Published online Jan 24, 2022. doi: 10.5306/wjco.v13.i1.9
Peer-review started: April 12, 2021
First decision: August 18, 2021
Revised: August 31, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: January 24, 2022
Processing time: 288 Days and 15.1 Hours
The cumulative evidence over the past decades has shown that the incidence of differentiated thyroid carcinoma (DTC) has exponentially increased. Approximately 10% of patients with DTC exhibit recurrent or metastatic disease, and about two-thirds of the latter will be defined as refractory to radioactive iodine (RAIR) treatment. Since this condition implies 10-year survival rates less than 10% after detection, using available treatments, such as systemic and targeted therapies, have become increasingly relevant. The initiation of these treatments aims to reach stabilization, tumor volume reduction, and/or symptom impro
Core Tip: The incidence of differentiated thyroid carcinoma has increased due to the rising detection of low-risk small carcinomas. Nevertheless, approximately 10% of patients exhibit advanced disease and two-thirds of the latter will be defined as radioactive iodine (RAI) refractory. After detection, 10-year survival rates are less than 10%, therefore the role of systemic and targeted therapy in these patients has become increasingly relevant in recent years. This review article aims to provide a summary of the current therapeutic strategies in iodine-refractory thyroid cancer, including approved target therapies as well as those for off-label use, RAI resensitization agents, and immunotherapy.
- Citation: Pitoia F, Jerkovich F, Trimboli P, Smulever A. New approaches for patients with advanced radioiodine-refractory thyroid cancer. World J Clin Oncol 2022; 13(1): 9-27
- URL: https://www.wjgnet.com/2218-4333/full/v13/i1/9.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i1.9
The cumulative evidence over the past decades has shown that the incidence of differentiated thyroid carcinoma (DTC) has increased exponentially, probably due to the rising detection of low-risk small carcinomas[1]. Nevertheless, approximately 10% of patients with DTC exhibit a more aggressive behavior in which persistent or recurrent distant metastatic disease is developed, and about two-thirds of them will be defined as refractory to radioactive iodine (RAI) treatment[2]. This condition cannot be defined by a single criterion, but it rather comprises a spectrum of scenarios included into any of the following: (1) Lack of initial RAI uptake in all or some of the metastatic foci in a whole-body scan (diagnostic or following a therapeutic dose) or lose of the ability to take up RAI after previous evidence of uptake; (2) Disease progression in a patient who has received RAI; (3) Disease progression in a patient who has received 600 mCi of 131I of cumulative activity; and/or (4) Locally advanced disease for whom surgical resection is not feasible and RAI uptake status cannot be assessed[2]. After the detection of radioiodine refractory (RAIR) disease, 10-year survival rates may decrease to less than 10%[2]. Therefore, using second-choice treatments, such as systemic and targeted therapy, in these patients has become increasingly relevant in recent years. This review article aims to provide a summary of the current therapeutic strategies for patients with RAIR thyroid cancer, including approved target therapies as well as those prescribed for off-label use, RAI resensitization agents, and immunotherapy.
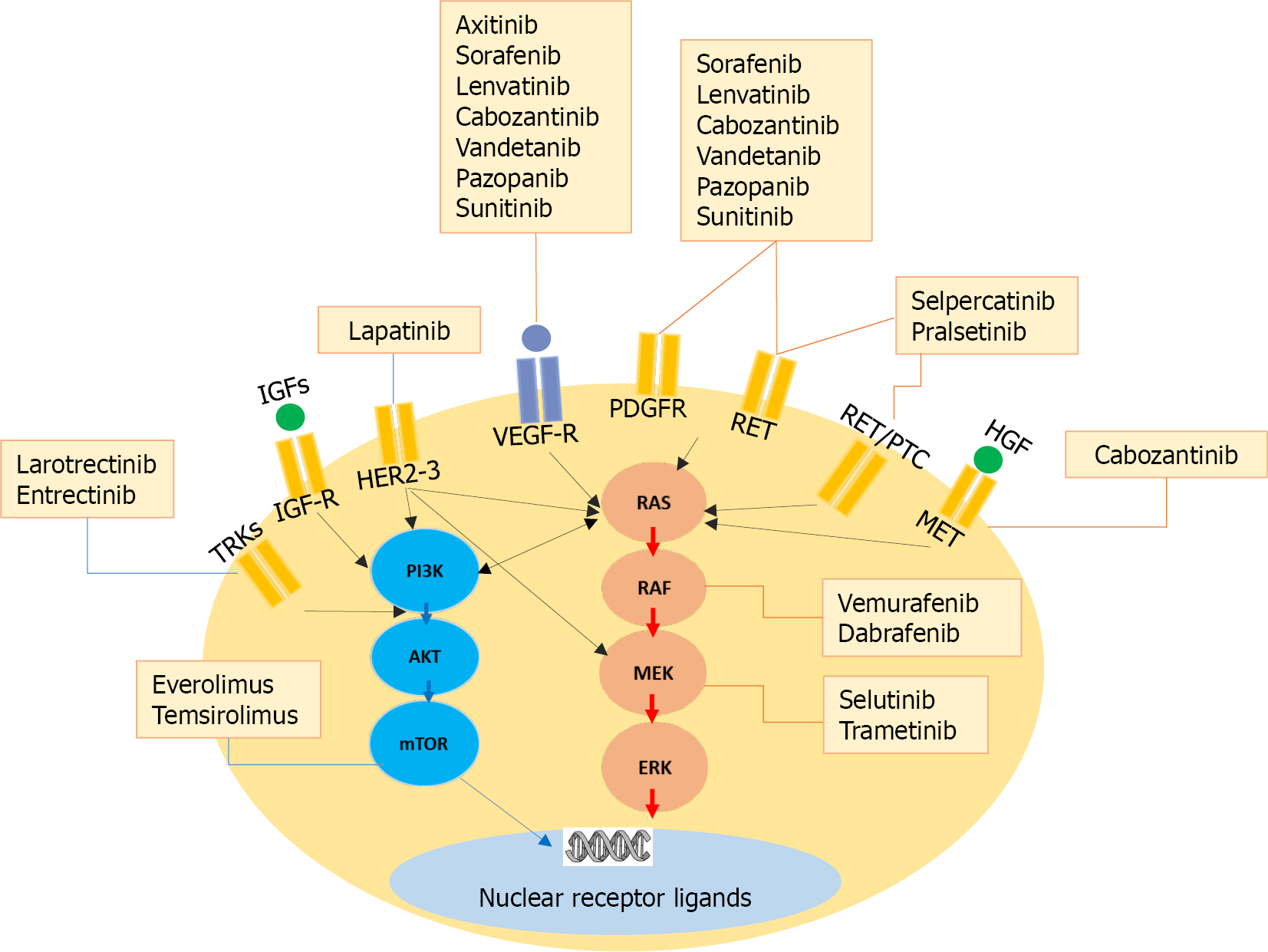
The underlying carcinogenic molecular pathways of differentiated thyroid cancer have been well defined. The MAPK signaling pathway is one of the most extensively studied[3]. Driver mutations such as in BRAF and RAS oncogenes, as well as fusions involving tyrosine kinase receptors, lead towards a constitutive activation of the downstream events resulting in cell proliferation, dedifferentiation, and cancer cell survival. These mutations could be targeted with specific therapies which result in cell growth inhibition[3,4]. Meanwhile, multikinase inhibitors (MKIs) confer their anti-tumor effect in radioiodine-refractory metastatic thyroid cancer by other effects, mainly through their anti-angiogenic activity[4]. The main molecular signaling pathways involved in thyroid carcinogenesis and the most significant inhibitors are summarized in Figure 1.
The initiation of health agencies approved systemic therapy or the enrollment of a patient in a clinical trial should be managed by highly specialized endocri
| Agent and national clinical trial number1 | Molecular target | Phase | Dosage | Enrolled patients (n) | PR (%) | mPFS (mo) | Common AEs | Serious AEs (grade ≥ 3) | Withdrawal due to AEs |
| Sorafenib[6]; NCT00984282 | VEGFR1–3, PDGFR, RET, c-kit, BRAF | III | 400 mg orally twice daily | 207 | 10.8 | 12.2 | Hand– foot skin reaction (76%), diarrhea (69%), alopecia (67%), rash (50%) | Hand-foot skin reaction (20%), hypertension (10%), weight loss (6%) | 19% |
| Lenvatinib[7]; NCT01321554 | VEGFR1–3, FGFR1–4, PDGFR, RET, c-kit | III | 24 mg per d in 28-d cycles | 261 | 63.2; 65 (4 complete response + 165 partial response) | 18.3 | Hypertension (68%), diarrhea (59%), fatigue (59%), decreased appetite (50%), decreased weight (46%), nausea (41%) | Hypertension (42%), proteinuria (10%), decreased weight (10%), fatigue (9%), diarrhea (8%) | 14% |
| Cabozantinib[28]; NCT01811212 | VEGFR2, MET, FLT3, RET, c-kit | II | 60 mg/d orally | 25 | 40 | 12.7 | Fatigue (44%), weight loss (36%), diarrhea (36%), hand– foot skin reaction (32%), hypertension (24%) | Hypophosphatemia (16%), lipase/amylase increase, neutropenia, fatigue, weight loss (12%) | |
| Axitinib[71]; NCT00094055 | VEGFR, PDGFR, c-kit | II | 5 mg twice daily | 60 | 30 | 18.1 | Fatigue (50%), diarrhea (48%), nausea (33%), anorexia (30%), hypertension (28%), stomatitis (25%), weight loss (25%), and headache (22%) | Hypertension (12%), proteinuria (5%), fatigue (5%) | |
| Vandetanib[72]; NCT00537095 | VEGFR2/3, EGFR, RET | II | 300 mg/d | 72 | 8.3 | 11.1 | Diarrhea (74%), hypertension (34%), acne (27%), asthenia, anorexia (26%), nausea, rash (25%), fatigue, QTc prolongation (23%) | QTc prolongation (14%), diarrhea (10%), asthenia (7%), fatigue (5%) | 33% |
| Sunitinib[73]; NCT00381641 | PDGFR, FLT3, c-kit, VEGFR, RET | II | 37.5 mg/d orally | 35 | 31 | 12.8 | Neutropenia (34%), leukopenia (31%), fatigue (26%), HFS (26%), diarrhea (26%) | Neutropenia (34%), leukopenia (31%), diarrhea, hand/foot syndrome (17%), fatigue (11%) | 11% |
| Pazopanib[74]; NCT00625846 | VEGFR, PDGFR, c-kit | II | 800 mg/d orally in 4-wk cycle | 37 | 49 | 11.7 | Fatigue (78%), skin and hair hypopigmentation (75%), diarrhea (73%), nausea (73%) | Raised alanine aminotransferase level (11%) | 5% |
| Dovitinib[75]; NCT02964144 | FGFR, VEGFR | II | 500 mg/d orally for five days, followed by a 2-d rest every week | 40 | 20.5 | 5.4 | Diarrhea (54%), anorexia (36%), vomiting (26%), fatigue (23%), and nausea (21%) | Neutropenia (13%) | 20% |
| Apatinib[31]; NCT03167385 | VEGFR2, c-Kit, c-SRC | II | 750 mg/d orally (n = 10, group I) - 500 mg/d orally (n = 10, group II) | 20 | 90 (I); 70 (II) | 18.4 | Hand– foot skin reaction (95%), proteinuria (90%) and hypertension (80%) | ||
| Lapatinib[76]; NCT01947023 | HER2/3 | I | 750 mg initial dose, escalated to 500 mg daily; + Dabrafenib 150 mg twice daily | 13 | 60 | 15 | Lymphocytic toxicity (7%) | ||
| Vemurafenib[58]; NCT01286753 | BRAF V600E | II | 960 mg orally twice daily | 51 | VEGFR naive: 39%; Previous VEGFR: 27% | VEGFR naive: 18.8; Previous VEGFR: 8.9 | Rash (73%), fatigue (69%), alopecia, dysgeusia (54%), creatinine increase, weight decrease (50%), arthralgia, anorexia, nausea, skin papilloma (46%) | Skin squamous cell carcinoma (23.5%), lymphopenia, and increased γ-glutamyl-transferase (8%) | 27% |
| Dabrafenib[57]; NCT00880321 | BRAF V600E | I | 150 mg twice daily | 13 | 29 | 11.3 | Skin papillomas (57%), hyperkeratosis (36%), alopecia (29%) | Elevated lipase, elevated amylase, fatigue, febrile neutropenia and squamous cell carcinoma (7%) | 0% |
| Selumetinib[66]; NCT00559949 | MEK-1/2, RAS, BRAF V600E | II | 100 mg twice daily for 28-d cycles | 39 | 3 | 8 | Rash (77%), fatigue (49%), diarrhea (49%), peripheral edema (36%) | Rash (18%), fatigue (8%) | 15% |
| Larotrectinib[33]; NCT02122913 | NTRK fusions | II | 100 mg twice daily | 153 | 129 (95%); 24 (16%) complete response | 28.3 | Fatigue (30%), cough, constipation (27%), dizziness, alanine aminotransferase increase (25%) | Anemia (10%), decreased neutrophil count (5%) | 2% |
| Entrectinib[36]; NCT02097810 (STARTRK-1) NCT02568267 (STARTRK-2) | NTRK fusions | II | 600 mg/d orally | 54 | 50 | 10 | Dysgeusia (47%), fatigue, constipation (28%), diarrhea (27%), edema peripheral, dizziness (24%) | Anemia (12%), weight gain (10%) | 4% |
| Everolimus[62]; NCT01118065 | mTOR | II | 10 mg/d orally | 33 | 3 | 12.9 | Mucositis, acneiform rash, fatigue, cough | Fatigue (8%), weight loss, infection (6%) | |
| Temsirolimus[63]; NCT01025453 | mTOR | II | Temsirolimus (25 mg IV weekly) + sorafenib (200 mg twice daily) | 36 | 22 | 12 | Hyperglycemia (19%), fatigue (13%), anemia (11%), oral mucositis, alanine aminotransferase increased (8%) | 14% |
Multikinase inhibitors block several signaling pathways responsible for tumor proliferation and survival, with varying degrees of potency[2]. However, the main target for MKIs is the vascular endothelial growth factor receptor (VEGFR) and therefore the inhibition of tumor angiogenesis[3]. That is why they are also called antiangiogenic MKIs. These MKIs have demonstrated in phase III trials, an increase in the median progression free-survival (mPFS) from 11 to 18 mo, and objective responses of 12% to 64%[6,7]. We should consider that these drugs are usually tumoristatic and will eventually lose their effect due to on-target or off-target resistance, after which, another therapy will be needed. To date, only one post-hoc analysis of the SELECT study has demonstrated improved overall survival in a subgroup of patients receiving an Lenvatinib vs placebo[8].
Sorafenib inhibits the VEGFR 1, 2, and 3, platelet-derived growth factor, RET, c-kit, and less potently, BRAF kinases[9]. In the phase III DECISION trial, patients treated with sorafenib (n = 207) had a significantly longer PFS over patients receiving placebo (n = 209) (10.8 vs 5.8 mo, respectively; HR, 0.587; 95%CI: 0.45–0.76; P < 0.0001)[6]. The clinical benefit rate (CR + PR + SD > 6 mo) was 54%, with a PR rate of 12.2% and an SD > 6 mo of 41.8%[6]. The median duration of PR was 10.2 mo. An improvement in OS could not be demonstrated, probably because a large proportion of patients in the placebo arm (71%) crossed over to treatment[6]. In the last metanalysis that included 636 patients from 15 studies receiving sorafenib, 26% of patients (95%CI: 0.19-0.34) achieved a PR, and 44% (98%CI: 0.39-0.48) an SD[10]. PFS time ranged from 9 to 21.3 mo and OS ranged from 10 to 56 mo[10]. In an exploratory analysis of the phase III trial, patients who received open-label sorafenib after progression under the placebo arm achieved a comparable PFS to those receiving sorafenib from the beginning of the trial (9.6 vs 10.8 mo)[11]. This could suggest that delaying the initiation of sorafenib could not have a significant impact on the effectiveness. Also, in the same analysis, patients who continued receiving sorafenib after progression had a still longer PFS in comparison to patients who initially received placebo (6.7 vs 5.8)[11], meaning that sorafenib could still be an option in patients when an alternative drug is not available or not possible. In our real-life experience with sorafenib (n = 18), 72% had SD ≥ 6 mo and 11% demonstrated PR with a PFS of 16.5 mo[12].
The most frequent adverse events during sorafenib treatment were hand-foot skin reaction, diarrhea, fatigue, alopecia, weight loss, and rash[6,10,12]. HFS reaction and hypertension were the most frequent grade 3-4 AEs, reported from to and from to, respectively[6,10,12]. As reported with other MKIs, dose reductions and interruptions were frequent, however, drug withdrawal was uncommon[6,10,12]. The recommended initial dose of sorafenib is 400 mg twice a day[13]. In an exposure-response model, initial lower doses of sorafenib (600 or 400 mg/d) were associated with improved tolerability but reduced PFS. However, a strategy of 800 mg/d for an initial two cycles followed by dose reductions seemed likely to maintain efficacy while possibly mitigating some AEs[14]. The summary of the efficacy and safety of sorafenib in patients with thyroid cancer reported by clinical trials is shown in Table 2.
| Ref. | n | Type | PR, % | SD, % | Median | Median | Most frequent AE | Most frequent grade 3-4 AE |
| PFS (mo) | OS (mo) | |||||||
| Gupta-Abramson et al[77], 2008 | 27 | DTC | 26 | 59 | 19 | - | HFS, 93% | Hypertension, 13% |
| Kloos et al[78], 2009 | 33 | PTC | 15 | 57 | 16 | 23 | Fatigue, 85% | Fatigue, 16% |
| Hoftijzer et al[79], 2009 | 31 | DTC | 25 | 34 | 14.5 | - | HFS, 66% | HFS, 18% |
| Cabanilas et al[59], 2010 | 13 | DTC | 20 | 60 | 19 | HFS, 60% | - | |
| Keefe et al[80], 2011 | 47 | DTC/PD | 38 | 47 | 22 | 32.4 | - | - |
| Ahmed et al[81], 2011 | 19 | DTC | 16 | - | - | - | Dermatology (other than HFS), 88% | HFS, 44% |
| Chen et al[82], 2011 | 9 | DTC | 33 | 44 | 10.5 | - | Alopecia, 100% | - |
| Marotta et al[83], 2012 | 17 | DTC | 30 | 41 | 9 | 10 | HFS, 88% | |
| Schneider et al[84], 2012 | 31 | DTC | 31 | 42 | 18 | 34.5 | HFS, 71% | HFS, 22% |
| Capdevilla et al[85], 2012 | 16 | DTC | 19 | 50 | 13.3 | 23.6 | HFS and diarrhea, 62% | HFS, 23% |
| Brose et al[6], 2014 | 207 | DTC | 12 | 42 | 10.8 | . | HFS, 73.6% | HFS, 20.3% |
| Benekli et al[86], 2014 | 14 | DTC | - | 43 | 21.3 | - | - | HFS, 22% |
| Dadu et al[87], 2008 | 51 | DTC | - | - | - | 56 | - | - |
| Luo et al[88], 2014 | 8 | DTC | 50 | 37 | 9.4 | 12.8 | Alopecia, 75% | Hypocalcemia and serum amylase increased, 12.5% |
| Gallo et al[89], 2015 | 20 | DTC | 25 | 40 | 8.2 | 28.4 | Fatigue, 95% | Gastrointestinal symptoms, 15% |
| Kim et al[90], 2018 | 98 | DTC | 25 | 37 | 9.7 | - | HFS, 76% | HFS, 41% |
| Jerkovich et al[12], 2019 | 18 | DTC | 11 | 72 | 16.5 | - | HFS, 67% | HFS, 14% |
Lenvatinib inhibits FGFR1, -2, -3, -4, PDGFR, VEGFR1, -2, -3, RET, and KIT kinases[15]. In phase III clinical trial SELECT, median PFS was significantly longer in patients treated with lenvatinib in comparison to those receiving placebo (18.3 vs 3.6 mo, respectively; HR, 0.21; 99%CI: 0.14-0.31; P < 0.001)[7]. The response rate was 64.8% (CR 1.5% and PR 63.2%), with a median time to response of only 2 mo[7]. Real-life studies published afterward had reported PR from 31% to 69%, SD from 20% to 60%, and PFS from 10 to 13.8 mo[16-23]. This apparent lower efficiency of lenvatinib in observational data could be explained by the fact that these studies included patients with more than one prior MKI treatment, ECOG PS ≥ 3, more comorbidities, and patients who did not start with a full dose (24 mg per day). In fact, in our experience with lenvatinib (n = 22), when we excluded patients that would have not met the SELECT inclusion criteria, PR increased from 31.8% to 50% and PFS from 13.7 to 22 mo[23]. Hypertension was the most common adverse event (63%-83%) in almost all studies[7,16-19,21,23] and the most frequent grade 3-4 adverse event, occurring in 31%-42% of cases[7,23]. Other adverse effects include diarrhea, fatigue, decreased appetite, and decreased weight[7,16-23]. The recommended initial dose is 24 mg per day[13]. A lower initial dose and longer dose interruptions led to lower response rates and shorter progression-free survival[24,25]. A summary of the efficacy and safety of lenvatinib in patients with thyroid cancer reported by phase III clinical trial and real-life studies is shown in Table 3.
| Ref. | n | Patients with prior TKIs % | CR % | PR % | SD % | Median | Median | Most frequent AE | Most frequent grade 3-4 AE |
| PFS (mo) | OS (mo) | ||||||||
| Schlumberger et al[7] 2015 | 261 | 25 | 1 | 63 | 23 | 18.3 | - | Hypertension, 68% | Hypertension, 42% |
| Berdelou et al[16], 2017 | 75 | 68 | 0 | 31 | 51 | 10 | - | Fatigue, 75% | Hypertension 35% |
| Jasim et al[17], 2017 | 25 | 31 | 0 | 50 | 28 | - | - | Hypertension 64% | Hypertension 40% |
| Sugino et al[18], 2018 | 29 | 13 | 0 | 69 | 21 | - | - | Hypertension 76% | - |
| Locati et al[19], 2019 | 94 | 64 | 0 | 36 | 41 | 10.8 | 23.8 | Fatigue, 13% | Fatigue, 8% |
| Lee et al[20], 2019 | 57 | 89 | 0 | 38 | 60 | 5.1 | 19.3 | General weakness 43% | |
| Masaki et al[21], 2019 | 42 | 10 | 0 | 62 | 24 | 13.8 | - | Hypertension, 83% | Proteinuria, 36% |
| Aydemirli et al[22], 2020 | 39 | 77 | 2 | 33 | 37 | 9.7 | 18.3 | Hypertension and fatigue, 64% | Hypertension, 28% |
| Jerkovich et al[23], 2020 | 22 | 59 | 4 | 32 | 32 | 13.7 | - | Hypertension, 64% | Hypertension, 23% |
Cabozantinib is a RET, vascular endothelial growth factor receptor-2 (VEGFR2), and MET kinases inhibitor agent currently approved for the treatment of advanced medullary thyroid cancer[26]. However, it has also been studied in 15 patients with RAI-refractory DTC in a phase I clinical trial, with promising efficacy[27]. Ten of the included patients were previously treated with VEGF inhibitors, mostly sorafenib. Cabozantinib was administered at a starting dose of 140 mg daily. A partial response was observed in 8 (53%) patients, 5 with prior VEGF inhibitors treatment. On the other hand, a phase II trial is currently ongoing, which involves a cabozantinib therapy in RAIR-DTC patients who experienced disease progression after second- or third-line VEGFR-targeted therapy[28]. Partial response was reached in 10 (40%) of the 25 enrolled patients, with a starting dose of 60-80 mg daily. The median PFS and OS were 12.7 and 34.7 mo, respectively[28].
Exelixis announced by the end of 2020 that, at a planned interim analysis, the phase III COSMIC-311 pivotal trial met the co-primary endpoint, demonstrating a significant reduction in the risk of disease progression or death of 78% of patients receiving cabozantinib compared to placebo (HR, 0.22, 96%CI: 0.13-0.36; P < 0.0001) in patients with RAIR differentiated thyroid cancer who have progressed after up to two prior VEGFR-targeted therapies. The safety profile was consistent with that previously observed for cabozantinib. In 2021, Exelixis® announced that the United States Food and Drug Administration (FDA) approved cabozantinib as a second/third line additional treatment for patients with RAIR thyroid cancer[29]. With this third MKI approved, there will surely be a change in defining first and second line of treatment according to the drug potency.
Apatinib, also known as rivoceranib, is a tyrosine kinase inhibitor that selectively inhibits the VEGFR2. Apatinib inhibits VEGF-mediated endothelial cell migration and proliferation thus blocking new blood vessel formation in tumor tissue. This agent also mildly inhibits c-Kit and c-SRC tyrosine kinases[30]. A recent phase II study performed in 20 patients with advanced thyroid cancer showed promising results with an objective response rate (ORR) of 80%, a median PFS of 18.4 mo (95%CI: 9.2-36.8 mo) and a median OS of 51.6 mo (95%CI: 29.2-not reached). The most common adverse events included palmar-plantar erythrodysaesthesia syndrome (19/20), proteinuria (18/20) and hypertension (16/20)[31].
Neurotrophic tropomyosin receptor kinase (NTRK) fusions have been reported in variable percentages of patients with DTC (2%-25%)[32]. Larotrectinib and Entrectinib are highly selective inhibitors of TRK receptors and have been approved by the FDA for the treatment of any solid tumor-bearing an NTRK1-3 fusion mutation (tumor-agnostic indication). Entrectinib also inhibit altered oncogenic expression of ALK and ROS1, which are much less frequent in DTC[32].
In a pooled analysis of three-phase 1/2 clinical trials, out of 24 patients with DTC bearing an NTRK fusion who received larotrectinib, 79% experienced an objective response[33]. This drug showed durable responses with a median time of 35 mo in the overall group of patients with solid tumors[33]. Also, larotrectinib seems to be active within the central nervous system (CNS)[33], which makes it an indispensable option when brain metastases are present in patients harboring this fusion, knowing that they have a worse outcome in patients with differentiated thyroid cancer[34]. Most frequent adverse events were primarily grade 1 and 2 and included fatigue (30%), cough, constipation (27%), dizziness (25%), and alanine aminotransferase increase (25%). The most common grade 3 or worse treatment-emergent adverse events (regardless of attribution) were anemia (10%) and decreased neutrophil count (5%)[33]. We recently showed our experience with Larotrectinib in a patient with RAIR DTC who had a rapid progression on MKI therapy (sorafenib and lenvatinib), and who had a complete response to treatment including the disappearance of multiple CNS metastasis[35].
Entrectinib also blocks ROS1 and ALK and was specifically designed to have systemic activity and cross the blood–brain barrier. In an analysis of three-phase I or II trials, two out of four patients had a PR with entrectinib[36]. Most AE were grade 1-2 and included dysgeusia (47%), fatigue (28%), and constipation (28%). The most common grade 3 or 4 treatment-related AE were anemia (12%) and weight gain (10%)[36].
TRK fusion-positive cancers can develop resistance to TRK inhibition[37]. This resistance can be classified into off-target (new additional mutations that may occur in the tumor) or on-target (within the same altered receptor, due point mutations that lead to amino acid substitutions in the solvent front, the gatekeeper residue or the xDFG motif)[38]. Mutations in the NTRK kinase domain cause resistance to TRK inhibitors by interfering with binding of the inhibitor, altering the kinase domain conformation or altering ATP-binding affinity[38].
New drugs are currently in development for those patients who develop on-target resistance, among them, selitrectinib and repotrectinib. Due to their small size, these low molecular weight molecules are able to engage the ATP-binding pocket while avoiding the steric penalties of kinase domain substitutions[39,40]. Selitrectinib is currently the drug with which the most experience has been gained. Thirty-one patients with solid tumors with NTRK fusions, previously treated with a TRK inhibitor (larotrectinib, entrectinib or PLX7486) with a median duration of prior therapy of 11 mo (range 2-30 mo) received treatment with selitrectinib. In patients with TRK kinase domain mutations (the majority of which involved the solvent front), the ORR was 45%[41].
RET/PTC rearrangements are present in 5%-25% of papillary thyroid carcinomas[42], although the occurrence of these mutations may be less frequent in advanced DTC[43]. Selpercatinib and pralsetinib, are kinase inhibitors that selectively target RET kinase, and were approved by the FDA for the treatment of advanced or metastatic RET fusion-positive thyroid cancer. In the phase 1/2 trial LIBRETTO-001, among 19 RET fusion-positive, non-medullary thyroid cancer patients, objective response was reported in 79%[44]. At 1 year, 71% of responses were ongoing, and 64% of the patients were free of progression[44]. The most common grade 3 or 4 adverse events included hypertension (21%), increased alanine aminotransferase (11%), increased aspartate aminotransferase (9%), hyponatremia (8%), and diarrhea (6%)[44].
In the phase 1/2 ARROW trial, praseltinib demonstrated objective responses in 75% (9/12), with a median duration of response of 14.5 mo, and 67% of responding patients continuing treatment[45]. Most treatment-related adverse events were grade 1-2, and included increased aspartate aminotransferase (31%), anemia (22%), increased alanine aminotransferase (21%), constipation (21%) and hypertension (20%)[45].
Mutation-specific kinase inhibitors -RET and NTRK inhibitors, as well as BRAF inhibitors-produced higher and durable objective responses[32,33,42,43]. Although prolongation of progression-free survival has not yet been demonstrated in phase III clinical trials, they seem to be promising options for RAIR thyroid cancer patients. In line with this, the implementation of molecular screening strategies seems to be necessary to improve the clinical course of these patients.
Evidence on acquired resistance mechanisms to RET, both on target and off-target, is recently arising. Selpercatinib and pralsetinib were oriented to target gatekeeper mutations, such as RET V804 and S904F which was associated with resistance to RET-targeted kinase inhibitors, as vandetanib[46]. Nevertheless, five RET kinase domain mutations at three non-gatekeeper residues were identified from selpercatinib and pralsetinib-resistant medullary thyroid cancer cell lines in a recent experimental study[47]. Information on acquired resistance to these drugs obtained from studies on non-small cell lung cancer (NSCLC) is slightly more extensive. For example, it was found acquired RET G810R/C/S/V mutations in RET fusion-positive tumors from patients who developed resistance to selpercatinib[48] and pralsetinib treatments[49]. Other reports of acquired selpercatinib resistance with MET amplification were demon
These experimental findings have shown the imperative need to develop next-generation targeted RET agents focused on both gatekeeper and non-gate keeper mutations for on- and off-target resistance in order to develop and validate combination therapies.
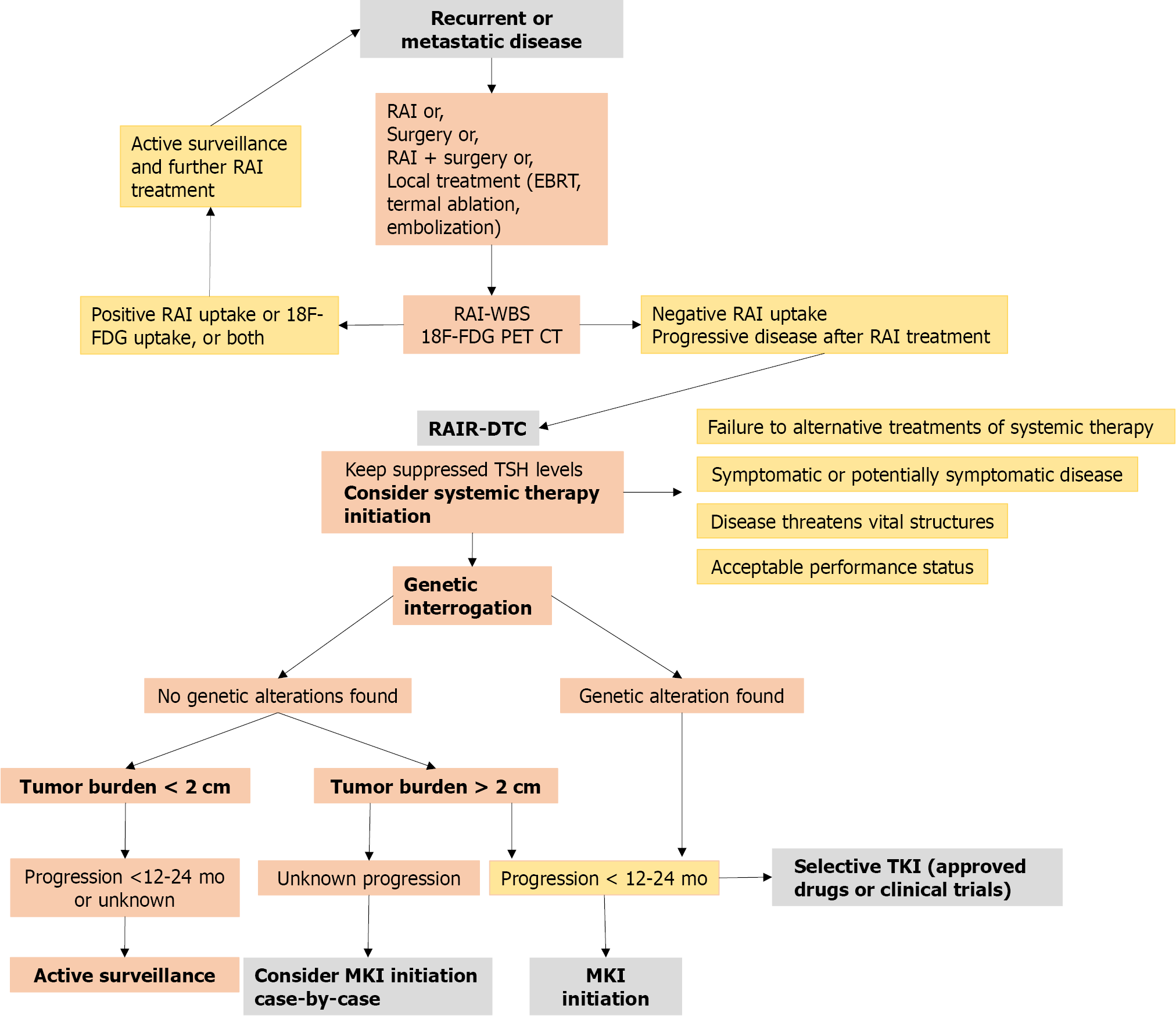
Considering the time-limited benefits of FDA-approved kinase inhibitors treatment in RAI-refractory thyroid cancer, it became necessary to develop additional new therapeutic lines that would enhance compatibility with individual patient needs by improving efficacy and adverse events profile. Several targeting agents are being studied in advanced differentiated thyroid cancers, but none of them have been approved yet (Table 1). A summary of some relevant ongoing clinical trials for the treatment of advanced RAIR-DTC are shown in Table 4.
| NCT number | Title | Status | Interventions | Characteristics | Population | Dates | Locations |
| NCT04554680 | Clinical Trial in RAI-Refractory Thyroid Carcinoma Evaluating BRAF & MEK Blockade for Redifferentiation Therapy | Recruiting | Drug: Dabrafenib and trametinib | Study type: Interventional | Enrollment: n = 5 | Study start: December 30, 2020 | National University Hospital, Singapore, Singapore |
| Phase: Phase 2 | Age: 21-99 yr | Study completion: April 2022 | |||||
| Study design: Allocation: N/A; Intervention model: Single group assignment; Masking: None (open label); Primary purpose: Treatment outcome; Measures: The proportion of participants attaining at least one tumor lesion with lesional dosimetry of ≥ 2000 cGy with I-131 dose of = | Sex: All | ||||||
| NCT01709292 | Vemurafenib Neoadjuvant Trial in Locally Advanced Thyroid Cancer | Active, not recruiting | Drug: Vemurafenib (all groups) | Study type: Interventional | Enrollment: n = 24 Age: 18 yr and older | Study start: November 7, 2012 | University of Texas MD Anderson Cancer Center, Houston, Texas, United States |
| Drug: Vemurafenib (Post Surgery) - Group A + C Other: Post Surgery - Group B | Phase: Phase 2 | Sex: All | Study completion: November 30, 2020 | ||||
| Study design: Allocation: NonRandomized intervention; Model: Parallel assignment; Masking: None (open label); Primary purpose: Treatment outcome; Measures: Percent change in ERK (extracellular-signal regulated kinase) phosphorylation and tumor size, objective response rate | |||||||
| NCT03167385 | Phase 2 Trial of Apatinib Mesylate in Locally Advanced/ Metastatic Differentiated Thyroid Carcinoma | Unknown | Drug: Apatinib mesylate | Study type: Interventional | Enrollment: n = 20 Age: 18 to 75 yr | Study start: March 22, 2017 | Tianjin Medical University Cancer Institute and Hospital, Tianjin, Tianjin, China |
| Phase: Phase 2 | Sex: All | Study completion: December 31, 2020 | |||||
| Study design: Allocation: N/A; Intervention model: Single group; assignment; Masking: None (open label); Primary purpose: Treatment outcome; Measures: Disease control rate, progression free survival, overall survival, objective response rate | |||||||
| NCT03753919 | Durvalumab Plus Tremelimumab for the Treatment of Patients With Progressive, Refractory Advanced Thyroid Carcinoma - The DUTHY Trial | Recruiting | Drug: Durvalumab Drug: Tremelimumab | Study type: Interventional | Enrollment: 46 Age: 18 yr and older | Study start: April 2 | Instituto Catalán de Oncología de Hospitalet, L'Hospitalet de Llobregat, Barcelona, Spain; Hospital Provincial de Castellón, Castelló, Valencia, Spain; Hospital Clínic Barcelona, Barcelona, Spain; Hospital Universitari Vall d'Hebron, Barcelona, Spain; MD Anderson Cancer Center, Madrid, Spain; Hospital Clínico San Carlos, Madrid, Spain; Hospital Universitario 12 de Octubre, Madrid, Spain; Hospital Universitario HM Sanchinarro, Madrid, Spain; Hospital Universitario La Paz, Madrid, Spain; Hospital Universitario Ramón y Cajal, Madrid, Spain; and 5 more |
| Phase: Phase 2 study | Sex: All | Study completion: July 2021 | |||||
| Design: Allocation: N/A; Intervention model: Single group assignment; Masking: None (open label); Primary purpose: Treatment outcome; Measures: Progression-free survival rate at 6 mo, overall survival rate at 6 mo, overall response rate, duration of response, median progression-free survival, incidence of treatment, emergent adverse events (safety and tolerability), median overall survival, response status after start of study treatment | |||||||
| NCT00537095 | Efficacy and Safety of Vandetanib (ZD6474) in Patients With Metastatic Papillary or Follicular Thyroid Cancer | Active, not recruiting | Drug: Vandetanib Other: Placebo | Study type: Interventional | Enrollment: n = 165 Age: 18 yr and older | Study start: September 29, 2007 | Research Site, Brussels, Belgium; Research Site, Odense, Denmark; Research Site, Angers Cedex 9, France Research Site, Angers Cedex, France; Research Site, Bordeaux Cedex, France; Research Site, Caen Cedex 5, France; Research Site, Caen Cedex, France; Research Site, Lyon Cedex, France; Research Site, Lyon, France; Research Site, Marseille Cedex 9, France; and 12 more |
| Phase: Phase 2 | Sex: All | Study completion: December 2021 | |||||
| Study design: Allocation: Randomized; Intervention model: Parallel assignment; Masking: Double (participant, investigator); Primary purpose: Treatment outcome; Measures: Time to tumor progression, disease control rate at 6 mo, objective response rate, time to death | |||||||
| NCT03602495 | Donafenib in 131I-Refractory Differentiated Thyroid Cancer | Recruiting | Drug: Donafenib Drug: Placebo | Study type: Interventional | Enrollment: n = 204 Age: 18 yr and older | Study start: August 29, 2018 | Peking Union Medical College Hospital, Beijing, Beijing, China |
| Phase: Phase 3 | Sex: All | Study completion: December 2021 | |||||
| Study design: Allocation: Randomized; Intervention model: Parallel assignment; Masking: Double (participant, investigator); Primary purpose: Treatment outcome; Measures: Progression-free survival, overall survival, objective response rate, disease control rate, time to disease progression |
BRAF oncogene mutations are present in approximately 50% of PTCs, while it has been observed that it rises to over 90% when an anaplastic transformation emerges from a prior history of PTC[52]. Under this premise, a clinical study using the combination dabrafenib 150 mg twice daily + trametinib 2 mg daily (selective inhibitors of BRAF V600E kinase and MEK1-2 kinase, respectively) in 23 patients with locally advanced, unresectable, or metastatic ATC[53], prompted the rapidly FDA approval for these patients. This study showed an overall response rate of 61%, with complete and partial response rates of 4% and 57%, respectively. Progression free survival for at least 6 mo was seen in 64% of these patients and overall survival was 80% at 1 year. The most common adverse events were fatigue (44%), fevers (31%), and nausea (31%), and the most common grade 3 and 4 adverse event was anemia (13%)[53].
In our setting, where access to molecular tests and target therapies is not widely available yet, we have reported the cases of two patients with metastatic and locally unresectable ATC, in whom the use of dabrafenib-trametinib (D-T) provided a dramatic reduction of the cervical mass with a minimal residual loco-regional disease, and even allowed surgical resection on one of them. Besides, a partial and complete response to the pulmonary metastatic disease was also observed[54,55].
The combination of D-T was studied in a phase II clinical trial that included 53 patients with BRAF mutated RAIR-PTC with disease progression within the last year[56]. The participants were randomized to Arm A (dabrafenib 300 mg daily, n = 26) or Arm B (dabrafenib 150 mg daily + trametinib 2 mg daily, n = 27). Cross-over to Arm B was allowed at the time of progression. Out of 25% of patients had prior therapy with multi-kinase inhibitors. Preliminary results exhibited partial responses in 10 (38%) and 9 (33%) patients from Arm A and B, respectively. Progression-Free Survival for patients who received D-T was 11.4 mo, with a median follow-up of 13 mo. The treatment-related adverse events were similar to previously reported trials[56].
Dabrafenib and vemurafenib have been approved as single agents for the treatment of advanced melanoma, but they also have been evaluated in phase 2 trials in patients with BRAF V600E–mutated PTC[57,58]. Both BRAF inhibitors are effective also in papillary carcinoma, although the outcomes have not been as robust as for ATC, so neither are currently approved for this use. In general terms, objective responses were seen for up to half of patients treated with either vemurafenib or dabrafenib in different trials and clinical experiences[57-59]. Among them, a randomized, multi-institutional, open-label phase 2 trial was conducted over two arms of patients with BRAF V600E–mutated PTC[57]. Arm A employed dabrafenib as a single agent and arm B, the combination of dabrafenib with trametinib. Partial responses were reached in 10 of 26 patients (38%) from arm A, and 9 of 17 (33%) from arm B, with median PFS of 11.4 and 15.1 mo, respectively. Common adverse events included fever, diarrhea, anemia, fatigue, nausea, alopecia and skin reactions[57]. Meanwhile, a non-randomized, open-label, multicenter phase 2 vemurafenib trial was conducted in two cohorts of patients with BRAF V600E–mutated PTC. Cohort 1 was comprised of 26 patients who had never received multikinase VEGFR inhibitors, in which the best overall response (partial response) was reached in 12 patients (38%), with a median duration of PFS of 18.8 mo (14.2–26), and the median OS had yet to be reached. In cohort 2 were included 25 patients who previously received MKIs treatment. Partial response rates were seen in 27.3%, with a median PFS of 8.9 mo. The most common adverse events reported were rash, fatigue, weight loss, dysgeusia, and alopecia. Serious adverse events were seen in 62% and 68% of the patients in cohort 1 and 2, respectively, including benign and malignant skin lesions and cerebrovascular accidents, among others[58].
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that exerts as an essential regulator of cell growth-related processes[60]. Everolimus and temsirolimus are two mTOR inhibitors that demonstrated clinical benefits in other cancers like advanced renal carcinoma, metastatic breast cancer, and pancreatic neuroendocrine tumors, in which they were approved by the FDA[61]. Since the mTOR pathway is over-activated in thyroid cancer, some studies have tested these agents' effects on advanced thyroid cancer, with promising outcomes[62,63].
Everolimus was evaluated in a single-arm, multicentric phase II study that included 31 patients with aggressive RAIR-DTC, among other thyroid tumor histologies. There was one PR (3%) but 27 patients (82%) had SD, for a clinical benefit rate of 84.8% and a median PFS for 12.9 mo. Median OS was not reached and 2-year OS was 73.5%[62]. For its part, a phase 2 study that enrolled 36 patients with metastatic RAIR-DTC evaluated the efficacy of the combination of oral sorafenib (200 mg twice daily) and intravenous temsirolimus (25 mg weekly)[63]. A partial response was reached in 8 patients (22%), while stable and progression disease was seen in 21 (58%) and 1 (2%) patients, respectively. The mPFS at one year was 30.5% and the most common toxicities included hyperglycemia, fatigue, anemia, and oral mucositis. The authors concluded that this combination appears to have better response rates in patients with RAI-refractory thyroid cancer who received no prior treatment, regardless of whether RAS or RAF mutation was present[63].
It has been well described that activating BRAF mutations induce loss of differentiated features required for response to radioiodine treatment, while its blockade would restore radioiodine uptake in experimental models[64]. In patients with radioiodine-refractory differentiated carcinoma with somatic BRAF or RAS mutations, treatment with the specific targeted inhibitors may restore radioiodine responsiveness in up to two-thirds of patients, permitting iodine therapeutic administration leading to tumor shrinkage in up to one-third[57-59]. On the other hand, constitutive activation of MAPK pathway causes inhibition of a variety of thyroid genes, including NIS, leading to the investigation of selective MAPK blocking agents as Selumetinib, as redifferentiation agent[64,65].
Selumetinib is a MEK1–2, RAS and BRAF V600E inhibitor which efficacy was evaluated in 32 RAIR-DTC patients enrolled in a multicenter, open-label, phase II trial[66]. There were 1 partial response (3%), 21 stable disease (54%), and 11 progressive diseases (28%). Median PFS was 32 wk, and it was seen that BRAF V600E mutants had a longer median PFS compared with patients with BRAF wild-type cancer (33 vs 11 wk, respectively). This suggest a potential beneficence of Selumetinib based on underlying genetic disorders. The most common adverse events included rash, fatigue, diarrhea, and peripheral edema[66]. A phase III trial is currently in progress which continues to explore selumetinib's redifferentiation benefits in a larger number of participants[66] (Table 2).
In recent years, there has been significant progress in the field of oncological immunotherapy. Several immunotherapeutic agents have now been approved by the FDA for the treatment of a variety of malignancies, including melanoma, non-small cell lung cancer, renal and breast carcinomas, among others[67]. In this line, several phase I studies research the use of immunotherapy in the treatment of advanced differentiated thyroid cancer focuses on restoring immune surveillance[68]. The recent identification of blocking antibodies of CTLA-4 and PD-1 to their corresponding ligands (CD80/86 and PD-L1/PD-L2 respectively) enhances the effector T cells and inhibits the regulatory suppressor cells. Thus, the evidence of PD-1 (+) T cell in thyroid tumors involved lymph nodes in PTC patients suggests the potential utility of immune checkpoint inhibitors like pembrolizumab (as a single agent or in combination with MKIs) for advanced thyroid cancers[68]. Only a few immunotherapy trials in patients with thyroid cancer have been published to date, but several trials are ongoing.
Pembrolizumab is an anti–PD-1 monoclonal antibody that exhibits antitumor activity by blocking interaction between PD-1 and its ligands[68]. Patients with advanced thyroid cancer were enrolled in the nonrandomized, phase Ib KEYNOTE-028 trial conducted to evaluate its safety and antitumor activity in 22 patients with advanced papillary or follicular thyroid cancer. Pembrolizumab 10 mg/kg was administered every 2 wk up to 24 mo or until confirmed progression or intolerable toxicity. SD was achieved by 57% (4/7) of patients with follicular histology and 60% (9/15) of patients with papillary histology and two patients reached partial response for 8 and 20 mo. Median PFS was 7 mo and median overall survival was not reached. Diarrhea and fatigue were the most common adverse events[69]. This study suggests that pembrolizumab may be effective and have a favorable safety profile in PD-L1–positive thyroid cancer, providing a baseline for future research[69].
Other ongoing single-arm multicenter phase II study combine lenvatinib and pembrolizumab in patients with RAIR-DTC[70]. Patients were excluded if they had received previous VEGFR-directed multikinase therapy. The lenvatinib starting dose was 20 mg/d orally and pembrolizumab was 200 mg IV every 3 wk. The preliminary results showed that out of 29 evaluable patients, 18 (62%) had a partial response, 10 (35%) had stable disease and the clinical benefit rate was 97%. The PFS at 12 mo was 74%, and median PFS was not yet reached. The most common adverse events were hypertension (47%), weight loss (13%), maculopapular rash (13%), leukopenia (7%), diarrhea (7%), and oral mucositis (7%)[70]. While the results are promising, the continuation of this study will help determine the magnitude of the responses.
In conclusion, therapeutic options for patients with advanced radioiodine-refractory differentiated thyroid carcinoma have been increasingly evolving and fine-tuned. While the introduction of new therapies for multiple molecular targets has made it possible to extend progression-free survival, their impact on overall survival is still unclear. Based on the improving knowledge of the underlying molecular mechanisms in these patients, novel agents under study bring us a new scope for the near future. Thus, increasingly tailored therapy focused on critical molecular pathways will be offered, allowing to overcome drug evasion mechanisms, enhance efficacy, minimize adverse events, and finally achieve an overall survival improvement in these patients.
| 1. | Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, Ogilvie JB, Terris DJ, Netterville J, Wong RJ, Randolph G; AACE Endocrine Surgery Scientific Committee. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract. 2015;21:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, Pacini F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 3. | Fallahi P, Ferrari SM, Galdiero MR, Varricchi G, Elia G, Ragusa F, Paparo SR, Benvenga S, Antonelli A. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin Cancer Biol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Weitzman SP, Sherman SI. Novel Drug Treatments of Progressive Radioiodine-Refractory Differentiated Thyroid Cancer. Endocrinol Metab Clin North Am. 2019;48:253-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Rao SN, Cabanillas ME. Navigating Systemic Therapy in Advanced Thyroid Carcinoma: From Standard of Care to Personalized Therapy and Beyond. J Endocr Soc. 2018;2:1109-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1169] [Article Influence: 97.4] [Reference Citation Analysis (1)] |
| 7. | Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib vs placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1418] [Article Influence: 128.9] [Reference Citation Analysis (0)] |
| 8. | Brose MS, Worden FP, Newbold KL, Guo M, Hurria A. Effect of Age on the Efficacy and Safety of Lenvatinib in Radioiodine-Refractory Differentiated Thyroid Cancer in the Phase III SELECT Trial. J Clin Oncol. 2017;35:2692-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3187] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 10. | Feng G, Luo Y, Zhang Q, Zeng F, Xu J, Zhu J. Sorafenib and radioiodine-refractory differentiated thyroid cancer (RR-DTC): a systematic review and meta-analysis. Endocrine. 2020;68:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Paschke R, Schlumberger M, Nutting C, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Shong YK, Sherman SI, Smit J, Kappeler C, Molnar I, Brose MF. Exploratory analysis of outcomes for patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-RDTC) receiving open label sorafenib post-progression on the phase III DECISION trial. Exper Clin Endocrin Diabetes. 2015;123:03-05. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Jerkovich F, García Falcone MG, Pitoia F. The experience of an Endocrinology Division on the use of tyrosine multikinase inhibitor therapy in patients with radioiodine-resistant differentiated thyroid cancer. Endocrine. 2019;64:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 10245] [Article Influence: 1024.5] [Reference Citation Analysis (1)] |
| 14. | Grevel J, Jentsch G, Austin R, Prins NH, Lettieri J, Mitchell D, Huang F, Brose MS, Schlumberger M, Meinhardt G, Peña CEA, Ploeger BA. Exposure-Response Modeling and Simulation of Progression-Free Survival and Adverse Events of Sorafenib Treatment in Patients With Advanced Thyroid Cancer. Clin Transl Sci. 2019;12:459-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459-5465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 420] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 16. | Aashiq M, Silverman DA, Na'ara S, Takahashi H, Amit M. Radioiodine-Refractory Thyroid Cancer: Molecular Basis of Redifferentiation Therapies, Management, and Novel Therapies. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Jasim S, Iniguez-Ariza NM, Hilger CR, Chintakuntlawar AV, Ryder MM, Morris JC 3rd, Bible KC. Optimizing lenvatinib therapy in patients with metastatic radioactive iodine-resistant differentiated thyroid cancers. Endocr Pract. 2017;23:1254-1261. [PubMed] [DOI] [Full Text] |
| 18. | Sugino K, Nagahama M, Kitagawa W, Ohkuwa K, Uruno T, Matsuzu K, Suzuki A, Masaki C, Akaishi J, Hames KY, Tomoda C, Ogimi Y, Ito K. Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr J. 2018;65:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Locati LD, Piovesan A, Durante C, Bregni M, Castagna MG, Zovato S, Giusti M, Ibrahim T, Puxeddu E, Fedele G, Pellegriti G, Rinaldi G, Giuffrida D, Verderame F, Bertolini F, Bergamini C, Nervo A, Grani G, Rizzati S, Morelli S, Puliafito I, Elisei R. Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur J Cancer. 2019;118:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Lee EK, Kim SM, Kim BH, Kim MJ, Lim DJ, Kim MH, Shin DY, Kang HC, Ahn BC, Kim SW, Ahn HY, Park YJ. Lesion-Based Evaluation Predicts Treatment Response to Lenvatinib for Radioactive Iodine-Refractory Differentiated Thyroid Cancer: A Korean Multicenter Retrospective Study. Thyroid. 2019;29:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Masaki C, Sugino K, Saito N, Akaishi J, Hames KY, Tomoda C, Suzuki A, Matsuzu K, Uruno T, Ohkuwa K, Kitagawa W, Nagahama M, Ito K. Efficacy and Limitations of Lenvatinib Therapy for Radioiodine-Refractory Differentiated Thyroid Cancer: Real-World Experiences. Thyroid. 2020;30:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Aydemirli MD, Kapiteijn E, Ferrier KRM, Ottevanger PB, Links TP, van der Horst-Schrivers ANA, Broekman KE, Groenwold RHH, Zwaveling J. Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data. Eur J Endocrinol. 2020;182:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Jerkovich F, Califano I, Bueno F, Carrera JM, Giglio R, Abelleira E, Pitoia F. Real-life use of lenvatinib in patients with differentiated thyroid cancer: experience from Argentina. Endocrine. 2020;69:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Tahara M, Brose MS, Wirth LJ, Suzuki T, Miyagishi H, Fujino K, Dutcus CE, Gianoukakis A. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer. 2019;106:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. |
Brose MS.
A multicenter, randomized, double-blind, phase II study of lenvatinib (LEN) in patients (pts) with radioiodine-refractory differentiated thyroid cancer (RR-DTC) to evaluate the safety and efficacy of a daily oral starting dose of 18 mg |
| 26. | Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639-3646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 882] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 27. | Cabanillas ME, Brose MS, Holland J, Ferguson KC, Sherman SI. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid. 2014;24:1508-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Cabanillas ME, de Souza JA, Geyer S, Wirth LJ, Menefee ME, Liu SV, Shah K, Wright J, Shah MH. Cabozantinib As Salvage Therapy for Patients With Tyrosine Kinase Inhibitor-Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase II International Thyroid Oncology Group Trial. J Clin Oncol. 2017;35:3315-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Exelixis Inc. Exelixis Announces Breakthrough Therapy Designation Granted to Cabozantinib for the Treatment of Patients with Previously Treated Radioactive Iodine-Refractory Differentiated Thyroid Cancer. [cited 25 February 2021]. Available from: https://ir.exelixis.com/news-releases/news-release-details/exelixis-announces-breakthrough-therapy-designation-granted. |
| 30. | Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Lin YS, Zhang X, Wang C, Liu YQ, Guan WM, Liang J. Long-Term Results of a Phase II Trial of Apatinib for Progressive Radioiodine Refractory Differentiated Thyroid Cancer. J Clin Endocrinol Metab. 2021;106:e3027-e3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Chen Y, Chi P. Basket trial of TRK inhibitors demonstrates efficacy in TRK fusion-positive cancers. J Hematol Oncol. 2018;11:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020; 21(4), 531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 753] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 34. | Zunino A, Pitoia F, Faure E, Reyes A, Sala M, Sklate R, Ilera V, Califano I; Thyroid Department of Sociedad Argentina de Endocrinología y Metabolismo. Unusual metastases from differentiated thyroid carcinoma: analysis of 36 cases. Endocrine. 2019;65:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Pitoia F. Complete response to larotrectinib treatment in a patient with papillary thyroid cancer harboring an ETV6‐NTRK3 gene fusion. Clin Case Rep. 2021;9:1905-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1227] [Article Influence: 175.3] [Reference Citation Analysis (0)] |
| 37. | Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 1094] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 38. | Russo M, Misale S, Wei G, Siravegna G, Crisafulli G, Lazzari L, Corti G, Rospo G, Novara L, Mussolin B, Bartolini A, Cam N, Patel R, Yan S, Shoemaker R, Wild R, Di Nicolantonio F, Bianchi AS, Li G, Siena S, Bardelli A. Acquired Resistance to the TRK Inhibitor Entrectinib in Colorectal Cancer. Cancer Discov. 2016;6:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 39. | Drilon A, Nagasubramanian R, Blake JF, Ku N, Tuch BB, Ebata K, Smith S, Lauriault V, Kolakowski GR, Brandhuber BJ, Larsen PD, Bouhana KS, Winski SL, Hamor R, Wu WI, Parker A, Morales TH, Sullivan FX, DeWolf WE, Wollenberg LA, Gordon PR, Douglas-Lindsay DN, Scaltriti M, Benayed R, Raj S, Hanusch B, Schram AM, Jonsson P, Berger MF, Hechtman JF, Taylor BS, Andrews S, Rothenberg SM, Hyman DM. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017;7:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 40. | Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, Zhu VW, Ahn MJ, Camidge DR, Nguyen J, Zhai D, Deng W, Huang Z, Rogers E, Liu J, Whitten J, Lim JK, Stopatschinskaja S, Hyman DM, Doebele RC, Cui JJ, Shaw AT. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018;8:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 41. | Hyman DM, Kummar S, Farago AF. Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). In Proceedings of the 110th Annual Meeting of the American Association for Cancer Research; 2019 March 29–3 April 2019. Abstract CT127 2019. Atlanta (GA), Philadelphia (PA): AACR. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Tirrò E, Martorana F, Romano C, Vitale SR, Motta G, Di Gregorio S, Massimino M, Pennisi MS, Stella S, Puma A, Gianì F, Russo M, Manzella L, Vigneri P. Molecular Alterations in Thyroid Cancer: From Bench to Clinical Practice. Genes (Basel). 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Mady LJ, Grimes MC, Khan NI, Rao RH, Chiosea SI, Yip L, Ferris RL, Nikiforov YE, Carty SE, Duvvuri U. Molecular Profile of Locally Aggressive Well Differentiated Thyroid Cancers. Sci Rep. 2020;10:8031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux S, Godbert Y, Barlesi F, Morris JC, Owonikoko TK, Tan DSW, Gautschi O, Weiss J, de la Fouchardière C, Burkard ME, Laskin J, Taylor MH, Kroiss M, Medioni J, Goldman JW, Bauer TM, Levy B, Zhu VW, Lakhani N, Moreno V, Ebata K, Nguyen M, Heirich D, Zhu EY, Huang X, Yang L, Kherani J, Rothenberg SM, Drilon A, Subbiah V, Shah MH, Cabanillas ME. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med. 2020;383:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 548] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 45. | Subbiah V, Hu MI, Gainor JF, Mansfield AS, Alonso G, Taylor MH, Weijia Zhu V, Garrido Lopez P, Amatu A, Doebele RC, Cassier PA, Keam B, Schuler MH, Zhang H, Clifford C, Palmer M, Green J, Turner CD, Curigliano G. Clinical activity of the RET inhibitor pralsetinib (BLU-667) in patients with RET fusion+ solid tumors. J Clin Oncol. 2020;38:109. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 47. | Subbiah V, Shen T, Terzyan SS, Liu X, Hu X, Patel KP, Hu M, Cabanillas M, Behrang A, Meric-Bernstam F, Vo PTT, Mooers BHM, Wu J. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol. 2021;32:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 48. | Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, Tuch BB, Yoda S, Gainor JF, Sequist LV, Oxnard GR, Gautschi O, Drilon A, Subbiah V, Khoo C, Zhu EY, Nguyen M, Henry D, Condroski KR, Kolakowski GR, Gomez E, Ballard J, Metcalf AT, Blake JF, Dawson SJ, Blosser W, Stancato LF, Brandhuber BJ, Andrews S, Robinson BG, Rothenberg SM. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J Thorac Oncol. 2020;15:541-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 49. | Gainor J, Curigliano G, Doebele RC, Lin JJ, H. Ou S, Miller S, Turner CD, Subbiah V. Analysis of resistance mechanisms to pralsetinib in patients with RET fusion-positive non-small cell lung cancer (NSCLC) from the ARROW study. IASLC 2020 North American Conference on Lung Cancer (October 16-17, 2020) 2020; Abstract OA05.02. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Rosen EY, Johnson ML, Clifford SE, Somwar R, Kherani JF, Son J, Bertram AA, Davare MA, Gladstone E, Ivanova EV, Henry DN, Kelley EM, Lin M, Milan MSD, Nair BC, Olek EA, Scanlon JE, Vojnic M, Ebata K, Hechtman JF, Li BT, Sholl LM, Taylor BS, Ladanyi M, Jänne PA, Rothenberg SM, Drilon A, Oxnard GR. Overcoming MET-Dependent Resistance to Selective RET Inhibition in Patients with RET Fusion-Positive Lung Cancer by Combining Selpercatinib with Crizotinib. Clin Cancer Res. 2021;27:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 51. | Zhu VW, Madison R, Schrock AB, Ou SI. Emergence of High Level of MET Amplification as Off-Target Resistance to Selpercatinib Treatment in KIF5B-RET NSCLC. J Thorac Oncol. 2020;15:e124-e127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, Gunn GB, Lu C, Ferrarotto R, Lai SY, Cabanillas ME. Patterns of Treatment Failure in Anaplastic Thyroid Carcinoma. Thyroid. 2017;27:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 53. | Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol. 2018;36:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 662] [Article Influence: 73.6] [Reference Citation Analysis (1)] |
| 54. |
Smulever A, Barrio Lower Daniele S, Damiano G, Pitoia F.
Re: "Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in |
| 55. | Bueno F, Abelleira E, von Stecher F, de Lima AP, Pitoia F. Dramatic clinical response to dabrafenib plus trametinib in anaplastic thyroid carcinoma and the challenges faced during the COVID-19 pandemic. Arch Endocrinol Metab. 2021;65:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Manisha H Shah, Lai Wei, Lori J Wirth, Gregory A Daniels, Jonas A De Souza, Cynthia Dawn Timmers, Jennifer L Sexton, Mamdouh Beshara, Debra Nichols, Norka Snyder, Catherine E Devine, Bhavana Konda, Naifa Lamki Busaidy. Results of randomized phase II trial of dabrafenib vs dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J Clin Oncol. 2017;35:6022. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Falchook GS, Millward M, Hong D, Naing A, Piha-Paul S, Waguespack SG, Cabanillas ME, Sherman SI, Ma B, Curtis M, Goodman V, Kurzrock R. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid. 2015;25:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 58. | Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, Sherman SI, Sherman EJ. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:1272-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 59. | Dadu R, Shah K, Busaidy NL, Waguespack SG, Habra MA, Ying AK, Hu MI, Bassett R, Jimenez C, Sherman SI, Cabanillas ME. Efficacy and tolerability of vemurafenib in patients with BRAF(V600E) -positive papillary thyroid cancer: M.D. Anderson Cancer Center off label experience. J Clin Endocrinol Metab. 2015;100:E77-E81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4260] [Cited by in RCA: 4487] [Article Influence: 224.4] [Reference Citation Analysis (0)] |
| 61. | Falkowski S, Woillard JB. Therapeutic Drug Monitoring of Everolimus in Oncology: Evidences and Perspectives. Ther Drug Monit. 2019;41:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Hanna GJ, Busaidy NL, Chau NG, Wirth LJ, Barletta JA, Calles A, Haddad RI, Kraft S, Cabanillas ME, Rabinowits G, O'Neill A, Limaye SA, Alexander EK, Moore FD Jr, Misiwkeiwicz K, Thomas T, Nehs M, Marqusee E, Lee SL, Jänne PA, Lorch JH. Genomic Correlates of Response to Everolimus in Aggressive Radioiodine-refractory Thyroid Cancer: A Phase II Study. Clin Cancer Res. 2018;24:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 63. | Sherman EJ, Dunn LA, Ho AL, Baxi SS, Ghossein RA, Fury MG, Haque S, Sima CS, Cullen G, Fagin JA, Pfister DG. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer. 2017;123:4114-4121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, Tosi E, Cavaliere A, Gulino A, Filetti S, Russo D. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 65. | Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 691] [Cited by in RCA: 598] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 66. | Hayes DN, Lucas AS, Tanvetyanon T, Krzyzanowska MK, Chung CH, Murphy BA, Gilbert J, Mehra R, Moore DT, Sheikh A, Hoskins J, Hayward MC, Zhao N, O'Connor W, Weck KE, Cohen RB, Cohen EE. Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18:2056-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current Perspectives in Cancer Immunotherapy. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 68. | French JD, Kotnis GR, Said S, Raeburn CD, McIntyre RC Jr, Klopper JP, Haugen BR. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:E934-E943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 69. | Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. 2019;19:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 70. | Haugen B, French J, Worden FP, Konda B, Sherman EJ, Dadu R, Gianoukakis AG, Wolfe EG, Foster NR, Bowles DW, Wirth LJ. Lenvatinib plus pembrolizumab combination therapy in patients with radioiodine-refractory (RAIR), progressive differentiated thyroid cancer (DTC): Results of a multicenter phase II international thyroid oncology group trial. J Clin Oncol. 2020;38:6512-6512. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 472] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 72. | Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, Gómez JM, Bonichon F, Leenhardt L, Soufflet C, Licour M, Schlumberger MJ. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 73. | Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260-5268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 310] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 74. | Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC 3rd, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C; Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 75. | Lim SM, Chung WY, Nam KH, Kang SW, Lim JY, Kim HG, Shin SH, Sun JM, Kim SG, Kim JH, Kang CW, Kim HR, Cho BC. An open label, multicenter, phase II study of dovitinib in advanced thyroid cancer. Eur J Cancer. 2015;51:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Sherman EJ, Ho AL, Baxi SS, Dunn L, Korte SH, Haque S, Ghossein RA, Chen HX, Pfister DG. Combination of dabrafenib (DAB). J Clin Oncol. 2017;35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714-4719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 499] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 78. | Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 399] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 79. | Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, Weijers K, Pereira AM, Huijberts M, Kapiteijn E, Romijn JA, Smit JW. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 80. | Keefe SM, Troxel AB, Rhee S, Puttaswamy K, O'Dwyer PJ, Loevner LA, Mandel SJ, Brose MS. Phase II trial of sorafenib in patients with advanced thyroid cancer. J Clin Oncol. 2011;29:5562. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Ahmed M, Barbachano Y, Riddell A, Hickey J, Newbold KL, Viros A, Harrington KJ, Marais R, Nutting CM. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 82. | Chen L, Shen Y, Luo Q, Yu Y, Lu H, Zhu R. Response to sorafenib at a low dose in patients with radioiodine-refractory pulmonary metastases from papillary thyroid carcinoma. Thyroid. 2011;21:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Marotta V, Ramundo V, Camera L, Del Prete M, Fonti R, Esposito R, Palmieri G, Salvatore M, Vitale M, Colao A, Faggiano A. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin Endocrinol (Oxf). 2013;78:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 84. | Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol. 2012;167:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Capdevila J, Iglesias L, Halperin I, Segura A, Martínez-Trufero J, Vaz MÁ, Corral J, Obiols G, Grande E, Grau JJ, Tabernero J. Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer. 2012;19:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Benekli M, Yalcin S, Ozkan M, Elkiran ET, Sevinc A, Cabuk D, Coskun HS, Oksuzoglu B, Bayar B, Akbulat A, Ozet A. Efficacy of sorafenib in advanced differentiated and medullary thyroid cancer: experience in a Turkish population. Onco Targets Ther. 2015;8:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Dadu R, Waguespack SG, Sherman SI, Hu MI, Busaidy NL, Jimenez C, Habra MA, Ying AK, Bassett RL, Cabanillas ME. Efficacy and tolerability of different starting doses of sorafenib in patients with differentiated thyroid cancer. Oncologist. 2014;19:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Luo Y, Shi Y, Xing P, Wang L, Feng Y, Han X, He X. Sorafenib in metastatic radioactive iodine-refractory differentiated thyroid cancer: A pilot study. Mol Clin Oncol. 2014;2:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Gallo M, Michelon F, Castiglione A, Felicetti F, Viansone AA, Nervo A, Zichi C, Ciccone G, Piovesan A, Arvat E. Sorafenib treatment of radioiodine-refractory advanced thyroid cancer in daily clinical practice: a cohort study from a single center. Endocrine. 2015;49:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Kim M, Kim TH, Shin DY, Lim DJ, Kim EY, Kim WB, Chung JH, Shong YK, Kim BH, Kim WG; Korean Thyroid Cancer Study Group (KTCSG). Tertiary Care Experience of Sorafenib in the Treatment of Progressive Radioiodine-Refractory Differentiated Thyroid Carcinoma: A Korean Multicenter Study. Thyroid. 2018;28:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casella C S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ