Published online Mar 24, 2019. doi: 10.5306/wjco.v10.i3.149
Peer-review started: November 10, 2018
First decision: November 14, 2018
Revised: December 17, 2018
Accepted: January 8, 2019
Article in press: January 9, 2019
Published online: March 24, 2019
Processing time: 134 Days and 16.8 Hours
Hypoxia-inducible factor 1α (HIF-1α) is a gene that regulates tumor survival, neovascularization and invasion. Overexpression of HIF-1α correlates with poor prognosis in hepatocellular carcinoma (HCC). RO7070179 is a HIF-1α inhibitor that decreases HIF-1α mRNA and its downstream targets, it could be a potential treatment in HCC.
To evaluate safety and preliminary activity of RO7070179 in patients with previously treated HCC, with focus on a patient with prolonged response to RO7070179.
In the preclinical study of RO7070179 in a HCC xenograft model, the mice were separated into 4 groups with each group received doses of 0, 3, 10 and 30 mg/kg for total 10 doses. HCC patients who failed at least one line of systemic treatment, received RO7070179 as a weekly infusion, each cycle is 6 wk. We evaluated the safety and HIF-1α mRNA levels of RO7070179.
Preclinical evaluation of RO7070179 in orthotopic HCC xenograft model showed no significant differences in HCC tumor weight between the 3 and 10 mg/kg groups. However, dose of 10 mg/kg of RO7070179, has shown 76% reduction of the amount of HIF-1α mRNA in HCC tissue. In the phase 1b study of RO7070179 in previously treated HCC patients, 8 out of 9 were evaluable: 1 achieved PR and 1 SD. The patient with PR responded after 2 cycles treatments, which has been maintained for 12 cycles. This patient also showed reduction in perfusion of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) after 1 cycle of treatment. After 1 cycle of treatment, both patients with PR and SD showed decrease in HIF-1α mRNA at the root of biopsies (each biopsy was divided into 2 specimens, the tip and the root).
RO7070179 can reduce HIF-1α mRNA level in HCC patients with SD or PR. It is well tolerated at 10 mg/kg, with transaminitis as the dose of increased toxicity. This study indicates that RO7070179 might benefit HCC patients, and an early signal for clinical benefit can potentially be predicted through changes in either mRNA level or DCE-MRI within 1 cycle of therapy.
Core tip: Overexpression of hypoxia-inducible factor 1α (HIF-1α) correlates with poor prognosis in hepatocellular carcinoma (HCC) patients. HIF-1a inhibitor decreases HIF-1a mRNA and its downstream targets, it could be a potential treatment in HCC.
- Citation: Wu J, Contratto M, Shanbhogue KP, Manji GA, O’Neil BH, Noonan A, Tudor R, Lee R. Evaluation of a locked nucleic acid form of antisense oligo targeting HIF-1α in advanced hepatocellular carcinoma. World J Clin Oncol 2019; 10(3): 149-160
- URL: https://www.wjgnet.com/2218-4333/full/v10/i3/149.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i3.149
Hypoxia-inducible factor 1α (HIF-1α) is a transcription factor that mediates adaptive responses to changes in tissue oxygenation. HIF-1α regulates genes that encode proteins controlling tumor metabolism, pH, neovascularization, drug resistance, invasion, autophagy, and cell survival[1-5]. Overexpression of HIF-1α protein has been found in multiple malignant tumors including hepatocellular carcinoma (HCC). Significant association between HIF-1α overexpression and patient mortality has been shown in HCC[6]. HCC indicates a strong unmet medical need, as sorafenib had been the only approved medication for 10 years from 2007-2017[7]. Overexpression of HIF-1α could be one of the mechanisms for resistance. Targeting HIF-1α can be a useful approach in HCC.
RO7070179 (EZN-2968) is an antisense oligonucleotide targeting HIF-1α in a synthetic locked nucleic acid form that are more resistant to degradation while maintaining the binding specificity. Previous phase I dose finding studies suggest that it is tolerated at a dose of 18 mg/kg IV once a week for three consecutive weeks followed by 3-wk off in a 6-wk cycle; or up to 13 mg/kg if dosed weekly. The dose limiting toxicity is hepatotoxicity as manifested by increase of liver function tests aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT). RO7070179 is accumulated in liver and kidney, and effectively suppresses HIF-1α mRNA level HIF-1α in post-treatment HCC tumor biopsies. However, a proof of mechanism study in patients with various solid tumors leads to an inconsistent result among the down-regulation of HIF-1α mRNA, HIF-1α protein, mRNAs of several HIF-1α target genes and tumor perfusion. This study is designed to focus on one single type of tumor HCC for a reevaluation RO7070179’s target and downstream effectors, based on the rationale that liver is one of the organs with the most abundant RO7070179 accumulation. Various translational endpoints were designed to study the mechanism including HIF-1α mRNA levels, protein levels of key HIF-1α targets, angiogenesis, tumor perfusion and therapeutic efficacy after 1 cycle of treatment with RO7070179.
Hep3B HCC cells were used to inject orthotopically into the liver of SCID-beige mice. Without treatment, nearly all mice died in 8 wk. Two weeks after Hep3B tumor cells were implanted, mice were separated into 4 groups (10 mice for each group) and received RO7070179 subcutaneous injection at doses of 0, 3, 10 and 30 mg/kg for a total of 10 doses (days 0, 1, 2, 3.5, 7, 10.5, 14, 17.5, 21 and 24.5). All mice were harvested at 6th weeks after HCC implantation for analysis. Tumor and normal liver were weighted, and then RNA extracted for examination of HIF-1α mRNA by qRT-PCR (Taqman) and tissue RO7070179 oligo levels. Other analysis of the liver and tumor specimens include genome-wide microarrays, proteins with IHC, angiogenesis with CD31 staining, Ki67, tumor apoptosis marker Casp-3.
The AllPrep DNA/RNA/miRNA universal kits were used for preparation of RNA. First, 250 ng of total RNA was prepped using the high capacity cDNA reverse transcription kit (Life Technologies) and final product normalized to 100 µL. Among them, 4 µL was used for qPCR by the TaqMan gene expression assay (Life Technologies) specific to HIF1α transcripts and a panel of 4 endogenous controls (GAPDH, UBE2D2, SFRS4 and CTBP1). To analyze the relative expression level of HIF-1α, the mean Ct value (Ct - cycle threshold, is a DNA quantification measurement in real time PCR that contains the number of cycles where the fluorescence surpasses the threshold of background level) of each gene expression assay was determined from 3 replicates. The linear range of Ct values was determined by the validation method. Delta Ct of HIF1α and the geometric mean of endogenous controls were calculated. The Delta Ct of each specimen and a Universal Human Reference RNA (UHRR) were used to determine the relative expression level of HIF-1α (Delta Ct) of each specimen.
Immunohistochemistry analysis
Immunohistochemistry (IHC) was performed per standard procedures using the following antibodies; HIF-1α (Cat#LS-C177574, from LS Bio); HIF-2α (#PA1-16510, Thermo); VEGF (#ab52917, Abcam); CD31 (#ab28364, Abcam); Ki67 (#VP-RM04, Vector); cleaved Caspase 3-Asp175 (#9664s, Cell Signaling).
The quantification of the oligo levels of RO7070179 is conducted with a hybridization-ELISA procedure for the determination of RO7070179. The method involves hybridization of the antisense oligonucleotide (containing phosphorothioate modifications in its backbone and LNA modification to some of its sugar moieties) to complementary oligonucleotide probes, one (capture probe) complementary to the 5’-half of the RO7070179 molecule, and one detection probe complementary to the 3’-half of the molecule. The capture probe is conjugated with biotin which mediates non-covalently binding to the surface of a Reacti-Bind Neutravidin coated black plate, whereas the detection probe is conjugated with digoxygenin. Measurement of the surface bound analyte is accomplished by the addition of a Fab-fragment of an antibody directed against digoxigenin. This Fab-fragment is coupled to an alkaline phosphatase which converts AttoPhos® to a fluorescent product that is directly related to the amount of bound RO7070179.
A phase 1b clinical trial was also conducted in 6 sites. Patients with advanced HCC who fulfill the criteria of HCC diagnosis according to the American Association for the Study of Liver Disease criteria and have failed at least one line of systemic therapy were enrolled. Other key eligibility criteria include Child-Pugh score A, ECOG 0-1, adequate organ function with liver function AST/ALT no higher than 3 X upper limit of normal (ULN) and platelet above 60000/µL, bilirubin and creatinine no higher than 2 X ULN. RO7070179 was administered as a 2-h weekly IV infusion in 6-wk cycles after two loading doses of 13 mg/kg RO7070179 administered on Days 1 and 4 in Week 1 of Cycle 1. If unacceptable toxicities were observed in 30% of existing patients, subsequent patients would have a dose reduction to 10 mg/kg weekly or 6 mg/kg if still not tolerable.
Extensive translational analyses were included in for the study. All patients should have a dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to analyze the tumor perfusion in baseline and 1 cycle after treatment to evaluate tumor perfusion using Ktrans and other parameters. The result of DCE-MRI is used to select a region in tumor for biopsy in baseline. The region selected correlates to no evidence of necrosis and relatively lower blood flow, hence potentially higher HIF-1α expression. Patients also had a second biopsy after 1 cycle of treatment at the same site for comparison. Clinical efficacy was assessed by contrast CT every 3 mo until progression or death. The biopsy was targeted to have a core of 20 mm in length, with tips of both ends saved in cryovials for RNA extraction, and the next 6 mm for fixation in 10% formalin for histology examination and IHC. The remaining specimens were snap frozen for measuring of drug levels as part of the tumor PK analysis.
Four groups of mice were treated with RO7070179 at the doses of 0, 3, 10 and 30 mg/kg for total 10 doses. Mice in the group that received the highest dose at 30 mg/kg/dose failed to tolerate treatment with 2 deaths at day 6, 2 deaths at day 7 and 3 deaths at day 8. The remaining 3 mice of this group were then sacrificed ahead of schedule on day 9. The liver enzymes AST/ALT/ALP (alkaline phosphatase) of these 3 mice all showed significant elevation above 800, consistent with hepatotoxicity from the study medication. Mice in the remaining 3 groups were sacrificed at the scheduled time at 1 cycle. AST/ALT in blood of mice receiving 3 and 10 mg/kg RO7070179 also revealed a dose-dependent increase, consistent with RO7070179-related liver toxicity. Liver in the three remaining groups were dissected first and compared in weights. The weights of the control and those receiving 3 mg/kg RO7070179 showed no difference, but that receiving 10 mg/kg had a statistical increase compared with the other two groups, which is potentially related to a compensatory proliferation due to hepatotoxicity. Liver tumors were then dissected and weighted, and no statistical difference was observed.
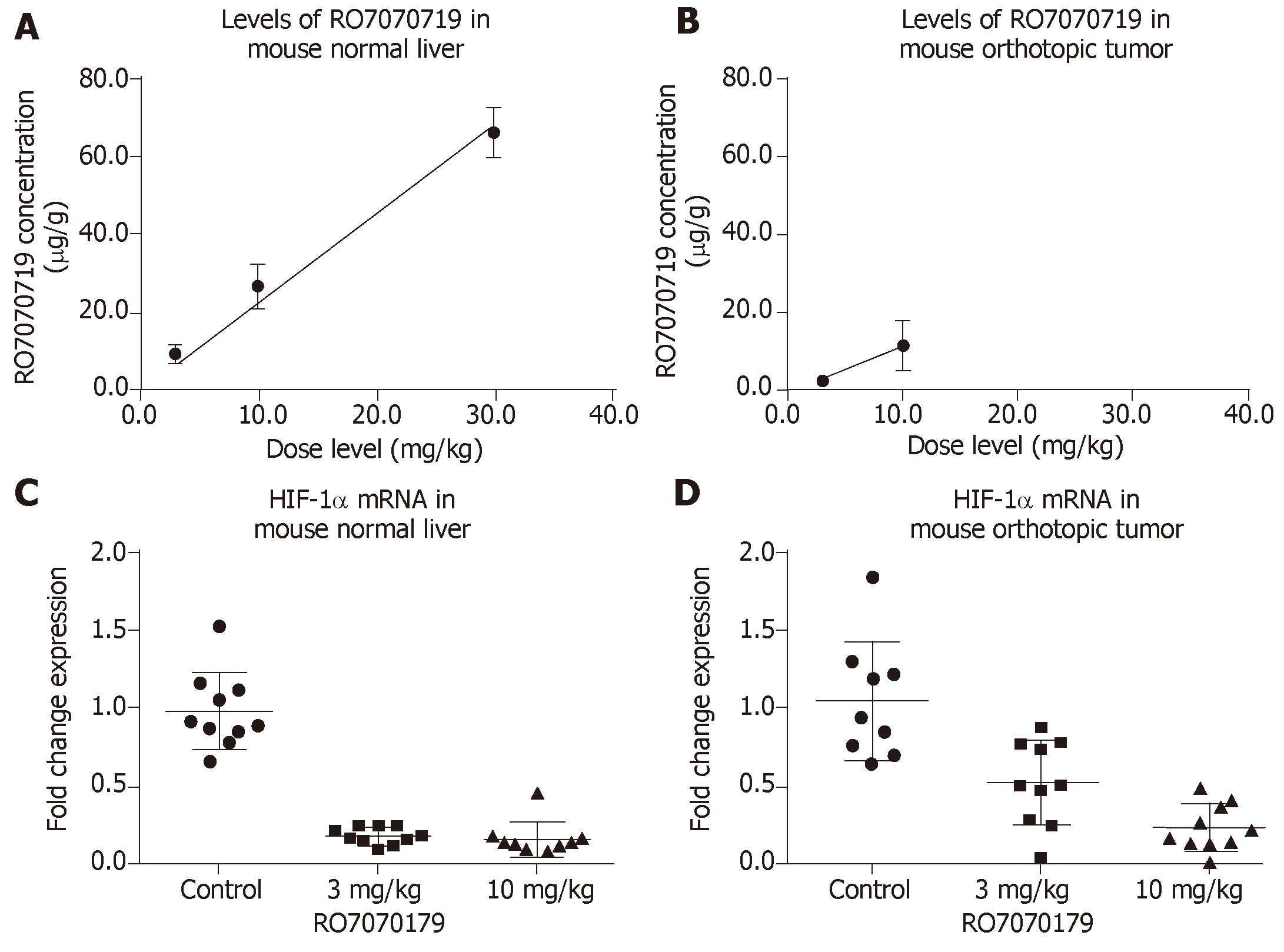
Concentrations of RO7070719 in plasma, tumor and healthy liver followed dose-linear kinetics over this dose range. The mean accumulated liver concentrations were 9.92, 27.1 and 66.6 µg/g tissue at the 3, 10, and 30 mg/kg/administration, respectively (Figure 1A). The mean concentration in tumors was 2.38 and 11.5 µg/g tissue at 3.0 and 10.0 mg/kg/administration, respectively, indicating that tumors were less exposed to RO7070719 than normal liver tissue (Figure 1B).
Based on the TaqMan qPCR analysis of HIF-1α mRNA (the primary target of RO7070179) in normal liver, the groups received 3 and 10 mg/kg achieved 82% and 84%, decreases in the HIF1α mRNA level when compared to that of the control group (Figure 1C). When compared to control in the orthotopic tumor the levels of HIF-1α mRNA showed a 53% decrease in the group of 3 mg/kg compared to control, and a 76% of decrease in the group of 10 mg/kg (Figure 1D). This result support the target engagement of RO7070179 in both normal liver and orthotopic tumor, and normal liver is more sensitive to RO7070179 than the orthotopic tumor.
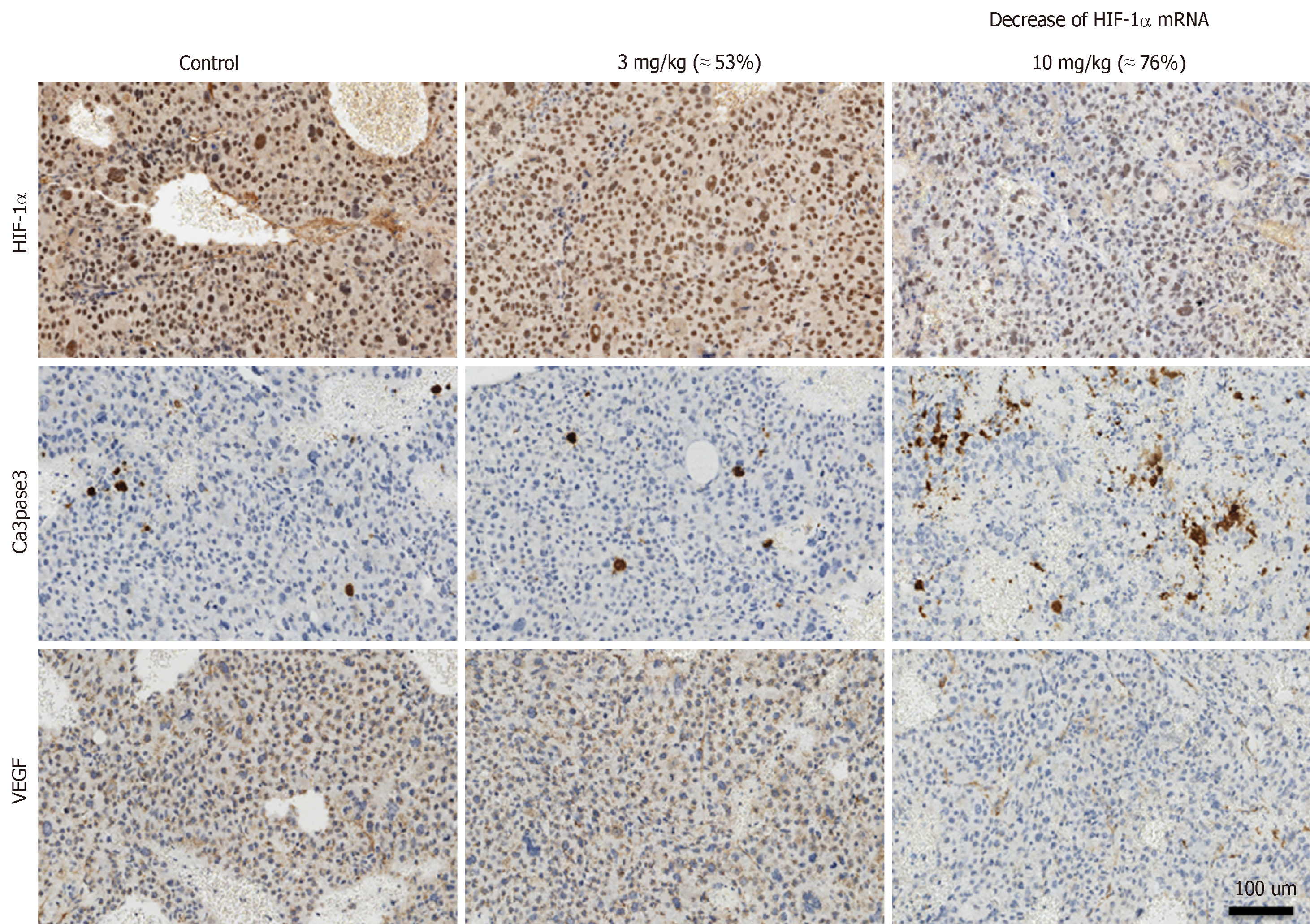
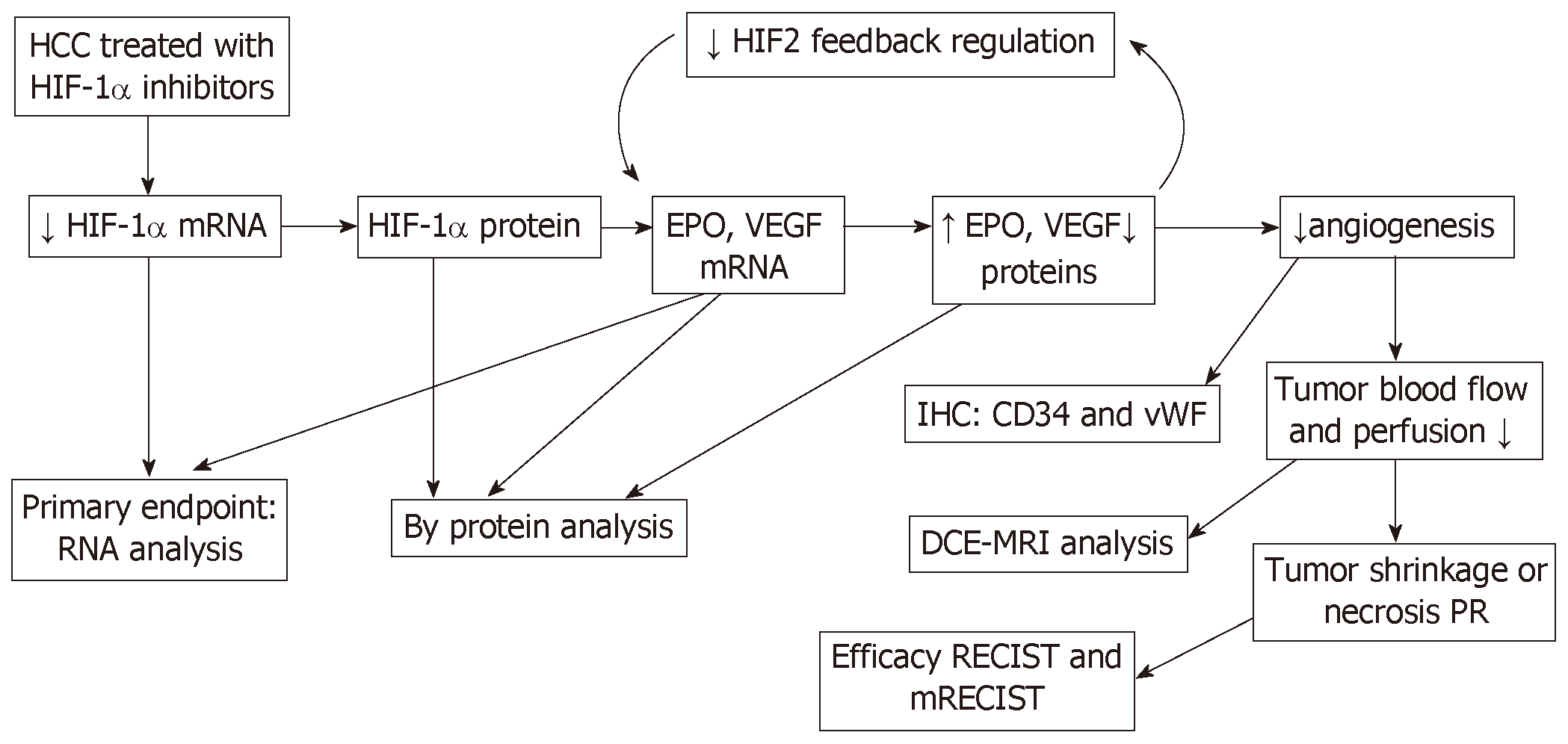
Immunohistochemical analysis was conducted in tumor to examine the protein levels of HIF-1α and its downstream effector VEGF, apoptosis marker activated Caspase 3 as shown in Figure 2. The levels of HIF-1α showed a barely visible decrease in protein level in the group receiving 3 mg/kg RO7070179, and an obvious decrease in the group receiving 10 mg/kg RO7070179, which is consistent with the HIF-1α mRNA decrease of 53% and 76%, respectively. Similarly, minimal change was noticed in the levels of VEGF and activated Caspase 3 in the tumor treated with 3 mg/kg; whereas an obvious decrease in VEGF and an increase of activated Caspase 3 were observed in tumors treated with 10 mg/kg, which is consistent with those of the HIF-1α mRNA. These results indicate that the biological effect of HIF-1α decrease can be further extended to VEGF and eventually to an induction of caspase cascade. We created the biomarker analysis flow chart to illustrate the mechanism of action of HIF-1α inhibitors in HCC (Figure 3)[8].
| Demographic and baseline patient characteristics (n = 8 patients, 100%) | |
| Age (years old) | |
| < 65 | 3 (37.5%) |
| ≥ 65 | 5 (62.5%) |
| Race | |
| Asian | 2 (25%) |
| White | 3 (37.5%) |
| African | 3 (37.5%) |
| American | |
| Ethnicity | |
| Hispanic | 2 (25%) |
| Non-Hispanic | 6 (75%) |
| Weight (kg) | |
| Minimum | 56.7 |
| Median | 71.95 |
| Maximum | 111.3 |
| Height (cm) | |
| Minimum | 161 |
| Median | 170 |
| Maximum | 179 |
| BMI (kg/m2) | |
| Minimum | 19.2 |
| Median | 26.68 |
| Maximum | 39.9 |
The positive animal study prompted a clinical trial to investigate the activity of RO7070179 in HCC patients who have failed at least one line of systemic therapy, predominately sorafenib. A total of 9 male patients were enrolled in the study with age group between 45 to 75 years. Three of 8 patients (37.5%) were below 65 years and the remaining 5 patients (62.5%) were more than 65 years, the median age was 69 years. Two of 8 patients (25.0%) were Asian, 3 of 8 patients (37.5%) were Black or African American and 3 of 8 patients (37.5%) were white. Two of 8 patients (25.0%) were from Hispanic or Latino origin, while six of 8 patients (75.0%) were from non-Hispanic or Latino origin. The median weight (kg) of patients at baseline was 71.95. The minimum and maximum weight of patients enrolled for study was 56.7 and 111.3 respectively. The median height (cm) of patients at baseline was 170. The minimum and maximum height of patients enrolled for study was 161 and 179 respectively. The median BMI (kg/m2) of patients at baseline was 26.68. The minimum and maximum BMI (kg/m2) of patients enrolled for study was 19.2 and 39.9 respectively.
The study started with a dose at 13 mg/kg IV at days 1 and 4 in week 1 for loading, followed by 13 mg/kg weekly afterwards. The dose was reduced to 10 mg/kg afterwards due to observation of increased toxicity in 3 of the first 6 patients. The toxicity of RO7070179 was considered related to drug accumulation in liver, and HCC patients have less liver reserve compared to those with other malignancies tested in prior phase I studies. Subsequently dose was reduced to 10 mg/kg weekly and it was well tolerated in the next 3 patients.
The most common adverse events (AEs) were ALT increased (5 patients, 55.6%), AST increased (5 patients, 55.6%), blood creatinine increased (4 patients, 44.4%), fatigue (4 patients, 44.4%), chills (3 patients, 33.3%) and decreased appetite (3 patients, 33.3%). The most common grade 3 or 4 AEs were ALT increased (4 patients, 44.4%) and AST increased (4 patients, 44.4%).
Only 3 of the 9 patients were able to complete 2 cycles of treatments and had efficacy evaluation by a follow-up CT scan (Figure 4). One patient, who had a prior surgical resection and recurred in the surgical margin with metastatic to lung, had a robust efficacy. The pretreatment liver lesion was as large as 6.7 cm in size with multiple lung metastasis. With 2 cycles of treatments, this patient achieved PR by mRECIST criteria, and the lesion in liver decreased to 3.7 cm along with reduction in size and number of lung lesions. As of today, this patient has completed the study (12 cycles) and stayed in PR as of May 14, 2018 of CT scan. The lung lesions complete disappeared and liver lesion down to 2.3 cm without contrast enhancement. Two other patients also completed 2 and 4 cycles, respectively. One had SD and one with PD at the first assessment post cycle 2.
The mRNA of HIF-1α was first examined by qPCR. Specimens 1 are the tumor specimens located at the tip of the biopsy cores and specimens 2 are those at the other end of biopsy, called root so that different locations of biopsies can be investigated to detect any potential spatial heterogeneity. The results are shown in Table 2. Unfortunately, no definitive trend can be detected for either specimens 1 or 2, or before and after RO7070179 treatment. This suggests a significant temporal and spatial relationship exist in tumor.
As of safety cut-off date, 28 Feb 2017, efficacy data is represented as “spaghetti plots” for HIF-1α in specimens obtained prior to the administration (screening) of the HIF-1α inhibitor and after cycle 1 of (6 wk) treatment. Dots represent individual specimens; lines connect specimens from individual patients taken at consecutive time interval. Expression is shown as the difference in the cycle threshold (Ct) value between the HIF-1α gene and the mean Ct of the reference genes, higher values correspond to stronger expression.
Table 2 shows gene expression as delta Ct value of specimen 01 in patient. The super-responder patient was observed with lower delta Ct value as compared at screening corresponding to up regulation expression of gene as compared at screening. It showed down regulation of HIF-1α gene.
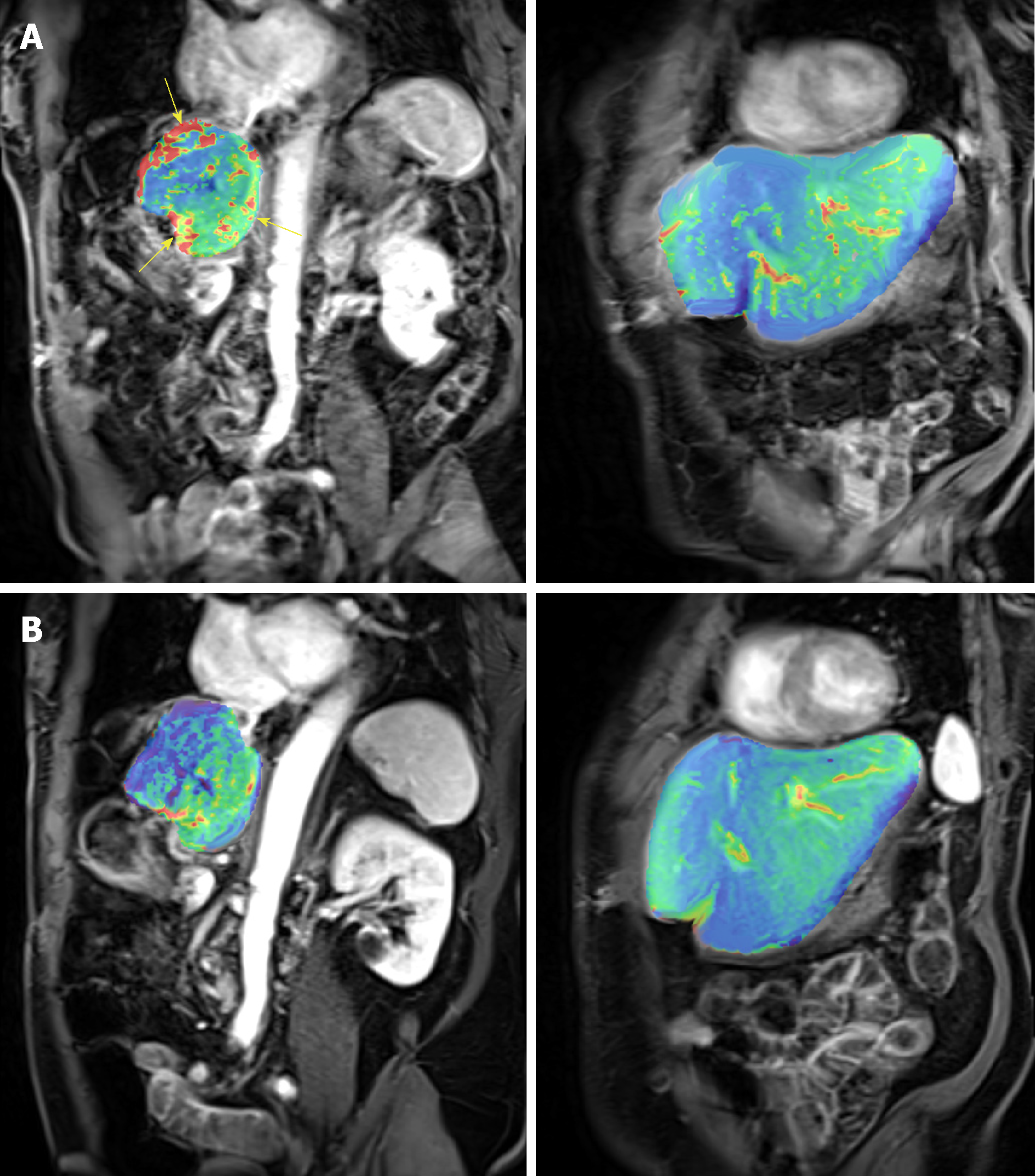
The DEC-MRI scans of the patient who achieved durable PR were examined in details. Figure 5 shows the heat map of the perfusion scan in liver and tumor before (Figure 5A) and after the first cycle of treatment (Figure 5B), and a clear reduction was demonstrated after treatment. The Ktrans, a parameter representing perfusion, was calculated in tumor as shown in Table 3.
| Pre-treatment | Post-treatment |
| Tumor | |
| Ktrans pre-treatment: 849 × 10-3/min | Ktrans post-treatment: 122 × 10-3/min |
| Liver (left lobe) | |
| Ktrans pre-treatment: 245 × 10-3/min | Ktrans post-treatment: 255 × 10-3/min |
High level of HIF-1α mRNA indicates poor prognosis in HCC[9,10]. During hypoxia, HIF-1α does not go through degradation by enzyme prolyl hydroxylase, instead it is stabilized and accumulated in the nucleus. HIF-1α then activates the transcription of target genes such as vascular endothelial growth factor (VEGF) that contributes angiogenesis. In addition, it stimulates transcriptions of growth factors such as insulin-like growth factor-2 and transforming growth factor-α that lead to cell proliferation. It also induces epithelial-mesenchymal transition by inhibiting E-Cadherin, which maintains the epithelial integrity, thus stimulates tumor invasion. HIF-1α therefore leads to neovascularization, proliferation, and metastasis in various tumors, including HCC[11]. RO7070179, a HIF-1α inhibitor has been studied in HCC xenograft models and later on in HCC patients.
In the orthotopic HCC mice model, the absorption of RO7070179 is higher in normal liver compared to HCC tissue. Therefore, after treatment with RO7070179, the level of HIF-1α mRNA (the target of RO7070179 inhibition) is lower in normal liver than in HCC. In order to achieve adequate reduction of HIF-1α protein or mRNA level in HCC, a high dose of RO7070179 is required. This was illustrated in the preclinical study using orthotopic HCC xenograft mice. The groups that received 3 and 10 mg/kg exhibited dose dependent-increase of AST/ALT level, while the highest dose of 30 mg/kg led to liver toxicity (7 out of 10 mice died, and the remaining had excessively high level of the AST/ALT beyond 800).
No difference in HCC tumor weights were shown between the two groups, indicating that the lower dose (3 mg/kg) had produced similar tumor volume reduction compared to the higher dose (10 mg/kg). Liver toxicities were explained by the pharmacokinetics (PK) data, which showed that RO7070179 achieved a higher concentration in normal liver than the tumor, in a dose dependent manner.
At a dose of 10 mg/kg, RO7070179 decreased the level of HIF-1α mRNA by 76%, which was sufficient to reduce the protein level of HIF-1α and the downstream target VEGF of HIF-1α and induction of apoptosis (shown by activated caspase 3) (Figure 2). However, at the dose of 3 mg/kg, RO7070179 was able to reduce mRNA level by 53%, but HIF-1α protein level did not change, nor was the downstream target of HIF-1α mRNA (VEGF) or apoptosis based on activated caspase 3. These findings suggest that it is necessary to suppress at least 75% of the mRNA level of HIF-1α in order to accomplish adequate inhibition of HCC tumor clinically.
In our phase 1b study of RO7070179 in previously treated HCC patients, the primary end point of HIF-1α mRNA level change after 1 cycle of treatment was not met, and the study was terminated early. The secondary end point of this study regarding the safety, efficacy, and pharmacokinetics of RO7070179 could not be done due to the small number of patient enrolled. However, the extra-ordinary response observed in one single patient warrant us to undergo detailed investigation of this this patient to learn any lesion from this patient. We will focus our discussion on the super-responder.
DCE-MRI changes correlate with response: This super-responder achieved PR after 2 cycles of treatment (Figure 5) and this response is still maintained after 12 cycles. The response indicates that RO7070179 induces a rapid response as early as 2 cycles which can be potentially long lasting. In consistent with the MRI tumor shrinkage, Ktrans in DCE-MRI also exhibit a significant decrease after 1 cycle treatment (Table 3). This indicated that the reduction of tissue perfusion in the tumor after 1 cycle of treatment reflected true decrease in tissue perfusion, as liver background perfusion did not change from the baseline. Reduction in tissue perfusion on DCE-MRI correlates with an early response to therapy.
In a prospective study of 31 HCC patients who were treated with sorafenib and tegafur/uracil, the progression free survival (PFS) was 29.1 wk in patients with reduction of Ktrans ≥ 40% vs 8.7 wk in patients with reduction of Ktrans < 40%. This change was demonstrated as early as 14 d on treatment[12]. The overall survival (OS) is also longer in patients with Ktrans ≥ 40% compared to patients with Ktrans < 40% (53 wk vs 14.9 wk, respectively)[12]. Even though the patient with PR has not reached PFS as of January 27, 2018 (18 mo), our study is consistent with previously published literature that Ktrans ≥ 40% (Table 3) can lead to longer PFS.
In the super-responder patient with PR, HIF-1α mRNA levels both decreased after 1 cycle of treatment. Interestingly, this change was much more pronounced in specimen 2. We attribute this to tumor heterogeneity as different part of tumor could have spatial and temporal variation in tumor blood perfusion, resulting in different levels of HIF-1α. This explains the usage of tumor biopsy for analysis of HIF-1α mRNA or protein levels is not reliable and poorly correlated with the clinical outcome. Similar variation in HIF-1α mRNA was observed in other patients. For example in the patient who achieved SD, specimen 1 was high after 1 cycle treatment, while specimen 2 is lower than specimen 1. Both patients with PR and SD showed decrease of the HIF-1α mRNA after 1 cycle treatment at specimen 2. On the other hands, patient with progression of disease (PD) showed increase level of HIF-1α mRNA at specimen 2.
Each HIF-1α inhibitor has a slight different mechanism of action. For instance, PX-478 works in transcriptional and translation level, causes reduction both in HIF-1α mRNA and protein level[13]. Other HIF-1α inhibitors only decrease the HIF-1α mRNA level such as GL331[14]. It works by inhibiting the target genes such as VEGF. RO7070179, an antisense oligonucleotide that also only decrease HIF-1α mRNA level with minimal effect on HIF-1α protein level. HIF-1α inhibitors can cause target gene downstream changes by inhibiting the tumor-induced angiogenesis, regardless if there is a decrease in HIF-1α mRNA alone or in both mRNA and protein level[15,16]. This also illustrates the difficulty in using HIF-1α mRNA or protein as the biomarkers for clinical prediction.
RO7070179 achieved PR in 1 out of 9 HCC patients, an early response can potentially be predicted through either mRNA level change or reduction in tissue perfusion on DCE-MRI within 1 cycle of therapy using the root of the biopsy specimen.
HIF-1α promotes tumor survival, neovascularization and tumor invasion especially in HCC. RO7070179 is an HIF-1α inhibitor that is well tolerated at 10 mg/kg, with transaminitis as the dose limiting toxicities. It reduces HIF-1α mRNA level and downstream targets, it could be a potential treatment in HCC. In a super-responder HCC patient, HIF-1α mRNA level decreased at both specimen 1 (tip of biopsy) and specimen 2 (root of biopsy) after 1 cycle (6 wk) of RO7070179 treatment. It also showed an early signal response from DCE MRI analysis by decrease of perfusion in HCC tumor after 1 cycle of treatment. In a super-responder HCC patient who achieved PR response at 2 cycle (12 wk), continued until 12 cycles. This study indicates that RO7070179 might benefit HCC patients and its response can be predicted through early 6 wk of DCE MRI and biopsy of tumor. This result will require more patients for validation.
Hypoxia-inducible factor 1α (HIF-1α) is a gene that regulates tumor survival, neovascularization and invasion. Overexpression of HIF-1α correlates with poor prognosis in hepatocellular carcinoma (HCC). RO7070179 is an HIF-1α inhibitor that decreases HIF-1α mRNA and its downstream targets, it could be a potential treatment in HCC.
HIF-1α inhibitor such as RO7070179 has a potential benefit for HCC patients in the future. Eventhough, in this study there was only one super-responder HCC patients that showed PR with RO7070179. It needs validation in a large population and studies.
The purpose of this study is to evaluate safety and preliminary activity of RO7070179 in patients with previously treated HCC, with focus on a patient with prolonged response to RO7070179.
The research methods is a prospective study in preclinical study of RO7070179 in a HCC xenograft model, the mice were separated into 4 groups with each group received doses of 0, 3, 10 and 30 mg/kg for total 10 doses. HCC patients who failed at least one line of systemic treatment, received RO7070179 as a weekly infusion, each cycle is 6 wk. We evaluated the safety and HIF-1α mRNA levels of RO7070179.
Preclinical evaluation of RO7070179 in orthotopic HCC xenograft model showed no significant differences in HCC tumor weight between the 3 and 10 mg/kg groups. However, dose of 10 mg/kg of RO7070179, has shown 76% reduction of the amount of HIF-1α mRNA in HCC tissue. In the phase 1b study of RO7070179 in previously treated HCC patients, 8 out of 9 were evaluable: 1 achieved PR and 1 SD. The patient with PR responded after 2 cycles treatments, which has been maintained for 12 cycles. This patient also showed reduction in perfusion of DCE-MRI after 1 cycle of treatment. After 1 cycle of treatment, both patients with PR and SD showed decrease in HIF-1α mRNA at the root of biopsies (each biopsy was divided into 2 specimens, the tip and the root).
RO7070179 can reduce HIF-1α mRNA level in HCC patients with SD or PR. It is well tolerated at 10 mg/kg, with transaminitis as the dose of increased toxicity. This study indicates that RO7070179 might benefit HCC patients, and an early signal for clinical benefit can potentially be predicted through changes in either mRNA level or DCE-MRI within 1 cycle of therapy.
RO7070179 has shown encouraging activity in previously treated HCC patients, however such activity needs to be further evaluated in a phase II study with more patients.
| 1. | Huang GW, Yang LY, Lu WQ. Expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in hepatocellular carcinoma: Impact on neovascularization and survival. World J Gastroenterol. 2005;11:1705-1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1877] [Cited by in RCA: 1969] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 4. | Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 481] [Article Influence: 18.5] [Reference Citation Analysis (3)] |
| 5. | Jiang BH, Agani F, Passaniti A, Semenza GL. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328-5335. [PubMed] |
| 6. | Dai CX, Gao Q, Qiu SJ, Ju MJ, Cai MY, Xu YF, Zhou J, Zhang BH, Fan J. Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer. 2009;9:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10523] [Article Influence: 584.6] [Reference Citation Analysis (9)] |
| 8. | Greenberger LM, Horak ID, Filpula D, Sapra P, Westergaard M, Frydenlund HF, Albaek C, Schrøder H, Ørum H. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol Cancer Ther. 2008;7:3598-3608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Zheng SS, Chen XH, Yin X, Zhang BH. Prognostic significance of HIF-1α expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e65753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Xiong XX, Qiu XY, Hu DX, Chen XQ. Advances in Hypoxia-Mediated Mechanisms in Hepatocellular Carcinoma. Mol Pharmacol. 2017;92:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Cohen RB, Olszanski A, Figueroa J, Hurwitz H, Lokiec F, Rezai K, Berkowitz N, Buchbinder A. Down-modulation of mRNA by EZN-2968, an hypoxia-inducible factor-1α (HIF-1α) mRNA antagonist, administered in adult patients with advanced solid tumors. Cancer Res. 2011;71:LB-407-LB-407. [DOI] [Full Text] |
| 12. | Hsu CY, Shen YC, Yu CW, Hsu C, Hu FC, Hsu CH, Chen BB, Wei SY, Cheng AL, Shih TT. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011;55:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Chang H, Shyu KG, Lee CC, Tsai SC, Wang BW, Hsien Lee Y, Lin S. GL331 inhibits HIF-1alpha expression in a lung cancer model. Biochem Biophys Res Commun. 2003;302:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Ding L, Chen X, Jing K, Wang H, Zhang W. Inhibition of the VEGF expression and cell growth in hepatocellular carcinoma by blocking HIF-1alpha and Smad3 binding site in VEGF promoter. J Huazhong Univ Sci Technolog Med Sci. 2006;26:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Yasuda S, Arii S, Mori A, Isobe N, Yang W, Oe H, Fujimoto A, Yonenaga Y, Sakashita H, Imamura M. Hexokinase II and VEGF expression in liver tumors: correlation with hypoxia-inducible factor 1 alpha and its significance. J Hepatol. 2004;40:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
CONSORT 2010 Statement: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Morelli F, Ozyigit G, Zhou J S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ