Published online Jul 24, 2021. doi: 10.5306/wjco.v12.i7.522
Peer-review started: February 27, 2021
First decision: March 31, 2021
Revised: April 11, 2021
Accepted: June 18, 2021
Article in press: June 18, 2021
Published online: July 24, 2021
Processing time: 143 Days and 21.7 Hours
The long-term success of standard anticancer monotherapeutic strategies has been hampered by intolerable side effects, resistance to treatment and cancer relapse. These monotherapeutic strategies shrink the tumor bulk but do not effectively eliminate the population of self-renewing cancer stem cells (CSCs) that are normally present within the tumor. These surviving CSCs develop mechanisms of resistance to treatment and refuel the tumor, thus causing cancer relapse. To ensure durable tumor control, research has moved away from adopting the monotreatment paradigm towards developing and using combination therapy. Combining different therapeutic modalities has demonstrated significant therapeutic outcomes by strengthening the anti-tumor potential of monotreatment against cancer and cancer stem cells, mitigating their toxic adverse effects, and ultimately overcoming resistance. Recently, there has been growing interest in combining natural products from different sources or with clinically used che
Core Tip: There has been great interest in integrating thymoquinone (TQ) in combi
- Citation: Fatfat Z, Fatfat M, Gali-Muhtasib H. Therapeutic potential of thymoquinone in combination therapy against cancer and cancer stem cells. World J Clin Oncol 2021; 12(7): 522-543
- URL: https://www.wjgnet.com/2218-4333/full/v12/i7/522.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i7.522
Cancer incidence and mortality are still growing worldwide despite the monumental efforts and the significant progress made in developing therapeutic strategies and improving detection techniques for combatting this disease. Around 19 million new cases and nearly 10 million deaths are estimated globally in 2020[1]. The conventional therapeutic strategies used to treat cancer are surgery, radiotherapy and chemo
Over the last few decades, there has been increased interest in combining cancer treatments rather than using single therapeutic agents. A monotherapeutic strategy having one mode of action eradicates only one subpopulation of tumor cells. Other subpopulations which are less sensitive can escape the treatment and reform a re
Thymoquinone (TQ), the major bioactive compound extracted from Nigella sativa essential oil, has shown promising antitumor activity in vitro and in vivo against a wide range of cancer types[18]. What makes TQ an attractive therapeutic agent is its safe profile. It was found to be non-toxic to several normal cells including normal mouse kidney cells[19], normal human lung fibroblasts[20] and normal human intestinal cells[21]. TQ exerts its antineoplastic effects through several modes of action, and its exact molecular target is not known yet. It inhibits cancer cell proliferation and blocks the cell cycle progression. In addition, TQ induces apoptosis by generating reactive oxygen species (ROS), causing DNA damage, upregulating pro-apoptotic factors, activating caspases and causing poly (ADP-ribose) polymerases (PARP) cleavage, disrupting mitochondrial membrane integrity besides modulating several pathways such as p53, wingless/integrated (Wnt), mitogen-activated protein kinase, signal transducer and activator of transcription 3 (STAT3)[22]. It also interrupts metastasis by downregulating the epithelial to mesenchymal transition transcription factors twist-related protein 1 (TWIST1) and E-Cadherin, and inhibits angiogenesis by suppressing the nuclear factor kappa B (NFkB) pathway[22]. Interestingly, TQ was found to inhibit the proliferation of several chemoresistant cancer cells and induce apoptosis in colon CSCs that are resistant to the conventional chemotherapeutic drug 5-fluorouracil (5-FU)[23,24].
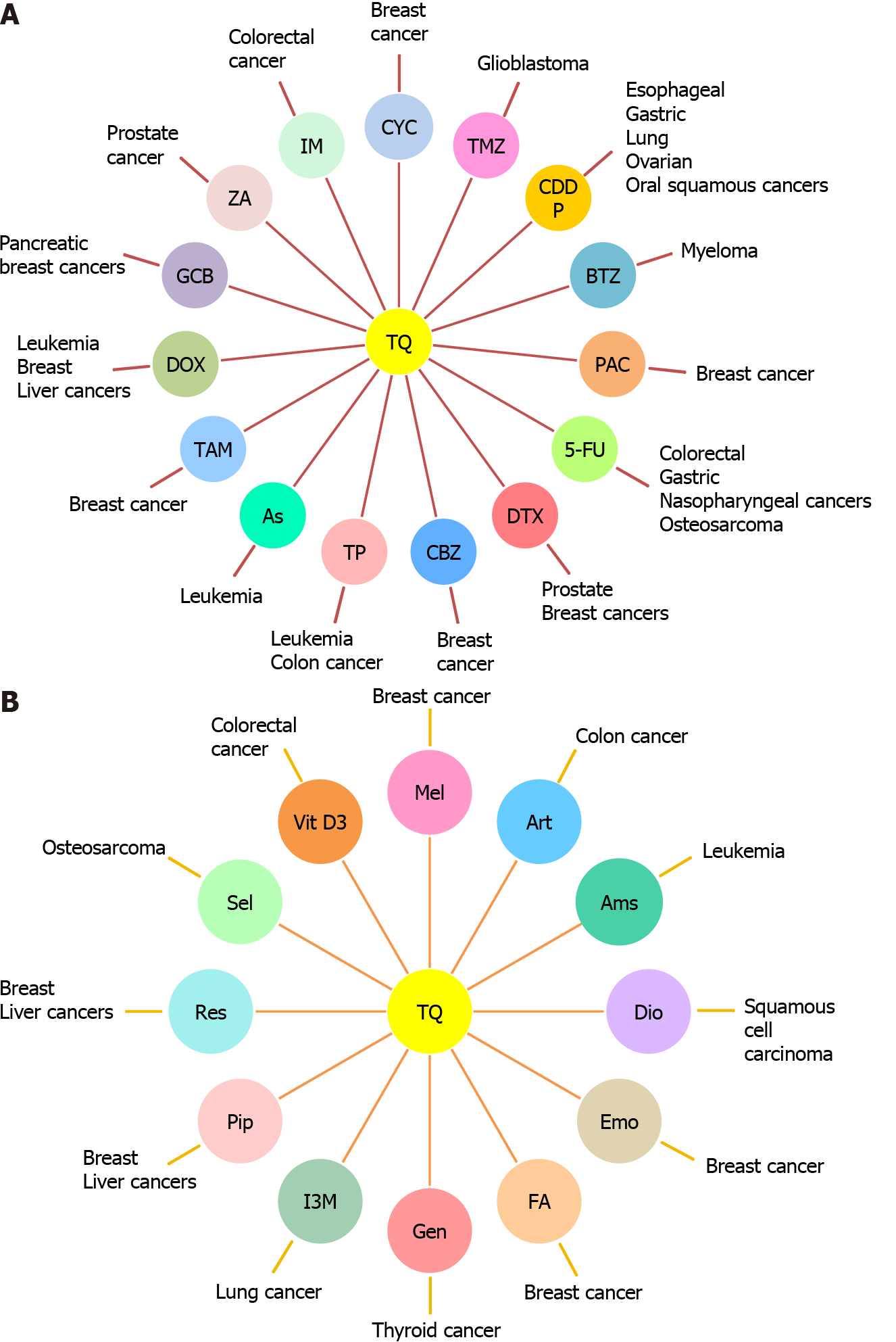
These effective anticancer properties of TQ made it an interesting therapeutic candidate for combination therapy with standard therapeutic agents or other natural products to improve cancer treatment efficacy and safety (Figure 1). Here, we shed light on the combinatorial effects of TQ on the activity of these therapeutic agents used in treating CSCs and cancer cells.
The mode of action of each chemotherapeutic agent as well as the cellular and molecular mechanisms of action of the combination treatment are presented in Table 1.
| Chemotherapeutic agent | Mode of action | Patients or animal model or cell lines | Cellular and molecular mechanism of action of the combination treatment | Ref. |
| Cyclophosphamide | Alkylates guanine base and causes the formation of DNA crosslinks leading to cell death | SKBR-3 and MDA-231 breast cancer cells | Increases the percentage of cells in G1 and sub- G1 phases. Downregulates the phosphorylation of Akt and the expression of cyclin D1 and upregulates PTEN | Emadi et al[25], Khan et al[27] |
| Temozolomide | Methylates DNA at specific sites on guanine and adenine bases causing cell demise | U87MG human glioblastoma multiforme cells | Increases the mitochondrial membrane potential disruption, cytochrome c release, ROS generation, DNA fragmentation and Bax/Bcl-2 ratio. Activates p53, caspases 9 and 3 and reduces NO and GSH levels. Reduces the expression and secretion of MMP-2 and MMP-9. Downregulates beclin-1 and ATG-7 | Stupp et al[29], Khazaei et al[32], Pazhouhi et al[33], Pazhouhi et al[35] |
| Cisplatin | Interacts with purine bases and forms DNA crosslinks resulting in cell death | ID8-NGL mouse ovarian cancer cells. OVCAR3 and NCI/ADR-RES human ovarian cancer cells. BL/6 mice injected with ID8-NGL cells | Increases the level of Bax, pH2AX (ser139), cleaved caspase 3 and PARP. Decreases the level of PCNA and Ki67 | Siddik et al[36], Wilson et al[39] |
| Eca-109 human esophageal cancer cells. BALB/c nude mice inoculated with Eca-109 cells | Decreases the expression of p-STAT3, p-JAK2, Bcl-2, survivin and cyclin D1. Increases the expression of Bax and activates caspases 3, 7 and 9. Induces chromatin condensation and nuclear fragmentation | Hu et al[40] | ||
| NCI-H460 non-small lung cancer cells. SCID mice injected with NCI-H460 cancer cells | Reduces the ratio of phosphor-Ser529 NFkB/NFkB | Jafri et al[42] | ||
| UMSCC-14C head and neck squamous cancer cells and normal oral epithelial cells | Increases p53 and caspase 9 expression. Decreases Bcl-2 expression | Alaufi et al[43] | ||
| SGC-7901 human gastric cancer cells. BALB/c mice implanted with gastric cancer cells | Increases the level of Bax, AIF, cytochrome c, cleaved caspases 9 and 3. Decreases the level of cyclin D1, Bcl-2, procaspases 9 and 3. Inhibits PI3K/Akt signaling pathway and downregulates P-gp by upregulating PTEN | Ma et al[44] | ||
| 5-Fluorouracil | A pyrimidine analogue inhibiting the activity of thymidylate synthase enzyme causing the disruption of DNA synthesis and cell death | BGC-823, SGC-7901, MGC-803 and HGC-27 human gastric cancer cells. BALB/c athymic nude mice inoculated with gastric cancer cells | Increases the release of mitochondrial cytochrome c and the level of Bax, caspases 3 and 9. Decreases the level of Bcl-2 and induces nuclear fragmentation and chromatin condensation | Wilson et al[45], Lei et al[48] |
| Azoxymethane-induced colorectal tumors in Wistar rats | Increases the expression of DKK-1, CDNK-1A, TGF-β1, TGF-βRII, Smad4 and GPx. Decreases the expression of Wnt, β-catenin, NFκB, COX-2, iNOS, VEGF and TBRAS | Kensara et al[49] | ||
| HCT116, HT29 and SW620 human colon cancer cellsSW837 rectal cancer cells. Normal human intestinal epithelial cells. CAM tumors derived from HCT116 cells | Downregulates Wnt/β-catenin and PI3K/Akt pathways | Ndreshkjana et al[50] | ||
| FADU nasopharyngeal cancer cells | Decreases the level of GSH | Williams et al[51] | ||
| MG63 human osteosarcoma cells | Sarman et al[52] | |||
| Gemcitabine | A deoxycytidine analog preventing chain elongation during DNA synthesis causing cell death | PANC-1 and MIA PaCa-2 human pancreatic cancer cells | Downregulates PKM2 and decreases the expression of procaspase 3 and PARP | Moysan et al[53], Pandita et al[56] |
| PANC-1, BxPC-3, and AsPC-1 human pancreatic cancer cell lines. BALB/c nude mice injected with PANC-1 cells | Downregulates Notch1, NICD, Bcl-2, Bcl-xL and XIAP. Inactivates Akt/mTOR/S6 signaling pathway and decreases the phosphorylation and nuclear translocation of p65. Upregulates PTEN, caspases 3 and 9 and Bax and increases cytochrome c release | Mu et al[57] | ||
| MCF-7 and T47D human breast cancer cells | Increases pre-G1 cell population | Bashmail et al[58] | ||
| Paclitaxel | Inhibits microtubules disassembly and induces mitotic arrest | 4T1 mouse breast cancer cells. Ehrlich tumor cells. Balb/c mice injected with Ehrlich tumor ascites cells | Increases the level of full length and cleaved caspases 3, 7 and 12 and PARP. Reduces phosphorylated p65 and Akt1. Modulates genes involved in apoptosis, cytokine -cytokine receptor interaction, Fas signaling, p53 signaling and JAK/STAT signaling | Ojima et al[59], Şakalar et al[63] |
| MCF-7 and T47D human breast cancer cells | Increases pre-G1 cell population. Increases the level of cleaved caspase 3 and PARP and the expression of beclin-1 and LC3-II | Bashmail et al[64] | ||
| MCF-7 human breast cancer cells | Soni et al[65] | |||
| Docetaxel | Inhibits microtubules disassembly and induces mitotic arrest | DU-145 human prostate cancer cells | Blocks PI3K/Akt signaling pathway and induces DNA fragmentation | Ojima et al[59], Dirican et al[69] |
| DU-145 and C4-2B human prostate cancer cells | Inhibits PI3K/Akt signaling pathway. Increases the expression of Bax, Bid, caspase 3 and PARP and decreases the expression of Bcl-xL | Singh et al[70] | ||
| MCF-7 and MDA-MB-231 human breast cancer cells | Induces DNA damage, cells shrinkage, nuclear fragments, apoptotic bodies and cytoplasmic vacuolation | Alkhatib et al[71] | ||
| MCF-7 and MDA-MB-231 human breast cancer cells | Zafar et al[72] | |||
| MCF-7 and MDA-MB-231 human breast cancer cells. Balb/c mice healthy or injected with Ehrlich ascites carcinoma cells | Induces nuclear fragmentation and restores the levels of oxidative stress parameters MDA, SOD and GSH. Prevents the alteration of blood cell count and serum biochemical parameters AST, ALT, creatinine and BUN | Zafar et al[73] | ||
| MCF-7 breast cancer cells | Odeh et al[74] | |||
| Cabazitaxel | Inhibits microtubules disassembly and induces mitotic arrest | MCF-7 and MDA-MB-231 human breast cancer cells | Induces DNA fragmentation and increases the sub-G1 population | Ojima et al[59], Kommineni et al[78] |
| Doxorubicin | Intercalates DNA, inhibits topoisomerase II, forms free radicals when reduced leading to cell cycle arrest and cell death | Human HTLV-1 positive (HuT-102) and HTLV-1 negative (Jurkat) CD4+ malignant T-cell lines. NOD/SCID mice inoculated with HuT-102 tumor cells | Increases the sub-G1 population and induces ROS production. Disrupts the mitochondrial membrane potential. Downregulates the expression of NFkΒ and Ki67 and increases the phosphorylation of p53 | Meredith et al[79], Fatfat et al[83] |
| HL-60 acute myeloid leukemia cells. Dox resistant HT-29 colon carcinoma cells. MCF-7/TOPO multi-drug resistant breast cancer cells | Induces caspases 3 and 8 activity and ROS generation. Disrupts the mitochondrial membrane potential | Effenberger-Neidnicht et al[84] | ||
| BALB/c OlaHsd-foxn1 nude mice injected with MDA-MB-231 breast cancer cells | Induces p38 MAPK phosphorylation and inhibit the expression of XIAP, survivin, Bcl-xL and Bcl-2 | Woo et al[85] | ||
| SMMC-7721 and HepG2 hepatocarcinoma cells and human normal liver cells HL-7702 | Increases caspase 3 and PARP cleavage | Jehan et al[86] | ||
| MDA-MB-231 human breast cancer cells. MCF-10A and 3T3 non-neoplastic cells | Induces cell shrinkage, membrane blebbing and apoptotic bodies and disrupts the cell membrane. Increases the Sub-G0 population | Ibiyeye et al[87] | ||
| MCF-7 human breast adenocarcinoma and HEPG2 human hepatocellular carcinoma. Albino mice implanted with Heps murine liver cancer cells | Decreases NFkB level and increases that of caspase 3. Increases the level of renal antioxidant enzymes SOD and catalase. Modulates the level of renal oxidative stress biomarkers GSH and MDA. Decreases the level of nephrotoxicity biomarkers BUN and serum creatinine | Zidan et al[88] | ||
| Albino transplanted with Ehrlich carcinoma cells | Upregulates p53 and reduces the level of Bcl-2. Decreases the level of cardiac MDA. Decreases the serum level of cardiac markers lactate and creatine | El-Ashmawy et al[89] | ||
| Topotecan | Inhibits DNA topoisomerase I and causes the formation of irreversible DNA double stranded breaks resulting in cell death. Inhibits hypoxia-inducible factor 1α | U937 acute myelogenous leukemia cells | Increases the sub-G1 population. Increases the expression level of Bax/Bcl-2, p53 and p21 and the cleavage of caspases 3 and 9 | Robati et al[90], Khalife et al[95] |
| HT-29 human colon cancer cells | Increases the sub-G1 population. Has no effect on p53, Bax and Bcl-2 expression | Khalife et al[96] | ||
| Bortezomib | Inhibits the proteasome | U266, H929, KMS, RPMI-8226, RPMI-8226-Dox-6 (doxorubicin-resistant clone), RPMI-8226-LR-5 (a melphalan-resistant clone) human multiple myeloma cells. Balb/c mice implanted with U266 cells | Increases the sub-G1 population and the cleavage of caspase 3 and PARP. Reduces the phosphorylation of NFkB (p65) and the expression of Ki67, VEGF, Bcl-2 and the serum levels of IL-6 and TNF-α | Siveen et al[99] |
| Imatinib | Inhibits tyrosine kinase | HCT116 human colorectal cancer cells | Decreases the expression of ABCB1, ABCG2 and hOCT1. Increases the uptake/efflux ratio of imatinib | Thabet et al[103] |
| Tamoxifen | Competes with estrogen and estradiol for the binding to their receptors and modulates their signaling pathway | MCF-7 and MDA-MB-231 human breast cancer cells | Day et al[104], Ganji-Harsini et al[106] | |
| MCF-7, MDA-MB-231, MDA-MB-468, T47D, NIH/3T3 and HaCaT human breast cancer cells. Athymic BALB/c mice injected with MDA-MB-231 cells | Decreases the expression of XIAP and the level of p-Akt, p-Bad, p-MAPK and p-GSK-3β and downregulates the expression of Bcl-xL, Bcl-2 and Ki67. Increases the cleavage of caspase 9 and PARP and induces the expression of Bax, AIF, cytochrome c and p27. Increases the percentage of cells in sub-G1 phase and the fragmentation of DNA | Rajput et al[107] | ||
| Breast cancer patients | Increases the tumor tissue catalase, SOD and caspase 3. Decreases the tumor tissue Bcl-2, TGF-β1, MDA, TNF-α and IL-6 | Kabel et al[108] | ||
| Zoledronic acid | Inhibits osteoclast-mediated bone resorption | PC-3 and DU- 145 human prostate cancer cells | Increases DNA fragmentation and activates caspases 3 and 7 | Polascik et al[109], Dirican et al[112] |
| Arsenic trioxide | Human HTLV-I positive (HuT-102 and C91) and HTLV-I negative (CEM and Jurkat) malignant T-cell lines. NOD SCID mice inoculated with HuT-102 cells | Increases the percentage of cells in Pre-G1 phase, the disruption of the mitochondrial membrane potential and the cleavage of PARP and caspase 3. Upregulates p53, Bax and downregulates XIAP and Bcl- 2 | Houssein et al[117] |
Cyclophosphamide[25]: Cyclophosphamide has been used in treating a broad spectrum of cancers including leukemia, lymphoma, breast and ovarian cancers[26]. In a study conducted by Khan et al[27], TQ was found to amplify the growth inhibitory effects of low doses of cyclophosphamide in breast cancer cells. This combination upregulated the expression of phosphatase and tensin homolog (PTEN) and downregulated the phosphorylation of its downstream signaling molecule Akt in addition to decreasing the expression of cyclin D1. The PTEN/phosphatidylinositol-3-kinase (PI3K)/Akt pathway is known to be an important tumorigenic pathway responsible for cell cycle progression, survival, and migration of malignant cells[28].
Temozolomide[29]: Temozolomide (TMZ) has been approved by the Food and Drug Administration for the treatment of glioblastoma multiforme[30]. However, the anti
Cisplatin[36]: Cisplatin (CDDP) is one of the most used chemotherapeutic drugs in the treatment of a wide range of cancer types[37]. The primary dose-limiting side effect of CDDP is the dose- dependent nephrotoxicity, which restricts the use of high doses of CDDP to increase its anticancer activity[38]. Numerous studies have demonstrated the anti-neoplastic efficacy of combining TQ with CDDP in different types of cancers as an alternative way to increase CDDP potency. In ovarian cancer, these two agents were found to synergize to induce apoptosis in vitro and in a mouse syngeneic model. The combination was more effective in increasing the levels of Bcl-2-associated X protein (Bax), phospho-histone 2AX on serine 139, cleaved caspase 3 and PARP and in down
5-FU[45]: 5-FU is the third most frequently used chemotherapeutic drug in the treat
Gemcitabine[53]: Gemcitabine (GCB) has been approved for treating different types of cancer including pancreatic and breast cancers[54]. The therapeutic application of GCB was compromised by several drawbacks including its short half-life in the blood circulation, poor membrane permeability in addition to the development of chemoresistance[55]. TQ and GCB were found to induce synergistic apoptosis in GCB sensitive and resistant pancreatic cancer cells by downregulating pyruvate kinase M2 expre
Paclitaxel[59]: Paclitaxel (PAC) is widely used for the treatment of several cancer types including breast, ovary, colorectal and lung cancers[60]. The major challenges that restrict its curative effect are chemoresistance and adverse effects that are mainly caused by the polyethylated castor oil that is usually added to its formulation to increase its solubility[61,62]. Three studies have evaluated the potential of the combinatorial effect of TQ and PAC in breast cancer. TQ-PAC combination produced a synergistic anticancer activity through the modulation of genes involved in apoptosis, cytokine-cytokine receptor interaction, Fas signaling, p53 signaling and JAK/STAT signaling[63]. In another study, combining TQ with PAC augmented the necrotic and caspase dependent- apoptotic responses in T47D breast cancer cells compared to PAC alone. While in the apoptosis defective MCF-7 cells, both individual and combined treatments induced significant cell death by autophagy[64]. The co-encapsulation of TQ and PAC in polymeric biodegradable poly (lactide-co-glycolide) nanoparticles lowered PAC effective anticancer dose and reduced cancer cell viability more effec
Docetaxel: Docetaxel (DTX) has been approved for the treatment of different type of tumors including prostate cancer and breast cancer[66]. However, low water solu
Cabazitaxel: Cabazitaxel (CBZ) was approved as the second line therapy for metastatic castration-resistant prostate cancer[75]. However, its low aqueous solubility, poor membrane permeability, and severe side effects like neutropenia and anemia are the challenging drawbacks for successful cancer management[76,77]. Combining TQ with CBZ caused synergistic apoptotic effects in breast cancer cells. To address the drug delivery challenge, TQ and CBZ were co-loaded in lipospheres. The combined drugs loaded lipospheres had enhanced apoptotic effects compared to the drug combination in solution[78].
Doxorubicin[79]: Doxorubicin (DOX) is a primarily adopted chemotherapeutic agent for treating a wide spectrum of solid and liquid tumors[80]. Despite the robust anti
Topotecan[90]: Topotecan (TP) was approved for the second-line treatment of small cell lung cancer and was recommended to treat platinum resistant ovarian cancer[91,92]. The instability of the chemical structure of TP in aqueous solutions and in the plasma reduces its anticancer efficacy and causes side effects[93,94]. TQ was found to boost the anti-proliferative and apoptotic effects of non-cytotoxic doses of TP in acute myelogenous leukemia and in colon cancer cells. This effect was exerted by upregulation of p53 and Bax, downregulation of Bcl-2, increase in the cleavage of caspases 9 and 3 in leukemia cells and through p53- and Bax/Bcl-2-independent mechanisms in colon cancer cells. In addition, pretreatment of leukemia cells with TQ followed by TP was found to be more effective than the simultaneous application of both therapeutic agents[95,96].
Bortezomib: Bortezomib (BTZ) was approved for the treatment of multiple myeloma[97]. It acts by inhibiting NFkB pathway known to be constitutively activated in multiple myeloma due to genetic aberrations in its components[98]. TQ was found to augment the apoptotic activity of BTZ in multiple myeloma cells in vitro by enhancing caspase 3 activation and PARP cleavage. In a xenograft multiple myeloma mouse model, TQ potentiated the anti-neoplastic effects of BTZ by further suppressing NFkB and consequently downregulating the proliferative (Ki67), anti-apoptotic (Bcl-2), angiogenic (VEGF) and inflammatory (interleukin-6 and tumor necrosis factor-α) effectors. The authors further showed that TQ reduced the proliferation of BTZ resistant multiple myeloma cells[99].
Imatinib: Imatinib (IM) is a potent tyrosine kinase inhibitor that was approved for treating chronic myeloid leukemia and gastrointestinal stromal tumors[100]. Resis
Tamoxifen[104]: Tamoxifen (TAM) is one of the first-line therapies for hormone receptor-positive breast cancer patients[105]. A synergistic apoptotic effect was observed by combining TQ and TAM in breast cancer cells in vitro regardless of hormone receptor status[106]. Apoptosis was induced through synergistic inhibition of X-linked inhibitor of apoptosis protein (XIAP) resulting in caspase 9 activation and PARP cleavage along with PI3K/Akt pathway inhibition, which caused the downregulation of Bcl-xL, Bcl-2, and upregulation of Bax, apoptosis inducing factor, cyto
Zoledronic acid: Zoledronic acid is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. It was approved to prevent and reduce the progression of skeletal complications associated with bone metastasis from solid tumors including prostate cancer[109]. Besides its anti-resorption activity, preclinical and clinical data demonstrated its anti-tumor effects in different types of cancer[110,111]. TQ intensified the apoptotic activity of zoledronic acid in PC-3 (hormone resistant and chemotherapy sensitive) and DU-145 (hormone and chemotherapy resistant) prostate cancer cell lines through a synergistic increase in DNA fragmen
Arsenic trioxide was approved for the treatment of acute promyelocytic leukemia[113]. The combination of arsenic trioxide (As) with interferon alpha (IFN-α) was found to have an effective anti-neoplastic activity in ATL. As and IFN-α synergistically induced apoptosis in ATL leukemia cells in vitro and cured murine ATL[114,115]. A phase II trial involving patients with relapsed/refractory adult T-cell leukemia/lym
Radiotherapy is a mainstay therapeutic modality for the treatment of early and advanced solid cancers. Nearly 50% of cancer patients receive radiotherapy during their treatment course[118]. However, its therapeutic potency was found to be compromised by the damage of the surrounding healthy tissue in addition to the development of radioresistance[119]. To overcome these challenges and enhance radiotherapy efficacy, exploring radiosensitizers, molecules that make cancer cells more susceptible to radiations, has attracted great attention[120]. Several studies demonstrated the radiosensitizing role of TQ on cancer cells in vitro. TQ augmented the anti-proliferative and apoptotic effects of ionizing radiation and further enriched the sub-G1 population in breast cancer cells[121]. In addition, sensitization with TQ prevented the radiation-induced metastatic progression of breast cancer cells through the restoration of the levels of TGF-β and its downstream effectors in addition to epithelial and mesenchymal markers[122]. In melanoma, TQ enhanced the apoptotic responses of low doses of gamma knife irradiation by further inhibiting the phos
| Therapeutic agent | Animal model or cell line | Cellular and molecular mechanism of action of the combination treatment | Ref. |
| Radiation | MCF-7 and T47D human breast cancer cells | Increases the percentage of cells in sub-G1 phase | Velho-Pereira et al[121] |
| MCF-7 and MDA-MB-231 human breast cancer cells | Restores the expression levels of TGF-β and its downstream molecules NFkB, Smad2, Snail and Twist, adhesion molecules E-cadherin and cytokeratin 19, mesenchymal markers integrin αV, MMP-9, and MMP-2 | Rajput et al[122] | |
| B16-F10 melanoma cells | Inhibits the phosphorylation of JAK2 and STAT3. Increases the expression of caspase 3 and Bax. Reduce the expression of Bcl-2 and survivin and the level of VEGF-A, MCP-1, TGF-β1, RANTES and IL-1β. Induces DNA damage | Hatiboglu et al[123] | |
| microRNA-34a | BT-549 metastatic breast cancer cells | Targets and downregulates TWIST1 and ZEB1 | Imani et al[126] |
| Akt-siRNA | Akt-overexpressing MCF-7 and T47D. Tamoxifen resistant MCF-7 and T47D breast cancer cells. BALB/c mice injected with MCF-7/TAM cells | Reduces Akt expression and MDM-2 activation. Activates p53, increases the level of Bax and Bim and decreases the level of Bcl-2 and Ki67 | Rajput et al[127] |
| Vitamin D3 | Azoxymethane-induced colorectal tumors in Wistar rats | Reduces the level of Wnt, β-catenin, NFkB, COX-2, iNOS, VEGF and HSP-90 and increases that of DKK-1, CDNK-1A, TGF-β1, TGF-β/RII and Smad4 | Mohamed et al[131] |
| Melatonin | EMT6/P mouse breast cancer cells. Balb/C mice transplanted with EMT6/P cells | Reduces the expression of VEGF and the serum level of AST and ALT. Increases the serum level of IFN-α and decreases that of IL-4 | Odeh et al[134] |
| Artemisinin | CCRF-CEM and multidrug-resistant CEM/ADR5000 human leukemia cells. Healthy human foreskin fibroblasts | Fröhlich et al[136] | |
| Artesunic acid | HCT116, HT29, Caco-2, DLD-1 colon cancer cells. HCEC nonmalignant colon epithelial cells | Induces ROS generation, DNA damage, PARP and caspase 9 cleavage. Increases the level of ɣ-H2AX | Fröhlich et al[137] |
| Diosgenin | A431 and Hep2 human squamous cell carcinoma. Swiss albino mice injected with sarcoma 180 cells | Induces DNA fragmentation and cytoskeletal changes. Decreases the expression of CD31 and Ki67 | Das et al[138] |
| Emodin | MCF-7, MDA-MB-231, MDA-MB-468 and T47D human breast cancer cells. CAM inoculated with MCF-7 cells | Increases the percentage of cells in sub-G1 phase. Increases ROS generation, cytochrome c release, expression levels of p53, Bax and cleaved caspase 3. Reduces Bcl-2, pFAK and integrinβ1 expression level. Induces nuclear fragmentation, shrinkage, apoptotic body formation, chromatin condensation and membrane blebbing | Bhattacharjee et al[140] |
| Ferulic acid | MDA-MB-231 human breast cancer cells | Al-Mutairi et al[143] | |
| Genistein | CALC-62 and ACC448 human thyroid cells derived from anaplastic carcinoma CGTH-W1, ACC360 derived from follicular carcinoma | Reduces the expression level of human telomerase reverse transcriptase, VEGF-A and NFkB. Increases the expression level of PTEN and p21 and activates caspase 3 | Ozturk et al[145] |
| Indirubin-3-monoxime | A549 human lung cancer cells. HFL-1 human fetal lung fibroblast. CD1-nude mice injected with A549 cells | Increases the percentage of cells in Sub-G0 phase. Reduces Bcl-2/Bax ratio, TNF-α release and p-Akt (s473), p-mTOR, NFkB/p65, caspase3 and p53 expression level | Dera et al[147] |
| Piperine | EMT6/P mouse mammary cancer cells. Balb/C female mice injected with EMT6/P cancer cells | Reduces VEGF expression. Increases IFN-γ and IL-2 level and caspase 3 activity | Talib et al[149] |
| HepG2 human hepatocellular cancer cells | Increase ROS generation and decreases GSH and NADPH level | Das et al[151] | |
| Resveratrol | HepG2 human hepatocellular cancer cells | Increases caspase 3 activity. Decreases GSH and MDA level | Ismail et al[153] |
| EMT6/p mouse epithelial breast cancer cells. MCF-7 and T47D human epithelial breast cancer cells kidney epithelial cells. Balb/C mice injected with EMT6/p cancer cells | Induces DNA fragmentation and increases IFN-γ and IL-4 level. Reduces VEGF expression | Alobaedi et al[154] | |
| Selenium | MG-63 human osteosarcoma cell line | Increases cellular damage, and decreases the level of alkaline phosphatase and GSH | Barron et al[156] |
Gene therapy is a modern therapeutic approach that demonstrated immense and impressive potential against cancer. It consists of delivering therapeutic genetic materials such as small interfering RNA (siRNA), microRNA, and anti-sense oligonucleotides into cancer cells to restore target gene expression, which is modulated and associated with tumorigenesis[124]. miR-34a is a tumor-suppressive microRNA found to be downregulated in numerous human cancers including breast cancer[125]. Re-introducing miR-34a in metastatic breast cancer cells targeted and inhibited the expression of epithelial to mesenchymal transition-associated proteins TWIST1, zinc finger E-box binding homeobox 1 and NOTCH1 and suppressed breast cancer cell migration and invasion. Moreover, combining TQ with miR-34a synergistically downregulated TWIST1 and zinc finger E-box binding homeobox 1, suggesting the promising therapeutic potential of this combination against breast cancer metastasis[126]. In another study, multilamellar gold niosomes were developed for the co-delivery of therapeutic Akt-siRNA and TQ to overcome chemotherapeutic resistance induced by Akt overexpression in breast cancer. TQ-siRNA dual loaded niosomes produced stronger anti-proliferative and apoptotic effects in breast cancer in vitro and in vivo compared to free TQ and TQ loaded niosomes. The mechanism of the combination treatment involved an effective decrease of the cellular level of Akt which sensitized breast cancer cells to TQ toxicity leading to inhibition of mouse double minute 2 and therefore induction of p53-dependent apoptosis[127].
Vitamins: Vitamin D3, the active metabolite of vitamin D, was reported to have potent chemopreventive effects against colorectal cancer in vitro and in vivo[128,129]. In addition, vitamin D supplementation was demonstrated to have clinically positive effects on survival outcomes in patients with colorectal cancer[130]. TQ was found to enhance the chemopreventive effect of vitamin D3 in suppressing the initiation and progression of colon tumors in an azoxymethane-induced rat model of colon cancer. The combination treatment significantly attenuated the number of grown tumors and large aberrant crypts foci. In addition, it decreased the level of pro-oncogenic (Wnt, β-catenin, NFkB, heat shock protein 90 HSP-90) and angiogenic (VEGF, iNOS and COX2) biomarkers and increased the expression of anti-oncogenic (DKK-1, CDNK-1A, TGF-β1, TGF-β/RII and Smad4) biomarkers compared with individual treatments[131].
Melatonin: Melatonin is a natural hormone involved in different biological activities including regulating the circadian rhythm[132]. Ample evidence revealed that melatonin exerts powerful anti-tumor effects through different modes of action including the activation of anticancer immune responses[133]. The combination of TQ with melatonin in breast cancer bearing mice resulted in 60% of cure in treated mice and produced a stronger apoptotic, necrotic and anti-angiogenetic response in addition to a more potent activation of T helper 1 mediated anticancer immune res
Numerous studies have tested the anti-neoplastic efficacy of combining TQ with other plant-derived molecules in different types of cancer. Artemisinin is a sesquiterpene lactone extracted from the Chinese medicinal plant Artemisia annua[135]. Fröhlich et al[136,137] linked each of Artemisinin and its semisynthetic derivative artesunic acid with TQ via covalent bonds and tested the anticancer efficacy of the formed hybrid molecules in vitro. They found that the ether-linked artemisinin-TQ hybrid exhibited a potent and selective anti-proliferative activity that was superior to that of the conventional drug DOX against sensitive and multidrug-resistant leukemia cells without being toxic to normal human foreskin fibroblasts[136]. They also found that the ester-linked artesunic acid-TQ hybrid promoted apoptosis mediated by ROS-induced DNA damage in colon cancer cells while being non-toxic to normal colon epithelial cells. The hybrid’s effect was found superior to each of the conventional drug 5-FU, the dual and individual treatments[137]. In another study, Das et al[138] demonstrated the syner
TQ was found to potentiate the effects of each of GCB and PAC in depleting the CD44+/CD24- CSCs population within MCF-7 and T47D breast cancer cells[58,64]. In another study, the co-delivery of DOX and TQ in ACNP effectively eradicated breast CSCs enriched from MDA-MB-231 cells cultured in 3D compared to single drug loaded ACNP and drug combinations in solution. The combined drugs loaded ACNP effi
The combined treatment of TQ and emodin improved the elimination of breast CSCs as demonstrated by the enhanced reduction in mammospheres forming efficiency and in CD44+/CD24- CSCS population compared to single treatments. Moreover, it down
We have emphasized the tremendous potential of TQ in augmenting the anti-neoplastic effects of different therapeutic modalities against a wide range of cancer cells. TQ sensitized cancer cells to radiotherapy and improved outcomes of cancer resistance to conventional chemotherapeutic agents. The use of TQ in combination therapy also lowered the effective doses of standard chemotherapies which helped reduce their associated toxicities while maintaining their therapeutic effectiveness. The combination of TQ with other plant-derived molecules has shown interesting results and merits further investigation to introduce them as potential candidates for treating cancer. Although the studies investigating TQ potency in eliminating CSC in combination therapy are scarce, their results demonstrated great promise. Involving TQ in combination therapy could possibly further eliminate CSCs from tumors and prevent regrowth of neoplasms.
Despite its remarkable anticancer activity, studies reporting TQ anticancer therapeutic potential in clinical settings are still limited due mainly to its hydropho
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68596] [Article Influence: 13719.2] [Reference Citation Analysis (201)] |
| 2. | Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F, Gong Z, Guo C, Li X, Deng H, Cao K, Zhou M, Xiang B, Li Y, Li G, Xiong W, Zeng Z. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res. 2018;37:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 316] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 3. | Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ, Ibrahim ME, Rauch C. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 450] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 4. | Haque MM, Desai KV. Pathways to Endocrine Therapy Resistance in Breast Cancer. Front Endocrinol (Lausanne). 2019;10:573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Sabnis AJ, Bivona TG. Principles of Resistance to Targeted Cancer Therapy: Lessons from Basic and Translational Cancer Biology. Trends Mol Med. 2019;25:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 6. | Mao JJ, Chung A, Benton A, Hill S, Ungar L, Leonard CE, Hennessy S, Holmes JH. Online discussion of drug side effects and discontinuation among breast cancer survivors. Pharmacoepidemiol Drug Saf. 2013;22:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 2542] [Article Influence: 282.4] [Reference Citation Analysis (3)] |
| 8. | Park SY, Gönen M, Kim HJ, Michor F, Polyak K. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J Clin Invest. 2010;120:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Assenov Y, Brocks D, Gerhäuser C. Intratumor heterogeneity in epigenetic patterns. Semin Cancer Biol. 2018;51:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (3)] |
| 10. | Jolly MK, Celià-Terrassa T. Dynamics of Phenotypic Heterogeneity Associated with EMT and Stemness during Cancer Progression. J Clin Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Pribluda A, de la Cruz CC, Jackson EL. Intratumoral Heterogeneity: From Diversity Comes Resistance. Clin Cancer Res. 2015;21:2916-2923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Prieto-Vila M, Takahashi RU, Usuba W, Kohama I, Ochiya T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 13. | Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8:38022-38043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 1718] [Article Influence: 214.8] [Reference Citation Analysis (0)] |
| 14. | Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3023] [Cited by in RCA: 2672] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 15. | Taylor WF, Jabbarzadeh E. The use of natural products to target cancer stem cells. Am J Cancer Res. 2017;7:1588-1605. [PubMed] |
| 16. | Sauter ER. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev Clin Pharmacol. 2020;13:265-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 17. | Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2277] [Cited by in RCA: 4161] [Article Influence: 693.5] [Reference Citation Analysis (0)] |
| 18. | Majdalawieh AF, Fayyad MW, Nasrallah GK. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit Rev Food Sci Nutr. 2017;57:3911-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Park EJ, Chauhan AK, Min KJ, Park DC, Kwon TK. Thymoquinone induces apoptosis through downregulation of c-FLIP and Bcl-2 in renal carcinoma Caki cells. Oncol Rep. 2016;36:2261-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Gurung RL, Lim SN, Khaw AK, Soon JF, Shenoy K, Mohamed Ali S, Jayapal M, Sethu S, Baskar R, Hande MP. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS One. 2010;5:e12124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Mahmoud YK, Abdelrazek HMA. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed Pharmacother. 2019;115:108783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Schneider-Stock R, Fakhoury IH, Zaki AM, El-Baba CO, Gali-Muhtasib HU. Thymoquinone: fifty years of success in the battle against cancer models. Drug Discov Today. 2014;19:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Ballout F, Monzer A, Fatfat M, Ouweini HE, Jaffa MA, Abdel-Samad R, Darwiche N, Abou-Kheir W, Gali-Muhtasib H. Thymoquinone induces apoptosis and DNA damage in 5-Fluorouracil-resistant colorectal cancer stem/progenitor cells. Oncotarget. 2020;11:2959-2972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 708] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 26. | Falzone L, Salomone S, Libra M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front Pharmacol. 2018;9:1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 578] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 27. | Khan A, Aldebasi YH, Alsuhaibani SA, Khan MA. Thymoquinone Augments Cyclophosphamide-Mediated Inhibition of Cell Proliferation in Breast Cancer Cells. Asian Pac J Cancer Prev. 2019;1153-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 316] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 29. | Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2:552-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Dehdashti AR, Hegi ME, Regli L, Pica A, Stupp R. New trends in the medical management of glioblastoma multiforme: the role of temozolomide chemotherapy. Neurosurg Focus. 2006;20:E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3:198-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 842] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 32. | Khazaei M, Pazhouhi M. Temozolomide-Mediated Apoptotic Death Is Improved by Thymoquinone in U87MG Cell Line. Cancer Invest. 2017;35:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Pazhouhi M, Sariri R, Khazaei MR, Moradi MT, Khazaei M. Synergistic effect of temozolomide and thymoquinone on human glioblastoma multiforme cell line (U87MG). J Cancer Res Ther. 2018;14:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol Cancer Ther. 2018;17:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 504] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 35. | Pazhouhi M, Sariri R, Rabzia A, Khazaei M. Thymoquinone synergistically potentiates temozolomide cytotoxicity through the inhibition of autophagy in U87MG cell line. Iran J Basic Med Sci. 2016;19:890-898. [PubMed] |
| 36. | Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265-7279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2561] [Article Influence: 111.3] [Reference Citation Analysis (1)] |
| 37. | van den Berg JH, Beijnen JH, Balm AJ, Schellens JH. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat Rev. 2006;32:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019;26:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 333] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 39. | Wilson AJ, Saskowski J, Barham W, Yull F, Khabele D. Thymoquinone enhances cisplatin-response through direct tumor effects in a syngeneic mouse model of ovarian cancer. J Ovarian Res. 2015;8:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Hu X, Ma J, Vikash V, Li J, Wu D, Liu Y, Zhang J, Dong W. Thymoquinone Augments Cisplatin-Induced Apoptosis on Esophageal Carcinoma Through Mitigating the Activation of JAK2/STAT3 Pathway. Dig Dis Sci. 2018;63:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1338] [Cited by in RCA: 1712] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 42. | Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010;29:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Alaufi OM, Noorwali A, Zahran F, Al-Abd AM, Al-Attas S. Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci Rep. 2017;7:13131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Ma J, Hu X, Li J, Wu D, Lan Q, Wang Q, Tian S, Dong W. Enhancing conventional chemotherapy drug cisplatin-induced anti-tumor effects on human gastric cancer cells both in vitro and in vivo by Thymoquinone targeting PTEN gene. Oncotarget. 2017;8:85926-85939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11:282-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 46. | Sara JD, Kaur J, Khodadadi R, Rehman M, Lobo R, Chakrabarti S, Herrmann J, Lerman A, Grothey A. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10:1758835918780140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 47. | Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 549] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 48. | Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X, Dong W. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem Biophys Res Commun. 2012;417:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Kensara OA, El-Shemi AG, Mohamed AM, Refaat B, Idris S, Ahmad J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des Devel Ther. 2016;10:2239-2253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Ndreshkjana B, Çapci A, Klein V, Chanvorachote P, Muenzner JK, Huebner K, Steinmann S, Erlenbach-Wuensch K, Geppert CI, Agaimy A, Ballout F, El-Baba C, Gali-Muhtasib H, Roehe AV, Hartmann A, Tsogoeva SB, Schneider-Stock R. Combination of 5-fluorouracil and thymoquinone targets stem cell gene signature in colorectal cancer cells. Cell Death Dis. 2019;10:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Williams S, Tucci MA, Benghuzzi HA. The effect of combination treatments of epigallocatechin-3-gallate, thymoquinone, and 5-Fluorouracil on fadu nasopharyngeal carcinoma cells. Biomed Sci Instrum. 2014;50:361-366. [PubMed] |
| 52. | Sarman H, Bayram R, Benek SB. Anticancer drugs with chemotherapeutic interactions with thymoquinone in osteosarcoma cells. Eur Rev Med Pharmacol Sci. 2016;20:1263-1270. [PubMed] |
| 53. | Moysan E, Bastiat G, Benoit JP. Gemcitabine versus Modified Gemcitabine: a review of several promising chemical modifications. Mol Pharm. 2013;10:430-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 54. | Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Future Oncol. 2005;1:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 55. | Amrutkar M, Gladhaug IP. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 56. | Pandita A, Kumar B, Manvati S, Vaishnavi S, Singh SK, Bamezai RN. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS One. 2014;9:e107154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Mu GG, Zhang LL, Li HY, Liao Y, Yu HG. Thymoquinone Pretreatment Overcomes the Insensitivity and Potentiates the Antitumor Effect of Gemcitabine Through Abrogation of Notch1, PI3K/Akt/mTOR Regulated Signaling Pathways in Pancreatic Cancer. Dig Dis Sci. 2015;60:1067-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Bashmail HA, Alamoudi AA, Noorwali A, Hegazy GA, AJabnoor G, Choudhry H, Al-Abd AM. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci Rep. 2018;8:11674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 59. | Ojima I, Lichtenthal B, Lee S, Wang C, Wang X. Taxane anticancer agents: a patent perspective. Expert Opin Ther Pat. 2016;26:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 60. | Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 381] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 61. | Galletti E, Magnani M, Renzulli ML, Botta M. Paclitaxel and docetaxel resistance: molecular mechanisms and development of new generation taxanes. ChemMedChem. 2007;2:920-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 62. | Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1266] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 63. | Şakalar Ç, İzgi K, İskender B, Sezen S, Aksu H, Çakır M, Kurt B, Turan A, Canatan H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. 2016;37:4467-4477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Bashmail HA, Alamoudi AA, Noorwali A, Hegazy GA, Ajabnoor GM, Al-Abd AM. Thymoquinone Enhances Paclitaxel Anti-Breast Cancer Activity via Inhibiting Tumor-Associated Stem Cells Despite Apparent Mathematical Antagonism. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Soni P, Kaur J, Tikoo K. Dual drug-loaded paclitaxel–thymoquinone nanoparticles for effective breast cancer therapy. J Nanopart Res. 2015;17:1-12. |
| 66. | Ramaswamy B, Puhalla S. Docetaxel: a tubulin-stabilizing agent approved for the management of several solid tumors. Drugs Today (Barc). 2006;42:265-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Akhtartavan S, Karimi M, Karimian K, Azarpira N, Khatami M, Heli H. Evaluation of a self-nanoemulsifying docetaxel delivery system. Biomed Pharmacother. 2019;109:2427-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Baker J, Ajani J, Scotté F, Winther D, Martin M, Aapro MS, von Minckwitz G. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009;13:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 69. | Dirican A, Atmaca H, Bozkurt E, Erten C, Karaca B, Uslu R. Novel combination of docetaxel and thymoquinone induces synergistic cytotoxicity and apoptosis in DU-145 human prostate cancer cells by modulating PI3K-AKT pathway. Clin Transl Oncol. 2015;17:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Singh SK, Apata T, Gordetsky JB, Singh R. Docetaxel Combined with Thymoquinone Induces Apoptosis in Prostate Cancer Cells via Inhibition of the PI3K/AKT Signaling Pathway. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 71. | Alkhatib MH, Bawadud RS, Gashlan HM. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci Rep. 2020;10:18124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Zafar S, Akhter S, Ahmad I, Hafeez Z, Alam Rizvi MM, Jain GK, Ahmad FJ. Improved chemotherapeutic efficacy against resistant human breast cancer cells with co-delivery of Docetaxel and Thymoquinone by Chitosan grafted lipid nanocapsules: Formulation optimization, in vitro and in vivo studies. Colloids Surf B Biointerfaces. 2020;186:110603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 73. | Zafar S, Akhter S, Garg N, Selvapandiyan A, Kumar Jain G, Ahmad FJ. Co-encapsulation of docetaxel and thymoquinone in mPEG-DSPE-vitamin E TPGS-lipid nanocapsules for breast cancer therapy: Formulation optimization and implications on cellular and in vivo toxicity. Eur J Pharm Biopharm. 2020;148:10-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Odeh F, Naffa R, Azzam H, Mahmoud IS, Alshaer W, Al Bawab A, Ismail S. Co-encapsulation of thymoquinone with docetaxel enhances the encapsulation efficiency into PEGylated liposomes and the chemosensitivity of MCF7 breast cancer cells to docetaxel. Heliyon. 2019;5:e02919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Paller CJ, Antonarakis ES. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des Devel Ther. 2011;5:117-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Mahajan M, Khurana RK, Sahajpal NS, Utreja P, Sankar R, Singh B, Jain SK. Emerging Strategies and Challenges for Controlled Delivery of Taxanes: A Comprehensive Review. Curr Drug Metab. 2015;16:453-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Kommineni N, Mahira S, Domb AJ, Khan W. Cabazitaxel-Loaded Nanocarriers for Cancer Therapy with Reduced Side Effects. Pharmaceutics. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Kommineni N, Saka R, Bulbake U, Khan W. Cabazitaxel and thymoquinone co-loaded lipospheres as a synergistic combination for breast cancer. Chem Phys Lipids. 2019;224:104707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Meredith AM, Dass CR. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol. 2016;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 80. | Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267-3285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 1054] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 81. | Al-Malky HS, Al Harthi SE, Osman AM. Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. J Oncol Pharm Pract. 2020;26:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 82. | Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 663] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 83. | Fatfat M, Fakhoury I, Habli Z, Mismar R, Gali-Muhtasib H. Thymoquinone enhances the anticancer activity of doxorubicin against adult T-cell leukemia in vitro and in vivo through ROS-dependent mechanisms. Life Sci. 2019;232:116628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Effenberger-Neidnicht K, Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol. 2011;67:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Woo CC, Hsu A, Kumar AP, Sethi G, Tan KH. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One. 2013;8:e75356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 86. | Jehan S, Zhong C, Li G, Zulqarnain Bakhtiar S, Li D, Sui G. Thymoquinone Selectively Induces Hepatocellular Carcinoma Cell Apoptosis in Synergism With Clinical Therapeutics and Dependence of p53 Status. Front Pharmacol. 2020;11:555283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 87. | Ibiyeye KM, Nordin N, Ajat M, Zuki ABZ. Ultrastructural Changes and Antitumor Effects of Doxorubicin/Thymoquinone-Loaded CaCO3 Nanoparticles on Breast Cancer Cell Line. Front Oncol. 2019;9:599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Zidan AA, El-Ashmawy NE, Khedr EG, Ebeid EM, Salem ML, Mosalam EM. Loading of doxorubicin and thymoquinone with F2 gel nanofibers improves the antitumor activity and ameliorates doxorubicin-associated nephrotoxicity. Life Sci. 2018;207:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | El-Ashmawy NE, Khedr EG, Ebeid EM, Salem ML, Zidan AA, Mosalam EM. Enhanced anticancer effect and reduced toxicity of doxorubicin in combination with thymoquinone released from poly-N-acetyl glucosamine nanomatrix in mice bearing solid Ehrlish carcinoma. Eur J Pharm Sci. 2017;109:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Robati M, Holtz D, Dunton CJ. A review of topotecan in combination chemotherapy for advanced cervical cancer. Ther Clin Risk Manag. 2008;4:213-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Kim YH, Mishima M. Second-line chemotherapy for small-cell lung cancer (SCLC). Cancer Treat Rev. 2011;37:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Abushahin F, Singh DK, Lurain JR, Grendys EC, Rademaker AW, Schink JC. Weekly topotecan for recurrent platinum resistant ovarian cancer. Gynecol Oncol. 2008;108:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Vali AM, Toliyat T, Shafaghi B, Dadashzadeh S. Preparation, optimization, and characterization of topotecan loaded PEGylated liposomes using factorial design. Drug Dev Ind Pharm. 2008;34:10-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Kollmannsberger C, Mross K, Jakob A, Kanz L, Bokemeyer C. Topotecan - A novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology. 1999;56:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Khalife R, El-Hayek S, Tarras O, Hodroj MH, Rizk S. Antiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemia. Clin Lymphoma Myeloma Leuk. 2014;14 Suppl:S46-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Khalife R, Hodroj MH, Fakhoury R, Rizk S. Thymoquinone from Nigella sativa Seeds Promotes the Antitumor Activity of Noncytotoxic Doses of Topotecan in Human Colorectal Cancer Cells in Vitro. Planta Med. 2016;82:312-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 505] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 98. | Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy JD Jr, Kuehl WM, Staudt LM. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 793] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 99. | Siveen KS, Mustafa N, Li F, Kannaiyan R, Ahn KS, Kumar AP, Chng WJ, Sethi G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget. 2014;5:634-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 100. | Westerdijk K, Desar IME, Steeghs N, van der Graaf WTA, van Erp NP; Dutch Pharmacology and Oncology Group (DPOG). Imatinib, sunitinib and pazopanib: From flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br J Clin Pharmacol. 2020;86:258-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 101. | Nestal de Moraes G, Souza PS, Costas FC, Vasconcelos FC, Reis FR, Maia RC. The Interface between BCR-ABL-Dependent and -Independent Resistance Signaling Pathways in Chronic Myeloid Leukemia. Leuk Res Treatment. 2012;2012:671702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | Zhang Q, Li Z, Xu K, Qian Y, Chen M, Sun L, Song S, Huang X, He Z, Li F, Zhang D, Yang L, Wang Y, Xu H, Xu Z. Intracellular concentration and transporters in imatinib resistance of gastrointestinal stromal tumor. Scand J Gastroenterol. 2019;54:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Thabet NA, El-Khouly D, Sayed-Ahmed MM, Omran MM. Thymoquinone chemosensitizes human colorectal cancer cells to imatinib via uptake/efflux genes modulation. Clin Exp Pharmacol Physiol. 2021;48:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Day CM, Hickey SM, Song Y, Plush SE, Garg S. Novel Tamoxifen Nanoformulations for Improving Breast Cancer Treatment: Old Wine in New Bottles. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 105. | Zhang T, Feng F, Zhao W, Tian J, Yao Y, Zhou C, Dong S, Wang C, Zang C, Lv Q, Sun C. Effect of first-line endocrine therapy in patients with hormone-sensitive advanced breast cancer: a network meta-analysis. Onco Targets Ther. 2018;11:2647-2656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Ganji-Harsini S, Khazaei M, Rashidi Z, Ghanbari A. Thymoquinone Could Increase The Efficacy of Tamoxifen Induced Apoptosis in Human Breast Cancer Cells: An In Vitro Study. Cell J. 2016;18:245-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 107. | Rajput S, Kumar BN, Sarkar S, Das S, Azab B, Santhekadur PK, Das SK, Emdad L, Sarkar D, Fisher PB, Mandal M. Targeted apoptotic effects of thymoquinone and tamoxifen on XIAP mediated Akt regulation in breast cancer. PLoS One. 2013;8:e61342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 108. | Kabel AM, Rashidy MAE, Omar MS. Ameliorative Potential of Tamoxifen/Thymoquinone Combination in Patients with Breast Cancer: A Biochemical and Immunohistochemical. J Can Sci Res. 2016;1:102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag. 2008;4:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Green J, Lipton A. Anticancer properties of zoledronic acid. Cancer Invest. 2010;28:944-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Avilés A, Nambo MJ, Neri N, Castañeda C, Cleto S, Huerta-Guzmán J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol. 2007;24:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Dirican A, Erten C, Atmaca H, Bozkurt E, Kucukzeybek Y, Varol U, Oktay Tarhan M, Karaca B, Uslu R. Enhanced cytotoxicity and apoptosis by thymoquinone in combination with zoledronic acid in hormone- and drug-resistant prostate cancer cell lines. J BUON. 2014;19:1055-1061. [PubMed] |
| 113. | Wang QQ, Jiang Y, Naranmandura H. Therapeutic strategy of arsenic trioxide in the fight against cancers and other diseases. Metallomics. 2020;12:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 114. | El-Sabban ME, Nasr R, Dbaibo G, Hermine O, Abboushi N, Quignon F, Ameisen JC, Bex F, de Thé H, Bazarbachi A. Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood. 2000;96:2849-2855. [PubMed] |
| 115. | El Hajj H, El-Sabban M, Hasegawa H, Zaatari G, Ablain J, Saab ST, Janin A, Mahfouz R, Nasr R, Kfoury Y, Nicot C, Hermine O, Hall W, de Thé H, Bazarbachi A. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J Exp Med. 2010;207:2785-2792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 116. | Hermine O, Dombret H, Poupon J, Arnulf B, Lefrère F, Rousselot P, Damaj G, Delarue R, Fermand JP, Brouet JC, Degos L, Varet B, de Thé H, Bazarbachi A. Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol J. 2004;5:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 117. | Houssein M, Fatfat M, Habli Z, Ghazal N, Moodad S, Khalife H, Khalil M, Gali-Muhtasib H. Thymoquinone synergizes with arsenic and interferon alpha to target human T-cell leukemia/lymphoma. Life Sci. 2020;251:117639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1209] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 119. | Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1293] [Cited by in RCA: 1611] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 120. | Linam J, Yang LX. Recent developments in radiosensitization. Anticancer Res. 2015;35:2479-2485. [PubMed] |
| 121. | Velho-Pereira R, Kumar A, Pandey BN, Jagtap AG, Mishra KP. Radiosensitization in human breast carcinoma cells by thymoquinone: role of cell cycle and apoptosis. Cell Biol Int. 2011;35:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 122. | Rajput S, Kumar BN, Banik P, Parida S, Mandal M. Thymoquinone restores radiation-induced TGF-β expression and abrogates EMT in chemoradiotherapy of breast cancer cells. J Cell Physiol. 2015;230:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 123. | Hatiboglu MA, Kocyigit A, Guler EM, Akdur K, Khan I, Nalli A, Karatas E, Tuzgen S. Thymoquinone Enhances the Effect of Gamma Knife in B16-F10 Melanoma Through Inhibition of Phosphorylated STAT3. World Neurosurg. 2019;128:e570-e581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Goswami R, Subramanian G, Silayeva L, Newkirk I, Doctor D, Chawla K, Chattopadhyay S, Chandra D, Chilukuri N, Betapudi V. Gene Therapy Leaves a Vicious Cycle. Front Oncol. 2019;9:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 242] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 125. | Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P, Tassone P, Caraglia M. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014;3:e194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 406] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 126. | Imani S, Wei C, Cheng J, Khan MA, Fu S, Yang L, Tania M, Zhang X, Xiao X, Fu J. MicroRNA-34a targets epithelial to mesenchymal transition-inducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget. 2017;8:21362-21379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 127. | Rajput S, Puvvada N, Kumar BN, Sarkar S, Konar S, Bharti R, Dey G, Mazumdar A, Pathak A, Fisher PB, Mandal M. Overcoming Akt Induced Therapeutic Resistance in Breast Cancer through siRNA and Thymoquinone Encapsulated Multilamellar Gold Niosomes. Mol Pharm. 2015;12:4214-4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 128. | Murillo G, Matusiak D, Benya RV, Mehta RG. Chemopreventive efficacy of 25-hydroxyvitamin D3 in colon cancer. J Steroid Biochem Mol Biol. 2007;103:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 129. | Tangpricha V, Spina C, Yao M, Chen TC, Wolfe MM, Holick MF. Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr. 2005;135:2350-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 130. | Vaughan-Shaw PG, Buijs LF, Blackmur JP, Theodoratou E, Zgaga L, Din FVN, Farrington SM, Dunlop MG. The effect of vitamin D supplementation on survival in patients with colorectal cancer: systematic review and meta-analysis of randomised controlled trials. Br J Cancer. 2020;123:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 131. | Mohamed AM, Refaat BA, El-Shemi AG, Kensara OA, Ahmad J, Idris S. Thymoquinone potentiates chemoprotective effect of Vitamin D3 against colon cancer: a pre-clinical finding. Am J Transl Res. 2017;9:774-790. [PubMed] |
| 132. | Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M, Canesi L, Papa S, Galli F. Melatonin signaling and cell protection function. FASEB J. 2010;24:3603-3624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 133. | Vinther AG, Claësson MH. [The influence of melatonin on the immune system and cancer]. Ugeskr Laeger. 2015;177:V10140568. [PubMed] |
| 134. | Odeh LH, Talib WH, Basheti IA. Synergistic effect of thymoquinone and melatonin against breast cancer implanted in mice. J Cancer Res Ther. 2018;14:S324-S330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 135. | Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 902] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 136. | Fröhlich T, Reiter C, Saeed MEM, Hutterer C, Hahn F, Leidenberger M, Friedrich O, Kappes B, Marschall M, Efferth T, Tsogoeva SB. Synthesis of Thymoquinone-Artemisinin Hybrids: New Potent Antileukemia, Antiviral, and Antimalarial Agents. ACS Med Chem Lett. 2018;9:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 137. | Fröhlich T, Ndreshkjana B, Muenzner JK, Reiter C, Hofmeister E, Mederer S, Fatfat M, El-Baba C, Gali-Muhtasib H, Schneider-Stock R, Tsogoeva SB. Synthesis of Novel Hybrids of Thymoquinone and Artemisinin with High Activity and Selectivity Against Colon Cancer. ChemMedChem. 2017;12:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 138. | Das S, Dey KK, Dey G, Pal I, Majumder A, MaitiChoudhury S, kundu SC, Mandal M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS One. 2012;7:e46641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 139. | Sethi G, Shanmugam MK, Warrier S, Merarchi M, Arfuso F, Kumar AP, Bishayee A. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 140. | Bhattacharjee M, Upadhyay P, Sarker S, Basu A, Das S, Ghosh A, Ghosh S, Adhikary A. Combinatorial therapy of Thymoquinone and Emodin synergistically enhances apoptosis, attenuates cell migration and reduces stemness efficiently in breast cancer. Biochim Biophys Acta Gen Subj. 2020;1864:129695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 141. | Dong X, Fu J, Yin X, Cao S, Li X, Lin L; Huyiligeqi; Ni J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother Res. 2016;30:1207-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 551] [Article Influence: 55.1] [Reference Citation Analysis (7)] |
| 142. | Mahendra P, Bisht S. Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn Rev. 2012;6:141-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 143. | Al-Mutairi A, Rahman A, Rao MS. Low Doses of Thymoquinone and Ferulic Acid in Combination Effectively Inhibit Proliferation of Cultured MDA-MB 231 Breast Adenocarcinoma Cells. Nutr Cancer. 2021;73:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 144. | Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 446] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 145. | Ozturk SA, Alp E, Yar Saglam AS, Konac E, Menevse ES. The effects of thymoquinone and genistein treatment on telomerase activity, apoptosis, angiogenesis, and survival in thyroid cancer cell lines. J Cancer Res Ther. 2018;14:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 146. | Damiens E, Baratte B, Marie D, Eisenbrand G, Meijer L. Anti-mitotic properties of indirubin-3'-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene. 2001;20:3786-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 147. | Dera AA, Rajagopalan P, Al Fayi M, Ahmed I, Chandramoorthy HC. Indirubin-3-monoxime and thymoquinone exhibit synergistic efficacy as therapeutic combination in in-vitro and in-vivo models of Lung cancer. Arch Pharm Res. 2020;43:655-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 148. | Meghwal M, Goswami TK. Piper nigrum and piperine: an update. Phytother Res. 2013;27:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 149. | Talib WH. Regressions of Breast Carcinoma Syngraft Following Treatment with Piperine in Combination with Thymoquinone. Sci Pharm. 2017;85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 150. | Thombare N, Jha U, Mishra S, Siddiqui MZ. Guar gum as a promising starting material for diverse applications: A review. Int J Biol Macromol. 2016;88:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 151. | Das S, Bera D, Pal K, Mondal D, Karmakar P, Das S, et al. Guar gum micro-vehicle mediated delivery strategy and synergistic activity of thymoquinone and piperine: An in vitro study on bacterial and hepatocellular carcinoma cells. J Drug Deliv Sci Technol. 2020;60:101994. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 152. | Perrone D, Fuggetta MP, Ardito F, Cottarelli A, De Filippis A, Ravagnan G, De Maria S, Lo Muzio L. Resveratrol (3,5,4'-trihydroxystilbene) and its properties in oral diseases. Exp Ther Med. 2017;14:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 153. | Ismail N, Abdel–Mottaleb Y, Eissa Ahmed AA, El-Maraghy NN. Novel combination of thymoquinone and resveratrol enhances anticancer effect on hepatocellular carcinoma cell line. Futur J Pharm Sci. 2018;4:41-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 154. | Alobaedi OH, Talib WH, Basheti IA. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac J Trop Med. 2017;10:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 155. | Kieliszek M. Selenium⁻Fascinating Microelement, Properties and Sources in Food. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 156. | Barron J, Benghuzzi H, Tucci M. Effects of thymoquinone and selenium on the proliferation of mg 63 cells in tissue culture. Biomed Sci Instrum. 2008;44:434-440. [PubMed] |
| 157. | Ibiyeye KM, Zuki ABZ. Cockle Shell-Derived Aragonite CaCO3 Nanoparticles for Co-Delivery of Doxorubicin and Thymoquinone Eliminates Cancer Stem Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yuan YY