients was advised to discontinue hormone therapy, however, decided to continue it against medical advice. No length of follow up or recurrence was reported[34]. However, the authors present an interesting debate as to what the acceptable balance of risk vs benefit is for cessation of hormone therapy in this group of patients given the often competing oncologic vs gender affirming interests[34].
Patients not taking GAHs: One paper identified a TGGD patient who underwent gender affirming breast augmentation surgery without prior hormone therapy treatment[32]. This patient was diagnosed with BIA-ALCL and subsequently treated with bilateral implant removal and capsulectomy of the affected side. The patient did not receive any radiation or chemotherapy and was tumor-free 10 mo post-operatively[32].
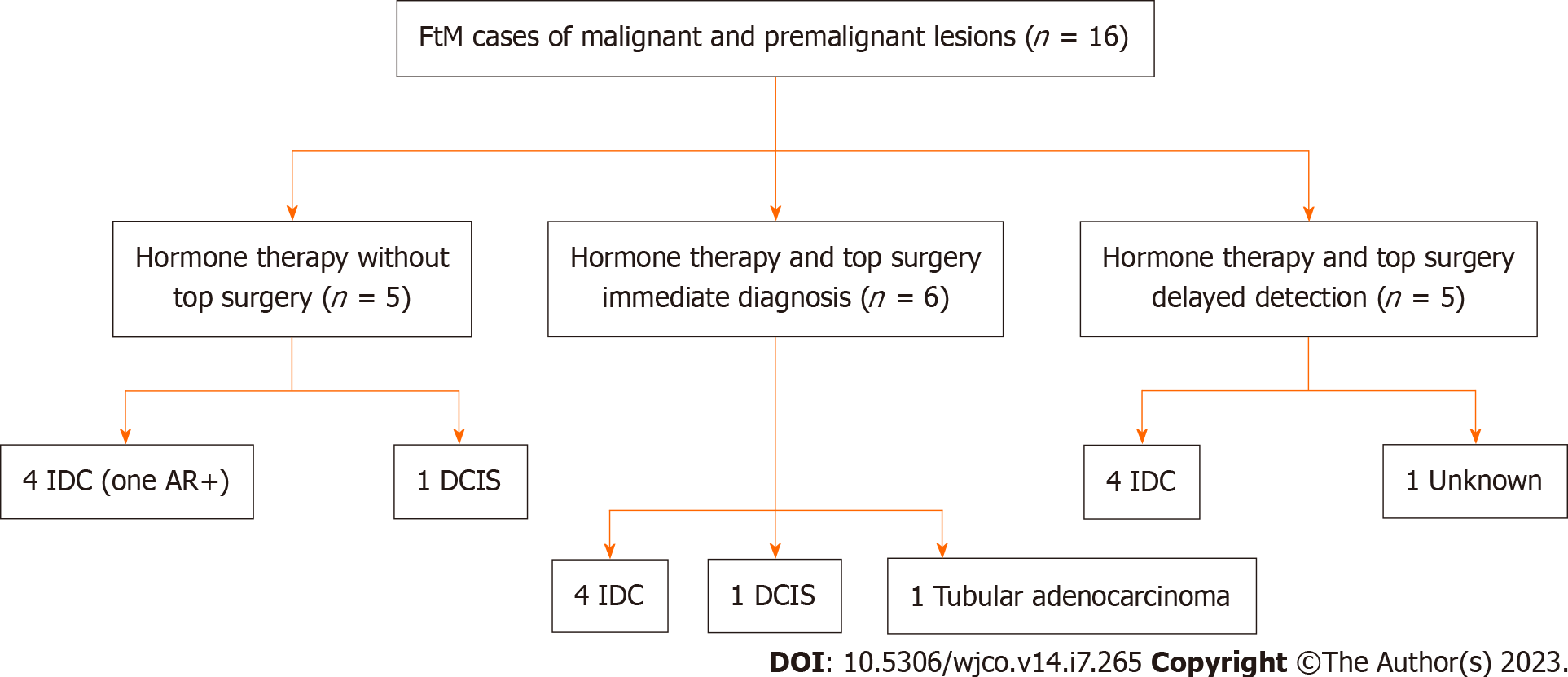
There was a total of 16 female to male gender affirming surgery patients among 12 case studies. Each group was further categorized according to hormonal status, gender affirming surgery, and the timing of detection (immediate or delayed) (Figure 4). Immediate detection describes patients whose breast cancer was discovered at the time of gender-affirming top surgery. Delayed detection describes cases of breast cancer that were detected after top surgery. Patients that did not undergo surgery or hormone therapy were excluded as we were largely interested in understanding how these factors influenced breast cancer detection and management. Patients who were not diagnosed with breast cancer were excluded (Figure 2B).
Four patients (out of 12 patients) among three papers were identified with intramuscular testosterone usage and development of breast cancer prior to top surgery (mastectomies)[26,35,36]. All four patients developed an IDC. There was a mix of hormone receptor positivity with no specific trend. The average time on intramuscular testosterone therapy was 4.7 years. The average age at diagnosis was 46.3 years old. Four patients among two papers were excluded due to no hormone therapy used[21,26].
One patient with ER+/PR+/HER2+/AR+ IDC was treated with bilateral mastectomies with right sentinel lymph node biopsy, nipple-areolar grafting, neoadjuvant chemotherapy, and continuation of testosterone therapy survived an unknown amount of time. A second patient diagnosed with ER+/PR-/HER2+ IDC was treated with unilateral mastectomy and adjuvant chemotherapy. Management with testosterone therapy was unknown. The patient expired within two years. A third patient with ER+/PR+ IDC was treated with lumpectomy, followed by bilateral nipple-sparing mastectomies 1 year later and unknown management of testosterone therapy after diagnosis was in remission at least 10 years. The fourth patient with ER-/PR+ IDC bilateral managed with nipple sparing mastectomy, adjuvant and neoadjuvant chemotherapy with permanent discontinuation of hormone therapy was in remission at least 5 years. Unfortunately, the sparse number of cases studied and incomplete patient history and follow up in these patients do not provide a good platform to draw conclusions for hormone continuation, surgical management, or survival.
One of the patients developed a clinically interesting finding of an androgen receptor (AR) positive IDC[37,38]. The authors of this paper emphasized the importance of testing for AR sensitivity in TGGD patients as some of the patients may be taking testosterone and stopping the hormone may impact their gender dysphoria. However, continuing GAH therapy could lead to progression or recurrence of the cancer after treatment given the cancer’s responsiveness to the AR sensitivity.
One patient developed DCIS, a premalignant lesion, in the setting of testosterone therapy. This is an interesting finding as even a premalignant lesion is a risk later down the line for these patients and begs the question of needing oncologic mastectomy to completely mitigate the risk. It is important to mention that the DCIS in the cis gender individual, not on androgen therapy, can undergo a risk reduction with hormone blockers and wide local excision and may not particularly necessitate a mastectomy. Had this pathology not been caught in the pre-operative setting, this patient could have been found to have a cancer or DCIS later during the top surgery or even in rare occasions in the post top surgery setting i.e., in case the residual breast tissue will keep the burden of DCIS pathology. Therefore, this situation emphasizes the importance reevaluating GAH dosing or discussing discontinuing the hormone altogether.
Immediate detection (surgical pathology): Five patients (out of 9 total patients) were found to have cancer based on surgical specimens sent for histologic evaluation during their top surgery. Four of these patients had invasive ductal carcinoma, one developed tubular adenocarcinoma[21,38,39]. In addition, one patient’s pathology revealed a high grade DCIS[40]. The mean time on intramuscular testosterone therapy was 11.2 years. The average age at diagnosis was 45.4 years old. There were no patients that were found to have cancer during top surgery that did not take hormone therapy beforehand.
One patient with ER+/PR-/HER2- IDC was treated with bilateral mastectomies along with axillary lymph node dissection and chemotherapy. Later that patient presented with recurrence and underwent re-excision, radiotherapy, and tamoxifen treatment with unknown management of testosterone therapy after diagnosis with remission. Another patient with ER+/PR-/HER2+ IDC was treated after bilateral mastectomy with sentinel lymph node dissection plus chemotherapy. After a temporary discontinuation of testosterone therapy, the patient went into full remission. A third patient who was ER+/PR+/HER2+ IDC was treated with bilateral mastectomy with axillary lymph node dissection plus chemotherapy. After temporary discontinuation of testosterone, the patient had unknown survival. A fourth patient with ER+/PR+/HER2- IDC was treated after partial mastectomy breast reduction with full left mastectomy with sentinel node sampling with anastrozole plus radiation. After permanent discontinuation of testosterone, the patient had unknown survival. Finally, the last patient with ER+/PR+/HER2- tubular adenocarcinoma was treated with mastectomy and had a negative sentinel node biopsy. They did not discontinue testosterone, and received no further treatment, but had an unknown survival.
Overall, this section emphasizes the potential impact of having pathology sent for specimens at the time of surgery as earlier intervention on these patients could only improve the survival. All of these patients opted for full mastectomy (if not already done), whether unilateral or bilateral, for treatment of the cancer. Unfortunately, we are unable to draw clear conclusions from this subgroup for guidance on hormone discontinuation and survival outcome. However, one retrospective review comments on the increased risk of premalignant lesions and cancer found in surgical specimens of 193 bilateral mastectomies for TGGD patients both with and without hormones and reported an incidence of 8.8% of atypical lesions requiring further investigation[41]. Thus, even if no malignancy is anticipated in these patients, they would benefit from sending surgical specimens for pathologic evaluation.
Delayed detection (no surgical pathology): Four patients among five papers were found to have cancer based on screening post mastectomy. The mean amount of time post mastectomy for cancer diagnosis was 10 years. Four patients developed invasive ductal carcinoma, one patient’s diagnosis was unknown[42-44]. The average time on intramuscular testosterone therapy was 7.7 years. The mean time after the first breast surgery was 10 years. The average age at diagnosis was 46.2 years old.
One patient with ER+/PR+/HER2 equivocal metastatic IDC discovered 20 years after bilateral mastectomy with free nipple grafts with unknown testosterone hormone management post diagnosis was treated with letrozole and had unknown survival. A second patient with ER+/PR+/AR+/HER2- IDC discovered 12 years post mastectomy, was treated with breast partial resection, sentinel lymph node dissection, radiation therapy and aromatase inhibitors (patient refused tamoxifen due to feminization effects) with unknown testosterone hormone management post diagnosis and had unknown survival. A third patient who was diagnosed with triple negative IDC discovered seven years after bilateral mastectomy was treated with lumpectomy and adjuvant chemotherapy. This patient had unknown testosterone hormone management and had survived at least two years after treatment. A fourth patient with ER-/PR- metastatic IDC discovered one year after bilateral subcutaneous nipple sparing mastectomy was treated with neoadjuvant chemotherapy and radical mastectomy with axillary dissection. This patient had unknown testosterone hormone management post diagnosis and had unknown survival.
This subgroup of patients poses an interesting discussion of reduced risk of cancer from previous mastectomy, yet development of cancer in residual breast tissue shows such risk reduction not to be absolute. This would be due to the incomplete removal of breast tissue and pre-pectoral fascia in those that go for gender affirmation mastectomies vs oncologic mastectomies. The question as to whether we should offer a completion of (full oncologic) mastectomies (removing the pectoral fascia and the nipples) for such GAS patients remains uncertain. However, since the nature of these patients is higher loss to follow up and noncompliance with traditional screening, in addition to taking hormones, this population could be at higher risk than others for surreptitious development of cancer. Thus, they might benefit from a prophylactic oncologic mastectomy rather than a gender affirmation (subcutaneous) mastectomy. Clearly, this needs to be weighed against the cosmetic benefits of a subcutaneous mastectomy with nipple-areolar preservation and the quality-of-life implications that it affords.
Of the 30 case studies, four patients were identified who were positive for BRCA2[23,24,33]. These four patients were transgender women. In cis-gender females with BRCA 1 or 2 mutation the lifetime risk of developing breast cancer is 55%-72%, while the lifetime risk in cis-females in 13%. In cis-gender men with a BRCA2 gene mutation, the lifetime risk of breast cancer is approximately 7 to 8 percent, while the lifetime risk of male breast cancer in the general population is approximately 0.1 percent[45,46].
In one case report, the patient, transgender woman, underwent bilateral skin-sparing mastectomy after confirming they were BRCA2 positive. A second patient, transgender woman, developed IDC two years after starting hormone therapy. She had bilateral mastectomies with immediate expander reconstruction and right sentinel lymph node sampling as well as adjuvant radiation therapy and then subsequently tested positive for BRCA2. A third patient, transgender woman, developed IDC after seven years on hormone therapy and underwent a right simple mastectomy with sentinel lymph node biopsy. There was recurrence 30 mo post-mastectomy, so radiation therapy and adjuvant chemotherapy were given as treatment. A fourth patient was identified as BRCA2 positive but had not developed cancer yet.
One of the most important points to be made about this subgroup analysis is that all six patients discontinued gender-affirming hormone therapy upon diagnosis with BRCA mutations. This seems to be the current standard of practice for management of these patients yet many patients choose not to discontinue hormone therapy. In fact, our review came across a few arguments against cessation, namely the history of treating advanced breast cancer with low dose estrogens and the deleterious effects of cessation on the mental well-being of TGGD patients[24,26,33]. More research is required to determine if there is a true therapeutic benefit to cessation of GAHs. Our systematic review also identified recommendations such as offering TGGD women who are BRCA1/2 positive risk-reducing mastectomies prior to breast augmen
Additionally, this brings up an interesting discussion of whether we should routinely test these patients for BRCA before undergoing surgical intervention, or even prior to hormone initiation. This patient population would inherently benefit from more prophylactic interventions given the higher loss to follow-up and screening. Recommendations for the surgical management of the BRCA+ TGGD patients follow similar guidelines to the cis-gender individual for risk-reducing bilateral mastectomies over conservative, primarily aesthetic breast reductions. Overall, more studies need to be done to elucidate and strengthen further recommendations with regard to BRCA management in TGGD.
Our search yielded seven cases of breast implant associated-anaplastic large cell lymphoma in transgender women[32,48]. There is a known increased risk of developing BIA-ALCL in cis-gender women with textured implants[32]. Thus, this risk is conferred in TGGD females as well. Loss of follow up and willing to seek medical attention may be further exacerbated by lack of provider knowledge on gender friendly language ultimately leading to delayed recognition and diagnosis[32]. Avoidance of gender specific language such as “breast” instead of “chest” as reference for anatomical parts may assist with patient willingness for follow-up and screening.
One patient underwent unilateral mastectomy (implant previously removed) with resection of pectoral muscle, and axillary node dissection and received chemotherapy. The second one underwent bilateral en-bloc resection of capsule and implant. The third one underwent en-bloc resection of implant, capsule, and mass (resection included part of pectoral muscle) plus chemotherapy. The fourth one underwent bilateral en-bloc resection of capsule and implant plus sentinel lymph node biopsy, excision of active lymph node and chemotherapy. The fifth patient underwent en-bloc resection of the capsule and implant. The sixth one underwent bilateral en-bloc resection of capsule and implant plus sentinel lymph node biopsy, along with chemotherapy and adjuvant radiation therapy. The seventh patient underwent bilateral en-bloc resection of capsule, implant and tumor plus chemotherapy. The average time to diagnosis was 13.4 years, which is slightly more delayed yet comparable to cis-gender timeline of 9.75 years[49].
As BIA-ALCL is becoming more common in TGGD patients, surgeons should be aware of this and encourage follow up. Often patients experienced symptoms at least 2 years before going to their followed up, and with less frequency than cis-gender individuals[32]. Education of “signs and symptoms patients should look out for” may go a long way in improving rates of follow up as it makes patients aware of the dangers and gives them agency and involvement in their treatment.
Although it has been declared illegal since 1970s due to high number of complications, unfortunately free silicone injection has been and continues to be performed as a mode of breast augmentation in the TGGD individuals[50]. Secondary breast reconstruction after silicone injections is relevant to chest feminization. In one study, the incidence of prior silicone breast injections was 7.3%. In their cohort of 41 chest feminization surgery patients, there was only one patient with minor complications which healed without surgical intervention[51]. This study concluded that careful evaluation and planning can minimize the risk of complications in secondary breast reconstruction post silicone injections. Another study reported a case of TGGD patient with a false-positive axillary lymph nodes due to silicone adenitis from silicone leakage[52]. A final case reports two incidences TGGD patients with breast inflammation and necrosis as a result of silicone and paraffin injections[53].
A carefully performed history and physical exam are critical to planning reconstruction options. One point that was not discussed in these case reports is how silicone will affect breast imaging and routine cancer screening by obscuring the gland tissue. This has been addressed in the American College of Radiology (ACR) guidelines in more detail. Overall, successful reconstruction is possible as long as one familiarizes themselves with silicone usage and how it can mimic other pathologies. Patients with silicone may require further workup to ensure etiology of pathology before surgical planning can safely begin.
Figure 5 includes the PRISMA flow diagram regarding endometrial and cervical cancer studies. A lack of endometrial screening protocols for TGGD people on HRT, lead providers to follow the guidelines currently in place for cis-gender women. There is currently no evidence-based indication to perform prophylactic screening for endometrial cancer in cis-gender women. As such, diagnostic procedures like an endometrial biopsy or transvaginal ultrasound are not routinely recommended for transgender men regardless of hysterectomy status. Abnormal vaginal discharge and bleeding serve as signs to seek screening measures. The ACS recommends educating TGGD individuals with a vagina on the topic of unusual vaginal bleeding and to explore instances both pre and post hysterectomy. This may be difficult as TGGD individuals often avoid regular visits to their gynecologist, especially after undergoing a hysterectomy.
A uterine pathology study from Grimstad et al[54] reviewed 94 transgender men receiving testosterone therapy, reporting no case of endometrial cancer[55]. A similar pathologic analysis from Ralph et al[55] reported no evidence of malignant changes to the endometrium of transgender men in response to long-term testosterone treatment[55]. The uterine histological similarity to cis-gender women indicates regular endometrial screening is unlikely to be necessary for transgender men undergoing androgen therapy.
The literature documents one case of uterine cancer in a transgender man after he was found to have a mass noted during speculum examination for planned hysterectomy in preparation for GAS[56]. Post-operative pathology from a transmasculine person’s radical hysterectomy revealed stage IIIC endometrioid adenocarcinoma of the uterus. The diagnosis included involvement of the parametrium and lymph nodes. The patient was treated with 6 cycles of chemotherapy (carboplatin and paclitaxel) before declining additional treatment. Two years later, the patient had evidence of recurrent disease and underwent additional chemotherapy. Follow up beyond this date is lost. The authors note the potential importance of evaluating the endometrium prior to undergoing a hysterectomy as the surgery could have been altered to more effectively treat the adenocarcinoma.
At present, there are no specific guidelines for transgender men regarding cervical cancer screening. As such, providers currently follow the guidelines created for cis-gender women when conducting screening on transgender men. The current recommendation indicates any cis-woman over 21 years old should have a Pap smear performed every 3 years or a human papillomavirus (HPV) test performed every 5 years. Screening may stop if the patient no longer has a cervix or if the patient is 65 years old and testing had been normal over the previous 10 years. A partial or supracervical hysterectomy preserves the cervix, indicating that not all transgender men with hysterectomies should stop receiving regular cervical cancer screenings.
In some cases, a routine postoperative histology workup may reveal cervical carcinoma in situ. Dysplasia of the cervix can spread to the vagina, which indicates the need for continued screening of the vaginal fornices even post-hysterectomy. It follows that convincing TGGD patients to continue regular cancer screenings after their hysterectomy poses a challenge, most importantly when partial cervix tissue remains[57].
A possible solution exists, such as increasing the availability of self-collected HPV DNA tests. Goldstein et al[58] reported a 2-fold increase in transgender men receiving HPV testing after introducing self-collected HPV swabbing options[58]. Additional research shows self-collected HPV tests have a 71.4% sensitivity when compared to provider-collected HPV tests[59]. The efficacy is consistent with the rates seen in the cis-gender women population choosing to use self-collected swabs.
Adding to the complexity of cancer screening is the inconsistent correlation between exogenous testosterone use and the risk of carcinoma in the female reproductive system. There is a report of TGGD patients on androgens having higher rates of unsatisfactory or abnormal Pap smear results when compared to cis-gender women[60]. Contradicting this study are two recent publications that showed no significant difference between rates of epithelial cell abnormalities between transgender and cis-gender women receiving Pap tests[61,62]. Further studies must be done to determine the extent to which exogenous testosterone treatment can influence cell growth in cervical tissue.
There are 3 reported cases of cervical cancer in transgender men documented in the literature. Driák and Šamudovský[63] report a case of localized squamous carcinoma, which was detected during a pathologic analysis of the cervix post- abdominal hysterectomy[63]. The patient had been on androgen therapy for the previous 4 years and did not need any oncological treatment beyond the hysterectomy. A case presented by Urban et al[56] follows a transgender man diagnosed with invasive stage IB adenoma malignum after receiving a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy[56]. The patient reported vaginal bleeding for a 2-year period prior to the surgery, but contributed this to androgen therapy, which he had been on for the previous 7 years. He was subsequently treated with weekly cisplatin, external beam pelvic radiation, and intracavitary radiation to the upper vagina, which left him without evidence of disease. The most recent report of cervical carcinoma comes from Beswick et al[64], who present a trans
Current screening guidelines state that there is no unique recommendation for TGGD individuals with ovaries. It is recommended that they follow the same guidelines established for cis-gender women: routine age-appropriate surveillance, a gynecological evaluation at least every 3 years (particularly for patients with a strong family history associated to ovarian cancer) with a pelvic examination, and routine ovarian cancer screening is not recommended[2,65].
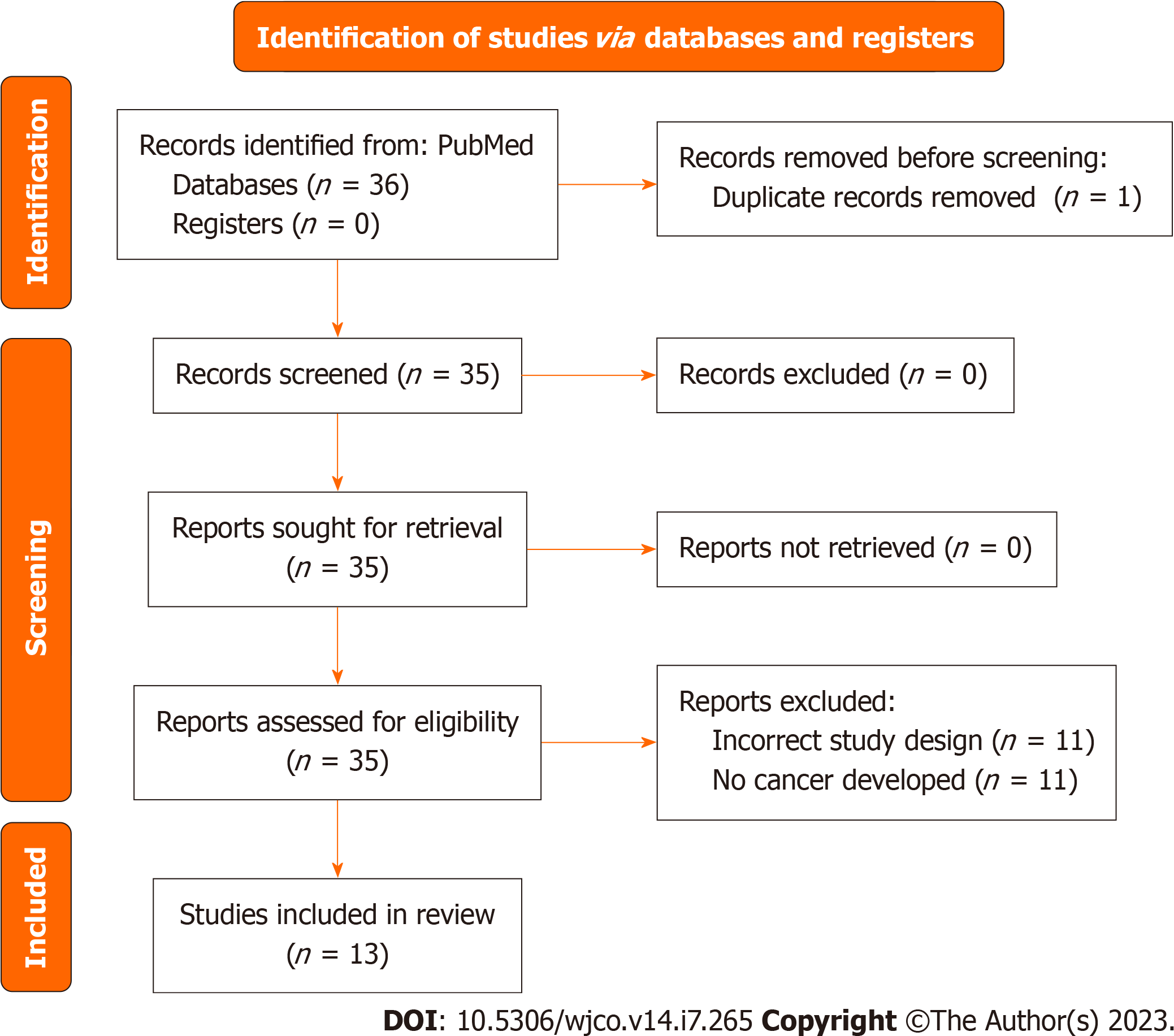
Of the 13 articles included in our systematic review, seven described cases of ovarian cancer amongst TGGD and their respective management. There have been eight cases reported in the literature regarding cases of ovarian cancer amongst TGGD individuals, seven of whom had taken gender-affirming hormone therapy[66-72].
Cases in literature and their respective management: Figure 6 includes the PRISMA flow diagram regarding ovarian cancer studies. Hage et al[66] published the first journal article discussing two case reports about TGGD individuals who were diagnosed with ovarian cancer. Patient A was diagnosed with papillary cystadenocarcinoma and underwent a laparotomy, supracolic omentectomy, and left oophorectomy followed by adjuvant combination chemotherapy with taxol, epirubicin, cis-platinum. Patient B was diagnosed with papillary borderline tumor in the left ovary, which was discovered as the patient was admitted to undergo a hysterectomy and bilateral salpingo-oophorectomy. Patient B eventually underwent a laparotomy and resection of multicystic mass. No radiotherapy or chemotherapy was required. In both cases reported, Patient A and Patient B had a history of hormone therapy reported[66].
The next case was reported by Dizon et al[67], which described a 46-year-old transgender man who was diagnosed with endometrioid adenocarcinoma arising in the left ovary and fallopian tube. This patient underwent a total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic and para-aortic node dissection, and peritoneal staging biopsies. Following surgery, chemotherapy was completed, consisting of carboplatin and paclitaxel. This case report also noted that the patient had used hormone therapy as part of their gender affirming surgery, but it was discontinued following surgery[67].
Another case report by Ferreira et al[68] described a 23-year-old transgender man with a history of testosterone therapy who was diagnosed with bilateral serous borderline ovarian tumor and underwent a total hysterectomy and bilateral salingo-oophorectomy. There was no discussion about subsequent chemotherapy[68].
Aubrey et al[69] published a similar case report about a 36-year-old transgender man diagnosed with stage IIA ovarian endometrium cancer who underwent a bilateral salpingo-oophorectomy followed by six cycles of chemotherapy. This patient also was using hormone therapy, which was discontinued after surgery[69].
Stevens and Abrahm[70] published another case report of a 67-year-old who was diagnosed with metastatic ovarian cancer and used exogenous testosterone. There was no mention about chemotherapy or surgery, and the patient remained in the hospital and received palliative care[70].
Bilash and Walker[71] published an article discussing Bilash’s personal experiences as a transgender individual who was diagnosed with polycystic ovarian syndrome in his early 20s and underwent a bilateral oophorectomy and total hysterectomy at the age of 30 for stage III ovarian cancer. Bilash and Walker[71] began using testosterone therapy following surgery[71].
Millington et al[72] presented a case report about a 17-year-old transgender adolescent who was diagnosed with serous borderline ovarian tumor. The patient began subcutaneous testosterone cypionate 12 wk prior to the diagnosis. For treatment, the patient elected a right salpingo-oophorectomy. Post-operatively, testosterone was restarted two months following the procedure and surveillance of the remaining ovary was continued and eventually unremarkable over time[72].
Prevention and Management: As demonstrated by both the case reports and the current literature, there have been discussions about the possible relationship between using testosterone supplements and a potential increased risk of ovarian cancer. However, it has been repeatedly emphasized that there is currently a lack of reported cases and data in the TGGD community to prove the possible mitogenic effects of long-term exposure to exogenous androgens on ovaries[66,68,69,72,73].
This correlates to another topic that has been debated in the literature, which is the use of bilateral salpingo-oophorectomies as a possible preventative measure of ovarian cancer in the TGNC community. Currently, according to National Comprehensive Cancer Network guidelines for cis-gender women, TGGD who are carriers of the BRAA1 and BRCA2 mutation should be offered risk-reducing salpingo-oophorectomy. If patients chose to defer this procedure, serial monitoring is considered as an alternative[47]. Some articles explore the possibility of expanding preventative ovariectomies to TGGD patients who are eligible for gender-affirming surgery and are on hormone therapy (such as a simultaneous salpingo-oophorectomy for TGGD individuals who undergo hysterectomy)[66,74]. Other articles noted the lack of knowledge about the long-term effects of oophorectomy at the time of a hysterectomy and how oophorectomies affect the quality of life, gender dysphoria, and the risk reduction of ovarian cancer in the TGNC population[67,68,73,75]. Kwiatkowska et al[76] emphasizes the responsibility of the physician during hormone therapy, in which that gender-affirming surgeries must be beneficial for the overall well-being of the patient, which continues to remain a gray area due to the lack of research about the impact of hormone therapy on the risk of ovarian cancer and the benefits of prophylactic bilateral salpingo-oophorectomy in TGGD individuals using hormone therapy[76].
Overall current guidelines state TGGD individuals neither require routine ovarian cancer screening nor additional surveillance and prophylactic oophorectomies are not needed as TGGD individuals are not at an increased risk of ovarian cancer[2].
The healthcare needs of TGGD individuals are unique due to gender-affirming hormonal therapy and or surgical interventions. The most commonly used hormone therapies are antiandrogens combined with Estrogen. Subsequently, after 18–36 mo of hormone therapy[77], transgender women can undergo vaginoplasty, including orchiectomy. The Prostate usually is not removed during feminizing genital GAS (fgGAS) (vaginoplasty or vulvoplasty) due to potential significant complications such as incontinence. The permanence of the prostate after fgGAS poses a continued risk for prostate cancer.
Antiandrogen and estrogen therapy with or without orchiectomy is theorized to have a lower incidence of prostate cancer in transgender women compared to cisgender men[78]. The main goal of hormone therapy is the regression of adult male sexual characteristics while inducing female sexual development in a transgender women with minimal long-term risk. While Estrogen has a short-term risk of thrombosis, the long-term risk of estrogen use is unclear[79]. Recent research has shown estrogen receptor–a, may have carcinogenic effects on the Prostate alone. A higher estradiol to dihydrotestosterone ratio may promote stromal cell growth in the prostate as well[79].
As part of antiandrogen treatment in male to female TGNC patients Prostate-specific antigen (PSA) and human glandular kallikrein (hK2) have been found to be elevated in plasma and urine after antiandrogen treatment in transgender women[80]. Both of these molecules are mainly produced by the Prostate, and androgens regulate their genes through the AR. Currently, screening guidelines for the TGGD population with prostates are the same as cis men. Transgender women 50 years and older should undergo annual prostate evaluation, consisting of digital rectal examination (DRE). Annual PSA evaluation might still have pertinence in prostate cancer screening and follow up. de Nie et al[81] performed a prostate biopsy in a transgender woman diagnosed with prostate cancer who had undergone orchiectomy with estrogen treatment[81]. The biopsy produced positive staining for prostate acid phosphatase (PAP) and prostate-specific antigen (PSA), showing that natural prostate activity persists in the castrated individuals and that this activity does not rely solely on androgens. As PSA is usually highly suppressed in these individuals following bilateral orchiectomy, any PSA value greater than 1.0 ng/mL should be regarded as concerning[82]. Further research on the adequate PSA monitoring threshold is required for this subset of patients.
A biopsy is a primary tool for diagnosing prostate cancer and determining a Gleason score for prognosis. Some studies have shown the difficulty of assigning a correct Gleason score due to morphologic changes to the Prostate induced by androgen deprivation adding a layer of complexity when interpreting results in the TGGD population[83]. For both cis men and transgender women diagnosed with prostate cancer multiple treatments are available. Amongst them include gonadotropin-releasing hormone (GnRH) agonists/antagonists, radiotherapy, chemotherapy, robotic-assisted laparoscopic prostatectomy, and cystoprostatectomy. New therapies such as abiraterone, enzalutamide, sipuleucel-T and cabazitaxel have been introduced to treat hormone-resistant prostate cancer[82].
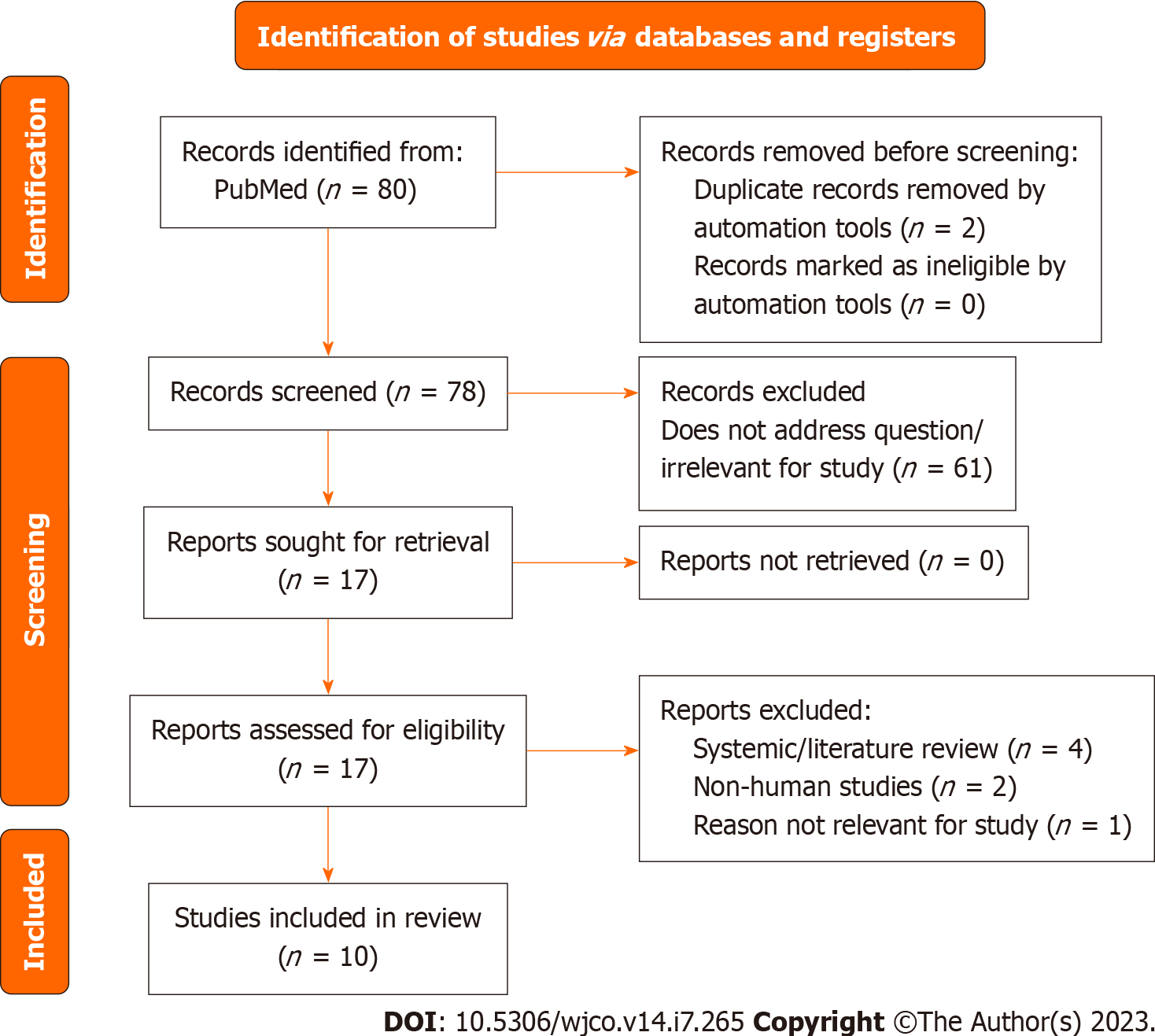
In our review, we identified 14 TGGD individuals diagnosed with Prostate Cancer. Figure 7 includes the PRISMA flow diagram regarding prostate cancer studies. Of those 14, three underwent chemotherapy using estramustine, mitoxan
The absence of TGGD-specific screening guidelines, unconfirmed effects of gender-affirming hormone therapy on prostate cancer, change of the pelvis anatomy following the surgery, and barriers of care by the healthcare providers and system can delay cancer diagnosis and treatment. The combination of factors may lead to poorer prognosis in this population[79]. Yet, although the incidence is lower in TGGD women, Jackson et al[79] have indicated that prostate cancer could be more aggressive amongst TGGD population with increased mortality amongst TGGD women. Incidence of prostate cancer after prolonged use of gender-affirming hormone therapy raises questions about the “protective” role of castrating status in cancer pathogenesis[85]. Further study regarding the effects of gender-affirming hormone therapy and orchiectomy is needed to shape the screening and treatment of Prostate cancer in TGGD women.
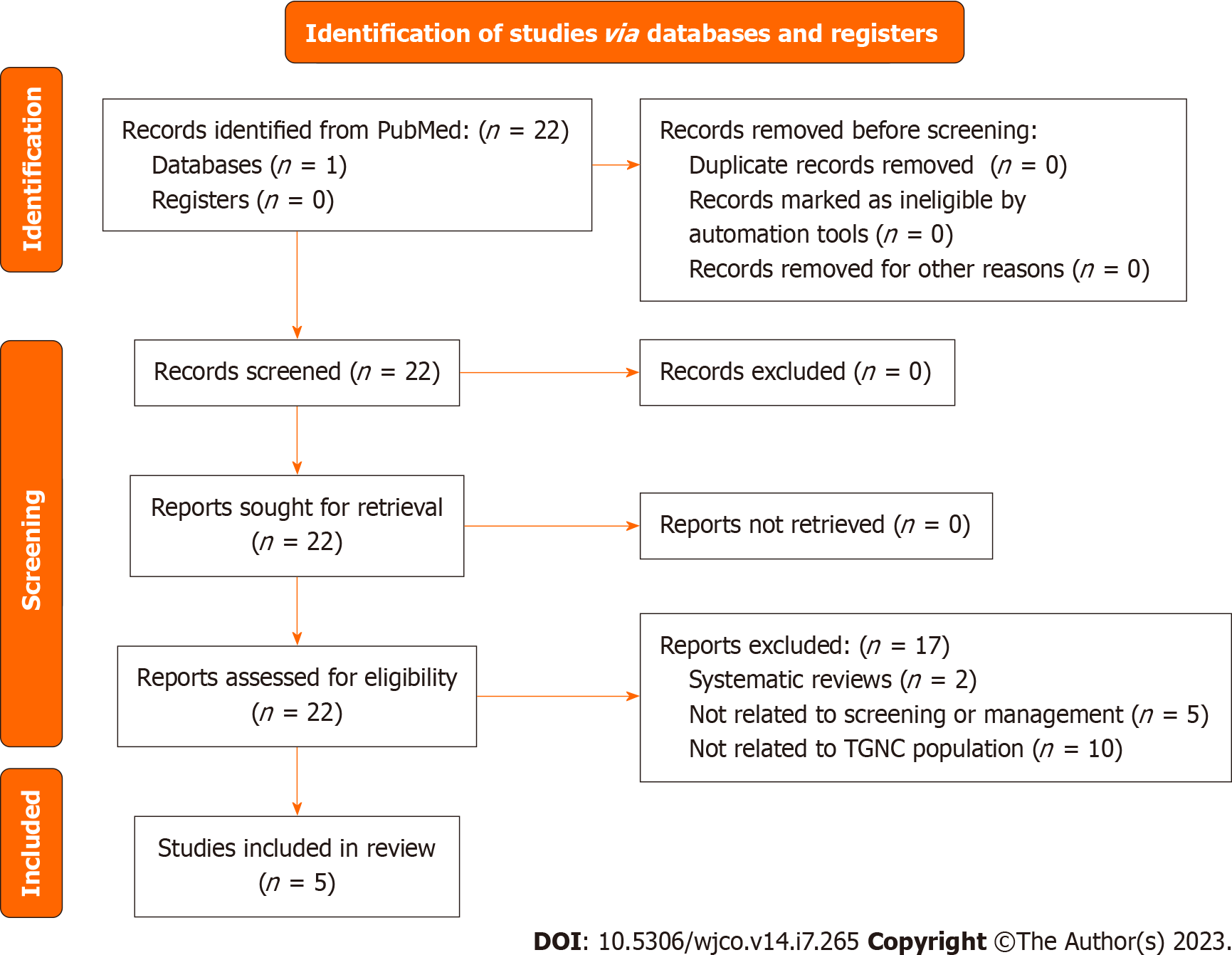
Currently, the USPSTF recommends against regular screening for Testicular Cancer in cis-gender men and has no recommendations for TGGD population. Figure 8 includes the PRISMA flow diagram regarding testicular cancer studies. Some societies recommend annual self-examinations. In TGGD population, hormonal therapy (primarily estradiol) is instituted with the goal to develop female secondary sex characteristics. Estrogen is thought to be a risk factor for development of testicular cancer although no large-scale studies have been done that show a link.
Standard management of testicular cancer involves tumor markers (β- human chorionic gonadotropin, Lactate Dehydrogenase (LDH) and alpha-fetoprotein), computed tomography scan of chest/abdomen/pelvis followed by radical orchiectomy. Tumor markers can help differentiate the type of cancer present, although standard of care involves a radical orchiectomy up front. Our review resulted in 5 cases of testicular cancer found in the TGGD population. Two cases were found when testosterone levels failed to suppress despite hormonal therapy. One case reported by Wolf-Gould and Wolf-Gould[86] was found to have an intratubular germ cells neoplasia (carcinoma in site), embryonal cell carcinoma[86]. Another case reported by Elshimy et al[87] was found to have a B-HCG secreting seminoma[87]. One case of seminoma reported by Kvach et al[88], was discovered incidentally after penile-inversion vaginoplasty[88]. A case by Chandhoke et al[89], reported a 38-year-old transgender woman with a testicular mass and a retroperitoneal tumor that was too morbid to resect[89]. The patient underwent radical orchiectomy followed by maintenance on chemotherapy and surveillance with serial imaging. An interesting case by Kobori et al[90] was revealed to have a mature testicular teratoma with positive estrogen receptor expression while undergoing hormonal therapy with estrogen and progesterone[90]. The authors note that although receptor expression does not necessarily imply causation, the contribution of estrogen cannot be ruled out. The patient elected to stop hormonal therapy in this case.
All patients underwent radical orchiectomy with chemotherapy reserved for patients who met criteria per cis-gender guidelines. Four patients elected to stop estrogen therapy; however, this was after an extensive discussion with the patient on the social and psychological effects of cessation.
Prior to breast cancer screening guidelines for the TGGD patient from the American College of Radiology in November 2021, no formal cancer screening guidelines were made for the TGGD population. In most instances, screening guidelines for the TGGD population default to cis-gender screening recommendations and management. Further, guidelines are needed to address non binary patients as existing literature in this select population is also lacking. Although screening suggestions based on this systematic review are alluded to in each organ section, the discussion of organ specific screening centers on a call to action for better research.
Discussion on cancer management is provided in each organ section in more detail. However, some overarching themes hold true for all cancer management. Provider education in the communication skills with the TGGD population in the form of gender friendly language is paramount to improve the existing barriers of care, improve healthcare accessibility and increase provider options for these patients. Addressing the limitations of care and actively participating in scientific research for this population will allow for earlier detection of cancer, improved treatment adherence, improved patient care accessibility and ultimately improved patient follow up and satisfaction.
Currently, a comprehensive guideline for cancer screening in the TGGD population is lacking. Prior to breast cancer screening guidelines for this population from the ACR in November 2021, no formal cancer screening guidelines were made for the TGGD population. In most instances, screening guidelines defaulted to cis gender screening recommendations and management. However, caring for the TGGD population undergoing gender affirming surgery is highly individualized and requires consideration of factors such as the age at which they commenced hormonal therapy, the stage of transition, and the disproportionate social determinants of health these patients are subject to. For all these reasons, these patients are at higher risk of developing cancer and or having their cancer detected at a later, more aggressive stage because they do not have access to the appropriate and comprehensive care they require.
This study performed systematic review of the current literature surrounding both the screening and management of cancer in the transgender and gender diverse population whom are considering gender affirming surgery. In addition to calling for better education and evidence based guidelines for physicians to follow, this paper is a call to action for physicians to openly address the limitations of care and to actively participating in scientific research for this population to allow for earlier detection of cancer, improved treatment adherence, improved patient care accessibility and ultimately improved patient satisfaction.
Lack of screening and management guidelines in the transgender and gender diverse (TGGD) and non binary population.
A comprehensive guideline for cancer screening in the TGGD population is lacking. Caring for the TGGD population undergoing Gender Affirmation Surgery is highly individualized and requires consideration for the whole, integral patient including the physical and psychological realm. Communication and access to care should strive for inclusion and avoid potential discrimination from misgendering. Once diagnosed with cancer, TGGD patients should receive care at institutions capable of providing a multi-disciplinary approach. This collective approach will ensure record upkeep and help delay any unnecessary delays in care. Resolving the lack of guidelines, improving inclusion, and diminishing the barriers of care will ultimately lead to more timely and efficient care for the TGGD population.
Literature is lacking regarding screening and management guidelines in the TGGD and non binary population. Barriers of care are present and need to be addressed to improve access and quality of care for this population.
A systematic review utilizing the preferred reporting items for systematic reviews and meta-analyses guidelines was used. Rayyan software was used to organize and collaborate on articles for reviewers. A systematic search of PubMed on January 5th, 2022, with the following terms: “TGNC”, OR “transgender”, OR “gender non-conforming”, OR “gender nonbinary” AND “cancer screening”, AND “breast cancer”, AND “cervical cancer”, AND “uterine cancer”, AND “ovarian cancer”, AND “prostate cancer”, AND “testicular cancer”, AND “surveillance”, AND “follow-up”, AND “management”. After eliminating review articles, duplicates, abstracts, articles not relevant to the section topic or opinion pieces a total of 70 studies with original data were obtained. Articles relevant to the section topic, including the search terms were included in this systematic review. Search parameters were performed according to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Two independent reviewers Araya S and Nannapaneni S carried out independent abstract revisions on January 11th, 2022, using systematic review software “Rayyan” registered in Cambridge Massachusetts.
Literature is lacking regarding screening and management guidelines in the TGGD and non binary population. Barriers of care are present and need to be addressed to improve access and quality of care for this population.
Caring for the TGGD and nonbinary patients is a complex process and requires understanding of three key points – care is highly individual, it depends on stage of gender affirming surgery, and it is centered on proper provider education and training. An understanding of the biopsychosocial model of health, where illness must be considered from not only the physical body, but also from the psychological and social aspects is required. Prior to breast cancer screening guidelines for the TGGD patient from the American College of Radiology in November 2021, no formal cancer screening guidelines were made for the TGGD population. In most instances, screening guidelines for the TGGD population default to cis gender screening recommendations and management. Further, guidelines are needed to address non binary patients as existing literature in this select population is also lacking. Although screening suggestions based on this systematic review are alluded to in each organ section, the discussion of organ specific screening centers on a call to action for better research. Discussion on cancer management is provided in each organ section in more detail. However, some overarching themes hold true for all cancer management. Provider education in the communication skills with the TGGD population in the form of gender friendly language is paramount to improve the existing barriers of care, improve healthcare accessibility and increase provider options for these patients. Addressing the limitations of care and actively participating in scientific research for this population will allow for earlier detection of cancer, improved treatment adherence, improved patient care accessibility and ultimately improved patient follow up and satisfaction.
Creating specific cancer screening and management guidelines for the TGGD and non binary population while improving barriers to care.
| 1. | Flores AR, Herman JL, Gates GJ, Brown TNT. How Many Adults Identify as Transgender in the United States. The Williams Institute; 2016. [cited 1 June 2023]. Available from: https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/. |
| 2. | Sterling J, Garcia MM. Cancer screening in the transgender population: a review of current guidelines, best practices, and a proposed care model. Transl Androl Urol. 2020;9:2771-2785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 14342] [Article Influence: 1434.2] [Reference Citation Analysis (1)] |
| 4. | Patel JM, Dolitsky S, Bachman GA, Buckley de Meritens A. Gynecologic cancer screening in the transgender male population and its current challenges. Maturitas. 2019;129:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kiran T, Davie S, Singh D, Hranilovic S, Pinto AD, Abramovich A, Lofters A. Cancer screening rates among transgender adults: Cross-sectional analysis of primary care data. Can Fam Physician. 2019;65:e30-e37. [PubMed] |
| 6. | Deebel NA, Morin JP, Autorino R, Vince R, Grob B, Hampton LJ. Prostate Cancer in Transgender Women: Incidence, Etiopathogenesis, and Management Challenges. Urology. 2017;110:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Nelson B. A cancer screening crisis for transgender patients: Discrimination, patient unease, provider ignorance, and a highly gendered health care system are impeding cancer screening and risk assessment in the transgender population. In this article, the first of a 2-part series, we explore how clinicians can begin to address those barriers. Cancer Cytopathol. 2019;127:421-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Clarke CN, Cortina CS, Fayanju OM, Dossett LA, Johnston FM, Wong SL. Breast Cancer Risk and Screening in Transgender Persons: A Call for Inclusive Care. Ann Surg Oncol. 2022;29:2176-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Snow A, Cerel J, Loeffler DN, Flaherty C. Barriers to Mental Health Care for Transgender and Gender-Nonconforming Adults: A Systematic Literature Review. Health Soc Work. 2019;44:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Unger CA. Care of the transgender patient: a survey of gynecologists' current knowledge and practice. J Womens Health (Larchmt). 2015;24:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Gatos KC. A Literature Review of Cervical Cancer Screening in Transgender Men. Nurs Womens Health. 2018;22:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Stenzel AE, Moysich KB, Ferrando CA, Starbuck KD. Clinical needs for transgender men in the gynecologic oncology setting. Gynecol Oncol. 2020;159:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | James S, Herman J, Rankin S, Keisling M, Mottet L, Anafi MA. The report of the 2015 US transgender survey. 2016. [cited 1 June 2023]. Available from: https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf. |
| 14. | Jarrett BA, Peitzmeier SM, Restar A, Adamson T, Howell S, Baral S, Beckham SW. Gender-affirming care, mental health, and economic stability in the time of COVID-19: a global cross-sectional study of transgender and non-binary people. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Loo S, Almazan AN, Vedilago V, Stott B, Reisner SL, Keuroghlian AS. Understanding community member and health care professional perspectives on gender-affirming care-A qualitative study. PLoS One. 2021;16:e0255568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 12193] [Article Influence: 2438.6] [Reference Citation Analysis (6)] |
| 17. | de Blok CJM, Wiepjes CM, Nota NM, van Engelen K, Adank MA, Dreijerink KMA, Barbé E, Konings IRHM, den Heijer M. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. BMJ. 2019;365:l1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 18. | Luehmann N, Ascha M, Chwa E, Hackenberger P, Termanini K, Benning C, Sama D, Felt D, Beach LB, Gupta D, Kulkarni SA, Jordan SW. A Single-Center Study of Adherence to Breast Cancer Screening Mammography Guidelines by Transgender and Non-Binary Patients. Ann Surg Oncol. 2022;29:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Tabaac AR, Sutter ME, Wall CSJ, Baker KE. Author Response to "Letter to the Editor Regarding 'Gender Identity Disparities in Cancer Screening Behaviors'". Am J Prev Med. 2019;56:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Brown A, Lourenco AP, Niell BL, Cronin B, Dibble EH, DiNome ML, Goel MS, Hansen J, Heller SL, Jochelson MS, Karrington B, Klein KA, Mehta TS, Newell MS, Schechter L, Stuckey AR, Swain ME, Tseng J, Tuscano DS, Moy L; Expert Panel on Breast Imaging. ACR Appropriateness Criteria® Transgender Breast Cancer Screening. J Am Coll Radiol. 2021;18:S502-S515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Gooren L, Bowers M, Lips P, Konings IR. Five new cases of breast cancer in transsexual persons. Andrologia. 2015;47:1202-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Stafford A, Shobieri A, Stamatakos M, Edmiston K. Ductal carcinoma in situ in the male-to-female transgender population. Breast J. 2020;26:2439-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Corman V, Potorac I, Manto F, Dassy S, Segers K, Thiry A, Bours V, Daly AF, Beckers A. Breast cancer in a male-to-female transsexual patient with a BRCA2 mutation. Endocr Relat Cancer. 2016;23:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Sieberg R, Soriano K, Zuurbier R. A rare case of breast cancer in a transgender woman. Radiol Case Rep. 2021;16:3285-3288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995;82:341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Brown GR. Breast Cancer in Transgender Veterans: A Ten-Case Series. LGBT Health. 2015;2:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Nehlsen AD, Bhardwaj A, Weltz C, Green S. Triple Negative Breast Cancer in a Male to Female Transgender Patient: A Case Report and Literature Review. Adv Radiat Oncol. 2020;5:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Pattison ST, McLaren BR. Triple negative breast cancer in a male-to-female transsexual. Intern Med J. 2013;43:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Grabellus F, Worm K, Willruth A, Schmitz KJ, Otterbach F, Baba HA, Kimmig R, Metz KA. ETV6-NTRK3 gene fusion in a secretory carcinoma of the breast of a male-to-female transsexual. Breast. 2005;14:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Ali N, Sindhu K, Bakst RL. A Rare Case of a Transgender Female With Breast Implant-Associated Anaplastic Large Cell Lymphoma Treated With Radiotherapy and a Review of the Literature. J Investig Med High Impact Case Rep. 2019;7:2324709619842192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | de Boer M, van der Sluis WB, de Boer JP, Overbeek LIH, van Leeuwen FE, Rakhorst HA, van der Hulst RRWJ, Hijmering NJ, Bouman MB, de Jong D. Breast Implant-Associated Anaplastic Large-Cell Lymphoma in a Transgender Woman. Aesthet Surg J. 2017;37:NP83-NP87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Zaveri S, Yao A, Schmidt H. Breast Implant-Associated Anaplastic Large Cell Lymphoma Following Gender Reassignment Surgery: A Review of Presentation, Management, and Outcomes in the Transgender Patient Population. Eur J Breast Health. 2020;16:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Li JZ, Tu HYV, Avram R, Pinthus J, Bordeleau L, Hodgson N. Cancer prevention and screening in a BRCA2-positive male to female transgender patient. Breast J. 2018;24:1112-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Teoh ZH, Archampong D, Gate T. Breast cancer in male-to-female (MtF) transgender patients: is hormone receptor negativity a feature? BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Barghouthi N, Turner J, Perini J. Breast Cancer Development in a Transgender Male Receiving Testosterone Therapy. Case Rep Endocrinol. 2018;2018:3652602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Mingrino J, Wang Y. Apocrine ductal carcinoma in situ associated with testosterone therapy in a transgender individual. Breast J. 2021;27:475-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Fehl A, Ferrari S, Wecht Z, Rosenzweig M. Breast Cancer in the Transgender Population. J Adv Pract Oncol. 2019;10:387-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Fundytus A, Saad N, Logie N, Roldan Urgoiti G. Breast cancer in transgender female-to-male individuals: A case report of androgen receptor-positive breast cancer. Breast J. 2020;26:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Shao T, Grossbard ML, Klein P. Breast cancer in female-to-male transsexuals: two cases with a review of physiology and management. Clin Breast Cancer. 2011;11:417-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Eismann J, Heng YJ, Fleischmann-Rose K, Tobias AM, Phillips J, Wulf GM, Kansal KJ. Interdisciplinary Management of Transgender Individuals at Risk for Breast Cancer: Case Reports and Review of the Literature. Clin Breast Cancer. 2019;19:e12-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Jacoby A, Rifkin W, Zhao LC, Bluebond-Langner R. Incidence of Cancer and Premalignant Lesions in Surgical Specimens of Transgender Patients. Plast Reconstr Surg. 2021;147:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Chotai N, Tang S, Lim H, Lu S. Breast cancer in a female to male transgender patient 20 years post-mastectomy: Issues to consider. Breast J. 2019;25:1066-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Katayama Y, Motoki T, Watanabe S, Miho S, Kimata Y, Matsuoka J, Doihara H, Nanba Y. A very rare case of breast cancer in a female-to-male transsexual. Breast Cancer. 2016;23:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Nikolic DV, Djordjevic ML, Granic M, Nikolic AT, Stanimirovic VV, Zdravkovic D, Jelic S. Importance of revealing a rare case of breast cancer in a female to male transsexual after bilateral mastectomy. World J Surg Oncol. 2012;10:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 46. | Evans DG, Susnerwala I, Dawson J, Woodward E, Maher ER, Lalloo F. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Bedrick BS, Fruhauf TF, Martin SJ, Ferriss JS. Creating Breast and Gynecologic Cancer Guidelines for Transgender Patients With BRCA Mutations. Obstet Gynecol. 2021;138:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Patzelt M, Zarubova L, Klener P, Barta J, Benkova K, Brandejsova A, Trneny M, Gürlich R, Sukop A. Anaplastic Large-Cell Lymphoma Associated with Breast Implants: A Case Report of a Transgender Female. Aesthetic Plast Surg. 2018;42:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39:S3-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 50. | Murariu D, Holland MC, Gampper TJ, Campbell CA. Illegal silicone injections create unique reconstructive challenges in transgender patients. Plast Reconstr Surg. 2015;135:932e-933e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Tirrell AR, Abu El Hawa A, Bekeny JC, Del Corral G. Outcomes in chest feminization patients with a history of illicit hormone use and silicone injections. Breast J. 2021;27:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 52. | D'hulst L, Nicolaij D, Beels L, Gheysens O, Alaerts H, Van de Wiele C, Maes A. False-Positive Axillary Lymph Nodes Due to Silicone Adenitis on (18)F-FDG PET/CT in an Oncological Setting. J Thorac Oncol. 2016;11:e73-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Bjerno T, Basse PN, Siemssen PA, Møller TD. [Injection of high viscosity liquids. Acute or delayed excision?]. Ugeskr Laeger. 1993;155:1876-1878. [PubMed] |
| 54. | Grimstad FW, Fowler KG, New EP, Ferrando CA, Pollard RR, Chapman G, Gomez-Lobo V, Gray M. Uterine pathology in transmasculine persons on testosterone: a retrospective multicenter case series. Am J Obstet Gynecol. 2019;220:257.e1-257.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 55. | Ralph O, Shroff N, Christopher N, Ahmed A, Berner A, Barrett J, Sandison A, Ralph D. Mp59-16 response of endometrium to testosterone therapy in trans men and non-binary people undergoing hysterectomy. J Urol. 201:(Suppl 4). [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Urban RR, Teng NN, Kapp DS. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204:e9-e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Peitzmeier SM, Khullar K, Reisner SL, Potter J. Pap test use is lower among female-to-male patients than non-transgender women. Am J Prev Med. 2014;47:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 58. | Goldstein Z, Martinson T, Ramachandran S, Lindner R, Safer JD. Improved Rates of Cervical Cancer Screening Among Transmasculine Patients Through Self-Collected Swabs for High-Risk Human Papillomavirus DNA Testing. Transgend Health. 2020;5:10-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Reisner SL, Deutsch MB, Peitzmeier SM, White Hughto JM, Cavanaugh TP, Pardee DJ, McLean SA, Panther LA, Gelman M, Mimiaga MJ, Potter JE. Test performance and acceptability of self- versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients. PLoS One. 2018;13:e0190172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 60. | Adkins BD, Barlow AB, Jack A, Schultenover SJ, Desouki MM, Coogan AC, Weiss VL. Characteristic findings of cervical Papanicolaou tests from transgender patients on androgen therapy: Challenges in detecting dysplasia. Cytopathology. 2018;29:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Davis K, Kwon R, Graham A, White M, Maleki Z, Rodriguez E. Comparison of Cervical Cancer Screen Results on Female-to-Male Transgender Patients With Female Patients. Am J Clin Pathol. 2022;157:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 62. | Williams MPA, Kukkar V, Stemmer MN, Khurana KK. Cytomorphologic findings of cervical Pap smears from female-to-male transgender patients on testosterone therapy. Cancer Cytopathol. 2020;128:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Driák D, Samudovský M. Could a man be affected with carcinoma of cervix?--The first case of cervical carcinoma in trans-sexual person (FtM)--case report. Acta Medica (Hradec Kralove). 2005;48:53-55. [PubMed] |
| 64. | Beswick A, Corkum M, D'Souza D. Locally advanced cervical cancer in a transgender man. CMAJ. 2019;191:E76-E78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Beamer LC. Hereditary Breast and Hereditary Ovarian Cancer: Implications for the Oncology Nurse. Semin Oncol Nurs. 2019;35:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76:413-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Dizon DS, Tejada-Berges T, Koelliker S, Steinhoff M, Granai CO. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Obstet Invest. 2006;62:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Ferreira C, Fraga J, Antunes C, Gonçalo M, Donato P. Serous Borderline Tumor in Transgender Female-to-Male Individuals: A Case Report of Androgen Receptor-Positive Ovarian Cancer. Case Rep Radiol. 2021;2021:8861692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Aubrey C, Saad N, Köbel M, Mattatall F, Nelson G, Glaze S. Implications for management of ovarian cancer in a transgender man: Impact of androgens and androgen receptor status. Gynecol Oncol. 2021;161:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Stevens EE, Abrahm JL. Adding Silver to the Rainbow: Palliative and End-of-Life Care for the Geriatric LGBTQ Patient. J Palliat Med. 2019;22:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Bilash T, Walker LM. Spare Parts: Navigating Ovarian Cancer as a Transgender Man. J Clin Oncol. 2022;40:1027-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Millington K, Hayes K, Pilcher S, Roberts S, Vargas SO, French A, Veneris J, O'Neill A. A serous borderline ovarian tumour in a transgender male adolescent. Br J Cancer. 2021;124:567-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Harris M, Kondel L, Dorsen C. Pelvic pain in transgender men taking testosterone: Assessing the risk of ovarian cancer. Nurse Pract. 2017;42:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Cao CD, Amero MA, Marcinkowski KA, Rosenblum NG, Chan JSY, Richard SD. Clinical Characteristics and Histologic Features of Hysterectomy Specimens From Transmasculine Individuals. Obstet Gynecol. 2021;138:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Kwiatkowska A, Kułak K, Wertel I. Gender Dysphoria Disrupting the Course of Treatment of a Recurrent Juvenile Granulosa Cell Tumor in an Adolescent Female: A Case Report. Case Rep Oncol. 2020;13:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 77. | Gooren L, Morgentaler A. Prostate cancer incidence in orchidectomised male-to-female transsexual persons treated with oestrogens. Andrologia. 2014;46:1156-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 78. | Randolph JF Jr. Gender-Affirming Hormone Therapy for Transgender Females. Clin Obstet Gynecol. 2018;61:705-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Jackson SS, Han X, Mao Z, Nogueira L, Suneja G, Jemal A, Shiels MS. Cancer Stage, Treatment, and Survival Among Transgender Patients in the United States. J Natl Cancer Inst. 2021;113:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 80. | Obiezu CV, Giltay EJ, Magklara A, Scorilas A, Gooren L, Yu H, Diamandis EP. Dramatic suppression of plasma and urinary prostate specific antigen and human glandular kallikrein by antiandrogens in male-to-female transsexuals. J Urol. 2000;163:802-805. [PubMed] |
| 81. | de Nie I, de Blok CJM, van der Sluis TM, Barbé E, Pigot GLS, Wiepjes CM, Nota NM, van Mello NM, Valkenburg NE, Huirne J, Gooren LJG, van Moorselaar RJA, Dreijerink KMA, den Heijer M. Prostate Cancer Incidence under Androgen Deprivation: Nationwide Cohort Study in Trans Women Receiving Hormone Treatment. J Clin Endocrinol Metab. 2020;105:e3293-e3299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 82. | Ellent E, Matrana MR. Metastatic Prostate Cancer 35 Years After Sex Reassignment Surgery. Clin Genitourin Cancer. 2016;14:e207-e209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Dorff TB, Shazer RL, Nepomuceno EM, Tucker SJ. Successful treatment of metastatic androgen-independent prostate carcinoma in a transsexual patient. Clin Genitourin Cancer. 2007;5:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Sharif A, Malhotra NR, Acosta AM, Kajdacsy-Balla AA, Bosland M, Guzman G, Prins GS, Abern MR. The Development of Prostate Adenocarcinoma in a Transgender Male to Female Patient: Could Estrogen Therapy Have Played a Role? Prostate. 2017;77:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7:E544-E546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Wolf-Gould CS, Wolf-Gould CH. A Transgender Woman with Testicular Cancer: A New Twist on an Old Problem. LGBT Health. 2016;3:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Elshimy G, Tran K, Harman SM, Correa R. Unmasked Testicular Seminoma During Use of Hormonal Transgender Woman Therapy: A Hidden hCG-Secreting Tumor. J Endocr Soc. 2020;4:bvaa074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Kvach EJ, Hyer JS, Carey JC, Bowers M. Testicular Seminoma in a Transgender Woman: A Case Report. LGBT Health. 2019;6:40-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Chandhoke G, Shayegan B, Hotte SJ. Exogenous estrogen therapy, testicular cancer, and the male to female transgender population: a case report. J Med Case Rep. 2018;12:373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 90. | Kobori Y, Suzuki K, Iwahata T, Shin T, Sato R, Nishio K, Yagi H, Arai G, Soh S, Okada H. Mature Testicular Teratoma with Positive Estrogen Receptor Beta Expression in a Transgendered Individual on Cross-Sex Hormonal Therapy: A Case Report. LGBT Health. 2015;2:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ragosta S, United Kingdom; Withers R, United States S-Editor: Li L L-Editor: A P-Editor: Xu ZH