Published online Mar 24, 2020. doi: 10.5306/wjco.v11.i3.110
Peer-review started: October 22, 2019
First decision: November 27, 2019
Revised: January 31, 2020
Accepted: February 8, 2020
Article in press: February 8, 2020
Published online: March 24, 2020
Processing time: 146 Days and 11.1 Hours
Cancer constitutes the second leading cause of death globally and is considered to have been responsible for an estimated 9.6 million fatalities in 2018. Although treatments against gastrointestinal tumors have recently advanced, those interventions can only be applied to a minority of patients at the time of diagnosis. Therefore, new therapeutic options are necessary for advanced stages of the disease. Glycosylation of antitumor agents, has been found to improve pharmacokinetic parameters, reduce side effects, and expand drug half-life in comparison with the parent compounds. In addition, glycosylation of therapeutic agents has been proven to be an effective strategy for their targeting tumor tissue, thereby reducing the doses of the glycodrugs administered to patients. This review focusses on the effect of the targeting properties of glycosylated antitumor agents on gastrointestinal tumors.
Core tip: In nature, glycosylation has proven an effective strategy for expanding the biologic information of biomolecules by adding a new level of structural diversity. The high specificity of the interaction with carbohydrates and the overexpression of carbohydrate receptors in tumoral cells that can be specifically targeted by glycodrugs enable a selective administration of those agents to the tumor tissues. Accordingly, the glycosylation of antitumor agents has been found to improve pharmacokinetic parameters, reduce side effects, expand drug half-life, and reduce the dosage of the consequent glycoderivatives.
- Citation: Molejon MI, Weiz G, Breccia JD, Vaccaro MI. Glycoconjugation: An approach to cancer therapeutics. World J Clin Oncol 2020; 11(3): 110-120
- URL: https://www.wjgnet.com/2218-4333/full/v11/i3/110.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i3.110
Cancer is the second leading cause of death worldwide, having been responsible for an estimated of 9.6 million deaths in 2018[1,2]. Moreover, 21.6% of all tumors worldwide are gastrointestinal cancers at more than 26000 cases per year[3]. Despite considerable research efforts in recent years, the impact of conventional strategies - including surgery, radiotherapy, and chemotherapy - on the prognosis of tumors has been only moderate[4,5]. The anticancer drugs used in chemotherapy usually target cells that are proliferating. These chemodrugs can be grouped according to their main role: antitumor antibiotics, alkylating agents, topoisomerase inhibitors, DNA-complexing agents, mitotic inhibitors, hormones, and immunotherapeutic agents. The activity of those drugs is essential for predicting side effects; moreover, what is remarkable is that most of the available anticancer drugs have the disadvantage of lacking systems for delivery to the target organ or tissue. Consequently, the majority of the administered drug remains circulating in the bloodstream, thus increasing the side effects on noncancerous cells[6].
The selective targeting of therapeutic agents has several advantages, such as increasing the concentration of the drugs in the tumor and reducing the concentration in other tissues[7]. Carbohydrates per se, exhibit a high solubility in water, a low toxicity, and a high biocompatibility; thus constituting an attractive system for facilitating drug-delivery. Glycosylated compounds can be targeted to a broad range of cellular receptors because of the specificity of interaction with cell-surface carbohydrates. Accordingly, a number of glycoconjugated antitumoral agents have been reported to selectively deliver the parent drugs to the desired sites[8,9]. In 1971, Rogers et al[10] demonstrated for the first time the usefulness of targeting proteins that bear carbohydrates as ligands. To our knowledge, no therapeutic targeting system is currently on the market, though many candidates aimed at that end nevertheless exist. This review will therefore focus primarily on the synthesis of the most relevant glycosylated therapeutic agents and the effect of those derivatives on gastrointestinal tumors.
The most widely studied drug-transporting and receptor-targeting glycoligands are the glucose-transporters (GLUTs) and the lectin receptors as described below.
The energy produced from glucose metabolism is essential for sustaining mammalian-cell life. The end products of that metabolic pathway are lactate and, upon full oxidation in the mitochondria, CO2[11].
In tumors and other proliferating cells, the rate of glucose uptake increases considerably and lactate is produced, regardless of the availability of oxygen and functional mitochondria. The tendency of tumoral tissues to anaerobically metabolize large amounts of glucose, in comparison with noncancerous tissue, is known as the Warburg Effect[12,13].
The GLUT is a transport protein of the facilitator family involved in glucose translocation across the cell membrane. Although, GLUT transporters are expressed in almost every cell type[14], tumor cells express a large number of glucose transporters that are related to poor prognosis[15]. At present, 14 different GLUTs-1 to 14 - have been described[16]. GLUT-1, in particular, is known to be overexpressed in tumor cells, including those of liver, pancreas, and stomach[17]. These transporters can specifically recognize and transport different sugars, such as glucose, mannose, galactose, 2-deoxyglucose and glucosamine analogs[18].
Therefore, as a result of the Warburg effect, designing and developing glycosyl-based targeted drugs is a subject of high/considerable/widespread interest[19,20].
Lectins are defined as proteins, usually linked to carbohydrates[21-23], that are present in plants and animals and involved in many biologic processes - including cell growth, differentiation, signalling, adhesion and migration, and apoptosis[24-26]. Lectins can act as receptors, either for binding oligosaccharides to cell membranes or free-floating glycans involving monosaccharides in order to mediate signal transduction and/or drug transport[27].
Asialoglycoprotein receptors (ASGPRs), including asialoglycoprotein receptors 1 and 2-ASGPR1 and ASGPR2 - are expressed on the surface of hepatocytes and stomach and gallbladder epithelia[28] and preferentially interact with the sugars D-galactose and L-rhamnose. The molecular mechanism consists in the internalization of the receptor-ligand complex through a clathrin-mediated endocytosis. Once inside the cell, the ligands are released, enabling the recycling of ASGPR receptors back into the plasma membrane. The quick cycling of internalized receptors is the key process that maintains their concentration on the cell surface. Owing to the high specificity in the binding of galactose and rhamnose to ASGPR receptors, these interactions result a promising approach to drug targeting[29].
Among the lectin-based receptors, the rhamnose-binding lectin receptor ligates specifically to rhamnose and is highly expressed on various tumor-cell types, like the cell-culture lines KB (from a human squamous-cell carcinoma), PC3 (from a human prostatic small-cell carcinoma), HT-29 (from a human colon adenocarcinoma) and MCF-7 (a breast adenocarcinoma)[30]. Although the molecular mechanism involved in the transmission of messages by rhamnose-binding lectin receptor has not been yet studied in humans, the strategy for using rhamnosylated anticancer molecules as novel candidates for pharmacological applications has recently been explored[29].
Below we present several antitumoral agents as informative examples of such modifications - namely, ifosfamide, chlorambucil, and paclitaxel. The drugs were linked to various carbohydrate moieties and the antitumoral effects evaluated on gastrointestinal tumors. The cytotoxic activities of the synthesized glycoconjugates were then compared to those of the corresponding parent nonglycosylated molecules (Table 1).
| Agent | Activity | Sugar moiety | Efficacy glycoconjugates compared to aglicone |
| Ifosfamide | Alkalating agent | Glucose | In vitro (less toxic) and in vivo (reduced tumor size). Clinical trials ongoing. |
| Chlorambucil | Alkalating and DNA-complexing agent | D-threose | HT29 and HCT15 (showed 8-12 fold, and 15-fold, respectively, improved activities targeting cancers cell lines over the parent drug). |
| Geldanamycin | HSP90 inhibitor | Glucose | Glucose-GA showed anticancer activity with IC50 of 70.2-380.9 nM in SW620, HT29, MCF-7 and K562 cancer cells by-glucosidase activation inside of the tumor cells. |
| Geldanamycin | HSP90 inhibitor | Galactose | SW620, HT29, MCF-7 and K562 (anticancer activity of galactose-GE conjugate increased by 3- to 40-fold when incubated with galactosidase over the parent drug). |
| Emodin | Tyrosine kinase inhibitor | D-rhamnose | A594, HepG2, OVCAR-3, Hela, K562 and SGC-790 (cell proliferation was inhibited and EM-d-Rha conjugate displayed IC50 values in low μmolar ranges). |
| Paclitaxel | Mitotic inhibitor | Glucose | NCl-H838, MES-SA, HCT-116, and NPC-TW01 (cell proliferation was inhibited the conjugated presented higher cytotoxicity, induced tubulin aggregation and chromosomal condensation compared to paclitaxel). |
| Doxorubicin | Antitumor antibiotic | Galactose | In vitro viability of HepG2 (hepatocarcinoma) and MCF-7 (breast cancer) tumor cells incubated with DOX was higher than that of Gal-DOX. In vivo experiments showed that tumor size in Gal-DOX-treated groups was greatly reduced in comparison to the DOX-treated group. |
| Doxorubicin | Antitumor antibiotic | 2-amino-2-deoxy-D-glucose and succinic acid | In vitro and in vivo studies showed that 2DG-SUC-ADM induced a higher level of apoptosis and higher inhibition rates in MCF-7 and HepG2 tumoral cells than the parent aglycone ADM. |
Ifosfamide - whose cytotoxic metabolite of in plasma is ifosforamide - is an alkylating agent has been bound to β-D-glucose to form glufosfamide[31,32]. This glycoconjugate was the first molecule bearing a sugar to be explicitly designed and evaluated as a cancer-targeting cytotoxic compound. Within tumor cells, glufosfamide is metabolized by glucosidases to form ifosforamide. This cytotoxic metabolite, in turn, forms DNA crosslinks, therefore inhibiting DNA replication and cell growth[33]. Moreover, treatment with GLUT-1 inhibitors reduced the anticancer efficiency of glufosfamide, suggesting that the drug conjugate was internalized into cells via the GLUT-1 transporter. Finally, glufosfamide was less myelotoxic and presented a higher antitumour activity both in vitro and in vivo than the parent aglycone[32].
In 1997, the first human clinical trial to test glufosfamide was carried out in Europe, and the results obtained with 20 pancreatic-cancer patients were reported[34]. Two cases evidenced a good response to the treatment, 10 resulted in stable disease, while 8 patients failed to respond. More significantly, one pancreatic-cancer patient in a different trial experienced a complete remission for over 4 years. Since pancreatic-cancer biopsy samples had been found to overexpress GLUT-1; in 2010, Chiorean et al[35] performed a phase-II study of glufosfamide plus the nucleoside analog gemcitabine, the standard chemotherapeutic treatment in pancreatic cancer. Glufosfamide and gemcitabine in combination yielded a modest response in two trials on pancreatic-adenocarcinoma patients. In conclusion, glufosfamide appeared to constitute an effective cytotoxic agent exhibiting high antitumor selectivity that was due to an active interaction with the transporter GLUT-1.
Chlorambucil is an antineoplasic drug within the class of alkylating agents that is used to treat various forms of cancer[36]. The reactive radical ethylenimonium forms after alkylation that interferes in DNA, RNA, and protein synthesis. The first report of chlorambucil synthesis was over five decades ago[37]. Goff et al[38] (2010) synthesized and evaluated a 63-member library of chlorambucil-based neoglycosides in ten different human-carcinoma cell-culture systems, including lung, colorectal, liver, breast, prostate, central nervous system, and ovarian cell lines. The synthesis consists of several chemical steps to perform chlorambucil-based libraries for chemoselective glycosylation. On the basis of this study, the neoglycosides, D-glucuronolactonide and D-threoside were selected as the most potent antitumoral agents. D-threoside glycoside manifested an 8-fold higher efficacy in general, with a respective 12-fold, and 15-fold greater effectiveness in targeting the malignant cell lines HT-29, and HCT-15 (from colorectal adenocarcinomas) over the parent drug. The authors concluded that D-threoside was the most active chlorambucil neoglycoside among the compounds tested. In summary, a novel panel of glycoconjugates were designed and synthesized through the use of common metabolic carbohydrates that are preferentially recognized by cell-membrane receptors, therefore favoring the uptake of the glycodrugs[38]. The specific mechanisms and the receptors or transporters involved in this process remain to be elucidated.
Geldanamycin (GE) is a potent anticancer antibiotic that inhibits the heat-shock protein 90 (Hsp90)[39]. This protein is a molecular chaperon involved in the modulation of the activity of various protein kinases. Hsp90 has been found to be 2- to 10-fold more expressed in various human cancer cells than in normal tissues, suggesting the use of that protein as a possible target for cancer therapy[40]. Although GE has long been recognized as an inhibitor of tumor growth, the potential clinical utility of that agent is hampered by its severe side effects[41,42]. To circumvent this problem, an approach involving a binary chemical step was designed by Cheng et al[43] (2005), resulting in a series of glycosyl-GE derivatives. The enzyme-specific activation of these glycosylated prodrugs with galactose and glucose moieties was performed with α-galactosidase and ß-glucosidase, respectively. The effect of the resulting derivatives was evaluated in different cancer-cell lines, including SW620, HT-29, MCF-7, and K-562 leukemia cells. In particular, the glucose-GE exhibited antitumor activity after cleavage of the glucose moiety by the β-glucosidase inside the tumor cells. The anticancer activity of the galactose-GE conjugate increased by 3- to 40-fold when incubated with exogenous galactosidase in vitro, but remained inactive in the absence of the added enzyme because, unlike intracellular glucosidases, galactosidases are present in only low or undetectable levels in serum[43]. Consequently, the activation of the galactosyl-GE prodrugs by the exogenous enzyme would appear to be a necessary targeting approach, involving a strategy leading to a dual tactic consisting of the glycosylation of the enzyme as well as the drug, in order to direct the whole system to the target tissue[8,9].
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone) is a natural anthraquinone derivative found in the roots and rhizomes of numerous plants. As a tyrosine-kinase inhibitor, emodin inhibits cell growth in several types of tumor cells[44-46] and regulates the expression of genes involved in cell apoptosis, oncogenesis, cell proliferation, and cancer-cell invasiveness and metastasis[47-49]. The antitumor effects of emodin have been described, but the molecular mechanism has not been fully elucidated. The synthesis and design of emodin conjugated to D-rhamnose (EM-D-Rha), inhibited cell proliferation in a panel of different human-cancer-cell lines including A549 (lung carcinoma), HepG2 (hepatoma), OVCAR-3 (ovarian carcinoma), HeLa (cervical carcinoma) and K-562 (chronic myelogenous leukaemia) and SGC-790 (endocervical adenocarcinoma). EM-d-Rha also manifested lower IC50 values and was 10-fold more cytotoxically effective than the aglycone on HepG2 cells, leading to a decrease in mitochondrial transmembrane potential and to an upregulation of the expression of apoptosis elements[50].
Doxorubicin (DOX), the active compound in the trade drug named adriamycin (ADM), belongs to the anthracycline family, is one of the most powerful and widely used chemotherapeutic drugs, and as such is recognized by the World Health Organization[51]. Unfortunately, apart from the drug's activity as an intercalating agent and topoisomerase-II inhibitor, DOX causes severe toxic effects such as nephrotoxicity, hepatotoxicity, and alopecia, compromising mainly heart tissues and the gastrointestinal tract as a result of the compound's systemic action. In view of the many efforts that have been made order to overcome these limitations, an increase in drug efficiency through the conjugation of DOX to a carbohydrate ligand that can specifically recognize tumoral cells is a promising approach[52,53].
Ma et al[54] (2015) conjugated galactose to DOX covalently to form the prodrug Gal-DOX and then evaluated the derivative's tumor-targeting capability in different cancer-cell lines. They found that Gal-Dox treatment increased cell death in HepG2 and MCF-7 cells compared to DOX. These results were the opposite when they treated normal L02 hepatocytes. They proposed that this difference was caused by the high specific binding of Gal to ASGPR1 receptors, which were overexpressed on the surface of HepG2 and MCF-7 tumor cells. Consistent with that conclusion, since those nonmalignant hepatocytes contained lower plasma-membrane levels of ASGPR1, the L02 cells maintained high cell viability, thus suggesting low toxicity of Gal-DOX. As to side effects, Gal-DOX is accumulated in heart tissue to a lesser extent than DOX, thus diminishing myocardial damage. In vivo results indicated a reduction in tumor size in the Gal-DOX-treated group compared to the DOX-treated group. Of notable relevance here is that the survival of the Gal-DOX-treated group was 100%, whereas the rate of DOX-treated group was 50%, thus supporting the conclusion that Gal-Dox preferentially targeted the tumor cells. These findings suggest that Gal-DOX is a promising drug for tumor- directed therapy[54]. In another study, ADM was conjugated with 2-amino-2-deoxy-D-glucose and succinic acid (2DG–SUC–ADM). There the investigators found that the ternary derivative was highly specific for tumor cells (MCF-7 and HepG2) via the GLUT-1 transporter, while exerting no significant adverse effects on normal cells. The action of the glycoconjugate was also confirmed in vivo, thus demonstrating that the glycosylated molecule could specifically target tumoral cells as opposed to the aglycone. These results suggest 2DG–SUC–ADM as a promising drug for targeting cancer cells[55].
Paclitaxel is a cytotoxic chemotherapeutic drug, classified as a "plant alkaloid," a "taxane" and an "antimicrotubule agent"[55,56]. The molecular basis for the action of paclitaxel consists in a binding to tubulin subunits, thus disrupting mitosis and causing cell death[57-59]. Despite being widely used in the clinic, this drug has pronoinced side-effects; thus affecting both normal cells and tumors. Another problem with paclitaxel is its poor water solubility. Lin et al[60] (2008) designed glycan-based paclitaxel prodrugs, consisting of 2'-paclitaxel conjugated with glucosyl or glucuronyl residues by an ester or an ether linkage. These glycodrugs not only had increased solubility in water compared to the parent compound, but exhibited higher selectivity against targeted cancer cells as well. Glucosyl-paclitaxel displayed the higher cytotoxicity and could induce tubulin aggregation and chromosomal condensation in a tumor-cell line[60]. The cells overexpressing GLUTs favored the uptake of glucose-paclitaxel and facilitated the entrance of the bulky compounds. Therefore, the authors proposed the synthesis of glucoconjugates as an alternative approach for improving the directed delivery of drugs to cancer cells overexpressing GLUTs[60].
5-fluorouracil (5Fu) is part of a group of chemotherapeutic drugs known as antimetabolites. These compounds incorporate into normal macromolecules to produce a slightly different structure that interferes in the metabolism of the cancer cells. 5Fu is one of the most commonly used drugs to treat gastrointestinal and breast cancers, although the agent is mostly used in combination with other drugs like oxaliplatin[61-63].
Davis and coauthors synthesized the prodrugs DOX and 5Fu capped by L-rhamnose and evaluated their use in a lectin-directed–enzyme-activated-prodrug therapy (LEAPT) system. The LEAPT system uses biocatalysts for site-selective drug delivery through the construction of novel glycosylated enzymes and prodrugs. The drugs capped with rhamnose synthesized by Davis and coworkers were released by an α-rhamnosidase, which enzyme is by itself glycosylated with galactose. The glycosylated enzyme specifically targets the ASGPR hepatic-receptors. The biodistribution revealed that the glycosylated enzyme became quickly sequestered to the liver, and to a lower extent to the kidney and bladder. Of essential relevance was that the co-administration of the prodrugs did not interfere in the colocalization of the LEAPT system[8,9]. Finally, the authors demonstrated that the prodrug could be activated in the liver only by the presence of the prelocalized glycosylated enzyme[8].
Within glycoscience new approaches are being undertaken for cancer therapy. Glycan-based vaccines have been developed for the specific enhancement of the immune response. The more complex task in formulating a cancer vaccine would be the selection of the appropriate antigen, which molecule should be exclusively expressed by cancer cells. Tumor-associated carbohydrate antigens, ought to be cellular components that are essential for malignant-cell survival in order to prevent the downregulation of the antigen and thus maintain the immune response. Tumor-associated carbohydrate antigens can be divided into two classes: glycoprotein antigens: The Thomsen Nouveau (Tn; GalNAc-a1-O-Ser/Thr), Thomsen-Friendreich (TF), and sialyl-Tn (sTn) linked to the hydroxyl group of serine or threonine residues of proteins and glycolipids[64]. Several studies demonstrated that altered glycosylation and aberrant glycan structures helped tumor cells to circumvent immune surveillance. Since high-affinity T cells recognizing self-antigens are eliminated during development of the central immune system, tumor-associated carbohydrate antigen-directed cancer vaccines face the challenge of activating any remaining low-affinity T cells[65-69].
Glycan-based vaccines may prove to be beneficial because unusual glycan motifs on glycoproteins can lead to vaccines with high specificity[70]. Mucin 1 (MUC1) is an O-linked glycan transmembrane protein overexpressed in various tumors—such as lung, breast, pancreas, kidney, ovary, and colon—and has been demonstrated to be aberrantly glycosylated in cancer cells but highly glycosylated in normal cells. Up to the present, most vaccines have targeted nonglycosylated MUC1, although this approach did not prove to be cancer-specific. Thus, targeting cancer-associated glycopeptide epitopes in MUC1 would be a promising alternative possibility[71-73]. New attempts have been made to develop glycosylation-based vaccines that target MUC1. In 2012, Madsen et al[74] found that the addition of GalNAc residues to MUC1 to aid antigen uptake and major-histocompatability-complex–class-II presentation for the generation of a potent cancer-specific humoral response. In another investigation, Li et al[75] designed and synthesized two linear trivalent glycopeptides of the immune-dominant epitope of MUC1. The antibodies induced by glycosylated-MUC1–based vaccines had a stronger binding than those raised by nonglycosylated MUC1, thus, having the potential to overcome the weak immunogenicity of natural MUC1 glycopeptides[75]. Further studies demonstrated that the antibodies elicited by a vaccine composed of the immunoadjuvant (Pam3CysSK4), a peptide T-helper epitope, and an aberrantly glycosylated MUC1 peptide, were significantly more lytic and more effective in tumor prevention than the unglycosylated control[76]. Other glycan-based vaccines that lead to higher immune responses are presently under development. The most likely to be effective is a vaccine composed of MUC1 glycopeptide in combination with a T-helper peptide, while another uses a combination of MUC1 with the toll-like receptor 2. The last one is composed of MUC1, toll-like receptor 2/9, and a Th peptide[70].
No clinical trials are currently being undertaken on gastrointestinal tumors, although a phase-III clinical trial is being carried out in patients with metastatic breast cancer that involves the glycan-based vaccine, sTn-KLH. The sTn-KLH–vaccine group evidenced an improved survival, relative to the overall survival among the patients treated without the vaccine[77,78].
Many efforts have been made to develop carbohydrate-based vaccines, though until now, none have been approved for clinical use, and many cancer-vaccine candidates have failed in clinical trials. One reason could be the ability of tumor cells to escape from the endogenous immune response or downregulate the immune-target molecules. Nevertheless, an optimization of vaccine formulation in terms of receptor- or antigen-targeting delivery systems may lead to significant improvements in the utilization of currently available carbohydrate antigens so as to open new perspectives for cancer treatment.
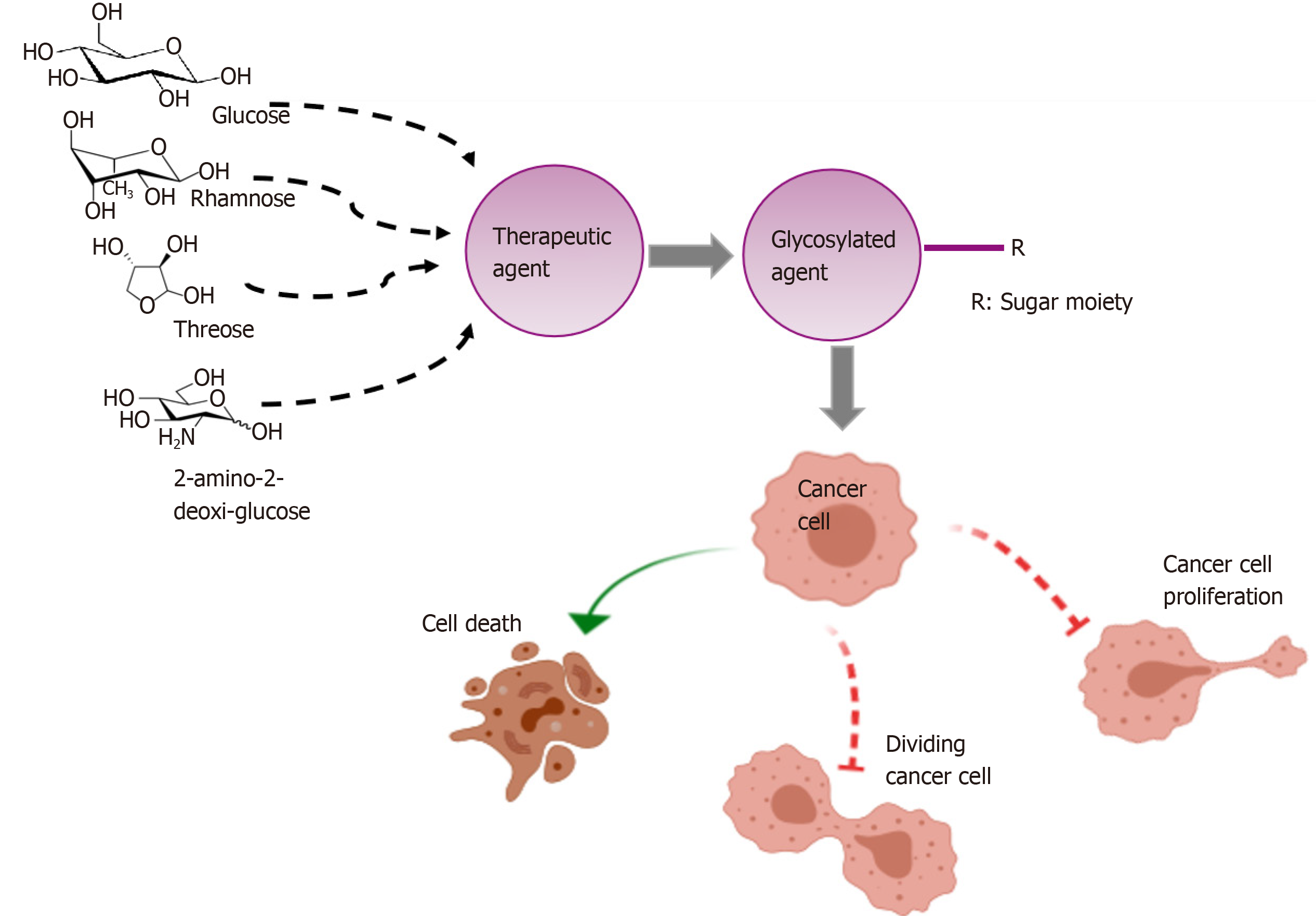
The conjugation of anticancer agents to carbohydrate-ligands that preferentially target tumor cells has resulted in the prediction that several conjugates would prove to have clinical efficacy. Specifically, glycoconjugation offers an improvement in targeting cancer cells, since are many sugar receptors are overexpressed in tumoral cells[79,80] (Figure 1). Furthermore, the addition of a sugar moiety (e.g., glucose, galactose, rhamnose) improves the water solubility and stability of the parent drugs. During recent years, this field has been emerging in translational medicine; but, in closing, we need to stress that glycodrug development is a rigorous process that still requires many steps to determine the true utility of that strategy.
The authors are grateful to Roxana Resnik, MSc, for her assistance in the language translation and editing of this manuscript. Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.
| 1. | Cancer Research UK. General cancer information. Available from: https://www.cancerresearchuk.org/about-cancer/cancer-in-general. |
| 2. | Global Burden of Disease Cancer Collaboration. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3033] [Article Influence: 337.0] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56668] [Article Influence: 7083.5] [Reference Citation Analysis (135)] |
| 4. | Taylor WF, Jabbarzadeh E. The use of natural products to target cancer stem cells. Am J Cancer Res. 2017;7:1588-1605. [PubMed] |
| 5. | Rao D, Parakrama R, Augustine T, Liu Q, Goel S, Maitra R. Immunotherapeutic advances in gastrointestinal malignancies. NPJ Precis Oncol. 2019;3:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Skeel RT. Antineoplastic drugs and biological response modifiers. In: Skeel RT. Handbook of cancer chemotherapy. Philadelpia: Lippincott Williams and Wilkins, 1999: 108-109. |
| 7. | Sathornsumetee S, Rich JN. Molecularly targeted therapy in neuro-oncology. In: Aminoff MJ, Boller F, Swaab DF, editors. Handbook of Clinical Neurology. Elsevier, 2012: 255-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Robinson MA, Charlton ST, Garnier P, Wang XT, Davis SS, Perkins AC, Frier M, Duncan R, Savage TJ, Wyatt DA, Watson SA, Davis BG. LEAPT: lectin-directed enzyme-activated prodrug therapy. Proc Natl Acad Sci USA. 2004;101:14527-14532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Garnier P, Wang XT, Robinson MA, van Kasteren S, Perkins AC, Frier M, Fairbanks AJ, Davis BG. Lectin-directed enzyme activated prodrug therapy (LEAPT): Synthesis and evaluation of rhamnose-capped prodrugs. J Drug Target. 2010;18:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Rogers JC, Kornfeld S. Hepatic uptake of proteins coupled to fetuin glycopeptide. Biochem Biophys Res Commun. 1971;45:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Annibaldi A, Widmann C. Glucose metabolism in cancer cells. Curr Opin Clin Nutr Metab Care. 2010;13:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 10191] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 13. | Gonzalez CD, Alvarez S, Ropolo A, Rosenzvit C, Bagnes MF, Vaccaro MI. Autophagy, Warburg, and Warburg reverse effects in human cancer. Biomed Res Int. 2014;2014:926729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 284] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141-E145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 702] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 17. | Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaíba MM, Begnami MD, Vilela RS, Paiva GR, Andrade RG, Soares FA. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo). 2011;66:965-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N. Crystal structure of the human glucose transporter GLUT1. Nature. 2014;510:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 600] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 19. | Calvaresi EC, Hergenrother PJ. Glucose conjugation for the specific targeting and treatment of cancer. Chem Sci. 2013;4:2319-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 20. | Coulibaly FS, Youan BC. Current Status of Lectin-Based Cancer Diagnosis and Therapy. AIMS Mol Sci. 2017;4:1–27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1159] [Cited by in RCA: 1099] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 22. | Thomas GB, Rader LH, Park J, Abezgauz L, Danino D, DeShong P, English DS. Carbohydrate modified catanionic vesicles: probing multivalent binding at the bilayer interface. J Am Chem Soc. 2009;131:5471-5477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Seeberger PH, Werz DB. Synthesis and medical applications of oligosaccharides. Nature. 2007;446:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 606] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 24. | Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R-62R. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 725] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 25. | Shahzad-ul-Hussan S, Cai M, Bewley CA. Unprecedented glycosidase activity at a lectin carbohydrate-binding site exemplified by the cyanobacterial lectin MVL. J Am Chem Soc. 2009;131:16500-16508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, Ju T, Molinaro RJ, Rivera-Marrero C, Xia B, Smith DF, Cummings RD. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med. 2010;16:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (8)] |
| 27. | Lerchen HG, Baumgarten J, Piel N, Kolb-Bachofen V. Lectin-Mediated Drug Targeting: Discrimination of Carbohydrate-Mediated Cellular Uptake between Tumor and Liver Cells with Neoglycoconjugates Carrying Fucose Epitopes Regioselectively Modified in the 3-Position. Angew Chem Int Ed Engl. 1999;38:3680-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Lis H, Sharon N. Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition. Chem Rev. 1998;98:637-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1328] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 29. | Xu L, Liu X, Li Y, Yin Z, Jin L, Lu L, Qu J, Xiao M. Enzymatic rhamnosylation of anticancer drugs by an α-L-rhamnosidase from Alternaria sp. L1 for cancer-targeting and enzyme-activated prodrug therapy. Appl Microbiol Biotechnol. 2019;103:7997-8008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Gao J, Gu G, Li G, Cui C, Sun B, Lou H. In situ RBL receptor visualization and its mediated anticancer activity for solasodine rhamnosides. Chembiochem. 2011;12:2418-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Stüben J, Port R, Bertram B, Bollow U, Hull WE, Schaper M, Pohl J, Wiessler M. Pharmacokinetics and whole-body distribution of the new chemotherapeutic agent beta-D-glucosylisophosphoramide mustard and its effects on the incorporation of [methyl-3H]-thymidine in various tissues of the rat. Cancer Chemother Pharmacol. 1996;38:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Pohl J, Bertram B, Hilgard P, Nowrousian MR, Stüben J, Wiessler M. D-19575--a sugar-linked isophosphoramide mustard derivative exploiting transmembrane glucose transport. Cancer Chemother Pharmacol. 1995;35:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Stenn KS. Metastasizing neoplastic cells. Am J Dermatopathol. 1979;1:375-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 34. | Briasoulis E, Judson I, Pavlidis N, Beale P, Wanders J, Groot Y, Veerman G, Schuessler M, Niebch G, Siamopoulos K, Tzamakou E, Rammou D, Wolf L, Walker R, Hanauske A. Phase I trial of 6-hour infusion of glufosfamide, a new alkylating agent with potentially enhanced selectivity for tumors that overexpress transmembrane glucose transporters: a study of the European Organization for Research and Treatment of Cancer Early Clinical Studies Group. J Clin Oncol. 2000;18:3535-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Chiorean EG, Dragovich T, Hamm J, Barrios CH, Gorini CF, Langmuir VK, Kroll S, Jung DT, Tidmarsh GT, Loehrer PJ. A phase 2 trial of glufosfamide in combination with gemcitabine in chemotherapy-naive pancreatic adenocarcinoma. Am J Clin Oncol. 2010;33:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Hartley JA, Bingham JP, Souhami RL. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards is preserved in intact cells. Nucleic Acids Res. 1992;20:3175-3178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Zhang HJ, Jinks RN, Wishart AC, Battelle BA, Chamberlain SC, Fahrenbach WH, Kass L. An enzymatically enhanced recording technique for Limulus ventral photoreceptors: physiology, biochemistry, and morphology. Vis Neurosci. 1994;11:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Goff RD, Thorson JS. Assessment of chemoselective neoglycosylation methods using chlorambucil as a model. J Med Chem. 2010;53:8129-8139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 40. | Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 340] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 370] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Sausville EA. Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters. Commentary re: P. Münster et al., Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. Clin. Cancer Res., 7: 2228-2236, 2001. Clin Cancer Res. 2001;7:2155-2158. [PubMed] |
| 43. | Cheng H, Cao X, Xian M, Fang L, Cai TB, Ji JJ, Tunac JB, Sun D, Wang PG. Synthesis and enzyme-specific activation of carbohydrate-geldanamycin conjugates with potent anticancer activity. J Med Chem. 2005;48:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH, Tseng SW. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002;64:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 45. | Su YT, Chang HL, Shyue SK, Hsu SL. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol. 2005;70:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 46. | Srinivas G, Anto RJ, Srinivas P, Vidhyalakshmi S, Senan VP, Karunagaran D. Emodin induces apoptosis of human cervical cancer cells through poly(ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol. 2003;473:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Huang Q, Shen HM, Shui G, Wenk MR, Ong CN. Emodin inhibits tumor cell adhesion through disruption of the membrane lipid Raft-associated integrin signaling pathway. Cancer Res. 2006;66:5807-5815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Kwak HJ, Park MJ, Park CM, Moon SI, Yoo DH, Lee HC, Lee SH, Kim MS, Lee HW, Shin WS, Park IC, Rhee CH, Hong SI. Emodin inhibits vascular endothelial growth factor-A-induced angiogenesis by blocking receptor-2 (KDR/Flk-1) phosphorylation. Int J Cancer. 2006;118:2711-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Cha TL, Qiu L, Chen CT, Wen Y, Hung MC. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65:2287-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Xing JY, Song GP, Deng JP, Jiang LZ, Xiong P, Yang BJ, Liu SS. Antitumor effects and mechanism of novel emodin rhamnoside derivatives against human cancer cells in vitro. PloS One. 2015;10:e0144781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | The WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3313] [Cited by in RCA: 3584] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 52. | Mahato R, Tai W, Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv Drug Deliv Rev. 2011;63:659-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 53. | de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 54. | Ma Y, Chen H, Su S, Wang T, Zhang C, Fida G, Cui S, Zhao J, Gu Y. Galactose as Broad Ligand for Multiple Tumor Imaging and Therapy. J Cancer. 2015;6:658-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Cao J, Cui S, Li S, Du C, Tian J, Wan S, Qian Z, Gu Y, Chen WR, Wang G. Targeted cancer therapy with a 2-deoxyglucose-based adriamycin complex. Cancer Res. 2013;73:1362-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev. 2004;56:53-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 351] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 57. | Abraham S, Guo F, Li LS, Rader C, Liu C, Barbas CF, Lerner RA, Sinha SC. Synthesis of the next-generation therapeutic antibodies that combine cell targeting and antibody-catalyzed prodrug activation. Proc Natl Acad Sci USA. 2007;104:5584-5589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Chatterjee SK, Zetter BR. Cancer biomarkers: knowing the present and predicting the future. Future Oncol. 2005;1:37-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | de Graaf M, Boven E, Scheeren HW, Haisma HJ, Pinedo HM. Beta-glucuronidase-mediated drug release. Curr Pharm Des. 2002;8:1391-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Wu J, Liu Q, Lee RJ. A folate receptor-targeted liposomal formulation for paclitaxel. Int J Pharm. 2006;316:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 61. | Lin YS, Tungpradit R, Sinchaikul S, An FM, Liu DZ, Phutrakul S, Chen ST. Targeting the delivery of glycan-based paclitaxel prodrugs to cancer cells via glucose transporters. J Med Chem. 2008;51:7428-7441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3255] [Cited by in RCA: 3781] [Article Influence: 164.4] [Reference Citation Analysis (1)] |
| 63. | Johnston PG, Kaye S. Capecitabine: a novel agent for the treatment of solid tumors. Anticancer Drugs. 2001;12:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Feng D, Shaikh AS, Wang F. Recent Advance in Tumor-associated Carbohydrate Antigens (TACAs)-based Antitumor Vaccines. ACS Chem Biol. 2016;11:850-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 65. | Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 512] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 66. | Carrascal MA, Severino PF, Guadalupe Cabral M, Silva M, Ferreira JA, Calais F, Quinto H, Pen C, Ligeiro D, Santos LL, Dall'Olio F, Videira PA. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol Oncol. 2014;8:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 67. | Pashov A, Monzavi-Karbassi B, Kieber-Emmons T. Immune surveillance and immunotherapy: lessons from carbohydrate mimotopes. Vaccine. 2009;27:3405-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Pashov A, Monzavi-Karbassi B, Raghava GP, Kieber-Emmons T. Bridging innate and adaptive antitumor immunity targeting glycans. J Biomed Biotechnol. 2010;2010:354068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 2325] [Article Influence: 211.4] [Reference Citation Analysis (0)] |
| 70. | Ho WL, Hsu WM, Huang MC, Kadomatsu K, Nakagawara A. Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma. J Hematol Oncol. 2016;9:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 71. | Sørensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, Burchell J, Clausen H. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Tarp MA, Sørensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, Clausen H. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 2007;17:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 349] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 73. | Ryan SO, Vlad AM, Islam K, Gariépy J, Finn OJ. Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice. Biol Chem. 2009;390:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Madsen CB, Petersen C, Lavrsen K, Harndahl M, Buus S, Clausen H, Pedersen AE, Wandall HH. Cancer associated aberrant protein O-glycosylation can modify antigen processing and immune response. PLoS One. 2012;7:e50139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Li M, Yu F, Yao C, Wang PG, Liu Y, Zhao W. Synthetic and immunological studies on trimeric MUC1 immunodominant motif antigen-based anti-cancer vaccine candidates. Org Biomol Chem. 2018;16:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ, Boons GJ. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci USA. 2012;109:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 77. | Miles D, Papazisis K. Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer. 2003;3 Suppl 4:S134-S138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | RodrÍguez E, Schetters STT, van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018;18:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 79. | Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 525] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 80. | Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 342] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo K, Norton PA S-Editor: Wang LY L-Editor: A E-Editor: Qi LL