Published online Jan 24, 2022. doi: 10.5306/wjco.v13.i1.49
Peer-review started: April 9, 2021
First decision: July 27, 2021
Revised: August 11, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 24, 2022
Processing time: 287 Days and 6.3 Hours
Individuals with Lynch syndrome (LS) and hereditary non-polyposis colorectal cancer (HNPCC) are at increased risk of both colorectal cancer and other cancers. The interplay between immunosuppression, a comorbid inflammatory condition (CID), and HNPCC on cancer risk is unclear.
To evaluate the impact of CIDs, and exposure to monoclonal antibodies and immunomodulators, on cancer risk in individuals with HNPCC.
Individuals prospectively followed in a hereditary cancer registry with LS/HNPCC with the diagnosis of inflammatory bowel disease or rheumatic disease were identified. We compared the proportion of patients with cancer in LS/HNPCC group with and without a CID. We also compared the proportion of patients who developed cancer following a CID diagnosis based upon exposure to immunosuppressive medications.
A total of 21 patients with LS/HNPCC and a CID were compared to 43 patients with LS/HNPCC but no CID. Cancer occurred in 84.2% with a CID compared to 76.7% without a CID (P = 0.74) with no difference in age at first cancer diagnosis 45.5 ± 14.6 vs 43.8 ± 7.1 years (P = 0.67). LS specific cancers were diagnosed in 52.4% with a CID vs 44.2% without a CID (P = 0.54). Nine of 21 (42.9%) patients were exposed to biologics or immunomodulators for the treatment of their CID. Cancer after diagnosis of CID was seen in 7 (77.8%) of exposed individuals vs 5 (41.7%) individuals unexposed to biologics/immunomodulators (P = 0.18). All 7 exposed compared to 3/5 unexposed developed a LS specific cancer. The exposed and unexposed groups were followed for a median 10 years and 8.5 years, respectively. The hazard ratio for cancer with medication exposure was 1.59 (P = 0.43, 95%CI: 0.5-5.1).
In patients with LS/HNPCC, the presence of a concurrent inflammatory condition, or use of immunosuppressive medication to treat the inflammatory condition, might not increase the rate of cancer occurrence in this limited study.
Core Tip: Individuals with hereditary non-polyposis colorectal cancer (HNPCC) are at increased risk of both colorectal cancer and other cancers. When they have a comorbid inflammatory condition (CID) that requires immunosuppression, clinicians may be hesitant to prescribe these medications due to concern of an elevated cancer risk. We show that individuals with HNPCC and CID have a similar cancer risk to those with HNPCC alone, and that the addition of immunosuppression does not increase overall cancer risk, but may increase the risk of LS-specific cancers.
- Citation: Faisal MS, Burke CA, Liska D, Lightner AL, Leach B, O’Malley M, LaGuardia L, Click B, Achkar J, Kalady M, Church J, Mankaney G. Association of cancer with comorbid inflammatory conditions and treatment in patients with Lynch syndrome. World J Clin Oncol 2022; 13(1): 49-61
- URL: https://www.wjgnet.com/2218-4333/full/v13/i1/49.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i1.49
Lynch syndrome (LS) is the most common hereditary cancer syndrome. Patients with LS have an increased cumulative lifetime risk of developing colorectal, endometrial, ovarian, stomach, small bowel, hepatobiliary, urothelial, and brain cancers[1]. This is explained by a germline pathogenic variant (PV) in one of the DNA mismatch repair (MMR) genes resulting in the translation of a defective enzyme unable to correct errors in base pairing during DNA replication[2]. The defective system leads to the accumulation of mutations, regulatory escape from the cell division cycle, and ultimately cancer[3]. LS accounts for 3% of all newly diagnosed colorectal and endometrial cancers[4]. Individuals who meet Amsterdam II criteria, have MSI-H colorectal cancers, but do not have a MMRPV are also have an elevated risk of colorectal cancer[5]. Hereditary non-polyposis colorectal cancer (HNPCC) is an umbrella term that, regardless of MMRPV status, includes individuals with MSI-H cancers and a suggestive family history.
Individuals with HNPCC may have co-existent systemic inflammatory conditions (CID) such as inflammatory bowel disease (IBD) and rheumatic diseases. A deregulated immune system contributes to the pathogenesis of these diseases and various inflammatory mediators and pathways have been implicated[6]. Treatment of moderate to severe inflammatory disease generally involves modulating the immune system with systemic immunosuppressive medications, in particular monoclonal antibodies and immunomodulators alone or in combination[7]. Because the immune system is known to protect against cancer by detecting neoantigens presented by cancer cells[8], of particular importance in HNPCC individuals with immunogenic MSI-H cancers[9,10], clinicians may be hesitant to prescribe these medications in patients with HNPCC due to concern of an elevated cancer risk. Limited data exists regarding the impact of CID and immunosuppressive medication exposure on the cancer risk in HNPCC.
Our primary aim was to evaluate the impact of CID on the cancer risk in HNPCC by comparing HNPCC individuals with and without a CID. Our secondary aim was to assess the effect of monoclonal antibody and/or immunomodulator exposure on cancer risk in HNPCC patients with concurrent CID.
This study was approved by Cleveland Clinic Institutional Review Board (IRB 2884). HNPCC individuals enrolled in the David G. Jagelman Hereditary Colorectal Cancer Registries at the Sanford R. M.D. Center for Hereditary Colorectal Neoplasia at the Cleveland Clinic from 1979 to 2019 who met inclusion criteria were included in the study. HNPCC was defined as individuals with an MSI-H tumor and belonging to a family fulfilling Amsterdam II criteria. Individuals with comorbid IBD including ulcerative colitis (UC) and Crohn’s disease (CD), and rheumatic diseases including rheumatoid arthritis and other inflammatory arthritides, psoriasis, ankylosing spondylitis, spondyloarthritis, systemic sclerosis, scleroderma, dermatomyositis, polymyositis, lupus, sarcoidosis, mixed connective tissue disease and undifferentiated connective tissue disease were included.
Variables extracted from the medical record included demographics, age at first cancer diagnosis and last follow up, MMRPV, sex, race, smoking history, personal and family history of LS-specific (colorectal, endometrial, urothelial, and small bowel) and other cancers, cancer stage and comorbid disease history (age at diagnosis, presenting signs and symptoms, treatments, exposure to biologics and/or immunomodulators with type, dose and duration of treatment noted for each medication).
The primary aim was to compare the proportion of individuals with HNPCC who develop cancer based on CID status. Cases (HNPCC with CID) were matched to controls (HNPCC without CID) in a 1:2 ratio. Controls were randomly chosen from the registry after matching for presence and type of MMRPV, age at last follow up, and gender. We compared the proportion of patients who had developed any cancer up to last follow up or death between the two groups. In a subgroup analysis, we then compared proportion of patients who developed colorectal cancer in HNPCC patients with and without IBD.
Our secondary aim was to compare the proportion of CID patients (n = 21) who developed cancer with and without exposure to a monoclonal antibody and/or immunomodulator therapy. Patients were divided into two groups based on any exposure to these medications. Duration of exposure was determined through the electronic medical record or paper chart review by duration of prescription length and provider notes. Immunosuppressive medications included anti-tumor necrosis factors (TNF) (infliximab, adalimumab, certolizumab pegol, etanercept and golimumab), anti-integrins (natalizumab and vedolizumab), anti-interleukins [anakinra, tocilizumab, sarilumab, ixekizumab, guselkumab, ustekimumab), janus kinase inhibitor (tofaci
Continuous variables are presented as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables are presented as number and percentage. Student t-test or Mann-Whitney-U test was used to compare continuous variables. Categorical variables were compared using chi-square test or Fisher exact test. For the primary aim, the proportion of individuals in each group with cancer was compared. For the secondary aim, we also carried out single variable cox proportional survival analysis to calculate hazard ratio (HR) for cancer and Kaplan Meier curve was constructed for the comparison between exposed and unexposed groups. Time to event started from the year of comorbid disease diagnosis to cancer diagnosis. Individuals who were lost to follow up are included until that time in analysis. All statistical work was done using SPSS v26.0.
64 HNPCC patients including 21 cases with a CID and 43 controls without CID were analyzed. Of the 14 patients with LS, MMRPV included MLH1 (23.8%), MSH2 (14.3%), MSH6 (9.5%) and PMS2 (19.6%). Seven (33%) did not have a MMRPV. Age at last follow up, gender, race, smoking history and family history of cancer did not differ between cases and controls (Table 1). CID in the 21 patients included CD (23.8%), UC (9.5%), inflammatory/rheumatoid arthritis (33.3%), psoriasis (14.3%), and one case each of psoriatic arthritis, dermatomyositis, ankylosing spondylitis, and sarcoidosis. The mean age at CID diagnosis was 39 ± 13 years.
| Inflammatory disease present, n = 21 | Controls, n = 43 | P value | |
| Current age (yr), mean ± SD | 53.9 ± 15.7 | 53.8 ± 7.8 | 0.97 |
| Gender, n (%) | |||
| Female | 12 (57.1) | 25 (58.1) | 0.94 |
| Race, n (%) | |||
| White | 17 (81.0) | 40 (93.0) | 0.34 |
| Black | 1 (4.8) | 1 (2.3) | |
| Others | 3 (14.3) | 2 (4.6) | |
| Smoking Status, n (%) | |||
| Former/current | 9 (42.9) | 20 (46.5) | |
| Never Smoker | 12 (57.1) | 23 (53.5) | 0.72 |
| Family History of Cancer, n (%) | |||
| Colon | 13 (61.9) | 33 (78.6) | 0.16 |
| LS Cancer | 11 (52.4) | 24 (57.1) | 0.72 |
| Other cancers | 12 (57.1) | 25 (58.1) | 0.94 |
| LS MMRPV, n (%) | 14 (66.7) | 21 (67.4) | 1 |
| MLH1 | 5 (23.8) | 10 (23.3) | |
| MSH2 | 3 (14.3) | 7 (16.3) | |
| MSH6 | 2 (9.5) | 4 (9.3) | |
| PMS2 | 4 (19.0) | 8 (18.6) | |
| No MMRPV | 7 (33.3) | 14 (32.6) | |
| Age of HNPCC diagnosis, mean ± SD | |||
| LS | 43.6 ± 14.0 | 45.4 ± 7.6 | 0.66 |
| No MMRPV | 49.0 ± 5.4 | 46.3 ± 2.2 | 0.59 |
| Screening colonoscopies | |||
| Median total number | 5 (IQR 3.0-6.0) | 4 (IQR 2.0-6.0) | 0.19 |
| Median years between colonoscopies | 1.0 (IQR 1.0-1.5) | 1.0 (IQR 1.0-1.6) | 0.87 |
| History of complete or partial colectomy | 15 (76.2) | 23 (53.5) | 0.08 |
| TAH-BSO (% of females in each group) | 10 (83.3) | 19 (76.0) | 0.8 |
| History of Prophylactic TAH-BSO (% of females in each group) | 6 (50.0) | 7 (28.0) | 0.27 |
| Proportion of patients with any cancer, n (%) | 16 (84.2) | 33 (76.7) | 0.74 |
| Cancer Incidence-10 yr follow up | 12 (57.1) | 20 (46.5) | 0.42 |
| Age at Diagnosis of first cancer (yr), mean ± SD | 45.5 ± 14.6 | 43.8 ± 7.1 | 0.67 |
The proportion of patients who had a history of cancer diagnosis at the time of their last follow up was 84.2% in cases and 76.7% in controls (P = 0.74). Age at first cancer diagnosis was 45.5 ± 14.6 years for cases and 43.8 ± 7.1 years for controls (P = 0.67). The proportion of patients who had developed cancer after diagnosis of CID in cases was 57.1% with a 10 year (6.0-16.5) median duration of follow-up and 46.5% in controls (P = 0.42) when also followed for 10 years prior to last follow up or death. Approximately half of the cancers were HNPCC-specific: 52.4% of cases vs 44.2% of controls (P = 0.54) (Table 2). The distribution of cancers based on MMRPV is presented in Table 3.
| Number of patients who developed a cancer | CID present, n = 21 | No CID, n = 43 |
| 12 (57.1) | 20 (46.5) | |
| Lynch syndrome specific cancers | ||
| Colorectal | 9 (42.9) | 12 (27.9) |
| Endometrial | 2 (9.5) | 8 (18.6) |
| Small Bowel | 0 (0) | 1 (2.3) |
| Urothelial | 1 (4.8) | 1 (2.3) |
| Non-Lynch Syndrome-specific cancers | ||
| Breast | 1 (4.8) | 0 (0) |
| Nasopharynx | 1 (4.8) | 0 (0) |
| Prostate | 1 (4.8) | 0 (0) |
| Ovarian | 0 (0) | 1 (2.3) |
| B-Cell Lymphoma | 1 (4.8) | 0 (0) |
| CID present | CID non present | |||||
| CRC | LS specific1 | All other | CRC | LS specific | All other | |
| MLH1 | 2 (40.0) | 2 (40.0) | 1 (20.0) | 2 (20.0) | 2 (20.0) | 0 (0) |
| MSH2 | 2 (66.7) | 0 (0) | 1 (33.3) | 2 (28.6) | 4 (57.1) | 1 (14.3) |
| MSH6 | 0 (0) | 0 (0) | 0 (0) | 2 (50.0) | 1 (25.0) | 0 (0) |
| PMS2 | 2 (50.0) | 0 (0) | 1 (25.0) | 0 (0) | 2 (25.0) | 0 (0) |
| No MMRPV | 3 (42.9) | 1 (14.3) | 1 (14.3) | 6 (42.9) | 1 (7.1) | 0 (0) |
Median total surveillance colonoscopies after HNPCC diagnosis were 5 (IQR 3.0-6.0) for cases and 4 (IQR 2.0-6.0, P = 0.19) for controls with a median 1 year interval for both groups. 42.9% of cases vs 46.5% of controls had partial colectomy and 33.3% of cases compared to 7.0% controls had total proctocolectomy. Overall, 83% of female cases and 76% of female controls had a history of total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO[MSF1]). 50.0% of cases and 28.0% of controls had the procedure prophylactically while the others had TAH-BSO for endometrial cancer. 1 (4.7%) female in case group had prophylactic TAH-BSO prior to the diagnosis of CID.
In a sub-group analysis, we compared the 7 individuals with IBD (5 CD and 2 UC) to the control group (n = 43) for the proportion of patients who developed specifically CRC. One CD individual had stricturing ileitis and no colonic disease or prior colon surgery and another developed CD in an ileal pouch following proctocolectomy and ileal pouch anal anastomosis for resection of a cecal adenocarcinoma. Both individuals were exposed to monoclonal antibodies/immunomodulators for treatment of CD. Of the other three CD patients, two had ileitis and one had mild segmental colitis. They did not receive monoclonal antibodies/immunomodulators for treatment. One UC patient had moderate pancolitis and received monoclonal antibodies/immunomodulator. The other UC patient had moderate left sided colitis and did not receive above mentioned immunosuppressive treatment. Mean duration of follow up for IBD in the exposed group was 19.2 ± 12.1 years compared to the unexposed group 16.0 ± 12.6 years, P = 0.64. All cases had intact colons at the time of CID diagnosis except the one CD individual mentioned above.
Four (57.1%) cases developed colorectal (CRC) cancer (P = 0.86) over a median 10-years (IQR 5-18) from IBD diagnosis compared to 53.5% of controls when followed for 10 years. Both UC and 2/5 CD (40%) patients developed CRC at a median age of 41.8 years (IQR 19-57) compared to 46.0 year (IQR 41-52) in the control group. Median stage of CRC cancer at the time of diagnosis was II in both groups.
For the secondary aim, we compared the proportion of individuals who developed cancer after diagnosis of a CID in the 9/21 (39.5%) individuals exposed to monoclonal antibodies and/or immunomodulators to the 12/21 (61.5%) unexposed. Mean age at end of follow-up for the exposed group was 54.2 ± 20.0 years compared to 53.7 ± 12.4 years for the unexposed group (P = 0.70). The exposed and unexposed groups were followed for a median 10 and 8.5 years, respectively.
No significant difference in age, gender, race, smoking, or family cancer history was observed between the groups (Table 4). 22.2% (2/9) of exposed and 16.7% (2/12) of the unexposed group had a history of cancer prior to CID diagnosis. One individual in the exposed group had a history of breast cancer while the other had a history of both pancreatic and colon cancer. In the unexposed group, one individual had history of colon cancer while the other had a history of both endometrial and colon cancer. Median duration of exposure to monoclonal antibodies and immunomodulators was 5.7 (3.4-8.3) years and 2.5 (0.8-8.0) years, respectively. Four patients on biologics also received combination therapy with an immunomodulator (Table 5).
| Characteristic | Exposed | Unexposed | P value |
| n = 9 (39.5) | n = 12 (60.5) | ||
| Current age (yr), mean ± SD | 54.2 ± 20 | 53.6 ± 12.4 | 0.7 |
| Gender, n (%) | |||
| Female | 4 (44.4) | 8 (66.7) | 0.4 |
| Race, n (%) | |||
| Caucasian | 7 (77.8) | 10 (83.3) | 1 |
| Others | 2 (22.2) | 2(16.7) | |
| Smoking Status, n (%) | |||
| Former/current | 4 (44.4) | 5 (41.7) | 1 |
| Never smoked | 4 (55.6) | 7 (58.3) | |
| Previous history of cancer, n (%) | 2 (22.2) | 2 (16.7) | 1 |
| Family history of cancer, n (%) | |||
| LS specific cancer | 6 (66.7) | 5 (41.7) | 0.39 |
| All other cancers | 6 (44.4) | 8 (66.7) | 0.4 |
| Duration of Follow up, (yr), median (IQR) | 10.0 (9.0-22.0) | 8.5 (5.3-17.3) | 0.38 |
| Comorbid disease, n (%) | 3 (33.3) | 4 (33.3) | 1 |
| Crohn’s disease | 2 (22.2) | 3 (25.0) | |
| Ulcerative colitis | 1 (11.1) | 1 (8.3) | |
| Rheumatic disease, n (%) | 6 (66.7) | 8 (66.7) | 1 |
| Pathogenic variant | |||
| MLH1 | 2 (22.2) | 3 (25.0) | |
| MSH2 | 2 (22.2) | 1 (8.3) | |
| MSH6 | 0 (0) | 2 (16.7) | 0.68 |
| PMS2 | 2 (22.2) | 2 (16.7) | |
| MSI-H | 3 (33.3) | 4 (33.3) | |
| Cancer after CID diagnosis | 7 (77.8) | 5 (41.7) | 0.18 |
| Time to Cancer After Diagnosis of CID, (yr), median (IQR) | 5.0 (2.0-16.0) | 5.0 (1.0-10.5) | 0.64 |
| Age at Diagnosis of first cancer (yr), median (IQR) | 49 (23.0-54.0) | 48 (44.0-50.0) | 0.99 |
| Disease | Genetic Diagnosis | Medication | Duration /mo | Dose/mg | Cancer type and stage | Age at diagnosis of cancer |
| Ulcerative colitis | PMS2 | Ustekinumab, | 12 | Colon II | 19 | |
| Golimumab | 6 | |||||
| Vedolizumab | 24 | |||||
| Crohn’s disease | MLH1 | 6MP | 24 | 50 | Colon I | 57 |
| Crohn’s disease | PMS2 | Adalimumab | 18 | Colon III | 17 | |
| Golimumab | 6 | |||||
| Vedolizumab | 12 | |||||
| Sarcoidosis | MLH1 | MTX | 60 | 15 | Renal I | 49 |
| Rheumatoid arthritis | LLS | Etanercept | 120 | Breast I, Colon IV | 76 | |
| Tofacitinib | 9 | |||||
| MTX | 35 | 15 | ||||
| Azathioprine | 72 | 50 | ||||
| Rheumatoid arthritis | MSH2 | MTX | 4 | 20 | Colon II | 51 |
| Rituximab | 72 | |||||
| Psoriatic arthritis | MSI-H | Adalimumab | 36 | Colon III | 44 | |
| Ustekinumab | 10 | |||||
| MTX | 36 | 15 | ||||
| Dermatomyositis | MSH2 | MTX | 12 | 15 | NA | NA |
| Rheumatoid arthritis | MSI-H | MTX | 120 | 10 | NA | NA |
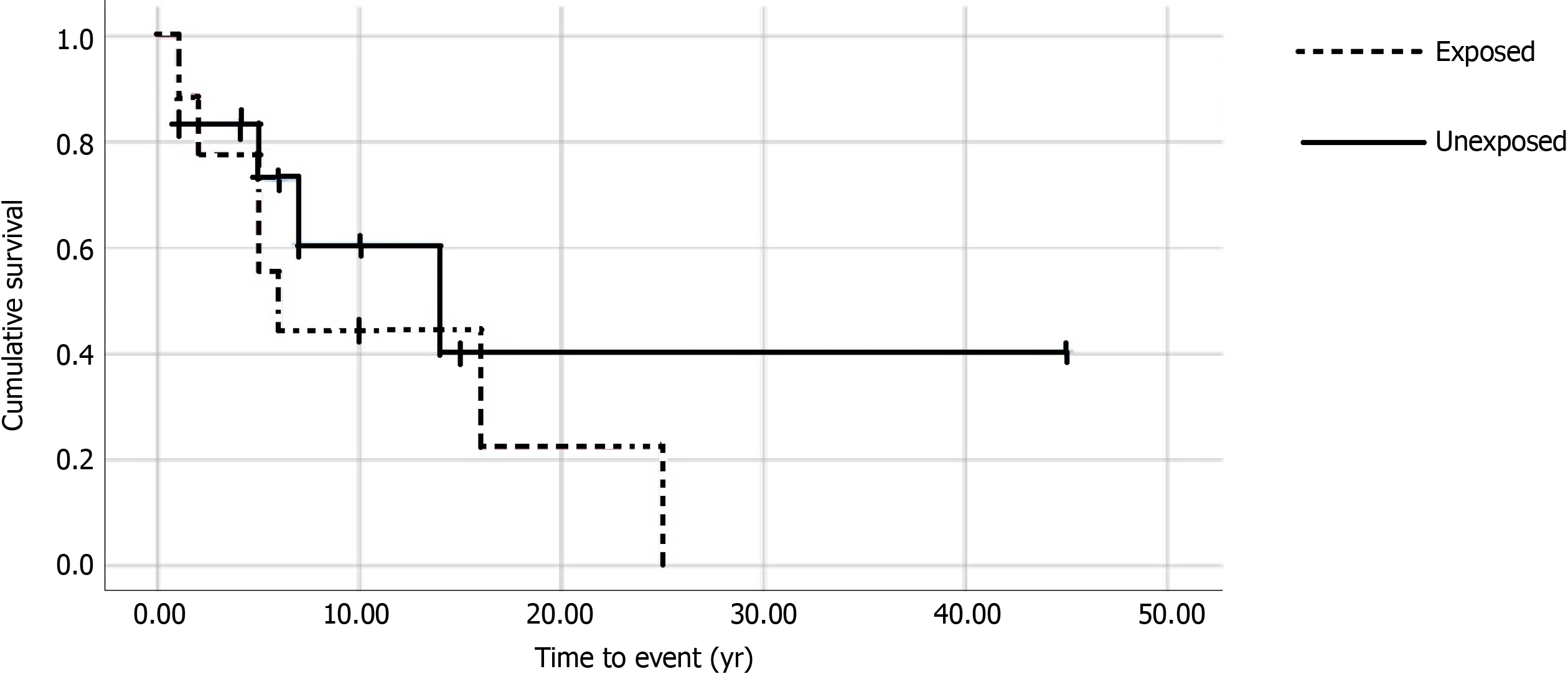
Seven of nine (77.8%) exposed compared to 5/12 (41.7%) unexposed patients developed any cancer after diagnosis of a CID (P = 0.18). The hazard ratio for cancer with medication exposure was calculated to be 1.59 (P = 0.43, 95%CI: 0.5-5.1). Figure 1 shows the Kaplan-Meier curve for cancer after diagnosis of CID. Median time to cancer after IBD or rheumatic disease diagnosis was 5.0 years (P = 0.64) in both groups. All 7 (77.8%) exposed individuals developed a LS-specific cancer compared to 3 (25%) unexposed. 9 total cancers developed in the 7 exposed individuals, including CRC (n = 6), and one each of breast, renal and endometrial cancer. The five unexposed individuals developed seven cancers in total, including CRC (n = 3) and one each of prostate, endometrial, nasopharynx and B cell lymphoma.
In individuals with IBD, 71.4% developed malignancy. All three (60.0%) exposed individuals with IBD developed cancer compared to two (40.0%) unexposed (P = 0.43). Fifty percent of rheumatic disease patients developed cancer. Four (57.1%) were exposed to immunosuppressive medications compared to three unexposed (42.9%). We found no significant difference in the proportion of individuals with cancer based on type of CID[MSF2] (IBD vs rheumatic disease) (P = 0.64). Mean age of diagnosis of CID was also similar in individuals who developed cancer (37.8 ± 14 years) compared to those who did not (40.3 ± 13.4 years, P = 0.39). The duration of CID was not associated with cancer incidence, 10.0 years (IQR 6.5-18.0) in those who developed cancer compared to 10.5 years (IQR 4-14.5) in those who did not develop cancer (P = 0.66).
When an inflammatory condition coexists with HNPCC, the clinician must evaluate the risk of cancer associated with the inflammatory disease itself, and any im
Epidemiological studies in the general population demonstrate that inflammatory diseases are associated with increased cancer risks. The standardized incidence ratio for CRC ranges from 1.1 to 7.0 in IBD and correlates with disease duration, anatomical extent, and severity[13]. A 10%-15% increased risk of cancer is observed in rheumatoid arthritis, particularly for lung cancer and lymphom[14]. Derikx et al[15] found that the incidence rate (4/15) of cancer in LS individuals with IBD was similar to LS without IBD, though they developed cancer at a younger age. Individuals were followed for 7 years and the impact of treatment was not evaluated. Another case series of 12 LS individuals with IBD found that 4 developed CRC between the ages of 32-47 years[16]. We also found a younger median age of cancer diagnosis at 42 years in the four of seven (57.1%) IBD patients but did not observe a difference in the overall cancer risk between those with IBD compared to controls. Interestingly, in the above quoted studies, most CRC cases occurred in those with UC, as both UC patients developed CRC in our study. However, we also found that two of five with colon-predominant CD developed CRC. We hypothesize that a colon-predominant inflammatory process in HNPCC individuals further compounds the risk of colon cancer as observed in IBD. This is further supported by the observation that MLH1 and MSH2 deficient mice who have experimentally induced colonic inflammation develop CRC at faster rates and younger ages than MMR-proficient mice[17,18].
Inflammatory diseases are associated with significant morbidity and poor quality of life if left untreated[19,20]. Therefore, professional societies recommend the use monoclonal antibodies and immunomodulators to treat moderate to severe disease[21-23]. A logical conclusion is that the treatment of an inflammatory disease decreases any associated cancer incidence. However, monoclonal antibodies and immunomodulators have been associated with increased cancer risk in the general population. Wolfe demonstrated increased risks of lymphoma OR 1.7 (95%CI: 1.3-2.2), melanoma OR 2.3 (95%CI: 0.9-5.4), and non-melanotic skin cancer (OR 1.5, 95%CI: 1.2-1.8) in individuals exposed to an anti-TNFs compared to the general population[24]. A meta-analysis from nine clinical trials that compared the cancer risk in rheumatoid arthritis patients exposed to TNF-α inhibitors vs placebo found an elevated OR for cancer of 3.3 (95%CI: 1.2-9.1) in the exposed group[25]. This risk was dose dependent. Similarly, a US Food and Drug Administration analysis from 1998 to 2008 demonstrated higher incidence rates of lymphomas and solid tumors in children and adolescents who received TNF-alpha inhibitors for IBD and juvenile rheumatoid arthritis[26]. The combination of a TNF-alpha inhibitor with a thiopurine immunomodulator further increased this cancer risk and was associated with an increased risk of non-melanoma skin cancer as well[27,28]. In addition, methotrexate has been implicated in lymphoproliferative disorders[29], and azathioprine and 6-mercaptopurine have been implicated in skin cancers and lymphomas[30].
Given the lack of difference in the proportion of cancer cases between HNPCC with and without CID, our secondary aim was to evaluate if medication exposure had any effect on the proportion of individuals who develop cancer. It has been described that TNF-alpha inhibitors play a major role by regulating the TNF Related Apoptosis Inducing Ligand, an important mediator in immune surveillance[31]. In addition, murine models demonstrate that immune suppression results in an increased risk of sporadic breast, lung, small intestine and colon cancers[32]. Suppression of the immune system’s antineoplastic role in MSI-H cancers could further compound these risks. Interestingly, we found that, though non-significant, there was an increased proportion of LS-specific cancers in the exposed group. The near doubling of LS-specific cancers (78% vs 42%) was primarily attributed to CRC cancer (66.6% vs 25%). Individuals with a colon-predominant IBD may benefit from medication sparing therapies and colonic resection may mitigate the elevated cancer risk as well.
The risk of CRC in IBD has historically been associated with severity and duration of the disease[33]. From this standpoint, immunosuppressive medications can potentially decrease CRC incidence in patients with IBD. In a metanalysis, Lu et al[34] describe an antineoplastic effect of thiopurines on colorectal neoplasia in patients with IBD, particularly amongst the patients with ulcerative colitis. However, these studies did not directly address individuals with LS. Genetic susceptibility to malignancy in LS adds another layer of complexity given the intricacy of balancing immunosuppression which decreases malignancy risk in inflammation but may also theoretically decrease immune surveillance.
There are limitations to our series worth mentioning, most of which are related to the inherent limitations seen in a retrospective study. Given the small subset of HNPCC patients with CID, we may not have captured a difference in cancer incidence between the exposed and unexposed groups when indeed there is one. However, this is the largest group of patients described with a length of follow up of at least 10 years. Furthermore, we are not able to specifically comment on the impact of each inflammatory disease based on severity and duration of illness. Moreover, the degree of immunosuppression was highly variable based on type, dose and duration of treatment which is described but not accounted for in the results. In our clinical experience, medications may be prescribed based on the severity of the inflammatory disease and personal history of malignancy. Though there is no data to guide specific management, the average duration of medication exposure exceeded one year. Drug trials also exclude patients with hereditary cancer syndromes, and it is unlikely that there will be a large enough population to evaluate each medication for each inflammatory condition. This study is the first to evaluate the impact of biologics and immunomodulators on cancer risk in HNPCC. Our study population was primarily Caucasian, however the malignancy risk associated with these medications have not been correlated with race. We also included individuals with HNPCC but without a MMRPV potentially masquerading a significant difference between exposed and unexposed groups, if any. However, these patients had MSI-H tumors and were followed in a similar fashion as those with LS due to an increased incidence of malignancy. The number of individuals without a MMRPV was also similar in both groups. Future studies should report cancer specific survival rates.
In our small cohort, CID does not appear to add any additional cancer risk to patients with HNPCC regardless of MMRPV status. The decision to start a biologic or immunomodulator in this cohort is understandably complex and should be individualized with consideration given to any colonic inflammatory disease.
In our small cohort, CID does not appear to add any additional cancer risk to patients with HNPCC regardless of MMRPV status. The decision to start a biologic or immunomodulator in this cohort is understandably complex and should be individualized with consideration given to any colonic inflammatory disease.
Patients with Lynch Syndrome and hereditary non polyposis colorectal cancer (HNPCC) have an increased cumulative lifetime risk of developing colorectal, endometrial, ovarian, stomach, small bowel, hepatobiliary, urothelial, and brain cancers. These individuals may have co-existent systemic inflammatory conditions such as inflammatory bowel disease (IBD) and rheumatic diseases. Treatment of moderate to severe inflammatory disease generally involves modulating the immune system with systemic immunosuppressive medications, in particular monoclonal antibodies and immunomodulators alone or in combination. Interaction of the inflammatory disease and immunosuppressive medications in individuals at increased risk of malignancy due to baseline genetic diagnosis is unknown.
The immune system is known to protect against cancer by detecting neoantigens presented by cancer cells, so clinicians may be hesitant to prescribe these medications in patients with HNPCC due to concern of an elevated cancer risk. This leads to significant morbidity for these individuals. Moreover, treatment with immunosuppressive medications might theretically place them at higher risk for cancer. There is limited existing data to guide clinicians in this regards.
The primary aim was to compare the proportion of individuals with Lynch syndrome and HNPCC who develop cancer based on comorbid inflammatory disease status. Lynch syndrome and HNPCC individuals with comorbid inflammatory disease (cases) were matched to controls (Lynch syndrome and HNPCC without comorbid inflammatory disease) in a 1:2 ratio. Our secondary aim was to compare the proportion of comorbid inflammatory disease patients (n = 21) who developed cancer with and without exposure to a monoclonal antibody and/or immunomodulator therapy in Lynch syndrome and HNPCC population.
Lynch Syndrome and HNPCC individuals enrolled in the David G. Jagelman Hereditary Colorectal Cancer Registries at the Sanford R. M.D. Center for Hereditary Colorectal Neoplasia at the Cleveland Clinic from 1979 to 2019 who met inclusion criteria were included in the study. Individuals with comorbid IBD including ulcerative colitis (UC) and Crohn’s disease (CD), and rheumatic diseases were included. For our primary aim, controls were randomly chosen from the registry after matching for presence and type of mismatch repair gene pathogenic variant, age at last follow up, and gender. We compared the proportion of patients who had developed any cancer up to last follow up or death between the two groups. For our secondary aim, patients were divided into two groups based on any exposure to these medications. Duration of exposure was determined through the electronic medical record or paper chart review by duration of prescription length and provider notes. The proportion of individuals who developed a cancer was calculated from the year of diagnosis of comorbid disease until last follow up or death.
64 HNPCC patients including 21 cases with a comorbid inflammatory disease and 43 controls without comorbid inflammatory disease were analyzed. The proportion of patients who had developed cancer after diagnosis of comorbid inflammatory disease in cases was 57.1% with a 10 year (6.0-16.5) median duration of follow-up and 46.5% in controls (P = 0.42) when also followed for 10 years prior to last follow up or death. Approximately half of the cancers were HNPCC-specific: 52.4% of cases vs 44.2% of controls (P = 0.54). For the secondary aim, we compared the proportion of individuals who developed cancer after diagnosis of a comorbid inflammatory disease in the 9/21 (39.5%) individuals exposed to monoclonal antibodies and/or immunomodulators to the 12/21 (61.5%) unexposed. Seven of nine (77.8%) exposed compared to 5/12 (41.7%) unexposed patients developed any cancer after diagnosis of a CID (P = 0.18). The hazard ratio for cancer with medication exposure was calculated to be 1.59 (P = 0.43, 95%CI: 0.5-5.1). This is the first study of its kind, attempting to address the interaction between genetic predisposition to cancer, inflammatory disease and immunosuppression. It remains to be seen whether these results are reproduced in larger multicenter studies.
In our small cohort, comorbid inflammatory disease does not appear to add any additional cancer risk to patients with HNPCC regardless of MMRPV status. The decision to start a biologic or immunomodulator in this cohort is understandably complex and should be individualized with consideration given to any colonic inflammatory disease.
We propose collaborative research to assess the risk of malignancy in lynch syndrome and HNPCC individuals on immunosuppressive medications. The risk of colorectal cancer in IBD has historically been associated with severity and duration of the disease. From this standpoint, immunosuppressive medications can potentially decrease colorectal incidence in patients with IBD. However, there are no studies that directly address individuals with genetic predisposition to cancer. Genetic susceptibility to malignancy adds another layer of complexity given the intricacy of balancing immunosuppression which decreases malignancy risk in inflammation but may also theoretically decrease immune surveillance.
| 1. | See WA. Commentary on "Risks of primary extracolonic cancers following colorectal cancer in Lynch syndrome." Win AK, Lindor NM, Young JP, Macrae FA, Young GP, Williamson E, Parry S, Goldblatt J, Lipton L, Winship I, Leggett B, Tucker KM, Giles GG, Buchanan DD, Clendenning M, Rosty C, Arnold J, Levine AJ, Haile RW, Gallinger S, Le Marchand L, Newcomb PA, Hopper JL, Jenkins MA, Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, Melbourne School of Population Health, The University of Melbourne, Victoria, Australia: J Natl Cancer Inst 2012;104(18):1363-72 [Epub 2012 Aug 28]. Urol Oncol. 2013;31:716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Koessler T, Oestergaard MZ, Song H, Tyrer J, Perkins B, Dunning AM, Easton DF, Pharoah PD. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Tannergård P, Lipford JR, Kolodner R, Frödin JE, Nordenskjöld M, Lindblom A. Mutation screening in the hMLH1 gene in Swedish hereditary nonpolyposis colon cancer families. Cancer Res. 1995;55:6092-6096. [PubMed] |
| 4. | Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, Hopper JL, Le Marchand L, Gallinger S, Newcomb PA, Haile R, Thibodeau SN, Gunawardena S, Jenkins MA, Buchanan DD, Potter JD, Baron JA, Ahnen DJ, Moreno V, Andreu M, Ponz de Leon M, Rustgi AK, Castells A; EPICOLON Consortium. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 409] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 5. | Ladabaum U. What Is Lynch-like Syndrome and How Should We Manage It? Clin Gastroenterol Hepatol. 2020;18:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O'Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1380] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 8. | Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1059] [Cited by in RCA: 1052] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 9. | Kloor M, von Knebel Doeberitz M. The Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer. 2016;2:121-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1201] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 11. | Candeias SM, Gaipl US. The Immune System in Cancer Prevention, Development and Therapy. Anticancer Agents Med Chem. 2016;16:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195:161-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 328] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P, Duclos B, Bourreille A, Faivre J, Peyrin-Biroulet L, Fléjou JF, Carrat F; CESAME Study Group. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166-175.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | Mellemkjaer L, Linet MS, Gridley G, Frisch M, Møller H, Olsen JH. Rheumatoid arthritis and cancer risk. Eur J Cancer. 1996;32A:1753-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 288] [Article Influence: 9.6] [Reference Citation Analysis (5)] |
| 15. | Derikx LA, Smits LJ, van Vliet S, Dekker E, Aalfs CM, van Kouwen MC, Nagengast FM, Nagtegaal ID, Hoogerbrugge N, Hoentjen F. Colorectal Cancer Risk in Patients With Lynch Syndrome and Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2017;15:454-458.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | McNamara KL, Aronson MD, Cohen Z. Is there a role for prophylactic colectomy in Lynch syndrome patients with inflammatory bowel disease? Int J Colorectal Dis. 2016;31:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Taniguchi K, Kakinuma S, Tokairin Y, Arai M, Kohno H, Wakabayashi K, Imaoka T, Ito E, Koike M, Uetake H, Nishimura M, Yamauchi K, Sugihara K, Shimada Y. Mild inflammation accelerates colon carcinogenesis in Mlh1-deficient mice. Oncology. 2006;71:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kohonen-Corish MR, Daniel JJ, te Riele H, Buffinton GD, Dahlstrom JE. Susceptibility of Msh2-deficient mice to inflammation-associated colorectal tumors. Cancer Res. 2002;62:2092-2097. [PubMed] |
| 19. | Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10:R45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 20. | Lönnfors S, Vermeire S, Greco M, Hommes D, Bell C, Avedano L. IBD and health-related quality of life -- discovering the true impact. J Crohns Colitis. 2014;8:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am J Gastroenterol. 2017;112:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 367] [Article Influence: 40.8] [Reference Citation Analysis (1)] |
| 22. | Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017;153:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 469] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 23. | Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poór G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1826] [Article Influence: 304.3] [Reference Citation Analysis (0)] |
| 24. | Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 385] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 25. | Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1816] [Cited by in RCA: 1830] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 26. | Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 2010;62:2517-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 27. | Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, Walsh C, Lawson R, Reynolds A, Emery P. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 28. | Amari W, Zeringue AL, McDonald JR, Caplan L, Eisen SA, Ranganathan P. Risk of non-melanoma skin cancer in a national cohort of veterans with rheumatoid arthritis. Rheumatology (Oxford). 2011;50:1431-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50:1740-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 450] [Article Influence: 20.5] [Reference Citation Analysis (6)] |
| 30. | Penn I. Cancers complicating organ transplantation. N Engl J Med. 1990;323:1767-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 356] [Article Influence: 9.9] [Reference Citation Analysis (4)] |
| 31. | Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature's TRAIL--on a path to cancer immunotherapy. Immunity. 2003;18:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2026] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 33. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 894] [Article Influence: 40.6] [Reference Citation Analysis (8)] |
| 34. | Lu MJ, Qiu XY, Mao XQ, Li XT, Zhang HJ. Systematic review with meta-analysis: thiopurines decrease the risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:318-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bogach J, Chiu CC, Yang BL S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ