Published online Mar 24, 2023. doi: 10.5306/wjco.v14.i3.117
Peer-review started: December 14, 2022
First decision: January 20, 2023
Revised: February 8, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 24, 2023
Processing time: 95 Days and 15.2 Hours
Medulloblastoma (MB) is considered the commonest malignant brain tumor in children. Multimodal treatments consisting of surgery, radiation, and chemo
Core Tip: Medulloblastoma (MB) is the most common malignant childhood tumor of the brain. Multi
- Citation: Kurdi M, Mulla N, Malibary H, Bamaga AK, Fadul MM, Faizo E, Hakamy S, Baeesa S. Immune microenvironment of medulloblastoma: The association between its molecular subgroups and potential targeted immunotherapeutic receptors. World J Clin Oncol 2023; 14(3): 117-130
- URL: https://www.wjgnet.com/2218-4333/full/v14/i3/117.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i3.117
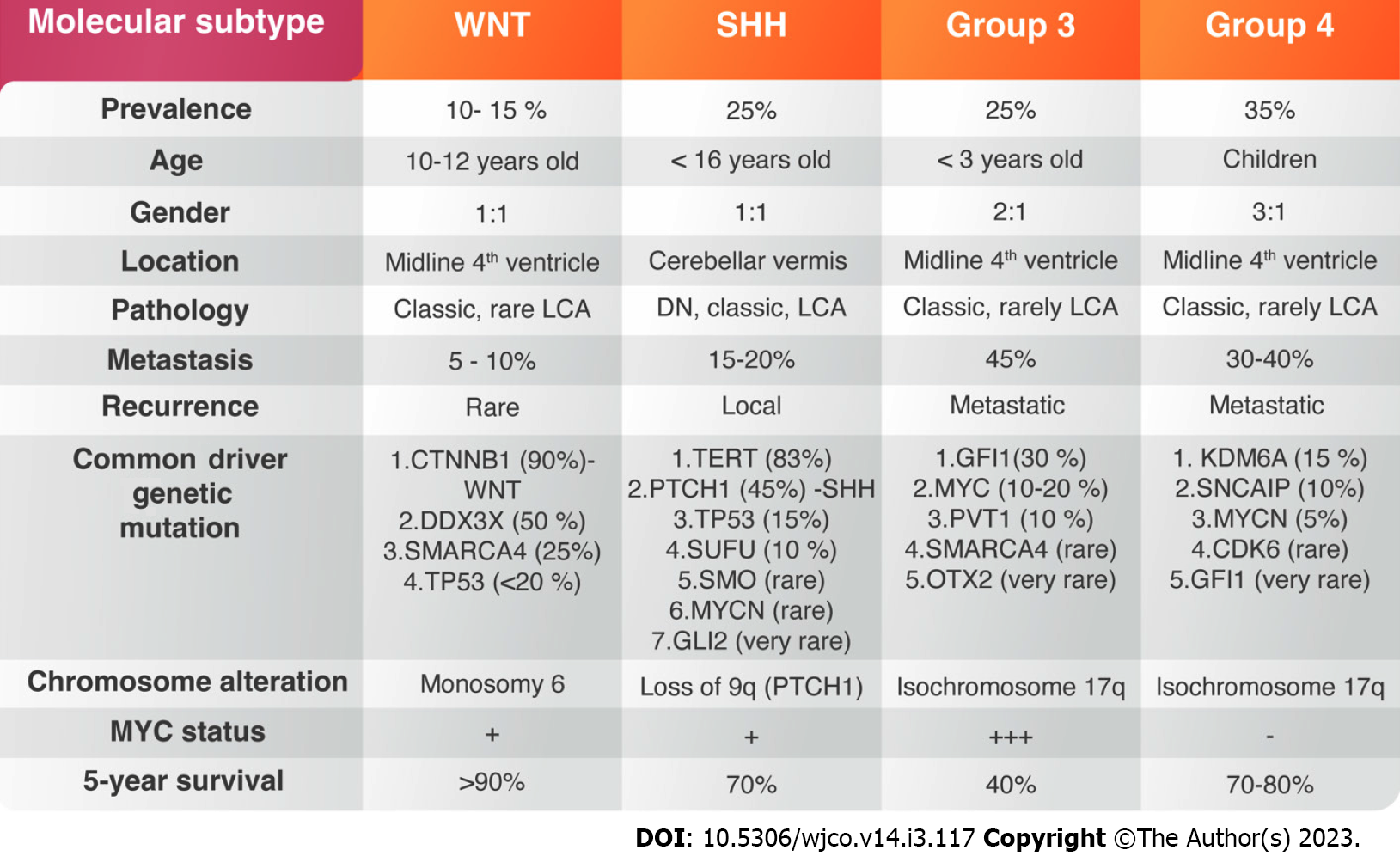
Brain tumors are the leading cause of oncological death during childhood, and medulloblastoma (MB) is the commonest malignant tumor of the brain, accounting for 20%-30% of all central nervous system (CNS) tumors[1]. Diverse treatment modalities consisting of surgery and chemoradiotherapy have improved the patient’s survival. Nevertheless, more than 1/3 of children with MB die within 5-years after diagnosis[2]. Late mortality remains a significant problem in disease consequences, which is attributed to tumour recurrence[3]. The persistent mortality, the failure of current drug therapies to extend life expectancy, and the serious complications of cytotoxic therapies indicate the necessity to explore new targeted treatments. Over the past decades, several tumor-centric studies have identified mutant genes and signaling pathways dysfunction that encourage MB growth. Most of MBs originate from the granular layer of cerebellum, which reside in the external granular layer and line the neocerebellum of newborns[4]. The existence of irregular biological signaling pathways created signaling dysregulation and genetic mutations affecting cerebellar development. Hence, the anatomical and cellular complexity of developing human tissues within the rhombic lip germinal zone produces glutamatergic neuronal lineages before its centralization. Molecular signatures encoded within a human rhombic-lip-derived lineage trajectory aligned with photoreceptor and unipolar cell profiles that are maintained in some medulloblastomas, suggesting a convergent basis. The advanced genomic studies over decades led to the assemblage of large amount of genetic information which resulted in four distinguishing molecular subgroups of MB including (Group 1) Wingless-activated (WNT-MB); (Group 2) Sonic-hedgehog-activated (SHH-MB); and Group 3 and Group 4[5] (Figure 1). Each group is characterized by distinct genetic abnormalities, methylation profiles, and clinical outcome. WNT- and SHH-type MBs are clearly detached from the other groups with lack of signaling pathway dysregulation identified in Group 3 and 4[5].
WNT-MB is the least common type, accounting for about 10%-15% of all MB patients. They are classically absent in infants and are seen more among children above 10 years of age[6-8] (Figure 1). The clinical outcome of the disease under 16-years of age is usually good, with 90% 5-year survival[8]. The genetic mutation of the Catenin Beta-1 (CTNNB1) gene is the most common genetic alteration accounting for 85% of all WNT-MBs[9,10]. A gene expression with methylation profiling performed on several MB cases in 2016 has divided WNT- MBs into two variants: WNT-α, which consists of patients with chromosome 6 monosomy and WNT-β, that occurs in adults with chromosomal diploidy[11,12]. CTNNB1 mutation usually occurs with other chromatin remodeling mutations such as Cyclic Adenosine Monophosphate Response Element Binding Protein (CREBBP), Mediator Complex Subunit 13 (MED13) and subunits of the nucleosome-remodeling complex such as SWI Matrix Associated, Actin Dependent Regulator of Chromatin, Subfamily A, Member 4 (SMARCA4), At-rich interaction Domain 1A (ARID1A)[9,10,13]. Most of WNT-MBs carries DEAD-Box Helicase 3 X-Linked (DDX3X) mutations, which participates in mRNA translation[12,14]. The germline mutation of antigen presenting cells (APC) on chromosome 5 as inherited Turcot syndrome and Anaplastic Lymphoma Kinase (ALK) gene also contribute to the development of WNT-MBs[9,15].
SHH-MB accounts for about 25% of all MBs with a 70% 5-years overall survival (OS). It is frequently seen in infants and adult patients[16,17]. The majority shows histologically nodular or desmoplastic morphology, which predicts a favourable prognosis[18]. TP53 mutation segregates SHH-MBs into tumors with TP53-wildtype, often seen in young children and associated with favorable prognosis, and TP53 mutant SHH-MB classically seen among older children and associated with poorer prognosis. SHH-MB with Protein Patch Homolog-1 (PTCH1) and Suppressor of Fused Homolog (SUFU) mutation are associated with Gorlin syndrome[19,20]. In children, TP53 mutations frequently occurs with GLI2 and MYCN-amplifications[9] (Figure 1).
Group 3 MB, a classical histological variant, accounts for 25% of all MBs and considered the deadliest subtype[7,21]. Tumours in this group with MYC-amplification carries a 20% risk of 5-years survival[22]. However, the most common cytogenetic abnormalities seen in Group 3 is the 17 ploss followed 16q and 9q losses[19]. Rare genetic variants in Group 3 MBs include Orthodenticle Homebox-2 (OTX2) and Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit (EZH2) amplifications and SMARCA4 mutations[23] (Figure 1).
Group 4 MB is the most frequent type among all MBs and often occurs in male more than females[6]. Isochromosome 17q is the most common cytogenetic aberration seen in this group. Other genetic variants include the loss of chromosome 8p, 10q, and the aberrations of 11p and 18q[2,17]. The clinical outcome is better in patients with chromosome 11 loss with an OS above 90%[19]. Zhou et al[24] reported that around 40% of Group 4 patients showed metastasis and treated as a high-risk disease. As we mentioned before, Group 3 and Group 4 MBs are genetically heterogeneous and not associated with germline mutations[25].
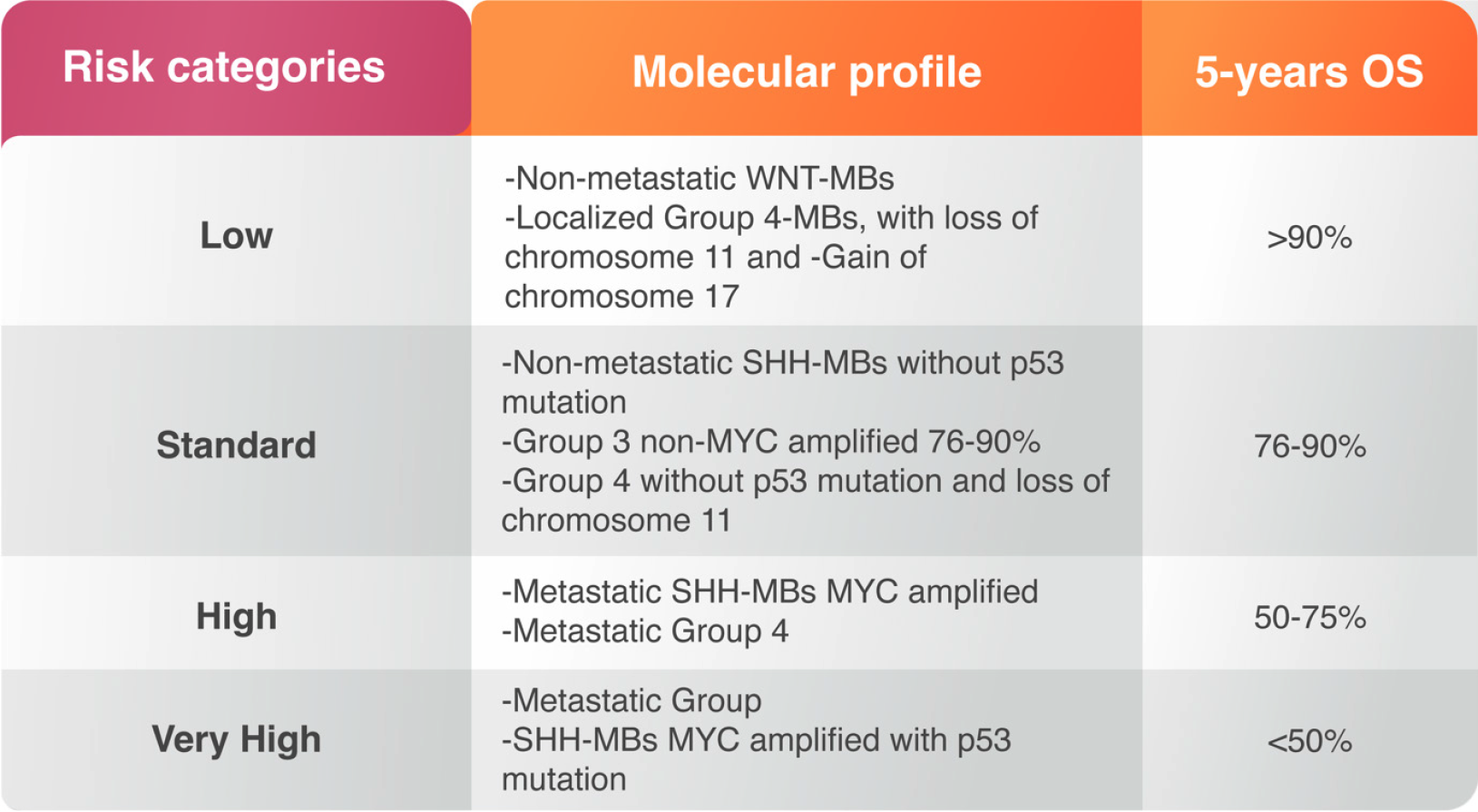
The magnitude of surgical resection in MB may not be as significant as earlier. After surgery, patients are treated with radiotherapy of the whole spinal axis with an additional boost targeting the tumor margins[26]. Radiotherapy usually starts 20-30 d after surgery however, delay of radiation may increase risk of recurrence and is therefore not recommended for patients older than 3 years[27,28]. Post-operative radiotherapy for children less than 3 years of old may increase risk of cognitive dysfunction[18]. Postoperative chemotherapy in MB patients is essential strategy to reduce the radiation effects and improve the survival, particularly in young children. The treatment varies based on the risk of drug toxicity and recurrence rate. Both risks are correlated with MB molecular alterations and considered as prognostic factors prior treatment. The risk of toxicity should be taken carefully in infants and children younger than three years of age while the recurrence is usually high in metastatic cases or cases undergoing subtotal resection. Anaplastic and large cell variants may have poor response and worsening outcome[29] (Figure 2). The high-risk group consists of SHH-MBs with MYCN-amplification; SHH-MB with metastatic dissemination and wildtype TP53, and metastatic Group 4 MBs[7]. High-risk population includes mutant TP53 SHH-MB patients and metastatic Group 3 MBs with MYCN-amplifications[7] (Figure 2).
Multi-modality treatments have been used in multiple clinical trials for ten years. The standard protocols included different chemotherapeutic agents with long-term or maintenance dose-related regime including ifosfamide, etoposide, methotrexate, cisplatin, and cytarabine, lomustine, and vincristine[30]. The maintenance regimen has improved the overall survival compared to the sandwich approach among patients with M0 or M1 disease[30,31]. Nonetheless, the most frequent and current treatment strategy includes risk-adapted radiotherapy followed by 4 cycles of cyclophosphamide, and a high dose of chemotherapy such as cisplatin, vincristine, followed by autologous stem cell transplantation. This protocol has improved the 5-year OS into 95%[16]. Additional clinical trials are ongoing to explore the efficacy of different treatment regimes in newly diagnosed MBs (Clini
| Clinical Trial | Trial objective | Samples | Targeted subgroup | Completion date |
| NCT01878617 | Clinical and molecular risk directed therapy of newly diagnosed MB | 660 | WNT, non-WNT, SHH | 2028 |
| NCT00089245 | Intrathecal radioimmunotherapy using I-8H9 | 120 | 8H9 reactive MB confirmed by IHC | 2024 |
| NCT02905110 | Simultaneous methotrexate/etoposide infusion | 10 | All MB subtypes | 2023 |
| NCT02962167 | Modified measles virus (MV-NIS) | 46 | All MB subtypes | 2024 |
| NCT02271711 | Expanded NK cells infusion with recurrent medulloblastoma | 12 | All MB subtypes | 2023 |
| NCT02359565 | Pembrolizumab in patient with recurrent medulloblastoma | 45 | All MB subtypes | 2023 |
| NCT03389802 | APX005M, a humanized IgG1κ monoclonal Ab that binds to CD40 | 45 | MB with CD40 activity | 2023 |
| NCT03299309 | PEP (CMV)-specific peptide vaccine in medulloblastoma | 30 | All MB subtypes | 2024 |
| NCT03598244 | Volitinib, a small molecule inhibitor of c-Met in recurrent MB | 50 | All MB subtypes | 2023 |
| NCT03173950 | Nivolumab, Immune check point inhibitor, in refractory MB | 180 | All MB subtypes | 2024 |
| NCT03500991 | HER2-Specific CAR T-cell locoregional immunotherapy | 48 | Her-2 expressed medulloblastoma | 2039 |
| NCT01356290 | Antiangiogenic therapy for recurrent medulloblastoma | 100 | All MB subtypes | 2026 |
| NCT03911388 | G207, an oncolytic herpes simplex virus-1 (HSV) | 15 | All MB subtypes | 2025 |
| NCT03638167 | EGFR806-specific CAR T-cell locoregional immunotherapy | 36 | EGFR positive tumours | 2040 |
| NCT03893487 | Fimepinostat, a small molecule inhibitor in young MB | 30 | All MB subtypes | 2027 |
| NCT03709680 | Palbociclib in combination with temozolomide and irinotecan | 184 | All MB subtypes | 2028 |
| NCT03904862 | CX-4945 inhibitor of casein kinase II (CK2) tolerability | 60 | SHH-medulloblastoma | 2028 |
| NCT03936465 | BMS-986158, a bromodomain inhibitor | 66 | MYCN amplification or BRD3 translocation MB | 2024 |
| NCT02650401 | Entrectinib (RXDX-101), a TRKA/B/C, ROS1, and ALK inhibitor | 68 | MB harboring- NTRK1/2/3, ROS1, ALK fusions | 2027 |
| NCT03210714 | Erdafitinib, an oral pan-FGFR inhibitor | 49 | Mutations in the FGFR1/2/3/4 pathway | 2024 |
| NCT03213678 | Samotolisib, a PI3K/mTOR inhibitor | 24 | PI3K/MTOR activating mutations | 2024 |
| NCT03213704 | Larotrectinib, NTRK fusion inhibitor for medulloblastoma | 49 | MB with NTRK fusions | 2024 |
| NCT03213665 | Tazemetostat, a small molecule EZH2 inhibitor | 20 | EZH2, SMARCB1, or SMARCA4 mutations | 2023 |
| NCT03233204 | Olaparib for refractory or aggressive medulloblastoma | 29 | Defects in DNA damage repair genes | 2024 |
| NCT04023669 | LY2606368, a molecularly targeted CHK1/2 inhibitor | 21 | Group3/Group4; SHH; indeterminate types | 2026 |
| NCT03526250 | Palbociclib (Pediatric MATCH treating trials | 49 | Rb positive solid tumours | 2025 |
| NCT02444546 | Wild-Type Reovirus in Combination with Sargramostim | 06 | All MB subtypes | 2026 |
| NCT04185038 | B7-H3-Specific CAR-T Cell Locoregional Immunotherapy | 90 | All MB subtypes | 2041 |
| NCT01601184 | Vismodegib combined with Temozolomide | 24 | SHH-MB group | 2023 |
| NCT03155620 | Targeted therapy directed by genetic testing | 2316 | All MB subtypes | 2027 |
| NCT00089245 | Iodine I 131 monoclonal antibody 8H9 | 120 | All MB subtypes | 2025 |
| NCT02271711 | Natural killer cell therapy | 12 | All MB subtypes | |
| NCT04315064 | Infusion of Panobinostat (MTX110) | 5 | All MB subtypes | 2024 |
| NCT04743661 | 131I-Omburtamab in recurrent medulloblastoma | 62 | All MB subtypes | 2030 |
| NCT03257631 | Pomalidomide onotherapy for recurrent or progressive MB | 53 | All MB subtypes | 2023 |
| NCT04320888 | Selpercatinib for treatment of advanced medulloblastoma | 49 | Tumour with activating RET alteration | 2027 |
All the previously mentioned clinical trials are stratified based on disease risk, molecular subgroups, patients age, and all are targeting tumour cells. The necessity to explore MB microenvironment is encouraged to help discovering new targeted receptors. The immune microenvironment of any cancer represents all types of cells surrounding the tumour cells including immune and none-immune cells. The relationship between these cells is mechanical and heterogeneous, by which they can facilitate in promoting or inhibiting tumor growth[32]. Because some studies have indicated that MBs have fewer immune cells than glioblastoma[33,34], the role of immune microenvironment in promoting or suppressing MB progression was found to be difficult to understand. Some cellular factors in tumour microenvironment may act against immune reaction and can promote tumour growth progression and angiogenesis. The infiltration of immune cells in MB might be limited due to the blood–brain barrier (BBB), which acts as physical barrier for immune cells infiltration[35]. Despite of some immune cells bypass across BBB, there may be an increase in trafficking toward the brain under certain conditions due to destruction of the BBB[36]. Some experimental models showed that the reactive astrocytes surrounding the tumour microenvironment form perivascular barriers to restrict the immune cells infiltration to the brain through BBB[37].
The presence of inflammatory cells in the tumor microenvironment has been scientifically accepted as an essential element in tumour progression. A study done by Gururangan et al[38] found that treated MB patients exhibited more CD4+T-cell lymphopenia. We can also presume that pre-operative and post-operative steroid treatment may induce systemic immunosuppression which prevents antitumor immunity in MB patients. Tumours with a low mutational burden respond less efficiently to immune checkpoint inhibitor compared to tumors with a high mutational burden[39]. Moreover, the acidification of the tumour microenvironment causing glycolytic activity can encourage macrophages infiltration through G protein coupled receptor, which in turn enhances vascular endothelial growth factor, thus promoting M2-like features of tumor-associated macrophage (TAM)[40].
APC, the immune cells in microenvironment, were proven to infiltrate malignant brain tumours in children. APCs is expressed by Major Histocompatibility Complex (MHC) class-I on tumor cells to allow them to be identified and killed by CD8 cytotoxic T- cells. MBs and atypical teratoid/rhabdoid tumors showed the lowermost cellular infiltration of this type among all malignant brain tumors[34]. Microglia, resident macrophages in the brain, are the most dominant APCs in brain tumors[35]. It is not clear if microglia promote anti-MB immune response. Mundt et al[41] showed that microglia are dispensable for T-cell entry into the brain and for local reactivation of T-cells. The loss of MHC class-I expression on tumor surface is also a common mechanism of immune escape in MB[42,43]. Because MHC class-I helps in the activation of CD8 cytotoxic T-cells, it acts as a passive regulator of natural killer (NK) cells. Thus, the loss of MHC-class I in tumor cells may increase tumour cell evasion[42,43].
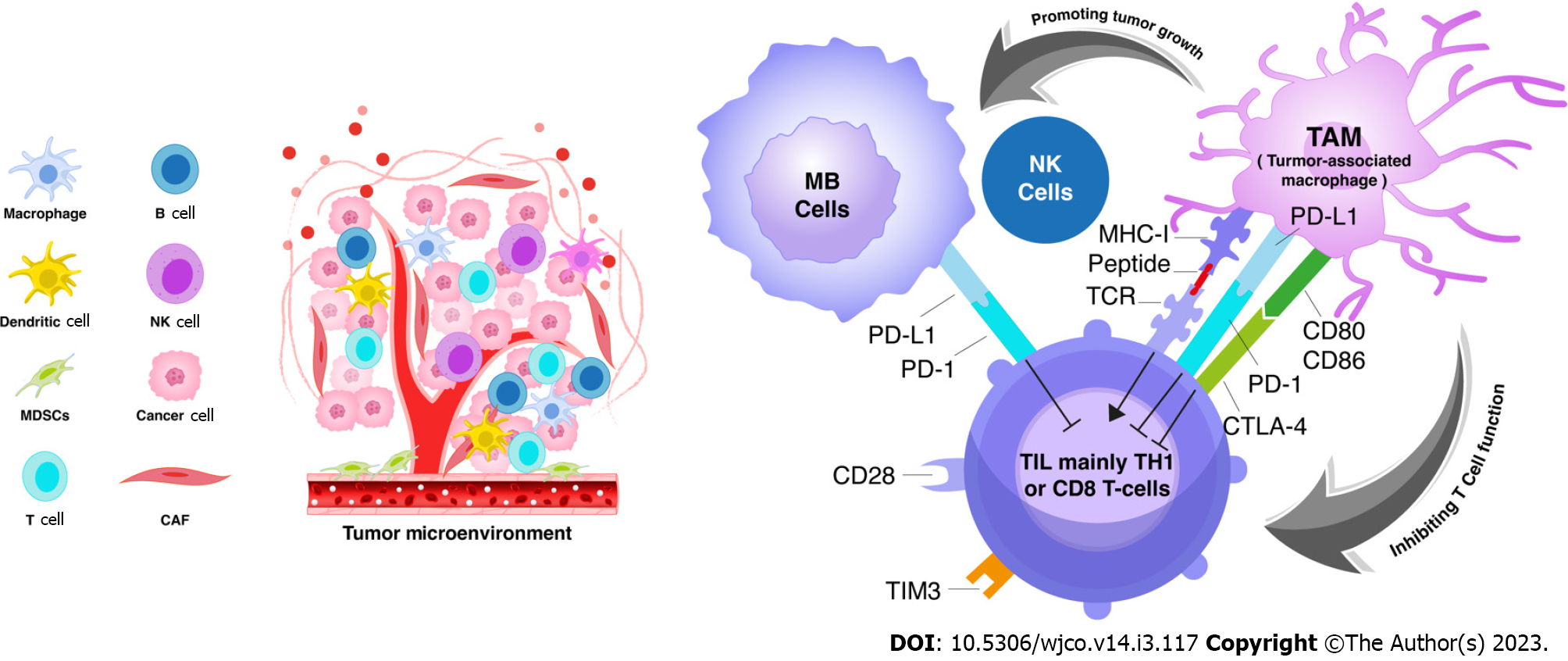
TAM is considered the major immune cell in the tumor microenvironment that can either support or inhibit tumor growth[44,45]. TAMs interact with tumour cells to promote tumour progression and invasion[46]. They are subclassified into two groups: (1) TAMs with M1 polarization, are induced by IFN-γ to release proinflammatory particles and are associated with some inflammatory response; and (2) TAMs with M2 polarization, are induced by interleukin-4 to release growth factors (e.g., epidermal growth factor, fibroblast growth factor-1, vascular endothelial growth factor) and involved in tumour progression and immunosuppression[47-49]. Uncontrolled activation of M1-polarzed TAM can shift towards M2-polarization in long term. However, the M2-like macrophages, which mimic TAMs in the tumour microenvironment, can be stimulated by cytokines[50]. EGF released by TAMs stimulate carcinogenesis, while VEGF regulates angiogenesis. These processes emphasize the actual immune-suppressive function of TAMs[51]. TAMs infiltration in the tumour microenvironment was proven to be a poor prognostic factor[50]. Clinical data have indicated that a large number of M2-polarized TAMs expressing CD163 and CD204 were correlated with a poor outcome of several body cancers[47] (Figure 3). Moreover, the presence of TAMs, mainly M2- type, has been also noted in many adult malignancies including CNS tumors[52-54]. In response to hypoxia, TAMs overexpress the PD-1 ligands[55]. PD-L1 overexpression in TAM has been reported in glioblastoma[56] but it has never been explored well in other brain tumours such as medulloblastoma.
The current role of TAMs in the prognosis of MB is still controversial. Despite of the molecular insights provided by MB subgroups, less information were reported about the role of TAMs in MBs[33]. The genetic alterations and the disease risk would make diverse effects on immune microenvironment[57]. Because TAMs are composed of variable amounts of microglia and macrophages, the composition of TAMs are different in all MB subgroups. Margol et al[58] and Zhang et al[59] reported that TAMs were significantly higher in SHH-MB compared to other MB subgroups. This may be due to the high expression of monocyte chemotactic protein-1 (MCP-1], which helps in TAM recruitment and M2 polarization[60]. Another possibility, SHH-MB may exhibit molecular signatures predictive for fibroblast, T-cells, and macrophage infiltration[34]. Nevertheless, the role of TAMs in this era is not clear and the previous reported studies did not reveal the prognostic connotations of TAMs in SHH-MBs[58].
CD163 expression was observed in the small number of SHH-MBs, which suggested that TAMs may play a dynamic role in SHH-MB formation[58,61]. Another study done by Crotty et al[62], revealed that less TAMs in microenvironment was associated with a low recurrence and low risk of metastasis. Lee et al[63] suggested that a large number of M1-polarized TAMs was associated with worsening outcome in SHH-MB patients. Lee and his group has also investigated the correlation between TAM recruitment and outcome, and they revealed that expressed M1-polarized TAMs predicted better progression-free survival but, TAMs showed no significant effect on OS[59]. Few studies showed that the immunoreactivity in MB microenvironment, regardless the subtype, is age-related[64]. In a study done by Zhang et al, they divided the patients into three age groups. They found that the group between 0-3 years of age and the group between 11-18 year of age had more TAMs than the group aged between 4-10 years. It implies that TAMs in MBs are crucial in different age groups[59]. Zhang et al[59] also found that TAMs, mainly M1-polarized type, are prevalent in MBs with metastatic disease.
Tumour recurrence and metastases are the major obstacle for treatment success, and the disease recurrence is responsible for 90% of MB mortality[65]. Group 3 and 4 patients develop spinal metastases regardless of the type of chemotherapy given after resection[2]. The presence of TP53-MYCN-alteration in these groups is associated with rapid tumour progression[66]. The ability of Group 3 and 4 to metastasize indicates that these tumor cells participate in the epithelial-to-mesenchymal transition (EMT), thus warranting additional investigations into EMT[67]. It is not yet known why tumor cells enter the EMT phase. A study done by Bonde et al[68] showed that TGFβ triggers the EMT phase, shifting the cancer cells to gain a mesenchymal phenotype. The lack of local nutrients, loss of supportive cells in microenvironment, and repeated mutations can all be reasons for this aggressive behavior. Funakoshi et al[69] found that loss of CDH1 allows tumour cells to detach from each other and can invade and metastasize.
Generally, increased T-cells trafficking in the brain has been reported in some neurological diseases. The activated T-cells have the role to alter the BBB, allowing for immune cells recruitment and entry to the brain parenchyma[70]. Tumour infiltrating lymphocytes (TIL) are considered signaling interacted cells between TAMs and tumour cells in the tumour microenvironment (Figure 3). The number of T-cells present in MB was found to be not significantly high compared to other control tissues[33]. Small amount of CD8 cytotoxic T-cells and NK cells suggest a less antitumor activity in MB[34]. However, a small percentage of helper T-cells (Th17) cells was also found at the site of the tumor but with uncertain significance[11]. Some experimental trials revealed that MB cells stimulate the release of the T-cells attractant (RANTES) from the endothelium, causing T-cell immigration[71]. Hence, increasing numbers of T-helper lymphocytes correlate with favourable prognosis in MB patients receiving chemotherapy[44].
T-regulatory cells (Tregs) control the activity of immune cells by releasing some anti-inflammatory cytokines such interleukin-10 (IL-10), and CTLA4-mediated trogocytosis[44]. Treg infiltration in MB microenvironment has been described by Gate et al[44]. Consequently, TGFβ drives the CD4 helper T-cells to Tregs, which in turn releases high levels of TGFβ. This process generates a feeding circuit to support immunosuppression. Elevated Treg in MBs can be therapy-induced, as Treg has been detected in the peripheral blood of some treated patients[38].
The interaction between TAMs and TILs were not scientifically explored in MB microenvironment (Figure 3). Kurdi et al[54] has explained the crosstalk between tumour cells, TAM sand TILs in glioblastoma. TAMs encircle cancer cells and supresses the killing action of T-cell thus, T-cells will not be able to help tumour cells against immune evasion. The TAMs accumulate in the microenvironment with less T-cells evolution[54]. Salsman et al[71] revealed that MB cell lines can interact with tumor endothelium to recruit T-cells to MB microenvironment, in particular macrophage migration inhibitory factor (MIF). MIF is the key molecule released by MB to stimulate the endothelial cells in the microenvironment to release more potent T-lymphocyte attractants[71].
Immune checkpoints represent a family of proteins on T-cells surface that interact with some ligands on APCs or tumour cells while they inhibit TCR-mediated ligands. Certain cancers (colorectal, ovarian and brain cancers) are resistant to immune checkpoint inhibitor[72]. The number of studies utilizing immunotherapy in the treatment approach of MB is limited. The approach had few selected options. Most of studies were observational and contained a small sample size. There are two clinical trials currently investigating the blockade of inhibitory checkpoint pathways in MB including pembrolizumab and nivolumab (NCT02359565) (NCT03173950). CD276, another immune check point inhibitor on T-cell, is also under investigation[73]. CD40 [a TNF receptor] expressed by antigen presenting cells and B-cells expresses cytokines, activates T-cells, and in turn timulate programmed cell death[74]. CD40 has a significant cytotoxic effect on tumor cells. APX005M, a humanized IgG1κ monoclonal antibody agonist of CD40 is currently evaluated in a phase I trial (NCT03389802) in patients with recurrent MBs. The recent actively recruiting clinical trials are summarized in (Table 1).
Numerous studies revealed that TAMs may interfere with some anti-tumor treatments such as chemotherapies and other antibody-based immunotherapies targeting some molecules such as PD-1/PD-1[50,72]. These findings emphasize that TAMs might be a promising target of novel anti-tumor treatment particularly in patient not responding to the standard treatment. The ability of TAMs to limit the efficacy of immune check point blockade has been previously investigated in several cancers[75,76]. TAMs express multiple ligands for checkpoint receptors, such as PD-L1/2, CD80/86, and CD204/CD206, and the current checkpoint inhibitors are different from the targeted receptors as they maintain a state of effective immunosuppression[77] (Figure 3). These legends, representing M2-polarized TAMs, have not been investigated in MB microenvironment. Martin et al[78] showed that MBs expressing reduced levels of PD-L1 can help tumour cells to evade from the immunity, suggesting that an inflamed tumor microenvironment is necessary for PD-1 pathway stimulation. However, the efficacy of PD-PD-L1 inhibitor has not been yet proven to be formally used in MB treatment.
Trogocytosis is a process involved in immune microenvironment concerned with the transfer of membrane fragments and cell surface proteins between cells. It is not known if induced iTregs can undergo trogocytosis. The trogocytosis of CD80/CD86 occurring in CTLA-4 or PDL1-independent approach plays a significant role in the immune suppression[79]. CD80/86 expression and trogocytosis have never been explored in MB microenvironment. As a key mechanism, Treg-linked CTLA-4 inhibits the CD80/CD86 molecules expression on APCs. Tekguc et al[80] revealed that blockade of CTLA-4 and PD-1/PD-L1 pathways may impede Treg-mediated immunosuppression, which in turn enhances anti tumour activity response. This novel exploration has not been investigated in MB. Several investigations have demonstrated that activation of PI3Kγ signaling in macrophages suppresses NF-κB, thereby stimulating immunosuppression. TAMs in cancers treated with chemotherapies are often responsible for chemoresistance as they are more susceptible to the cytotoxic effect of macrophages[81]. This process occurs when there is excessive recruitment of anti-apoptotic process in tumour microenvironment[82].
Understanding the molecular events in the mechanism of TAMs activation allows for the deve
CSF-1, a colony stimulating factor involved in the proliferation and the recruitment of monocytes-macrophages, is an essential target against TAM in tumour microenvironment. The expression of CSF-1 in tumour microenvirment was proven to be a poor prognosticator in multiple body cancers[85]. After treatment with CSF-1 inhibitor in one of clinical trials, the number of TAMs have depleted and there was an infiltration of CD8 cytotoxic T-cells in the tumor[86,87].
Reprogramming of TAM is another possible strategy to inhibit TAM activity. Several approaches attempted to switch M2-polarized TAMs into antitumor M1-like macrophages through monoclonal antibody inhibitors and Toll-like receptor (TLR) blockers. Alvarez-Arellano et al[88] revealed that TLR7 is a prognostic factor of survival in MB. Resiquimod, an agonist to TLR7/8, has shown an attention couple years ago for its efficacy to reprogram macrophages[89]. The CD47–SIRPα, involved in the regulation of phagocytosis, has never been used to reprogram TAMs. CD47 is expressed by tumor cells and interacts with the signal regulatory protein-α. Substantial evidence assumed that overexpression of CD47 in many cancers had a role in the phagocytic resistance[90]. However, this investigation has never been investigated in MB patients. Promising results were obtained in lymphoma patients in a combination of anti-CD47 with anti-CD20. Despite these results, the in vivo application of CD47 for the treatment of cancer is still limited.
Medulloblastoma is the most common malignant pediatric tumour in CNS that are subclassified into four distinguishing molecular subgroups. The current treatments failed to improve the patient’s survival significantly while the serious complications associated with these cytotoxic therapies warrant for exploring new therapeutic approaches targeting different immune receptors. The identification of tumour microenvironment has facilitated the scientists understanding how tumor growth and progression are regulated. TAMs and TILs, the main dominant immune cells in microenvironment, seem to have a major role in immune mechanism and tumor progression. Their infiltration in microenvironment has prompted researchers to evaluate the interaction of new targeted immune receptors with the current signaling pathways. Their infiltration in microenvironment may also be targeted through different reprogramming mechanisms. However, the ability of TAMs to limit the efficacy of immune check point blockade in MB requires further investigations. These strategic thoughts emphasize that TAMs might be a promising targeted treatment particularly in patients with recurrent or progressive MB. Further studies to explore new targeted receptors in tumour microenvironment and understanding the conventional relationship between TAMs, TILs and tumour cells are essential to develop new therapeutic approaches.
| 1. | Pollack IF, Boyett JM, Yates AJ, Burger PC, Gilles FH, Davis RL, Finlay JL; Children's Cancer Group. The influence of central review on outcome associations in childhood malignant gliomas: results from the CCG-945 experience. Neuro Oncol. 2003;5:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M, Dufour C, Vassal G, Milde T, Witt O, von Hoff K, Pietsch T, Northcott PA, Gajjar A, Robinson GW, Padovani L, André N, Massimino M, Pizer B, Packer R, Rutkowski S, Pfister SM, Taylor MD, Pomeroy SL. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 487] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 3. | Ning MS, Perkins SM, Dewees T, Shinohara ET. Evidence of high mortality in long term survivors of childhood medulloblastoma. J Neurooncol. 2015;122:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Altman J, Bayer SA. The generation, movements, and settling of cerebellar granule cells and the formation of parallel fibers in development of the cerebellar system in relation to its evolution structure and function. CRC Press 1997; New York pp. 334-361. |
| 5. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 11177] [Article Influence: 1117.7] [Reference Citation Analysis (0)] |
| 6. | Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1219] [Cited by in RCA: 1425] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 7. | Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 8. | Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, Taylor RE, Bailey S, Clifford SC, Gilbertson RJ. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 401] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 9. | Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, Gröbner S, Segura-Wang M, Zichner T, Rudneva VA, Warnatz HJ, Sidiropoulos N, Phillips AH, Schumacher S, Kleinheinz K, Waszak SM, Erkek S, Jones DTW, Worst BC, Kool M, Zapatka M, Jäger N, Chavez L, Hutter B, Bieg M, Paramasivam N, Heinold M, Gu Z, Ishaque N, Jäger-Schmidt C, Imbusch CD, Jugold A, Hübschmann D, Risch T, Amstislavskiy V, Gonzalez FGR, Weber UD, Wolf S, Robinson GW, Zhou X, Wu G, Finkelstein D, Liu Y, Cavalli FMG, Luu B, Ramaswamy V, Wu X, Koster J, Ryzhova M, Cho YJ, Pomeroy SL, Herold-Mende C, Schuhmann M, Ebinger M, Liau LM, Mora J, McLendon RE, Jabado N, Kumabe T, Chuah E, Ma Y, Moore RA, Mungall AJ, Mungall KL, Thiessen N, Tse K, Wong T, Jones SJM, Witt O, Milde T, Von Deimling A, Capper D, Korshunov A, Yaspo ML, Kriwacki R, Gajjar A, Zhang J, Beroukhim R, Fraenkel E, Korbel JO, Brors B, Schlesner M, Eils R, Marra MA, Pfister SM, Taylor MD, Lichter P. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547:311-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 560] [Cited by in RCA: 838] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 10. | Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, Chalhoub N, Baker SJ, Huether R, Kriwacki R, Curley N, Thiruvenkatam R, Wang J, Wu G, Rusch M, Hong X, Becksfort J, Gupta P, Ma J, Easton J, Vadodaria B, Onar-Thomas A, Lin T, Li S, Pounds S, Paugh S, Zhao D, Kawauchi D, Roussel MF, Finkelstein D, Ellison DW, Lau CC, Bouffet E, Hassall T, Gururangan S, Cohn R, Fulton RS, Fulton LL, Dooling DJ, Ochoa K, Gajjar A, Mardis ER, Wilson RK, Downing JR, Zhang J, Gilbertson RJ. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 695] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 11. | Zhao F, Ohgaki H, Xu L, Giangaspero F, Li C, Li P, Yang Z, Wang B, Wang X, Wang Z, Ai L, Zhang J, Luo L, Liu P. Molecular subgroups of adult medulloblastoma: a long-term single-institution study. Neuro Oncol. 2016;18:982-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B, Garzia L, Torchia J, Nor C, Morrissy AS, Agnihotri S, Thompson YY, Kuzan-Fischer CM, Farooq H, Isaev K, Daniels C, Cho BK, Kim SK, Wang KC, Lee JY, Grajkowska WA, Perek-Polnik M, Vasiljevic A, Faure-Conter C, Jouvet A, Giannini C, Nageswara Rao AA, Li KKW, Ng HK, Eberhart CG, Pollack IF, Hamilton RL, Gillespie GY, Olson JM, Leary S, Weiss WA, Lach B, Chambless LB, Thompson RC, Cooper MK, Vibhakar R, Hauser P, van Veelen MC, Kros JM, French PJ, Ra YS, Kumabe T, López-Aguilar E, Zitterbart K, Sterba J, Finocchiaro G, Massimino M, Van Meir EG, Osuka S, Shofuda T, Klekner A, Zollo M, Leonard JR, Rubin JB, Jabado N, Albrecht S, Mora J, Van Meter TE, Jung S, Moore AS, Hallahan AR, Chan JA, Tirapelli DPC, Carlotti CG, Fouladi M, Pimentel J, Faria CC, Saad AG, Massimi L, Liau LM, Wheeler H, Nakamura H, Elbabaa SK, Perezpeña-Diazconti M, Chico Ponce de León F, Robinson S, Zapotocky M, Lassaletta A, Huang A, Hawkins CE, Tabori U, Bouffet E, Bartels U, Dirks PB, Rutka JT, Bader GD, Reimand J, Goldenberg A, Ramaswamy V, Taylor MD. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell. 2017;31:737-754.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 899] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 13. | Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, Carneiro MO, Carter SL, Cibulskis K, Erlich RL, Greulich H, Lawrence MS, Lennon NJ, McKenna A, Meldrim J, Ramos AH, Ross MG, Russ C, Shefler E, Sivachenko A, Sogoloff B, Stojanov P, Tamayo P, Mesirov JP, Amani V, Teider N, Sengupta S, Francois JP, Northcott PA, Taylor MD, Yu F, Crabtree GR, Kautzman AG, Gabriel SB, Getz G, Jäger N, Jones DT, Lichter P, Pfister SM, Roberts TM, Meyerson M, Pomeroy SL, Cho YJ. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 625] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 14. | Schröder M. Human DEAD-box protein 3 has multiple functions in gene regulation and cell cycle control and is a prime target for viral manipulation. Biochem Pharmacol. 2010;79:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Trubicka J, Szperl M, Grajkowska W, Karkucińska-Więckowska A, Tarasińska M, Falana K, Dembowska-Bagińska B, Łastowska M. Identification of a novel inherited ALK variant M1199L in the WNT type of medulloblastoma. Folia Neuropathol. 2016;54:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol. 2014;11:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29:1424-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 588] [Cited by in RCA: 558] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 18. | Rutkowski S, Gerber NU, von Hoff K, Gnekow A, Bode U, Graf N, Berthold F, Henze G, Wolff JE, Warmuth-Metz M, Soerensen N, Emser A, Ottensmeier H, Deinlein F, Schlegel PG, Kortmann RD, Pietsch T, Kuehl J; German Pediatric Brain Tumor Study Group. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol. 2009;11:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Kool M, Jones DT, Jäger N, Northcott PA, Pugh TJ, Hovestadt V, Piro RM, Esparza LA, Markant SL, Remke M, Milde T, Bourdeaut F, Ryzhova M, Sturm D, Pfaff E, Stark S, Hutter S, Seker-Cin H, Johann P, Bender S, Schmidt C, Rausch T, Shih D, Reimand J, Sieber L, Wittmann A, Linke L, Witt H, Weber UD, Zapatka M, König R, Beroukhim R, Bergthold G, van Sluis P, Volckmann R, Koster J, Versteeg R, Schmidt S, Wolf S, Lawerenz C, Bartholomae CC, von Kalle C, Unterberg A, Herold-Mende C, Hofer S, Kulozik AE, von Deimling A, Scheurlen W, Felsberg J, Reifenberger G, Hasselblatt M, Crawford JR, Grant GA, Jabado N, Perry A, Cowdrey C, Croul S, Zadeh G, Korbel JO, Doz F, Delattre O, Bader GD, McCabe MG, Collins VP, Kieran MW, Cho YJ, Pomeroy SL, Witt O, Brors B, Taylor MD, Schüller U, Korshunov A, Eils R, Wechsler-Reya RJ, Lichter P, Pfister SM; ICGC PedBrain Tumor Project. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25:393-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 605] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 20. | Smith MJ, Beetz C, Williams SG, Bhaskar SS, O'Sullivan J, Anderson B, Daly SB, Urquhart JE, Bholah Z, Oudit D, Cheesman E, Kelsey A, McCabe MG, Newman WG, Evans DG. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32:4155-4161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12:818-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 512] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 22. | Sursal T, Ronecker JS, Dicpinigaitis AJ, Mohan AL, Tobias ME, Gandhi CD, Jhanwar-Uniyal M. Molecular Stratification of Medulloblastoma: Clinical Outcomes and Therapeutic Interventions. Anticancer Res. 2022;42:2225-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Menyhárt O, Giangaspero F, Győrffy B. Molecular markers and potential therapeutic targets in non-WNT/non-SHH (group 3 and group 4) medulloblastomas. J Hematol Oncol. 2019;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Zhou P, Sha H, Zhu J. The role of T-helper 17 (Th17) cells in patients with medulloblastoma. J Int Med Res. 2010;38:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Waszak SM, Northcott PA, Buchhalter I, Robinson GW, Sutter C, Groebner S, Grund KB, Brugières L, Jones DTW, Pajtler KW, Morrissy AS, Kool M, Sturm D, Chavez L, Ernst A, Brabetz S, Hain M, Zichner T, Segura-Wang M, Weischenfeldt J, Rausch T, Mardin BR, Zhou X, Baciu C, Lawerenz C, Chan JA, Varlet P, Guerrini-Rousseau L, Fults DW, Grajkowska W, Hauser P, Jabado N, Ra YS, Zitterbart K, Shringarpure SS, De La Vega FM, Bustamante CD, Ng HK, Perry A, MacDonald TJ, Hernáiz Driever P, Bendel AE, Bowers DC, McCowage G, Chintagumpala MM, Cohn R, Hassall T, Fleischhack G, Eggen T, Wesenberg F, Feychting M, Lannering B, Schüz J, Johansen C, Andersen TV, Röösli M, Kuehni CE, Grotzer M, Kjaerheim K, Monoranu CM, Archer TC, Duke E, Pomeroy SL, Shelagh R, Frank S, Sumerauer D, Scheurlen W, Ryzhova MV, Milde T, Kratz CP, Samuel D, Zhang J, Solomon DA, Marra M, Eils R, Bartram CR, von Hoff K, Rutkowski S, Ramaswamy V, Gilbertson RJ, Korshunov A, Taylor MD, Lichter P, Malkin D, Gajjar A, Korbel JO, Pfister SM. Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018;19:785-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 26. | Wolden SL, Dunkel IJ, Souweidane MM, Happersett L, Khakoo Y, Schupak K, Lyden D, Leibel SA. Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. J Clin Oncol. 2003;21:3079-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Schwalbe EC, Lindsey JC, Nakjang S, Crosier S, Smith AJ, Hicks D, Rafiee G, Hill RM, Iliasova A, Stone T, Pizer B, Michalski A, Joshi A, Wharton SB, Jacques TS, Bailey S, Williamson D, Clifford SC. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol. 2017;18:958-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 411] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 28. | Kann BH, Park HS, Lester-Coll NH, Yeboa DN, Benitez V, Khan AJ, Bindra RS, Marks AM, Roberts KB. Postoperative Radiotherapy Patterns of Care and Survival Implications for Medulloblastoma in Young Children. JAMA Oncol. 2016;2:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1020] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 30. | Hoff KV, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, Benesch M, Warmuth-Metz M, Soerensen N, Zwiener I, Goette H, Schlegel PG, Pietsch T, Kortmann RD, Kuehl J, Rutkowski S. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT'91. Eur J Cancer. 2009;45:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Franceschi E, Giannini C, Furtner J, Pajtler KW, Asioli S, Guzman R, Seidel C, Gatto L, Hau P. Adult Medulloblastoma: Updates on Current Management and Future Perspectives. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Quail DF, Joyce JA. The Microenvironmental Landscape of Brain Tumors. Cancer Cell. 2017;31:326-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 1330] [Article Influence: 147.8] [Reference Citation Analysis (5)] |
| 33. | Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, Wang M, Handler MH, Foreman NK. Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol. 2013;191:4880-4888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 34. | Bockmayr M, Mohme M, Klauschen F, Winkler B, Budczies J, Rutkowski S, Schüller U. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology. 2018;7:e1462430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Eisemann T, Wechsler-Reya RJ. Coming in from the cold: overcoming the hostile immune microenvironment of medulloblastoma. Genes Dev. 2022;36:514-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 528] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 37. | Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511-11522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 38. | Gururangan S, Reap E, Schmittling R, Kocak M, Reynolds R, Grant G, Onar-Thomas A, Baxter P, Pollack IF, Phillips P, Boyett J, Fouladi M, Mitchell D. Regulatory T cell subsets in patients with medulloblastoma at diagnosis and during standard irradiation and chemotherapy (PBTC N-11). Cancer Immunol Immunother. 2017;66:1589-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6065] [Cited by in RCA: 6489] [Article Influence: 589.9] [Reference Citation Analysis (0)] |
| 40. | Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 2330] [Article Influence: 194.2] [Reference Citation Analysis (0)] |
| 41. | Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci Immunol. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 42. | Raffaghello L, Nozza P, Morandi F, Camoriano M, Wang X, Garrè ML, Cama A, Basso G, Ferrone S, Gambini C, Pistoia V. Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res. 2007;67:5471-5478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Smith C, Santi M, Rajan B, Rushing EJ, Choi MR, Rood BR, Cornelison R, MacDonald TJ, Vukmanovic S. A novel role of HLA class I in the pathology of medulloblastoma. J Transl Med. 2009;7:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Haberthur K, Brennan K, Hoglund V, Balcaitis S, Chinn H, Davis A, Kreuser S, Winter C, Leary SE, Deutsch GH, Ellenbogen RG, Crane CA. NKG2D ligand expression in pediatric brain tumors. Cancer Biol Ther. 2016;17:1253-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Dehne N, Mora J, Namgaladze D, Weigert A, Brüne B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol. 2017;35:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 46. | Feng X, Szulzewsky F, Yerevanian A, Chen Z, Heinzmann D, Rasmussen RD, Alvarez-Garcia V, Kim Y, Wang B, Tamagno I, Zhou H, Li X, Kettenmann H, Ransohoff RM, Hambardzumyan D. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6:15077-15094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 47. | Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1630] [Article Influence: 181.1] [Reference Citation Analysis (0)] |
| 48. | Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest. 2017;97:498-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 49. | Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, Wang B, Yi L, Wang QL, Jiang XF, Yang L, Zhang P, Qian C, Cui YH, Zhang X, Bian XW. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 50. | Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1778] [Cited by in RCA: 3049] [Article Influence: 338.8] [Reference Citation Analysis (0)] |
| 51. | Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J, Prenen H, Ghesquière B, Carmeliet P, Mazzone M. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016;24:701-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 52. | Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 471] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 53. | Engler JR, Robinson AE, Smirnov I, Hodgson JG, Berger MS, Gupta N, James CD, Molinaro A, Phillips JJ. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS One. 2012;7:e43339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Kurdi M, Alghamdi B, Butt NS, Baeesa S. The relationship between CD204 (M2)-polarized tumour-associated macrophages (TAMs), tumour-infiltrating lymphocytes (TILs), and microglial activation in glioblastoma microenvironment: a novel immune checkpoint receptor target. Discov Oncol. 2021;12:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1182] [Cited by in RCA: 1733] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 56. | Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 378] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 57. | Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1769] [Article Influence: 196.6] [Reference Citation Analysis (0)] |
| 58. | Margol AS, Robison NJ, Gnanachandran J, Hung LT, Kennedy RJ, Vali M, Dhall G, Finlay JL, Erdreich-Epstein A, Krieger MD, Drissi R, Fouladi M, Gilles FH, Judkins AR, Sposto R, Asgharzadeh S. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin Cancer Res. 2015;21:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 59. | Zhang J, Yuan X, Wang Y, Liu J, Li Z, Li S, Liu Y, Gong X, Sun Y, Wu W, Sun L, Du S, Wang T. Tumor-Associated Macrophages Correlate With Prognosis in Medulloblastoma. Front Oncol. 2022;12:893132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | O'Connor T, Heikenwalder M. CCL2 in the Tumor Microenvironment. Adv Exp Med Biol. 2021;1302:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 61. | Maximov V, Chen Z, Wei Y, Robinson MH, Herting CJ, Shanmugam NS, Rudneva VA, Goldsmith KC, MacDonald TJ, Northcott PA, Hambardzumyan D, Kenney AM. Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma. Nat Commun. 2019;10:2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 62. | Crotty EE, Smith SMC, Brasel K, Pakiam F, Girard EJ, Connor YD, Zindy F, Mhyre AJ, Roussel MF, Olson JM. Medulloblastoma recurrence and metastatic spread are independent of colony-stimulating factor 1 receptor signaling and macrophage survival. J Neurooncol. 2021;153:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Lee C, Lee J, Choi SA, Kim SK, Wang KC, Park SH, Kim SH, Lee JY, Phi JH. M1 macrophage recruitment correlates with worse outcome in SHH Medulloblastomas. BMC Cancer. 2018;18:535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Patt S, Zimmer C. Age-related immunoreactivity pattern in medulloblastoma. Childs Nerv Syst. 1992;8:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Pizer BL, Clifford SC. The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials. Br J Neurosurg. 2009;23:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Hill RM, Kuijper S, Lindsey JC, Petrie K, Schwalbe EC, Barker K, Boult JK, Williamson D, Ahmad Z, Hallsworth A, Ryan SL, Poon E, Robinson SP, Ruddle R, Raynaud FI, Howell L, Kwok C, Joshi A, Nicholson SL, Crosier S, Ellison DW, Wharton SB, Robson K, Michalski A, Hargrave D, Jacques TS, Pizer B, Bailey S, Swartling FJ, Weiss WA, Chesler L, Clifford SC. Combined MYC and P53 defects emerge at medulloblastoma relapse and define rapidly progressive, therapeutically targetable disease. Cancer Cell. 2015;27:72-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 67. | Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2157] [Cited by in RCA: 2360] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 68. | Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 69. | Funakoshi Y, Sugihara Y, Uneda A, Nakashima T, Suzuki H. Recent advances in the molecular understanding of medulloblastoma. Cancer Sci. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 70. | Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 492] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 71. | Salsman VS, Chow KK, Shaffer DR, Kadikoy H, Li XN, Gerken C, Perlaky L, Metelitsa LS, Gao X, Bhattacharjee M, Hirschi K, Heslop HE, Gottschalk S, Ahmed N. Crosstalk between medulloblastoma cells and endothelium triggers a strong chemotactic signal recruiting T lymphocytes to the tumor microenvironment. PLoS One. 2011;6:e20267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Quaranta V, Schmid MC. Macrophage-Mediated Subversion of Anti-Tumour Immunity. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res. 2016;22:3425-3431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 74. | Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, Pittet MJ. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 518] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 76. | Gordon S, Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 464] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 77. | Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 378] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 78. | Martin AM, Nirschl CJ, Polanczyk MJ, Bell WR, Nirschl TR, Harris-Bookman S, Phallen J, Hicks J, Martinez D, Ogurtsova A, Xu H, Sullivan LM, Meeker AK, Raabe EH, Cohen KJ, Eberhart CG, Burger PC, Santi M, Taube JM, Pardoll DM, Drake CG, Lim M. PD-L1 expression in medulloblastoma: an evaluation by subgroup. Oncotarget. 2018;9:19177-19191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | Gu P, Gao JF, D'Souza CA, Kowalczyk A, Chou KY, Zhang L. Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell Mol Immunol. 2012;9:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 81. | Colotta F, Peri G, Villa A, Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. I. Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984;132:936-944. [PubMed] |
| 82. | Anfray C, Ummarino A, Andón FT, Allavena P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 83. | Canè S, Ugel S, Trovato R, Marigo I, De Sanctis F, Sartoris S, Bronte V. The Endless Saga of Monocyte Diversity. Front Immunol. 2019;10:1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 84. | Sánchez-Martín L, Estecha A, Samaniego R, Sánchez-Ramón S, Vega MÁ, Sánchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 85. | Koh YW, Park C, Yoon DH, Suh C, Huh J. CSF-1R expression in tumor-associated macrophages is associated with worse prognosis in classical Hodgkin lymphoma. Am J Clin Pathol. 2014;141:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1383] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 87. | Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, Brown JM. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 88. | Alvarez-Arellano L, Eguía-Aguilar P, Piña-Sánchez P, González-García N, Palma-Guzman A, Perezpeña-Diazconti M, Maldonado-Bernal C. High expression of Toll-like receptor 7 is a survival factor in pediatric medulloblastoma. Childs Nerv Syst. 2021;37:3743-3752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 89. | Thauvin C, Widmer J, Mottas I, Hocevar S, Allémann E, Bourquin C, Delie F. Development of resiquimod-loaded modified PLA-based nanoparticles for cancer immunotherapy: A kinetic study. Eur J Pharm Biopharm. 2019;139:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Murata Y, Kotani T, Ohnishi H, Matozaki T. The CD47-SIRPα signalling system: its physiological roles and therapeutic application. J Biochem. 2014;155:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 91. | Luzzi S, Giotta Lucifero A, Brambilla I, Semeria Mantelli S, Mosconi M, Foiadelli T, Savasta S. Targeting the medulloblastoma: a molecular-based approach. Acta Biomed. 2020;91:79-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial [CC BY-NC 4.0] license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mduma E, Tanzania; Ogino S, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH