Published online Apr 24, 2019. doi: 10.5306/wjco.v10.i4.166
- This article has been corrected.
- See: World J Clin Oncol. Aug 24, 2025; 16(8): 107778
Peer-review started: December 3, 2018
First decision: December 30, 2018
Revised: January 25, 2019
Accepted: February 27, 2019
Article in press: February 28, 2019
Published online: April 24, 2019
Processing time: 145 Days and 7.1 Hours
Aberrant activation of phosphorylated form of glycogen synthase kinase-3β [pS9GSK-3β (Serine 9 phosphorylation)] is known to trigger Wnt/β-catenin signal cascade but its clinicohistopathological implications in bladder carcinogenesis remain unknown.
To investigate the diagnostic and prognostic relevance of expressions of pS9GSK-3β, β-catenin and its target genes in the pathobiology of bladder cancer.
Bladder tumor tissues from ninety patients were analyzed for quantitative expression and cellular localization of pS9GSK-3β by immunohistochemical (IHC) staining. Real time-quantitative polymerase chain reaction and IHC were done to check the expression of β-catenin, Cyclin D1, Snail and Slug at transcriptome and protein level respectively. Clinicohistopathological variables were obtained from histology reports, follow up and OPD visits of patients. Expressions of the markers were statistically correlated with these variables to determine their significance in clinical setting. Results were analysed using SPSS 20.0 software.
Aberrant (low or no membranous/high nuclear/high cytoplasmic) expression of pS9GSK-3β was noted in 51% patients and found to be significantly associated with tumor stage and tumor grade (P = 0.01 and 0.04; Mann Whitney U test). Thirty one percent tumors exhibited aberrant co-expression of pS9GSK-3β and β–catenin proteins and showed strong statistical association with tumor stage, tumor type, smoking/tobacco chewing status (P = 0.01, 0.02 and 0.04, Mann-Whitney U test) and shorter overall survival probabilities of patients (P = 0.02; Kaplan Meier test). Nuclear immunostaining of Cyclin D1 in tumors with altered pS9GSK-3β/β–catenin showed relevance with tumor stage, grade and type.
β–catenin and pS9GSK-3β proteins are identified as markers of diagnostic/prognostic significance in disease pathogenesis. Observed histopathological association of Cyclin D1 identifies it as marker of potential relevance in tumors with altered pS9GSK-3β/β-catenin.
Core tip: Aberrant co-expression of phosphorylated form of glycogen synthase kinase-3β (pS9GSK-3β) and β–catenin proteins and their association with tumor stage, tumor type, smoking/tobacco chewing status and shorter overall survival probabilities of urinary bladder cancer patients validate them as potential markers of diagnostic/prognostic significance in disease pathogenesis. Observed histopathological association of nuclear Cyclin D1 identifies it as a marker of potential relevance in tumors with altered pS9GSK-3β/β-catenin proteins. These results rule out the possible post translational activation of target genes examined, by pS9GSK-3β/β-catenin/T-cell factor/lymphoid enhancer factor transcriptional complex in bladder tumorigenesis.
- Citation: Maurya N, Singh R, Goel A, Singhai A, Singh UP, Agrawal V, Garg M. Clinicohistopathological implications of phosphoserine 9 glycogen synthase kinase-3β/ β-catenin in urinary bladder cancer patients. World J Clin Oncol 2019; 10(4): 166-182
- URL: https://www.wjgnet.com/2218-4333/full/v10/i4/166.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i4.166
Urothelial carcinoma of bladder (UCB) is the fourth most common cancer in men and it ranks ninth most frequent cancer in women worldwide[1]. Intravesical immuno or chemotherapy and surgical resections are commonly employed modalities to treat non-muscle invasive cancer (NMIBC) and locally advanced cancer. Nevertheless, these tumors are prone for relapse and disease progresses with 50% to 90% rate of recurrence. Twenty five percent of patients, who are initially presented as muscle invasive bladder cancer (MIBC), undergo either radical cystectomy or radio-chemotherapy but fail to achieve complete therapeutic response[2]. Owing to heterogeneous clinical outcome of two distinct types of diseases (NMIBC and MIBC); limitations of standard cytopathology for low grade urothelial malignancies; and lack of mechanism for detecting early recurrence in high risk NMIBC and progression in MIBC, intense investigation of tumors using molecular diagnostics is a favorable model for urologic oncologists.
Wnt/β-catenin signaling plays pivotal role in the development of urogenital systemand hence, its abnormal activation plays significant role in bladder cancer pathogenesis[3]. β-catenin is a multifunctional protein, exists at the cell membrane in a complex with E-cadherin and α-catenin and is involved in cell-cell adhesion; maintenance of epithelial cell integrity; normal cell growth/differentiation, embryonic development, wound healing and apoptosis[4]. In normal cells, cytoplasmic β-catenin is usually maintained at low level; constitutively captured in a destruction complex [composed of adenomatous polyposis coli (APC), glycogen synthase kinase-3β (GSK-3β) and Axin]; phosphorylated by GSK-3β and casein kinases 1α (CK1α); and degraded via the ubiquitin proteasome pathway[5].
GSK-3 is a multifunctional serine/threonine kinase and is one of the essential components of Wnt/β-catenin signal cascade[6]. It is involved in multicellular processes, including proliferation, differentiation, cell cycle regulation, glycogen metabolism, cell mobility and survival. Its two isoforms: (1) GSK-3α and (2) GSK-3β, exhibit 98% identity within their kinase domain, nevertheless, their functions are different[7]. Site-specific phosphorylations at tyrosine-216 (active form of GSK-3β) and at serine-9 (inactive form of GSK-3β) are responsible for enhanced and reduced kinase activity of GSK-3β respectively. This makes GSK-3β, an important molecule and allows it to function as either tumor promoter or tumor suppressor[8].
Phosphorylation of GSK-3β at serine 9 (pS9GSK-3β) attenuates its enzyme activity and its increased cytosolic levels prevent it from phosphorylating its downstream targets including β-catenin. This leads to stabilization and cytosolic accumulation of β-catenin[9]. Subsequently, it is shuttled to the nucleus, forms complexes with members of the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family of transcription factors, and thereby activates target genes including Cyclin D1, Snail and Slug. These genes are regulators of cell proliferation and are involved in tumorigenesis[10]. Cyclin D1 is an important regulator of cell cycle progression and contributes to the neoplastic transformation of cells[11]. Snail and Slug belong to Snail family of zinc-finger transcription factors, share highly conserved zinc finger domains and regulate the transcription and translation of molecules that orchestrate the process of epithelial-to-mesenchymal transition (EMT)[12,13]. EMT renders tumor cells to transiently adapt altered morphological traits with reduced requirements of cell-cell contact, immune evasion and allows tumor cells to undergo series of events characterized by metastatic cascade[14].
An aberrant activation of pS9GSK-3β has been reported to trigger on Wnt/β-catenin signal cascade and has been associated with cell proliferation, differentiation and increased aggressiveness in several major human cancers including pancreatic, colorectal, ovarian, prostate, cervical, breast and bladder cancer[15-18]. Nevertheless, functional implications of pS9GSK-3β in the pathogenesis/prognosis of bladder cancer remain unclear. The present study has been taken up to examine the quantitative expression and localization of pS9GSK-3β protein in urinary bladder tumors. Aberrant accumulation of stabilized β-catenin and transcriptional induction of target genes (Cyclin D1, Snail and Slug) via activated β-catenin signaling pathway were evaluated for their clinical significance in well-defined cases of bladder cancer.
Findings of the present study suggest that pS9GSK-3β may be involved in urothelial tumorigenesis by stabilizing/regulating β-catenin via Wnt/β-catenin signal cascade. Association of aberrantly co-expressed pS9GSK-3β and β-catenin proteins, with tumor stage, tumor type, smoking/tobacco chewing status and shorter overall survival probabilities of patients validates them as potential markers of clinical relevance in the pathobiology of UCB. Histopathological association validates Cyclin D1 as a marker of clinical significance, nevertheless, these results rule out the possible post translational activation of target genes by pS9GSK-3β/β-catenin /TCF/LEF transcriptional complex in urothelial tumorigenesis.
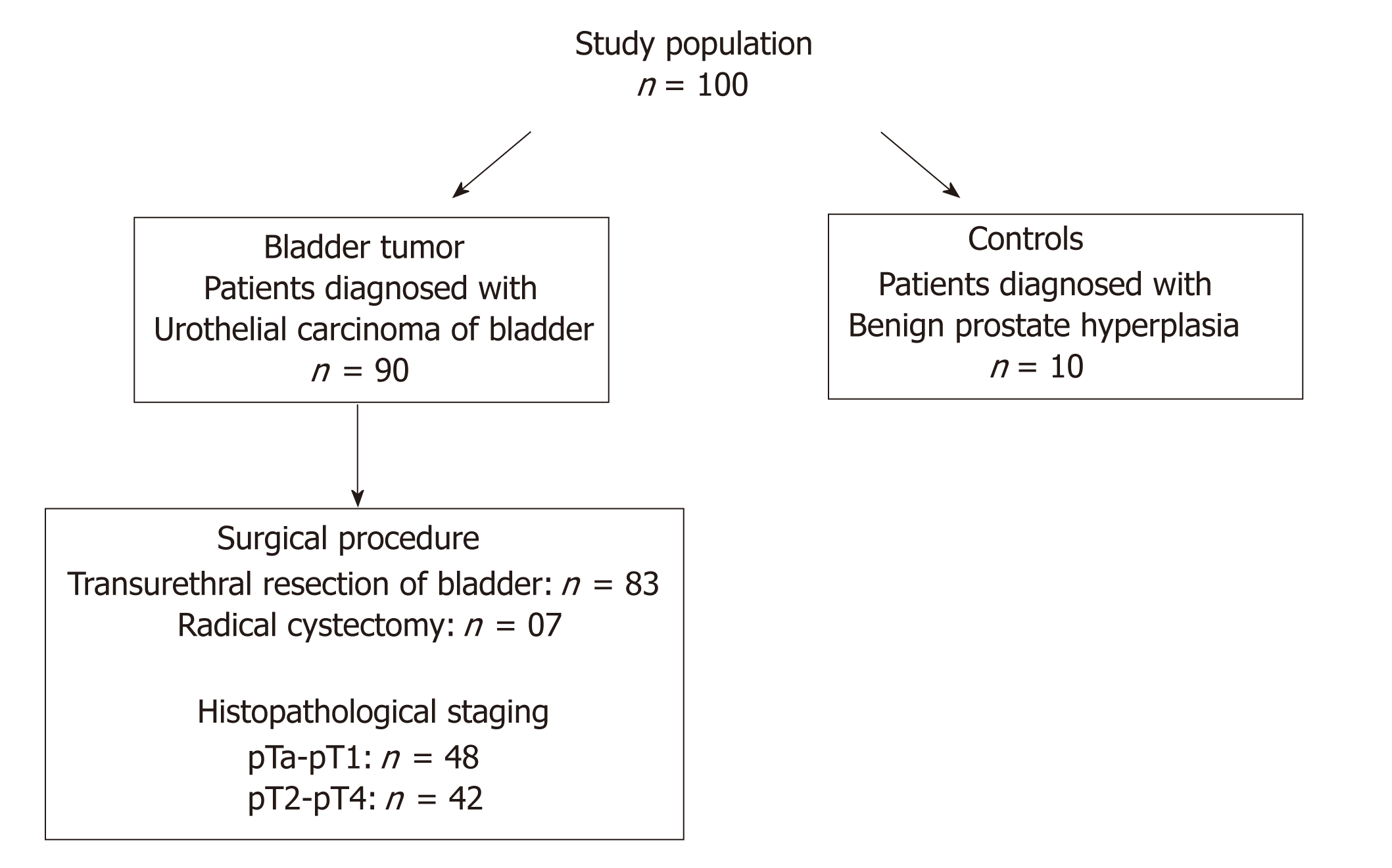
The study population consisted of ninety North Indian patients diagnosed with UCB were enrolled in the Department of Urology, King George Medical University (KGMU), Lucknow, India and Department of Urology, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS), Lucknow, India during 2014-2017 (Figure 1). All the patients examined with symptoms of hematuria followed by urinary frequency/irritative symptoms were assessed for primary tumor, which included bimanual examination under anesthesia before and after transurethral resection. Frozen tissue specimens were obtained from the patients at the time of transurethral resection/radical cystectomy. Matched formalin fixed and paraffin embedded tissue sections were archived from the Department of Pathology, KGMU and SGPGIMS, Lucknow, India. Studies were approved by the appropriate institutional research ethics committee and were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Ethical clearance was obtained from Bioethics Cell, Institutional Ethics Committee (IEC), KGMU (Reference No. 77th ECM II A/P14) and SGPGIMS (Reference no. 2014-166-CP-80), Lucknow, India.
Out of 90 patients, 48 were diagnosed with non-muscle invasive (stage pTa-pT1) and 42 had muscle invasive (stage pT2-pT4) tumors. Eighty three tumor tissue specimens were obtained from the patients who underwent transurethral resection of the bladder (TURB) and seven tumor tissue samples from the patients who underwent radical cystectomy. Ten tissue specimens of normal bladder mucosa were collected from patients with benign prostate hyperplasia after taking their signed and informed consent. These patients showed no obvious malignancy and had cold cup bladder biopsy. A small fraction of the excised bladder tumor or normal tissue was immersed in RNA later solution (Ambion; Thermo Fisher Scientific Life Sciences, Waltham, MA), and stored at -80 0C until further use.
The diagnoses and histopathological classification of the bladder tumor tissues were performed independently by pathologists according to WHO-ISUP, 2004 classification system[19]. Pathological stage was examined on the basis of degree of invasion of tumorous mass in lamina propria while, histological grade was determined by cell architecture, polarity, thickness and cohesiveness.
Quantitative expression and cellular localization analysis at protein level: Immunohistochemistry staining: Immunohistochemical (IHC) staining for putative biomarkers involved in GSK-3β and β-catenin signaling was performed using Ultra Vision Quanto detection system, horseradish peroxidase-diaminobenzidine (Thermo Fisher Scientific Life Sciences), according to the manufacturer’s instructions. Primary antibodies were used to stain biomarker proteins and these include antibodies to: (1) β-catenin (14; 224M-15; Cell Marque, Rocklin, CA) (1:40 dilution); (2) pS9GSK-3β (phospho S9; EPR2286Y; ab75814; Abcam, Cambridge, CA) (1:100 dilution); (3) Cyclin D1 (SP4; 241R-15; Cell Marque) (1:100 dilution); (4) Snail (Ab180714; Abcam, Cambridge, CA) (1:100 dilution); and (5) Slug (H-140; sc-15391; Santa Cruz Biotechnology, Santa Cruz, CA) (1:50 dilution). Three micron thick formalin fixed and paraffin embedded tumor and normal tissue sections from each sample were fixed on poly-L-lysine coated glass slides by heating at 70 0C for 3 h. Tissue sections were dewaxed using xylene and then subsequently washed with 100%, 70%, 50%, and 20% alcohol for 5 min each. After a brief wash, slides were then subjected to antigen retrieval with citrate buffer [low pH (citrate) buffer or high pH (Tris ethylene diamine tetra acetic acid) buffer] for 4 cycles of 5 min at full efficiency microwave system. Choice of buffer depends on the type of primary antibody. Sections were washed after blocking the activity of endogenous peroxidase by peroxide block (provided with the kit) for 10 min. Following this treatment, sections were incubated with the primary antibodies overnight at 4 0C followed by their incubation with primary antibody amplifier for 10 min at room temperature. Signals were detected by adding horseradish peroxidase polymer and diaminobenzidine, followed by counterstaining with Mayers’ hematoxylin for 1 min. Slides were dehydrated and mounted in dibutyl phthalate xylene medium, and visualized for the brown reaction product. Evaluation of IHC putative biomarkers for their cellular localization and expression at protein level was done using a Nikon Eclipse 50i light microscope (Nikon, Tokyo, Japan).
A total of 1000 cells were counted in consecutive fields at a magnification of 400× by two independent observers. Immunoreactivity of the tumor/normal/control tissue was analyzed as strong, weak/reduced, or no expression. Marker proteins were examined for their membranous, nuclear, and or cytoplasmic localization. Staining intensity was assessed on the scale of 1, 2 and 3 where 1 was assigned as reduced expression, 2 as moderate expression and 3 as strong expression. Overall IHC score was calculated by multiplying the percent positive cells stained and its staining intensity. Based on percent positive cells and their staining intensity, IHC score was assigned as low as 0 to as high as 300.
Nuclear immunostaining was examined for Cyclin D1, Snail and Slug. PhosphoS9GSK-3β and β-catenin were evaluated for no expression/low membranous expression; nuclear expression; cytoplasmic and; high membranous expression.
Quantitative expression analysis at transcriptome level: RNA extraction and real time–quantitative polymerase chain reaction: Total cellular RNA extraction was done from frozen tumor and control tissue specimens using 1 mL/mg TRIzol reagent (Ambion; Thermo Fisher Scientific Life Sciences). Extracted RNA was quantified by measuring its absorbance at 260 nm and purity was checked by taking its absorbance ratio at 260 nm and 280 nm. One microgram of RNA from each tissue/sample was reverse transcribed to cDNA using verso cDNA synthesis kit (Thermo Fisher Scientific Life Sciences) according to the manufacturer’s instructions. Subsequently, 10 ng of cDNA in 5 µL reaction mix was subjected to real-time quantitative polymerase chain reaction (RT-qPCR) for each tumor and normal tissue sample. RT-qPCR was performed in a Quant Studio 7 Flex Real-Time PCR platform (Applied Biosystems; Thermo Fisher Scientific Life Sciences) using Light Cycler 480 Syber green I master mix (TaKaRa, Clontech). Following primers were used to amplify the specific genes/putative biomarkers under study: (1) β-catenin, Forward: 5’GCTTGGAATGAGACTGCTGA3’ and Reverse: 5’CTGGCCATATCCACCAGAGT3’; (2) Cyclin D1, Forward: 5’TGCCACAGATGTGAAGTTCATT3’ and Reverse: 5’CAGTCCGGGTCACACTTGAT3’; (3) Slug, Forward: 5’TCGGACCCACACATTACC3’ and Reverse: 5’CCGAGATGGGGTTGATAATG3’; (4) Snail, Forward: 5’TCGTCCTTCTCCTCTACTTC3’ and Reverse: 5’TTCCTTGTTGCAGTATTTGC3’; and (5) β-actin as a housekeeping gene, Forward: 5’GGACTTCGAGCAAGAGATGG3’ and Reverse: 5’AGCACTGTGTTGGCGTACAG3’.
RT-qPCR cycling conditions include, initial denaturation at 95 0C for 5 min; followed by 35 cycles of denaturation at 94 0C for 30 s, annealing at 56 0C for 30 s, extension at 72 0C for 1 min; and final extension at 72 0C for 10 min. Relative levels of mRNA expression were quantified as ∆Ct values by comparing it with the mean Ct values of β-actin. β-actin was taken as reference/endogenous control gene (∆Ct = Ct target - Ct reference) to normalize any possible difference in the amount of total RNA. Furthermore, ∆∆Ct for each target gene in tumor sample was calculated using ∆Ct of normal tissue against respective gene (∆∆Ct = ∆Cttumor - ∆Ctnormal). Average fold change expression was then calculated by applying 2-∆∆Ct. Ct values for all the genes in each tissue sample were assayed and analyzed in triplicates.
Quantitative expression of putative biomarkers involved in β-catenin signaling at transcriptome and protein level was statistically correlated with clinical features including smoking/tobacco chewing status of patients and presence or absence of hematuria; and histopathological variables including tumor stage, tumor grade, tumor type (primary/recurrence), presence or absence of metastasis and lymph node involvement using SPSS, version 20.0 software; Microsoft, Redmond, WA. Kaplan-Meier along with log-rank test was employed to evaluate probable relation between biomarker expression and overall survival (OS)/progression free survival (PFS) of patients. Period of assessment for OS of patients includes the time period between the date of surgery/cystoscopy as an entry date and the date of last follow up/date of patients’ demise due to cancer. Time period between the date of surgery/cystoscopy as an entry date and date of tumor progression on last follow up/date of patients’ demise due to tumor progression was used for PFS studies. Quantitative data are presented as the mean ± SE. The P values less than 0.05 were considered as statistically significant.
Present study was performed on a cohort of 90 patients with UCB and 10 normal tissues. Median age of these patients was 60 years, ranging from minimum of 38 years to maximum of 82 years. Eleven percent (10/90) patients were female. Of total, 80% patients (72/90) were examined with gross painless hematuria as the primary clinical symptom. Smoking/tobacco chewing history of 76 patients was available; out of which 64.5% were regular smokers or tobacco chewers and 35.5% were non-smokers. Fifty three percent (48/90; stage pTa-pT1) patients were presented with non-muscle invasive disease, while 46.7% patients (42/90; stage pT2-pT4) were diagnosed with muscle invasive disease. Histologically, 34.4% tumors were classified as low grade carcinomas and 65.6% patients were presented with high grade carcinomas. Among the total 90 patients, 43.3% (39/90) patients were diagnosed with the tumor of recurrent type. As per clinicohistopathological records, only 6.7% patients were examined with lymph node metastasis that developed over a mean period of 42 mo since initial diagnosis. Follow up data was available in 71 patients. Of 71 patients, 69% patients survived and 28.2% patients showed progression over an average time line of 36 mo. Clinicohistopathological profiles of the patients included in the study are summarized in Table 1.
| Clinicohistopathological variables | n (%) |
| Total No. of patients | 90 (100) |
| Age (yr) median, range | 60, 38-82 |
| N < 60 | 41.1) |
| N ≥ 60 | 53 (58.9) |
| Gender | |
| Males | 88.9) |
| Females | 10 (11.1) |
| Hematuria | |
| Present | 80) |
| No information | 18 (20) |
| Smoking/tobacco chewing status | |
| Smokers/tobacco chewers | 54.4) |
| Non-smokers/non-tobacco chewers | 30) |
| No information | 14 ( 15.6) |
| Tumor type | |
| UCB | 100) |
| SCC/others | None |
| Tumor grade | |
| Low | 34.4) |
| High | 59 (65.6) |
| Tumor stage | |
| NMIBC (pTa-pT1) | 53.3) |
| MIBC (pT2-pT3) | 42 (46.7) |
| Lymph node involvement | |
| N0 | 91.1) |
| N1 | 8 (8.9) |
| Metastasis | |
| M0 | 91.1) |
| M1 | 8 (8.9) |
| Type | |
| Primary | 56.7) |
| Recurrent | 39 (43.3) |
| Surgical procedure | |
| TURBT | 92.2) |
| Radical cystectomy | (7.8) |
| Progression | 28.2) |
| Deaths due to cancer | 14 (19.7) |
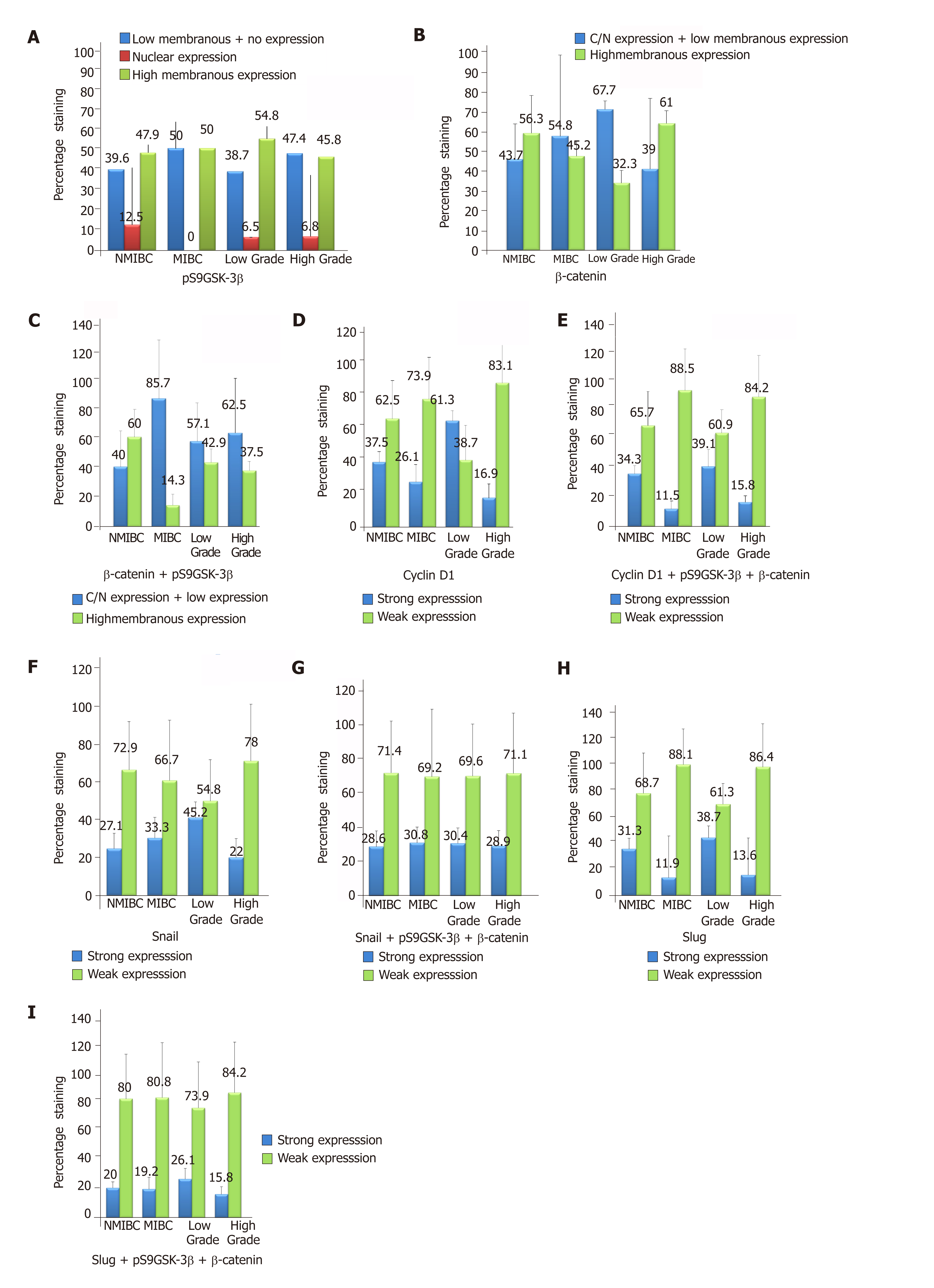
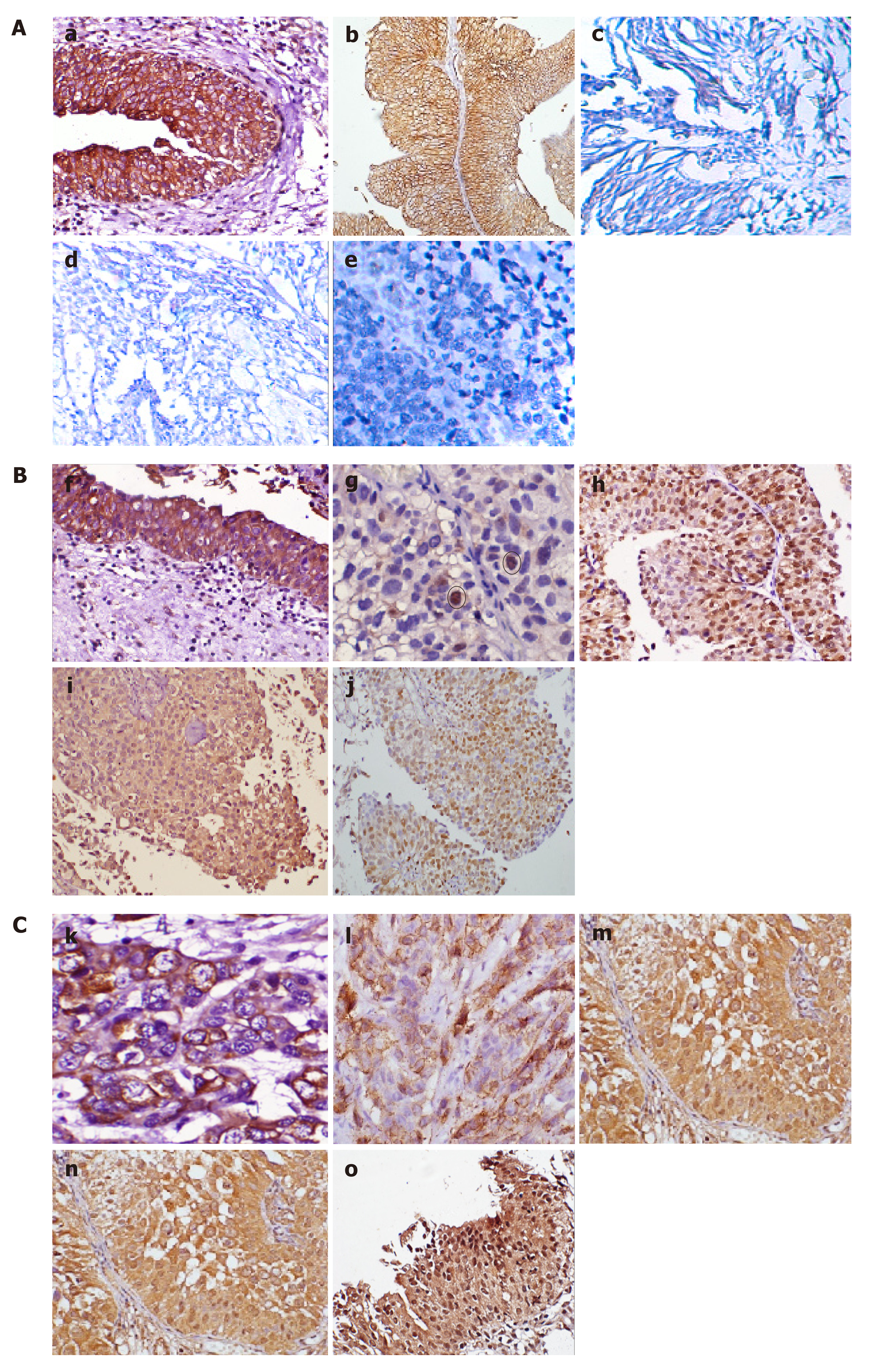
IHC staining was done to detect the expression of pS9GSK-3β in a cohort of 90 human bladder tumor sections and 10 normal bladder tissue sections. High membranous expression of pS9GSK-3β was observed in all the sections obtained from control bladder tissues. However, 51.1% (46/90) tumors exhibited its aberrant expression which includes low membranous (20% tumors with IHC score ≤ 200), nuclear/cytoplasmic (6.7% tumors with IHC score ≥ 1) or no expression (24.4% tumors IHC score = 0) (Figure 2A and Figure 3). Aberrant expression of pS9GSK-3β was observed in 52% (25/48) non-muscle invasive (low membranous: 20.8%, cytoplasmic/nuclear: 12.5% and no expression: 18.8%) and in 50% (21/42) muscle invasive tumors (low membranous: 19%, cytoplasmic/nuclear: 0% and no expression: 31%) (P = 0.01; Mann Whitney U test). Immunostaining of pS9GSK-3β was observed in 45.2% (14/31) low grade (low membranous: 25.8%, cytoplasmic/nuclear: 6.4% and no expression: 12.9%) and 54.2% (32/59) high grade tumors (low membranous: 16.9%, cytoplasmic/nuclear: 6.8% and no expression: 30.5%) (P = 0.04; Mann Whitney U test). Aberrant expression of pS9GSK-3β was noted in 59% (23/39) recurrent tumors (low membranous: 20.5%, cytoplasmic/nuclear: 12.8% and no expression: 25.6%); 49% (24/49) patients with smoking/ tobacco chewing habit (low membranous: 20.4%, cytoplasmic/nuclear: 8.2% and no expression: 20.4%); and 54.2% (39/72) patients with hematuria (low membranous: 22.2%, cytoplasmic/nuclear: 8.3% and no expression: 23.6%) (Table 2). Forty four tumors out of 90 cases exhibited strong membranous expression of pS9GSK-3β but did not show any statistical relevance with clinicohistopathological variables (data not shown).
| Characteristics | pS9GSK-3β (low membranous, no expression or N/C expression)(n = 46/90) |
| AGE 60 (38-78) | P = 0.87 |
| < 60 | 20/46 |
| ≥ 60 | 26/46 |
| Stage | P = 0.01a |
| Non muscle invasive | 25/46 |
| Muscle invasive | 21/46 |
| Grade | P = 0.04a |
| Low grade | 14/46 |
| High grade | 32/46 |
| Type | P = 0.35 |
| Primary | 23/46 |
| Recurrent | 23/46 |
| Smoking/tobacco chewing | P = 0.66 |
| Yes | 24/46 |
| No | 14/46 |
| Unknown | 8/46 |
| Hematuria | P = 0.64 |
| Present | 39/46 |
| Unknown | 7/46 |
| Metastasis | P = 0.58 |
| Present | 1/46 |
| Absent | 45/46 |
Immunostaining was done to check β-catenin expression at protein level and its subcellular localization in 90 bladder tumors (Figure 2B and Figure 3). Its strong membranous expression was seen in control/normal urothelium while out of 90 tumors, 44 showed aberrant expression of β–catenin. Out of these 44 tumors, 23 showed low membranous (25.6% with IHC score ≤ 200); 12 exhibited nuclear/cytoplasmic (13.3% with IHC score > 0); and 9 did not show any expression (10% with IHC score = 0). Forty four percent (21/48) non-muscle invasive (low membranous: 20.8%; cytoplasmic/nuclear: 16.7%; no expression: 6.2%); 54.8% (23/42) muscle invasive tumors (low membranous: 30.9%; cytoplasmic/nuclear: 9.5%; no expression: 14.3%); 67.7% (21/31) low grade (low membranous: 32.3%; cytoplasmic/nuclear: 25.8%; no expression: 9.7%); and 39% (23/59) high grade tumors (low membranous: 22%; cytoplasmic/nuclear: 6.8%; no expression: 10.3%) were examined to aberrantly express β–catenin (Figure 2B). Its altered expression was determined in 53.8% (21/39) recurrent tumors (low membranous: 38.5%; cytoplasmic/nuclear: 10.3%; no expression: 5.1%); 53.1% (26/49) patients with smoking/tobacco chewing history (low membranous: 30.6%; cytoplasmic/nuclear: 12.2%; no expression: 10.2%); and 45.8% (33/72) patients with hematuria (low membranous: 25%; cytoplasmic/nuclear: 12.5%; no expression: 8.3%).
Out of the total 90 tumors examined, 28 (31.1%) tumors co-expressed altered protein levels of pS9GSK-3β and β–Catenin proteins (Figure 2C). These proteins were observed to be aberrantly co-expressed in 67.9% (19/28) muscle invasive tumors (P = 0.01; Mann Whitney U test); 78.6% (22/28) high grade tumors; 60.7% (17/28) recurrent tumors (P = 0.02; Mann Whitney test); 60.7% (17/28) frequent smokers/tobacco chewers and 82.1% (23/28) tumors from hematuric patients (P = 0.04; Mann Whitney U test) (Table 3).
| Characteristics | pS9GSK-3β + β-Catenin (low membranous, no expression or N/C expression)(n = 28/90) |
| Age 60 (38-78) | P = 1.0 |
| < 60 | 11/28 |
| ≥ 60 | 17/28 |
| Stage | P = 0.01a |
| Non muscle invasive | 9/28 |
| Muscle invasive | 19/28 |
| Grade | P = 0.09 |
| Low grade | 6/28 |
| High grade | 22/28 |
| Type | P = 0.02a |
| Primary | 11/28 |
| Recurrent | 17/28 |
| Smoking/tobacco chewing | P = 0.04a |
| Yes | 17/28 |
| No | 9/28 |
| Unknown | 0/28 |
| Hematuria | P = 1.0 |
| Present | 23/28 |
| Unknown | 5/28 |
| Metastasis | P = 0.9 |
| Present | 1/28 |
| Absent | 27/28 |
Effect of altered levels of proteins in combination was investigated on patients’ survival. Follow up data of 71 patients was available over an average time period of 36 months. Progression and mortality due to cancer was observed in 28.2% (20/71) and 19.7% (14/71) patients, respectively.
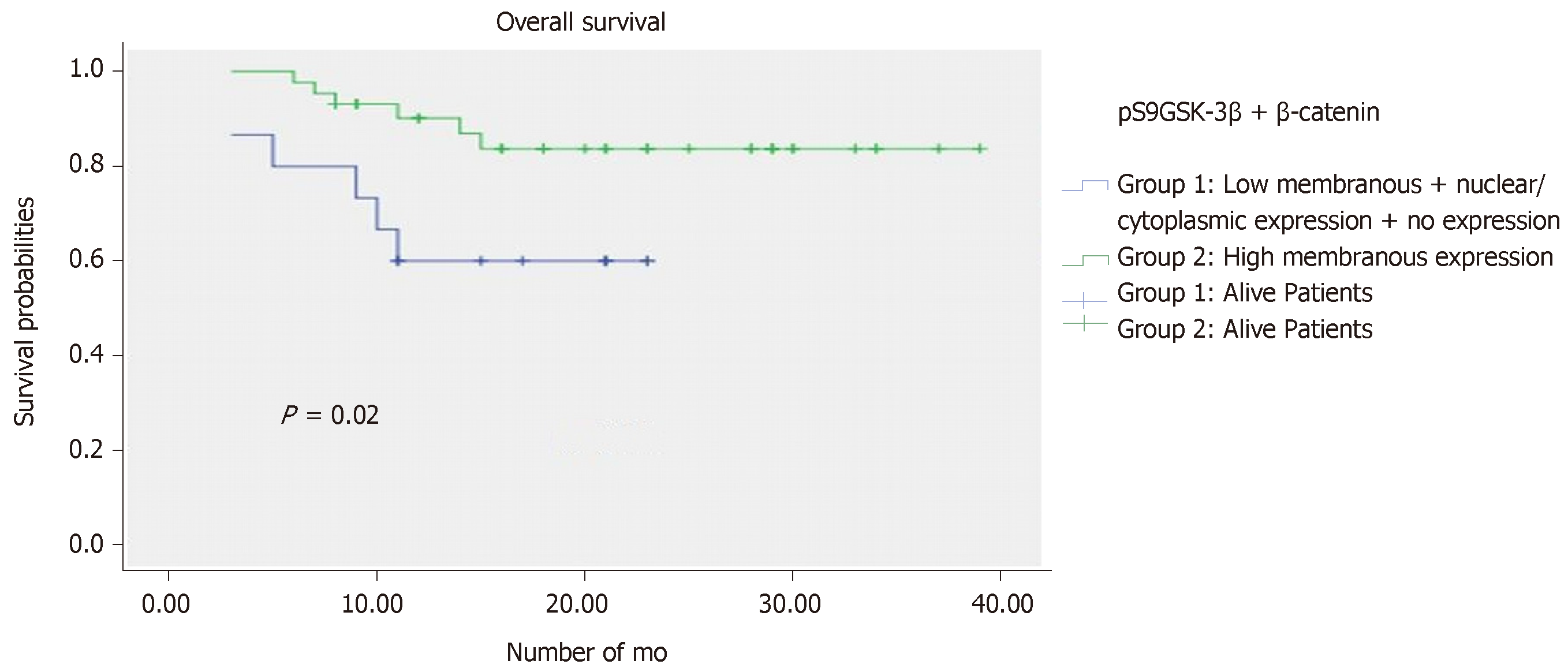
Kaplan Meier analysis along with log rank test identified the aberrantly co-expressed pS9GSK-3β and β–catenin, as significant prognostic factor for a short OS (P = 0.02) (Figure 4). However, PFS analysis failed to deduce any significant correlation in a given cohort.
Target genes’ expression and pS9GSK-3β/β-catenin levels: Their clinical implications
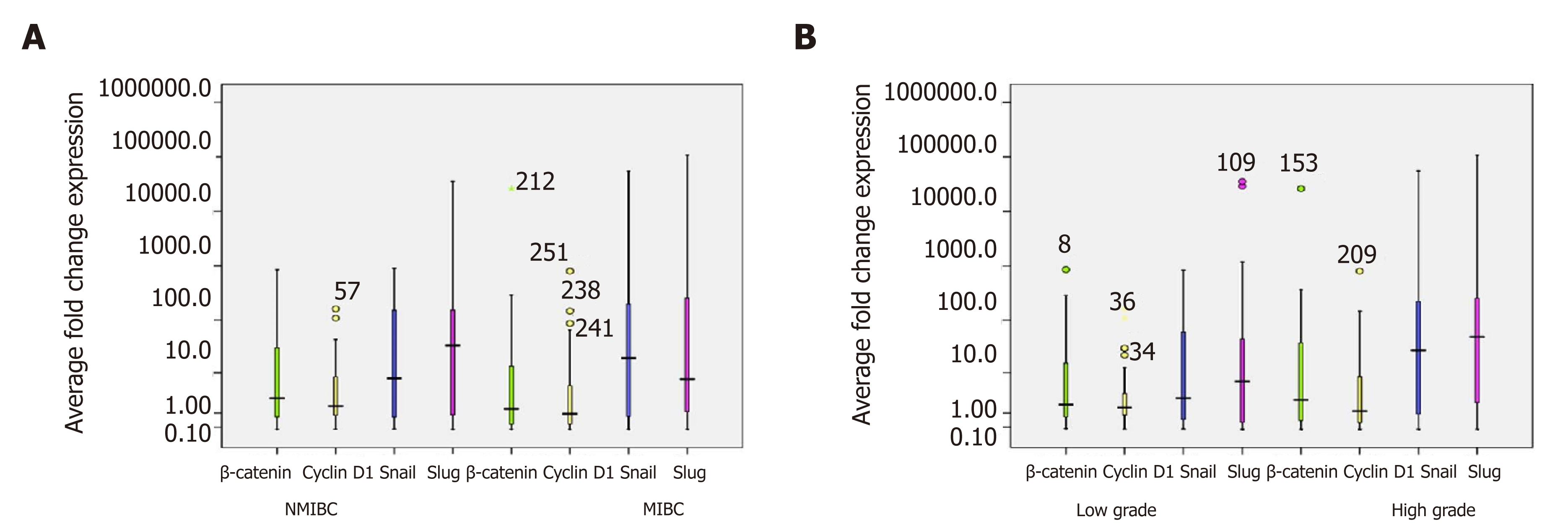
RT-qPCR study was done to evaluate total β-catenin expression at transcriptome level in 90 bladder tumors and its expression as fold change was calculated by comparing its levels in control bladder tissues (Figure 5A and B). Of 90 bladder tumors, 63.3% (57/90) tumors were analyzed to exhibit strong expression of total β–Catenin. These tumors were examined for quantitative expression of target genes including Cyclin D1, Slug and Snail at transcriptome level (Figure 5A, B and Table 4). Out of 57 tumors, 39 tumors showed strong expression of Cyclin D1 and found to be significantly correlated with increased total β–catenin (P = 0.017; Kendall’s tau-b correlation test). Excluded group revealed no significance. RT-qPCR results demonstrated stronger expression of Snail in 70.2% (40/57) tumors compared to normal urothelial tissue. Seventy four percent (42/57) tumors were found to express higher levels of Slug. Statistical studies showed strong correlation between higher levels of Slug and total β-catenin (P = 0.001; Pearson correlation test).
| β-Catenin | Cyclin D1 | Snail | Slug | |
| Characteristics | Strong expression (n = 57/90) | Strong expression (n = 39/57) | Strong expression (n = 40/57) | Strong expression (n = 42/57) |
| Age 60 (38-78) yr | p = 0.0071a | P = 0.851 | P = 0.585 | P = 0.021a |
| < 60 | 23/57 | 17/23 | 16/23 | 16/23 |
| ≥ 60 | 34/57 | 22/34 | 24/34 | 26/34 |
| Stage | P = 0.845 | P = 0.198 | P = 0.205 | P = 0.929 |
| Non muscle invasive | 34/57 | 26/34 | 25/34 | 25/34 |
| Muscle invasive | 23/57 | 13/23 | 15/23 | 17/23 |
| Grade | P = 0.137 | P = 0.373 | P = 0.420 | P = 0.893 |
| Low grade | 21/57 | 16/21 | 14/21 | 13/21 |
| High grade | 36/57 | 23/36 | 26/36 | 29/36 |
| Type | P = 0.289 | P = 0.806 | P = 0.62 | P = 0.787 |
| Primary | 32/57 | 21/32 | 22/32 | 22/32 |
| Recurrent | 25/57 | 18/25 | 18/25 | 20/25 |
| Smoking/tobacco chewing | P = 0.738 | P = 0.377 | P = 0.784 | P = 0.592 |
| Yes | 26/57 | 17/26 | 18/26 | 20/26 |
| No | 18/57 | 13/18 | 13/18 | 14/18 |
| Unknown | 13/57 | 9/13 | 5/13 | 8/13 |
| Hematuria | P = 0.397 | P = 0.740 | P = 0.362 | P = 0.685 |
| Present | 46/57 | 32/46 | 36/46 | 36/46 |
| Unknown | 11/57 | 7/3 | 4/3 | 6/3 |
| Metastasis | P = 0.84 | P = 0.39 | P = 0.49 | P = 0.12 |
| Present | 6/57 | 4/6 | 4/6 | 3/6 |
| Absent | 51/57 | 35/51 | 36/51 | 39/51 |
At protein level, immunostaining analysis identified nuclear staining of Cyclin D1 (IHC score > 0) in 32% (29/90) tumors and its association with aberrant levels of β-catenin with respect to tumor recurrence (P = 0.017; Kendall’s tau-b correlation test). Strong nuclear expression of Cyclin D1 was examined to be statistically associated with the aberrant expressions of either pS9GSK-3β or β-catenin with respect to tumor stage (P = 0.05; Mann Whitney U test) and tumor grade (P = 0.01; Mann Whitney U test) (Figure 2D, E, Figure 3 and Table 5).
| Aberrant protein expression of either pS9GSK-3β or β-Catenin: n = 61/90 | |||||||
| Characteristics | pS9GSK-3βorβ-Catenin (n = 61/90) | Cyclin D1 Strong nuclear expression (n = 15/61) | Cyclin D1 Weak nuclear expression (n = 46/61) | SnailStrong nuclear expression (n = 18/61) | Snail Weak nuclear expression (n = 43/61) | Slug Strong nuclear expression (n = 12/61) | Slug Weak nuclear expression (n = 49/61) |
| Age 60 (38-78) | P = 0.18 | P = 0.03 | P = 0.92 | P = 0.36 | P = 0.17 | P = 0.38 | P = 0.6 |
| < 60 | 30/61 | 9/30 | 21/30 | 7/30 | 23/30 | 6/30 | 24/30 |
| ≥ 60 | 31/61 | 6/31 | 25/31 | 11/31 | 20/31 | 6/31 | 25/31 |
| Stage | P = 0.45 | P = 0.05a | P = 0.28 | P = 0.96 | P = 0.37 | P = 0.19 | P = 0.47 |
| Non muscle invasive | 35/61 | 12/35 | 23/35 | 10/35 | 25/35 | 7/35 | 28/35 |
| Muscle invasive | 26/61 | 3/26 | 23/26 | 8/26 | 18/26 | 5/26 | 21/26 |
| Grade | P = 0.13 | P = 0.01a | P = 0.55 | P = 0.96 | P = 0.66 | P = 0.45 | P = 0.90 |
| Low grade | 23/61 | 9/23 | 14/23 | 7/23 | 16/23 | 6/23 | 17/23 |
| High grade | 38/61 | 6/38 | 32/38 | 11/38 | 27/38 | 6/38 | 32/38 |
| Type | P = 0.1 | P = 0.65 | P = 0.33 | P = 0.42 | P = 0.98 | P = 0.19 | P = 0.13 |
| Primary | 33/61 | 9/33 | 25/33 | 9/33 | 24/33 | 9/33 | 24/33 |
| Recurrent | 28/61 | 6/28 | 21/28 | 9/28 | 19/28 | 3/28 | 25/28 |
| Smoking/tobacco chewing | P = 0.7 | P = 0.29 | P = 0.56 | P = 0.75 | P = 0.16 | P = 0.14 | P = 0.26 |
| Yes | 40/61 | 11/40 | 32/40 | 13/40 | 27/40 | 6/40 | 32/40 |
| No | 19/61 | 4/19 | 12/19 | 5/19 | 14/19 | 4/19 | 17/19 |
| Unknown | 2/61 | 0/2 | 2/2 | 0/2 | 2/2 | 2/2 | 0/2 |
| Hematuria | P = 0.93 | P = 0.53 | P = 0.65 | P = 0.26 | P = 0.62 | P = 0.37 | P = 0.73 |
| Present | 46/61 | 12/46 | 34/46 | 15/46 | 31/46 | 9/46 | 37/46 |
| Unknown | 15/61 | 3/7 | 12/7 | 3/7 | 12/7 | 3/7 | 12/7 |
| Metastasis | P = 0.25 | P = 0.51 | P = 0.53 | P = 0.24 | P = 0.58 | P = 0.26 | P = 0.12 |
| Present | 5/61 | 2/5 | 3/5 | 3/5 | 2/5 | 2/5 | 3/5 |
| Absent | 56/61 | 13/56 | 43/56 | 15/56 | 41/56 | 10/56 | 46/56 |
Elevated levels of nuclear Snail (IHC score > 0) were observed in 27 out of 90 tumors (Figure 2F and Figure 3). Out of 61 tumors with aberrant expressions of either pS9GSK-3β or β-catenin, 29.5% tumors showed increased nuclear expression of Snail (Figure 2G). None of the clinicohistopathological variables showed significance with the expression levels of these proteins.
Ninety tumors were examined for nuclear immunostaining of Slug (IHC score > 0). Out of 90, 20 tumors were observed with strong expression (Figure 2H and Figure 3). Out of 61 tumors with aberrant immunoexpressions of either pS9GSK-3β or β-catenin, only 19.7% (12/61) tumors were found to exhibit nuclear expression of Slug in urothelium but no statistical association was observed (Figure 2I and Table 5).
Diverse cellular processes including proliferation, differentiation, glycogen metabolism, motility, cell survival are regulated by GSK-3β. Many important cellular functions of GSK-3β are known to be mediated by its influence on Wnt/β-catenin signal cascade. Being the essential component of the protein complex, GSK-3β phosphorylates and targets β-catenin for subsequent ubiquitin-proteasomal degradation, thereby maintains the normal cell physiology[20].
Serine 9 phosphorylation of GSK-3β leads to dephosphorylation of glycogen synthase; increases glycogen synthesis; and promotes protein and lipid biosynthesis. Its expression is restricted to umbrella cells of urinary tract, epithelial cells of breast and pancreaticobiliary duct, fallopian tube, epididymis, secretory cells of prostatic gland, distal nephron of kidney, gastrointestinal tract[20]. Expression of GSK-3β is known to be associated with differentiation of ectodermal derived tissues. Expression of phosphorylated form of GSK-3β (pS9GSK-3β) in epithelial cells of endodermal derived tissues in humans may validate its function to serve as an IHC marker for epithelial cells[20].
Strong membranous staining of pS9GSK-3β suggests functional and physiologic requirement of its expression in normal bladder urothelium/umbrella cells[20]. Normal urothelial cells were examined for strong membranous expression of pS9GSK-3β. Strong intensity of membranous pS9GSK-3β may indicate its association with the barrier function (barrier to urine, toxin and pathogen) of umbrella cells[21].
Phosphorylation of GSK-3β at Serine 9 inhibits its activity, causes dysregulation of the Wnt/β-catenin pathway; prevents β-catenin from degradation, allows its stabilization and accumulation in nucleus and cytosol, thus positively regulates cell proliferation and survival[22]. Aberrant expression of pS9GSK-3β examined by loss/no membranous expression or its nuclear/cytosolic accumulation was observed in 51.1% urothelial tumors and these tumors showed significant association with tumor grade and stage.
Activation of Wnt/β-catenin signaling pathway is known to be associated with altered pS9GSK-3β and cytosolic/nuclear accumulation of stabilized β-catenin in several human cancers[9]. Total β-catenin expression was noted in 63.3% (57/90) tumors at transcriptome level whereas 49% (44/90) tumors showed either its loss of membranous immunostaining or appearance of nuclear/cytosolic β-catenin protein. In accordance with earlier reports, strong membranous staining was confined to non-malignant group/normal urothelial cells[4,10]. Present study examines the comparative aberrant expression of β-catenin in non-muscle invasive (43.7%) and muscle invasive (53.8%) tumors, however, 67.7% low grade tumors and 39% high grade tumors showed its altered levels. Study by Elserafy et al[4] reports statistical association between nucleocytoplasmic expression of β-catenin with poor prognostic parameters (tumor grade, stage, lymph node metastasis, vascular and perineural invasion, mitosis, and microvessel density). Another study by Li et al[23], investigates the expression of either cytoplasmic or nuclear β-catenin as an independent prognostic factor for overall survival in non-small-cell lung cancer. Nevertheless, in concordance with the results of the work by Bilim et al[24], 2000, current study failed to derive any independent prognostic relevance of β-catenin in bladder tumorigenesis.
Tumors co-expressing aberrant levels of pS9GSK-3β and β-catenin proteins were examined and identified to exhibit statistical association with tumor stage; tumor type; smoking/tobacco chewing status of patients and poor overall survival probabilities. These results indicate that low membranous pS9GSK-3β and cytosolic/nuclear pS9GSK-3β participate in neoplastic transformation by stabilizing β-catenin during urothelial tumorigenesis and could be considered as potential prognostic marker.
Tumor promoting effects of nuclear pS9GSK-3β have been reported to be mediated by activating cell cycle and survival via inducing the expression of critical cell cycle regulators, such as Cyclin D1[25,26]. Increased cytosolic amount of β-catenin facilitates its translocation to nucleus where it binds to T-cell-specific transcription factors, TCF and LEF and stimulates the transcription of Cyclin D1[27]. Research studies reveal the clinical functions of overexpressed Cyclin D1 via activation of pS9GSK-3β/β-catenin signal cascade in tumor development in major human cancers[10,26,28-30]. Increased Cyclin D1 levels in bladder tumor tissues showed significant association with total β-catenin in recurrent tumors at transcriptome level. Immunostaining of Cyclin D1 in tumors with altered levels of either pS9GSK-3β or β-catenin proteins and its association with tumor stage and grade may validate it as a marker of prognostic significance but does not explain its possible post translational activation by pS9GSK-3β/β-catenin/TCF/LEF transcriptional complex in bladder cancer progression.
Membranous β-catenin complexes with E-Cadherin and α-catenin and maintains cell–cell adhesion and cell polarization[31]. Loss of membranous β-catenin results in dissociation of cells and promotes invasion and metastasis[32]. pS9GSK-3β mediated β-catenin stabilization followed by activation of β-catenin/TCF/LEF transcriptional complex is known to induce EMT and enhance cell invasion/migration by upregulating the expression of Snail and Slug transcription factors[31,33]. Experimental studies demonstrate the effect of β-catenin, Snail and Slug on EMT promoted cell invasion during bladder tumorigenesis and correlate them with poor prognosis[13,31,34,35]. Induced nuclear expressions of Snail and β-catenin by inactive form of GSK-3β and their functional interaction have been shown to regulate fibroblasts migration, EMT activation in non-tumorigenic breast epithelial cells and lung epithelial cells and correlate with poor prognosis in patients with gastric cancer and colorectal cancer[36-38]. Present study reports an inter-correlation between total β–catenin levels and increased Slug expression with tumor stage at transcriptome level. In disagreement with other studies, immunostaining results fail to exhibit any possible correlation between increased nuclear Snail/Slug and aberrant levels of pS9GSK-3β/β-catenin and their prognostic significance in a given cohort of bladder tumors. Conflicts in the results could be due to different sample size and their source and therefore, multicenter clinical studies are required for reliable conclusion.
Findings of the present study strongly support the involvement of pS9GSK-3β in urothelial tumorigenesis by stabilizing β-catenin via β-catenin signal cascade. Statistical association of aberrantly co-expressed pS9GSK-3β and β–catenin proteins with tumor stage, type, smoking/tobacco chewing status and shorter overall survival probabilities of patients validates them as potential markers of clinical relevance in the pathobiology of bladder cancer.
Phosphorylated GSK-3β mediated stabilization and activation of β-catenin signal cascade and its cross talk with other key pathways have been reported to be associated with cell proliferation, invasion, angiogenesis, and increased aggressiveness in several major human cancers. To validate the clinical functions of pS9GSK-3β and its downstream effects through β-catenin signaling in the pathobiology of bladder cancer, expression levels of pS9GSK-3β, stabilized/activated β-catenin and its target genes, Cyclin D1, Snail and Slug were examined in well-defined cases of bladder cancer. Results based on the association/correlation studies between the expression levels of marker proteins and clinicohistopathological variables indicate that aberrantly expressed pS9GSK-3β participate in neoplastic transformation by stabilizing β-catenin during urothelial tumorigenesis and could be considered as potential prognostic marker. Histopathological association of nuclear Cyclin D1 in tumors expressing either pS9GSK-3β or β-catenin validates it as a prognostic marker in urothelial tumorigenesis. Nevertheless, absence of any significance rules out the possible post translational activation of Cyclin D1, Snail or Slug by pS9GSK-3β/β-catenin/TCF/LEF transcriptional complex in bladder cancer progression.
Aberrant activation of phosphorylated form of glycogen synthase kinase-3β [pS9GSK-3β (Serine 9 phosphorylation)] is known to trigger Wnt/β-catenin signal cascade.
The clinicohistopathological implications of glycogen synthase kinase-3β [pS9GSK-3β (Serine 9 phosphorylation)]/β-catenin in bladder carcinogenesis remain unknown.
The aim of the present study is to investigate the diagnostic and prognostic relevance of expressions of pS9GSK-3β, β-catenin and its target genes in the pathobiology of bladder cancer.
Cellular localization and the expression of pS9GSK-3β proteins were examined by immunohistochemical (IHC) staining in ninety tumor tissues resected from patients diagnosed with bladder cancer. Real time-quantitative polymerase chain reaction and IHC were done to check the expression of β-catenin, Cyclin D1, Snail and Slug at transcriptome and protein level respectively. Expressions of the markers were statistically correlated with these variables to determine their significance in clinical setting.
Aberrant (low or no membranous/high nuclear/high cytoplasmic) expression of pS9GSK-3β was noted in 51% patients and found to be significantly associated with tumor stage and tumor grade. Thirty one percent tumors exhibited aberrant co-expression of pS9GSK-3β and β–catenin proteins and showed strong statistical association with tumor stage (P = 0.01), tumor type (P = 0.02), smoking/tobacco chewing status (0.04) and shorter overall survival probabilities of patients (P = 0.02).
Findings of the present study strongly support the involvement of pS9GSK-3β in urothelial tumorigenesis by stabilizing β-catenin. Statistical association of aberrantly co-expressed pS9GSK-3β and β–catenin proteins with patients’ variables validates them as potential markers of clinical relevance in the pathobiology of bladder cancer.
Results based on the association/correlation studies between the expression levels of marker proteins and clinicohistopathological variables indicate that aberrantly expressed pS9GSK-3β participates in neoplastic transformation by stabilizing β-catenin during urothelial tumorigenesis and could be considered as potential prognostic marker.
Authors, Niharika Maurya and Rinni Singh are thankful to University grant commission (UGC), Govt. of India for providing research fellowship.
| 1. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9585] [Article Influence: 798.8] [Reference Citation Analysis (3)] |
| 2. | Garg M. Urothelial cancer stem cells and epithelial plasticity: current concepts and therapeutic implications in bladder cancer. Cancer Metastasis Rev. 2015;34:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Hrbácek J, Brisuda A, Babjuk M. Involvement of epithelial-mesenchymal transition in urinary bladder cancer progression. A review. Debates on Bladder Cancer. 2011;3:1-6. |
| 4. | Elserafy FA, Elbaky TMA, Elsherif EAR, Nor-Eldin-Elkady NM, Dawood MM, Ei Gharabawy MS. Evaluation of β -Catenin expression in muscle-invasive urothelial bladder carcinoma. Menoufia Med J. 2015;27:507–513. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1107] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 6. | Woodgett JR, Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta. 1984;788:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1168] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 8. | Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A, Kato T, Tomita Y. Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res. 2010;16:5124-5132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Urakami S, Shiina H, Enokida H, Kawakami T, Tokizane T, Ogishima T, Tanaka Y, Li LC, Ribeiro-Filho LA, Terashima M, Kikuno N, Adachi H, Yoneda T, Kishi H, Shigeno K, Konety BR, Igawa M, Dahiya R. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clin Cancer Res. 2006;12:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Liang X, Men QL, Li YW, Li HC, Chong T, Li ZL. Silencing of Armadillo Repeat-Containing Protein 8 (ARMc8) Inhibits TGF-β-Induced EMT in Bladder Carcinoma UMUC3 Cells. Oncol Res. 2017;25:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Liu B, Miyake H, Nishikawa M, Fujisawa M. Expression profile of epithelial-mesenchymal transition markers in non-muscle-invasive urothelial carcinoma of the bladder: correlation with intravesical recurrence following transurethral resection. Urol Oncol. 2015;33:110.e11-110.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Singh R, Ansari JA, Maurya N, Mandhani A, Agrawal V, Garg M. Epithelial-To-Mesenchymal Transition and Its Correlation With Clinicopathologic Features in Patients With Urothelial Carcinoma of the Bladder. Clin Genitourin Cancer. 2017;15:e187-e197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Garg M. Epithelial plasticity and metastatic cascade. Expert Opin Ther Targets. 2018;22:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1294] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 16. | Wang C, Jin H, Wang N, Fan S, Wang Y, Zhang Y, Wei L, Tao X, Gu D, Zhao F, Fang J, Yao M, Qin W. Gas6/Axl Axis Contributes to Chemoresistance and Metastasis in Breast Cancer through Akt/GSK-3β/β-catenin Signaling. Theranostics. 2016;6:1205-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Shin S, Im HJ, Kwon YJ, Ye DJ, Baek HS, Kim D, Choi HK, Chun YJ. Human steroid sulfatase induces Wnt/β-catenin signaling and epithelial-mesenchymal transition by upregulating Twist1 and HIF-1α in human prostate and cervical cancer cells. Oncotarget. 2017;8:61604-61617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Chen Z, Zhou L, Wang L, Kazobinka G, Zhang X, Han X, Li B, Hou T. HBO1 promotes cell proliferation in bladder cancer via activation of Wnt/β-catenin signaling. Mol Carcinog. 2018;57:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | World Health Organization Classification of Tumours. Pathology and genetics of male genital organs.Eble JN, Sauter G, Epstein JE, Sesterhenn IA (eds).. Lyon, France: IARC Press 2004; Available from: https://www.iarc.fr/wp-content/uploads/2018/07/BB7.pdf. |
| 20. | Lee H, Ro JY. Differential expression of GSK3β and pS9GSK3β in normal human tissues: can pS9GSK3β be an epithelial marker? Int J Clin Exp Pathol. 2015;8:4064-4073. [PubMed] |
| 21. | Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Xu X, Zou L, Yao Q, Zhang Y, Gan L, Tang L. Silencing DEK downregulates cervical cancer tumorigenesis and metastasis via the DEK/p-Ser9-GSK-3β/p-Tyr216-GSK-3β/β-catenin axis. Oncol Rep. 2017;38:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Li XQ, Yang XL, Zhang G, Wu SP, Deng XB, Xiao SJ, Liu QZ, Yao KT, Xiao GH. Nuclear β-catenin accumulation is associated with increased expression of Nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med. 2013;11:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Bilim V, Kawasaki T, Katagiri A, Wakatsuki S, Takahashi K, Tomita Y. Altered expression of beta-catenin in renal cell cancer and transitional cell cancer with the absence of beta-catenin gene mutations. Clin Cancer Res. 2000;6:460-466. [PubMed] |
| 25. | Ryves WJ, Harwood AJ. The interaction of glycogen synthase kinase-3 (GSK-3) with the cell cycle. Prog Cell Cycle Res. 2003;5:489-495. [PubMed] |
| 26. | Hafeez BB, Ganju A, Sikander M, Kashyap VK, Hafeez ZB, Chauhan N, Malik S, Massey AE, Tripathi MK, Halaweish FT, Zafar N, Singh MM, Yallapu MM, Chauhan SC, Jaggi M. Ormeloxifene Suppresses Prostate Tumor Growth and Metastatic Phenotypes via Inhibition of Oncogenic β-catenin Signaling and EMT Progression. Mol Cancer Ther. 2017;16:2267-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Najdi R, Holcombe RF, Waterman ML. Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog. 2011;10:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 28. | Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262-4266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 633] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 29. | Huang B, Zhang J, Zhang X, Huang C, Hu G, Li S, Xie T, Liu M, Xu Y. Suppression of LETM1 by siRNA inhibits cell proliferation and invasion of bladder cancer cells. Oncol Rep. 2017;38:2935-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Yuan H, Yu S, Cui Y, Men C, Yang D, Gao Z, Zhu Z, Wu J. Knockdown of mediator subunit Med19 suppresses bladder cancer cell proliferation and migration by downregulating Wnt/β-catenin signalling pathway. J Cell Mol Med. 2017;21:3254-3263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875-4887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 32. | White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 403] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 33. | Zheng H, Li W, Wang Y, Liu Z, Cai Y, Xie T, Shi M, Wang Z, Jiang B. Glycogen synthase kinase-3 beta regulates Snail and β-catenin expression during Fas-induced epithelial-mesenchymal transition in gastrointestinal cancer. Eur J Cancer. 2013;49:2734-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Stemmer V, de Craene B, Berx G, Behrens J. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene. 2008;27:5075-5080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Wu K, Zeng J, Zhou J, Fan J, Chen Y, Wang Z, Zhang T, Wang X, He D. Slug contributes to cadherin switch and malignant progression in muscle-invasive bladder cancer development. Urol Oncol. 2013;31:1751-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol. 2004;24:1505-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 38. | Nagarajan D, Melo T, Deng Z, Almeida C, Zhao W. ERK/GSK3β/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition. Free Radic Biol Med. 2012;52:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kupeli S, Morelli F, Su CC S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ