Published online Oct 24, 2022. doi: 10.5306/wjco.v13.i10.789
Peer-review started: August 30, 2022
First decision: September 26, 2022
Revised: October 8, 2022
Accepted: October 12, 2022
Article in press: October 12, 2022
Published online: October 24, 2022
Processing time: 50 Days and 21.7 Hours
Natural orifice specimen extraction (NOSE) via the anus or vagina replaces conventional transabdominal specimen retrieval via the transabdominal route through a limited mid-line laparotomy or Pfannenstiel incision. Reducing the number of laparoscopic ports further decreases operative abdominal wall trauma. These techniques reduce the surgical wound size as well as the risk of incision-related morbidity.
To compare short-term outcomes following 3-port NOSE surgery with a matched cohort of conventional non-NOSE colorectal cancer surgery.
Patients who underwent elective 3-port laparoscopic colorectal NOSE surgery between February to October 2021 were identified. Selection criteria for NOSE surgery was adapted from the 2019 International Consensus on Natural Orifice Specimen Extraction Surgery for colorectal cancer. Patients with clinical T4 or N2 tumors on staging computed tomography were also excluded. The propensity score-matched cohort was identified amongst patients who underwent conventional laparoscopic colorectal surgery from January 2019 to December 2020. Matching was performed in the ratio of 1:4 based on age, gender, type of resec
Over the eight-month study duration, 14 consecutive cases (nine female, five male) of elective 3-port laparoscopic surgery with NOSE were performed for colorectal cancer. Median age and body mass index were 70 (range 43-82) years and 24.1 (range 20.0-31.7) kg/m2 respectively. Six patients underwent transanal NOSE and eight had transvaginal NOSE. Median operative time, intraoperative blood loss and postoperative length of stay were 208 (range 165-365) min, 30 (range 10-150) mL and 3 (range 2-6) d respectively. Two (14%) suffered minor postoperative compilations not attributable to the NOSE procedure. Median follow-up duration was 12 (range 8-15) mo. No instances of mortality, local or distant disease recurrence were recorded in this cohort. Compared to the conventional surgery cohort of 56 patients, the 3-port NOSE cohort had significantly quicker mean return of bowel function (2.6 vs 1.2 d, P < 0.001), reduced postoperative pain and patient-controlled analgesia use, and decreased length of hospital stay (6.4 vs 3.4 d, P < 0.001). There were no statistical differences in surgical duration and perioperative complication rates between the NOSE and non-NOSE cohorts.
3-port laparoscopic colorectal surgery with NOSE is a feasible technique, augmenting the minimally invasive nature of surgery and producing good outcomes. Appropriate patient selection and expertise in conventional laparoscopy are required.
Core Tip: This paper demonstrates the benefit of reduced port laparoscopic colorectal surgery with natural orifice specimen extraction compared to conventional laparoscopic colorectal surgery. This technique represents a natural progression towards scarless surgery - the holy grail of minimally invasive surgery.
- Citation: Seow-En I, Chen LR, Li YX, Zhao Y, Chen JH, Abdullah HR, Tan EKW. Outcomes after natural orifice extraction vs conventional specimen extraction surgery for colorectal cancer: A propensity score-matched analysis. World J Clin Oncol 2022; 13(10): 789-801
- URL: https://www.wjgnet.com/2218-4333/full/v13/i10/789.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i10.789
Minimal access abdominopelvic surgery has come a long way since the advent of laparoscopic colorectal surgery in the early 90 s. New technologies and platforms have been introduced, including robotic and transanal minimally invasive surgery. The primary objective remains the same - complete tumor extirpation along with the draining lymphatic tissue. Without deviating from the principles of surgical oncology, increasing experience and expertise of laparoscopic surgeons has encouraged continued surgical innovation, resulting in improved operative technique and patient outcomes.
Natural orifice specimen extraction (NOSE) is a logical progression in the evolution of minimally invasive colorectal surgery. Removal of the surgical specimen via a natural bodily orifice such as the vagina or anus replaces the need for conventional specimen extraction (CSE) via the transabdominal route through a limited mid-line laparotomy or Pfannenstiel incision. This greatly reduces the surgical wound size as well as the risk of incision-related morbidity.
The first use of NOSE in colorectal surgery was reported in 1993 by Franklin et al[1], who described laparoscopic colectomy with transanal specimen retrieval. There has been continued interest in this technique almost three decades later. Three meta-analyses comparing laparoscopic colorectal resection with NOSE vs CSE have been published in the last two years[2-4]. These studies consistently demonstrate the benefits of NOSE, in terms of overall complications, incision-related complications, intraoperative blood loss, postoperative pain, return of gastrointestinal function and length of hospital stay. However, NOSE required a longer operating time than CSE. No significant differences were observed for cancer-specific outcomes, including local and distant recurrences, 3- and 5-year disease-free survival and overall survival[2-4].
Furthermore, patients who underwent NOSE colectomy were found to have better perception of body image and cosmetic appearance compared to CSE at a median follow-up of approximately 3-years after surgery[5]. Quality of life and gastrointestinal function following NOSE were also found to be superior to a propensity score-matched cohort of CSE at 3-mo post-surgery[6]. We recently demonstrated the feasibility of NOSE following combined colectomy and liver resection[7].
Conventional laparoscopic colorectal surgery is performed using 4 or 5 ports: 1 camera port, 2 operator ports and 1 or 2 assistant ports. Reducing the number and size of the ports further decreases the operative trauma to the abdominal wall. 3-port colorectal surgery with 1 camera port and 2 operator working ports has previously been demonstrated to be feasible[8-10]. A recent study showed equivalent long-term oncologic outcomes with 3-port right hemicolectomy compared to the conventional 5-port technique; the former was also associated with significantly less operative blood loss[11].
Logically, the minimally invasive nature of surgery is augmented utilizing 3-port surgery in addition to NOSE, enhancing the overall benefit to the patient. In this study we aimed to compare the short-term outcomes following 3-port NOSE surgery with a matched cohort of conventional laparoscopic non-NOSE surgery across a variety of colorectal cancer resection types. We also discuss the in-depth technical approach to NOSE surgery.
From 1 February to 1 October 2021, all cases of elective 3-port laparoscopic colorectal surgery with NOSE for colorectal cancer were included in the study. Selection criteria for NOSE surgery was adapted from the 2019 International Consensus on Natural Orifice Specimen Extraction Surgery for colorectal cancer[12]. Colectomy for benign diagnoses were excluded from the analysis. Patients with clinical T4 or N2 tumors on staging computed tomography were also excluded. Final decision to proceed with the NOSE procedure was only made following laparoscopic assessment.
The propensity score-matched cohort was identified amongst anonymized subjects who underwent elective laparoscopic colorectal surgery with CSE for colorectal cancer from January 2019 to December 2020. Matching was performed in the ratio of 1:4 based on age, gender, type of resection, and p - tumor node metastasis staging. Statistical analysis was performed using R statistical software (version 4.1.2). Continuous variables were compared with the Mann-Whitney U test and independent t-test, while dichotomous variables with compared using chi-squared test.
Ethics approval for the study was granted by the SingHealth centralized institutional review board (reference number 2022/2114), conforming to the provisions of the Declaration of Helsinki. All patients who underwent NOSE surgery provided written informed consent for participation in the study.
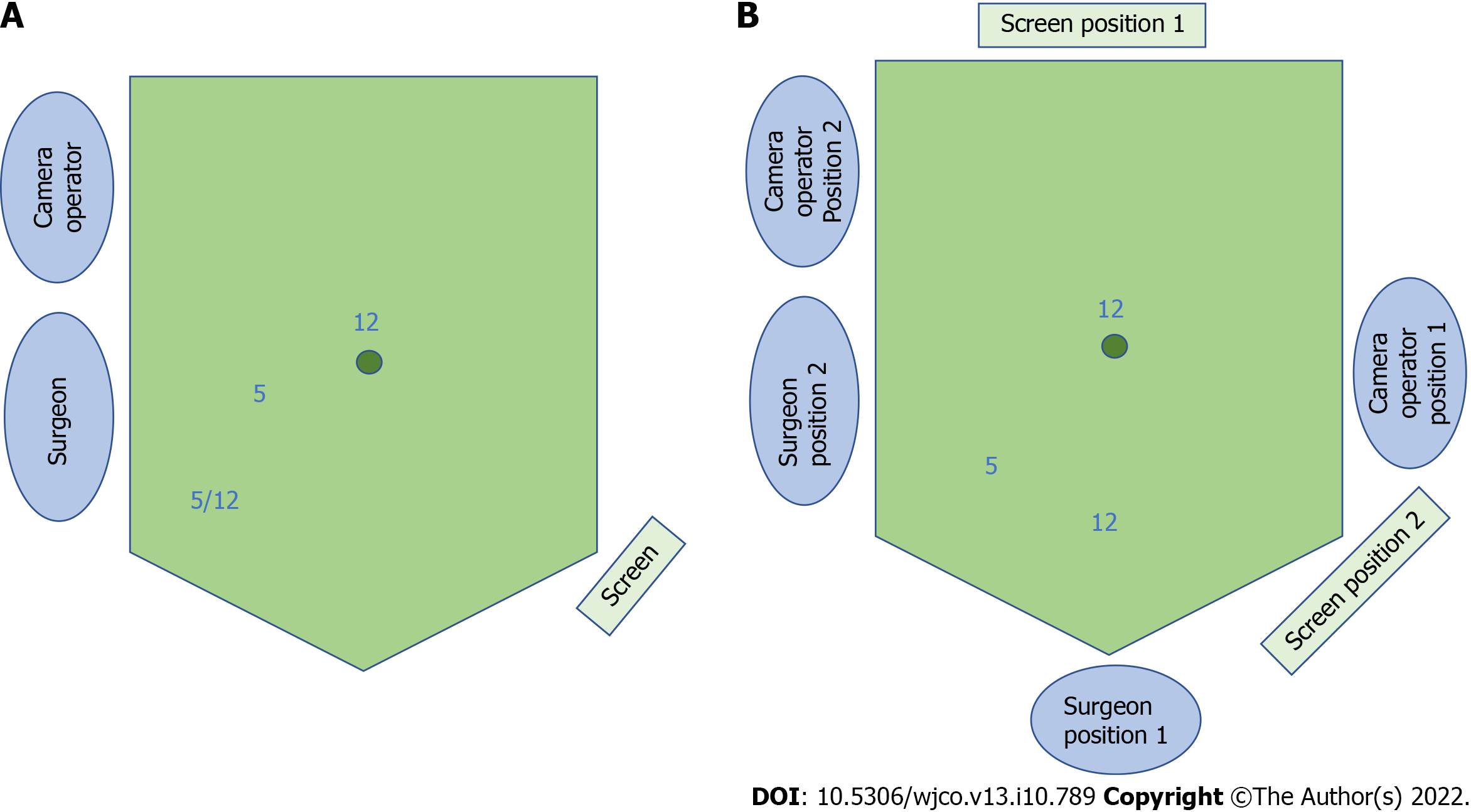
The 3-port laparoscopic NOSE technique involves 3 phases: (1) Standard laparoscopic bowel mobilization and oncologic resection; (2) Natural orifice specimen extraction; and (3) Intestinal reconstruction. We utilized the port placements and operative set-up as shown in Figure 1.
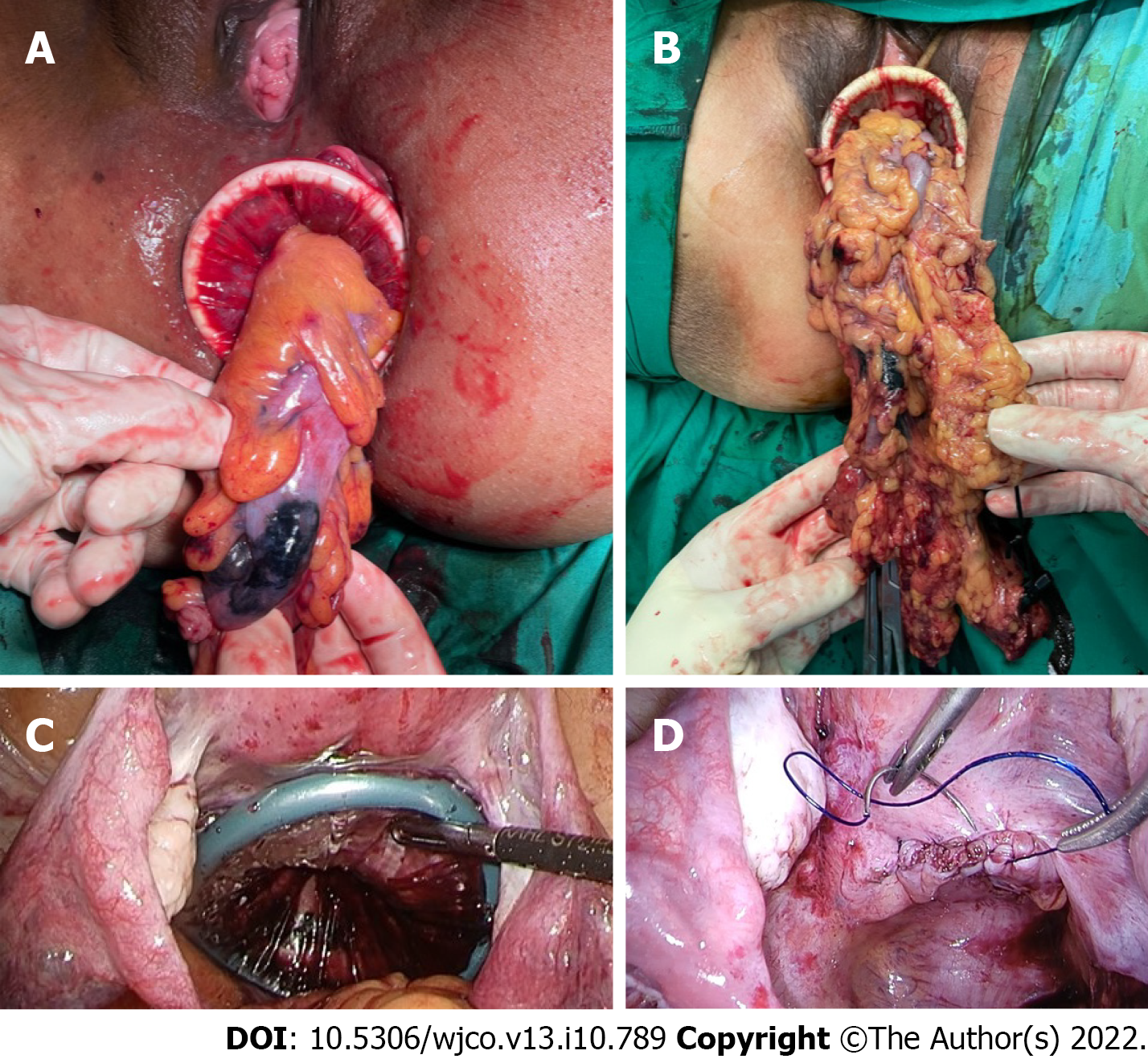
NOSE procedure: For left-sided resections, transanal NOSE was the only possible natural orifice extraction method in males, and preferable over transvaginal NOSE in females to avoid an additional vaginal incision. The transvaginal route via a posterior vaginotomy was chosen to allow retrieval of larger specimens due to the increased elasticity of the vagina[11]. For both transanal and transvaginal NOSE, the specimen was delivered through an extra small Alexis® dual-ring wound protector with the inner ring inserted fully into intraperitoneal space and the outer ring opened against the perineum to shorten the length of the channel (Figure 2). Reducing the length of the channel for extraction is of particular importance for sigmoid cancer surgery where the full length of the rectum is preserved.
For right-sided resections, only females were selected for the NOSE procedure. All specimens were thus extracted transvaginally. We recently reported our technique for 3-port laparoscopic D3 right hemicolectomy with transvaginal NOSE[13]. Transanal NOSE has been successfully performed and described following right-sided colonic surgery, in both male and female patients[14,15]. However, this approach requires an additional rectal incision and was avoided in our cohort, due to the added risk of luminal content spillage.
Care was taken to ensure surgical specimens were delivered complete (Figure 2) and did not tear or rupture during the extraction process. Following transvaginally delivery, the posterior vaginotomy was closed continuously with a barbed suture (Figure 2).
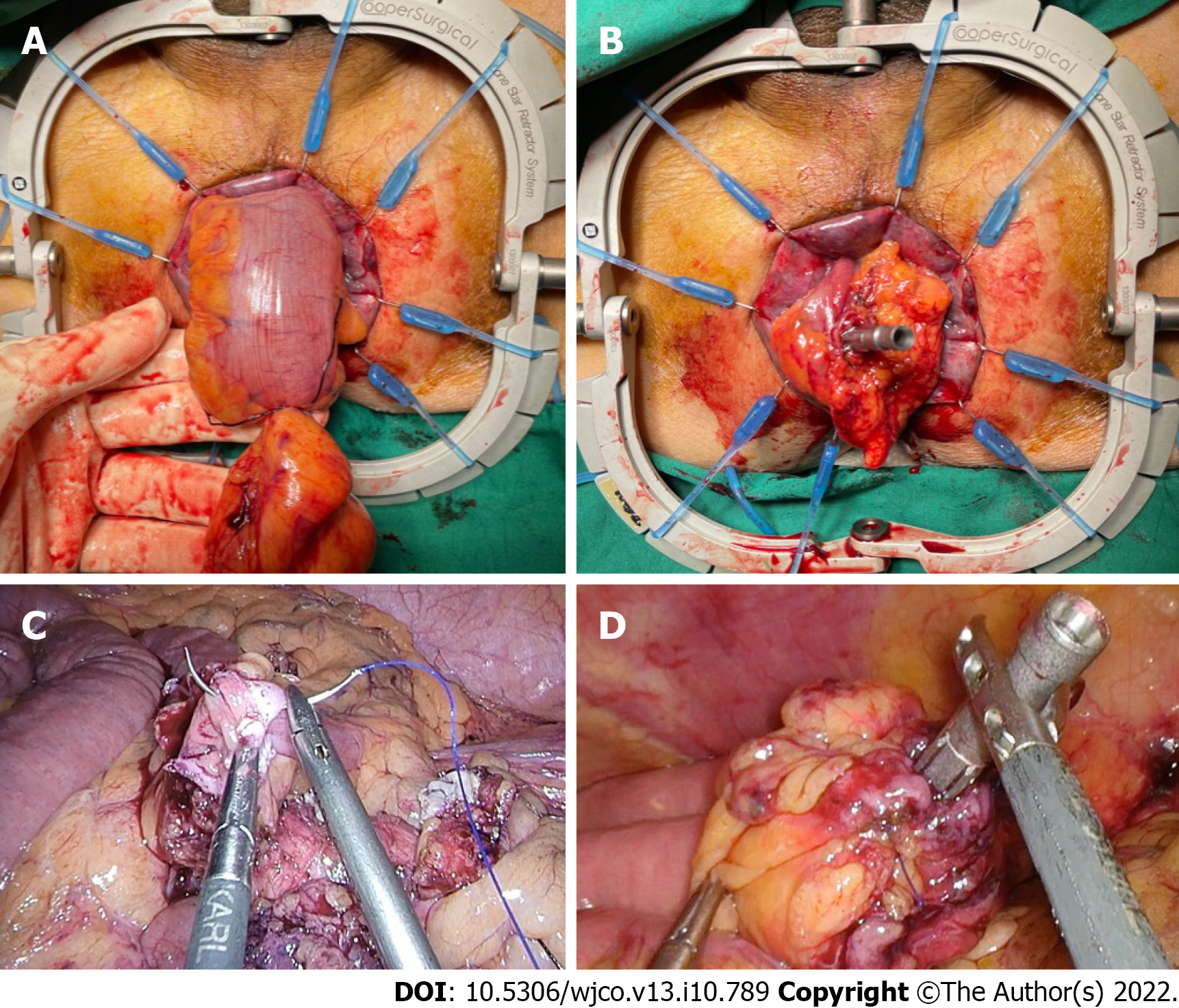
Intestinal reconstruction: Restoration of intestinal continuity following left-sided NOSE surgery requires management of the proximal and distal bowel ends prior to anastomosis, which was performed with a circular stapler.
Two methods were used to secure the anvil to the proximal bowel. The first involved transanal or transvaginal colonic pull-through to allow extracorporeal anvil application (Figure 3). This required complete mobilization of splenic flexure for length. In our cohort, medial-to-lateral splenic flexure takedown did not require additional port placement. The second technique involved securing the anvil to cut end of the proximal bowel using an intracorporeal purse-string suture (Figure 3). This approach required less colonic mobilization but had a theoretical risk of luminal content spillage in a poorly bowel-prepped patient.
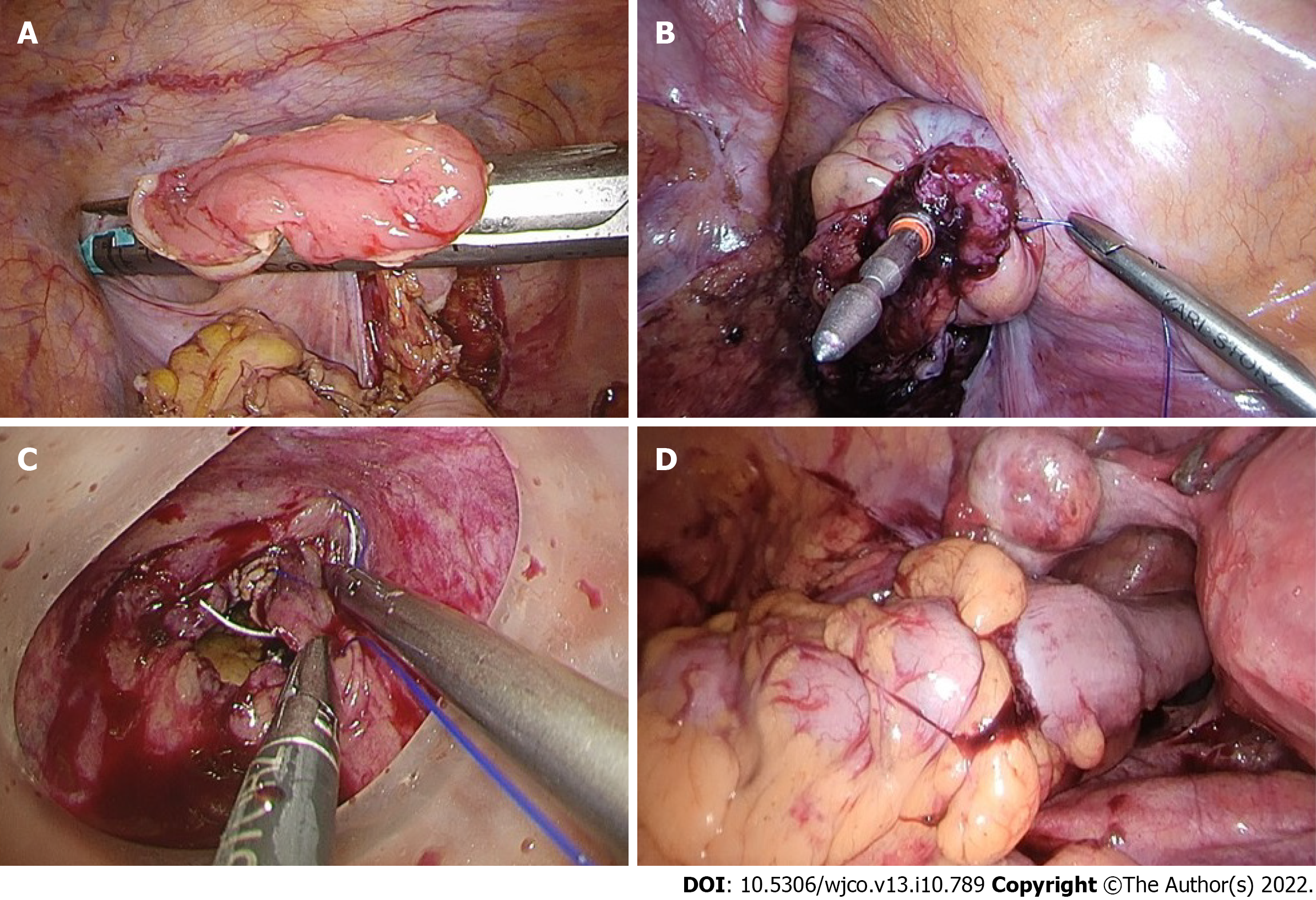
Rectal stump closure was performed using several techniques. The first was conventional distal transection with a linear stapler, where transvaginal NOSE was planned. A linear stapler was also used to seal the open rectal stump following transanal NOSE (Figure 4). Otherwise, a purse-string suture could be applied to the rectal stump and anchored to the spike of circular stapler. For high anastomoses, the purse-string could be applied laparoscopically. For low rectal anastomoses, transanal application of the purse-string was preferred, with the aid of a transanal minimally invasive surgery access device (Figure 4).
The hypothetical advantage of rectal purse-string closure is the creation of a double purse-string single-stapled anastomosis (Figure 4). This method eliminates the “dog-ears” of the anastomosis, with theoretical points of weakness at the corners of the linear staple line and the cross-stapled junctions between the linear and circular staple lines[16]. Furthermore, the double purse-string anastomosis enabled the use of a smaller 5 mm port instead of 12 mm, as a linear stapler was not required (Figure 1).
Ileocolic anastomoses following right-sided resections were performed in an antiperistaltic side-to-side fashion, with the linear stapler introduced via the 12mm suprapubic port (Figure 1). This was previously demonstrated in a video correspondence[13].
Over the eight-month study duration, 14 consecutive cases (nine female, five male) of elective 3-port laparoscopic colorectal surgery with NOSE were performed by a single surgeon. Patient and surgical characteristics of these are shown in Table 1. Six patients underwent transanal NOSE and eight had transvaginal NOSE. Median age and body mass index (BMI) were 70 (range 43-82) years and 24.1 (range 20.0-31.7) kg/m2 respectively. All patients with left-sided resections underwent pre-operative bowel preparation with 2 L polyethylene glycol. No bowel preparation was administered for right-sided resections.
| Patient | Age | ASA | Sex | BMI (kg/m2) | Surgery | Indication |
| 1 | 80 | 3 | F | 29.1 | Anterior resection, transvaginal NOSE | Sigmoid cancer pT3N1M0 |
| 2 | 59 | 1 | F | 20.0 | Left hemicolectomy, transvaginal NOSE | Splenic flexure cancer pT3N1M0 |
| 3 | 82 | 3 | F | 22.4 | Anterior resection, transvaginal NOSE | Sigmoid cancer pT3N0M0 |
| 4 | 43 | 2 | F | 31.7 | Anterior resection, transanal NOSE | Sigmoid cancer pT1N0M0 |
| 5 | 78 | 3 | F | 21.6 | Right hemicolectomy (D3), transvaginal NOSE | Transverse colon cancer pT3N1M0 |
| 6 | 63 | 2 | F | 28.0 | Right hemicolectomy (D3), transvaginal NOSE | Hepatic flexure cancer pT1N0M0 |
| 7 | 77 | 2 | M | 20.3 | Anterior resection with DI, transanal NOSE | Mid rectal cancer pT3N1M0 |
| 8 | 50 | 2 | F | 28.0 | Anterior resection, transvaginal NOSE | Sigmoid cancer pT3N0M0 |
| 9 | 77 | 2 | M | 24.3 | Anterior resection with DI, transanal NOSE | Mid rectal cancer pT3N1M0 |
| 10 | 79 | 3 | M | 24.3 | Anterior resection, transanal NOSE | Upper rectal cancer pT3N1M0 |
| 11 | 73 | 3 | M | 22.4 | Anterior resection, transanal NOSE | Sigmoid cancer pT4N2M1 |
| 12 | 58 | 2 | F | 23.5 | Anterior resection, transvaginal NOSE | Sigmoid cancer pT2N0M0 |
| 13 | 67 | 2 | M | 27.6 | Anterior resection, transanal NOSE | Sigmoid cancer pT2N1M0 |
| 14 | 58 | 2 | F | 23.8 | Left hemicolectomy, transvaginal NOSE | Splenic flexure cancer pT1N0M0 |
Operative data and postoperative outcomes are given in Table 2. Median operative time, intraoperative blood loss and postoperative length of stay were 208 (range 155-365) min, 30 (range 10-150) mL and 3 (range 2-6) d respectively. All patients recovered gastrointestinal function within the first two postoperative d, defined as passage of flatus and non-mucoid stool. All surgical margins were clear (R0) and all had more than 12 harvested lymph nodes.
| Patient | Operative time (min) | Blood loss (mL) | Time to first flatus/ BO (da) | Postoperative LOS (da) | Postoperative complications |
| 1 | 235 | 30 | 1/1 | 4 | Nil |
| 2 | 170 | 20 | 1/2 | 3 | Nil |
| 3 | 210 | 30 | 2/2 | 3 | Nil |
| 4 | 200 | 20 | 1/1 | 3 | Nil |
| 5 | 260 | 100 | 2/1 | 3 | Nil |
| 6 | 255 | 50 | 2/2 | 5 | Chylous ascites |
| 7 | 265 | 80 | 1/1 | 3 | Nil |
| 8 | 175 | 10 | 1/1 | 3 | Nil |
| 9 | 300 | 150 | 1/1 | 6 | High stoma output |
| 10 | 365 | 100 | 1/1 | 3 | Nil |
| 11 | 205 | 20 | 1/2 | 3 | Nil |
| 12 | 155 | 30 | 1/2 | 2 | Nil |
| 13 | 205 | 10 | 2/2 | 3 | Nil |
| 14 | 180 | 50 | 1/2 | 3 | Nil |
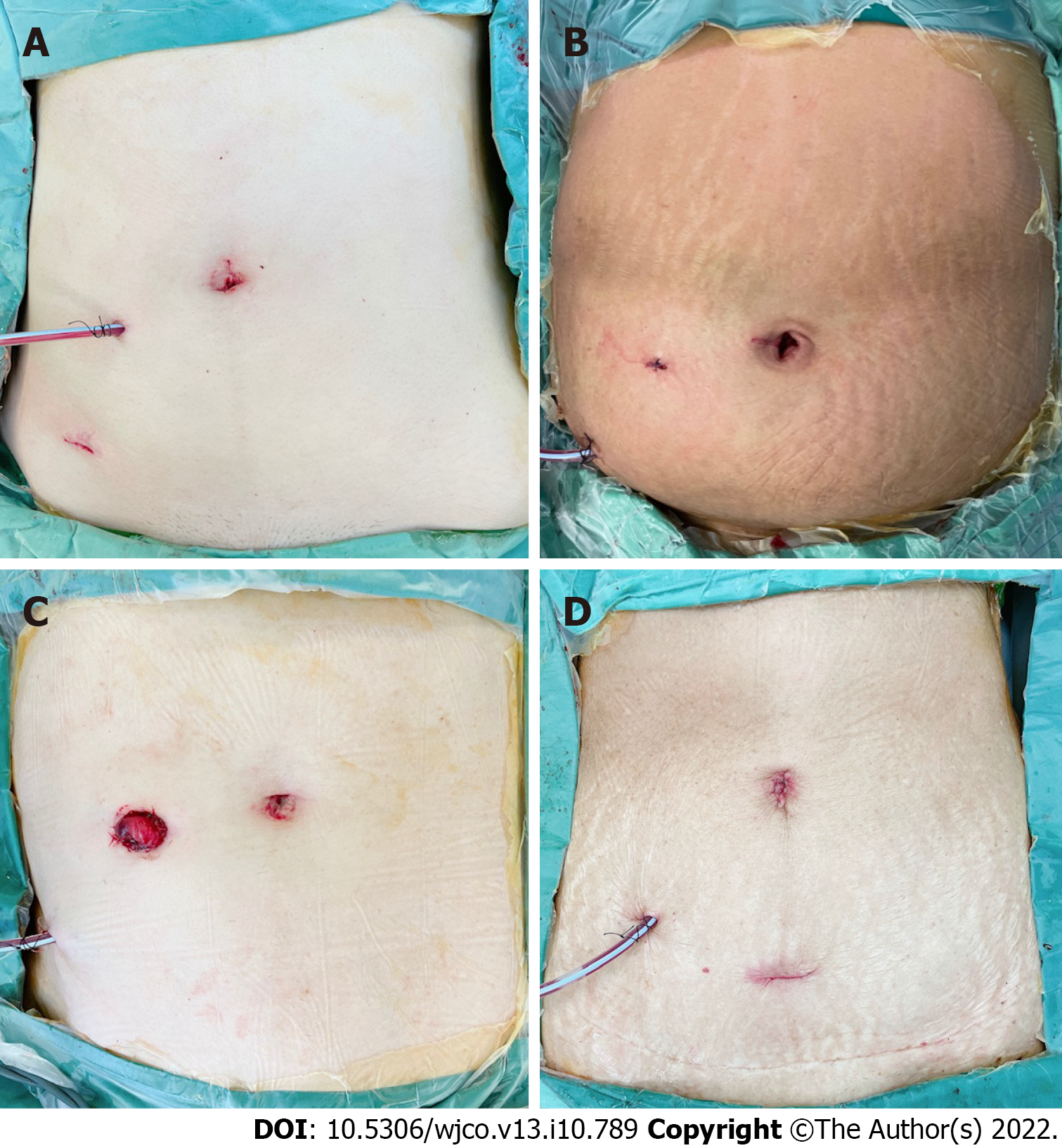
Overall complication rate was 14% (n = 2), although both were minor without requiring return to the operating theatre. One patient had low-volume chylous ascites (Clavien-Dindo grade I) and the other had high ileostomy output requiring antimotility agents (Clavien-Dindo grade II); the latter was re-admitted to hospital on postoperative day 18 for dehydration. Neither complication was attributable to the natural orifice extraction or reconstruction technique. Postoperative abdominal appearance following 3-port NOSE surgery is shown in Figure 5. Median follow-up duration was 12 (range 8-15) mo. No instances of mortality, local or distant disease recurrence were recorded.
Propensity score matching identified 56 patients from an anonymized, prospectively maintained, retrospective database, who underwent conventional laparoscopic colorectal surgery at our unit from 2019 to 2020. Comparisons of characteristics and perioperative outcomes between the NOSE and non-NOSE cohorts are shown in Table 3 and Table 4 respectively. Due to inconsistent documentation in the non-NOSE group, operative blood loss was not compared between the cohorts.
| Characteristic | NOSE, n = 14 Frequency (%) | Non-NOSE, n = 56 Frequency (%) | P value |
| Age, mean ± SD (yr) | 67.4 ± 12.4 | 73.1 ± 10.2 | 0.182 |
| Gender | |||
| Male | 5 (35.7) | 21 (37.5) | 0.765 |
| Female | 9 (64.3) | 35 (62.5) | |
| BMI, mean ± SD (kg/m2) | 24.8 ± 3.5 | 24.0 ± 4.1 | 0.526 |
| ASA score | |||
| 1 | 1 (7.1) | 1 (1.8) | 0.265 |
| 2 | 8 (57.1) | 43 (76.8) | |
| 3 | 5 (35.7) | 12 (21.4) | |
| Tumor location | |||
| Caecum to transverse colon | 2 (14.3) | 18 (32.1) | 0.219 |
| Splenic flexure to sigmoid | 9 (64.3) | 22 (39.3) | |
| Rectum | 3 (21.4) | 16 (28.6) | |
| Surgery | |||
| Anterior resection | 7 (50.0) | 23 (41.1) | 0.576 |
| Low anterior resection | 3 (21.4) | 13 (23.2) | |
| Left hemicolectomy | 2 (14.3) | 3 (5.4) | |
| Right hemicolectomy | 2 (14.3) | 17 (30.4) | |
| Defunctioning stoma creation | |||
| Yes | 3 (21.4) | 9 (16.1) | 0.634 |
| No | 11 (78.6) | 47 (83.9) | |
| AJCC pT stage | |||
| T1 | 4 (28.6) | 7 (12.5) | 0.527 |
| T2 | 2 (14.3) | 10 (17.9) | |
| T3 | 7 (50) | 33 (58.9) | |
| T4 | 1 (7.1) | 6 (10.7) | |
| AJCC pN stage | |||
| N0 | 6 (42.9) | 33 (58.9) | 0.236 |
| N1 | 7 (50.0) | 15 (26.8) | |
| N2 | 1 (7.1) | 8 (14.3) | |
| M stage | |||
| M0 | 13 (92.9) | 55 (98.2) | 0.282 |
| M1 | 1 (7.1) | 1 (1.8) | |
| Stage | |||
| I | 4 (28.6) | 13 (23.2) | 0.162 |
| II | 1 (7.1) | 20 (35.7) | |
| III | 8 (57.1) | 22 (39.3) | |
| IV | 1 (7.1) | 1 (1.8) |
| Outcome | NOSE n = 14 Frequency (%) | Non-NOSE n = 56 Frequency (%) | P value |
| Duration of surgery, mean ± SD (min) | 227 ± 55 | 261 ± 96 | 0.463 |
| Intraoperative complications | |||
| Yes | 0 (0) | 2 (3.6) | 0.473 |
| No | 14 (100) | 54 (96.4) | |
| 30-day postoperative complications | |||
| Yes | 2 (14.3) | 13 (23.2) | 0.466 |
| No | 12 (85.7) | 43 (76.8) | |
| Clavien-Dindo classification | 0.202 | ||
| 1 | 1 (7.1) | 5 (8.9) | |
| 2 | 1 (7.1) | 7 (12.5) | |
| 3 | 0 | 0 | |
| 4 | 0 | 1 (1.8) | |
| 5 | 0 | 0 | |
| Time to first bowel movement, mean ± SD (d) | 1.2 ± 0.4 | 2.6 ± 2.0 | <0.001 |
| Length of hospitalization stay, mean ± SD (d) | 3.4 ± 1.0 | 6.4 ± 5.3 | <0.001 |
| POD 1 highest pain scorea, mean ± SD | 1.5 ± 1.9 | 3.0 ± 2.0 | 0.012 |
| POD 2 highest pain scorea, mean ± SD | 0.7 ± 1.5 | 1.8 ± 2.2 | 0.066 |
| POD 1 PCA total morphine use, mean ± SD (mg) | 1.4 ± 3.3 | 6.7 ± 8.1 | 0.002 |
| POD 2 PCA total morphine use, mean ± SD (mg) | 0 | 2.5 ± 5.1 | 0.005 |
There were no statistical differences in surgical duration and perioperative complication rates between the NOSE and non-NOSE cohorts. The 3-port NOSE group had significantly quicker return of bowel function, reduced postoperative pain and analgesia use, with a mean use of zero mg of patient-controlled morphine on the second postoperative day. Notably, the average length of hospital stay was almost twice as long in the non-NOSE group compared to the NOSE group.
As recommended by the international NOSE surgery consensus, the maximum tumor dimension for transanal and transvaginal NOSE are 3 cm and 5 cm respectively[12]. While tumor size can be estimated on preoperative imaging, the decision to proceed with the NOSE procedure can often only be established intraoperatively, due radiological limitations on assessment of peritumoral desmoplastic reaction and mesocolic or mesorectal bulkiness, which may add considerably to the overall specimen diameter.
Moreover, while absolute diameter is an important consideration, the relative size of the specimen to the width of the pelvic outlet as well as the laxity of the chosen bodily orifice may be more crucial in determining the success or failure of the procedure. As illustrated by a recent series of NOSE following sigmoidectomy for volvulus, surgery for benign colorectal disease without a physical mass is ideal for NOSE[17].
BMI limits of 30 kg/m2 and 35 kg/m2 were suggested for transanal and transvaginal NOSE respectively[12]. Obese patients often possess a bulkier mesocolon or mesorectum which increases the difficulty of extraction. Nonetheless, the benefits of reduced incision may be more apparent in a patient with a thicker abdominal wall, who is at an increased risk of wound complications including infection and herniation. We previously demonstrated a successful transvaginal NOSE technique in a patient with BMI of 37 kg/m2[18]. A large retrospective Australian study also demonstrated the feasibility of NOSE in obese patients[19].
Unlike prior reports, NOSE did not significantly add to operative time in our experience, even with the removal of assistant ports[2-4]. Following our findings, routine postoperative patient-controlled opioid anaesthesia (PCA), a feature of our unit’s enhanced recovery program, was discontinued for NOSE patients in view of minimal use[20]. Furthermore, postoperative ileus was virtually eliminated in the studied cohort. This may be explained by the relative lack of extracorporeal bowel exposure, as well as quicker patient mobilization. There were also no infective complications recorded, despite known concerns regarding contamination during transanal specimen extraction[21].
While the reduction of several laparoscopic ports may ostensibly offer only minor improvement over traditional 4- or 5-port surgery, reduced-port colorectal surgery represents another incremental step towards the holy grail of scarless surgery. In the modern era of minimally invasive surgery, an accumulation of several small gains may be required to make meaningful clinical differences to patient outcomes. In our opinion, the reduced-port technique is synergistic with natural orifice specimen extraction techniques to further minimize abdominal wall trauma. Another advantage of reduced-port surgery is the removal of dependence on a surgical assistant, particularly in the setting of limited manpower resources.
Single incision laparoscopic surgery (SILS), and 2-port laparoscopic surgery (using a SILS multi-channel umbilical port and one separate working port), have been demonstrated in colorectal surgery[22-24]. While these techniques reduce the number of ports even further, a larger umbilical incision is generally required for insertion of a multi-channel access device, which offsets the decrease in overall number of ports. Considerable operative challenges can also be anticipated with a SILS access device, including clashing of the laparoscopic instruments with the endoscope, and operator discomfort due to awkward surgical posture. In our experience, the 3-port technique provides the optimal balance between minimizing abdominal trauma and allowing operator as well as cameraman comfort by enabling adequate optical and working port triangulation.
A technical learning curve exists for 3-port NOSE surgery, and the 3-port technique and natural orifice extraction each present with a separate set of challenges. The issue of lack of tissue traction by an assistant can be overcome via positional changes of the operating table. The uterus should be hitched to the anterior abdominal wall for all female patients (Figure 2), facilitating pelvic visualization during rectal mobilization or the NOSE procedure. Additional assistant ports should be used if difficulties are encountered. In event of a problematic natural orifice extraction, transabdominal specimen extraction can be performed instead of NOSE with minimal added detriment to the patient. Operators should be proficient in conventional laparoscopic colorectal surgery before attempting the 3-port NOSE technique.
Our study is limited by the small sample size in the NOSE cohort. Furthermore, the benefits shown in the 3-port NOSE group may have been largely contributed by the reduced abdominal incision, consistent with the findings from previous studies, rather than the reduced number of ports used[2-4]. Nonetheless, the feasibility and clinical applicability of the 3-port NOSE technique is still demonstrated across a range of colorectal resection types, with considerable improvements in short-term outcomes compared to conventional laparoscopy.
3-port laparoscopic colorectal surgery with NOSE is a feasible and safe technique, and together augment the minimally invasive nature of surgery producing excellent cosmesis and good outcomes. Appropriate patient selection and expertise in conventional laparoscopy are required. Larger studies are necessary to draw conclusive results.
Natural orifice specimen extraction (NOSE) via the anus or vagina replaces conventional transabdominal specimen retrieval via the transabdominal route through a limited mid-line laparotomy or Pfannenstiel incision. Reducing the number of laparoscopic ports further decreases operative abdominal wall trauma. These techniques reduce the surgical wound size as well as the risk of incision-related morbidity.
To our knowledge, the technique of 3-port colorectal cancer surgery with NOSE has never been evaluated or described in-depth.
To compare short-term outcomes following 3-port NOSE surgery with a matched cohort of conventional non-NOSE colorectal cancer surgery.
This was a retrospective cohort study of patients who underwent elective 3-port laparoscopic colorectal NOSE surgery between February to October 2021. The propensity score-matched cohort was identified amongst patients who underwent conventional laparoscopic colorectal surgery from January 2019 to December 2020. Matching was performed in the ratio of 1:4 based on age, gender, type of resection, and p - tumor node metastasis staging.
Our results showede no statistical differences in surgical duration and perioperative complication rates between the NOSE and non-NOSE cohorts. As hypothesized, the 3-port NOSE cohort had significantly quicker mean return of bowel function (2.6 vs 1.2 d, P < 0.001), reduced postoperative pain and patient-controlled analgesia use, and decreased length of hospital stay (6.4 vs 3.4 d, P < 0.001), compared to the conventional surgery cohort.
3-port laparoscopic colorectal surgery with NOSE is a feasible technique, augmenting the minimally invasive nature of surgery and producing good outcomes.
Studies with larger patient numbers are necessary to draw definitive conclusions. A defined criteria should be evaluated for more objective selection of patients who are considered for colorectal NOSE surgery.
| 1. | Franklin ME Jr, Ramos R, Rosenthal D, Schuessler W. Laparoscopic colonic procedures. World J Surg. 1993;17:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 130] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | He J, Yao HB, Wang CJ, Yang QY, Qiu JM, Chen JM, Shen Z, Yang GG. Meta-analysis of laparoscopic anterior resection with natural orifice specimen extraction (NOSE-LAR) vs abdominal incision specimen extraction (AISE-LAR) for sigmoid or rectal tumors. World J Surg Oncol. 2020;18:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Lin J, Lin S, Chen Z, Zheng B, Lin Y, Zheng Y, Liu Y, Chen SQ. Meta-analysis of natural orifice specimen extraction vs conventional laparoscopy for colorectal cancer. Langenbecks Arch Surg. 2021;406:283-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Chin YH, Decruz GM, Ng CH, Tan HQM, Lim F, Foo FJ, Tai CH, Chong CS. Colorectal resection via natural orifice specimen extraction vs conventional laparoscopic extraction: a meta-analysis with meta-regression. Tech Coloproctol. 2021;25:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 5. | Wolthuis AM, Meuleman C, Tomassetti C, D'Hooghe T, Fieuws S, de Buck van Overstraeten A, D'Hoore A. How do patients score cosmesis after laparoscopic natural orifice specimen extraction colectomy? Colorectal Dis. 2015;17:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Tang Q, Zhu Y, Xiong H, Sheng X, Hu Z, Hu H, Huang R, Zhang Q, Yuan Z, Xie L, Gao Z, Wang Y, Wang G, Wang X. Natural Orifice Specimen Extraction Surgery vs Conventional Laparoscopic-Assisted Resection in the Treatment of Colorectal Cancer: A Propensity-Score Matching Study. Cancer Manag Res. 2021;13:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Seow-En I, Koh YX, Tan EKW. Transvaginal natural orifice specimen extraction (NOSE) following laparoscopic combined D3 right hemicolectomy and liver resection. Dis Colon Rectum, 2022 [cited 20 September 2022]. Available from: https://www.researchgate.net/publication/354449205_Transvaginal_natural_orifice_specimen_extraction_surgery_NOSES_in_3D_laparoscopic_partial_or_radical_nephrectomy_a_preliminary_study. |
| 8. | Seow-En I, Tan KY, Mohd Daud MA, Seow-Choen F. Traditional laparoscopic colorectal resections can be performed effectively using a three-port technique. Tech Coloproctol. 2011;15:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Hasegawa F, Kawamura YJ, Sasaki J, Tsujinaka S, Konishi F. Oncological 3-port laparoscopic colectomy by 1 surgeon and 1 camera operator: a preliminary report. Surg Laparosc Endosc Percutan Tech. 2013;23:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Tsutsumi S, Morita H, Fujii T, Suto T, Yajima R, Takada T, Asao T, Kuwano H. Feasibility of Reduced Port Laparoscopic Colectomy for Colon Cancer. Hepatogastroenterology. 2015;62:873-875. [PubMed] |
| 11. | Zhang T, Zhang Y, Shen X, Shi Y, Ji X, Wang S, Song Z, Jing X, Ye F, Zhao R. LongTerm Outcomes of Three-Port Laparoscopic Right Hemicolectomy Versus Five-Port Laparoscopic Right Hemicolectomy: A Retrospective Study. Front Oncol. 2021;11:762716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Guan X, Liu Z, Longo A, Cai JC, Tzu-Liang Chen W, Chen LC, Chun HK, Manuel da Costa Pereira J, Efetov S, Escalante R, He QS, Hu JH, Kayaalp C, Kim SH, Khan JS, Kuo LJ, Nishimura A, Nogueira F, Okuda J, Saklani A, Shafik AA, Shen MY, Son JT, Song JM, Sun DH, Uehara K, Wang GY, Wei Y, Xiong ZG, Yao HL, Yu G, Yu SJ, Zhou HT, Lee SH, Tsarkov PV, Fu CG, Wang XS; International Alliance of NOSES. International consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep (Oxf). 2019;7:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 13. | Seow-En I, Koo CH, Chen LR, Tan EK. 3-port laparoscopic D3 right hemicolectomy with transvaginal natural orifice specimen extraction - A Video Vignette. Colorectal Dis. 2022;24:880-881. [PubMed] |
| 14. | Cheng CC, Hsu YR, Chern YJ, Tsai WS, Hung HY, Liao CK, Chiang JM, Hsieh PS, You JF. Minimally invasive right colectomy with transrectal natural orifice extraction: could this be the next step forward? Tech Coloproctol. 2020;24:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Sun P, Liu Z, Guan X, Luo S, Cai XH, Li JW, Wang XS. Laparoscopic radical right hemicolectomy with transrectal-specimen extraction: a novel natural-orifice specimen-extraction procedure. Gastroenterol Rep (Oxf). 2021;9:182-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Saurabh B, Chang SC, Ke TW, Huang YC, Kato T, Wang HM, Tzu-Liang Chen W, Fingerhut A. Natural Orifice Specimen Extraction With Single Stapling Colorectal Anastomosis for Laparoscopic Anterior Resection: Feasibility, Outcomes, and Technical Considerations. Dis Colon Rectum. 2017;60:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Seow-En I, Chang SC, Ke TW, Shen MY, Chen HC, William Tzu-Liang Chen. Uncomplicated Sigmoid Volvulus Is Ideal for Laparoscopic Sigmoidectomy With Transrectal Natural Orifice Specimen Extraction. Dis Colon Rectum. 2021;64:e90-e93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Seow-En I, Khor SN, Tan KW. Laparoscopic 3-port anterior resection with transvaginal natural orifice specimen extraction (NOSE) in a patient with a high BMI: A video vignette [published online ahead of print]. Colorectal Dis 2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Chen MZ, Cartmill J, Gilmore A. Natural orifice specimen extraction for colorectal surgery: Early adoption in a Western population. Colorectal Dis 2021; 23: 937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Seow-En I, Wu J, Yang LWY, Tan JSQ, Seah AWH, Foo FJ, Chang M, Tang CL, Tan EKW. Results of a colorectal enhanced recovery after surgery (ERAS) programme and a qualitative analysis of healthcare workers' perspectives. Asian J Surg 2021; 44: 307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Ngu J, Wong AS. Transanal natural orifice specimen extraction in colorectal surgery: bacteriological and oncological concerns. ANZ J Surg. 2016;86:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Gu C, Wu Q, Zhang X, Wei M, Wang Z. Single-incision vs conventional multiport laparoscopic surgery for colorectal cancer: a meta-analysis of randomized controlled trials and propensity-score matched studies. Int J Colorectal Dis. 2021;36:1407-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Kim CW, Cho MS, Baek SJ, Hur H, Min BS, Kang J, Baik SH, Lee KY, Kim NK. Oncologic outcomes of single-incision vs conventional laparoscopic anterior resection for sigmoid colon cancer: a propensity-score matching analysis. Ann Surg Oncol. 2015;22:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Kang JH, Lee SY, Kim CH, Kim HR, Kwak HD, Ju JK, Kim YJ. Comparison of the short-term outcomes of reduced-port laparoscopic surgery and conventional multiport surgery in colon cancer: a propensity score matching analysis. Ann Surg Treat Res. 2018;94:147-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Colorectal Surgeons (Singapore).
Specialty type: Oncology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen WTL, Taiwan; Frascio M, Italy; Yotsov T, Bulgaria S-Editor: Wang LL L-Editor: A P-Editor: Wang LL