Published online Jan 24, 2020. doi: 10.5306/wjco.v11.i1.31
Peer-review started: March 26, 2019
First decision: September 2, 2019
Revised: October 23, 2019
Accepted: November 6, 2019
Article in press: November 6, 2019
Published online: January 24, 2020
Processing time: 278 Days and 3.6 Hours
Ameloblastomas are common benign epithelial odontogenic neoplasms that present an aggressive and unpredictable behavior that may modify treatment strategies. Different signaling pathways that participate in the progression of these tumors have been identified. B-raf proto-oncogene serine/threonine kinase (BRAF) is a protein involved in the behavior of ameloblastomas, and it is related to many cell mechanisms. BRAF gene mutations have been identified in ameloblastomas, of which the BRAF V600E (valine substituted by glutamic acid at amino acid 600) mutation has been the most common and can be present concomitantly with other mutations that may be involved in its behavior. Targeted therapies have been used as an alternative in the case of resistance or contraindications to conventional treatments.
To document the presence of BRAF V600E and additional mutations, their behavior, and targeted therapies in these tumors.
An electronic literature search was conducted according to PRISMA guidelines in PubMed/MEDLINE, Cochrane, EMBASE, and SpringerLink using the terms “ameloblastomas”, “BRAF V600E”, “additional mutations”, and “targeted therapies”. Ameloblastomas were classified according to WHO guidelines. Inclusion criteria were articles in English, published not more than 10 years ago, and studies with laboratory works related to BRAF V600E. Articles were evaluated by two independent reviewers and retrieved for full-text evaluation. The EBLIP Critical Appraisal Checklist was used to evaluate the quality of the eligible studies. Descriptive statistical analysis was performed.
Two independent reviewers, with a substantial concordance indicated by a kappa coefficient of k = 0.76, evaluated a total of 19 articles that were included in this study. The analysis registered 521 conventional ameloblastomas (AM), 81 unicystic ameloblastomas (UA), 13 ameloblastic carcinomas (AC), three metastatic ameloblastomas (MA), and six peripheral ameloblastomas (PA), of which the histopathological type, anatomic location, laboratory tests, expression of BRAF mutation, and additional mutations were registered. The BRAF V600E mutation was found in 297 AM (57%), 63 UA (77.7%), 3 AC (23%), 1 MA (50%), and 5 PA (83.3%). Follicular type predominated with a total of 116 cases (40%), followed by plexiform type with 63 cases (22.1%). Furthermore, both types presented additional mutations, in which alterations in JAK3 P132T, SMARCB1, PIK3CA, CTNNB1, SMO, and BRAF G606E genes were found. Four case reports were found with targeted therapy to BRAF V600E.
The identification of BRAF V600E and additional mutations as an aid in targeted therapies has been a breakthrough in alternative treatments of ameloblastomas where surgical treatments are contraindicated.
Core tip: Ameloblastoma is a common neoplasia that is developed from odontogenic epithelium. It is an aggressive and recurrent tumor that can present metastases or malignant transformation. This neoplasia is characterized by presenting different clinical and histological varieties as well as several mutations related to its behavior. Nowadays, there are several studies focused on targeted therapies against the mutations of this tumor, one of the most frequent ones being B-raf proto-oncogene serine/threonine kinase (BRAF) V600E, the treatment of which has been associated with good response. These targeted therapies are suitable for resistant tumors. This study focused on BRAF V600E mutations and its additional mutations and targeted therapies.
- Citation: González-González R, López-Verdín S, Lavalle-Carrasco J, Molina-Frechero N, Isiordia-Espinoza M, Carreón-Burciaga RG, Bologna-Molina R. Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review. World J Clin Oncol 2020; 11(1): 31-42
- URL: https://www.wjgnet.com/2218-4333/full/v11/i1/31.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i1.31
Ameloblastoma is defined by WHO as a benign epithelial odontogenic intraosseous neoplasia, which is characterized by expansion and a tendency for local recurrence[1]. It is an aggressive neoplasia formed by odontogenic epithelium with mature fibrous stroma and odontogenic ectomesenchyme[2]. Histologically, it is characterized by cyst like formations and tumor nests that remind the epithelial component of the enamel organ, as well as by the anastomosis of strands of odontogenic epithelium limited by columnar cells that lack of morphological pattern of reticulum stellate-like cells[1-3]. Its underlaying origins are epithelial rests of Malassez, remnants of Hertwig’s epithelial sheath, and dental lamina[3]. It is the most common odontogenic epithelial neoplasia, with severe clinical implications, capacity of malignant transformation, and metastases[2]. Ameloblastoma global incidence is 0.5 cases per million population, being more frequent in China and Africa, where it comprises 14% of all tumor and cystic lesions that appear in the maxilla and mandible[2]. The first therapeutic choice is total surgical resection, by which most of the cases achieve the total elimination of neoplasia[4]. In the appearance of recurrent ameloblastoma, either metastatic ameloblastoma (MA) or ameloblastic carcinoma (AC), adjuvant therapies are implemented, and they consist of radiotherapy (RT) and chemotherapy (CT), which are controversial in their use due to a high recurrence rate and unpredictable results when they are used as a first-line and sole therapy without surgical treatment[4,5]. Due to these facts, new strategies of targeted therapies have been implemented in order to knock down the signaling pathways that participate in the development of this neoplasia, highlighting the MAPK signaling pathway with its predominant B-raf proto-oncogene serine/threonine kinase (BRAF) V600E mutation and the presence of additional mutations[3]. Thus, the aim of this study was to produce a systematic review in which current concepts and advances in targeted therapies regarding the BRAF V600E mutation in ameloblastomas were evaluated.
This study was developed according to the criteria established in the guidelines recommended by Preferred Reporting Items for Systematic Reviews and Meta-analyses. The electronic search was conducted in PubMed/MEDLINE, Cochrane, EMBASE and SpringerLink. The employed keywords were the following, including abbreviations: Ameloblastoma mutations, BRAF, BRAF V600E, PIK3CA, JAK P132T, SMARCB1, SMO and associated mutations to BRAF and BRAF V600E, and treatments in ameloblastoma. Ameloblastomas were defined according to their variants: Ameloblastoma (AM) (follicular and plexiform), unicystic ameloblastoma (UA) (luminal/intraluminal and mural), MA, AC and peripheral ameloblastoma (PA). The Booleans and/or were employed in order to search for the following terms: “Ameloblastoma” and/or “targeted therapies” and/or “BRAF” and/or “associated mutations”, and/or “BRAF V600E”. The same strategy was employed for AM, UA, MA, AC and PA. After the screening of titles and abstracts, the studies with useful information were retrieved for full text evaluation. Open access and restricted access articles were reviewed and were retrieved by institutional support. All AM, UA, MA, AC and PA were classified according to WHO[1]. Different classifications were adapted.
Two independent evaluators reviewed the selected articles, which were considered eligible when they fulfilled all of the following criteria: (1) Studies whose content was clearly associated with targeted therapies against BRAF V600E mutation in AM, UA, MA, AC, or PA; (2) Articles that were published not more than 10 years before this study was conducted (January 1st 2009–2019); (3) Articles in the English language; (4) Articles with positive cases corroborated by molecular and immunohistochemical (IHC) techniques for the study of BRAF mutations and additional mutations; and (5) Articles included in PubMed/MEDLINE, Cochrane, EMBASE, and SpringerLink. Different types of studies were considered, such as systematic reviews, meta-analyses, and molecular studies whose objective was to evaluate targeted therapies in ameloblastomas.
Exclusion criteria were the following: (1) Review articles, book chapters, systematic reviews, meta-analyses, and molecular studies in a language other than English; (2) Articles which were published more than 10 years before the established date; (3) Studies of targeted therapies not directly related to the established signaling pathways; and (4) Articles that study isolated mutations which are not BRAF V00E-related.
The quality of the eligible studies was evaluated by two independent reviewers using the EBLIP Critical Appraisal Checklist[6]. Disagreements between reviewers were resolved by discussion. Both independent reviewers extracted the data required for this study from each article and finally evaluated and discussed the data to achieve concordance. All extracted data were registered in a table to eliminate possible mistakes.
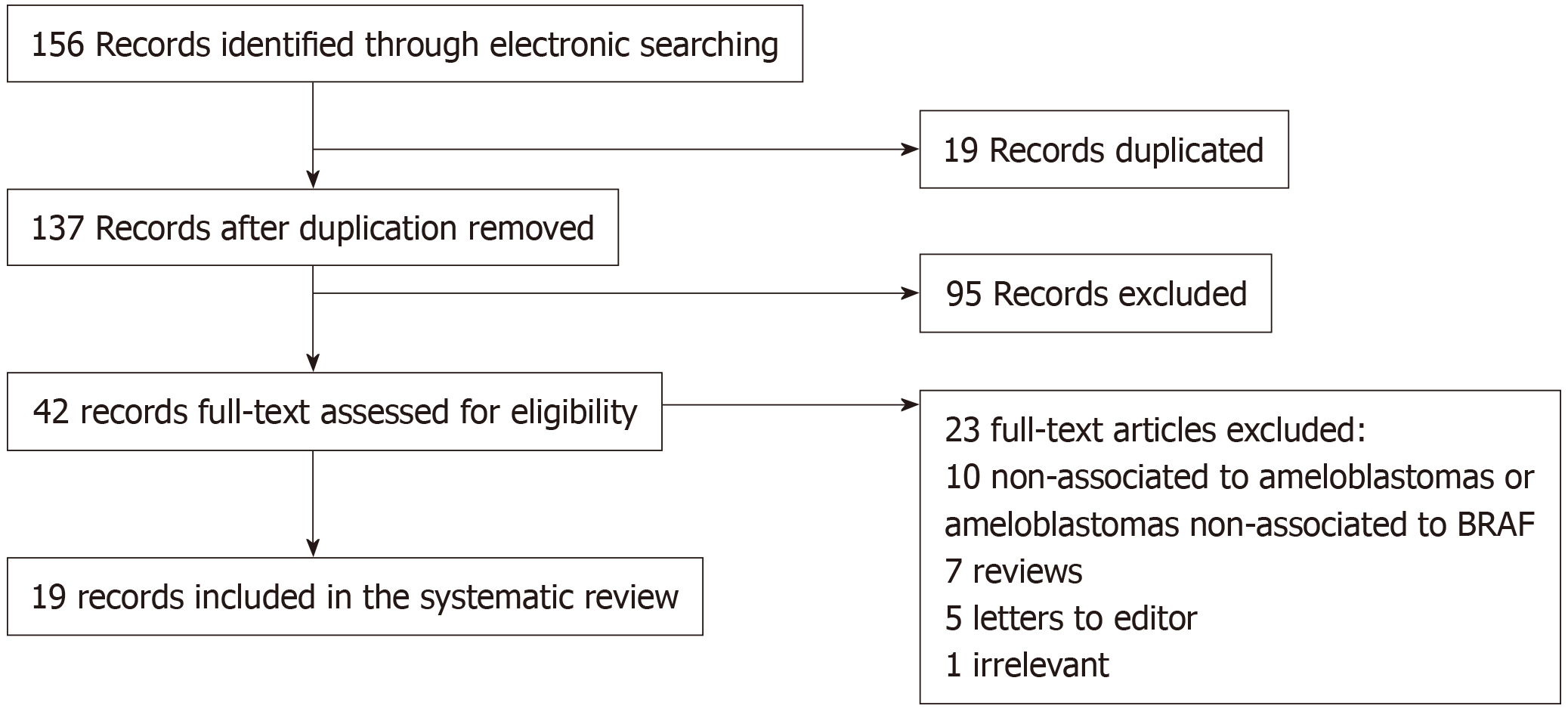
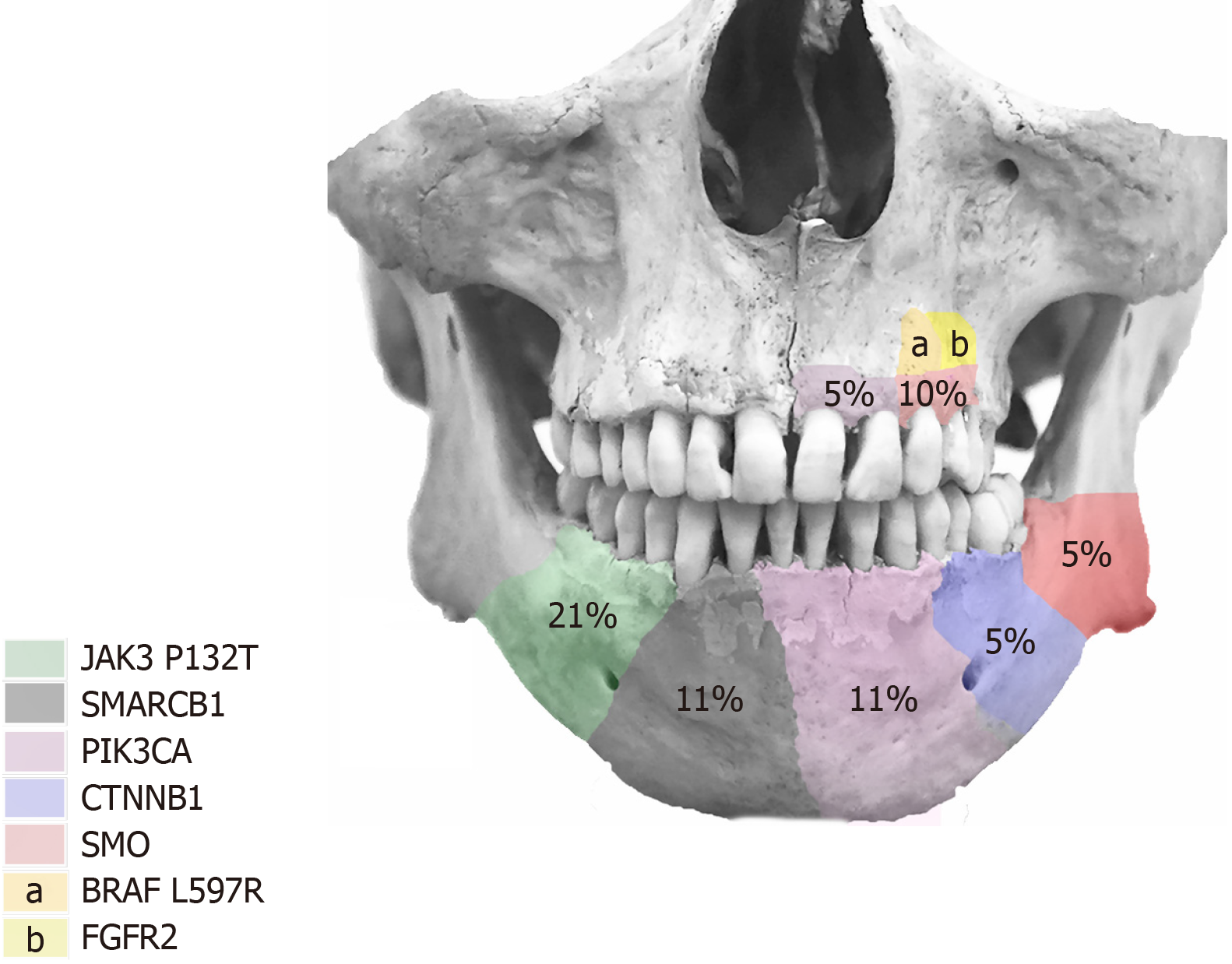
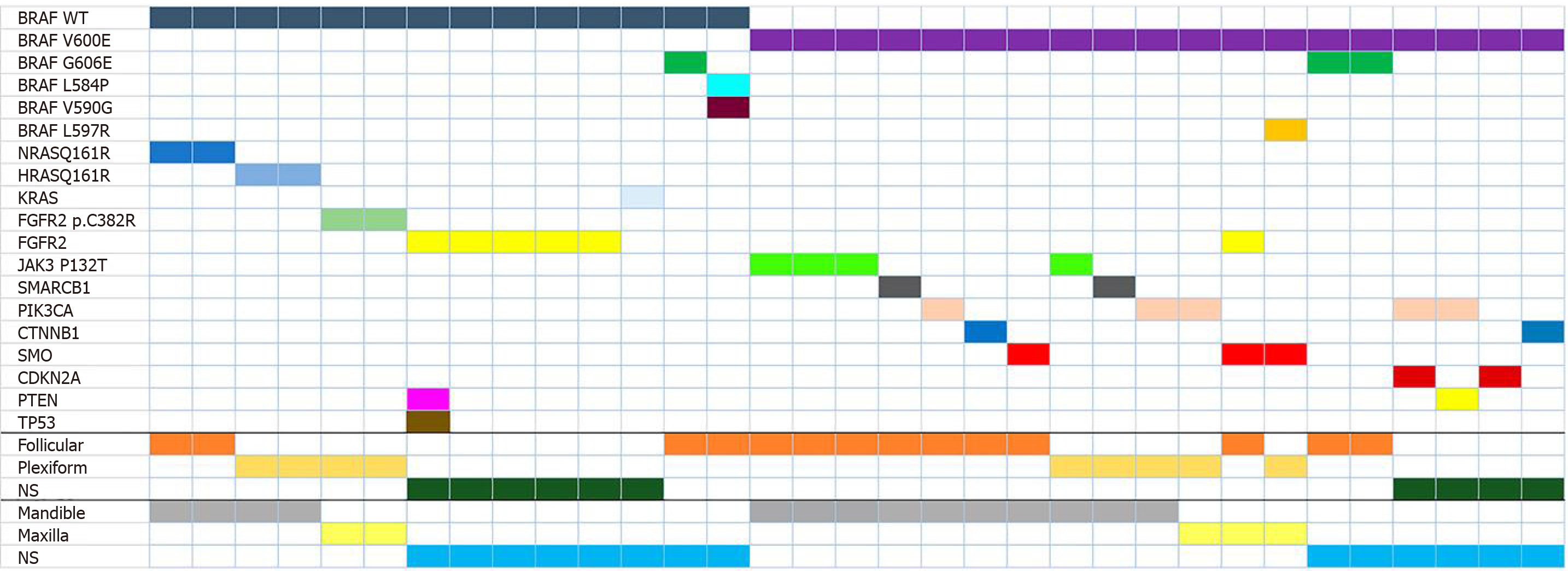
The literature search recorded a total of 156 articles. After the evaluation by two independent reviewers, with a substantial concordance indicated by a kappa coefficient of k = 0.76, 19 articles, which fulfilled the inclusion criteria, were included in this study. The other 137 articles were excluded as they did not accomplish the inclusion criteria. Figure 1 summarizes the selection of the articles that were considered in the elaboration of this systematic review. The reviewed articles registered 521 AM, 81 UA, 13 AC, 3 MA, and 6 PA, of which the histopathological type, anatomic location, laboratory tests, expression of BRAF mutation, and additional mutations were registered. A total of 39 AM, 10 UA, and 7 AC presented the expression of BRAF. For AM, follicular type was the most predominant type of this expression, with 15 out of 39 found cases. Follicular and plexiform types registered the highest quantity of additional mutations to BRAF expression, among which mutations of NRAS Q161R, HRAS Q161R, FGFR2, KRAS, and other variants of BRAF (G606E, L548P, V590G) were found to have mutated. Bartels et al[7] reported the unique case of additional mutations with BRAF expression in which FGFR2 presented concomitant mutation of PTEN and TP53. BRAF V600E mutation was found in 297 AM (57%), 63 UA (77.7%), 3 AC (23%), 1 MA (50%), and 5 PA (83.3%) cases. Follicular type predominated with a total of 116 cases (40%), followed by plexiform type with 63 (22.1%). Additionally, both types presented additional mutations, in which alterations in JAK3 P132T, SMARCB1, PIK3CA, CTNNB1, SMO, and BRAF G606E genes were found. Figure 2 describes the anatomic location of the additional mutations, and Figure 3 describes the relation between BRAF and BRAF V600E expression with additional mutation. The complete collected data and results can be found in Table 1. Four cases of targeted therapy of ameloblastomas with the presence of BRAF V600E were found. Reported cases with targeted therapies are described in Table 2.
| BRAF type | Ameloblastoma | Histological pattern | Mutations | Additional mutation | Anatomic location | ||
| Type | Cases with expression | Total cases | Histological pattern (cases) | Gene (cases/total) | Gene (cases) | Site (cases) | |
| BRAF WT | Ameloblastoma | 39 | 521 | Follicular (15) | NRAS Q161R (2/15) | Mandible (9) | |
| Maxilla (1) | |||||||
| G606E (1/15) | NS (5) | ||||||
| L584P, V590G (1/15) | |||||||
| Plexiform (14) | HRASQ161R (2/14) | Mandible (10) | |||||
| FGFR2 p.C382R (2/14) | Maxilla (1) | ||||||
| NS (3) | |||||||
| Mixed (4) | Mandible (4) | ||||||
| NS (6) | FGFR2 (4/12) | PTEN, TP53 (1) | NS (6) | ||||
| KRAS (1/12) | |||||||
| Unicystic ameloblastoma | 10 | 81 | Luminal/intraluminal (6) | Mandible (4) | |||
| Maxilla (2) | |||||||
| NS (4) | Mandible (1) | ||||||
| NS (3) | |||||||
| Ameloblastic carcinoma | 7 | 13 | Without cell variant (5) | Mandible (3) | |||
| Maxilla (2) | |||||||
| NS (2) | NS (2) | ||||||
| Metastasizing ameloblastoma | 0 | 2 | |||||
| BRAF V600E | Ameloblastoma | 297 | 521 | Follicular (121) | Mandible (91) | ||
| JAK3 P132T (3/121) | |||||||
| SMARCB1 (1/121 | |||||||
| PIK3CA (1/121) | |||||||
| CTNNB1 (1/121) | |||||||
| SMO (1/121) | |||||||
| SMO (1/121) | FGFR2 (1) | Maxilla (4) | |||||
| Left frontal bone (1) | |||||||
| G606E (2/121) | NS (25) | ||||||
| Plexiform (65) | JAK3 P132T (1/65) | Mandible (38) | |||||
| SMARCB1 (1/65) | |||||||
| PIK3CA (1/65) | |||||||
| PIK3CA (1/65) | Maxilla (2) | ||||||
| BRAF 597R (1/65) | SMO (1) | ||||||
| NS (25) | |||||||
| No follicular (20) | Mandíbula (20) | ||||||
| No plexiform (15) | NS (15) | ||||||
| Granular-cell (4) | Mandible (4) | ||||||
| Mixted (10) | Mandible (4) | ||||||
| NS (6) | |||||||
| Without cell variant (1) | Mandible (1) | ||||||
| Desmoplastic (2) | Mandible (1) | ||||||
| Maxilla (1) | |||||||
| NS (59) | FGF21 (23) | Mandible (4) | |||||
| FGFR11 (24) | Maxilla (1) | ||||||
| NS (45) | |||||||
| Cavernus sinus (1) | |||||||
| Coronoid process (1) | |||||||
| Unicistyc ameloblastoma | 63 | 81 | Luminal/Intraluminal (21) | Mandible (19) | |||
| Maxilla (2) | |||||||
| Mural (20) | Mandible (20) | ||||||
| NS (22) | Mandible (12) | ||||||
| Maxilla (1) | |||||||
| NS (9) | |||||||
| Peripheral ameloblastoma | 5 | 6 | NS (5) | NS (5) | |||
| Ameloblastic carcinoma | 3 | 13 | Without cell variant (3) | Mandible (3) | |||
| Metastasizing | 1 | 2 | Follicular (1) | Mandible (1) | |||
| NS | Acanthomatous (7) | Mandible (7) | |||||
| Granular cells (2) | Mandible (2) | ||||||
| NS (2) | PIK3CA, CDKN2A (1) | Mandible (1) | |||||
| PIK3CA, PTEN (1) | Mandible (1) | ||||||
| NS (2) | CDKN2A (1) | Mandible (1) | |||||
| CTNNB1 (1) | Mandible (1) | ||||||
| No mutations | Metastasizing Ameloblastoma | 1 | 2 | Plexiform (1) | Infratemporal fosa (1) | ||
| Ameloblastic Carcinoma | 3 | 13 | NS (3) | NS (3) | |||
| Unicistyc Ameloblastoma | 1 | 81 | NS (1) | ||||
| Ref. | Age | Evolu-tion tumor (yr) | Gender | Tumor | Locali-zation | Primary tumor/ recur-rent | Pre-vious treat-ments | Size | Course of the disea-ses | Muta-tion detected | Treat-ment | Evolu-tion | Follow-up |
| Kaye et al[27] | 40 | 30 | M | MA | Left jaw, bilateral neck mass, pulmo-nary metas-tases | Recurrent | Surgical resection and radiot-herapy | NS | Three recur-rences (13, 9, 7 yr), surgical resection (two recur-rences), RT (last recurrence), neck bilateral and lung metas-tasis. (imaging diagnosis CT) | BRAF V600E (gene profile and IHC) | BRAF/MEK inhibition (Dabra-fenib 150 mg twice daily + Trame-tinib 2 mg once daily) | Decrease of the tumor size and metas-tases in the first four days | 20 wk (tumor response to treatment, without toxicity) |
| Faden et al[26] | 83 | 16 | F | AM | Right jaw | Recurrent | Conser-vative surgery | 3.79 cm × 5.87 cm × 5.62 cm | Two recur-rences, difficult to nutrition, not suitable for surgery | BRAF V600E | Dabra-fenib 75 mg twice daily | Decrease of the tumor size | 12 wk (tumor response to treatment) |
| Fernandes et al[29] | 29 | 12 | F | AM | Ramus and left jaw | Recurrent | Surgical resection and radiot-herapy | 1.3 cm × 0.9 cm (residual tumor) | Tumor recur-rence by conser-vative surgery for 16 yr, metas-tases in cavernous sinus and tumorl extention to orbit | BRAF V600E (PCR allele-specific) | Vemurafenib 960 PO twice daily and analgesic treatment | Decrease of the tumor size with sympto-matology (anorexy, nausea and fatigue) | 11 wk, asympto=matic with treatment tolerance |
| Tan et al[28] | 85 | NS | M | AM | Left jaw | Primary | Surgical resection | 4 cm (CT Scan) | Tumor recur-rence with patho-logical fracture | BRAF V600E (PCR allele-specific) | Dabrafenib 150 mg PO twice daily | Decrea-sed of tumor size with develo-pment of actinic keratosis on face, back and scalp and thic-kening voice | 16 wk, notable decrease of tumor size |
BRAF is a protein that leads to different cell mechanisms, such as metabolism and proliferation. There are different signaling pathways that are activated in these mechanisms in an extracellular and intracellular manner.
More than 40 mutations of BRAF were identified in different neoplasms. The most frequent ones are missense mutations of the 600 residues of the BRAF gene, in which valine is replaced by glutamine (V600E) and results in the constitutive activation of MEK/ERK signaling in tumors[8,9]. The BRAF V600E mutation has been employed as a predictive, diagnostic, and prognostic biomarker in different tumors. In ameloblastomas, the presence of this mutation was first described by Brown et al[10] and Kurppa et al[11], who indicated its influence on the resistance to the targeted therapy of EGF receptors. Furthermore, Jhamb et al[8] described the relation between BRAF and the RAS/MAPK pathway in the pathogenesis of AM. Since these findings, several studies focusing on targeted therapies against BRAF mutations have been developed. Sweeney et al[12] found the BRAF V600E mutation in 46% of the analyzed AM. Moreover, they reported other variants of the BRAF mutation (L597R), which results in the substitution of leucine residue by arginine in the 597 BRAF position by an increment of kinase activity[13] and associations with SMO and FGFR2, found in the maxilla. SMO is of interest as its mutations are frequent in ameloblastomas of the maxilla and is, unlike BRAF, frequently not associated with other mutations. It is important to highlight that in this review different mutational variants associated with BRAF V600E (G606E and L597R) were found in follicular and plexiform AM, respectively. Although the functional implication of these variants regarding treatment has not been studied[14], and there are few reports of these mutations in ameloblastomas. Further studies may possibly establish the relation between these variants and their histological pathways, tumor behaviors, and treatment resistance. Based on these findings, it is possible that additional mutations of BRAF V600E are mainly associated with the histological pathway of AM. In the present study, more than 500 cases of AM were analyzed, including those of the Sweeney et al[12] study, in which more than 50% of the total cases presented the BRAF V600E mutation. Additionally, studies on the detection of BRAF with IHC have been done using BRAF antibody clone VE-1, which shows a high specificity and sensitivity in cells that express this mutation[15]. This expression was associated with recurrence, osseous disruptions, and multilocular radiographic pathways[16], and although these variables are not evaluated in this review, it is important to indicate that the presence of the BRAF mutation is related to clinical behavior[10,12,16]. The presence of BRAF V600E in AM is commonly associated with other mutations and can frequently be present in younger patients[2,10,17,18].
In the analysis of UA, no additional mutations to BRAF V600E were reported. This difference in UA may be related to: (1) BRAF expression being presented earlier in tumorigenesis of ameloblastomas, and through tumor evolution this acquires mutations additional to BRAF V600E, or (2) UA may be a prior neoplasia of AM, in which mutated BRAF is present from the beginning of the pathology. Another important fact is that BRAF V600E expression is found in the same proportion in luminal/intraluminal and mural types, which may indicate that this mutation is not entirely associated with tumor behavior. This assumption may be supported by the data found in AM, as most ameloblastomas with aggressive behavior present an association between BRAF V600E and other mutations, and it is possible that these additional mutations are related to behavior.
AC is classified as aggressive ameloblastic tumor with a tendency to metastasis, mainly to the lung, with a median survival rate that varies between 5 and 17.6 years according to global surveillance and those from the maxilla being more aggressive. BRAF mutations have been described the same as for AM[1].
In this analysis, a total of 13 AC cases were registered, of which 23% (7) presented the BRAF V600E mutation, which was the opposite to UA, where a great number of cases were registered with this mutation. Additionally, none of the cases presented any additional mutations, unlike AM, which presented a higher number of secondary mutations in the cases with BRAF V600E. These results may be associated mainly with the lack of cases reporting AC with BRAF analysis, which suggest that in further studies the presence of BRAF should be evaluated in order to establish a treatment strategy for more aggressive ameloblastomas, which may possibly lead to the discovery of new additional mutations.
Six cases of PA were registered, of which 83.3% (5) presented the BRAF V600E mutation, and one being an unspecified case. These ameloblastomas have a similar histological pathway as their opposite, the intraosseous type, which may support the expression of BRAF. However, the percentual difference is large (83.3% vs 57%) due to the low number of reported cases. However, because of their location, these ameloblastomas are not aggressive in comparison with their opposite, the intraosseous type.
MA is an ameloblastoma that develops metastases despite its benign appearance. Like most ameloblastomas, MA presents a higher predilection of metastasis to the mandible, and its metastatic nests are commonly found in the lung, followed by the lymph nodes. This neoplasia is usually of long latency before developing metastases and can be associated with repeated recurrences after surgical treatment. This is an uncommon entity and its outcome depends on the metastatic nests and surgical viability[1]. In this analysis, MA corresponds to less than 1% of the total cases registered, which indicates that it is an uncommon tumor and consequently poorly studied. Two of the three registered cases presented BRAF V600E (66.6%) without additional mutations. This allows us to conclude that secondary mutations of BRAF V600E are possibly exclusive to AM.
Overall, ameloblastomas have a predilection for the mandible, mainly located in the body, ramus, and symphysis[1,2,17]. Registered data showed that BRAF V600E mutations were more frequent in the mandible with an approximate ratio of 21:1, although this data is not precise as 79 (24.6%) cases with the mutation were registered without the specific anatomic location.
In this analysis, BRAF V600E cases without additional mutations corresponded to 93.7%, compared with those that presented multiple mutations at 6.23%. This explains why treatment based on the knockdown of BRAF V600E may be successful. Overall, in mandible 4.3% (10/231) of the registered cases presented multiple mutations, whereas in maxilla the percentage was higher (27.7%). This could be an interesting finding, as it is possible that targeted therapies to BRAF are more successful in mandibular tumors, whereas in the maxilla, combined therapies should be considered as the more frequently registered secondary mutation was SMO[19]. Additionally, SMO mutations can be isolated, which may modify the treatment strategy. This result is interesting as the recurrence risk is related to the mutational status and tumors with multiples mutations are associated with high recurrences[19]. Although there are few additional mutations of BRAF V600E in ameloblastomas, these were more frequently present in the mandible, and it is possible that these mutations are exclusive to AM. Additional mutations that were frequently registered in this review were JAK P132T, SMO, SMARCB1, PIK3CA, and CTNNB1 in the mandible and BRAF L597R, FGFR2, PIK3CA, and SMO in the maxilla. Other secondary mutations with unspecified anatomic location were BRAF G606E, CTNNB1, CDKN2A, and PTEN.
To date, there are no references to the association between JAK3 P132T presence and ameloblastomas. However, this gene has been studied in some neoplasia, such as head and neck squamous cell carcinoma (HNSCC), with a reported relation to racial predisposition and megakaryocytic leukemias related to leukemogenesis[20,21].
SMO is a protein whose upregulation is related to signaling of the Sonic Hedgehog (SHH) pathway participating in the pathogenesis of several malignant neoplasia[22]. However, the regulation of SMO in ameloblastomas is not well characterized, but it has been suggested that overexpression can lead to a constitutive activation of the SHH signaling pathway and finally contribute to cell survival and proliferation. The results observed in our analysis detected concomitant mutations of BRAF V600E with SMO and a higher predilection to the maxilla; thus, it is considered that this neoplasia may have a more aggressive behavior due the participation of SMO in the SHH signaling pathway and its relation to BRAF V600E. It is interesting that the presence of the isolated SMO mutation in maxillary ameloblastomas has been pointed out. This finding may be important, as it is possible that treatment of maxillary ameloblastomas should mainly focus on targeted therapies against SMO, and as this is present in the SHH signaling pathway, maxillary tumors may be more aggressive.
SMARCB1 is a gene that codifies for the SWI/SNF complex, which plays an important role in the regulation of transcriptional mechanisms. Its main function is the regulation of the cell cycle trough pRb and HDAC1. Furthermore, this gene is involved in the SWI/SNF complex that suppresses E2F activity knocking down the phase S. It is normally expressed in all the tissues. SMARCB1 alterations have been associated with rhabdoid malignant neoplasia as well as benign lesions such as schwannomatosis[23]. Of all the cases, 11% of additional mutations of BRAF V600E were associated with SMARCB1. Although the relation of this gene to ameloblastomas behavior has not been established.
PIK3CA is an important regulator of the signaling of the tyrosine kinase receptors that are crucial in the proliferation, growth, and differentiation mechanisms. It is one of the most frequently mutated oncogenes and is observed in different neoplasia[24]. The alterations in this gene have been observed in 10%–33% of HNSCC, whose amplification is related to a low expression of PTEN[24]. The findings in this study indicate the presence of the PIK3CA mutation in 11% of ameloblastomas with BRAF V600E, which may indicate that ameloblastomas with these mutations are more susceptible to recurrence.
Although a direct association between PIK3CA with recurrence was not found in this analysis, recurrence was involved in multiple mutations associated with BRAF V600E[19]. Thus, ameloblastomas related to BRAF V600E associated with multiple mutations are tumors that can acquire several characteristics from the additional mutated genes.
In vitro studies have been developed in order to evaluate the therapeutic use of treatments against the BRAF V600E mutation. Brown et al[10] evaluated the in vitro effects of vemurafenib in the activation of the MAPK signaling pathway and the proliferation in the AM-1 cell line. Their results demonstrated the knock-down of cell proliferation and indicated that vemurafenib treatment is a potential alternative in ameloblastomas whose surgical treatment is related with significant facial deformities and frequent recurrences. Furthermore, vemurafenib is recommended in MA, local aggressive tumors, and non-candidate patients as an alternative to surgical treatment[10]. Thus, patients who present BRAF V600E mutation are eligible for targeted therapies, using combined treatments related to knocking down BRAF-related signaling pathways. A great example is combined therapy with vemurafenib (BRAF inhibitor) and trametinib (MEK inhibitor) of which some reported cases have shown excellent results, especially in MA[10,24].
Although there are no reports of serial cases that indicate the success of targeted therapies in ameloblastomas, they have been used as adjuvant or neoadjuvant therapies in order to improve the outcome of treatments, to increase functional mobility, and to improve cosmetic results[25-27]. Thus, the use of targeted therapies has been limited as previously described.
This analysis demonstrates most of the molecular alterations of ameloblastomas and their relation to anatomic location and possible association with behavior. The identification of BRAF V600E and the additional mutations as an aid in targeted therapies has been a breakthrough in alternative treatments of ameloblastomas where surgical treatment is contraindicated. The analyzed studies evaluate several mutations and their possible association with the biology of these tumors. The findings are an important advancement in the study of ameloblastomas and alternative treatments, although the latter is limited to few case reports. Further studies are necessary in order to adequately determine the success of targeted therapies and resistance to treatment by the BRAF V600E mutation.
Ameloblastomas are benign tumors that arise from the odontogenic epithelium whose behavior is defined as aggressive, infiltrative, recurrent, with aesthetic implications and rarely propense to local and distant metastases. Recently B-raf proto-oncogene serine/threonine kinase (BRAF) V600E gene mutation has been reported in ameloblastomas. Thus, targeted therapies against this mutation have been evaluated as an alternative treatment. In this study, a systematic review was performed in order to evaluate the frequent mutation of BRAF and another associated mutations, as well as targeted therapies against them.
Performing a systematic review allows to know the reports of frequent mutations in ameloblastomas and alternative treatments against them, as well as evaluate therapeutic response.
The aim of this study was to evaluate the presence of BRAF V600E mutation and another related mutations in ameloblastomas and provide information about the role of the mutations in the behavior of ameloblastomas, as well as targeted therapies reported.
A literature research was carried out between January 1st 2009-2019 in order to perform a systematic review, of which 19 articles with relevant content regarding BRAF and its mutations in ameloblastomas were included, as well as targeted therapies against them.
A total of 624 ameloblastomas were evaluated, in which BRAF V600E was the most frequent mutation. Of the total of the included articles, four case reports registered targeted therapies against BRAF V600E.
In the current study, the most frequent mutation was BRAF V600E, which interestingly was frequently associated to other mutations that conferred more aggressiveness with recurrence and metastases. Regarding anatomic location, it is suggested that associated mutations to BRAF V600E are more common in the mandible. Targeted therapies against this mutation represented a significant outcome in patients that presented these types of tumors. Since this is the first systematic review developed about this subject, it could be suggested that the use of targeted therapies as adjuvant to surgical treatment may offer important outcome in the clinical evolution and the follow up, specially in recurrent, metastatic and malignant ameloblastic tumors.
The information obtained in this review demonstrates the current implementation of targeted therapies against BRAF V600E mutation in ameloblastic tumors.
| 1. | Vered M, Muller S, Heikinhemo K. Bening epithelial odontogenic tumors. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC), 2017: 215-218. |
| 2. | Effiom OA, Ogundana OM, Akinshipo AO, Akintoye SO. Ameloblastoma: current etiopathological concepts and management. Oral Dis. 2018;24:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | You Z, Liu SP, Du J, Wu YH, Zhang SZ. Advancements in MAPK signaling pathways and MAPK-targeted therapies for ameloblastoma: A review. J Oral Pathol Med. 2019;48:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Huang CM, Chen JY, Chen CH, Huang CJ. Radiotherapy for a repeatedly recurrent ameloblastoma with malignant transformation. Head Neck. 2014;36:E1-E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Amzerin M, Fadoukhair Z, Belbaraka R, Iraqui M, Boutayeb S, M'rabti H, Kebdani T, Hassouni K, Benjaafar N, El Gueddari BK, Errihani H. Metastatic ameloblastoma responding to combination chemotherapy: case report and review of the literature. J Med Case Rep. 2011;5:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Alcock L. A critical appraisal tool for library and information research. Library Hi Tech. 2016;24:387-99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Bartels S, Adisa A, Aladelusi T, Lemound J, Stucki-Koch A, Hussein S, Kreipe H, Hartmann C, Lehmann U, Hussein K. Molecular defects in BRAF wild-type ameloblastomas and craniopharyngiomas-differences in mutation profiles in epithelial-derived oropharyngeal neoplasms. Virchows Arch. 2018;472:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Jhamb T, Kramer JM. Molecular concepts in the pathogenesis of ameloblastoma: implications for therapeutics. Exp Mol Pathol. 2014;97:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (7)] |
| 10. | Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS, Elenitoba-Johnson KS, Betz BL. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20:5517-5526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Kurppa KJ, Catón J, Morgan PR, Ristimäki A, Ruhin B, Kellokoski J, Elenius K, Heikinheimo K. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol. 2014;232:492-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Sweeney RT, McClary AC, Myers BR, Biscocho J, Neahring L, Kwei KA, Qu K, Gong X, Ng T, Jones CD, Varma S, Odegaard JI, Sugiyama T, Koyota S, Rubin BP, Troxell ML, Pelham RJ, Zehnder JL, Beachy PA, Pollack JR, West RB. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet. 2014;46:722-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 13. | Lovly CM, William P, Sosman J. Vanderbilt-Ingram Cancer Center [cited 14 March 2019]. In: BRAF L597R. Available from: //www.mycancergenome.org/content/disease/melanoma/braf/138/. |
| 14. | Soltani M, Tabatabaiefar MA, Mohsenifar Z, Pourreza MR, Moridnia A, Shariati L, Razavi SM. Genetic study of the BRAF gene reveals new variants and high frequency of the V600E mutation among Iranian ameloblastoma patients. J Oral Pathol Med. 2018;47:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kwon JH, Jeong BK, Yoon YS, Yu CS, Kim J. Utility of BRAF VE1 Immunohistochemistry as a Screening Tool for Colorectal Cancer Harboring BRAF V600E Mutation. J Pathol Transl Med. 2018;52:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Fregnani ER, Perez DE, Paes de Almeida O, Fonseca FP, Soares FA, Castro-Junior G, Alves FA. BRAF-V600E expression correlates with ameloblastoma aggressiveness. Histopathology. 2017;70:473-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Heikinheimo K, Huhtala JM, Thiel A, Kurppa KJ, Heikinheimo H, Kovac M, Kragelund C, Warfvinge G, Dawson H, Elenius K, Ristimäki A, Baumhoer D, Morgan PR. The Mutational Profile of Unicystic Ameloblastoma. J Dent Res. 2019;98:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Pereira NB, Pereira KM, Coura BP, Diniz MG, de Castro WH, Gomes CC, Gomez RS. BRAFV600E mutation in the diagnosis of unicystic ameloblastoma. J Oral Pathol Med. 2016;45:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Gültekin SE, Aziz R, Heydt C, Sengüven B, Zöller J, Safi AF, Kreppel M, Buettner R. The landscape of genetic alterations in ameloblastomas relates to clinical features. Virchows Arch. 2018;472:807-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M, Goss VL, Lee KA, Eide CA, Wong MJ, Stoffregen EP, McGreevey L, Nardone J, Moore SA, Crispino J, Boggon TJ, Heinrich MC, Deininger MW, Polakiewicz RD, Gilliland DG, Druker BJ. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Guerrero-Preston R, Lawson F, Rodriguez-Torres S, Noordhuis MG, Pirini F, Manuel L, Valle BL, Hadar T, Rivera B, Folawiyo O, Baez A, Marchionni L, Koch WM, Westra WH, Kim YJ, Eshleman JR, Sidransky D. JAK3 Variant, Immune Signatures, DNA Methylation, and Social Determinants Linked to Survival Racial Disparities in Head and Neck Cancer Patients. Cancer Prev Res (Phila). 2019;12:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Gurgel CA, Buim ME, Carvalho KC, Sales CB, Reis MG, de Souza RO, de Faro Valverde L, de Azevedo RA, Dos Santos JN, Soares FA, Ramos EA. Transcriptional profiles of SHH pathway genes in keratocystic odontogenic tumor and ameloblastoma. J Oral Pathol Med. 2014;43:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Agaimy A. The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity. Adv Anat Pathol. 2014;21:394-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Al-Amri AM, Vatte C, Cyrus C, Chathoth S, Hashim TM, Mohamed YS, Al Ali R, Alsaid A, Al Ali A. Novel mutations of PIK3CA gene in head and neck squamous cell carcinoma. Cancer Biomark. 2016;16:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kreppel M, Zöller J. Ameloblastoma-Clinical, radiological, and therapeutic findings. Oral Dis. 2018;24:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Faden DL, Algazi A. Durable treatment of ameloblastoma with single agent BRAFi Re: Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Kaye FJ, Ivey AM, Drane WE, Mendenhall WM, Allan RW. Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J Natl Cancer Inst. 2015;107:378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Tan S, Pollack JR, Kaplan MJ, Colevas AD, West RB. BRAF inhibitor treatment of primary BRAF-mutant ameloblastoma with pathologic assessment of response. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:e5-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Fernandes GS, Girardi DM, Bernardes JPG, Fonseca FP, Fregnani ER. Clinical benefit and radiological response with BRAF inhibitor in a patient with recurrent ameloblastoma harboring V600E mutation. BMC Cancer. 2018;18:887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Uruguay
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin C, Kupeli S S-Editor: Zhang L L-Editor: A E-Editor: Qi LL