Published online Apr 24, 2022. doi: 10.5306/wjco.v13.i4.287
Peer-review started: November 15, 2021
First decision: February 8, 2022
Revised: February 22, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: April 24, 2022
Processing time: 158 Days and 0.6 Hours
The prognostic value of preoperative fluorine-18-fluorodeoxyglucose positron-emission tomography (18F-FDG PET) scan for determining overall survival (OS) in breast cancer (BC) patients is controversial.
To evaluate the OS predictive value of preoperative PET positivity after 15 years.
We performed a retrospective search of the Universitair Ziekenhuis Brussel patient database for nonmetastatic patients who underwent preoperative PET between 2002-2008. PET positivity was determined by anatomical region of interest (AROI) findings for breast and axillary, sternal, and distant sites. The prognostic role of PET was examined as a qualitative binary factor (positive vs negative status) and as a continuous variable [maximum standard uptake value (SUVmax)] in multivariate survival analyses using Cox proportional hazards models. Among the 104 identified patients who received PET, 36 were further analyzed for the SUVmax in the AROI.
Poor OS within the 15-year study period was predicted by PET-positive status for axillary (P = 0.033), sternal (P = 0.033), and combined PET-axillary/sternal (P = 0.008) nodes. Poor disease-free survival was associated with PET-positive axillary status (P = 0.040) and combined axillary/sternal status (P = 0.023). Cox models confirmed the long-term prognostic value of combined PET-axillary/sternal status [hazard ratio (HR): 3.08, 95% confidence interval: 1.42-6.69]. SUVmax of ipsilateral breast and axilla as continuous covariates were significant predictors of long-term OS with HRs of 1.25 (P = 0.048) and 1.54 (P = 0.029), corresponding to relative increase in the risk of death of 25% and 54% per SUVmax unit, respectively. In addition, the ratio of the ipsilateral axillary SUVmax over the contralateral axillary SUVmax was the most significant OS predictor (P = 0.027), with 1.94 HR, indicating a two-fold relative increase of mortality risk.
Preoperative PET is valuable for prediction of long-term survival. Ipsilateral axillary SUVmax ratio over the uninvolved side represents a new prognostic finding that warrants further investigation.
Core Tip: In our study population of nonmetastatic breast cancer patients, preoperative fluorine-18-fluorodeoxyglucose positron-emission tomography (18F-FDG PET) scan provided valuable overall survival prognostic information. This retrospective study included the longest (15-year) follow-up observation period to date in a series of these patients. Data from anatomical regions of interest and statistical analyses determined that the ipsilateral axillary maximum standard uptake value (SUVmax) with reference to the contralateral uninvolved axilla was the strongest predictor of survival.
- Citation: Perrin J, Farid K, Van Parijs H, Gorobets O, Vinh-Hung V, Nguyen NP, Djassemi N, De Ridder M, Everaert H. Is there utility for fluorine-18-fluorodeoxyglucose positron-emission tomography scan before surgery in breast cancer? A 15-year overall survival analysis. World J Clin Oncol 2022; 13(4): 287-302
- URL: https://www.wjgnet.com/2218-4333/full/v13/i4/287.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i4.287
Breast cancer (BC) is the most commonly diagnosed cancer in the female population worldwide, as well as the most common cause of cancer deaths among women[1]. For staging and disease assessment, current guidelines acknowledge the use of fluorine-18-fluorodeoxyglucose (National Library of Medicine Medical Subject Headings [MeSH] entry term: 18F-FDG) positron-emission tomography [MeSH entry term: positron-emission tomography (PET) scan] before surgery for patients with a IIB or higher stage of BC, and reservedly for patients in stage IIA[2,3]. The potential staging advantage of PET combined with computed tomography (PET/CT) over CT scan alone or even bone scintigraphy is that PET/CT can more efficiently detect lymph node invasion and distant metastases[4].
Unfortunately, the published evidence to support the use of PET/CT to predict disease prognosis has been contradictory or of insufficient quality. Some studies have indicated that the specific survival rate is significantly shorter when metastases have been detected on PET[5]. One study determined PET-positive status for axillary nodes to be the foremost preoperative prognostic factor for disease-free survival (DFS) at 5 years[6]. Additionally, a few studies have suggested that the maximum standard uptake value (SUVmax) of the primary tumor could be predictive of survival; however, the threshold values have differed among studies[7,8].
The lack of consensus on the prognostic value of PET may be due to short follow-up times in the previous studies. Additionally, in a study that compared PET with various prognostic markers, the value of PET was overshadowed by assessment of lymph nodes obtained via axillary lymph node dissection (authors' reference cited in Data Sharing Statement). However, sentinel node biopsy has taken precedence over axillary dissection, and analyzing the data without axillary dissection would be more relevant to current practice. Furthermore, the potential role for SUVmax in prognosis has not yet been fully considered, and no consideration has been given to the restricted mean survival time (RMST), although it enjoys a growing acceptance rate among clinical practitioners as a preferred survival measurement[9]. Based on all these factors, renewed analyses into the prognostic role of preoperative PET positivity on the long-term outcome of BC needs to be addressed.
Patients with primary BC who underwent a PET scan prior to their originally recommended surgery as part of the multidisciplinary team management at the Universitair Ziekenhuis Brussel (UZ Brussel; https://www.uzbrussel.be/) between 2002-2008 were identified retrospectively. Patients who met any one or more of the following criteria were excluded: noncarcinoma histology; palliative surgery; or clinically-detected metastatic disease. Follow-up data was collected for each included patient, with the last update having occurred on January 31, 2020.
This study was approved by the institution’s ethics committee. All diagnostic and therapeutic procedures had been performed in accordance with the local national guidelines and the Declaration of Helsinki 1964. All patients had received appropriate information and provided informed consent to undergo the procedures. All clinical data were described previously[6], and the steps used in data acquisition are detailed at https://dx.doi.org/10.17504/protocols.io.bf7jjrkn. The study’s collective data are available at https://doi.org/10.17632/sfvtmrd8z9.2.
Patients had fasted for + 6 h, and the PET scan had been rescheduled if hyperglycemia was detected. For all, the tracer activity was in the range of 370-536 MBq (mean: 464 ± 56 MBq). Sixty minutes after tracer perfusion, each patient had been placed in a supine position with arms extended above the head. Whole-body images had then been acquired using attenuation correction and an interleaved protocol with an LSO PET camera (ECAT Accel; Siemens, Hoffman Estates, IL, United States). The parameters of emission data, 2-dimensional mode, and reconstruction-segmentation used were identical to the procedure described elsewhere[6]. Synchronous computed tomography had not been available at the time.
We abstracted the PET image data according to the anatomical regions of interest (AROIs) and according to the source of the available image media storage.
The AROI selected for this study conformed to anatomical regions considered in the radiotherapy of the breast[10], including the whole ipsilateral breast, whole contralateral breast, ipsilateral axillary-supraclavicular lymph node region (shortened to "axillary" in the remainder of the present report), contralateral axillary-supraclavicular lymph node region, sternal-mediastinal area (internal mammary chain), and the remaining body volume (i.e. distant, outside the breasts, axillary and sternal areas).
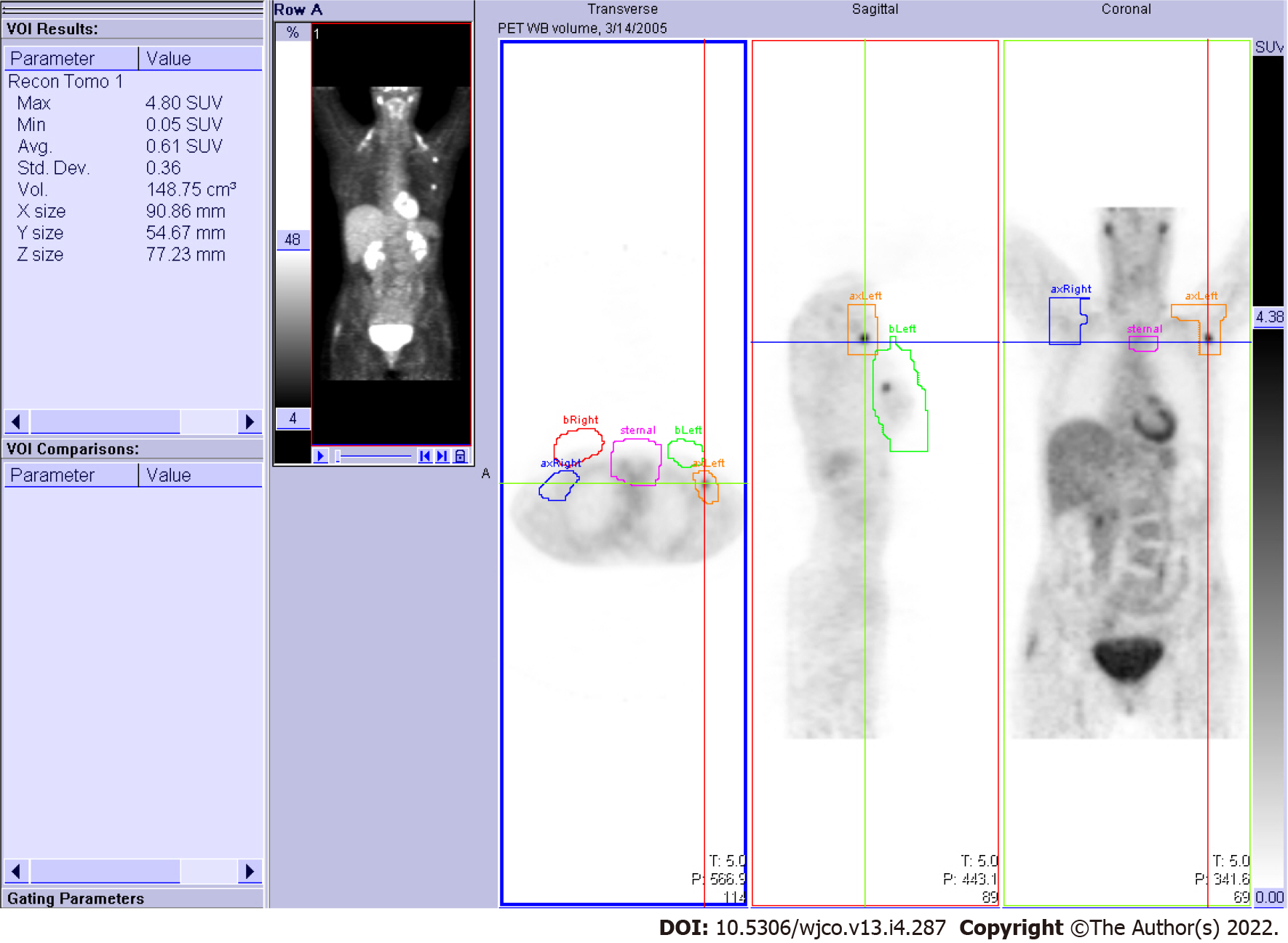
The image media storage comprised two forms: a medical FDG-PET diagnostic report with a screen image printout (for all patients) and image files archived on the PET server (for some patients). First, using the medical diagnostic report, the image in each AROI was assigned a binary score of positive or negative according to visibly increased activity or lack thereof in the AROI. Next, using the PET server workstation and accompanying free-hand volumetric drawing tool, the 3-dimensional AROIs were delineated on all available patient PET scan datasets. Ultimately, the SUVmax was abstracted for each patient from their AROIs (Figure 1).
Repeated PET scans were excluded. For bilaterally involved breasts, only the scan from the first side involved was retained, or from the side with more extensive axillary surgery if there was doubt about the diagnostic precedence.
Missing data were imputed using multivariate imputation by chained equations[11] for first histopathology finding (1 missing), HER2-neu score (1 missing), estrogen receptor (ER) and progesterone receptor (PR) status (1 missing), grade (4 missing), tumor size (1 missing), and number of examined and positive nodes (5 missing). Lymphovascular invasion was dichotomized as present vs no invasion or unknown (20 missing). HER2 fluorescence in situ hybridization (FISH) status was considered as nonimputable and was therefore excluded from the analysis (51 missing).
Survival analyses used times from the date of first pathological diagnosis to the date of last known status. The outcome event for overall survival (OS) was death from any cause. The outcome events for DFS were any local-regional or distant recurrence, secondary tumor, or death from any cause. Survival rates were computed using the Kaplan-Meier method[12]. The expectation of remaining years of life, or the RMST, up to a time horizon of 15 years was used to summarize the patients’ survival[9]. This value was calculated as the area below the survival curve. The area between survival curves measures the difference between RMSTs (∆), but the precision and statistical significance are affected by the amount of censoring. As in other tests comparing survival curves, the difference and the area between the two curves can appear large and still might be nonsignificant when there are few events. ∆ is expressed in years, in the same unit of time as the survival time, and indicates the impact of prognostic factors in terms of difference in life expectancy according to the prognostic levels.
The prognostic role of PET was examined as a qualitative binary factor (positive vs negative status) and as a continuous variable (SUVmax) in multivariate survival analyses using Cox proportional hazards models[13]. The models excluded pathological lymph node involvement, which is a strong predictor of survival that would otherwise confound and mask the significance of other factors (authors' reference cited in Data Sharing Statement). Long-term follow-up with OS and DFS at 15 years was used to validate models previously reported elsewhere[6]. The Nagelkerke index of explained variation (R2N) and the Royston-Sauerbrei index of prognostic separation (R2D) were computed to evaluate the Cox models[14].
All computations was carried out with the R statistical software, version 3.6.3[15], with the mice package for missing data imputation, and the survRM2 package for the RMSTs.
A total of 157 consecutive records were identified, out of which 53 cases were excluded for postsurgical PET performance (n = 17), breast surgery not specified in the original multidisciplinary team’s recommended management of the case (n = 12), history of previous cancer (n = 8), nonunique records (n = 5), no diagnosis of cancer (n = 2), axillary surgery not performed (n = 2), no histology data (n = 2), noncarcinoma (n = 2 sarcoma, n = 1 noninvasive tumor), and no information on the primary therapy (n = 2). Among the remaining 104 patients representing the study population, surgery had been performed at a median of 6 d after the PET imaging for the 85 (81.7%) patients who did not receive neoadjuvant therapy and at a median of 83 d after the PET imaging (range: 5-201 d, n = 1 date unknown) for the 19 (18.3%) patients who received neoadjuvant therapy. The type of breast surgery performed was lumpectomy in 26 (25.0%) patients, mastectomy in 77 (74.0%), and exclusive radiotherapy assimilated to mastectomy in 1 (1.0%). All lymph node surgeries were limited to the axilla, without exploration of the internal mammary chain. Table 1 summarizes the main characteristics of the study’s patient population.
| Characteristic | n | % |
| Sex | ||
| Male | 2 | 2.0 |
| Female | 102 | 98.0 |
| Age at diagnosis (yr) | ||
| Median (range) | 58.9 | (32.5–83.0) |
| < 40 | 8 | 7.7 |
| 40-59 | 51 | 49.0 |
| ≥ 60 | 45 | 43.3 |
| Histology | ||
| Invasive ductal carcinoma | 84 | 80.8 |
| Lobular carcinoma | 14 | 13.4 |
| Other | 6 | 5.8 |
| Tumor laterality | ||
| Bilateral | 5 | 4.8 |
| Left | 45 | 43.3 |
| Right | 54 | 51.9 |
| Tumor quadrant | ||
| Inner | 16 | 15.4 |
| Central | 14 | 13.5 |
| Outer | 64 | 61.5 |
| Other | 10 | 9.6 |
| Clinical T4 stage | 8 | 7.7 |
| Tumor size | ||
| 0-20 mm (TNM T1 classification[16]) | 37 | 35.6 |
| > 20 mm (one imputed as 30 mm) | 67 | 63.5 |
| Stage | ||
| I | 18 | 17.6 |
| IIA | 28 | 27.5 |
| IIB | 18 | 17.6 |
| III | 34 | 33.3 |
| IV | 4 | 3.9 |
| Unknown | 2 | – |
| Grade | ||
| 1 | 29 | 29.0 |
| 2 | 42 | 42.0 |
| 3 | 29 | 29.0 |
| Unknown | 4 | – |
| Hormone receptor status | ||
| ER+/PR+ | 67 | 64.4 |
| ER–/PR– | 20 | 19.2 |
| Other | 17 | 16.3 |
| Events | ||
| Loco-regional recurrence | 4 of 104 | 3.8 |
| Distant metastases | 31 of 104 | 29.8 |
| Death from any cause | 28 of 104 | 26.9 |
The patterns of PET observed among the total 104 patients were abstracted as binary score, and the SUV measurements of 36 patients for whom imaging could be retrieved are listed in Table 2 and Table 3, respectively. PET status of axillary, combined PET axillary/sternal, and combined PET all-sites were significantly related to tumor size (Table 2). SUVs were measured on the AROIs regardless of visual enhancement, making them available in all breast, axillary, and sternal sites. Ipsilateral local-regional uptake also correlated with tumor size (Table 3). For distant sites, the SUV measurements were carried out on visually hypermetabolic areas only, and therefore, the information was limited to 8 non-analyzable cases.
| Anatomical region of interest | Tumor ≤ 20 mm | Tumor > 20 mm | P |
| n = 37 | n = 67 | ||
| Breast ipsilateral | < 0.001 | ||
| PET negative | 12 (32.4) | 5 (7.5) | |
| PET positive | 25 (67.6) | 62 (92.5) | |
| Axillary ipsilateral | 0.006 | ||
| PET negative | 29 (78.4) | 34 (50.7) | |
| PET positive | 8 (21.6) | 33 (49.3) | |
| Sternal | 0.906 | ||
| PET negative | 35 (94.6) | 63 (94.0) | |
| PET positive | 2 (5.4) | 4 (6.0) | |
| Distant | 0.447 | ||
| PET negative | 32 (86.5) | 54 (80.6) | |
| PET positive | 5 (13.5) | 13 (19.4) | |
| Any of axillary or sternal | 0.019 | ||
| PET negative | 27 (73.0) | 33 (49.3) | |
| PET positive | 10 (27.0) | 34 (50.7) | |
| Any of axillary, sternal, or distant | 0.005 | ||
| PET negative | 26 (70.3) | 28 (41.8) | |
| PET positive | 11 (29.7) | 39 (58.2) |
| Anatomical region of interest | Tumor ≤ 20 mm | Tumor > 20 mm | P |
| n = 18 | n = 18 | ||
| Breast ipsilateral | |||
| SUVmax, mean (SD) | 2.3 (1.4) | 4.4 (3.1) | 0.012 |
| Breast contralateral | |||
| SUVmax, mean (SD) | 1.7 (1.3) | 1.7 (0.6) | 0.918 |
| Axillary ipsilateral | |||
| SUVmax, mean (SD) | 1.8 (0.9) | 2.8 (1.8) | 0.063 |
| Axillary contralateral | |||
| SUVmax, mean (SD) | 1.5 (0.5) | 1.8 (0.5) | 0.140 |
| Sternal | |||
| SUVmax, mean (SD) | 1.8 (0.5) | 2.1 (0.6) | 0.061 |
| All breast, axillary and sternal regions combined | |||
| SUVmax, mean (SD) | 2.7 (1.5) | 4.8 (3.0) | 0.014 |
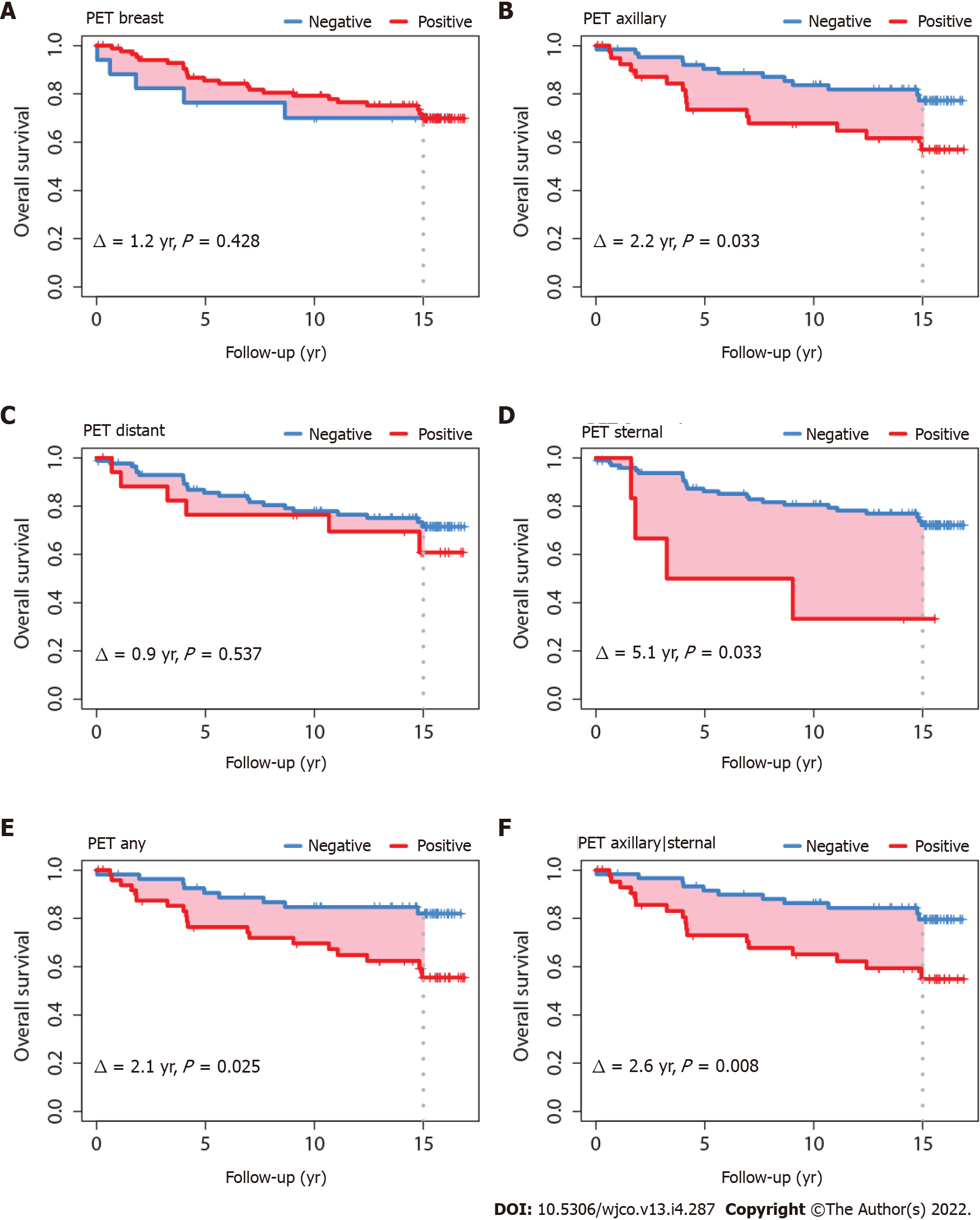
Surviving patients (no OS event) had a median follow-up of 15.1 years. The OS was significantly worse for those who had a PET-positive axillary nodal status (∆ = 2.2 years, P = 0.033); thus, at a time horizon of 15 years, patients with a PET-positive axillary status had a life expectancy that was 2.2 years shorter than patients with a PET-negative axillary status. Patients with a PET-positive sternal nodal status also had a shorter life expectancy, with ∆ of 5.1 years (P = 0.033) (Figure 2). When patients were considered as a group defined by PET-positive axillary status and/or sternal status but without distant positivity (i.e., regional positivity without distant positivity, regardless of breast local status), life expectancy was decreased, with ∆ of 2.6 years (P = 0.008). When patients were considered as a group defined by any PET-positive status for axillary, sternal or distant (i.e. any regional or distant positivity, regardless of breast local status), life expectancy was also decreased, with ∆ of 2.1 years (P = 0.025). Survival differences were not evident when comparing the PET-positive groups for breast or distant separately (Figure 2). Survival differences were also not evident on subgroup analyses for PET-positive axillary status for tumors ≤ 20 mm (TNM T1 classification[16]). For tumors > 20 mm, survival differences were evident when axillary and/or sternal status were PET-positive (i.e., regional positivity), with ∆ of 3.0 years (P = 0.015).
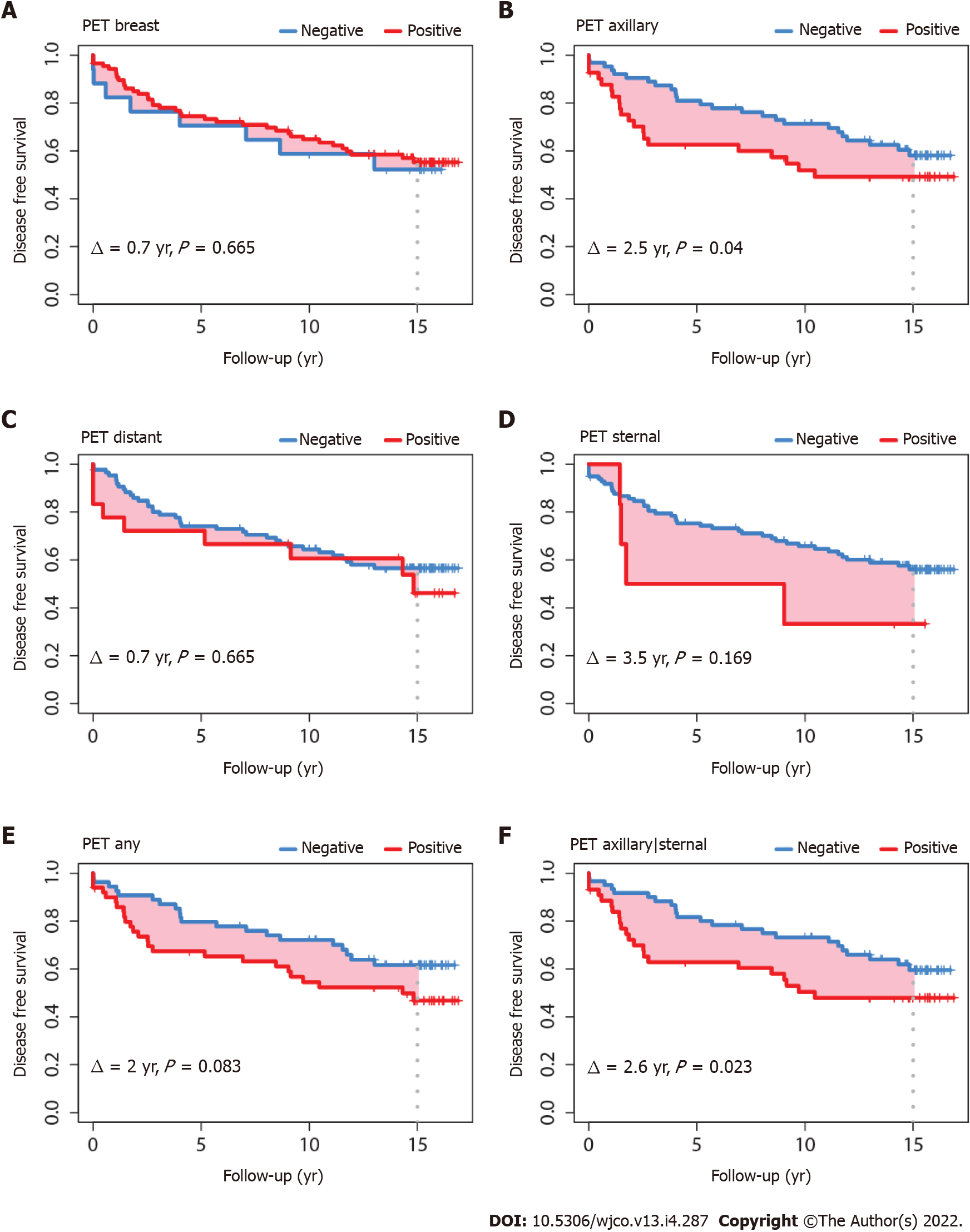
DFS was also worse for patients with a PET-positive axillary nodal status (∆ = 2.5 years, P = 0.040) and with a PET-positive status in any of the axillary and sternal regions (∆ = 2.6 years, P = 0.023) (Figure 3). DFS was not statistically different for patients with tumors ≤ 20 mm; however, in patients with tumors > 20 mm, DFS was significantly worse for those with PET-positive axillary status (∆ = 3.4 years, P = 0.017), sternal status (∆ = 5.9 years, P = 0.048), and combined axillary and sternal status (∆ = 3.9 years, P = 0.005). The pattern of the DFS survival curves according to tumor size ≤ 20 mm or > 20 mm (data not shown) were comparable to the global DFS’s shown in Figure 3.
Multivariate OS and DFS analyses used PET axillary|sternal status, age at diagnosis, and adjuvant hormone therapy covariates, which were the same parameters used previously in our short-term follow-up study[6]. In the earlier study, OS was inconclusive and was not reported, and DFS at 5 years of follow-up indicated significance for PET-positive axillary status only[6]. Table 4 shows a comparison of the previous short-term follow-up DFS hazard ratios (HRs) with the current OS and DFS HRs for the longer follow-up period. The DFS HRs for age and adjuvant hormone therapy at 15 years of follow-up were 1.03 and 0.51, respectively, comparable to the previous DFS HRs of 1.05 and 0.43, respectively, at 5 years of follow-up. That is, despite patients becoming older, from diagnosis at 58.9-years-old (Table 1) to near 75-years-old in the present study, age and adjuvant hormone therapy retained their prognostic value. Of note, however, the DFS HR for PET was 1.74 at 15 years of follow-up, as compared with 2.81 at 5 years of follow-up, suggesting some loss of prognostic value regarding DFS. In contrast, regarding the OS, the R2N of 0.077 and R2D of 0.159 for PET positivity at 15 years of follow-up (Table 4) indicate that the PET covariate provided prognostic information almost half that of the R2N of 0.189 and R2D of 0.396 from a full model that was computed by taking into account all three covariates together.
| Disease-free survival 5 yr of follow-up[6] | Disease-free survival 15 yr of follow-up, present study | Overall survival 15 yr of follow-up, present study | ||||||
| Variable | HR (95%CI) | R2N | HR (95%CI) | R2N | R2D | HR (95%CI) | R2N | R2D |
| PET axillary/sternal (positive vs negative) | 2.81 (1.17, 6.74) | 0.059 | 1.74 (0.96, 3.14) | 0.030 | 0.053 | 3.08 (1.42, 6.69) | 0.077 | 0.159 |
| Age at diagnosis (yr) | 1.05 (1.01, 1.09) | 0.046 | 1.03 (1.00, 1.06) | 0.047 | 0.075 | 1.06 (1.02, 1.10) | 0.109 | 0.227 |
| Adjuvant hormone therapy (yes vs no) | 0.43 (0.16, 1.13) | 0.030 | 0.51 (0.24, 1.08) | 0.025 | 0.049 | 0.46 (0.18, 1.18) | 0.020 | 0.083 |
| Full model | NA | 0.091 | 0.142 | 0.189 | 0.396 | |||
The impact of SUVmax on OS is shown in Table 5. The regions of interest were the tumor and ipsilateral breast, the ipsilateral axillary lymph node region, and the internal mammary chain (sternal). In addition to the absolute SUVmax, the ratios of ipsilateral breast SUVmax relative to the contralateral breast and ipsilateral axillary relative to the contralateral axillary SUVmax were computed.
| Region of interest | HR | P |
| Sternal | 3.50 | 0.080 |
| Breast ipsilateral | 1.25 | 0.048 |
| Breast contralateral | 0.72 | 0.528 |
| Axillary ipsilateral | 1.54 | 0.029 |
| Axillary contralateral | 1.03 | 0.969 |
| Combined breast/axillary/sternal | 1.27 | 0.032 |
| Ratio SUVmax breast ipsilateral/ SUVmax breast contralateral | 1.34 | 0.101 |
| Ratio SUVmax axillary ipsilateral/ SUVmax axillary contralateral | 1.94 | 0.027 |
| Ratio SUVmax in any of breast/axillary/sternal/lowest of SUVmax breast or axillary contralateral | 1.50 | 0.036 |
The absolute SUVmax of the uninvolved contralateral breast and contralateral axilla did not correlate with OS (P = 0.528). Likewise, SUVmax of the contralateral axillary site also did not correlate with OS (P = 0.969). In contrast, the SUVmax of the involved breast and the ipsilateral axillary were significantly associated with OS (P = 0.048 and P = 0.029, respectively). The SUVmax on all combined local-regional sites was also significant (P = 0.032). However, the ratio of SUVmax ipsilateral axilla over SUVmax contralateral was the most significant of all factors examined (P = 0.027), with HR of 1.94, indicating a two-fold relative increased risk of death (Table 5).
PET-positive axillary nodal and sternal status were found to be the predominant preoperative prognostic factor for OS, providing a stronger prognostic perspective than previously found[6]. Although the present study is limited by its retrospective design, it represents the longest observation period published for the prognostic role of PET scan status in OS of BC patients[7].
To place the present study in context, it started 20 years ago with the first patient diagnosed and receiving preoperative PET in 2002, while the principal investigator was working at his alma mater the UZ Brussel, a university hospital in Belgium. The first case series was presented at the San Antonio Breast Cancer Symposium in 2004 (Poster 2010, Breast Cancer Res Treat 2004; 88: S90, reference omitted). Among the patients who had received adjuvant radiotherapy, 32 had previously undergone a PET scan and we found an association between the PET nodal positivity and more extensive nodal involvement. We hypothesized that the larger number of nodes retrieved in PET-positive cases suggested lymphangiogenesis factors associated with an increased tumor metabolic activity. Obviously, preoperative PET would have served as an important tool to tailor radiation treatment fields in BC, as reported by Bral et al, who showed how ignorance of PET imaging could lead to failed targeting of radiation for hypermetabolic lymph nodes (Strahlenther Onkol 2008; 184(2): 100-4, reference omitted). It was, however, impossible to implement preoperative PET scan in our daily practice at the time of treatment of our study’s cases because of a Belgium healthcare restriction on PET scan. Only 13 facilities nationwide were allowed to implement PET and our cases fell beyond the defined population restrictions; moreover, BC was not a recognized indication for PET scan[17].
Despite these precluding logistical healthcare restrictions, the need for PET did not abate and we continued to observe a trickle of patients who had received PET. The 2004 study reported only on nodal pathology but had no follow-up. By simple logic, if PET correlates with lymph node involvement, then it would also correlate with survival. The study was reconducted in 2010 without funding, by investigators devoting volunteer time, accruing the present cohort of 104 patients. That study confirmed the nodal correlation and, indeed, provided evidence for the expected impact on early DFS. The analysis was published 2 years later, in 2012[6]. Meanwhile, guidelines did not change and still considered PET inappropriate for the early assessment of BC. To address that issue, we established a quasi-prospective protocol in 2015, with the intent to increase the number of observations with a second cohort of patients. The protocol is available at https://www.isrctn.com/ISRCTN17962845 through the linked file https://www.isrctn.com/editorial/retrieveFile/b3691bca-5277-4025-bd3e-ebea2701d143/38272.
As detailed in the protocol, the number of patients needed was 162. We expected 210 cases to be retrieved in 2015 to allow for comparison of patterns of practice between the cohorts from 2002-2008 (PET without CT) and 2009-2015 (PET with CT). The analysis was intended to be completed in 2020, with the intent to give precedence to explore the innovative concept of SUV ratios (protocol, link in ISRCTN17962845). As planned and reported herein, we updated the follow-up of the present cohort on January 31, 2020. Unexpectedly, however, when we returned to the PET server workstation, the 3-dimensional irregular free-hand volumetric measurement tool (Figure 1) had been wiped out by an upgrade to the system. As such, we could not verify the consistency of our earlier AROI delineations and SUVmax measurements. Repeated delineation measurement and extending the present study to a larger cohort have had to be deferred.
The focus of this study was on prognosis, rather than diagnostic accuracy. Nevertheless, a note on the latter is warranted. Out of the 63 patients who had PET-negative axillary status (Table 2, axillary ipsilateral), 29 (imputed) had histopathologically identified involved lymph nodes, and out of the 41 PET-positive axillary patients, 5 had no histopathologically identified node involvement. These data yield a sensitivity of 55% [95% confidence interval (CI): 43%-68%] and a specificity of 87% (95%CI: 73%-96%) which indicate that PET, like other imaging modalities, cannot replace pathology to determine microscopic involvement. This is concordant with findings from a United States’ multicentric prospective study for the detection of axillary nodal metastasis; the sensitivity and the specificity of PET were 61% (95%CI: 54%-67%) and 80% (95%CI: 79%-81%) respectively[18]. A meta-analysis of 19 studies including 1729 patients evaluated the performances of PET (with or without CT) for axillary detection and found that the sensitivity was 66% (95%CI: 50%-79%) and the specificity was 96% (95%CI: 90%-99%)[19]. Several tumor characteristics are known to correlate with higher rates of false negatives, such as low tumor grade or proliferation index, lobular histology, estrogen receptor- or progesterone receptor-positive status[20,21], in contrast to higher FDG uptake being less likely to cause false negativity in tumors exhibiting high proliferation rate and enhanced microvasculature[22].
In a previous study that focused on metastatic disease, the OS for 47 out of 189 patients with stage IIB and higher classified M1 by PET in a 3-year follow-up was significatively shorter (57% vs 88%, P < 0.0001)[23]. Other authors also noted that at stage IIB and higher, survival of patients was shorter when a distant metastasis was detected on PET-CT regardless of tumor phenotype[5]. However, several studies found that positive PET status is predictive of recurrence and survival, irrespective of metastatic status, most likely because of the correlation with poor clinicopathologic factors[24]. Our study was not designed to correlate between distant metastasis and survival. Metastatic patients were excluded from the selection, hence, there were few remaining cases detected afterwards with additional distant localizations (8 in this series).
PET-positive status was found to not be significantly predictive of OS and DFS for patients with tumors ≤ 20 mm, which can be explained by the small number of patients and the lower mortality in this subgroup. This could also be explained by the spatial resolution of PET for small tumors and small axillary node, especially data obtained in 2002 during which PET performance was lower than that of sentinel lymph node biopsy[19,25]. In the subgroup of tumors ≤ 20 mm, corresponding to stage I, a multicentric study concluded that out of the 325 women with a BC, only 13 had a PET-positive status, and of those, only 3 were confirmed and 10 were deemed false positives[25]. Considering the whole series, PET status in the breast was not prognostic. The contribution of primary breast tumor size, which affects PET detection, is a long-standing debate[26] (see also Claire Verschraegen, on the effect of tumor size in breast cancer, Ann Surg 2005; 241: 309-318, reference omitted). The issue, however, is beyond the scope of this report. We can only remark that most patients in our study (74%) received mastectomy.
Compared to the previous study involving 5 years of follow-up, PET-positive axillary and combined axillary and sternal status remained significant predictors of DFS at the extended follow-up of 15 years, although to a lesser extent (Figure 3 and Table 4). A diagnostic check of the DFS model revealed a violation of the proportional hazards assumption of the Cox model. The assumption requires that the HRs between two treatment groups be independent of time[27]. The assumption fails if the survival curves cross over or overlap for a long time, or when the treatment has an early effect but the initial separation gets smaller over time[27]. This latter pattern of violation is evidenced in Figure 3, where the differences between the pairs of DFS curves show a tendency to narrow with longer follow-up, in contrast to Figure 2 where the OS curves remain proportionally distinct. OS is the gold standard; several studies have shown that DFS is not always predictive of OS[28]. Also, OS has the advantage of being unambiguously defined, in contrast to DFS, which has multiple definitions depending on the type of study and cancer involved. DFS is frequently used because it requires less observation time and fewer patients. However, with longer survival, patients advance in age. They present with increasing comorbidities or with physical function deterioration and they are no longer willing or are unable to attend oncology consultations (typically when we call to enquire, patients would report a neurological, cardiac, respiratory, or joint and mobility problem) or in case they remain fit, the follow-up consultation is often discontinued after 10 years. Severe comorbidities can mask recurrence, and over time cancer surveillance loses priority for attending physicians. Consequently, less information on recurrence is available over time, whereas information on living or dead status can be obtained through national registries and is more reliable than disease status. These reasons likely explain why prognostication is better with DFS outcome in the short-term and better with OS outcome in the long-term.
Limitations of the study include bias inherent to its retrospective design. Beyond that, the investigators were not blinded to patients’ outcomes, which could have affected the scoring of the PET images; moreover, the scoring itself depended upon the visual appreciation of screen printouts and pre-defined rules to abstract images were not established. There was also no assessment of inter-observer agreement on the scores. The patients had been treated 15 years prior, which represented both a strength and a weakness, with the latter being related to medical management and treatment changes over that time. Most of the patients had presented with advanced tumors, for which a high prevalence of lymph node involvement and increased likelihood of PET positivity could be expected. Few patients presented with T1 tumors (Table 2); no conclusion could be drawn for these smaller tumors.
Molecular subtypes are known to affect PET positivity, as already mentioned[20-22]. However, the small study size (with only 28 events for OS; Table 1) precluded extensive analyses. By the one-in-ten rule of thumb (i.e. one variable for ten events[29]), it was decided to retain only the three-variables parsimonious model of Table 4 in the multivariate analysis, as built onto the precursor study[6]. Interestingly, despite the small study size, these three variables illustrate distinct facets relevant to BC management, specifically: PET as an indicator of disease aggressivity; age as a potential surrogate of increased risk of co-morbidity; and adjuvant hormone therapy as a surrogate of tumor subtype reflecting that hormone therapy is normally given only when the breast tumor expresses hormone receptors.
The present study innovates measurement of SUVmax based on a full-anatomical region of interest. The AROI’s were defined regardless of SUV pattern, avoiding the potential arbitrary selection of small presumably pathological areas; although, without dual acquisition of CT, delineation of the anatomical boundaries was uncertain.
Surprisingly, few studies have implemented PET image analysis using AROIs. Yoo et al[30] delineated the nipple-areolar complex on the ipsilateral index breast and the contralateral normal breast. The ratio of the ipsilateral over contralateral SUVmax in the delayed image phase PET was then found to be an independent predictor of nipple-areolar involvement. Other than study of PET for nononcological cognitive symptoms and dementia, where the interest was in the whole brain and subregions[31], we are not aware of any other BC study implementing full-AROI-based PET measurements. Reflecting on PET studies in the peer-reviewed literature, the European Association of Nuclear Medicine Guidelines define volumes of interest only as relative to tumor areas, without allowance to full organs or anatomic regions[32].
The prognostic value of FDG-PET in particular has been demonstrated in numerous disease conditions[33-38]. The present study shows that the prognostic role in BC is no different than that with other cancers, serving as an indicator of increased metabolism and therefore adverse survival outcome. The SUVmax in different anatomical regions was related to the overall risk of death. There is a growing recognition that quantitative continuous SUVmax and other SUV metrics have an important prognostic role[39]. Our study adds to the evidence that SUVmax as a continuous variable improves the power of the analysis to optimize research yield, which can be particularly important when the resources and number of patients are restricted. The study also contributes a new intuitive finding, in that using the uninvolved side as a reference for SUV measurements can improve PET prognostication; the observation, however, deserves further study.
This study confirms that PET-positive axillary status in preoperative BC is a significant predictor of OS after 15 years of follow-up, and more so with breast tumors > 20 mm. In view of the long-term survival impact, the finding argues that preoperative PET should be considered as a standard in all BC cases whenever the primary tumor size exceeds 20 mm. The role of preoperative PET in tumors ≤ 20 mm is less clear and warrants further investigation.
The role of preoperative fluorine-18-fluorodeoxyglucose positron-emission tomography (18F-FDG PET) scan (referred to hereafter as FDG-PET) in early operable breast cancer (BC) is considered controversial and is even discouraged by clinical guidelines.
In dissension with guidelines, the evidence indicates that FDG-PET is a metabolic indicator of aggressive disease, warranting reconsideration of its role in the preoperative evaluation of BC.
Long-term follow-up is needed to address the importance of any marker. The study evaluates the very long-term (15-year) prognostic role of preoperative FDG-PET.
The medical records of clinically nonmetastatic BC patients receiving preoperative FDG-PET were retrieved. Survivals were compared according to FDG-PET positive/negative status using the restricted mean survival time at a time horizon of 15 years. Multivariate analyses was performed with Cox proportional hazard models. In addition, the survival impact of absolute maximum standard uptake value (SUVmax) and ratios of SUVmax relative to the contralateral uninvolved side were evaluated.
Among 104 patients, regional FDG-PET positivity in the axillary or the sternal region was found to be a strong predictor of 15-year overall survival (P = 0.008). Patients with a positive regional PET status had an expected survival that was 2.6 years shorter than patients with negative regional PET status. Statistical significance was maintained for tumors > 20 mm, though not for tumors ≤ 20 mm. Cox models demonstrated the independent prognostic role. In addition, in a subgroup of 36 patients for whom quantitative SUV was available, representing 36 × 15 years = 540 patient-years follow-up and hence no lesser importance than a study of 189 patients but with only 3 years of follow-up, the ratio of ipsilateral axillary SUVmax vs uninvolved contralateral axillary SUVmax was the most significant among other SUV measures (P = 0.027).
This study involved the longest known follow-up of preoperative FDG-PET in early operable BC. It provides survival information heretofore unavailable. Predicting an expected survival difference of 2.6 years out of a time horizon of 15 years can be a major consideration in the initial management of BC. In addition, the SUVmax ratio of ipsilateral over uninvolved side might represent a new finding that warrants investigation.
FDG-PET might have a predominant role in the workup of BC. The present research did not have sufficient power to address the role of preoperative FDG-PET in tumors ≤ 20 mm. Future studies should consider accruing patients presenting with small tumors.
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, Aremu O, Artaman A, Asgedom SW, Assadi R, Atey TM, Avila-Burgos L, Awasthi A, Ba Saleem HO, Barac A, Bennett JR, Bensenor IM, Bhakta N, Brenner H, Cahuana-Hurtado L, Castañeda-Orjuela CA, Catalá-López F, Choi JJ, Christopher DJ, Chung SC, Curado MP, Dandona L, Dandona R, das Neves J, Dey S, Dharmaratne SD, Doku DT, Driscoll TR, Dubey M, Ebrahimi H, Edessa D, El-Khatib Z, Endries AY, Fischer F, Force LM, Foreman KJ, Gebrehiwot SW, Gopalani SV, Grosso G, Gupta R, Gyawali B, Hamadeh RR, Hamidi S, Harvey J, Hassen HY, Hay RJ, Hay SI, Heibati B, Hiluf MK, Horita N, Hosgood HD, Ilesanmi OS, Innos K, Islami F, Jakovljevic MB, Johnson SC, Jonas JB, Kasaeian A, Kassa TD, Khader YS, Khan EA, Khan G, Khang YH, Khosravi MH, Khubchandani J, Kopec JA, Kumar GA, Kutz M, Lad DP, Lafranconi A, Lan Q, Legesse Y, Leigh J, Linn S, Lunevicius R, Majeed A, Malekzadeh R, Malta DC, Mantovani LG, McMahon BJ, Meier T, Melaku YA, Melku M, Memiah P, Mendoza W, Meretoja TJ, Mezgebe HB, Miller TR, Mohammed S, Mokdad AH, Moosazadeh M, Moraga P, Mousavi SM, Nangia V, Nguyen CT, Nong VM, Ogbo FA, Olagunju AT, Pa M, Park EK, Patel T, Pereira DM, Pishgar F, Postma MJ, Pourmalek F, Qorbani M, Rafay A, Rawaf S, Rawaf DL, Roshandel G, Safiri S, Salimzadeh H, Sanabria JR, Santric Milicevic MM, Sartorius B, Satpathy M, Sepanlou SG, Shackelford KA, Shaikh MA, Sharif-Alhoseini M, She J, Shin MJ, Shiue I, Shrime MG, Sinke AH, Sisay M, Sligar A, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tessema GA, Topor-Madry R, Tran TT, Tran BX, Ukwaja KN, Vlassov VV, Vollset SE, Weiderpass E, Williams HC, Yimer NB, Yonemoto N, Younis MZ, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1105] [Cited by in RCA: 1194] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 2. | Miladinova D. Molecular Imaging in Breast Cancer. Nucl Med Mol Imaging. 2019;53:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Jeong YJ, Kang DY, Yoon HJ, Son HJ. Additional value of F-18 FDG PET/CT for initial staging in breast cancer with clinically negative axillary nodes. Breast Cancer Res Treat. 2014;145:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Liu Y. Role of FDG PET-CT in evaluation of locoregional nodal disease for initial staging of breast cancer. World J Clin Oncol. 2014;5:982-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ulaner GA, Castillo R, Wills J, Gönen M, Goldman DA. 18F-FDG-PET/CT for systemic staging of patients with newly diagnosed ER-positive and HER2-positive breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Vinh-Hung V, Everaert H, Lamote J, Voordeckers M, van Parijs H, Vanhoeij M, Verfaillie G, Fontaine C, Vees H, Ratib O, Vlastos G, De Ridder M. Diagnostic and prognostic correlates of preoperative FDG PET for breast cancer. Eur J Nucl Med Mol Imaging. 2012;39:1618-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Diao W, Tian F, Jia Z. The prognostic value of SUVmax measuring on primary lesion and ALN by 18F-FDG PET or PET/CT in patients with breast cancer. Eur J Radiol. 2018;105:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (4)] |
| 8. | Wen W, Xuan D, Hu Y, Li X, Liu L, Xu D. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with breast cancer: A systematic review and meta-analysis. PLoS One. 2019;14:e0225959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 724] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 10. | Nielsen MH, Berg M, Pedersen AN, Andersen K, Glavicic V, Jakobsen EH, Jensen I, Josipovic M, Lorenzen EL, Nielsen HM, Stenbygaard L, Thomsen MS, Vallentin S, Zimmermann S, Offersen BV; Danish Breast Cancer Cooperative Group Radiotherapy Committee. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013;52:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1635] [Cited by in RCA: 1995] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 12. | Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32610] [Cited by in RCA: 31450] [Article Influence: 462.5] [Reference Citation Analysis (0)] |
| 13. | Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model New York, NY Springer-Verlag 2000. [DOI] [Full Text] |
| 14. | Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23:723-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing 2020; R version 3.6.3. |
| 16. | American Joint Committee on Cancer; Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti Iii A. AJCC Cancer Staging Manual Seventh Edition. New York, Dordrecht, Heidelberg, London: Springer, 2010. |
| 17. | Cleemput I, Dargent G, Poelmans J, Camberlin C, Van den Bruel A, Ramaekers D. [HTA Positron Emission Tomography in Belgium]. KCE Reports. Brussels: Belgian Health Care Knowledge Centre (KCE), 2005. |
| 18. | Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S-150S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2916] [Cited by in RCA: 2893] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 19. | Cooper KL, Harnan S, Meng Y, Ward SE, Fitzgerald P, Papaioannou D, Wyld L, Ingram C, Wilkinson ID, Lorenz E. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2011;37:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Groheux D, Giacchetti S, Espié M, Vercellino L, Hamy AS, Delord M, Berenger N, Toubert ME, Misset JL, Hindié E. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011;52:1526-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Humbert O, Berriolo-Riedinger A, Riedinger JM, Coudert B, Arnould L, Cochet A, Loustalot C, Fumoleau P, Brunotte F. Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol. 2012;23:2572-2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA, Molthoff CF. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 312] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Groheux D, Hindié E, Delord M, Giacchetti S, Hamy AS, de Bazelaire C, de Roquancourt A, Vercellino L, Toubert ME, Merlet P, Espié M. Prognostic impact of (18)FDG-PET-CT findings in clinical stage III and IIB breast cancer. J Natl Cancer Inst. 2012;104:1879-1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Jo I, Zeon SK, Kim SH, Kim HW, Kang SH, Kwon SY, Kim SJ. Correlation of Primary Tumor FDG Uptake with Clinicopathologic Prognostic Factors in Invasive Ductal Carcinoma of the Breast. Nucl Med Mol Imaging. 2015;49:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Pritchard KI, Julian JA, Holloway CM, McCready D, Gulenchyn KY, George R, Hodgson N, Lovrics P, Perera F, Elavathil L, O'Malley FP, Down N, Bodurtha A, Shelley W, Levine MN. Prospective study of 2-[¹⁸F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012;30:1274-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Fisher B, Slack NH, Bross ID. Cancer of the breast: size of neoplasm and prognosis. Cancer. 1969;24:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Dehbi HM, Royston P, Hackshaw A. Life expectancy difference and life expectancy ratio: two measures of treatment effects in randomised trials with non-proportional hazards. BMJ. 2017;357:j2250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Adunlin G, Cyrus JW, Dranitsaris G. Correlation between progression-free survival and overall survival in metastatic breast cancer patients receiving anthracyclines, taxanes, or targeted therapies: a trial-level meta-analysis. Breast Cancer Res Treat. 2015;154:591-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 30. | Yoo J, Kim BS, Chung J, Yoon HJ. Clinical value of delayed 18F-FDG PET/CT for predicting nipple-areolar complex involvement in breast cancer: A comparison with clinical symptoms and breast MRI. PLoS One. 2018;13:e0203649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Perani D. FDG PET and cognitive symptoms of dementia. Clin Transl Imaging. 2013;1:247-260. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ; European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2053] [Cited by in RCA: 2448] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 33. | Cerci JJ, Linardi CC, Pracchia LF, Junior JS, Trindade E, Delbeke D, Cerci RJ, Carr R, Meneghetti JC, Buccheri V. 2-[18F]-fluoro-2-desoxy-D-glucose positron emission tomography initial staging impacts on survival in Hodgkin lymphoma. World J Radiol. 2013;5:484-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Siddiqui F, Yao M. Application of fluorodeoxyglucose positron emission tomography in the management of head and neck cancers. World J Radiol. 2014;6:238-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Awan MJ, Siddiqui F, Schwartz D, Yuan J, Machtay M, Yao M. Application of positron emission tomography/computed tomography in radiation treatment planning for head and neck cancers. World J Radiol. 2015;7:382-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Detry O, Govaerts L, Deroover A, Vandermeulen M, Meurisse N, Malenga S, Bletard N, Mbendi C, Lamproye A, Honoré P, Meunier P, Delwaide J, Hustinx R. Prognostic value of (18)F-FDG PET/CT in liver transplantation for hepatocarcinoma. World J Gastroenterol. 2015;21:3049-3054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Abuodeh Y, Naghavi AO, Ahmed KA, Venkat PS, Kim Y, Kis B, Choi J, Biebel B, Sweeney J, Anaya DA, Kim R, Malafa M, Frakes JM, Hoffe SE, El-Haddad G. Prognostic value of pre-treatment F-18-FDG PET-CT in patients with hepatocellular carcinoma undergoing radioembolization. World J Gastroenterol. 2016;22:10406-10414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Lee JW, Lee MS, Chung IK, Son MW, Cho YS, Lee SM. Clinical implication of FDG uptake of bone marrow on PET/CT in gastric cancer patients with surgical resection. World J Gastroenterol. 2017;23:2385-2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Kitajima K, Miyoshi Y, Sekine T, Takei H, Ito K, Suto A, Kaida H, Ishii K, Daisaki H, Yamakado K. Harmonized pretreatment quantitative volume-based FDG-PET/CT parameters for prognosis of stage I-III breast cancer: Multicenter study. Oncotarget. 2021;12:95-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hou L, China; Lieto E, Italy; Lu H, China; Macruz CF, Brazil; Menendez-Menendez J, Spain S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ