Published online Oct 24, 2021. doi: 10.5306/wjco.v12.i10.935
Peer-review started: March 31, 2021
First decision: June 28, 2021
Revised: July 3, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 24, 2021
Processing time: 205 Days and 7.9 Hours
Clinical stage IV gastric cancer (GC) may need palliative procedures in the presence of symptoms such as obstruction. When palliative resection is not possible, jejunostomy is one of the options. However, the limited survival of these patients raises doubts about who benefits from this procedure.
To create a prognostic score based on clinical variables for 90-d mortality for GC patients after palliative jejunostomy.
We performed a retrospective analysis of Stage IV GC who underwent jejunostomy. Eleven preoperative clinical variables were selected to define the score categories, with 90-d mortality as the main outcome. After randomization, patients were divided equally into two groups: Development (J1) and validation (J2). The following variables were used: Age, sex, body mass index (BMI), American Society of Anesthesiologists classification (ASA), Charlson Comorbidity index (CCI), hemoglobin levels, albumin levels, neutrophil-lymphocyte ratio (NLR), tumor size, presence of ascites by computed tomography (CT), and the number of disease sites. The score performance metric was determined by the area under the receiver operating characteristic (ROC) curve (AUC) to define low and high-risk groups.
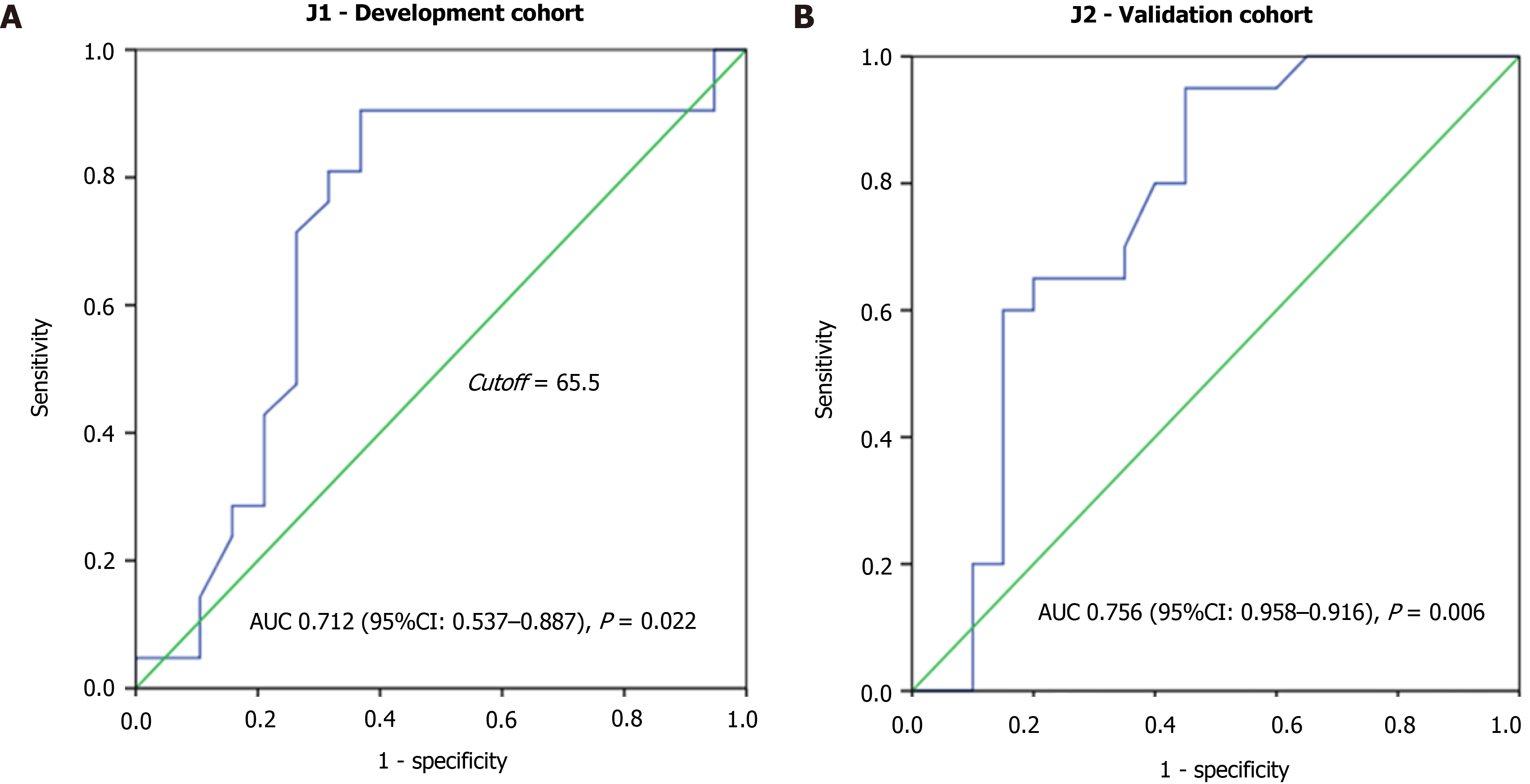
Of the 363 patients with clinical stage IVCG, 80 (22%) patients underwent jejunostomy. Patients were predominantly male (62.5%) with a mean age of 62.4 years old. After randomization, the binary logistic regression analysis was performed and points were assigned to the clinical variables to build the score. The high NLR had the highest value. The ROC curve derived from these pooled parameters had an AUC of 0.712 (95%CI: 0.537–0.887, P = 0.022) to define risk groups. In the validation cohort, the diagnostic accuracy for 90-d mortality based on the score had an AUC of 0.756, (95%CI: 0.598–0.915, P = 0.006). According to the cutoff, in the validation cohort BMI less than 18.5 kg/m2 (P < 0.001), CCI ≥ 1 (P = 0.001), ASA III/IV (P = 0.002), high NLR (P = 0.012), and the presence of ascites on CT exam (P = 0.004) were significantly associated with the high-risk group. The risk groups showed a significant association with first-line (P = 0.012), second-line chemotherapy (P = 0.009), 30-d (P = 0.013), and 90-d mortality (P < 0.001).
The scoring system developed with 11 variables related to patient’s performance status and medical condition was able to distinguish patients undergoing jejunostomy with high risk of 90 d mortality.
Core Tip: This is a retrospective study to evaluate the outcomes of jejunostomy in clinical stage IV gastric cancer patients, and create a scoring system based on clinical variables to identify the best candidates for this approach and avoid futile procedures. We analyzed 80 patients divided into a development and validation cohort. The score had an accuracy of 75.6% in the validation cohort, and was able to properly identify the cases with high risk of 90-d mortality.
- Citation: Ramos MFKP, Pereira MA, Dias AR, Sakamoto E, Ribeiro Jr U, Zilberstein B, Nahas SC. Jejunostomy in the palliative treatment of gastric cancer: A clinical prognostic score . World J Clin Oncol 2021; 12(10): 935-946
- URL: https://www.wjgnet.com/2218-4333/full/v12/i10/935.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i10.935
Gastric cancer (GC) represents the third leading cause of cancer mortality worldwide[1]. Surgical treatment remains the standard of care for patients with resectable GC. Nevertheless, many patients at the time of diagnosis have already locally unresectable tumors or signs of systemic disease (clinical stage IV GC patients). As a result, palliative procedures are indicated in the presence of symptoms, including bleeding, perforation, or obstruction.
Palliative gastrectomy is the preferred treatment option for locally advanced GC with gastric outlet obstruction whenever it is feasible. However, if the tumor cannot be resected, the placements of endoscopic stents or gastrojejunostomy are additional options for distal lesions, according to the patient's life expectancy[2,3]. Unfortunately, some tumors may be too large or located in the upper third of the stomach, not allowing performing the gastrojejunostomy proximal to the tumor. In this scenario, a nasoenteric tube or jejunostomy are the main alternatives. Although jejunostomy enables the use of enteral nutrition, the patient persists with the obstruction and does not tolerate a full oral diet, with negative impact on quality of life.
Most studies evaluate the outcomes concerning placing a feeding jejunostomy at the time of gastroesophageal resection[4,5]. There are few reports concerning the influence of clinical and treatment variables on the outcome of palliative jejunostomy. Questions about improving survival and quality of life, in contrast to the morbidity and mortality rates in these cases, remain unanswered. Consequently, there are still doubts about who benefits from jejunostomy.
Thus, the aim of this study was to analyze the clinical characteristics and prognosis of stage IV patients who underwent palliative jejunostomy due to obstructive GC, and create a prognostic score for mortality based on variables related to survival and worse outcomes.
We conducted a retrospective study of patients with stage IV who underwent jejunostomy for obstructive GC at the Cancer Institute between 2009 and 2020. The indications for jejunostomy and inclusion in the study were: Adenocarcinoma histology, obstructive GC that could not be resected, diffuse or proximal gastric lesion that could not be submitted to gastrojejunostomy.
Abdominal and pelvis computed tomography (CT), endoscopy, and laboratory tests were assessed preoperatively in all patients. The clinical data collected from our prospectively-maintained database included body mass index (BMI), laboratory blood test, the clinical performance by the American Society of Anesthesiologists (ASA) classification[6], the presence of comorbidities using the Charlson Comorbidity index (CCI)[7] (without the inclusion of age and GC as comorbidity), and Lauren histological type. GCs were staged according to the TNM 8th edition.
All patients were operated in a high-volume center by specializes surgeons. Postoperative complications (POC) were graded according to Clavien-Dindo's classification[8] and major POC were defined as Clavien III-V.
Palliative chemotherapy (CMT) consisted of a doublet containing fluoropyrimidine (capecitabine or 5-fluorouracil) and a platin (oxaliplatin or cisplatin) as the preferred systemic regimen for the first line. Irinotecan and cisplatin chemotherapy was chosen in some cases to avoid the use of infusion pumps or in those patients with difficulty swallowing capecitabine tablets. Paclitaxel or irinotecan was used in the second line. In our center monoclonal antibodies (trastuzumab or ramucirumab), as well as immunotherapy, are not usually available for GC treatment[9-11].
Postoperative follow-up was performed every month or in shorter period if necessary. Absence in appointments for more than 12 mo was considered as a loss of follow-up. The study was approved by the hospital ethics committee (NP1681/20) and registered online (https://plataformabrasil.saude.gov.br/; CAAE: 31626220.8. 0000.0068).
To create a scoring system, patients were randomized into two groups (1:1) by computer using the statistical software (SPSS). The score was developed with half of the patients and further evaluated in a validation cohort with the remaining patients. The score was built with 90-d mortality as the main outcome.
As predictors, 11 preoperative clinical variables were selected and classified in a dichotomous way to define the scoring system categories. Clinical and baseline variables—generally used in clinical practice that reflect the general patient´s status—and oncological variables related to the GC, that would have an impact on the prognosis and survival of patients undergoing palliative care, were selected to compose the score[12-15]. The following variables were used: Age, sex, BMI, ASA, CCI, hemoglobin (Hb) levels, albumin levels, neutrophil-lymphocyte ratio (NLR), tumor size, presence of ascites by tomography, and number of disease sites.
The points assigned to each category were determined by binary logistic regression analysis, and the score was calculated for each patient. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the performance metric of the score, as explained below.
Statistical analyses were carried out using SPSS software, version 20.0 (SPSS Inc, Chicago, IL). Chi-square or Fisher's Exact test, and t-test were used to evaluate categorical and continuous variables, respectively. Factors related to 90-d mortality were analyzed by binary logistic regression analysis, and the odds ratios (ORs) with 95% confidence interval (95%CI) were calculated. The score performance metric was determined by the AUC. An AUC > 0.7 was considered to indicate high diagnostic accuracy, given that at least 70% of the patients were classified correctly as the high-risk group for 90-d mortality. The optimal cutoff values were determined by maximizing Youden’s index (sensitivity + specificity - 1). Survival was estimated using the method of Kaplan–Meier, and the log-rank test was employed for comparisons between the curves. Overall survival (OS) was calculated from the date of surgery until the date of death. P value less than 0.05 was considered statistically significant.
During the referred period, 363 patients with clinical stage IV GC underwent surgical procedures. Among these, jejunostomy was performed in 80 (22%) patients. The clinical characteristics of all jejunostomy patients are summarized in SupplementaryTable 1.
Patients were predominantly male (62.5%) with a mean age of 62.4 (SD ± 12.8, range 24-84.6) years, and a mean BMI of 21.0 (SD ± 3.9). Most tumors affected the middle third of the stomach (67.4%), with a median diameter of 7.2 cm on the longest axis.
The presence of disease in one or more sites, in addition to the primary, was observed in 70% of the cases: 63.7% had peritoneal metastasis and 17.5% had distant metastasis.
After randomization, jejunostomy patients were divided equally into two groups: (J1) score development group; and (J2) score validation group.
Considering the J1 group, the 90-d mortality rate was 52.5%. To develop the predictive score of mortality in 90-d, variables related to the worst outcome were selected for the design of the model. After the binary logistic regression analysis (SupplementaryTable 2), 11 variables were included in the model, and points were assigned as shown in Table 1, for a maximum score value of 100. The following variables with the respective defined categories were used: Age (< 65 vs ≥ 65 years), sex (female vs male), BMI (< 18.5 kg/m2vs ≥ 18.5 kg/m2), ASA (I/II vs III/IV), CCI (0 vs ≥ 1), Hb levels (≤ 11 g/dL vs > 11 g/dL, which represents the lower limit between mild and moderate anemia for men and women), albumin levels (< 3.5 g/dL vs ≥ 3.5 g/dL), NLR (< 2.5 vs ≥ 2.5), tumor size (≤ 7 cm vs > 7 cm), number of disease sites (one vs two or more, with represents locoregional, distant, or peritoneal) and the presence of ascites by tomography (absent vs present).
| Variables | Category | Points |
| Sex | Female | 7 |
| Age (years) | ≥ 65 | 4 |
| BMI (kg/m2) | BMI < 18.5 | 1 |
| CCI | CCI ≥ 1 | 7 |
| ASA classification | III/IV | 9 |
| Hb (g/dL) | Hb ≤ 11 | 1 |
| Alb (g/dL) | Alb < 3.5 | 3 |
| NLR | NLR ≥ 2.5 | 50 |
| Presence of ascites on CT | Present | 5 |
| Tumor size (cm) | > 7 cm | 5 |
| Number of disease sites | Two or more | 8 |
| Total | 100 |
The highest score value was assigned to the high NLR category and the lowest value to the low levels of Hb and BMI. After, a value was assigned to each of the 40 patients, and the performance metric of the score was assessed through the construction of the ROC curve (Figure 1A). The AUC was 71.2% (AUC 0.712, 95%CI: 0.537–0.887, P = 0.022), with an estimated value of 65.5 for optimal cutoff.
Based on the cutoff, the J1 patients were divided in two risk groups for 90-d mortality: Low-risk (score < 65.5), with 14 (35%) patients; and high-risk (score ≥ 65.5) with 26 (65%) patients. The characteristics of the groups are shown in Table 2.
| Variables | J1-score development cohort | P value | |
| Low-risk | High-risk | ||
| n = 14 (%) | n = 26 (%) | ||
| Sex | 0.507 | ||
| Female | 6 (42.9) | 14 (53.8) | |
| Male | 8 (57.1) | 12 (46.2) | |
| Age (years) | 0.954 | ||
| mean (SD) | 62.0 (15.0) | 61.7 (12.9) | |
| BMI (kg/m2) | 0.101 | ||
| mean (SD) | 20.4 (2.7) | 22.4 (3.9) | |
| CCI | 0.885 | ||
| 0 | 10 (71.4) | 18 (69.2) | |
| ≥ 1 | 4 (286) | 8 (30.8) | |
| ASA class | 0.507 | ||
| I/II | 8 (57.1) | 12 (46.2) | |
| III/IV | 6 (42.9) | 14 (53.8) | |
| Hemoglobin (g/dL) | 0.456 | ||
| mean (SD) | 11.1 (1.9) | 10.5 (2.0) | |
| Albumin (g/dL) | 0.123 | ||
| mean (SD) | 3.7 (0.6) | 3.3 (0.6) | |
| NLR | 0.001 | ||
| mean (SD) | 3.03 (1.58) | 7.46 (5.22) | |
| Tumor size (cm) | 0.18 | ||
| mean (SD) | 6.9 (2.1) | 8.3 (3.4) | |
| Presence of ascites on CT | 0.48 | ||
| Absent | 6 (42.9) | 7 (26.9) | |
| Present | 8 (57.1) | 19 (73.1) | |
| Lauren type | 1 | ||
| Intestinal | 4 (28.6) | 6 (23.1) | |
| Diffuse/mixed | 9 (64.3) | 17 (65.4) | |
| Undetermined | 1 (7.1) | 3 (11.5) | |
| Number of disease sites | 0.408 | ||
| One (only locoregional) | 6 (42.9) | 7 (26.9) | |
| Two or more | 8 (57.1) | 19 (73.1) | |
| POC | 0.075 | ||
| No POC/Clavien 1-2 | 14 (100) | 19 (73.1) | |
| Clavien 3-5 | 0 (0) | 7 (26.9) | |
| Length of hospital stay (days) | 0.452 | ||
| Median (IQR) | 4.5 (1.7-6.3) | 5 (2.7-9.5) | |
| Palliative treatment-1st line | 0.257 | ||
| No | 6 (42.9) | 16 (61.5) | |
| Yes | 8 (57.1) | 10 (38.5) | |
| 30-d mortality | 0.013 | ||
| No | 13 (92.9) | 13 (50) | |
| Yes | 1 (7.1) | 13 (50) | |
| 90-d mortality | < 0.001 | ||
| No | 12 (85.7) | 7 (26.9) | |
| Yes | 2 (14.3) | 19 (73.1) | |
Sex, age, stage, ASA, and the presence of comorbidities showed no statistically significant differences between the groups. High NLR (P = 0.001), albumin levels lower than 3.5 g/dL (P = 0.026), and tumor size greater than 7 cm (P = 0.001) were related to the high-risk group. The risk groups showed a significant association with both 30 and 90-d mortality (P = 0.013 and P < 0.001, respectively).
Using the previously constructed scoring system, the score value was calculated for each patient in the validation cohort (J2 group, n = 40). The rate of 90-d mortality in this group was 50%.
As shown in Figure 1B, the risk score had superior diagnostic accuracy for 90-d mortality in the validation cohort (AUC 0.756, 95%CI: 0.598–0.915, P = 0.006). Using the previously established cutoff value (65.5 points), 21 (52.5%) and 19 (47.5%) patients were classified as low-risk and high-risk groups, respectively (Table 3).
| Variables | J2-score validation cohort | P value | |
| Low-risk | High-risk | ||
| n = 21 (%) | n = 19 (%) | ||
| Sex | 0.721 | ||
| Female | 6 (28.6) | 4 (21.1) | |
| Male | 15 (71.4) | 15 (78.9) | 0.072 |
| Age (years) | |||
| mean (SD) | 59.7 (12.4) | 66.7 (11.4) | |
| BMI (kg/m2) | 0.074 | ||
| mean (SD) | 21.4 (3.5) | 19.0 (4.7) | |
| CCI | 0.001 | ||
| 0 | 21 (100) | 11 (57.9) | |
| ≥ 1 | 0 (0) | 8 (42.1) | |
| ASA class | 0.002 | ||
| I/II | 16 (76.2) | 5 (26.3) | |
| III/IV | 5 (23.8) | 14 (73.7) | |
| Hemoglobin (g/dL) | 0.345 | ||
| mean (SD) | 11.4 (2.3) | 10.6 (2.4) | |
| Albumin (g/dL) | 0.047 | ||
| mean (SD) | 3.6 (0.6) | 3.1 (0.6) | |
| NLR | 0.012 | ||
| mean (SD) | 3.65 (3.45) | 8.81 (7.64) | |
| Tumor size (cm) | 0.731 | ||
| mean (SD) | 6.6 (2.6) | 6.9 (3.4) | |
| Presence of ascites on CT | 0.004 | ||
| Absent | 14 (66.7) | 4 (21.1) | |
| Present | 7 (33.3) | 15 (78.9) | |
| Lauren type | 0.753 | ||
| Intestinal | 5 (23.8) | 7 (36.8) | |
| Diffuse/mixed | 13 (61.9) | 9 (47.4) | |
| Undetermined | 3 (14.3) | 3 (15.8) | |
| Number of disease sites | 0.385 | ||
| One (only locoregional) | 7 (33.3) | 4 (21.1) | |
| Two or more | 14 (66.7) | 15 (78.9) | |
| POC | 1 | ||
| No POC/Clavien 1-2 | 19 (90.5) | 17 (89.5) | |
| Clavien 3-5 | 2 (9.5) | 2 (10.5) | |
| Length of hospital stay (days) | 0.455 | ||
| Median (IQR) | 3 (3-7.5) | 4 (4-8) | |
| Palliative treatment-1st Line | 0.012 | ||
| No | 5 (23.8) | 12 (63.2) | |
| Yes | 16 (76.2) | 7 (36.8) | |
| 30-d mortality | 0.049 | ||
| No | 18 (85.7) | 11 (57.9) | |
| Yes | 3 (14.3) | 8 (42.1) | |
| 90-d mortality | 0.027 | ||
| No | 14 (66.7) | 6 (31.6) | |
| Yes | 7 (33.3) | 13 (68.4) | |
BMI less than 18.5 kg/m2 (P < 0.001), CCI ≥ 1 (P = 0.001), ASA III/IV, (P = 0.002), high NLR (P = 0.012), and the presence of ascites on CT exam (P = 0.004) were significantly associated with the high-risk group.
Considering the postoperative outcomes, there were no differences in POC rate and length of hospital stay between the groups. However, mortality at 30 and 90 d were significantly higher in the high-risk group, demonstrating the performance of the score in predicting mortality based on the adopted cutoff value (P = 0.049 and P = 0.027, respectively). Besides, the rate of patients who received first and second-line palliative treatment was higher in the low-risk group (P = 0.012 and P = 0.009, respectively).
The median follow-up time for all patients was 2.1 mo (interquartile range = 0.6-5.9, mean of 4.4 mo). At the time of this study, 8 patients were alive, and 72 patients died (J1 = 36 and J2 = 36). The median OS for the entire jejunostomy cohort was 2.7 mo, compared to a median OS of 8 mo for the other clinical stage IV GC patients undergoing other surgical procedures (resection, bypass, or diagnostic laparoscopy) (P < 0.001) (Supplementary Figure 1).
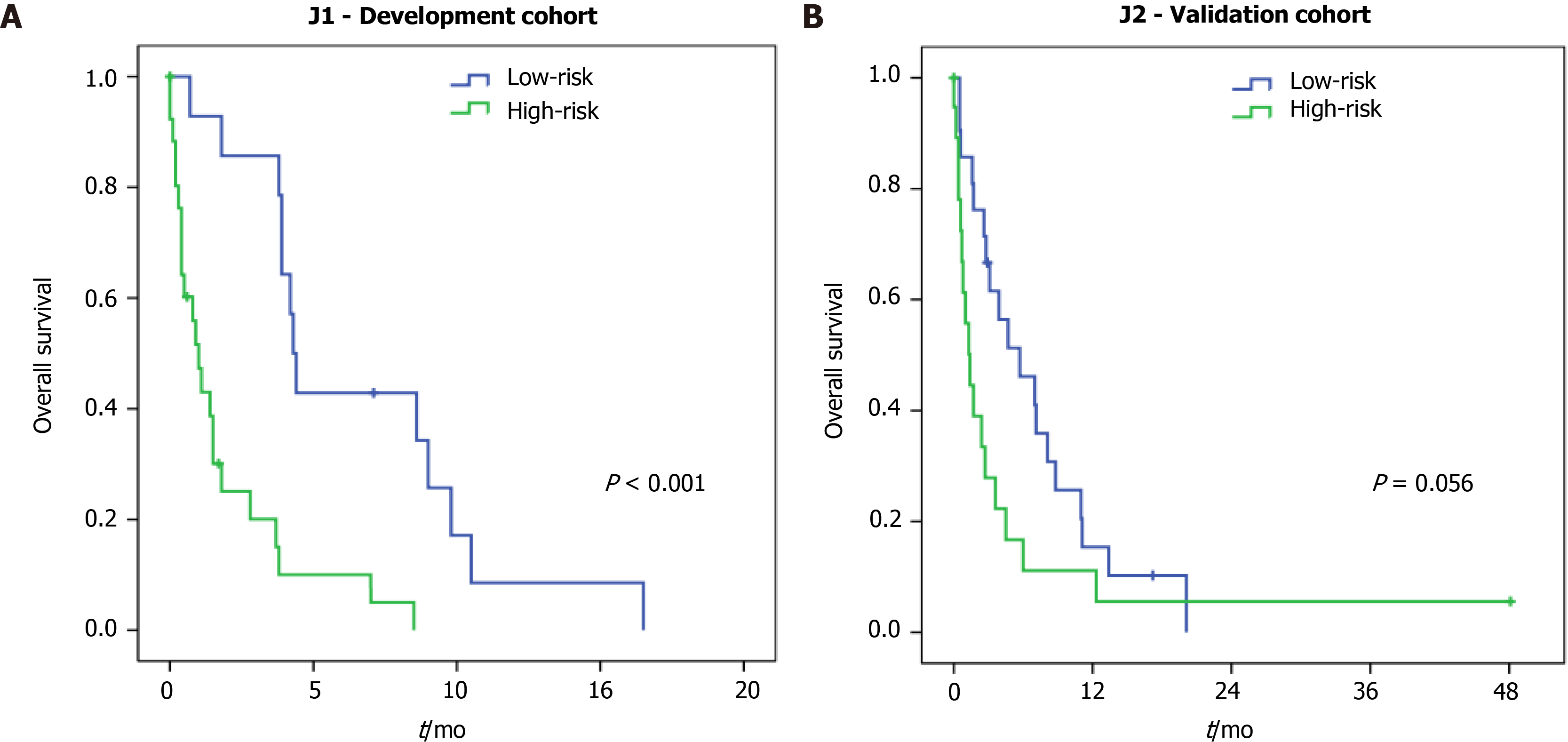
Regarding the risk groups in J1 patients (development cohort), the median OS for high and low-risk was 1.0 mo and 4.3 mo, respectively (P < 0.001) (Figure 2A). For the J2 patients (validation cohort), the median OS was 1.4 mo for the high-risk compared to 5.7 mo in the low-risk group (P = 0.059) (Figure 2B).
In the present study, we performed an analysis of a cohort of stage IV GC, not amenable to surgical resection, who underwent palliative jejunostomy, and we developed a prognostic score for 90-d mortality. Jejunostomy represented the therapeutic choice in 22% of stage IV GC patients. We provided a simple and feasible scoring system with variables easily available in the clinical assessment of patients. The score demonstrated an accuracy of 75.6% in the validation cohort and was associated with mortality rates.
As is widely known, palliative gastric resection is the best option for obstructive lesions, but some patients are unable to undergo this procedure. Proximal and bulky tumors also do not allow gastrojejunostomy to be performed to restore gastrointestinal continuity. Thus, in these cases, jejunostomy or nasoenteric tube are often indicated as a palliative measure to allow maintenance of nutritional enteral support[16]. Once the nutritional emergency is solved, patients may receive palliative CMT and resume oral intake in cases with good response.
The nasoenteric tube causes discomfort in the nasopharynx and oropharynx besides its visual uncomfortable aspect. On the other hand, jejunostomy involves performing an invasive surgical procedure on a patient who is already frail and debilitated[17].
Since these patients have a restricted prognosis, and particular clinical and oncological conditions, the rationale of developing the score was to take into account characteristics that can impact survival to properly identify the patients that are likely to benefit from the procedure.
Building a score involves the appropriate choice of variables since a wide variety of characteristics must be considered in GC cases for the management decision. In our model, we included 11 common usage variables related to the survival outcomes with broad external validity. The variables were categorized in a dichotomous way, which facilitates their use and reproducibility. Additionally, to provide an internal validation, the cohort was randomized into two samples: One for the construction of the model, and the second for the validation of the model.
Considering the variables that support the score, the impact of the NLR on the outcome was noteworthy, since the highest score was achieved by this parameter. The NLR represents a prognostic marker used in different solid tumors, including GC, and its correlation with survival is widely reported. Furthermore, the incidence of POC and their relation to NLR is also described in GC patients[12,18].
The rationale for this result is likely complex. Neutrophils present a pro-tumor behavior, as they promote angiogenesis, damage DNA, inhibit T-cell activity against tumor cells, and facilitate the metastatic process. Inversely, lymphocytes exert an anti-tumor function when they recognize tumor cell antigens, promoting cytolytic activity against these cells. Thus, the high NLR reflects a proinflammatory systemic status, leading to a protumoral environment that allows rapid tumor progression and development[19]. Therefore, NLR may also reflect a more advanced disease stage.
Regarding the outcome used to assign the score, we chose the 90-d mortality because it is considered a good parameter to reflect the value of a palliative procedure in patients with limited survival. Another outcome option to consider would be the ability to receive palliative CMT in the postoperative setting. However, this variable reflects not only the patient's performance after the procedure, but also their decision to perform palliative CMT. Some patients choose to adopt only best support care. Even so, in the validation court, low-risk patients received more frequently first and second lines of CMT.
Regarding complications related to jejunostomy, most of the reports are related to its performance after major gastrointestinal procedures for postoperative nutritional support[4,5,20,21]. Common surgical complications include loss or obstruction of the tube and local leakage of its content. The findings of some studies have shown that jejunostomy placed during GC resection is associated with increased complications. The analysis of this research included only palliative patients already with limited survival. Therefore, it is difficult to assess the impact of possible complications related to the procedure.
As previously mentioned, it is noteworthy that our score could distinguish low-risk patients, which received significantly more palliative CMT and had better median OS. Wang et al[22] analyzed 545 palliative procedures for GC treatment. Of these, 77 were considered as intubation procedures (17 gastrostomies and 60 jejunostomies), with a surgical mortality rate of 23.4%. The median OS in this group was 3.8 mo, and patients who underwent intubation procedures receive less chemotherapy compared to resected patients. In the study performed by Schmidt et al[23], the intubation group accounted for 12 out of 110 patients. These patients, combined with 52 other patients who underwent surgical procedures that did not involve the removal of the tumor, had a median OS of 9.2 mo. In our study, the median OS for the entire population was 2.7 mo. These results highlight the limited survival of these patients and the need for a good selection of patients for palliative jejunostomy.
Thus, with the application of the score, we were able to distinguish patients with a more limited survival: A high-risk group, with 1 mo and 1.4 mo in J1 and J2 cohorts, respectively; compared to the low-risk group, with 4.3 and 5.7 mo in J1 and J2 cohorts, respectively. This difference was significant in the development cohort but not in the validation cohort. Perhaps, the presence of one outlier with extremely high survival may have influenced the result in the validation cohort. As the score was developed to predict 90-d mortality, it did not affect the performance of the model. Furthermore, the score performance was higher in the validation cohort compared to the development group (AUC 0.756 vs AUC 0.712, respectively).
Some limitations in the present study should be mentioned. Unfortunately, due to its retrospective design, other outcomes related to the effectiveness of nutritional therapy provided by jejunostomy have not been evaluated. Analysis of weight curve, performance status, and quality of life could provide other relevant information. Also, the number of cases is a limitation. Even in a referral center, jejunostomy has a limited role in cancer treatment. This leads to a low volume of reports in the literature concerning its results, and maintains doubts as to its real benefit in cancer care and survival of these patients.
Accordingly, we believe that our series can provide data to assist in the decision of choosing the best way to maintain enteral nutrition. Since our score is easy to perform, it can be applied in other centers—including retrospectively—to evaluate their reproducibility and impact on the oncological outcomes of jejunostomy in these patients.
The scoring system developed with 11 variables related to patient’s performance status and medical condition was able to distinguish patients submitted to jejunostomy with a high risk of 90 d mortality. In addition, the score identified patients who were able to receive more CMT in the validation cohort.
Palliative gastrectomy is the initial treatment option for locally advanced gastric cancer (GC) with gastric outlet obstruction whenever it is feasible. Unfortunately, in some cases, nasoenteric tube or jejunostomy becomes the therapeutic alternative to allow maintenance of nutritional enteral support.
The limited survival of these patients raises doubts about who benefits from jejunostomy.
This study aimed to create a prognostic score for 90-d mortality for stage IV GC patients who underwent jejunostomy based on the clinical variables related to survival.
We conducted a retrospective analysis of 80 stage IV patients who underwent jejunostomy for obstructive GC. To create a scoring system, patients were randomized into two groups (1:1) by computer using statistical software. The score was developed with half of the patients and further evaluated in a validation cohort with the remaining patients. The score was developed with 90-d mortality as the main outcome.
We provided a simple and feasible score system with 11 variables easily available in the clinical assessment of patients. The score demonstrated an accuracy of 75.6% in the validation cohort and was associated with the mortality rates in patients who underwent jejunostomy.
The scoring system developed with variables related to patient’s performance status and medical condition was able to distinguish patients submitted to jejunostomy with a high risk of 90 d mortality.
The results of our series may contribute to identifying stage IV GC with unresectable tumors who can obtain better results with the jejunostomy. In addition, the score may contribute to the selection of patients who were able to receive chemotherapy, and thereby improving their survival.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56614] [Article Influence: 7076.8] [Reference Citation Analysis (134)] |
| 2. | Ramos MFKP, Barchi LC, de Oliveira RJ, Pereira MA, Mucerino DR, Ribeiro U Jr, Zilberstein B, Cecconello I. Gastric partitioning for the treatment of malignant gastric outlet obstruction. World J Gastrointest Oncol. 2019;11:1161-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 371] [Article Influence: 23.2] [Reference Citation Analysis (2)] |
| 4. | Torres Júnior LG, de Vasconcellos Santos FA, Correia MI. Randomized clinical trial: nasoenteric tube or jejunostomy as a route for nutrition after major upper gastrointestinal operations. World J Surg. 2014;38:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Sun Z, Shenoi MM, Nussbaum DP, Keenan JE, Gulack BC, Tyler DS, Speicher PJ, Blazer DG 3rd. Feeding jejunostomy tube placement during resection of gastric cancers. J Surg Res. 2016;200:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA Class). StatPearls. Treasure Island FL: StatPearls Publishing LLC., 2017. |
| 7. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 39557] [Article Influence: 1014.3] [Reference Citation Analysis (0)] |
| 8. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26034] [Article Influence: 1183.4] [Reference Citation Analysis (1)] |
| 9. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1713] [Article Influence: 95.2] [Reference Citation Analysis (1)] |
| 10. | Ilson DH, Saltz L, Enzinger P, Huang Y, Kornblith A, Gollub M, O'Reilly E, Schwartz G, DeGroff J, Gonzalez G, Kelsen DP. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol. 1999;17:3270-3275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, Esaki T, Nagase M, Fujitani K, Yamaguchi K, Ura T, Hamamoto Y, Morita S, Okamoto I, Boku N, Hyodo I. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (5)] |
| 12. | Ramos MFKP, Pereira MA, Yagi OK, Dias AR, Charruf AZ, Oliveira RJ, Zaidan EP, Zilberstein B, Ribeiro-Júnior U, Cecconello I. Surgical treatment of gastric cancer: a 10-year experience in a high-volume university hospital. Clinics (Sao Paulo). 2018;73:e543s. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Ben Kridis W, Marrekchi G, Mzali R, Daoud J, Khanfir A. Prognostic factors in metastatic gastric carcinoma. Exp Oncol. 2019;41:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Tan HL, Chia CS, Tan GHC, Choo SP, Tai DW, Chua CWL, Ng MCH, Soo KC, Teo MCC. Metastatic gastric cancer: Does the site of metastasis make a difference? Asia Pac J Clin Oncol. 2019;15:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6:41370-41382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 16. | Cotogni P, Stragliotto S, Ossola M, Collo A, Riso S; On Behalf Of The Intersociety Italian Working Group For Nutritional Support In Cancer. The Role of Nutritional Support for Cancer Patients in Palliative Care. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Bozzetti F. Is there a place for nutrition in palliative care? Support Care Cancer. 2020;28:4069-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Mohri Y, Tanaka K, Toiyama Y, Ohi M, Yasuda H, Inoue Y, Kusunoki M. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. Medicine (Baltimore). 2016;95:e3125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 20. | Blakely AM, Ajmal S, Sargent RE, Ng TT, Miner TJ. Critical analysis of feeding jejunostomy following resection of upper gastrointestinal malignancies. World J Gastrointest Surg. 2017;9:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Brenkman HJF, Roelen SVS, Steenhagen E, Ruurda JP, van Hillegersberg R. Postoperative complications and weight loss following jejunostomy tube feeding after total gastrectomy for advanced adenocarcinomas. Chin J Cancer Res. 2017;29:333-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Wang CS, Chao TC, Jan YY, Jeng LB, Hwang TL, Chen MF. Benefits of palliative surgery for far-advanced gastric cancer. Chang Gung Med J. 2002;25:792-802. [PubMed] |
| 23. | Schmidt B, Look-Hong N, Maduekwe UN, Chang K, Hong TS, Kwak EL, Lauwers GY, Rattner DW, Mullen JT, Yoon SS. Noncurative gastrectomy for gastric adenocarcinoma should only be performed in highly selected patients. Ann Surg Oncol. 2013;20:3512-3518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng XC S-Editor: Fan JR L-Editor: A P-Editor: Li JH