Published online Dec 24, 2021. doi: 10.5306/wjco.v12.i12.1202
Peer-review started: April 15, 2021
First decision: June 17, 2021
Revised: June 29, 2021
Accepted: November 18, 2021
Article in press: November 18, 2021
Published online: December 24, 2021
Processing time: 252 Days and 16.9 Hours
Gastric cancer (GC) is one of the most common malignant tumors worldwide. Tensin 4 (TNS4) is an adhesive protein belonging to the tensin family. This protein is located in focal adhesion sites. The TNS4 gene is considered an oncogene in numerous cancers. This protein plays an important role in adhesion, migration and proliferation of cells.
To evaluate expression of TNS4 protein in GC tissues and analysis of the clinical and histopathological parameters as well as the overall survival rate of patients.
The expression of TNS4 was assessed in 89 patients using immunohistochemistry.
Positive expression of TNS4 was observed in 49 of 89 patients (55.06%). Higher TNS4 expression was more common in GC tumors with a diameter ≥ 5 cm (P = 0.040). We demonstrated that an increase in TNS4 expression was more frequent in tumors of the histological type without mucinous components than in tumors from mucosal cancers (P = 0.023). Furthermore, TNS4 expression was higher in moderately differentiated tumors than in poorly differentiated and non-differentiated tumors (P = 0.002). Increased TNS4 expression was also noted in the intestinal type of GC according to Lauren’s classification (P = 0.020). No statistically significant correlation was found between the expression of TNS4 and the overall survival rate of patients.
TNS4 expression was significantly higher in tumors with a diameter ≥ 5 cm of the moderately differentiated intestinal type (according to Lauren’s classification) of GC without a mucinous component. Therefore, increased TNS4 expression is related to the histological type of GC with a better prognosis.
Core Tip: Tensin 4 (TNS4) is an adhesive protein belonging to the tensin family that plays an important role in cell adhesion, migration, and proliferation. These processes are important in cancer development and may limit cancer cell growth and improve patient survival. By applying immunohistochemistry, we investigated TNS4 expression in gastric cancer (GC) tissues and discovered that TNS4 expression was significantly higher in tumors with a diameter greater than 5 cm, in tumors of the moderately differentiated intestinal type (according to Lauren’s classification) and in GC without a mucinous component. We concluded that enhanced TNS4 expression was associated with the histological type of GC with a better prognosis.
- Citation: Nizioł M, Zińczuk J, Zaręba K, Guzińska-Ustymowicz K, Pryczynicz A. Increased tensin 4 expression is related to the histological type of gastric cancer. World J Clin Oncol 2021; 12(12): 1202-1214
- URL: https://www.wjgnet.com/2218-4333/full/v12/i12/1202.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i12.1202
In 2018, more than one million people were diagnosed with gastric cancer (GC), accounting for 5.7% of all malignant cancers worldwide; this cancer is ranked 5th in terms of incidence. Moreover, 8.2% of patients with GC died, which reveals the aggressive nature of this disease. GC was diagnosed twice as often in men than in women[1]. The development of this cancer is a multistage process, and there are numerous environmental and genetic factors that contribute to its progression, including infection with Helicobacter pylori (H. pylori) bacteria and Epstein-Barr virus, consumption of excess salt and alcohol or smoking[2]. An alarming phenomenon is the incidence of this type of cancer in young people under 40 years of age, most often as a result of a genetic predisposition. A previous report showed that 30% of patients under 30 had GC in their family history[3]. The diagnosis of this cancer at early stages of its progression is of key importance. Preoperative chemotherapy followed by surgical removal of the tumor mass in early cancer results in a 90% 5-year survival rate[4]. Therefore, it is crucial to search for molecular and biochemical changes typical of early stages of cancer development to inhibit tumor progression and increase the survival rate of patients with this type of cancer.
Tensin 4 (TNS4), also known as C-terminal tensin-like protein, belongs to the tensin family, which also includes tensins 1–3. The TNS4 gene is located on the long arm of chromosome 17q12-21. The TNS4 protein has a molecular weight of 77 kDa. In terms of structure, TNS4 is composed of the Src homology 2 domain (SH2) and the phosphotyrosine-binding domain (PTB). The SH2 domain allows the TNS4 protein to participate in the transmission of intracellular signals, while the PTB domain binds to membrane integrator receptors. Unlike tensins 1–3, TNS4 does not have an actin-binding domain[5]. Intracellularly, TNS4 is located in focal adhesion sites, where it enables signaling between the extracellular matrix and the cell interior. From a physiological perspective, the expression of TNS4 has been detected in the prostate and placenta[6]. Pathologically increased TNS4 expression appears in cancerous tumors. TNS4 was originally classified as a suppressor gene in prostate cancer[6], but as research on tensins progressed, its role as an oncogene was demonstrated in colorectal, breast, colon, esophagus, and lung cancers and thymoma, where this protein was overexpressed[7-15]. TNS4 plays an important role in biological processes connected with carcinogenesis, such as proliferation, migration, cell adhesion and invasiveness[16].
The aim of our study was to evaluate the IHC expression of TNS4 protein in GC (stages I-IV) as well as to assess the relationship between protein expression and selected clinical and pathological parameters and the overall survival rate of patients.
The study was conducted on a group of 89 patients diagnosed with GC who were treated surgically in the 2nd Clinical Department of General and Gastroenterological Surgery of the Medical University of Bialystok in the years 2005–2015. The tissue material was obtained from the archives of the Academic Center for Pathomorphological and Genetic-Molecular Diagnostics in Bialystok. The inclusion criterion was diagnosed adenocarcinoma at any stage of its progression; the exclusion criteria were diagnosed squamous cell carcinoma and other non-epithelial cancers, metastases of other cancers to the stomach and the lack of complete medical records. The normal mucous membrane was used as the control tissue. The research was approved by the Bioethics Committee of the Medical University of Bialystok, permission No. R-I-002/29/2019. The study was conducted in accordance with the World Medical Association Declaration of Helsinki for ethical principles for medical research involving human subjects. The characteristics of the study group are presented in Table 1.
| Parameter | Number of cases |
| Age | |
| < 60 | 29 (32.58%) |
| ≥ 60 | 60 (67.42%) |
| Gender | |
| Female | 29 (32.58%) |
| Male | 60 (67.42%) |
| Tumor diameter | |
| < 5 cm | 18 (20.22%) |
| ≥ 5 cm | 71 (79.78%) |
| Tumor localization | |
| Upper 1/3 | 17 (19.10%) |
| Middle1/3 | 33 (37.08%) |
| Lower 1/3 | 16 (17.98%) |
| Whole stomach | 23 (25.84%) |
| Histological type | |
| Adenocarcinoma | 54 (60.67%) |
| Adenocarcinoma mucinosum | 35 (39.33%) |
| Histological differentiation | |
| Moderately differentiated | 25 (28.09%) |
| Poorly differentiated | 35 (39.33%) |
| Non differentiated | 29 (32.58%) |
| Depth of invasion | |
| T1 | 6 (6.74%) |
| T2 | 7 (7.87%) |
| T3 | 66 (74.16%) |
| T4 | 10 (11.23%) |
| Lymph node metastasis | |
| Absent | 17 (19.10%) |
| Present | 72 (80.90%) |
| Distant metastasis | |
| Absent | 61 (68.54%) |
| Present | 28 (31.46%) |
| Blood vessel infiltration | |
| Absent | 48 (84.21%) |
| Present | 9 (15.79%) |
| Lymphatic vessel infiltration | |
| Absent | 21 (31.82%) |
| Present | 45 (68.18%) |
| Perineural infiltration | |
| Absent | 28 (34.15%) |
| Present | 54 (65.85%) |
| Peritumoral inflammation | |
| Absent | 42 (50.00%) |
| Present | 42 (50.00%) |
| Desmoplasia | |
| Small | 55 (66.27%) |
| Large | 28 (33.73%) |
| Helicobacter pylori infection | |
| Absent | 61 (73.49%) |
| Present | 22 (26.51%) |
| Lauren’s classification | |
| Intestinal | 44 (55.00%) |
| Diffuse | 36 (45.00%) |
Tissues collected during the operation were fixed in 4% buffered formalin and embedded in paraffin. Paraffin blocks were then cut on a microtome into approximately 4-µm-thick sections and stained with hematoxylin and eosin. A routine histopathological examination included the assessment of the histological type of cancer, malignancy grade (G), anatomoclinical stage (pT) and presence of lymph node metastases (pN), infiltration of blood and lymphatic vessels, perineural infiltration, peritumoral inflammation and degree of desmoplasia. Moreover, H. pylori infection was assessed by Giemsa staining. The following information was selected from the records with histopathological diagnosis: age and sex of patients, diameter and location of the tumor, presence of distant metastases and the type of cancer according to Lauren’s classification.
Immunohistochemical (IHC) staining was performed on 89 GC tissues. Paraffin blocks were cut on a microtome into approximately 4-µm-thick sections and mounted onto silanized slides. The microscopic sections were incubated overnight at 60 °C and then deparaffinized in xylene solutions and hydrated in a series of alcohol solutions of decreasing concentration (2 × 99.9%, 96%, 70%). The next step was blocking end
We particularly focused on obtaining reliable results of TNS4 IHC staining. To optimize the TNS4 staining procedure, we used positive and negative controls, and selected primary antibody dilutions (1:50, 1:75, 1:100, 1:200) and incubation times (30 min, 60 min, 120 min) were tested. For the control, antigen retrieval was also performed in buffers with pH = 6.0 and pH = 9.0.
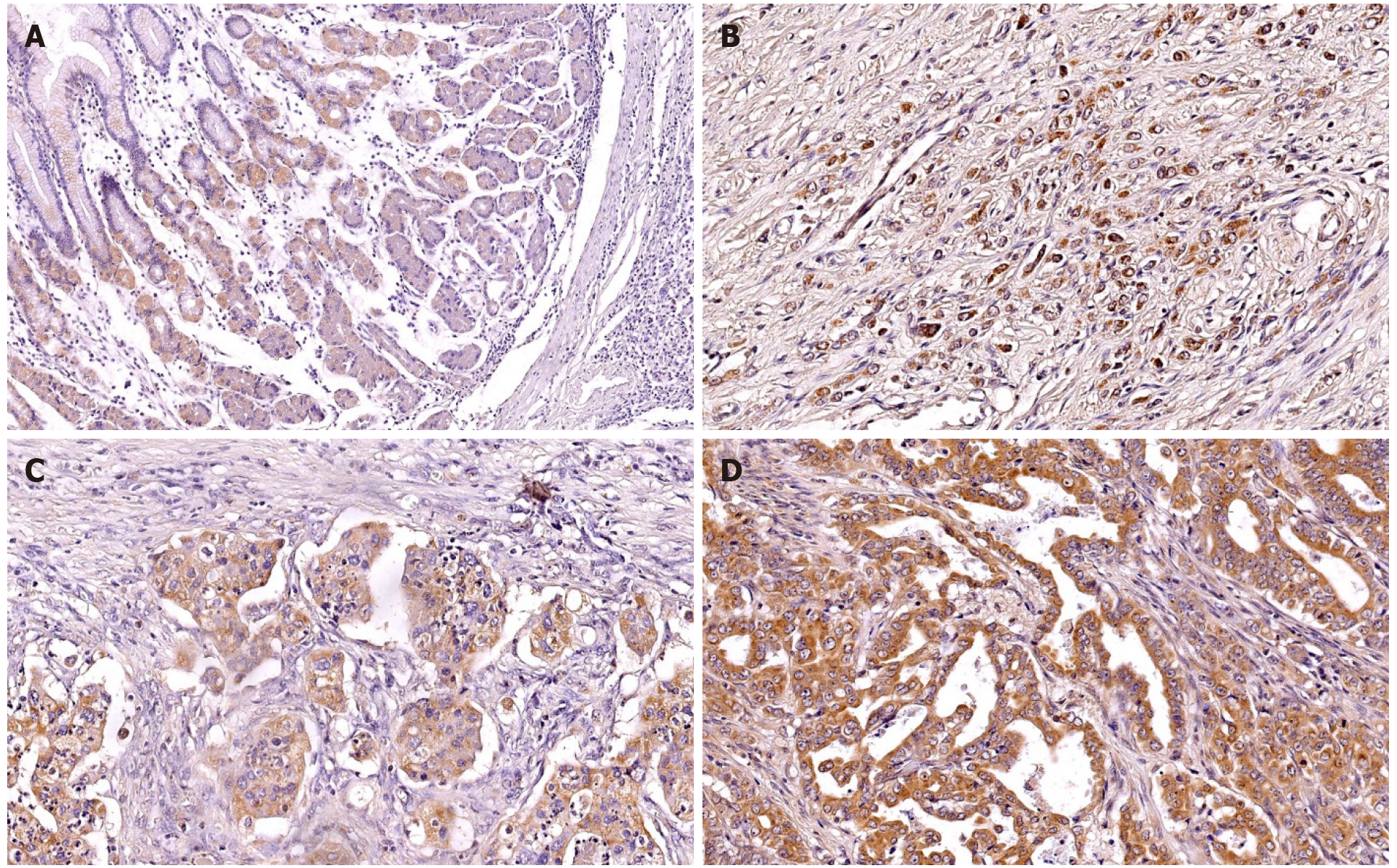
The preparations were examined under an Olympus BX41 light microscope by two independent pathomorphologists. TNS4 protein expression was evaluated under 400× magnification in 10 representative fields of view. In each field of view, 100 or more cancer cells were assessed. Protein expression was observed in both the cell membrane and the cytoplasm. The presence of TNS4 protein in ≥ 20% of neoplastic cells was considered positive expression.
The comparison of TNS4 expression with the selected clinicopathological parameters was carried out by the Mann-Whitney U test for two groups and the Kruskal-Wallis test for 3 or more groups. Dunn’s multiple comparison post hoc tests were conducted for the Kruskal-Wallis test. A value of P < 0.05 was considered statistically significant. The overall survival analysis was based on the Kaplan-Meier test. The program Statistica 13 (Statsoft, Krakow, Poland) was used for the analysis. Missing data were removed in pairs.
TNS4 protein expression was tested immunohistochemically in 89 GC samples and 20 normal stomach tissues. Statistical analysis demonstrated that positive TNS4 expression in cancer cells occurred in 49 of 89 patients (55%). In neoplastic cells, the expression of TNS4 was observed in the cell membrane and cytoplasm (Figure 1)
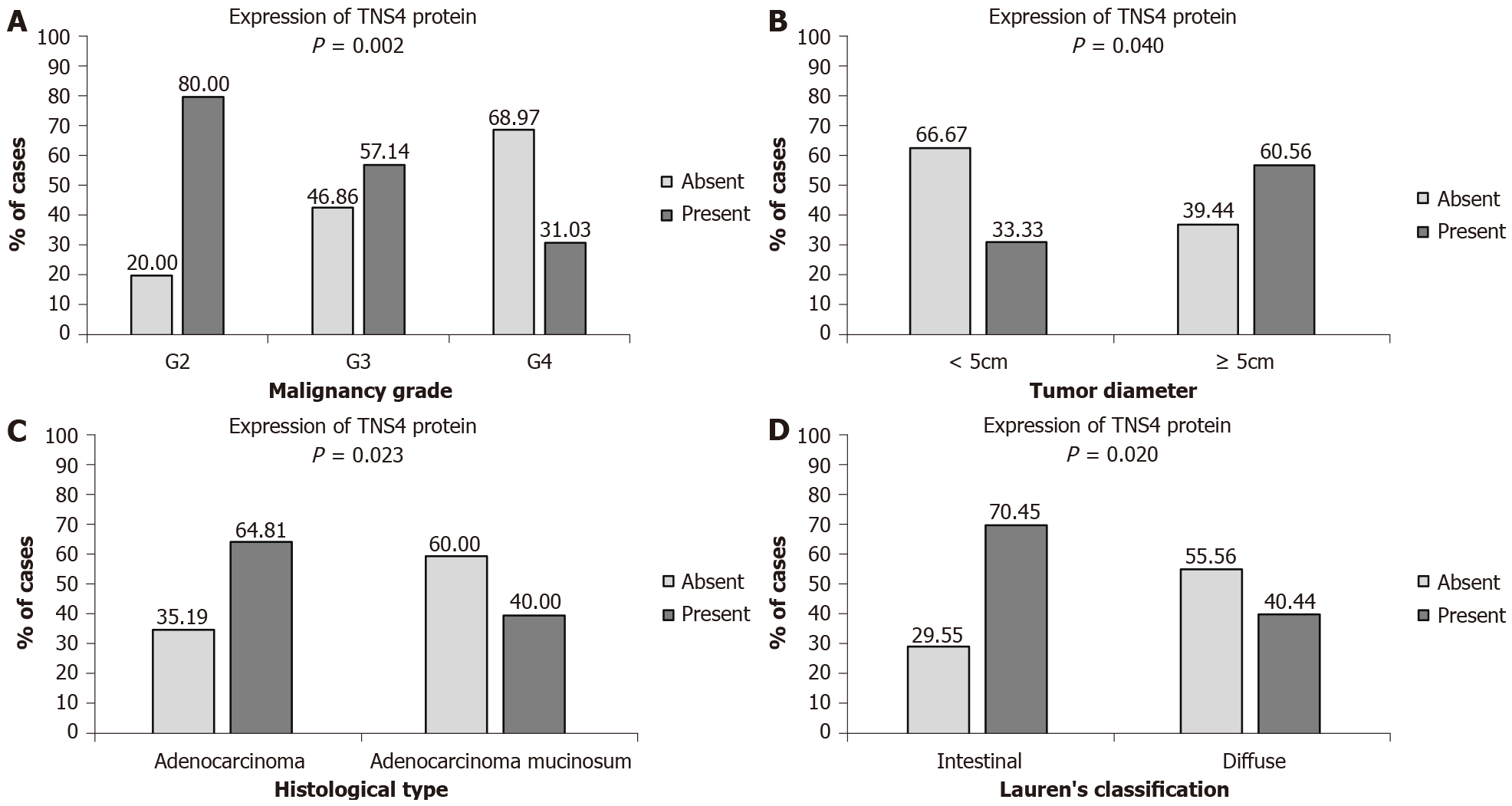
The statistical analysis did not show any significant relationships between TNS4 protein expression and the age and sex of patients, tumor location, anatomoclinical stage–pT, presence of lymph node and distant metastases, blood and lymphatic vessel infiltration, perineural infiltration, peritumoral inflammation, desmoplasia or H. pylori infection. However, a significant correlation was found between TNS4 expression and tumor diameter. Tumors with a diameter of < 5 cm were present in 20.22% of the patients, while tumors with a diameter of ≥ 5 cm were found in 79.78% of the patients. Positive expression of the TNS4 protein was more frequent in gastric tumors with a diameter of ≥ 5 cm (60.56% of the patients with positive expression) than in tumors < 5 cm in diameter (33.33% of the patients) (P = 0.040) (Figure 2A). Furthermore, a significant correlation with the histological type of tumor was demonstrated. Cancers without mucinous components were diagnosed in 60.67% of the patients, and mucosal tumors were diagnosed in 39.33% of the patients. A positive reaction to the TNS4 protein was considerably more frequent in the histological type without a mucinous component (64.81% of patients) than in mucinous adenocarcinomas (40.00% of patients) (P = 0.023) (Figure 2B). Statistical analysis also showed a significant correlation with the malignancy grade. In the study group, there were 28.09% moderately differentiated, 39.32% poorly differentiated and 32.59% non-differentiated tumors. The TNS4 protein occurred much more frequently in moderately differentiated cancers (80.00% of the patients) than in poorly differentiated (57.14%) and non-differentiated (31.03%) tumors (P = 0.002) (Figure 2C). It was also proven that TNS4 protein expression correlates with the type of tumor according to Lauren’s classification. The intestinal type was present in 55% of the patients, while 45% of the patients suffered from the diffuse type of GC. The presence of TNS4 was more frequently observed in intestinal-type tumors (70.45%) than in diffuse-type tumors (44.44%) (P = 0.020) (Figure 2D).
Table 2 presents a summary of the correlation between clinicopathological parameters and TNS4 protein expression found in our study.
| Parameter | Expression of tensin 4 protein | aP value | |
| Negative | Positive | ||
| Age | 0.666 | ||
| < 60 | 14 (48.28%) | 15 (51.72%) | |
| ≥ 60 | 26 (43.33%) | 34 (56.67%) | |
| Gender | 0.182 | ||
| Female | 16 (55.17%) | 13 (44.83%) | |
| Male | 24 (40.00%) | 36 (60.00%) | |
| Tumor diameter | 0.040 | ||
| < 5 cm | 12 (66.67%) | 6 (33.33%) | |
| ≥ 5 cm | 28 (39.44%) | 43 (60.56%) | |
| Tumor localization | 0.081 | ||
| Upper 1/3 | 10 (58.82%) | 7 (41.18%) | |
| Middle1/3 | 9 (27.27%) | 24 (72.73%) | |
| Lower 1/3 | 9 (56.25%) | 7 (43.75%) | |
| Whole stomach | 12 (52.17%) | 11 (47.83%) | |
| Histological type | 0.023 | ||
| Adenocarcinoma | 19 (35.19%) | 35 (64.81%) | |
| Adenocarcinoma mucinosum | 21 (60.00%) | 14 (40.00%) | |
| Histological differentiation | 0.002 (< 0.001) | ||
| Moderately differentiated | 5 (20.00%) | 20 (80.00%) | |
| Poorly differentiated | 15 (42.86%) | 20 (57.14%) | |
| Non differentiated | 20 (68.97%) | 9 (31.03%) | |
| Depth of invasion | 0.208 | ||
| T1 | 5 (83.33%) | 1 (16.67%) | |
| T2 | 2 (28.57%) | 5 (71.43%) | |
| T3 | 28 (42.42%) | 38 (57.58%) | |
| T4 | 5 (50.00%) | 5 (50.00%) | |
| Lymph node metastasis | 0.071 | ||
| Absent | 11 (64.71%) | 6 (35.29%) | |
| Present | 29 (40.28%) | 43 (59.72%) | |
| Distant metastasis | 0.794 | ||
| Absent | 28 (45.90%) | 33 (54.10%) | |
| Present | 12 (42.86%) | 16 (57.14%) | |
| Blood vessel infiltration | 0.980 | ||
| Absent | 21 (43.75%) | 27 (56.25%) | |
| Present | 4 (44.44%) | 5 (55.56%) | |
| Lymphatic vessel infiltration | 0.358 | ||
| Absent | 10 (47.62%) | 11 (52.38%) | |
| Present | 16 (35.56%) | 29 (64.44%) | |
| Perineural infiltration | 0.628 | ||
| Absent | 13 (46.43%) | 15 (53.57%) | |
| Present | 22 (40.74%) | 32 (59.26%) | |
| Peritumoral inflammation | 0.453 | ||
| Absent | 19 (46.34%) | 22 (53.66%) | |
| Present | 16 (38.10%) | 26 (61.90%) | |
| Desmoplasia | 0.710 | ||
| Small | 24 (43.64%) | 31 (56.36%) | |
| Large | 11 (39.29%) | 17 (60.71%) | |
| Helicobacter pylori infection | 0.824 | ||
| Absent | 26 (42.62%) | 35 (57.38%) | |
| Present | 10 (45.45%) | 12 (54.55%) | |
| Lauren’s classification | 0.020 | ||
| Intestinal | 13 (29.55%) | 31 (70.45%) | |
| Diffuse | 20 (55.56%) | 16 (44.44%) | |
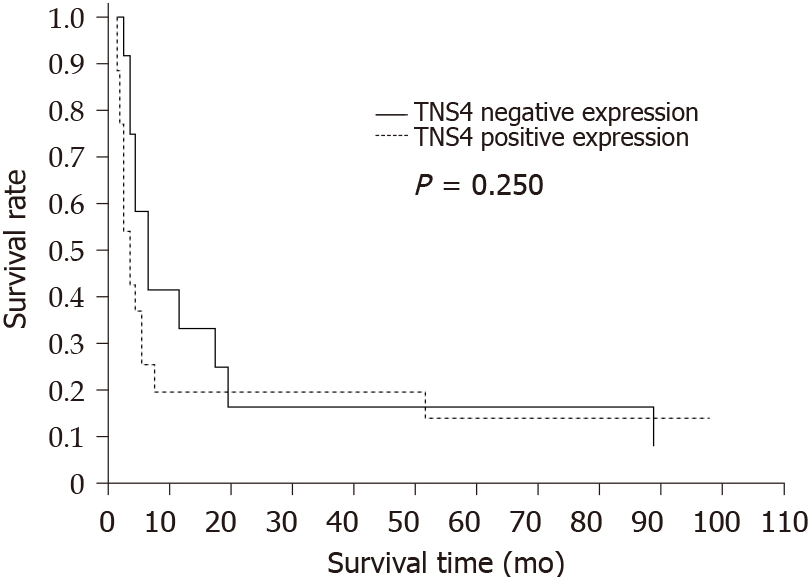
Statistical analysis did not reveal a significant correlation between TNS4 protein expression and overall patient survival (P = 0.25) (Figure 3).
In our study, we performed an IHC assessment of the expression of the TNS4 protein in GC patients diagnosed with stage I to IV disease. We observed that TNS4 expression was higher in tumor cells than in normal tissue, which is consistent with the results presented by Qi et al[17]. Sawazaki et al[18] evaluated the clinical significance of TNS4 mRNA expression in GC and demonstrated that its expression is significantly higher in tumor tissue, as confirmed by Sakashita et al[19]. Based on these observations, it can be concluded that changes in the expression of the TNS4 gene occur in tumor cells compared to normal gastric mucosa cells. This change is reflected in increased expression of TNS4 protein in GC.
In our research, we demonstrated that positive expression of TNS4 protein occurs twice as often in GC tumors with a diameter of ≥ 5 cm than in tumors with a diameter smaller than 5 cm. Similar observations were reported by other authors who noted that TNS4 protein expression increases with the size of breast tumors and hepatocellular carcinoma tumors[11,20]. This finding indicates a correlation between TNS4 expression and the progression of the tumor and may be associated with the activation of signaling pathways, including PI3/AKT and Ras/MAPK, the stimulation of which intensifies the processes of cell proliferation and migration[13]. During the activation of these pathways, TNS4 expression is increased under the influence of epidermal growth factor at the levels of mRNA transcription and protein translation. Increased TNS4 expression leads to enhanced migration and proliferative potential of cancer cells.
We observed significant correlations between the expression of TNS4 and malignancy grade, histological type and type of tumor according to Lauren’s classification. TNS4 protein was much more frequently present in moderately differentiated tumors than in poorly differentiated and non-differentiated tumors, in adenocarcinomas without mucinous components and in the intestinal type of GC. The classification of GC based on the degree of glandular cell differentiation is a prognostic factor. The more differentiated the gastric adenocarcinoma, the better the prognosis is for a patient. Poorly and non-differentiated adenocarcinoma cells differ phenotypically from each other, forming clusters without visibly formed glands[21]. There are no reports in the literature suggesting a significant relationship between TNS4 protein expression and the grade of tumor malignancy, which indicates the uniqueness of our study. In contrast, Sakashita et al[19] evaluated the TNS4 mRNA expression and observed that patients with moderately or poorly differentiated GC had higher levels of TNS4 expression. The differences in the results obtained by these researchers and us may be due to numerous mutations in the TNS4 gene in tumor cells, entailing changes in the structure of this protein. We demonstrated higher expression of this protein in moderately differentiated gastric adenocarcinoma, which suggests that TNS4 overexpression is associated with a better prognosis of the tumor.
Poorly differentiated GCs are characterized by weak or no developed glandular tubules. Under normal conditions, TNS4 is involved in glandular duct morphogenesis. This phenomenon was described by Wu et al[22] after their study on a 3D model of RWPE-1 cells of the prostate. These researchers demonstrated that a decrease in protein expression does not affect the phenotype of the newly formed glands, although their number decreased. The authors suggested that lowering TNS4 expression inhibits the proliferation of normal epithelial cells of the prostate by stopping the cell cycle. However, increased expression of TNS4 leads to disturbances in the formation of glandular ducts through β1-integrin/FAK pathway induction. Albasri et al[8] identified a link between TNS4 and the morphology of colorectal cancer cells. By means of confocal microscopy, these researchers demonstrated that induced expression of TNS4 affects the shape of cancer cells. Under the conditions of TNS4 overexpression, HCT116 cells assume a spindle shape compared to cells with physiologically low expression of TNS4.
In our research, we demonstrated significant differences in TNS4 expression between the two histological types of GC. We noted that positive expression of TNS4 protein was more frequent in GCs without mucinous components than in mucinous adenocarcinomas. Gastric mucosal adenocarcinomas have been linked to poorer prognosis, lower sensitivity to oncological treatment and increased risk of acquiring resistance to chemotherapy[23]. Currently, there are no data available to indicate a link between TNS4 expression and gastric adenocarcinomas without a mucinous component. Phenotypically, GC with a mucinous component is characterized by a considerable amount of mucin in the extracellular space (> 50%)[24].
In our study, we correlated the expression of TNS4 protein with the cancer type according to Lauren’s classification. Statistical analysis showed that positive TNS4 staining correlates with the intestinal type of tumor. The presence of this protein was more often observed in adenocarcinomas of the intestinal than diffuse type. The results presented by Chen et al[25] indicate that the intestinal type correlates with better prognosis and better clinical and pathological parameters than the diffuse type. From a histological point of view, intestinal-type GC is phenotypically characterized by cells forming glandular ducts in the lumen of which the cancer grows. Usually, these cells do not secrete mucus, but if it is produced, it remains in the lumen of the glands[26]. In the literature, we did not find any studies assessing TNS4 expression in relation to the histological type of GC according to Lauren’s classification. The positive correlation between the expression of TNS4 and the intestinal type of cancer suggests further directions of research as well as the need to investigate the role of this protein in the histogenesis of GC.
Our study did not show any correlation between the level of TNS4 protein expression and tumor progression (pT, pN, pM status), although the literature shows that TNS4 is located in focal adhesion sites and participates in cell migration, thus increasing the metastatic potential of tumor cells. Qi et al[17] observed a significant correlation between TNS4 expression and the tumor stage. Their results indicated a correlation between TNS4 expression and deeper infiltrating tumors as well as lymph node and distant metastases. We did not observe similar significant relationships in our study. Other researchers have shown that stimulated TNS4 expression results in an increased ability of cells to migrate, infiltrate into adjacent tissues and metastasize. TNS4 was shown to play an important role in epithelial-mesenchymal transition by stimulating several signaling pathways, including E-cadherin, Akt, Src, TGF-β/Smad and Snail[8,17,27-29]. Other signaling pathways that are stimulated under TNS4 overexpression are PI3K/Akt and Ras/MAPK. Their activation may intensify cell proliferation and migration[13]. Furthermore, in vitro studies revealed that TNS4 may promote colony formation, as demonstrated in a study on pancreatic cancer cells[30]. Another important signaling protein in cancer development is MET, which is stabilized by TNS4 and acts as a mediator in carcinogenesis[31]. The exact mechanisms regulating TNS4, e.g., a mutation that stabilizes this protein or amplifies the TNS4 gene, have not been discovered thus far. Thorpe et al[32] reported that TNS4 can be regulated by the EGFR/KRAS and STAT3 pathways. Other studies have indicated that the expression of this protein may be modulated by growth factors and cytokines, e.g., FGF2, PDGF, IGF-1, TGF-β, IL-6 and IL-13[33]. Other literature reports suggest that TNS4 may be engaged in tubulogenesis[34] as one of the stages of angiogenesis. The formation of blood vessel networks by tumor cells is the way in which the tumor microenvironment acquires nutrients and oxygen needed for tumor growth. Various publications have presented evidence for the oncogenic role of TNS4. In our research, we did not confirm the procancer role of the TNS4 protein. Our results prove that TNS4 may be related to the formation of a certain type of cancer, as observed in prostate cancer[35].
Moreover, we did not demonstrate any significant correlations between TNS4 expression level and the patients’ overall survival rate. According to the works of Qi et al[17] and Sakashita et al[19], TNS4 overexpression correlates with shorter survival time and worse prognosis for patients with GC. In our study, the lack of this correlation may result from a small number of evaluated patients and the lack of data on the survival of many of these patients, which is a limitation of our research and does not allow us to draw broader conclusions about the participation of TNS4 in GC carcinogenesis. Additionally, it is possible that differences between the mRNA level in the study by Sakashita et al[19] and the protein expression level in our research are because the regulation of TNS4 transcription depends on the type of cells or tissues as well as on potential mutations in the TNS4 gene in GC cells. Thus, the expression level of this protein in different tissues is different. The control of this gene expression may also result from epigenetic modifications[36].
The expression of TNS4 was significantly higher in tumors with a diameter of ≥ 5 cm, of a moderately differentiated type of GC without a mucinous component, and of the intestinal type according to Lauren’s classification. Increased levels of TNS4 expression are linked to the histological type of GC with a better prognosis.
Gastric cancer (GC) is still one of the most common malignant neoplasms worldwide in terms of incidence and cancer motility. The development of GC is a multistage process. Tensin 4 (TNS4) belongs to the tensin family. This protein can participate in the transmission of intracellular signals. TNS4 plays an important role in biological processes connected with carcinogenesis, such as proliferation, migration, cell adhesion and invasiveness. It is very important to search for new biomarkers that could help to diagnose GC at the early stages of its development.
We wanted to evaluate the role of the TNS4 protein in the development of GC. This protein may be a promising biomarker in the diagnosis of GC at the early stages of its development.
The study objective was to show the expression of TNS4 in GC tissues and assess the relationship between protein expression and clinical and pathological parameters and the overall survival rate of patients.
In our study, we used immunohistochemistry to evaluate the expression of the TNS4 protein. The research was conducted on a group of 89 patients.
We observed that higher TNS4 expression was more often observed in GCs with a larger diameter (P = 0.040). Our results also showed that an increase in TNS4 expression was more frequently observed in tumors without mucinous components than in tumors from mucosal cancers (P = 0.023). Furthermore, higher TNS4 expression was demonstrated in moderately differentiated tumors (P = 0.002). Increased TNS4 expression was also noted in the intestinal type of GC according to Lauren’s classification (P = 0.020). No significant correlation was found between the expression of TNS4 and the overall survival rate of patients.
The expression of TNS4 was significantly higher in tumors with a diameter of ≥ 5 cm, of a moderately differentiated type of GC without a mucinous component, and of the intestinal type according to Lauren’s classification. Increased levels of TNS4 expression are linked to the histological type of GC with a better prognosis.
It is possible to develop this study with cell line methods. It will be possible to investigate the potential role of TNS4 in GC cell proliferation.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56614] [Article Influence: 7076.8] [Reference Citation Analysis (134)] |
| 2. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 382] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 3. | Rona KA, Schwameis K, Zehetner J, Samakar K, Green K, Samaan J, Sandhu K, Bildzukewicz N, Katkhouda N, Lipham JC. Gastric cancer in the young: An advanced disease with poor prognostic features. J Surg Oncol. 2017;115:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 672] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 5. | Lo SH. Tensin. Int J Biochem Cell Biol. 2004;36:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Lo SH, Lo TB. Cten, a COOH-terminal tensin-like protein with prostate restricted expression, is down-regulated in prostate cancer. Cancer Res. 2002;62:4217-4221. [PubMed] |
| 7. | Liao YC, Chen NT, Shih YP, Dong Y, Lo SH. Up-regulation of C-terminal tensin-like molecule promotes the tumorigenicity of colon cancer through beta-catenin. Cancer Res. 2009;69:4563-4566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Albasri A, Seth R, Jackson D, Benhasouna A, Crook S, Nateri AS, Chapman R, Ilyas M. C-terminal Tensin-like (CTEN) is an oncogene which alters cell motility possibly through repression of E-cadherin in colorectal cancer. J Pathol. 2009;218:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Liao YC, Lo SH. Tensins - emerging insights into their domain functions, biological roles and disease relevance. J Cell Sci. 2021;134:jcs254029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Aratani K, Komatsu S, Ichikawa D, Ohashi T, Miyamae M, Okajima W, Imamura T, Kiuchi J, Nishibeppu K, Kosuga T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Tsuda H, Otsuji E. Correction: Overexpression of CTEN relates to tumor malignant potential and poor outcomes of adenocarcinoma of the esophagogastric junction. Oncotarget. 2019;10:5726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Albasri A, Aleskandarany M, Benhasouna A, Powe DG, Ellis IO, Ilyas M, Green AR. CTEN (C-terminal tensin-like), a novel oncogene overexpressed in invasive breast carcinoma of poor prognosis. Breast Cancer Res Treat. 2011;126:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Albasri A, Al-Ghamdi S, Fadhil W, Aleskandarany M, Liao YC, Jackson D, Lobo DN, Lo SH, Kumari R, Durrant L, Watson S, Kindle KB, Ilyas M. Cten signals through integrin-linked kinase (ILK) and may promote metastasis in colorectal cancer. Oncogene. 2011;30:2997-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Chan LK, Chiu YT, Sze KM, Ng IO. Tensin4 is up-regulated by EGF-induced ERK1/2 activity and promotes cell proliferation and migration in hepatocellular carcinoma. Oncotarget. 2015;6:20964-20976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Sasaki H, Moriyama S, Mizuno K, Yukiue H, Konishi A, Yano M, Kaji M, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y. Cten mRNA expression was correlated with tumor progression in lung cancers. Lung Cancer. 2003;40:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Sasaki H, Yukiue H, Kobayashi Y, Fukai I, Fujii Y. Cten mRNA expression is correlated with tumor progression in thymoma. Tumour Biol. 2003;24:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Albasri A, Fadhil W, Scholefield JH, Durrant LG, Ilyas M. Nuclear expression of phosphorylated focal adhesion kinase is associated with poor prognosis in human colorectal cancer. Anticancer Res. 2014;34:3969-3974. [PubMed] |
| 17. | Qi X, Sun L, Wan J, Xu R, He S, Zhu X. Tensin4 promotes invasion and migration of gastric cancer cells via regulating AKT/GSK-3β/snail signaling pathway. Pathol Res Pract. 2020;216:153001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Sawazaki S, Oshima T, Sakamaki K, Aoyama T, Sato T, Shiozawa M, Yoshikawa T, Rino Y, Imada T, Masuda M. Clinical Significance of Tensin 4 Gene Expression in Patients with Gastric Cancer. In Vivo. 2017;31:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Sakashita K, Mimori K, Tanaka F, Kamohara Y, Inoue H, Sawada T, Hirakawa K, Mori M. Prognostic relevance of Tensin4 expression in human gastric cancer. Ann Surg Oncol. 2008;15:2606-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Chen J, Zhang Y, Deng G, Ma J, Wu X, Qu Y, Zeng S. [Correlation between the expression of C-terminal tensin-like protein and the prognosis of hepatocellular carcinoma]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer. 2000;89:1418-1424. [PubMed] |
| 22. | Wu WM, Liao YC. Downregulation of C-Terminal Tensin-Like Protein (CTEN) Suppresses Prostate Cell Proliferation and Contributes to Acinar Morphogenesis. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Machlowska J, Pucułek M, Sitarz M, Terlecki P, Maciejewski R, Sitarz R. State of the art for gastric signet ring cell carcinoma: from classification, prognosis, and genomic characteristics to specified treatments. Cancer Manag Res. 2019;11:2151-2161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Jass JR, Sobin LH, Watanabe H. The World Health Organization's histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res. 2016;22:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 26. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4385] [Article Influence: 146.2] [Reference Citation Analysis (1)] |
| 27. | Asiri A, Toss MS, Raposo TP, Akhlaq M, Thorpe H, Alfahed A, Asiri A, Ilyas M. Cten promotes Epithelial-Mesenchymal Transition (EMT) in colorectal cancer through stabilisation of Src. Pathol Int. 2019;69:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Lu X, Gao J, Zhang Y, Zhao T, Cai H, Zhang T. CTEN induces epithelial-mesenchymal transition (EMT) and metastasis in non small cell lung cancer cells. PLoS One. 2018;13:e0198823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Thorpe H, Asiri A, Akhlaq M, Ilyas M. Cten promotes epithelial-mesenchymal transition through the post-transcriptional stabilization of Snail. Mol Carcinog. 2017;56:2601-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Al-Ghamdi S, Cachat J, Albasri A, Ahmed M, Jackson D, Zaitoun A, Guppy N, Otto WR, Alison MR, Kindle KB, Ilyas M. C-terminal tensin-like gene functions as an oncogene and promotes cell motility in pancreatic cancer. Pancreas. 2013;42:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Muharram G, Sahgal P, Korpela T, De Franceschi N, Kaukonen R, Clark K, Tulasne D, Carpén O, Ivaska J. Tensin-4-Dependent MET Stabilization Is Essential for Survival and Proliferation in Carcinoma Cells. Dev Cell. 2014;29:629-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Thorpe H, Akhlaq M, Jackson D, Al Ghamdi S, Storr S, Martin S, Ilyas M. Multiple pathways regulate Cten in colorectal cancer without a Tensin switch. Int J Exp Pathol. 2015;96:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Hung SY, Shih YP, Chen M, Lo SH. Up-regulated cten by FGF2 contributes to FGF2-mediated cell migration. Mol Carcinog. 2014;53:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Kwon SH, Nedvetsky PI, Mostov KE. Transcriptional profiling identifies TNS4 function in epithelial tubulogenesis. Curr Biol. 2011;21:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Li Y, Mizokami A, Izumi K, Narimoto K, Shima T, Zhang J, Dai J, Keller ET, Namiki M. CTEN/tensin 4 expression induces sensitivity to paclitaxel in prostate cancer. Prostate. 2010;70:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Yang K, Wu WM, Chen YC, Lo SH, Liao YC. ΔNp63α Transcriptionally Regulates the Expression of CTEN That Is Associated with Prostate Cell Adhesion. PLoS One. 2016;11:e0147542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Noncommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work noncommercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huang Y S-Editor: Zhang H L-Editor: A P-Editor: Zhang H