Published online May 24, 2020. doi: 10.5306/wjco.v11.i5.283
Peer-review started: December 31, 2019
First decision: March 15, 2020
Revised: April 16, 2020
Accepted: May 16, 2020
Article in press: May 16, 2020
Published online: May 24, 2020
Processing time: 145 Days and 4.9 Hours
Invasive lobular carcinomas (ILC) form 5%-10% of breast cancer and rarely show overexpression of human epidermal growth factor receptor 2 (HER2).
To describe the prevalence and prognostic factors of HER2 positive (HER2+) ILC in an Asian population.
A retrospective review of patients with ILC seen between January 1985 and March 2018 at various SingHealth medical institutions was conducted. Demographic and clinical data were collected from medical records. We examined clinicopathological characteristics and survival in relation to HER2 status.
A total of 864 patients were included. Prevalence of HER2 positivity was 10.1% (87 patients). Compared with HER2 negative (HER2-) ILC, HER2+ ILC was associated with a higher proportion of estrogen receptor negative (24.4% vs 5.9%, P < 0.001), progesterone receptor negative (PR-) (40.2% vs 24%, P = 0.002) and grade 3 tumours (Grade 3, 29.0% vs 10.2%, P < 0.001). Overall survival rate was poorer in patients with HER2+ compared to HER2- ILC (56.7% vs 72.9% alive at 10 years; hazard ratio 1.87, 95% confidence interval: 1.21-2.90, P = 0.004). Based on multivariate analysis, negative prognostic factors for overall survival included HER2 positivity, PR negativity, older age, Indian ethnicity and higher tumour stage.
Prevalence of HER2+ ILC was 10.1%. HER2+ ILC was more likely to have poorer prognostic features such as estrogen receptor negative, PR- and higher tumour grade, and have a poorer survival.
Core tip: We conducted a retrospective review of 864 patients with invasive lobular breast carcinoma (ILC) and examined the clinicopathological characteristics and survival in relation to human epidermal growth factor receptor 2 (HER2) status. Interestingly, our cohort reports a higher prevalence of HER2 positive ILC (10.1%) as compared to some previous studies. HER2 positive ILC was more likely to have poorer prognostic features such as estrogen receptor negative, progesterone receptor negative and higher tumour grade, and these patients have a poorer survival compared to those with HER2 negative ILC.
- Citation: Kee GJ, Tan RYC, Rehena S, Lee JJX, Zaw MWW, Lian WX, Yeong J, Tan SM, Lim SH, Tan BKT, Yap YS, Dent RA, Wong FY, Lee GE. Human epidermal growth factor receptor 2 positive rates in invasive lobular breast carcinoma: The Singapore experience. World J Clin Oncol 2020; 11(5): 283-293
- URL: https://www.wjgnet.com/2218-4333/full/v11/i5/283.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i5.283
Invasive lobular carcinomas (ILC) represent about 5%-10% of breast cancer[1-3]. Prevalence of overexpression of human epidermal growth factor receptor 2 (HER2) in breast cancer has been reported at 4.8%-5.1%[4,5]. The clinicopathological characteristics of HER2 positive (HER2+) invasive ductal carcinomas (IDC) are known to differ from that of HER2 negative (HER2-) IDC. HER2+ IDC is associated with estrogen receptor negativity (ER-), progesterone receptor negativity (PR-) and higher histologic grade[4,6]. A number of reports suggest that these associations are also present in ILC and that HER2 positivity may be a prognostic factor[7-13]. However, there remains a paucity of research examining the characteristics of HER2+ as opposed to HER2- ILC, particularly in Asian populations. This study aims to investigate the prevalence and prognostic clinicopathological factors of HER2+ ILC.
A retrospective review of patients with ILC seen between January 1985 and July 2018 at National Cancer Centre Singapore, Singapore General Hospital, Changi General Hospital and KK Women’s and Children’s Hospital was conducted. We obtained the clinical and pathological data of ILC patients from the Joint Breast Cancer Registry, our prospective database. Clinical variables included patient demographic factors such as age at diagnosis, gender, ethnicity, disease factors such as tumour side, size, grade, stage, nodal status, ER, PR and HER2 status, as well as treatment given such as chemotherapy, radiotherapy, surgery and anti-HER2 therapy. The study was reviewed and approved by the SingHealth Institutional Review Board CIRB Ref: 2019/2419.
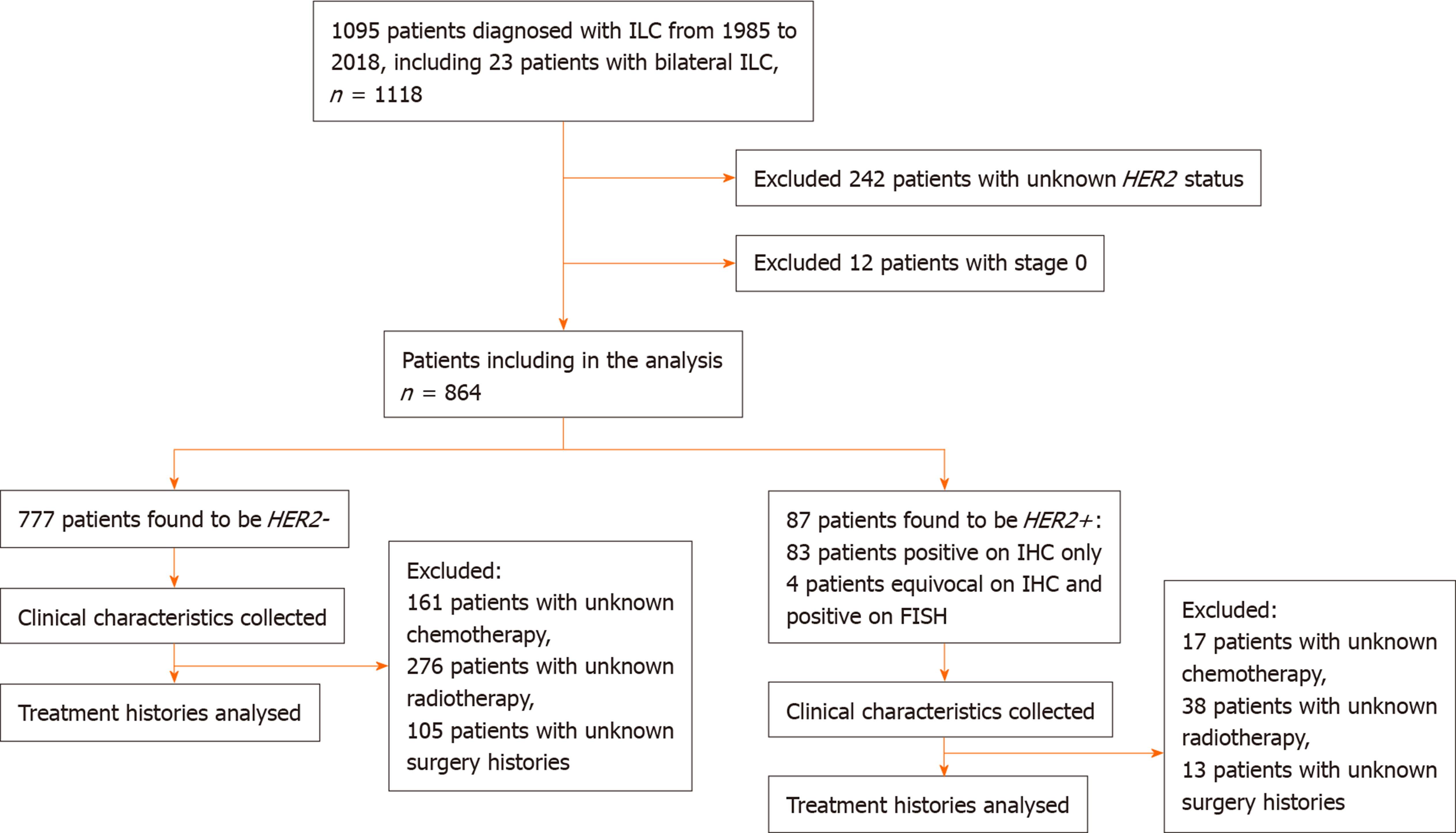
From 1985 to 2018, 1095 patients were diagnosed with ILC. Of these, 242 patients with unknown HER2 status were excluded from the study. Twelve patients with pathological stage 0 breast cancer were also excluded from the study. The remaining 864 patients were analysed (Figure 1).
Histopathological diagnoses of ILC were made by pathologists at various SingHealth medical institutions, namely Singapore General Hospital, Singapore; Changi General Hospital and KK Women’s and Children’s Hospital. Pathologic variables collected included ER, PR and HER2 status. ASCO-CAP guidelines were used to define positivity cut-offs for the tumours as follows: A positive ER/PR result was defined as the presence of at least 1% of tumour cell nuclei displaying unequivocal staining of any intensity, and for HER2, tumour positivity was defined as > 10% of tumour cells exhibiting 3+ membrane staining. Ambiguous HER2 cases were tested and confirmed by fluorescence in situ hybridization testing based on the ASCO-CAP guidelines[6-9]. In the Joint Breast Cancer Registry database, tumours were also classified into a molecular subtype as follows: Basal (ER-, PR- and HER2-); HER2+ (ER-, PR- and HER2+); Luminal A (ER- or PR- and HER2-); Luminal B (ER+ or PR+ and HER2+).
All demographic and clinicopathological characteristics were summarized in terms of HER2 status, as HER2+ and HER2- ILC. Categorical and continuous variables were summarized as frequency with percentage and median [interquartile range (IQR)] respectively. Differences between HER2+ and HER2- ILC were tested using chi-squared test for categorical variables and Mann-Whitney U test for continuous variables.
The primary outcome overall survival (OS) was treated as time-to-event data and survival time was defined as time from date of diagnosis to date of death or date last seen. Secondary outcomes included disease-free survival (DFS) and breast cancer-specific overall survival (BCSS). DFS was treated as time-to-event data and duration of DFS was defined as duration from date of last treatment to date of relapse or date last seen or date of mortality. BCSS was treated as time-to-event data and duration of BCSS was defined as duration from date of last treatment to date last seen or date of mortality if cause of death was attributed to breast cancer. OS, DFS and BCSS were analysed for HER2+ and HER2- status using Kaplan-Meier survival analysis and were tested using log-rank test.
Univariate and multivariate Cox proportional hazard (CPH) regression analysis were used to find associations between OS and other prognostic factors in these patients with ILC. The following clinicopathological characteristics were investigated in the model: Age, ethnicity, ER status, PR status, HER2 status, tumour size, stage, grade and treatment modalities such as chemotherapy, radiotherapy and surgery. Variables with P < 0.03 in the univariate CPH model were selected for multivariable model. Final multivariate CPH model was selected using stepwise, forward and backward variable selection method. Quantitative association from CPH regression model was expressed in terms hazard ratio with corresponding 95% confidence interval. Three separate CPH models were used for OS, DFS and BCSS. All statistical tests were two-sided and P < 0.05 was considered statistically significant. Analyses were performed using SAS Institute Inc 2013. SAS/ACCESS® 9.4 Interface to ADABAS (SAS Institute Inc., Cary, NC, United States).
A total of 864 patients with ILC were included in the analysis. Study population characteristics are shown in Table 1. Of note, a total of 87 (10.1%) were diagnosed with HER2+ ILC. Compared with HER2- ILC, HER2+ ILC was associated with a higher proportion of ER- (24.4% vs 5.9%, P < 0.001), PR- negative (40.2% vs 24%, P = 0.002) and grade 3 tumours (Grade 3, 29.0% vs 10.2%, P < 0.001) (Table 1).
| Characteristics | HER2+ (n = 87) | HER2- (n = 777) | Total (n = 864) | P value | |||
| Age (yr) | 1.000 | ||||||
| ≤ 50 | 30 (34.5) | 272 (35.0) | 302 (35.0) | ||||
| > 50 | 57 (65.5) | 505 (65.0) | 562 (65.0) | ||||
| Ethnicity | 0.594 | ||||||
| Chinese | 68 (78.2) | 558 (72.1) | 626 (72.7) | ||||
| Indian | 4 (4.6) | 60 (7.8) | 64 (7.4) | ||||
| Malay | 8 (9.2) | 68 (8.8) | 76 (8.8) | ||||
| Others | 7 (8.0) | 88 (11.4) | 95 (11.0) | ||||
| ER | < 0.001 | ||||||
| Negative | 21 (24.4) | 46 (5.9) | 67 (7.8) | ||||
| Positive | 65 (75.6) | 730 (94.1) | 795 (7.8) | ||||
| PR | 0.002 | ||||||
| Negative | 35 (40.2) | 185 (24.0) | 220 (25.6) | ||||
| Positive | 52 (59.8) | 587 (76.0) | 639 (74.4) | ||||
| Tumour size | 0.765 | ||||||
| 0.1-2 cm | 21 (41.2) | 230 (38.7) | 251 (38.9) | ||||
| > 2 cm | 30 (58.8) | 365 (61.3) | 395 (61.1) | ||||
| Tumour grade | <0.001 | ||||||
| Grade 1 | 7 (10.1) | 148 (22.5) | 155 (21.3) | ||||
| Grade 2 | 42 (60.9) | 443 (67.3) | 485 (66.7) | ||||
| Grade 3 | 20 (29.0) | 67 (10.2) | 87 (12.0) | ||||
| Tumour stage | 0.066 | ||||||
| Stage 1 | 20 (24.1) | 216 (30.3) | 236 (29.7) | ||||
| Stage 2 | 25 (30.1) | 267 (37.5) | 292 (36.7) | ||||
| Stage 3 | 27 (32.5) | 179 (25.1) | 206 (25.9) | ||||
| Stage 4 | 11 (13.3) | 50 (7.0) | 61 (7.7) | ||||
| Treatment | |||||||
| Chemotherapy1 | 50 (66.7) | 390 (54.2) | 440 (55.3) | 0.038 | |||
| With HER2 therapy | 47 (54.0) | - | - | 47 (54.0) | |||
| No HER2 therapy | 12 (13.8) | - | - | 12 (13.8) | |||
| Unknown if any HER2 therapy | 28 (32.2) | - | - | 28 (32.2) | |||
| Radiotherapy2 | 47 (62.7) | 404 (56.1) | 451 (56.7) | 0.276 | |||
| Surgery3 | 73 (92.4) | 690 (92.1) | 763 (92.1) | 0.929 | |||
Among the 87 patients with HER2+ ILC, 47 (54.0%) received HER2-directed therapy, 12 (13.8%) did not receive HER2-directed therapy and treatment data was not available for the remaining 28 (32.2%) patients. Of the patients who did not receive HER2-directed therapy, reasons cited upon review of clinical charts included cardiac comorbidities, poor performance status, very early stage cancer, refusal of therapy or lack of access to therapy in the years prior to the availability of HER2-directed therapy.
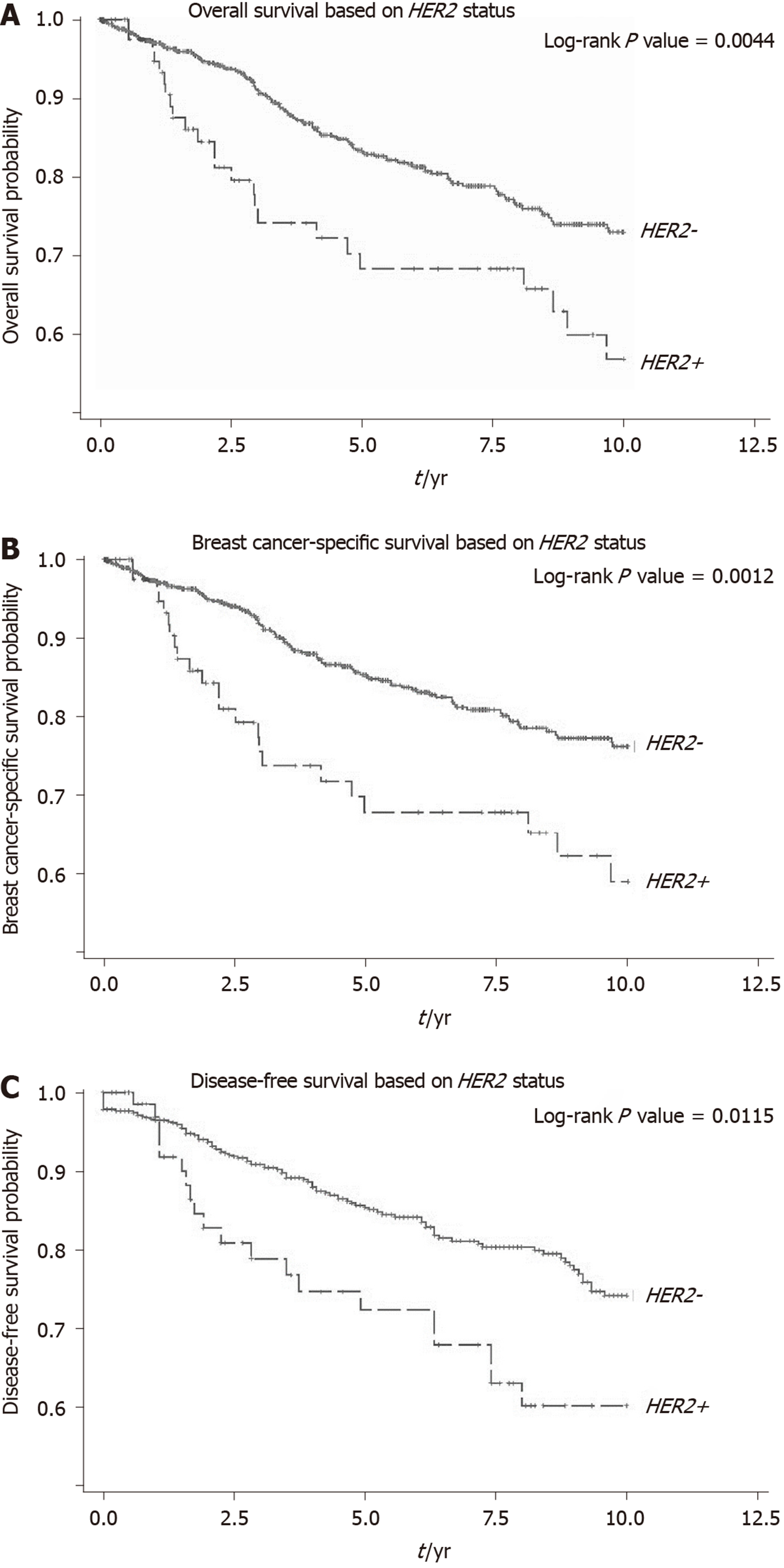
The median survival time was 2.95 (IQR: 1.89-8.87) years and 4.16 (IQR: 1.84-8.32) years respectively for HER2+ and HER2– ILC patients (P = 0.315). The 5-year and 10-year OS rates were 68.3% (59/87 patients) and 56.7% (49/87 patients) respectively in HER2+ patients and 83.4% (648/777 patients) and 72.9% (566/777 patients) respectively in HER2- patients (log-rank P = 0.004). The 5-year and 10-year BCSS and DFS rates in HER2+ and HER2- ILC patients are also shown in Figure 2.
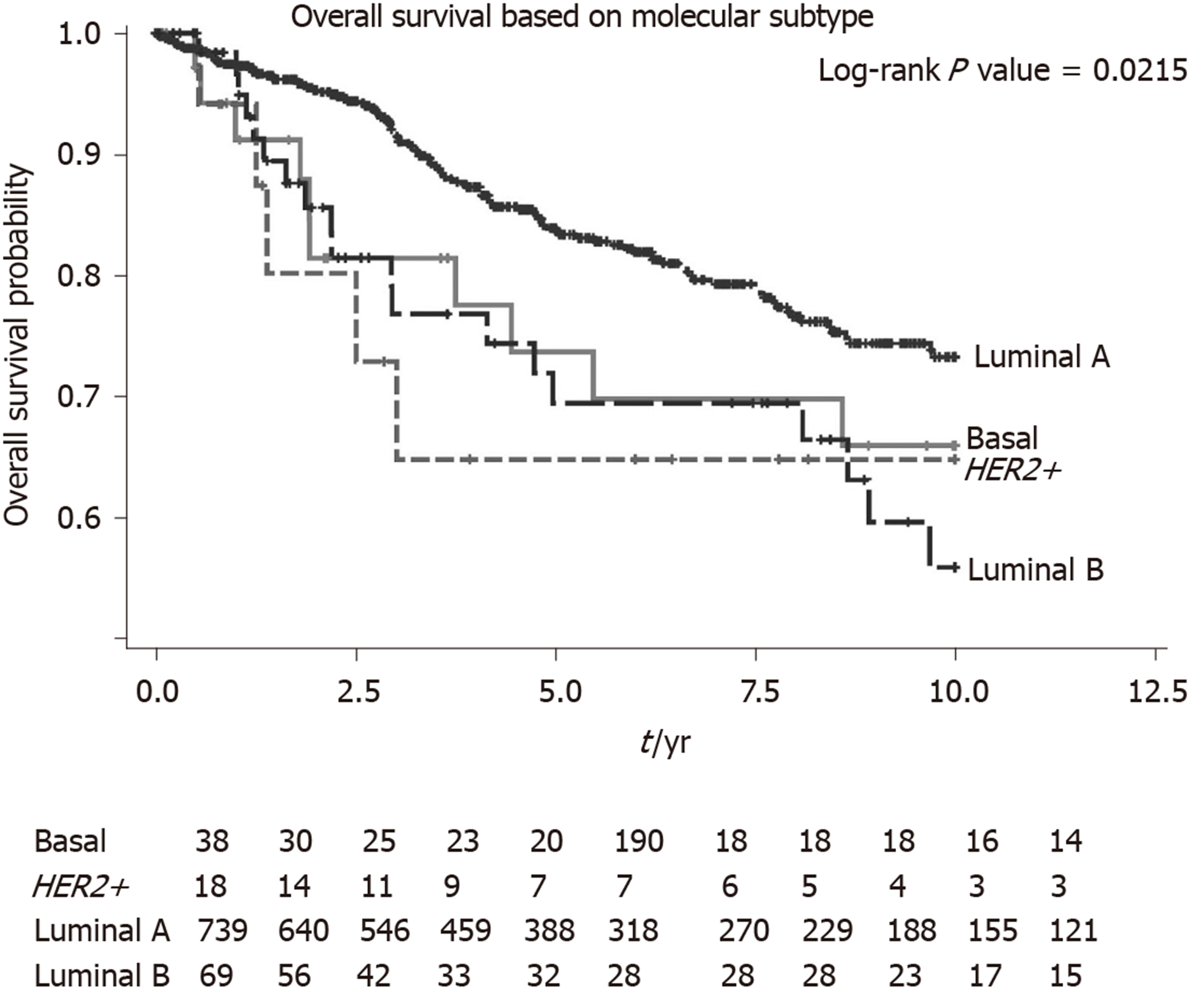
We performed a univariate and multivariate CPH regression analysis of OS in all 864 ILC patients. Based on the multivariate analysis, significant negative prognostic factors were HER2+, age, ethnicity and stage. HER2+ and luminal B molecular subtypes also had also notably poorer OS compared to Luminal A subtype (Table 2, Figure 3). Additional univariate and multivariate CPH regression analyses of BCSS and DFS demonstrated that HER2 positivity remained a significant negative prognostic factor for BCSS and DFS on both the univariate and multivariate analysis (Tables 3 and 4).
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (reference: ≤ 50 yr) | ||||||
| > 50 yr | 2.32 | 1.68-3.20 | < 0.001 | 2.17 | 1.37-3.44 | < 0.001 |
| Ethnicity (reference: Chinese) | < 0.0011 | 0.0011 | ||||
| Indian | 2.53 | 1.62-3.94 | < 0.001 | 3.41 | 1.78-6.54 | < 0.001 |
| Malay | 0.95 | 0.50-1.82 | 0.889 | 0.98 | 0.42-2.29 | 0.961 |
| Others | 0.40 | 0.15-1.08 | 0.070 | 0.64 | 0.19-2.12 | 0.462 |
| ER (reference: Negative) | ||||||
| Positive | 0.74 | 0.44-1.24 | 0.255 | |||
| PR (reference: Negative) | ||||||
| Positive | 0.62 | 0.44-0.87 | 0.005 | 0.57 | 0.35-0.91 | 0.018 |
| HER2 (reference: Negative) | ||||||
| Positive | 1.87 | 1.21-2.90 | 0.005 | 2.14 | 1.16-3.95 | 0.016 |
| Tumour size (reference: ≤ 2 cm) | ||||||
| > 2 cm | 2.43 | 1.45-4.06 | < 0.001 | |||
| Tumour stage (reference: Stage 1) | < 0.0011 | < 0.0011 | ||||
| Stage 2 | 2.33 | 1.09-4.99 | 0.030 | 1.75 | 0.76-4.03 | 0.191 |
| Stage 3 | 6.98 | 3.42-14.25 | < 0.001 | 4.52 | 2.06-9.89 | < 0.001 |
| Stage 4 | 61.82 | 29.73-128.57 | < 0.001 | 41.74 | 17.95-97.04 | < 0.001 |
| Tumor grade (reference: Grade 1) | < 0.0011 | 0.0751 | ||||
| Grade 2 | 1.45 | 0.83-1.89 | 0.190 | 1.05 | 0.57-1.93 | 0.877 |
| Grade 3 | 4.72 | 2.55-8.74 | < 0.001 | 1.89 | 0.93-3.84 | 0.079 |
| Chemotherapy (reference: No) | ||||||
| Yes | 0.97 | 0.69-1.37 | 0.866 | |||
| Surgery (reference: No) | ||||||
| Yes | 0.06 | 0.04-0.09 | < 0.001 | |||
| Radiotherapy (reference: No) | ||||||
| Yes | 0.89 | 0.63-1.27 | 0.518 | |||
| Molecular subtype (reference: Luminal A) | 0.0251 | 0.0021 | ||||
| Basal | 1.52 | 0.79-2.90 | 0.206 | 1.13 | 0.38-3.29 | 0.830 |
| HER2 positive | 2.08 | 0.85-5.10 | 0.108 | 4.21 | 1.43-12.44 | 0.009 |
| Luminal B | 1.89 | 1.16-3.07 | 0.011 | 2.52 | 1.41-4.49 | 0.002 |
| Characteristics | Univariate analysis | Multivariate analysis | ||||||
| HR | 95%CI | P value | HR | 95%CI | P value | |||
| Age (reference: ≤ 50 yr) | ||||||||
| > 50 yr | 2.16 | 1.53-3.05 | < 0.001 | |||||
| Ethnicity (reference: Chinese) | < 0.0011 | 0.0041 | ||||||
| Indian | 2.60 | 1.63-4.14 | < 0.001 | 2.55 | 1.28-5.05 | 0.008 | ||
| Malay | 0.89 | 0.43-1.82 | 0.744 | 1.07 | 0.43-2.67 | 0.885 | ||
| Others | 0.32 | 0.10-1.02 | 0.054 | 0.19 | 0.04-0.84 | 0.028 | ||
| ER (reference: Negative) | ||||||||
| Positive | 0.72 | 0.42-1.26 | 0.255 | |||||
| PR (reference: Negative) | ||||||||
| Positive | 0.61 | 0.42-0.88 | 0.008 | 0.40 | 0.23-0.70 | 0.001 | ||
| HER2 (reference: Negative) | ||||||||
| Positive | 2.08 | 1.32-3.26 | 0.002 | |||||
| Molecular subtype (reference: Luminal A) | 0.0111 | 0.0041 | ||||||
| Basal | 1.49 | 0.72-3.07 | 0.281 | 1.16 | 0.36-3.77 | 0.801 | ||
| HER2+ | 2.34 | 0.95-5.74 | 0.064 | 3.74 | 1.26-11.09 | 0.018 | ||
| Luminal B | 2.08 | 1.26-3.44 | 0.004 | 2.79 | 1.44-5.37 | 0.002 | ||
| Tumour size (reference: ≤ 2 cm) | ||||||||
| > 2 cm | 2.76 | 1.53-4.97 | < 0.001 | |||||
| Tumour stage (reference: Stage 1) | < 0.0011 | < 0.001 | ||||||
| Stage 2 | 3.11 | 1.09-8.92 | 0.034 | 2.19 | 0.74-6.49 | 0.159 | ||
| Stage 3 | 13.02 | 4.89-34.68 | < 0.001 | 6.49 | 2.35-17.89 | < 0.001 | ||
| Stage 4 | 117.79 | 43.5-317.87 | < 0.001 | 56.27 | 18.44-171.68 | < 0.001 | ||
| Tumor grade (reference: Grade 1) | < 0.0011 | 0.0011 | ||||||
| Grade 2 | 1.89 | 0.96-3.75 | 0.066 | 1.63 | 0.78-3.44 | 0.196 | ||
| Grade 3 | 7.10 | 3.44-14.64 | < 0.001 | 4.16 | 1.80-9.62 | 0.001 | ||
| Chemotherapy (reference: No) | ||||||||
| Yes | 1.23 | 0.84-1.80 | 0.290 | |||||
| Surgery (reference: No) | ||||||||
| Yes | 0.06 | 0.04-0.08 | < 0.001 | 0.23 | 0.11-0.51 | < 0.001 | ||
| Radiotherapy (reference: No) | ||||||||
| Yes | 0.94 | 0.65-1.37 | 0.758 | |||||
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (reference: ≤ 50 yr) | ||||||
| > 50 yr | 1.60 | 1.11-2.30 | 0.012 | 1.63 | 1.04-2.55 | 0.033 |
| Ethnicity (reference: Chinese) | 0.001 | |||||
| Indian | 2.61 | 1.52-4.48 | < 0.001 | |||
| Malay | 0.99 | 0.48-2.05 | 0.984 | |||
| Others | 1.99 | 1.10-3.58 | 0.022 | |||
| ER (reference: Negative) | ||||||
| Positive | 1.04 | 0.57-1.90 | 0.886 | |||
| PR (reference: Negative) | ||||||
| Positive | 0.97 | 0.65-1.43 | 0.876 | |||
| HER2 (reference: Negative) | ||||||
| Positive | 1.68 | 1.04-2.71 | 0.03 | |||
| Molecular subtype (reference: Luminal A) | 0.2171 | |||||
| Basal | 0.98 | 0.45-2.12 | 0.965 | |||
| HER2+ | 1.69 | 0.62-4.61 | 0.304 | |||
| Luminal B | 1.67 | 0.98-2.83 | 0.058 | |||
| Tumour size (reference: ≤ 2 cm) | ||||||
| > 2 cm | 2.02 | 1.26-3.25 | 0.004 | |||
| Tumour stage (reference: Stage 1) | < 0.0011 | < 0.0011 | ||||
| Stage 2 | 1.92 | 1.05-3.53 | 0.035 | 1.66 | 0.83-3.28 | 0.149 |
| Stage 3 | 5.66 | 3.21-9.98 | < 0.001 | 5.26 | 2.76-10.03 | < 0.001 |
| Stage 4 | 0.62 | 0.04-10.84 | 0.745 | 0.71 | 0.04-12.61 | 0.813 |
| Tumor grade (reference: Grade 1) | < 0.0011 | 0.0131 | ||||
| Grade 2 | 1.79 | 1.02-3.16 | 0.044 | 1.32 | 0.73-2.40 | 0.357 |
| Grade 3 | 3.72 | 1.89-7.34 | < 0.001 | 2.69 | 1.32-5.50 | 0.007 |
| Chemotherapy (reference: No) | ||||||
| Yes | 1.64 | 1.12-2.42 | 0.011 | |||
| Surgery (reference: No) | ||||||
| Yes | 0.14 | 0.08-0.23 | < 0.001 | |||
| Radiotherapy (reference: No) | ||||||
| Yes | 1.57 | 1.05-2.34 | 0.028 | |||
Interestingly, although most ILC patients have HER2- tumours, our cohort reports a higher prevalence of HER2+ ILC (10.1%) as compared to some previous studies[1-5]. The largest known study to date of 85048 ILC patients in the United States SEERS database found a HER2+ prevalence of only 4.8%[5]. Given that our study is one of the first few to describe prevalence of HER2+ ILC in Asian populations, this may suggest differences across ethnic and geographical populations, although further studies are required to validate this finding.
In our cohort, HER2+ ILC was significantly associated with ER negativity, PR negativity and higher tumour grade. This affirms findings in a previous study which concluded that HER2 positivity had an inverse relationship with ER and PR expression in ILC[10]. In the same study, PR negativity was notably more common than ER negativity in HER2+ ILC. This was also seen in our study with the frequency of PR- being nearly twice that of ER- in the HER2+ population. Our study reports a higher tumour grade in HER2+ ILC patients. This is not consistent with findings from previous studies which did not find significant associations with HER2 positivity and tumour grade or size[11-14]. We hypothesize that this may be due the smaller sample sizes in those studies and the heterogeneity of HER2+ ILC[15,16].
Our study also demonstrates poorer survival rates in HER2+ ILC as compared to HER2- ILC for OS, BCSS and DFS. On exploratory analyses of molecular subtypes, both HER2+ and luminal B molecular subtypes reflected this poorer OS, corroborating with a separate study which showed similar survival outcomes for the different molecular subtypes of ILC[17]. One possible biological explanation for poorer survival rates in HER2+ ILC is a synergistic effect of HER2 and cadherin 1 mutations which promotes tumourigenesis and early relapses in HER2+ ILC[18]. The finding of Indian ethnicity being a poorer prognostic factor for ILC on multivariate analysis also deserves further validation in a larger sample size as they formed < 5% of patients in this cohort, making it challenging to draw definitive conclusions.
Due to the retrospective nature of this study, missing data limited our ability to perform analyses on treatments received with regards to survival outcomes. Prospective studies with larger long-term follow-up sample sizes are needed to validate our observations in this study.
In conclusion, our study demonstrates the prevalence of HER2+ ILC to be 10.1%. HER2+ ILC patients were more likely to have poorer prognostic features such as ER-, PR- and higher tumour grade. Lastly, patients with HER2+ ILC had poorer OS, BCSS and DFS compared to those with HER2- ILC. These findings warrant further prospective studies to validate observation and investigate the benefit of various treatment modalities to improve outcomes in HER2+ ILC.
Invasive lobular carcinomas (ILC) represent about 5%-10% of breast cancer. Prevalence of overexpression of human epidermal growth factor receptor 2 (HER2) in breast cancer has been reported at 4.8%-5.1%. The clinicopathological characteristics of HER2 positive (HER2+) invasive ductal carcinomas are known to differ from that of HER2 negative (HER2-) invasive ductal carcinomas. However, there remains a paucity of research examining the characteristics of HER2+ as opposed to HER2- ILC, particularly in Asian populations.
This study compares the clinicopathological characteristics of HER2+ and HER2- ILC to assess the differences in survival probability between the two groups.
This study aims to investigate the prevalence and prognostic clinicopathological factors of HER2+ ILC in an Asian population.
A retrospective review of patients with ILC seen between January 1985 and March 2018 at various SingHealth medical institutions was conducted. Demographic and clinical data were collected from medical records. We examined clinicopathological characteristics and survival in relation to HER2 status. Differences between HER2+ and HER2- ILC were tested using chi-squared test for categorical variables and Mann-Whitney U test for continuous variables. Overall survival (OS), disease-free survival (DFS) and breast cancer-specific overall survival (BCSS) were analyzed for HER2+ and HER2- status using Kaplan-Meier survival analysis and were tested using log-rank test. All statistical tests were two-sided and P < 0.05 was considered statistically significant.
Interestingly, although most ILC patients have HER2- tumours, our cohort reports a higher prevalence of HER2+ ILC (10.1%) as compared to some previous studies. The median survival time was 2.95 (interquartile range: 1.89-8.87) years and 4.16 (interquartile range: 1.84-8.32) years respectively for HER2+ and HER2- ILC patients (P = 0.315). Based on the multivariate analysis, significant negative prognostic factors were HER2+, age, ethnicity and Stage. HER2+ and Luminal B molecular subtypes also had also notably poorer OS compared to Luminal A subtype. Additional univariate and multivariate Cox proportional hazard regression analyses of BCSS and DFS demonstrated that HER2 positivity remained a significant negative prognostic factor for BCSS and DFS on both the univariate and multivariate analysis.
In conclusion, our study demonstrates the prevalence of HER2+ ILC to be 10.1%. HER2+ ILC patients were more likely to have poorer prognostic features such as estrogen receptor negativity, progesterone receptor negativity and higher tumour grade. Lastly, patients with HER2+ ILC had poorer OS, BCSS and DFS compared to those with HER2- ILC.
The findings from our study warrant further prospective studies to validate observation and investigate the benefit of various treatment modalities to improve outcomes in HER2+ ILC.
| 1. | Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, Holmberg SB, Lindtner J, Snyder R, Thürlimann B, Murray E, Viale G, Castiglione-Gertsch M, Coates AS, Goldhirsch A; International Breast Cancer Study Group. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26:3006-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 2. | Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 398] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 3. | Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev. 2007;16:2773-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Desmedt C, Zoppoli G, Gundem G, Pruneri G, Larsimont D, Fornili M, Fumagalli D, Brown D, Rothé F, Vincent D, Kheddoumi N, Rouas G, Majjaj S, Brohée S, Van Loo P, Maisonneuve P, Salgado R, Van Brussel T, Lambrechts D, Bose R, Metzger O, Galant C, Bertucci F, Piccart-Gebhart M, Viale G, Biganzoli E, Campbell PJ, Sotiriou C. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J Clin Oncol. 2016;34:1872-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Chen Z, Yang J, Li S, Lv M, Shen Y, Wang B, Li P, Yi M, Zhao X, Zhang L, Wang L, Yang J. Invasive lobular carcinoma of the breast: A special histological type compared with invasive ductal carcinoma. PLoS One. 2017;12:e0182397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1250] [Cited by in RCA: 1701] [Article Influence: 212.6] [Reference Citation Analysis (0)] |
| 7. | Fan YS, Casas CE, Peng J, Watkins M, Fan L, Chapman J, Ikpatt OF, Gomez C, Zhao W, Reis IM. HER2 FISH classification of equivocal HER2 IHC breast cancers with use of the 2013 ASCO/CAP practice guideline. Breast Cancer Res Treat. 2016;155:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 863] [Article Influence: 71.9] [Reference Citation Analysis (1)] |
| 9. | Yeong J, Lim JCT, Lee B, Li H, Ong CCH, Thike AA, Yeap WH, Yang Y, Lim AYH, Tay TKY, Liu J, Wong SC, Chen J, Lim EH, Iqbal J, Dent R, Newell EW, Tan PH. Prognostic value of CD8 + PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer. 2019;7:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Yu J, Dabbs DJ, Shuai Y, Niemeier LA, Bhargava R. Classical-type invasive lobular carcinoma with HER2 overexpression: clinical, histologic, and hormone receptor characteristics. Am J Clin Pathol. 2011;136:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Ariga R, Zarif A, Korasick J, Reddy V, Siziopikou K, Gattuso P. Correlation of her-2/neu gene amplification with other prognostic and predictive factors in female breast carcinoma. Breast J. 2005;11:278-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Wasif N, Maggard MA, Ko CY, Giuliano AE. Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol. 2010;17:1862-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Adachi Y, Ishiguro J, Kotani H, Hisada T, Ichikawa M, Gondo N, Yoshimura A, Kondo N, Hattori M, Sawaki M, Fujita T, Kikumori T, Yatabe Y, Kodera Y, Iwata H. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Da Ros L, Moretti A, Querzoli P, Pedriali M, Lupini L, Bassi C, Carcoforo P, Negrini M, Frassoldati A. HER2-Positive Lobular Versus Ductal Carcinoma of the Breast: Pattern of First Recurrence and Molecular Insights. Clin Breast Cancer. 2018;18:e1133-e1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, Ellis IO. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol. 2005;18:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Park C, Park K, Kim J, Sin Y, Park I, Cho H, Yang K, Bae BN, Kim KW, Ahn S, Gwak G. Prognostic values of negative estrogen or progesterone receptor expression in patients with luminal B HER2-negative breast cancer. World J Surg Oncol. 2016;14:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, Rotmensz N, Montagna E, Dellapasqua S, Veronesi P, Galimberti V, Luini A, Goldhirsch A, Colleoni M, Viale G. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat. 2012;133:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Desmedt C, Zoppoli G, Sotiriou C, Salgado R. Transcriptomic and genomic features of invasive lobular breast cancer. Semin Cancer Biol. 2017;44:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ding MX, Ieni A, Lim SC, Wada R S-Editor: Yan JP L-Editor: A E-Editor: Liu JH