Published online Oct 24, 2021. doi: 10.5306/wjco.v12.i10.960
Peer-review started: April 21, 2021
First decision: July 16, 2021
Revised: July 26, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: October 24, 2021
Processing time: 183 Days and 14.1 Hours
Myeloid sarcoma (MS) is a rare hematologic malignancy defined as an extramedullary tumor of immature granulocytic cells. It can occur as primary or de novo and be associated with myelodysplasia or myeloproliferative neoplasms. The most frequent locations are the skin, lymph nodes and bones. The case of a patient with a diagnosis of primary granulocytic de novo gastric MS is reported.
A 19-year-old female patient with MS, whose abdominal computed tomography showed a bulky tumor of 16.5 cm in the gastric chamber with infiltration in the retroperitoneal, pancreatic and bile duct region; the histological study showed gastric mucosa diffusely infiltrated by mononucleated cells and the immunohistochemistry expressed myeloperoxidase. After receiving induction chemotherapy based on the 3 + 7 regimen (daunorubicin/cytarabine), the patient developed severe hematological toxicity and neutropenic typhlitis which required a prolonged medical treatment. She presented a rapid disease progression. Although she received supportive treatment, the patient died.
Gastric primary de novo MS is a rare and aggressive course neoplasm, fostering knowledge is very important to decide its management and to promote more approaches focused on understanding this pathology and its particularities in our population.
Core Tip: This case report describes a gastric primary de novo myeloid sarcoma (MS) which is a very rare hematological neoplasm with poor prognosis in a young and symptomatic patient. After receiving chemotherapy, she presented severe toxicity (neutropenic typhlitis) and rapid disease progression. This case highlights the importance of detecting gastric primary MS as a rare form of extramedullary myeloid leukemia presentation. Moreover, management of gastric primary MS could lead to interventions to avoid deterioration of gastrointestinal system during treatment. There is limited information of management and outcomes regarding gastric primary MS. Furthermore, there is very limited data about de novo MS in Peruvian patients.
- Citation: Rioja P, Macetas J, Luna-Abanto J, Tirado-Hurtado I, Enriquez DJ. Gastric myeloid sarcoma: A case report. World J Clin Oncol 2021; 12(10): 960-965
- URL: https://www.wjgnet.com/2218-4333/full/v12/i10/960.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i10.960
Myeloid sarcoma (MS) is defined as a myeloblast tumor produced in an anatomical place different from the bone marrow that destroys the original architecture of the local tissue. These tumors are also known as granulocytic sarcomas, chloromas or extramedullary myeloid tumors[1,2]. MS occurs more frequently in males and young individuals; its location is variable, and cerebral, mammary, testicular, gastrointestinal involvement has been reported, among other visceral organs, and it appears more frequently in the skin, lymph nodes and bones[3].
MS occurrence is frequently associated with acute myeloid leukemia (AML), affecting between 2.5% and 9% of patients. When the disease is detected without clinical signs of leukemia and in association with a negative bone marrow biopsy, it is classified as de novo MS[2], the incidence of which is 2 cases per million adults[2,4]. Gastrointestinal presentation is uncommon and shows nonspecific symptoms related to the effect of tumor mass[3,5].
The patient was a 19-year-old female without a relevant history.
The patient was admitted to the hospital with nausea, vomiting and early satiety of 5 mo of evolution associated with an episode of hematemesis.
The patient showed low body weight, a regular general condition, distended abdo
Laboratory tests at admission revealed moderate anemia (Hb: 9 g/dL), hypoalbuminemia (albumin: 2.5 g/dL), grade 4 hyperbilirubinemia (total bilirubin: 2.5 mg/dL, indirect bilirubin: 1.8 g/dL), grade 2 hypertransaminasemia (aspartate aminotransferase: 90 IU/L, alanine aminotransferase: 100 IU/L), and elevated alkaline phosphatase (360 IU/L).
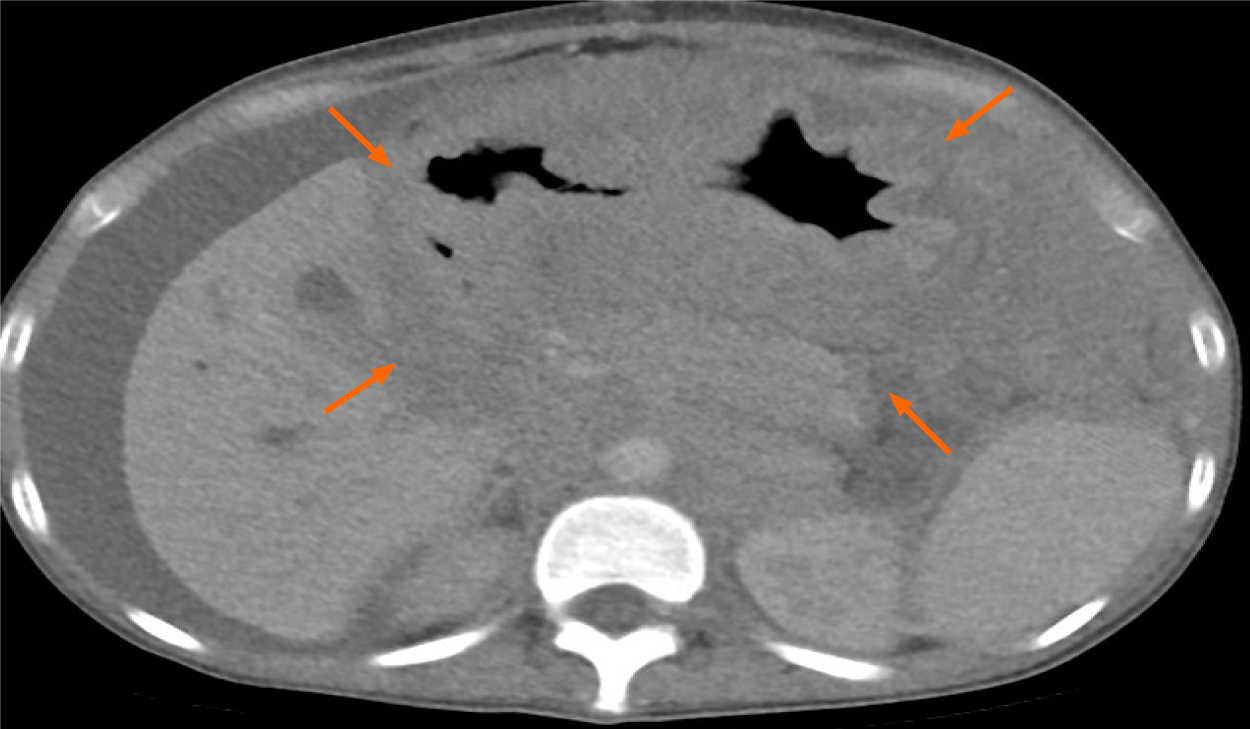
Abdominal computed tomography showed a bulky tumor of 16.5 cm in the gastric chamber, which infiltrated the retroperitoneal, pancreatic and bile duct regions, with dilation of the latter. In addition, peritoneal thickening, free fluid, splenomegaly and bilateral hydronephrosis were observed (Figure 1). Upper digestive endoscopy was reported at the level of the infiltrated gastric mucosal body with decreased con
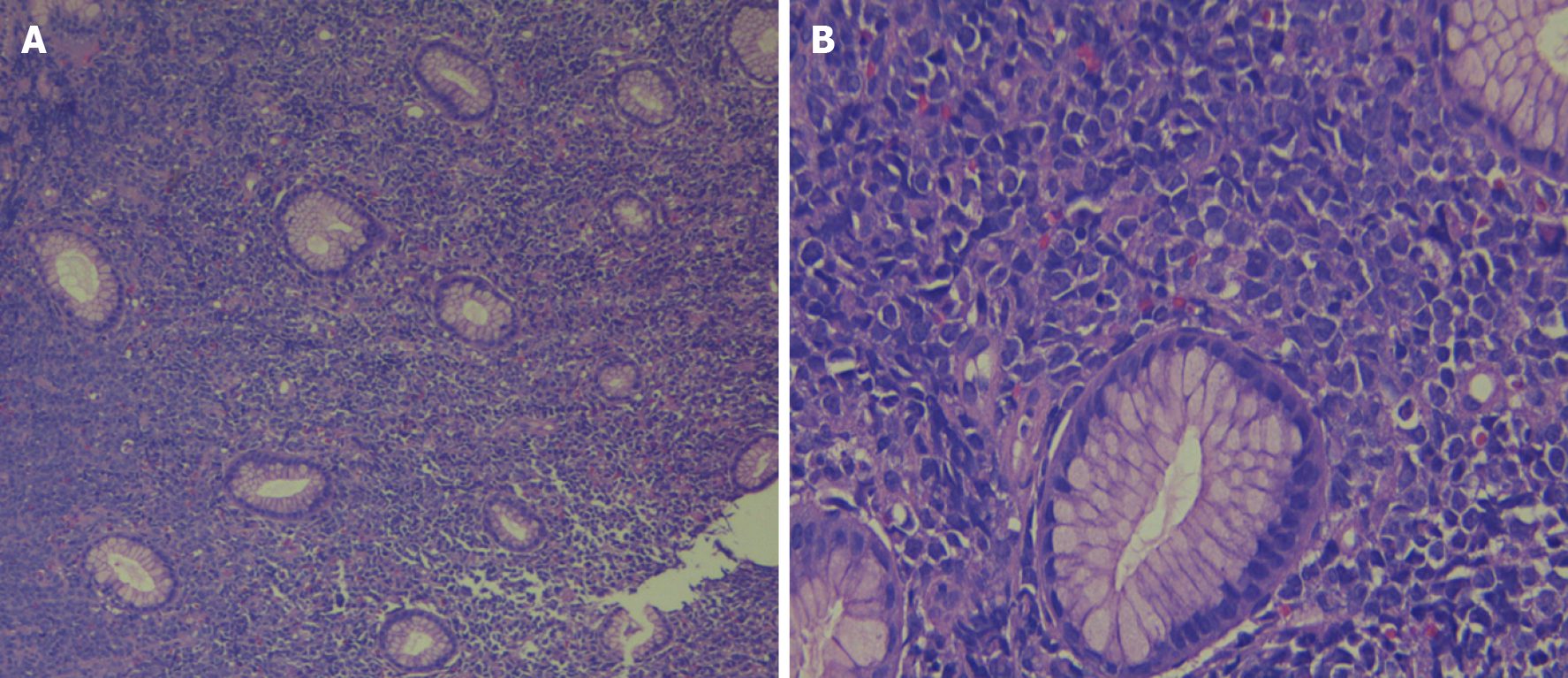
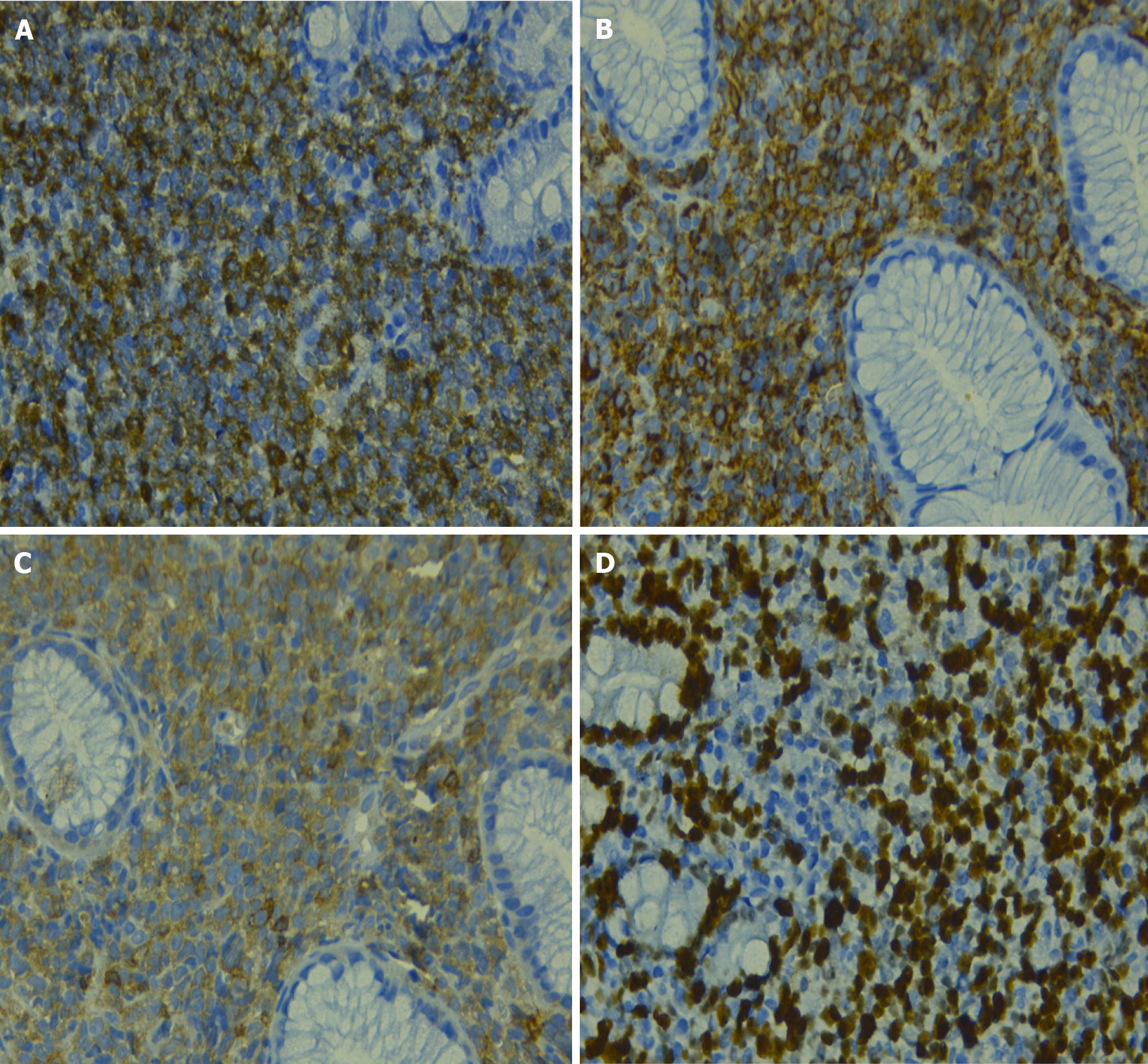
The histological study showed gastric mucosa diffusely infiltrated by mononucleated cells, which were intermediate in size with eosinophilic cytoplasm and blast-like nuclei (Figure 2). Immunohistochemistry indicated that these cells expressed myeloperoxidase (MPO) (+), CD117 (+) CD34 (+), CD20 (-), CD3 (-), CD68 (-), CD38 (-), CD30 (-), DTT (-), and Ki67: 80% (Figure 3). Bone marrow analysis by cytomorphology and flow cytometry was negative for infiltration by myeloid blasts. The bone marrow karyotype was 46 XX, and it was not possible to demonstrate the presence of the AML1-ETO, CBFB-MYH11, NPM1 mut A, and FLT3-ITD genes or mutations in exons 8 and 17 of the c-kit gene in bone marrow and the primary tumor.
This case final diagnosis is primary gastric de novo granulocytic MS.
The patient began induction treatment based on the 3 + 7 regimen (daunorubicin 60 mg/m2 for 3 d + citarabine 200 mg/m2 for 7 d). She developed severe hematological toxicity and neutropenic typhlitis requiring antibiotic and antifungal coverage, pare
In the reassessment of the disease 30 d after treatment, the patient reached a partial response. A second cycle of 3 + 7 regimen treatment was scheduled, and regular tolerance and rapid disease progression were observed, thus she received supportive treatment; however, the patient died.
The case of a 19-year-old woman with a final diagnosis of gastric granulocytic type MS is presented. The symptoms were similar to those reported in other cases of gastric MS, which was conditioned by the extensive intra-abdominal involvement of the neoplasm (Figure 1)[2,6,7]. The differential histopathological diagnosis was based on morphological and immunohistochemical characteristics, which included non-Hodgkin lymphoma, Burkitt lymphoma, diffuse large B-cell lymphoma, lymphoblastic lymphoma, and Ewing's sarcoma, among others[3,8]. The present case had a blast type histology. Classically, MS expresses myeloid markers such as CD13, CD33 and MPO, but these markers are not present in all cases. For example, the expression of MPO, one of the most frequent markers, has been reported to be between 83.6% and 64.1% in different series[9]. On the other hand, the expression of CD68, a marker related to lymphocyte lineage and lysosome leakage, was positive in our patient; its presence has been reported in up to 100% of patients with MS[8]. Other markers, such as CD33, CD13 and related to line B, are less consistent in these patients[4,8,9]. It can be distinguished from other round cell neoplasms, such as Ewing sarcoma or neuroendocrine tumors, with specific markers, such as CD99-specific neuronal enolase and CD99, respectively. It has been reported that the anomaly of the nucleus binding factor (translocation between chromosomes 8 and 21) is the most frequent in patients with de novo MS; this has been reported in 38% of cases in an North American study[8]. In contrast, translocation 8; 21 was only found in 3% of patients in an Italian series[9]. This translocation was not found in the present case, and there is probably a different frequency of it in our population.
Due to the rarity of MS, there are no prospective studies that guide its mana
In conclusion, gastric primary de novo MS is a rare neoplasm with an aggressive course. The differential diagnosis depends on the histological and immunohistochemical characteristics. Chemotherapy is the standard treatment, and important results have been reported with bone marrow transplantation. However, further collaborative studies are necessary to understand this pathology and its particularities in our population.
| 1. | Klco JM, Welch JS, Nguyen TT, Hurley MY, Kreisel FH, Hassan A, Lind AC, Frater JL. State of the art in myeloid sarcoma. Int J Lab Hematol. 2011;33:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 2. | Huang XL, Tao J, Li JZ, Chen XL, Chen JN, Shao CK, Wu B. Gastric myeloid sarcoma without acute myeloblastic leukemia. World J Gastroenterol. 2015;21:2242-2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk. 2017;17:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 4. | Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D, Bisceglia M, Ponzoni M, Gentile A, Rinaldi P, Franco V, Vincelli D, Pileri A Jr, Gasbarra R, Falini B, Zinzani PL, Baccarani M. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 461] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 5. | Ravulapati S, Siegel C, Dara A, Lionberger JM. Myeloid sarcoma without circulating leukemia mimicking gastrointestinal malignancy and lymphoma. Hematol Rep. 2018;10:7040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Bhat I. A Chloroma at the Gastric Cardia. Am J Gastroenterol. 2016;111:1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Yu T, Xu G, Xu X, Yang J, Ding L. Myeloid sarcoma derived from the gastrointestinal tract: A case report and review of the literature. Oncol Lett. 2016;11:4155-4159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Vachhani P, Bose P. Isolated gastric myeloid sarcoma: a case report and review of the literature. Case Rep Hematol. 2014;2014:541807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, Ohshima K. Clinicopathological, Cytogenetic, and Prognostic Analysis of 131 Myeloid Sarcoma Patients. Am J Surg Pathol. 2016;40:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Movassaghian M, Brunner AM, Blonquist TM, Sadrzadeh H, Bhatia A, Perry AM, Attar EC, Amrein PC, Ballen KK, Neuberg DS, Fathi AT. Presentation and outcomes among patients with isolated myeloid sarcoma: a Surveillance, Epidemiology, and End Results database analysis. Leuk Lymphoma. 2015;56:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Peru
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta S S-Editor: Gao CC L-Editor: A P-Editor: Wu RR