Published online May 24, 2022. doi: 10.5306/wjco.v13.i5.417
Peer-review started: January 5, 2022
First decision: February 15, 2022
Revised: April 1, 2022
Accepted: May 5, 2022
Article in press: May 5, 2022
Published online: May 24, 2022
Processing time: 138 Days and 18.6 Hours
As underlined in the minireview by Blomstrand et al, given the poor prognosis and the paucity of data on a therapeutic sequence in pancreatic ductal adenocarcinoma (PDAC), additional randomized controlled trials and real-world evidence studies addressing current and novel regimens are needed. The real-world outcomes of first-line chemotherapy regimens such as FOLFIRINOX and gemcita
Core Tip: The present letter is intended to contribute to the collection of data on pancreatic ductal adenocarcinoma (PDAC) treatments in second-line settings through our experience with the promising data of efficacy and safety of a small group of study patients treated with second-line liposomal irinotecan (nal-IRI) plus 5-fluorouracil and leucovorin. We also focused on highlighting the subgroups of PDAC patients who might benefit from target treatments, such as a small proportion of mutated BRCAs, and to identify comorbidities or characteristics that impact the prognosis of PDAC patients through our retrospective analysis that demonstrate a correlation between type II diabetes mellitus and improved overall survival.
- Citation: Pretta A, Spanu D, Mariani S, Liscia N, Ziranu P, Pusceddu V, Puzzoni M, Massa E, Scartozzi M, Lai E. How to improve metastatic pancreatic ductal adenocarcinoma patients’ selection: Between clinical trials and the real-world. World J Clin Oncol 2022; 13(5): 417-422
- URL: https://www.wjgnet.com/2218-4333/full/v13/i5/417.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i5.417
We read with great interest the review by Pretta et al[1], entitled “Real-world evidence on first- and second-line palliative chemotherapy in advanced pancreatic cancer”. This review provides a comprehensive overview on real-life data of metastatic pancreatic ductal adenocarcinoma (PDAC) patients treated in the first- and second-line setting. The authors critically compare the results obtained in a real-world population with those provided by randomised clinical trials (RCT), highlighting that the outcomes are consistent and similar, especially on first-line with FOLFIRINOX and gemcitabine -nab-paclitaxel.
We agree with the authors that PDAC patients enrolled in RCT represent a highly selected population and that despite real-world outcomes derive from different countries, with various regulatory agencies and health care systems, all data available in the literature seems to confirm the effectiveness and safety of chemotherapy regimens in real-life settings. Moreover, since no strong data on second-line settings are available, we greatly appreciate the authors’ effort to analyse this topic.
At our Centre, we conducted a retrospective analysis in a real-world population of metastatic PDAC patients treated with second-line liposomal irinotecan (nal-IRI) plus 5-fluorouracil and leucovorin (5FU/LV) in a compassionate use program, in order to assess clinical outcome, tolerability and potential prognostic factors. Statistical analysis was performed with the MedCalc package. The association between categorical variables was estimated by chi-square test. Survival distribution was estimated by the Kaplan-Meier method and survival curves comparison was evaluated with the log-rank test. The cut off value for laboratory parameters was identified with ROC curves. The study was approved by the Ethical Committee of the Cagliari University Hospital (Prot. PG /2018/7339, June 1, 2018) and was performed in accordance with the ethical principles stated in the Declaration of Helsinki and in the International Conference on Harmonization (ICH) Note for Guidance on Good Clinical Practice (GCP, ICH E6, 1995) and all applicable regulatory requirements.
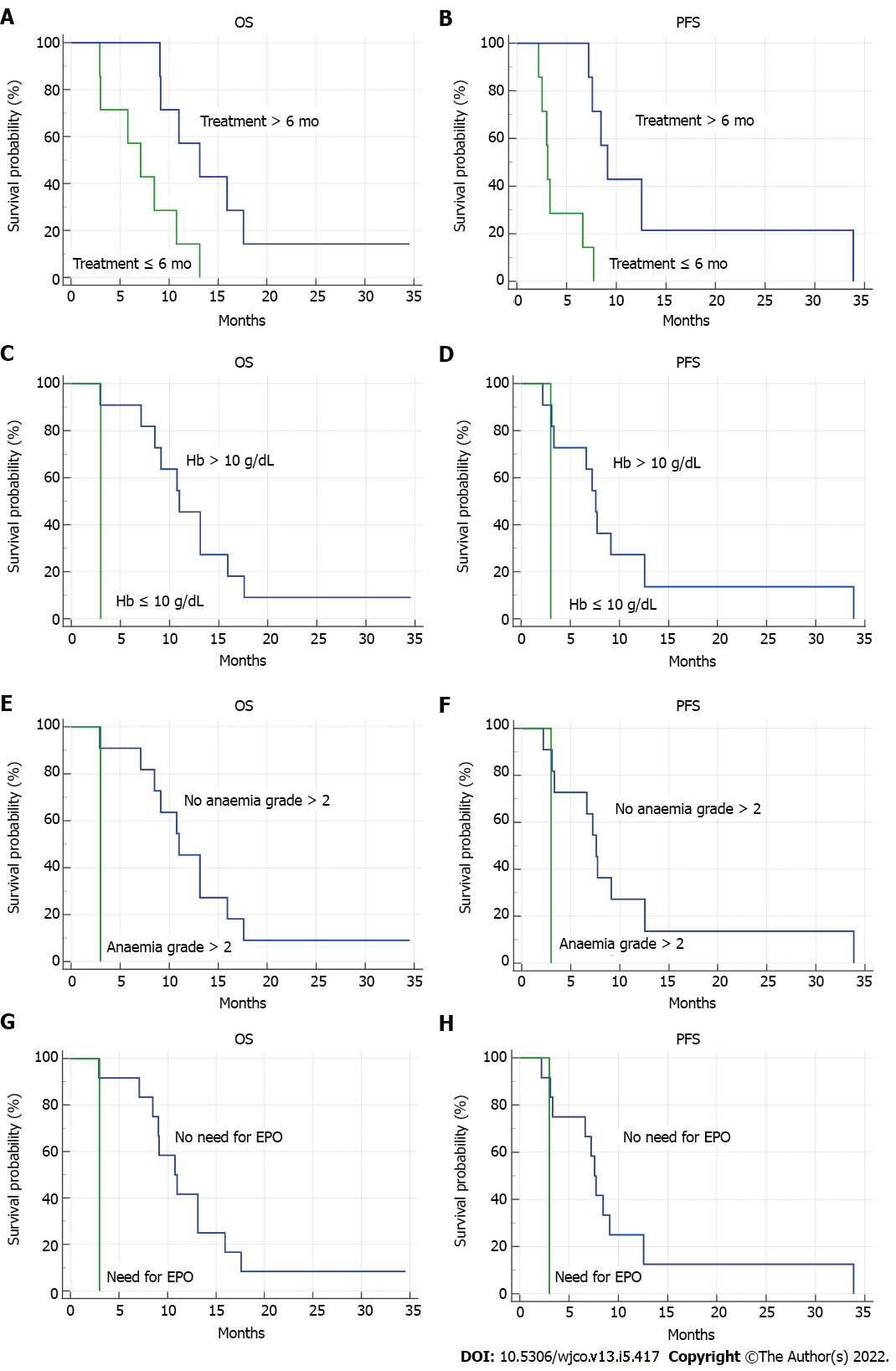
Globally, 14 patients treated with nal-IRI and 5FU/LV from June 2016 to November 2018 were included in our analysis. Baseline characteristics are shown in Table 1. Median overall survival (OS) was 9.1 (95%CI: 5.8-13.1) mo. Median progression free survival (PFS) was 7.2 mo (95%CI: 3.0-33.8). Three patients achieved a partial response, two had stable disease, and nine developed progression. Median duration of treatment with nal-IRI plus 5FU/LV was 6 mo; treatment longer than 6 mo was related to improved OS [13.1 mo (95%CI: 9-17) vs 7.1 mo (95%CI: 2.9-13.1), P = 0.0127, HR = 0.18] and PFS [9.1 mo (95%CI: 7.2-33.8) vs 3.1 mo (95%CI: 2.2-7.7), P = 0.0011, HR = 0.08]. Baseline haemoglobin levels equal or lower than 10 g/dL were associated with worse prognosis [OS: 11 mo (95%CI: 1-15.9) vs 3 mo, P = 0.0384, HR = 0.005; PFS: 7.6 mo (95%CI: 3.1-33.8) vs 3 mo, P = 0.0384, HR = 0.005]. 64% of patients developed grade > 2 toxicity. The occurrence of grade > 2 anaemia was related to shorter OS [3 mo vs 11 mo (95%CI: 7.1-15.9), P = 0.0384, HR = 0.005] and PFS [3 mo vs 7.6 mo (95%CI: 3.1-33.8), P = 0.0384, HR = 0.005]. As shown in Figure 1, the need for erythropoietin administration was associated with poorer OS [3 mo vs 10.7 mo (95%CI: 7.1-15.9), P = 0.0287, HR = 0.003] and PFS [3 mo vs 7.6 mo (95%CI 3.1-33.8), P = 0.0287, HR = 0.003]. In our real-world retrospective analysis, nal-IRI plus 5FU/LV confirmed its efficacy and tolerability, consistently with NAPOLI-1 RCT[2,3]. Longer duration of treatment was related to improved survival, whereas lower baseline haemoglobin levels, anaemia occurrence and the necessity of erythropoietin were negative prognostic factors. These results are consistent with some findings of a previous Italian large real-world analysis[4].
| Baseline characteristics | Patients, % |
| Sex | |
| Male | 71 |
| Female | 29 |
| Age, yr | |
| < 65 | 36 |
| ≥ 65 | 64 |
| Location of primary tumour | |
| Head-uncinate process | 57 |
| Body | 21.5 |
| Tail | 21.5 |
| Previous surgery | |
| Yes | 28.5 |
| No | 71.5 |
| Number of metastatic sites | |
| Single site | 36 |
| Multiple sites | 64 |
| Location of metastatic sites | |
| Lymph nodes | 78.5 |
| Liver | 57 |
| Peritoneum | 42.8 |
| Lung | 35.7 |
| First-line chemotherapy regimen | |
| Gemcitabine-nab-paclitaxel | 92.8 |
| Other | 7.2 |
As underlined by Blomstrand et al[1], there is an urgent need for prognostic and predictive biomarkers to improve the therapeutic management of PDAC patients and to prolong survival as well as more effective treatment strategies. Indeed, intensive research efforts and substantial progress in the understanding of the PDAC genetic background and molecular biology have not yet been matched either by the successful development of novel agents or by the identification of predictive biomarkers that could increase the effectiveness of existing therapies[5]. In contrast to other solid tumours, immunotherapy strategies have failed to yield any notable impact in PDAC. This is likely related to the critical role of the tumour microenvironment as a physical barrier and its inhibitory immune signalling. The most promising therapeutic strategy seems to be combination of immunotherapeutics with other targeted treatments[6].
To our knowledge, the only successful biomarker-driven phase III RCT so far is the POLO trial, which showed improved PFS with maintenance Olaparib, a PARP-inhibitor, vs placebo in germline BRCA-mutant PDAC not progressing after first-line platinum-based chemotherapy[7]. Notably, BRCA-mutant PDAC represents a unique entity with specific disease features that are still to be fully understood and BRCA1/2 alterations are the most explored targetable mutations. Moreover, in this patients’ setting, the sensitivity to platinum chemotherapy requires further research and confirmations. Recently, the concept of “BRCAness” has gained increasing importance. This term refers to high-grade genomic instability of non-BRCA-mutant cancers and represents a phenotype of defective homologous recombination to which somatic mutations in genes like BRCA1/2, ATM, PALB2, CHEK1, RAD51, and FANCA, can contribute. For these reasons, “BRCAness” is under evaluation as a biomarker for DNA-damaging agents and PARP inhibitors[8].
Finally, we believe that in a real-life focusing-approach, a particular focus should be deserved to the assessment of the impact of comorbidities on the PDAC patients’ prognosis. In a retrospective analysis that we conducted on 164 advanced PDAC patients, we demonstrated a correlation between type II diabetes mellitus and improved OS, both in the exploratory and in the validation cohort at univariate analysis (16 vs 10 mo; P = 0.004 and 11 vs 6 mo; P = 0.01, respectively). Moreover, in multivariate analysis, insulin-treated patients compared with non-diabetic patients had a significantly increased survival of 4.6 mo (P = 0.03). Surely, the correlation between OS and insulin-treated type II diabetes mellitus should be confirmed in prospective clinical trials[9].
In conclusion, in the era of precision medicine, larger and prospective studies in the real-world population, with the focus on specific PDAC subtypes (e.g., BRCA-mutant), would further clarify the impact of available and innovative treatment strategies on PDAC patients and help identify potential biomarkers to improve patients’ selection and prognosis.
We acknowledge all the authors whose publications are referred in our article.
| 1. | Blomstrand H, Batra A, Cheung WY, Elander NO. Real-world evidence on first- and second-line palliative chemotherapy in advanced pancreatic cancer. World J Clin Oncol. 2021;12:787-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Wang-Gillam A, Hubner RA, Siveke JT, Von Hoff DD, Belanger B, de Jong FA, Mirakhur B, Chen LT. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur J Cancer. 2019;108:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 3. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 879] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 4. | Pellino A, Manai C, Merz V, Scartozzi M, Milella M, De Vita F, Antonuzzo L, Zichi C, Satolli MA, Panebianco M, Noventa S, Giordano G, Nappo F, Zecchetto C, Puzzoni M, Vaccaro V, Pappalardo A, Giommoni E, Melisi D, Lonardi S. Observational retrospective evaluation of treatment with liposomal irinotecan plus fluorouracil/leucovorin for metastatic pancreatic cancer patients: An Italian large real-world analysis. J Clin Oncol. 2020;38:660-660. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Lai E, Puzzoni M, Ziranu P, Pretta A, Impera V, Mariani S, Liscia N, Soro P, Musio F, Persano M, Donisi C, Tolu S, Balconi F, Pireddu A, Demurtas L, Pusceddu V, Camera S, Sclafani F, Scartozzi M. New therapeutic targets in pancreatic cancer. Cancer Treat Rev. 2019;81:101926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Pretta A, Lai E, Persano M, Donisi C, Pinna G, Cimbro E, Parrino A, Spanu D, Mariani S, Liscia N, Dubois M, Migliari M, Impera V, Saba G, Pusceddu V, Puzzoni M, Ziranu P, Scartozzi M. Uncovering key targets of success for immunotherapy in pancreatic cancer. Expert Opin Ther Targets. 2021;25:987-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1759] [Article Influence: 251.3] [Reference Citation Analysis (0)] |
| 8. | Lai E, Ziranu P, Spanu D, Dubois M, Pretta A, Tolu S, Camera S, Liscia N, Mariani S, Persano M, Migliari M, Donisi C, Demurtas L, Pusceddu V, Puzzoni M, Scartozzi M. BRCA-mutant pancreatic ductal adenocarcinoma. Br J Cancer. 2021;125:1321-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Pretta A, Ziranu P, Puzzoni M, Lai E, Orsi G, Liscia N, Molinaro E, Mariani S, Riggi L, Rovesti G, Dubois M, Migliari M, Persano M, Saba G, Impera V, Musio F, Batzella E, Demurtas L, Pusceddu V, Astara G, Faloppi L, Casadei Gardini A, Andrikou K, Cascinu S, Scartozzi M. Retrospective survival analysis in patients with metastatic pancreatic ductal adenocarcinoma with insulin-treated type 2 diabetes mellitus. Tumori. 2021;107:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Valente R, Sweden; Yang ZG, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ