Published online Dec 24, 2020. doi: 10.5306/wjco.v11.i12.1029
Peer-review started: September 21, 2020
First decision: October 21, 2020
Revised: November 5, 2020
Accepted: November 28, 2020
Article in press: November 28, 2020
Published online: December 24, 2020
Processing time: 88 Days and 5.7 Hours
Mucoepidermoid carcinoma (MEC) is a rare malignancy of the head and neck; however, it accounts for a majority of the tumors of the salivary glands. This study used a national population-based registry to describe the pre-treatment and treatment-related prognostic factors that influence survival in patients with MEC of the major salivary glands. To our knowledge, this is the largest population-based study examining predictors of both overall and cause-specific survival of MEC of the major salivary glands.
To identify prognostic factors influencing overall survival (OS) and cause-specific survival (CSS) of patients with MEC of the major salivary glands.
We used the Surveillance, Epidemiology and End-Results Database of the National Cancer Institute to investigate a variety of factors that could influence survival of patients diagnosed with mucoepidermoid carcinoma of the major salivary glands. A total of 2210 patients diagnosed with MEC of the major salivary glands during the years of 1975-2016 were studied. The primary endpoints were OS and CSS. Cox regression analysis was used to perform univariate and multivariate analyses of clinical variables such as age at diagnosis, diagnosis year, sex, race, tumor size, stage, grade, treatment with or without surgical excision, and adjuvant radiotherapy treatment.
A total of 2210 patients diagnosed with MEC of the major salivary glands met inclusion criteria. In this study, 95% of patients underwent surgical excision and 41% received adjuvant radiation therapy. Median OS time for Grade I, II, and III/IV was 401 mo (± 48.25, 95%CI), 340 mo (± 33.68, 95%CI) and 55 mo (± 11.05, 95%CI), respectively. Univariate analysis revealed that lack of surgical excision was associated with decreased OS [hazard ratio (HR) 4.26, P < 0.0001] and that patients with localized disease had improved OS compared to both regional and distant disease (HR 3.07 and 6.96, respectively, P < 0.0001). Additionally, univariate analysis demonstrated that male sex, age over 50 at diagnosis, Grade III tumors, and increasing tumor size were associated with worsened OS (P < 0.0006). Univariate analysis of CSS similarly revealed that lack of surgical excision and Grade III carcinoma conferred decreased CSS (HR 4.37 and 5.44, respectively, P < 0.0001). Multivariate analysis confirmed that increasing age, in 10-year age bands, advanced tumor stage, increasing tumor size, Grade III carcinoma, male sex, and lack of surgical excision were associated with a statistically significant decrease in OS and CSS (P < 0.04). Of note, multivariate analysis revealed that the use of adjuvant radiation therapy was not associated with improved OS or CSS.
Multivariate analysis demonstrated increasing age, advanced tumor stage, increasing tumor size, Grade III carcinoma, male sex, and lack of surgical excision were associated with decreased OS and CSS (P < 0.04).
Core Tip: Mucoepidermoid carcinoma (MEC) of the major salivary glands is a rare cancer with a limited number of studies with high statistical power. The purpose of this study was to identify prognostic factors effecting overall survival (OS) and cause-specific survival (CSS) of individuals diagnosed with MEC of the major salivary glands. By using de-identified information from the Surveillance, Epidemiology and End-Results Program, we concluded that younger age at diagnosis, female sex, smaller tumor size, lower tumor grade, localized tumor growth, and more recent year of diagnosis were positive predictors of statistically significant improvements in OS and CSS.
- Citation: Taylor ZC, Kaya EA, Bunn JD, Guss ZD, Mitchell BJ, Fairbanks RK, Lamoreaux WT, Wagner AE, Peressini BJ, Lee CM. Overall and cause-specific survival for mucoepidermoid carcinoma of the major salivary glands: Analysis of 2210 patients. World J Clin Oncol 2020; 11(12): 1029-1044
- URL: https://www.wjgnet.com/2218-4333/full/v11/i12/1029.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i12.1029
Salivary gland malignancies are very rare, accounting for less than 5% of all head and neck cancers[1]. Within the larger group of salivary gland neoplasms, there are two subclassifications; the major and minor salivary gland cancers. The major salivary glands are comprised of the parotid gland and submandibular glands. In contrast to most other head and neck cancers, which are characterized predominantly by squamous cell carcinoma, the major salivary gland malignancies are categorized into a dozen or more histological subtypes, the most common of which is mucoepidermoid carcinoma[1-3].
Mucoepidermoid carcinoma (MEC) was first described by Stewart et al[4] in 1945 as salivary gland tissue that is comprised of epidermoid, mucous-secreting and intermediate cells. Since then, several grading systems have been developed in order to assign a histologic grade to the tumor upon pathologic evaluation and MECs are broken down into either low-, intermediate-, or high-grade malignancies depending on their level of invasion and differentiation[5-7]. This histopathological grading of the tumor is important as it is reportedly predictive of prognosis, where low-grade tumors have more favorable survival outcomes than high-grade tumors and intermediate-grade tumors fall in the middle[5,6,8-16].
Several studies have investigated potential risk factors for the development of MEC of the salivary glands. While smoking is a risk factor in a dose-dependent manner for all other major salivary gland cancer subtypes, it seems to be protective in MEC[17]. Furthermore, it has been discovered that prior radiation to the head or neck is a major risk factor for developing MEC in any of the salivary glands[5,18,19].
In contrast to most head and neck cancers in which TNM staging drives the primary treatment plan, the histopathological grading of MEC often directs the treatment regimen. Surgical resection, when feasible, is the cornerstone of therapy for MEC. Treatment options for mucoepidermoid carcinoma depend on grade of the tumor and resectability[7-9,15,20-23]. In most of these studies, the low-grade nature of Grade I tumors has allowed for surgical excision alone to be effective. Conversely, the high-grade tumors or tumors with positive surgical margins typically receive surgical excision and post-operative radiation[9,15,16,21,22,24,25]. This clear division of low-grade and high-grade with respect to treatment regimen has prompted some groups to attempt to characterize intermediate-grade neoplasms as more closely related to either the low- or high-grade tumors in order to drive treatment recommendations. However, there is disagreement amongst the academic community on this topic. Those that lump intermediate-grade in with low-grade affirm that there is no significant difference in clinical behavior or prognosis between the two grades[8,9,13,22,23], while others find that there is a significant difference in prognosis and intermediate-grade is closer in behavior to high-grade neoplasms[15,26].
Several other prognostic factors that have been studied at the institutional level include age, gender, degree of invasion, presence of positive surgical margins and the role of post-operative radiation therapy. Of note, both age and gender have presented themselves as independent factors that affect the overall prognosis of patients with MEC. Multiple groups have found that increasing age at diagnosis corresponds with worse prognosis across all tumor grades, although it is unclear what role other comorbidities play in this finding[5,7,14,23]. Additionally, males tend to have both higher grade neoplasms at diagnosis and worse overall survival[13-15,22,27,28]. Finally, post-operative radiation has been found to improve prognosis and extend overall survival in those patients with high-grade MEC or patients with positive margins following surgical excision, compared to surgery alone[8,9,13,20-22]. Importantly, these correlations have been made by evaluating small patient cohorts at individual institutions, owing to the rarity of MEC. Because of this, the statistical strength and ability to extrapolate to larger cohorts across the country is limited.
Our study aims to utilize data from the National Cancer Institute’s Surveillance, Epidemiology and End-Results (SEER) Program to evaluate pretreatment clinical factors like age at diagnosis, decade of diagnosis, sex, race, tumor size, tumor stage, and tumor grade as well as treatment protocols and their effect on overall survival (OS) and cause-specific survival (CSS) for mucoepidermoid carcinoma of the major salivary glands.
All data were acquired from the 1973-2016 database of the SEER program of the United States National Cancer Institute (NCI). The SEER database contains data from geographically specified United States locations that spans a population of approximately 30 million people. Registry data are submitted without personal identifiers; therefore, patient informed consent and ethics committee approval were not required to perform this analysis. The primary endpoints were OS and CSS. For this analysis we examined 2210 patients with a diagnosis of cancer of the major salivary glands and primary tumor histology of mucoepidermoid neoplasms. Our inclusion criteria included patients treated from 1973 to 2016 whose de-identified tumor information was included in the SEER database, patients with MEC as the primary tumor histology, patients who had info on size of tumor, regional nodal involvement or metastatic disease, both sexes and all ages. The patients were then grouped by age at diagnosis, tumor stage, tumor size, tumor grade, patient race, patient gender, whether the patient received radiation, whether the patient received surgery, and diagnosis year.
Survival curves were estimated using the Kaplan-Meier method and used to compare age at diagnosis, tumor stage, tumor size, tumor grade, patient race, patient gender, whether the patient received radiation, whether the patient received surgery, and diagnosis year. Then, 95% confidence intervals for the median survival time of the groups were constructed. Approximate confidence intervals for the log hazard-ratio were calculated using the estimate of standard error (se):
se=\sqrt{\sum_{i=1}^{k}\frac{1}{e_{ij}}}
-where eiis the extent of exposure to risk of death for group i of k at the jth distinct observed time for group i of k [Armitage P, Berry G. Statistical Methods in Medical Research (3rd edition). Blackwell 1994]. Log-rank tests were employed to determine if there is statistical evidence of differences between the survival curves of the groups. Finally, the Cox proportional hazard model was used in a multivariate analysis of the treatment groups, age groups, KPS groups, and primary tumor histology groups. All statistical analyses utilized StatsDirect Version 3.2.8 (StatsDirect Ltd., Altrincham, United Kingdom) and SigmaPlot Version 12.3 (SYSTAT Software, Inc., San Jose, CA). The statistical methods of this study were reviewed by Ben Peressini from DataWorks Northwest, LLC.
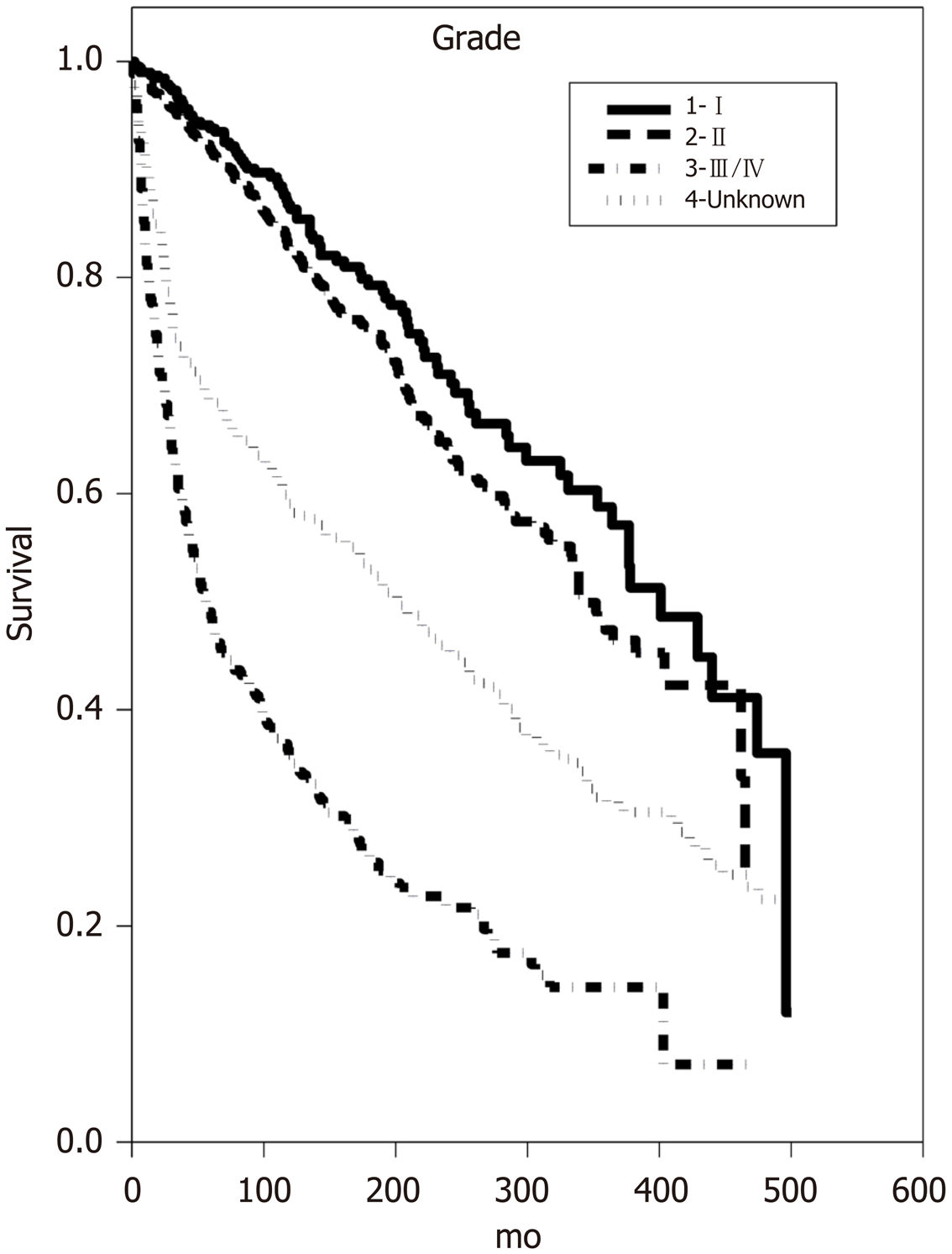
A total of 2210 patients with MEC of the major salivary glands met our inclusion criteria. There was not a sex preference as the prevalence of MEC for males and females was 1117 and 1093, respectively. Additionally, nearly 95% of patients in the study underwent surgery of some kind to have their tumor removed; however, only 46% of patients received radiation at any point in their treatment regimen. Median OS time for Grade I, II, and III/IV was 401 mo (± 48.25, 95%CI), 340 mo (± 33.68, 95%CI) and 55 mo (± 11.05, 95%CI), respectively (Table 1) Grade I correlates to low-grade MEC, grade II correlates to intermediate-grade MEC, and Grade III/IV correlates to high-grade MEC.
| n | Median survival | Univariate hazard ratio | Multivariate hazard ratio | |||
| 95%CI | Estimate | 95%CI | Estimate | 95%CI | ||
| Age bands | ||||||
| 00-09 | 11 | Cannot estimate | Cannot estimate | Cannot estimate | ||
| 10-19 | 86 | Cannot estimate | 0.12a | 0.02-0.37 | 0.13c | 0.06-0.32 |
| 20-29 | 174 | Cannot estimate | 0.16a | 0.07-0.32 | 0.18c | 0.12-0.26 |
| 30-39 | 275 | Cannot estimate | 0.62b | 0.42-0.92 | 0.73c | 0.68-0.78 |
| 40-49 | 319 | 401 ± 38.42 | Reference | Reference | ||
| 50-59 | 431 | 248 ± 13.93 | 2.55a | 1.93-3.39 | 2.51c | 2.33-2.69 |
| 60-69 | 381 | 167 ± 26.25 | 5.00a | 3.75-6.75 | 4.31c | 3.28-5.67 |
| 70-79 | 336 | 70 ± 17.94 | 11.38a | 8.25-15.95 | 7.52c | 6.91-8.18 |
| 80+ | 197 | 35 ± 6.87 | 20.02a | 13.67-29.92 | 12.24c | 11.58-12.95 |
| Stage | ||||||
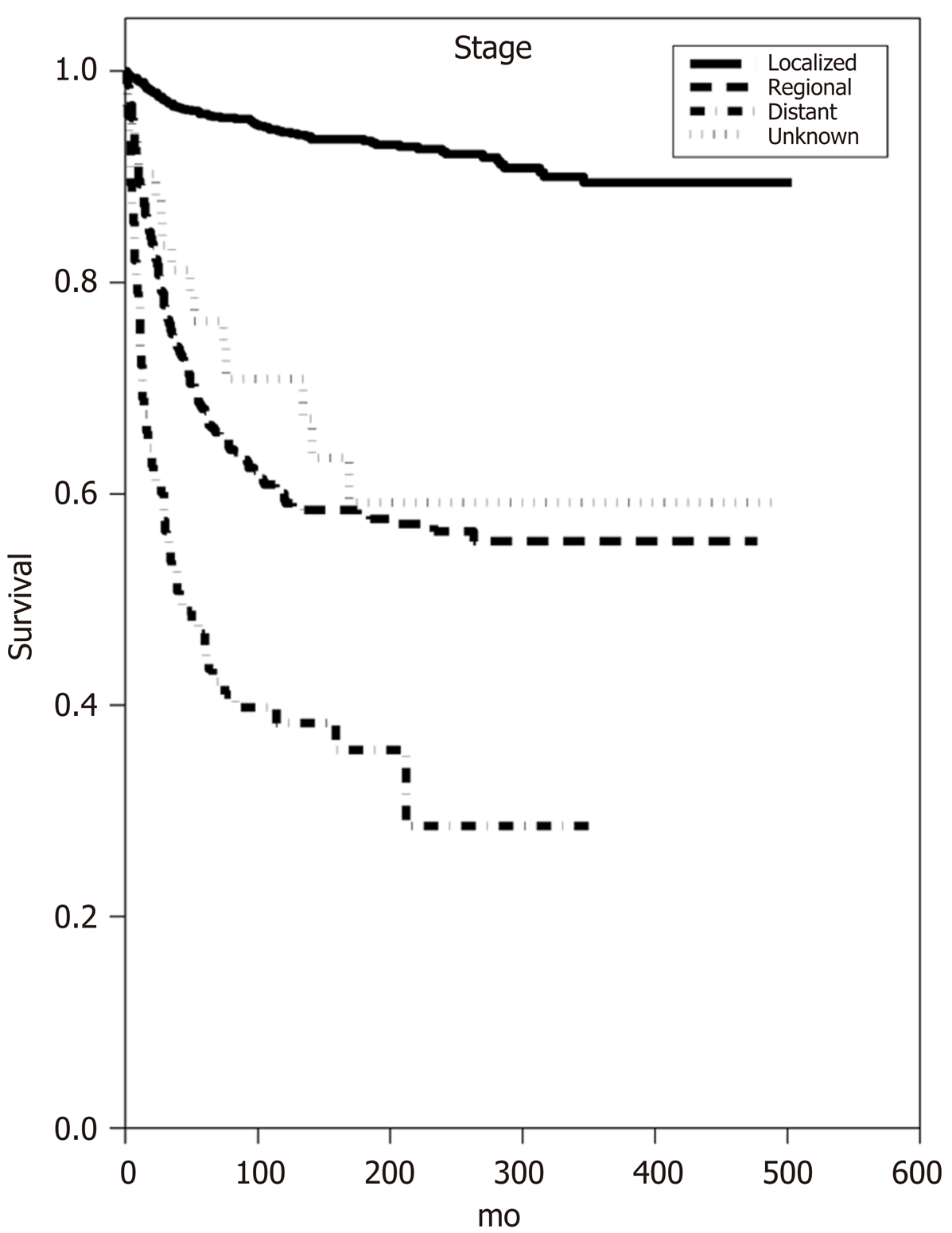
| Localized | 1344 | 342 ± 26.24 | Reference | Reference | ||
| Regional | 591 | 99 ± 19.59 | 3.07a | 2.66-3.54 | 1.95c | 1.95-1.96 |
| Distant | 143 | 29 ± 9.64 | 6.96a | 5.51-8.75 | 2.84c | 2.73-2.95 |
| Unknown | 132 | 118 ± 61.69 | 2.69a | 1.86-3.80 | 1.6b | 1.11-2.31 |
| Primary tumor size (mm) | ||||||
| 0-10 | 209 | Cannot estimate | Reference | Reference | ||
| 11-20 | 576 | 331 ± 59.73 | 1.95c | 1.30-3.01 | 1.64c | 1.39-1.94 |
| 21-30 | 355 | 248 ± 54.66 | 3.15a | 2.08-4.91 | 2.07c | 1.36-3.13 |
| 31-40 | 150 | 141 ± 57.38 | 4.63a | 2.97-7.40 | 2.23c | 1.98-2.52 |
| 41-50 | 57 | 46 ± 38.76 | 9.26a | 5.56-15.59 | 2.66c | 1.52-2.81 |
| > 50 | 87 | 52 ± 36.21 | 9.14a | 5.67-15.05 | 2.87c | 2.63-3.13 |
| Unknown/unspecific | 776 | 220 ± 26.3 | 3.43a | 2.35-5.18 | 2.09c | 1.73-2.51 |
| Grade | ||||||
| I | 403 | 401 ± 48.25 | Reference | Reference | ||
| II | 850 | 340 ± 33.68 | 1.26 | 0.98-1.63 | 1.30b | 1.02-1.67 |
| III/IV | 538 | 55 ± 11.05 | 5.44a | 4.27-6.99 | 2.10c | 1.98-2.23 |
| Unknown | 419 | 201 ± 39.44 | 2.49a | 1.94-3.20 | 1.61c | 1.52-1.70 |
| Race | ||||||
| American Indian/Alaska Native | 16 | Cannot estimate | 0.47 | 0.13-1.20 | 1.64 | 0.61-4.45 |
| Asian or Pacific Islander | 206 | 308 ± 66.3 | 0.70b | 0.53-0.91 | 0.93 | 0.71-1.21 |
| Black | 239 | Cannot estimate | 0.59a | 0.45-0.76 | 1.00 | 0.78-1.29 |
| Unknown | 23 | Cannot estimate | 0.10c | 0.00-0.54 | 0.31 | 0.04-2.26 |
| White | 1726 | 226 ± 21.22 | Reference | Reference | ||
| Sex | ||||||
| Female | 1093 | 327 ± 31.14 | Reference | Reference | ||
| Male | 1117 | 187 ± 22.69 | 1.76a | 1.54-2.01 | 1.26b | 1.10-1.44 |
| Radiation | ||||||
| No/unknown | 1200 | 340 ± 34.89 | 0.46a | 0.40-0.53 | 1.07 | 0.70-1.63 |
| Yes | 1010 | 147 ± 23.39 | Reference | Reference | ||
| Sequence | ||||||
| Not applicable | 1260 | 327 ± 29.07 | Reference | Reference | ||
| Radiation after surgery | 902 | 162 ± 25.62 | 1.82a | 1.59-2.08 | 1.18 | 0.76-1.83 |
| Radiation before surgery | 48 | 162 ± 56.48 | 1.67b | 1.12-2.40 | 1.17c | 1.10-1.25 |
| Surgery | ||||||
| No | 77 | 27 ± 11.81 | 4.26a | 3.16-5.64 | 2.09c | 1.40-3.10 |
| Unknown | 28 | 16 ± 10.37 | 4.43a | 2.87-6.57 | 2.41c | 1.43-4.05 |
| Yes | 2105 | 263 ± 23.24 | Reference | Reference | ||
| Diagnosis year | ||||||
| 1975-1995 | 960 | 221 ± 23.77 | 1.31c | 1.13-1.52 | 1.38c | 1.16-1.64 |
| 1996-2016 | 1250 | Cannot estimate | Reference | Reference | ||
| Surgery type | ||||||
| Excision | 2085 | 261 ± 23.23 | Reference | Variable not used in multivariate analysis | ||
| Limited surgical procedure/biopsy | 20 | Cannot estimate | 1.01 | 0.52-1.96 | ||
| None | 77 | 27 ± 11.81 | 4.28a | 3.17-5.67 | ||
| Unknown | 28 | 16 ± 10.37 | 4.44a | 2.88-6.59 | ||
| Diagnosis year | ||||||
| 1975-1984 | 405 | 207 ± 31.31 | 1.77a | 1.39-2.26 | Variable not used in multivariate analysis | |
| 1985-1994 | 497 | 230 ± 36.99 | 1.50c | 1.18-1.91 | ||
| 1995-2004 | 564 | Cannot estimate | 1.32b | 1.04-1.68 | ||
| 2005-2016 | 744 | Cannot estimate | Reference | |||
Upon univariate analysis, increasing age at diagnosis demonstrated a statistically significant decrease in OS (P < 0.016) (Table 1). A similar trend was seen upon analysis of tumor size, where increasing tumor size was associated with decreased OS (P < 0.0006). Not undergoing surgical excision of MEC appears to be a major predictor of OS [hazard ratio (HR) 4.26 (P <0.0001)], as does having distant tumor involvement upon diagnosis [HR 6.96 (P < 0.0001)]. Of note, male sex was also associated with decreased OS [HR 1.76 (P < 0.0001)], with a median OS time of 187 mo (± 22.69, 95%CI) compared to the median OS time for females of 327 mo (± 31.14, 95%CI).
Multivariate analysis of overall survival confirmed many of the associations seen upon univariate analysis. Increasing age at diagnosis was once again associated with decreased OS (P < 0.001) as was increasing tumor size (P < 0.001) (Table 1). Both regional and distant tumor involvement showed a decrease in OS (HR 1.95 and 2.84, respectively, P < 0.001). Tumor grade was also an independent predictor of OS as Grade II and III/IV tumors demonstrated decreased OS (HR 1.3 and 2.1, respectively, P < 0.04) (Figure 1). Male sex (Figure 2) and lack of surgical excision once again conferred a significant decrease in OS (HR 1.26 and 2.09, P < 0.001). Notably, neither race nor receipt of adjuvant radiation demonstrated significant increases or decreases in OS on multivariate analysis.
Univariate analysis of cause-specific survival revealed that age over 50 at diagnosis was associated with a decrease in CSS (P < 0.0001) (Table 2). Very strong predictors of decreased CSS upon univariate analysis were regional and distant tumor involvement (HR 7.46 and 16.71, respectively, P < 0.0001), as well as Grade III/IV neoplasms (HR 15.48, P < 0.0001). Lack of surgery was also associated with decreased CSS (HR 4.37, P < 0.0001). Finally, as was seen upon analysis of OS, male sex was once again associated with decreased CSS (HR 2.38, P < 0.0001).
| n | Median survival | Univariate hazard ratio | Multivariate hazard ratio | |||
| 95%CI | Estimate | 95%CI | Estimate | 95%CI | ||
| Age bands | ||||||
| 00-09 | 11 | Cannot estimate | Cannot estimate | Cannot estimate | ||
| 10-19 | 86 | Cannot estimate | 0.23b | 0.03-0.90 | 0.28b | 0.10-0.74 |
| 20-29 | 174 | Cannot estimate | 0.23b | 0.06-0.67 | 0.29c | 0.16--0.51 |
| 30-39 | 275 | Cannot estimate | 0.71 | 0.37-1.34 | 0.95 | 0.85-1.06 |
| 40-49 | 319 | Cannot estimate | Reference | Reference | ||
| 50-59 | 431 | Cannot estimate | 2.50a | 1.62-3.96 | 1.99c | 1.30-3.06 |
| 60-69 | 381 | Cannot estimate | 3.34a | 2.17-5.29 | 2.47c | 2.18-2.80 |
| 70-79 | 336 | Cannot estimate | 5.41a | 3.48-8.67 | 3.00c | 2.63-3.43 |
| 80+ | 197 | Cannot estimate | 7.67a | 4.69-12.92 | 3.47c | 3.17-3.79 |
| Stage | ||||||
| Localized | 1344 | Cannot estimate | Reference | |||
| Regional | 591 | Cannot estimate | 7.46a | 5.78-9.70 | 3.90c | 3.77-4.04 |
| Distant | 143 | 41 ± 23.43 | 16.71a | 11.95-23.34 | 5.79c | 5.77-5.81 |
| Unknown | 132 | Cannot estimate | 6.27a | 3.62-10.38 | 3.01c | 1.79-5.05 |
| Primary tumor size (mm) | ||||||
| 0-10 | 209 | Cannot estimate | Reference | Reference | ||
| 11-20 | 576 | Cannot estimate | 1.87 | 0.86-4.62 | 1.4b | 1.01-1.92 |
| 21-30 | 355 | Cannot estimate | 5.08a | 2.41-12.34 | 2.13c | 1.48-3.06 |
| 31-40 | 150 | Cannot estimate | 8.18a | 3.75-20.31 | 2.27b | 1.04-4.95 |
| 41-50 | 57 | Cannot estimate | 17.24a | 7.48-44-63 | 3.19c | 2.44-4.17 |
| > 50 | 87 | 89 ± 108.69 | 18.24a | 8.30-45.63 | 3.53c | 2.58-4.83 |
| Unknown/unspecific | 776 | Cannot estimate | 6.25a | 3.10-14.69 | 2.57c | 1.73-3.82 |
| Grade | ||||||
| I | 403 | Cannot estimate | Reference | Reference | ||
| II | 850 | Cannot estimate | 1.75 | 0.98-3.33 | 1.56 | 0.88-2.76 |
| III/IV | 538 | Cannot estimate | 15.48a | 9.17-28.16 | 4.35c | 2.52-7.52 |
| Unknown | 419 | Cannot estimate | 7.02a | 4.06-13.01 | 2.92c | 1.67-5.10 |
| Race | ||||||
| American Indian/Alaska Native | 16 | Cannot estimate | 1.24 | 0.33-3.20 | 4.29c | 2.18-8.48 |
| Asian or Pacific Islander | 206 | Cannot estimate | 0.64b | 0.41-0.96 | 0.79 | 0.52-1.19 |
| Black | 239 | Cannot estimate | 0.66b | 0.44-0.95 | 1.06 | 0.94-1.19 |
| Unknown | 23 | Cannot estimate | 0.00b | 0.00-0.90 | Cannot estimate | |
| White | 1726 | Cannot estimate | Reference | Reference | ||
| Sex | ||||||
| Female | 1093 | Cannot estimate | Reference | Reference | ||
| Male | 1117 | Cannot estimate | 2.38a | 1.92-2.96 | 1.36b | 1.09-1.69 |
| Radiation | ||||||
| No/unknown | 1200 | Cannot estimate | 0.27a | 0.22-0.34 | 2.10 | 0.43-1.49 |
| Yes | 1010 | Cannot estimate | Reference | Reference | ||
| Sequence | ||||||
| Not applicable | 1260 | Cannot estimate | Reference | Reference | ||
| Radiation after surgery | 902 | Cannot estimate | 2.66a | 2.15-3.30 | 0.96 | 0.50-1.84 |
| Radiation before surgery | 48 | Cannot estimate | 2.79c | 1.55-4.70 | 1.05 | 0.47-2.33 |
| Surgery | ||||||
| No | 77 | Cannot estimate | 4.37a | 2.89-6.38 | 2.17b | 1.18-3.98 |
| Unknown | 28 | 74 ± 7.67 | 4.84a | 2.54-8.44 | 1.62c | 1.49-1.76 |
| Yes | 2105 | Cannot estimate | Reference | Reference | ||
| Diagnosis year | ||||||
| 1975-1995 | 960 | Cannot estimate | 1.40b | 1.14-1.73 | 1.33b | 1.03-1.72 |
| 1996-2016 | 1250 | Cannot estimate | Reference | Reference | ||
| Surgery type | Variable not used in multivariate analysis | |||||
| Excision | 2085 | Cannot estimate | Reference | |||
| Limited surgical procedure/biopsy | 20 | Cannot estimate | 1.50 | 0.48-3.55 | ||
| None | 77 | Cannot estimate | 4.41a | 2.92-6.44 | ||
| Unknown | 28 | 74 ± 7.67 | 4.89a | 2.57-8.53 | ||
| Diagnosis year | Variable not used in multivariate analysis | |||||
| 1975-1984 | 405 | Cannot estimate | 2.09a | 1.53-2.88 | ||
| 1985-1994 | 497 | Cannot estimate | 1.47b | 1.06-2.04 | ||
| 1995-2004 | 564 | Cannot estimate | 1.44b | 1.05-1.98 | ||
| 2005-2016 | 744 | Cannot estimate | Reference | |||
Multivariate analysis of CSS confirmed that age over 50 at diagnosis was associated with a decrease in CSS (P < 0.001) and that increasing tumor size conferred a decrease in CSS (P < 0.04) (Table 2). Regional and distant tumor involvement (Figure 3) as well as Grade III/IV tumors at diagnosis were associated with decreased CSS (P < 0.001), just as was seen upon univariate analysis. Both lack of surgery and male sex demonstrated decreased CSS (HR 2.17 and 1.36, respectively, P < 0.01). Finally, American Indian/Alaska Native race conferred decreased CSS (HR 4.29, P < 0.001), though there were only 16 patients in this subgroup making the conclusions difficult to extrapolate to a larger population.
Mucoepidermoid carcinoma accounts for the majority of the major salivary gland malignancies and represents just one of the many histological subtypes that are responsible for such malignancies[1-3].
In this study, advanced age at diagnosis stood out as a very strong independent predictor of OS. Patients in the 50-59 years old age band had worse prognosis compared to younger age ranges (multivariate HR 2.51, P < 0.001) and this progressed in a stepwise fashion for each successive 10-year age band where patients who were 80 years or older had the worst OS (multivariate HR 12.24, P < 0.001). This relationship was mirrored in the multivariate analysis of CSS, where there was a decrease in CSS as patient age at diagnosis increased and patients who were greater than 80 years old at diagnosis had the worst CSS (multivariate HR 3.47, P < 0.001). These findings are in agreement with results from several other groups showing that age was a significant predictor of prognosis[5,7,12,14,23,27-29].
The role of sex as a predictive variable for determining MEC disease outcome has been explored on several different levels. Cheung et al[29] described improved 5-year survival rates for women compared to men in an analysis of all salivary gland malignancies. Several other studies of just MEC have evaluated and confirmed this improved survival for women[5,12-15,22,27,28]. In fact, several other groups explored this trend more in depth and noted that men presented with higher grade MEC upon diagnosis, providing a possible explanation for the favorable outcomes for women seen in other studies[7,12,15,22,27,29,30]. Our data showed that while there were nearly equal numbers of men and women diagnosed with MEC (1093 women and 1117 men), the median survival time was 1.75 times longer for women compared to men. Additionally, the multivariate analysis showed that men had a worse overall survival prognosis (multivariate HR 1.26, P = 0.001). Interestingly, Boukheris et al[1] showed an age-specific crossing pattern with respect to gender and incidence of MEC. When comparing both age and gender together, they discovered the incidence rate of MEC in men to be 72% that of women under the age of 50 (P < 0.05) [1]. However, this trend switched after the 50-year-old mark where the incidence rate of MEC in men was 157% that of women over the age of 50 (P < 0.05)[1].
Histopathologic grade of MEC has long been recognized as an independent predictor of prognosis. Even in 1970, Healey et al[6] described worsening 5-year overall survival rates for those with high-grade (Grade III) malignancies (31% OS for Grade III compared with 90% OS for Grade I). Since then, multiple groups have reaffirmed the negative impact of having a high-grade MEC malignancy on overall survival[5,8-11,13-16]. In fact, Seethala[31] asserts that there is no other salivary gland malignancy in which prognosis and treatment rely so heavily on histologic grading. Traditionally, low-grade tumors are treated with definitive surgery while high-grade MEC requires surgery and adjuvant radiation therapy. However, there is disagreement about how to treat patients with intermediate-grade MEC, due primarily to underlying disagreement about whether intermediate-grade malignancies behave more similarly to low-grade or high-grade neoplasms[26-28,32].
This differential classification of intermediate-grade tumors is due in part to the existence of several histologic grading systems which are used to varying extents by pathologists assigning a grade to the malignancies. In fact, it has been suggested that many pathologists refrain from using any of these grading criteria because they are cumbersome to use and there is a lack of consensus on which criteria is best[31,32]. Instead, pathologists use their own intuition in assigning a histologic grade, leading to further confusion about which tumors are truly low-grade, intermediate-grade or high-grade[31,32]. The four major grading criteria proposed for use for grading MEC of the major salivary glands include the modified Healey et al[6], Brandwein et al[5], Memorial Sloan-Kettering Cancer Center (MSKCC)[32] and Armed Forces Institute of Pathology (AFIP) criteria[7,30]. Both the AFIP and Brandwein models assign varying point values to the specific features that they observed as characteristic of more aggressive growth behavior and set ranges for what is considered low-grade, intermediate-grade, or high-grade[5,7,30]. Batsakis et al[33] described a subjective, three-tier grading system that took what Healey et al[6] proposed in 1970 and incorporated growth patterns and levels of cell differentiation into the criteria. The MSKCC system, proposed in 2014 to try and alleviate some of the confusion of the other three systems, similarly relies on a subjective analysis by the pathologist to determine whether the tumor specimen most closely aligns with their descriptions of low-, intermediate-, or high-grade MEC[32].
The AFIP grading system, though endorsed by the WHO[3], tends to downgrade the severity of MEC malignancies, meaning that it will classify more aggressive tumors as low-grade[31,32,34]. This has possible negative implications since treatment for low-grade tumors is strictly surgical excision which has proven to be insufficient for controlling high-grade MEC of the major salivary glands. On a similar note, the Brandwein system tends to upgrade the severity of malignancies, meaning that it will classify more indolent tumors as high-grade[32,34]. This too has possible negative implications for the patient who may undergo radical excision of the major salivary gland and surrounding structures and subsequent radiation for a tumor that could have been managed with local excision and less disfigurement. Despite this, some still recommend either the AFIP or Brandwein criteria because of ease of use, reproducibility, and the fact that a formalized system of some kind is better than no system at all, where the alternative is strictly subjective assessment[27,31].
The differential assignment of grade by each grading system was put on display as Qannam et al[34] used the varying criteria to classify MEC of a small patient subset. Histologic grading of 19 primary minor salivary gland tumors using the MSKCC, AFIP, modified Healey and Brandwein criterion only showed 32% agreement[34]. However, the level of disagreement was profound and most pronounced when looking at the distribution of malignancies that each grading system assigned as intermediate-grade[34]. A similar histologic grading disagreement was seen when Katabi et al[32] applied the four grading systems to a population of 52 patients with MEC of the major salivary glands. Here, there was disagreement between the histologic grading systems in 23 (or 44%) of the cases[32].
Intermediate-grade MEC is arguably the most important grade to assign correctly since grade plays into treatment decisions so heavily. Importantly, some of the grading systems describe IMG MEC as more closely related to LG MEC than HG MEC, while some say the opposite. However, our findings are in line with the majority of studies, as our multivariate analysis demonstrated that the OS of those diagnosed with Grade II (IMG) MEC was more similar to the OS of those with Grade I (LG) MEC upon diagnosis (HR 1.3, P < 0.04) than it was to Grade III/IV (HG) MEC (HR 2.1, P < 0.001). Furthermore, the median OS time for Grade I, II, and III/IV was 401 mo (± 48.25, 95%CI), 340 mo (± 33.68, 95%CI) and 55 mo (± 11.05, 95%CI), respectively, once again showing that intermediate-grade MEC behaves more similarly to low-grade MEC.
Larger MEC tumor size at diagnosis has uniformly conferred with worsening overall survival[7,11,12]. Our multivariate analysis of OS similarly demonstrated that increasing size of the primary tumor at the time of diagnosis corresponded to progressively worsening OS. Patients with tumors between 11-20 mm at diagnosis had worse OS than those with tumors between 0-10 mm (multivariate HR 1.64, P < 0.001) and this trend progressed for each successive 10mm size band where those with tumors greater than 50mm at diagnosis had the worst OS (multivariate HR 2.87, P < 0.001). Analysis of CSS also demonstrated decreased survival with increasing size of tumor at diagnosis where those with tumors between 0-10 mm had the best CSS, and those with tumors greater than 50 mm had the worst CSS (multivariate HR 3.53, P < 0.001).
As is seen in most other cancers throughout the body, more advanced MEC TNM staging at diagnosis is associated with worse overall and cause-specific survival. In 1975 and 1976, Spiro et al[35,36] made recommendations on ways to clinically stage cancers of the major salivary glands that included size, number, mobility, CN VII involvement and nodal status. Several years later, Spiro et al[12] looked at MEC of the salivary glands and found that while their assigned stage and histologic grade were very frequently in agreement, when there was a discrepancy (high grade, stage I or low grade, stage III), survival outcome was most impacted by stage and not histologic grade. This is contrary to Seethala’s[31] assertion that histologic grading of MEC dictates prognosis and treatment plans. However, a possible explanation for this disagreement lies in the fact that Seethala’s study, written in 2008, was using the more modern histologic grading systems of either AFIP, Brandwein, or modified Healey which all include some criteria that are normally part of staging criteria including angiolymphatic and perineural invasion. In other words, some factors that were historically part of staging criteria are now built into the commonly used grading criteria for MEC of the salivary glands leading to the difference in opinion between Spiro et al[35] and Seethala[31]. As such, it is very likely that the newer histologic grading being used at diagnosis is more important than clinical stage for the patient’s overall prognosis and treatment plan moving forward.
That being said, tumor stage is still considered an independent predictor of disease outcome, where increasing T stage as well as nodal involvement corresponds to decreased OS and CSS[8,9,11-14,16,22,27,28,32,37-39]. In fact, the current National Comprehensive Cancer Network (NCCN) guidelines continue to primarily rely on tumor staging for treatment recommendations related to all salivary gland neoplasms with some consideration for tumor grade. In our study, we generalized the staging to include local (N0M0), regional (N1M0), and distant (NxM1) disease. Our data demonstrate that, perhaps as expected, localized MEC confers a better OS than either regional (multivariate HR 1.95, P < 0.001) or distant (multivariate HR 2.84, P < 0.001) tumor involvement. The relationship between stage and prognosis was even more pronounced when looking at CSS data where once again, localized MEC conferred better CSS than both regional (multivariate HR 3.9, P < 0.001) and distant (multivariate HR 5.79, P < 0.001) MEC involvement.
Low-grade MEC of the major salivary glands has traditionally been treated with local excision of the tumor only. On the other hand, high-grade MEC is traditionally treated with wide local excision ± lymphadenectomy if lymph nodes are involved followed by adjuvant radiation. The treatment of intermediate-grade MEC is less clear and this is due in part to the confusion of whether it is more closely related in behavior to low-grade or high-grade MEC. As addressed above, our data confirm that intermediate-grade typically has a more indolent disease course and survival plots are more similar to those of low-grade neoplasms. The less aggressive behavior of these tumors allows for local excision only unless they exhibit characteristics of high-grade malignancy, defined by the NCCN Head and Neck Cancer Guidelines of 2020 as close or positive surgical margins, perineural invasion, or angiolymphatic invasion. In these specific cases, the NCCN Guidelines recommend undergoing adjuvant radiation therapy.
Some have suggested that radiation therapy does not improve patient survival. While this may be true, there is a possible confound that not all groups correct for in their analysis, and that is that those receiving radiation therapy are likely diagnosed with high-grade MEC or have intermediate-grade MEC with some high-grade characteristics including positive surgical margins or extracapsular extension. These patients already have much lower overall survival and disease-free survival (DFS) rates. Nance et al[27]. controlled for histologic grade in his study of 50 patients with MEC of the salivary glands and was still able to demonstrate that radiation does not confer any survival benefit in patients with high-grade MEC, and actually showed worse DFS rates in those with intermediate-grade MEC that were treated with radiation compared to those that were not. Ferrell et al[39] also looked specifically at adjuvant radiation therapy following a primary resection for high- and low-grade MEC and showed improved OS for high-grade MEC and no statistically significant difference in OS compared to surgery alone for low-grade MEC. While our adjuvant radiation outcome data was not analyzed separately for each grade of tumor, the multivariate data did show that adjuvant radiation has no statistically significant difference in OS or CSS compared to surgery alone when looking at all grades of MEC. This is an area that deserves more attention as there are currently opposing views on the role of adjuvant radiation in the treatment plans for the different grades of MEC[27,39].
Another possible confound in the data on adjuvant radiation therapy is that newer technology has changed the safety profile of radiation therapy significantly. Prior to the major rollout of intensity-modulated radiation therapy in the late-90s, 3D conformal imaging was used. And prior to either of these methods, a simple 2D X-ray was all that was used to map a patient’s organ location prior to therapy, leading to a significant amount of off-target radiation of healthy tissue. Now, with CT mapping and technology that delivers precise radiation doses to the cancerous tissue and permitted doses to the healthy surrounding tissue, the risk of off-target radiation damage is much lower. This is especially true of radiation for head and necks cancers in general, where one of the mainstays of treatment besides surgery has been radiation[40]. This trend in improved targeting of the tumor bed and protection of critical structures in accordance with the newer methods of radiation therapy could be accounted for in our multivariate analysis that shows both decreased OS (multivariate HR 1.38, P < 0.001) and CSS (multivariate HR 1.33, P < 0.04) in patients diagnosed and treated between 1975-1995 compared to those diagnosed and treated between 1996-2016. Another contributing factor to the improved OS and CSS is the past several decades is the improved safety of the surgical excision of tumors. Whether it be through an enhanced understanding of surgical technique at or around the salivary glands or fewer post-surgical complications as a result of facial nerve sparing, improvements in the mainstay of treatment for MEC have certainly had a positive impact on prognosis.
The SEER database does not collect information about chemotherapy use or dosing, so we are unable to comment directly on the benefits or drawbacks of such treatment for MEC of the major salivary glands. However, previous studies have made clear that chemotherapy has a poor ability to control MEC of the salivary glands and is even potentially detrimental to OS[14,39]. Rajasekaran et al[14] demonstrated a lower 5-year OS for surgery, chemotherapy and radiation combined when compared to surgery alone or surgery plus radiation for MEC of the parotid gland. Additionally, Ferrell et al[39] showed that surgery plus chemoradiation conferred worse OS than surgery alone when looking at all salivary gland cancers.
There is no survival difference based on race, ethnicity, or socioeconomic status in any salivary gland malignancy, which is in contrast to other cancers of the head and neck that show an increased incidence and mortality for African Americans[29]. When looking at our multivariate analyses, we similarly did not see an overall survival difference based on race. There was a worse prognosis noted for American Indian/Alaska Native patients when looking at cause-specific survival [multivariate hazard ratio of 4.29 (P < 0.001)], however the power of the conclusion drawn from that is not strong as only 16 patients identified as American Indian/Alaska Native.
Since our data is from the SEER database, the limitations of our study are the same as those that are inherent to the database itself. There is a lack of a centralized pathology review to confirm histopathologic diagnoses, and therefore, histologic misclassification is a possibility. Additionally, there is a lack of information about which histologic grading system was used to grade the MEC malignancies of each patient, which is an important distinguishing factor for MEC of the major salivary glands. The SEER database also does not provide information about local control of disease which makes interpretation of the benefit of adjuvant radiation therapy difficult. Adjuvant radiation therapy is primarily used for local control of disease, however SEER only provides information about OS and CSS.
While mucoepidermoid carcinoma is the most common histological subtype of the major salivary glands, it is still a rare tumor with a paucity of studies providing conclusions with high statistical power. This study is one of the largest population-based studies of MEC of the major salivary glands focused on identifying prognostic factors effecting OS and CSS. Younger age at diagnosis, female sex, smaller tumor size, lower tumor grade, localized tumor growth, and more recent year of diagnosis were positive predictors of statistically significant improvements in OS and CSS. This study also focused on the role of adjuvant radiation for treatment of MEC of the major salivary glands. Multivariate analysis did not show any statistically significant improvement in OS or CSS with adjuvant radiation following surgery. However, we did not analyze the role of adjuvant radiation for each different histologic grade of MEC and there are currently dissenting opinions in the literature about whether or not adjuvant radiation therapy plays a role in high-grade MEC of the major salivary glands[27,39]. For this reason, we believe further research should focus on the role of adjuvant radiation for low-, intermediate- and high-grade MEC.
While mucoepidermoid carcinoma (MEC) is a rare cancer, it is the most common histologic subtype of the major salivary glands. Despite this, there is a paucity of studies with high statistical power that provide conclusions on pretreatment and treatment related factors that affect survival. This study is one of the largest population-based studies of mucoepidermoid carcinoma of the major salivary glands focused on identifying prognostic factors effecting overall survival (OS) and cause-specific survival (CSS).
While mucoepidermoid carcinoma is a rare cancer, it is the most common histologic subtype of the major salivary glands. Despite this, there is a paucity of studies with high statistical power that provide conclusions on pretreatment and treatment related factors that affect survival. By identifying prognostic factors that affect both overall OS and CSS, we hope this study can help provide information to guide and inform treatment plans for patients diagnosed with MEC of the major salivary glands.
This study is one of the largest population-based studies of MEC of the major salivary glands and sought to identify prognostic factors influencing OS and CSS of patients with MEC of the major salivary glands.
De-identified cancer registry data from the Surveillance, Epidemiology and End-Results (SEER) Database of the National Cancer Institute was used to investigate a variety of factors that could influence survival of patients diagnosed with mucoepidermoid carcinoma of the major salivary glands. The primary endpoints were OS and CSS. Cox regression analysis was used to perform univariate and multivariate analyses of clinical variables such as age at diagnosis, diagnosis year, sex, race, tumor size, stage, grade, treatment with or without surgical excision, and adjuvant radiotherapy treatment.
A total of 2210 patients diagnosed with MEC of the major salivary glands met inclusion criteria. The clinical factors that were associated with statistically significant improvements in both OS and CSS include younger age at diagnosis, smaller tumor size, lower tumor grade, localized tumor growth, female sex, and more recent year of diagnosis. Importantly, no statistically significant improvement in OS or CSS was noted with adjuvant radiation therapy following surgery.
This study identified a variety of factors that affect OS and CSS for patients with mucoepidermoid carcinoma of the major salivary glands. These factors can help inform and guide treatment planning for mucoepidermoid carcinoma of the major salivary glands. Additionally, this study provided commentary on the debate between cancer staging vs histologic grading being more predictive of clinical outcome as well as which histologic grading system should be utilized for these cancers, something that was possible due to the improved statistical power of this study.
Further research is needed to better delineate the role of adjuvant radiation for low-, intermediate-, and high-grade MEC in order to better guide treatment planning. This study did not find a statistically significant improvement in OS or CSS for patients who received adjuvant radiation therapy, though we did not analyze the effect of radiation on OS and CSS for each histologic grade or tumor stage, nor did we analyze local control of disease from adjuvant radiation therapy due to the constraints of the SEER database. Furthermore, there are currently dissenting opinions about the role of adjuvant radiation for high-grade MEC of the major salivary glands.
The completion of this project would not have been possible without the support of my supervisor (Dr. Christopher Lee), my fellow medical school classmate (Erin Kaya), and the hard work of our biostatistician (Ben Peressini) as well as all the other surgeons and oncologists that provided both insight and editorial advice on this manuscript.
| 1. | Boukheris H, Curtis RE, Land CE, Dores GM. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:2899-2906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology & Genetics. Head and Neck Tumours. International Agency for Research on Cancer (IARC).; 2005. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Pathology-And-Genetics-Of-Head-And-Neck-Tumours-2005. |
| 4. | Stewart FW, Foote FW, Becker WF. Muco-Epidermoid Tumors of Salivary Glands. Ann Surg. 1945;122:820-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 340] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 328] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Healey WV, Perzin KH, Smith L. Mucoepidermoid carcinoma of salivary gland origin. Classification, clinical-pathologic correlation, and results of treatment. Cancer. 1970;26:368-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Park G, Lee SW. Postoperative radiotherapy for mucoepidermoid carcinoma of the major salivary glands: long-term results of a single-institution experience. Radiat Oncol J. 2018;36:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Chen AM, Lau VH, Farwell DG, Luu Q, Donald PJ. Mucoepidermoid carcinoma of the parotid gland treated by surgery and postoperative radiation therapy: clinicopathologic correlates of outcome. Laryngoscope. 2013;123:3049-3055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Chen MM, Roman SA, Sosa JA, Judson BL. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head Neck. 2014;36:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Hicks MJ, el-Naggar AK, Byers RM, Flaitz CM, Luna MA, Batsakis JG. Prognostic factors in mucoepidermoid carcinomas of major salivary glands: a clinicopathologic and flow cytometric study. Eur J Cancer B Oral Oncol. 1994;30B:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Spiro RH, Huvos AG, Berk R, Strong EW. Mucoepidermoid carcinoma of salivary gland origin. A clinicopathologic study of 367 cases. Am J Surg. 1978;136:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 189] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | McHugh CH, Roberts DB, El-Naggar AK, Hanna EY, Garden AS, Kies MS, Weber RS, Kupferman ME. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer. 2012;118:3928-3936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Rajasekaran K, Stubbs V, Chen J, Yalamanchi P, Cannady S, Brant J, Newman J. Mucoepidermoid carcinoma of the parotid gland: A National Cancer Database study. Am J Otolaryngol. 2018;39:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Liu S, Ow A, Ruan M, Yang W, Zhang C, Wang L, Zhang C. Prognostic factors in primary salivary gland mucoepidermoid carcinoma: an analysis of 376 cases in an Eastern Chinese population. Int J Oral Maxillofac Surg. 2014;43:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Chen AM, Granchi PJ, Garcia J, Bucci MK, Fu KK, Eisele DW. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 2007;67:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Sawabe M, Ito H, Takahara T, Oze I, Kawakita D, Yatabe Y, Hasegawa Y, Murakami S, Matsuo K. Heterogeneous impact of smoking on major salivary gland cancer according to histopathological subtype: A case-control study. Cancer. 2018;124:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Saku T, Hayashi Y, Takahara O, Matsuura H, Tokunaga M, Tokunaga M, Tokuoka S, Soda M, Mabuchi K, Land CE. Salivary gland tumors among atomic bomb survivors, 1950-1987. Cancer. 1997;79:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Whatley WS, Thompson JW, Rao B. Salivary gland tumors in survivors of childhood cancer. Otolaryngol Head Neck Surg. 2006;134:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Kaur J, Goyal S, Muzumder S, Bhasker S, Mohanti BK, Rath GK. Outcome of surgery and post-operative radiotherapy for major salivary gland carcinoma: ten year experience from a single institute. Asian Pac J Cancer Prev. 2014;15:8259-8263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Zenga J, Yu Z, Parikh A, Chen JX, Lin DT, Emerick KS, Faquin WC, Varvares MA, Deschler DG. Mucoepidermoid Carcinoma of the Parotid: Very Close Margins and Adjuvant Radiotherapy. ORL J Otorhinolaryngol Relat Spec. 2019;81:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Hosokawa Y, Shirato H, Kagei K, Hashimoto S, Nishioka T, Tei K, Ono M, Ohmori K, Kaneko M, Miyasaka K, Nakamura M. Role of radiotherapy for mucoepidermoid carcinoma of salivary gland. Oral Oncol. 1999;35:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Rapidis AD, Givalos N, Gakiopoulou H, Stavrianos SD, Faratzis G, Lagogiannis GA, Katsilieris I, Patsouris E. Mucoepidermoid carcinoma of the salivary glands. Review of the literature and clinicopathological analysis of 18 patients. Oral Oncol. 2007;43:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Garden AS, el-Naggar AK, Morrison WH, Callender DL, Ang KK, Peters LJ. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys. 1997;37:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Kaszuba SM, Zafereo ME, Rosenthal DI, El-Naggar AK, Weber RS. Effect of initial treatment on disease outcome for patients with submandibular gland carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Aro K, Leivo I, Mäkitie AA. Management and outcome of patients with mucoepidermoid carcinoma of major salivary gland origin: a single institution's 30-year experience. Laryngoscope. 2008;118:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Nance MA, Seethala RR, Wang Y, Chiosea SI, Myers EN, Johnson JT, Lai SY. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer. 2008;113:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Pires FR, de Almeida OP, de Araújo VC, Kowalski LP. Prognostic factors in head and neck mucoepidermoid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Cheung MC, Franzmann E, Sola JE, Pincus DJ, Koniaris LG. A comprehensive analysis of parotid and salivary gland cancer: worse outcomes for male gender. J Surg Res. 2011;171:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer. 1992;69:2021-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol. 2009;3:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Katabi N, Ghossein R, Ali S, Dogan S, Klimstra D, Ganly I. Prognostic features in mucoepidermoid carcinoma of major salivary glands with emphasis on tumour histologic grading. Histopathology. 2014;65:793-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Batsakis JG, Luna MA. Histopathologic grading of salivary gland neoplasms: I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol. 1990;99:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 109] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Qannam A, Bello IO. Comparison of histological grading methods in mucoepidermoid carcinoma of minor salivary glands. Indian J Pathol Microbiol. 2016;59:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Spiro RH, Hajdu SI, Strong EW. Tumors of the submaxillary gland. Am J Surg. 1976;132:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Spiro RH, Huvos AG, Strong EW. Cancer of the parotid gland. A clinicopathologic study of 288 primary cases. Am J Surg. 1975;130:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 182] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Mahmood U, Koshy M, Goloubeva O, Suntharalingam M. Adjuvant radiation therapy for high-grade and/or locally advanced major salivary gland tumors. Arch Otolaryngol Head Neck Surg. 2011;137:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol. 2006;13:293-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Ferrell JK, Mace JC, Clayburgh D. Contemporary treatment patterns and outcomes of salivary gland carcinoma: a National Cancer Database review. Eur Arch Otorhinolaryngol. 2019;276:1135-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Hong TS, Ritter MA, Tomé WA, Harari PM. Intensity-modulated radiation therapy: emerging cancer treatment technology. Br J Cancer. 2005;92:1819-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zahran M S-Editor: Fan JR L-Editor: A P-Editor: Zhang YL