Published online Feb 24, 2019. doi: 10.5306/wjco.v10.i2.52
Peer-review started: September 25, 2018
First decision: November 1, 2018
Revised: November 8, 2018
Accepted: January 5, 2019
Article in press: January 6, 2019
Published online: February 24, 2019
Processing time: 153 Days and 17.3 Hours
Continuous inhibition of angiogenesis beyond progression is an emerging treatment concept in the management of metastatic colorectal cancer patients with prior bevacizumab exposure. Treatment options include the continuation or reintroduction of bevacizumab during the second-line chemotherapy or switching to a different antiangiogenic monoclonal antibody such as aflibercept or ramucirumab. In the selection of treatment, patient-based factors such as performance status, age, tumor burden, and tolerance and sensitivity to the first-line bevacizumab-based therapy, as well as treatment-related factors such as toxicity, efficacy, and cost, should be taken into consideration.
Core tıp: Anti-angiogenic treatment is an essential part of the current armamentarium against metastatic colorectal cancer (mCRC). For now, bevacizumab is the only drug licensed for the treatment of chemotherapy-naïve patients with mCRC. However, patients undergoing first-line bevacizumab-based therapy eventually develop disease progression and become candidates for second-line chemotherapy. In this manuscript, we discuss the available anti-angiogenic therapeutic strategies that have been proven to be useful in the treatment of patients with mCRC in whom first-line bevacizumab-based therapy was ineffective.
- Citation: Kanat O, Ertas H. Existing anti-angiogenic therapeutic strategies for patients with metastatic colorectal cancer progressing following first-line bevacizumab-based therapy. World J Clin Oncol 2019; 10(2): 52-61
- URL: https://www.wjgnet.com/2218-4333/full/v10/i2/52.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i2.52
The medical treatment of metastatic colorectal cancer (mCRC) has become more diversified over the past few decades owing to the successful integration of targeted therapy agents, which block either epidermal growth factor signaling pathway or angiogenesis, into cytotoxic drug combinations[1]. Concordantly, a dramatic improvement in survival has been achieved among patients suffering from mCRC. Moreover, extensive preclinical efforts were able to identify additional targetable molecular alterations in these patients such as BRAF mutation, human epidermal growth factor receptor 2 amplification, and microsatellite instability[2-4]. The clinical application of compounds that can inhibit signaling pathways in cancer cells activated by these genetic events seems to provide additional survival gains in selected patients with mCRC.
Among the molecular targets mentioned above, tumor-driven angiogenesis is still an attractive target in mCRC[5-7]. The United States Food and Drug Administration has approved a total of four drugs that block angiogenesis (bevacizumab, aflibercept, ramucirumab, and regorafenib) in the treatment of mCRC (Table 1). Of these, bevacizumab is the only drug licensed for the treatment of chemotherapy-naïve patients with mCRC.
| Agent | Class | Target | Indication | Approved for | Recommended dose |
| Bevacizumab | Humanized Moab | VEGF-A | First- and second-line | Use in combination with oxaliplatin and irinotecan-based chemotherapy | 5 mg/kg or 10 mg/kg i.v. every 2 wk |
| Aflibercept | Fully human Moab | VEGF-A, -B, and PIGF | Second-line | Use in combination with FOLFIRI | 4 mg/kg i.v. every 2 wk |
| Ramucirumab | Fully human Moab | The extracellular domain of VEGFR-2 | Second-line | Use in combination with FOLFIRI | 8 mg/kg i.v. every 2 wk |
| Regorafenib | Oral multikinase inhibitor | VEGFR-1, -2, and -3 (in addition to RET, KIT, PDGFR, and FGFR | Beyond second-line | Single-use | 160 mg once daily, days 1-21 of 28-d cycle |
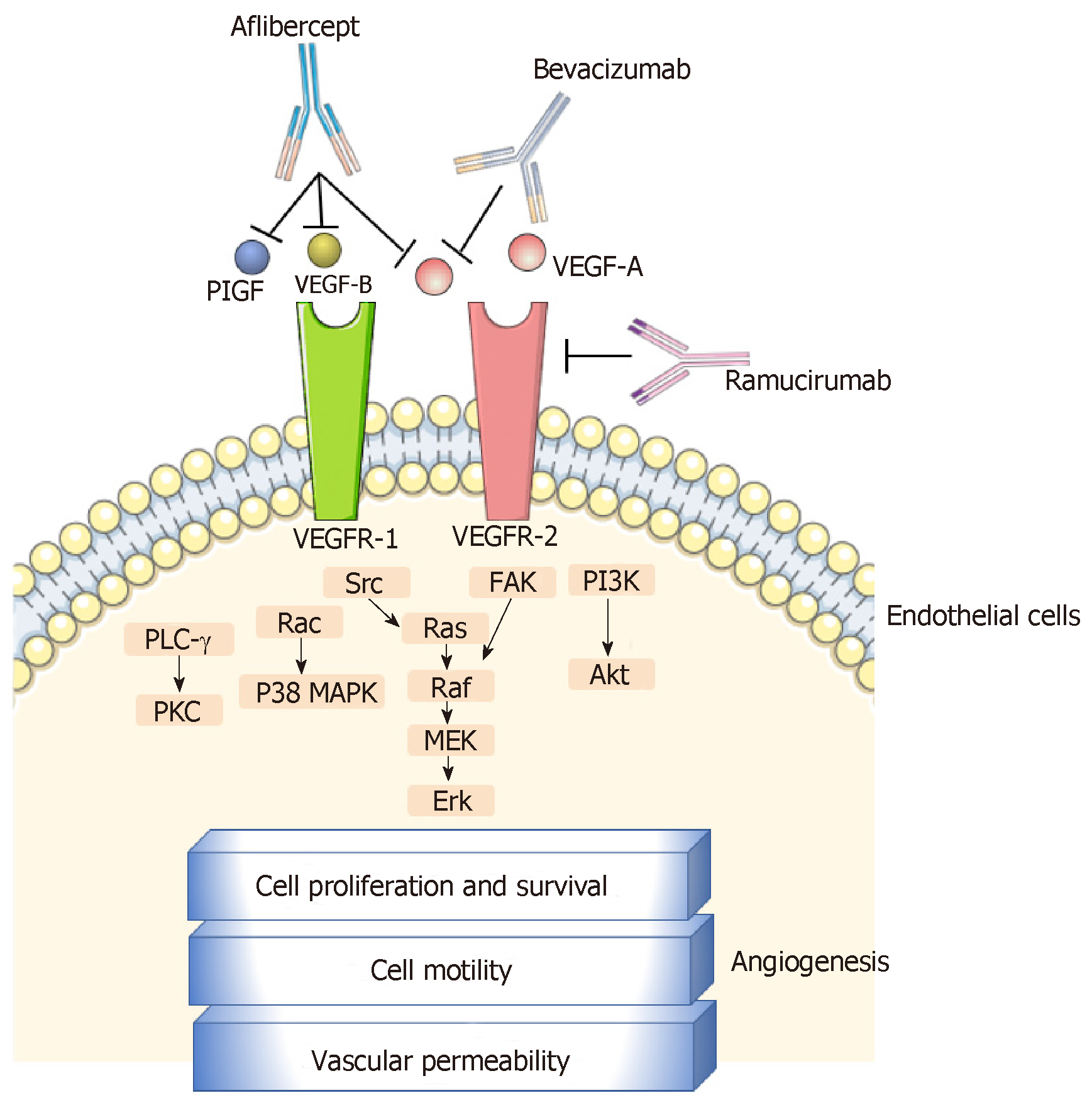
Bevacizumab is a murine-derived monoclonal antibody (muMAb A4.6.1) that inhibits angiogenesis by targeting the vascular endothelial growth factor (VEGF)-A. Belonging to the VEGF family, (VEGF)-A is a crucial angiogenic cytokine (Figure 1) that is produced by cancer and benign stromal cells, particularly in a hypoxia-inducible factor-1-dependent manner. It triggers angiogenic signals via interaction with endothelial cell-surface tyrosine kinase receptors [VEGF receptor-1 (VEGFR-1) and -2 (VEGFR-2)]. The binding of VEGF-A to the extracellular domain of these receptors induces their dimerization and autophosphorylation and the subsequent activation of intracellular pathways that contribute to cell proliferation (e.g., phospholipase-C-gamma and extracellular signal-regulated kinases 1/2 pathway), migration (e.g., focal adhesion kinase and p38 pathway), and survival (e.g., phosphatidylinositol 3-kinase/Akt pathway)[8-11]. Other members of the VEGF family, such as VEGF-B, -C, and -D, and placental growth factor (PIGF) play supporting roles in the process of angiogenesis[10,12].
Bevacizumab is conventionally administered in combination with oxaliplatin- or irinotecan-based doublet [i.e., FOLFOX (5-FU, leucovorin, and oxaliplatin) and FOLFIRI (5-FU, leucovorin, and irinotecan)] or triplet [i.e., FOLFOXIRI (5-FU, leucovorin, oxaliplatin, and irinotecan)] chemotherapy regimens. A recent meta-analysis of the first-line chemotherapy for mCRC confirmed that the addition of bevacizumab results in a significant improvement in progression-free survival [PFS; hazard ratio (HR) 0.66, P < 0.0001] and overall survival (OS; HR 0.84, P = 0.0001), compared with chemotherapy alone[13]. In addition, the clinical activity of bevacizumab is not influenced by currently validated predictors of treatment response and/or survival outcomes in mCRC, such as the mutational status (KRAS and BRAF genes) and anatomic location (left vs right side of the colon) of the primary tumor.
On the other hand, patients undergoing first-line bevacizumab-based therapy eventually develop disease progression (usually within 9 mo) and become candidates for second-line chemotherapy[13]. Available data strongly favor the continuous inhibition of angiogenesis (using maintenance bevacizumab therapy or switching to another antiangiogenic monoclonal antibody) during second-line chemotherapy to achieve a satisfactory clinical outcome[14,15]. In this article, we discuss therapeutic strategies that have been proven to be useful in the treatment of patients with mCRC in whom first-line bevacizumab-based therapy was ineffective.
Several United States-based non-randomized observational studies, such as the Bevacizumab Regimens: Investigation of Treatment Effects and Safety and the Avastin Registry: Investigation of Effectiveness and Safety, initially reported that the continuation of bevacizumab during second-line chemotherapy had a beneficial impact on the survival of patients with mCRC in whom first-line bevacizumab-based therapy was ineffective[16-18]. Further evidence in support of this treatment strategy was provided by the phase III ML18147 trial (Table 2)[19].
| Study | Type of study | The proportion of patients who received prior BEV | Treatment arms (No. of patients) | ORR (%) | mPFS (mo) | HR | mOS (mo) | HR |
| BRiTE[16] | Observational cohort | 100% | CT + BEV (642) | NA | 19.2 | 0.49 | 31.8 | 0.48 |
| CT alone (531) | NA | 9.5 | 19.9 | |||||
| No treatment (253) | NA | 3.6 | 2.05 | 12.6 | ||||
| ARIES[17] | Observational cohort | 100% | CT + BEV (438) | NA | 14.4 | 0.84 | NA | |
| CT alone (667) | NA | 10.6 | NA | |||||
| Cartwright et al[18] | Observational cohort | 100% | CT+ BEV (267) | NA | 14.6 | 0.74 | 27.9 | 0.76 |
| CT alone (306) | NA | 10.1 | 21.4 | |||||
| ML18147[19] | Phase 3 | 100% | FOLFOX/FOLFIRI + BEV (409) | 5 | 5.7 | 0.68 | 11.2 | 0.81 |
| FOLFOX/FOLFIRI + placebo (411) | 4 | 4.1 | 9.8 | |||||
| BEBYP[20] | Phase 3 | 100% | FOLFOX/FOLFIRI + BEV (92) | 21 | 6.8 | 0.70 | 15.5 | 0.77 |
| FOLFOX/FOLFIRI + placebo (92) | 17 | 5.0 | 14.4 | |||||
| VELOUR[27] | Phase 3 | 30% | FOLFIRI + Aflibercept (612) | 19.8 | 6.9 | 0.76 | 13.5 | 0.82 |
| FOLFIRI + placebo (614) | 11.1 | 4.7 | 12.0 | |||||
| RAISE[37] | Phase 3 | 100% | FOLFIRI + Ramucirumab (536) | 13.4 | 5.7 | 0.79 | 13.3 | 0.84 |
| FOLFIRI + placebo (536) | 12.5 | 4.5 | 11.7 |
The ML18147 trial was designed by German and Austrian investigators to evaluate the effectiveness of continuing with bevacizumab-based therapy following disease progression in patients with mCRC who had previously received irinotecan- and oxaliplatin-based chemotherapy regimens in combination with bevacizumab[19]. However, the study excluded patients who exhibited progression within the first 3 mo of first-line therapy (rapid progressors), those who showed progression 3 mo after the last bevacizumab administration, and those who received bevacizumab for < 3 consecutive months of first-line therapy. Overall, 820 patients were randomized to receive a novel chemotherapy regimen (fluoropyrimidine plus oxaliplatin or irinotecan) plus bevacizumab (equivalent of 2.5 mg/kg i.v. per week) or chemotherapy alone. Therapy was continued until the development of disease progression or intolerable toxicity. Patient stratification was conducted based on the first-line chemotherapy regimen, first-line PFS (≤ 9 mo vs > 9 mo), time from last bevacizumab administration (≤ 42 d vs > 42 d), and performance status (ECOG 0-1 vs 2).
In comparison with patients receiving chemotherapy alone, those receiving chemotherapy plus bevacizumab had a significantly longer median PFS (5.7 mo vs 4.0 mo; HR 0.63; P < 0.0001) and median OS [11.2 mo vs 9.8 mo; HR 0.81; 95% confidence interval (CI): 0.69-0.94; P = 0.0062]. Bevacizumab was consistently beneficial across all subgroups, although the response rates were relatively low in both groups (5% vs 4%). However, the disease control rate was significantly higher in the chemotherapy plus bevacizumab group (68% vs 54%, P < 0.0001). In addition, the chemotherapy plus bevacizumab group was not associated with increased toxicity, with the exception of specific bevacizumab-related (grade 3-5) side effects including bleeding/hemorrhage (2% vs < 1%), gastrointestinal perforation (2% vs < 1%), and venous thromboembolism (5% vs 3%). There were four treatment-related deaths in the chemotherapy plus bevacizumab group and three in the chemotherapy alone group.
The Bevacizumab Beyond Progression (BEBYP) phase III trial was designed by Italian researchers to investigate the clinical effectiveness of continuing bevacizumab or reintroducing it (after a bevacizumab-free interval of > 3 mo) in combination with second-line chemotherapy in patients with mCRC who developed disease progression following first-line bevacizumab-based therapy[20]. However, following the presentation of data from the ML18147 trial, the study was prematurely discontinued after inclusion of only 185 patients. These patients were randomized to receive second-line chemotherapy alone or in combination with bevacizumab and stratified into subgroups according to their performance status, (ECOG 0 vs 1-2), chemotherapy-free interval (> 3 mo vs < 3 mo), bevacizumab-free interval (> 3 mo vs < 3 mo), and the second-line chemotherapy regimen administered (FOLFIRI vs FOLFOX). The bevacizumab-free interval was longer than 3 mo in 50% of the patients in the chemotherapy plus bevacizumab group. After a median follow-up of 45.3 mo, when compared with chemotherapy alone, the continuation or reintroduction of bevacizumab with second-line chemotherapy was associated with a significantly higher median PFS (6.8 mo vs 5.0 mo; adjusted HR 0.70; 95%CI: 0.52–0.95; stratified log-rank P = 0.010) and median OS (15.5 mo vs 14.1 mo; adjusted HR 0.77; 95%CI: 0.56–1.06; stratified log-rank P = 0.043); this benefit was consistently observed across all patient subgroups. The response rates observed between the groups were not significantly different (17% vs 21%; P = 0.573). Subgroup analyses revealed an equivalent survival benefit regardless of whether bevacizumab was continued or reintroduced. The safety profile and frequency of adverse events were also similar in the treatment groups.
Aflibercept is a recombinant protein that is constructed from the second extracellular ligand-binding domain of VEGFR-1 and the third extracellular ligand-binding domain of VEGFR-2, fused to the constant region of a human immunoglobulin G1 molecule[21-25]. In contrast to bevacizumab that only inhibits VEGF-A, aflibercept can bind to other angiogenic cytokines (e.g., VEGF-B and PIGF) that are thought to play a role in resistance to bevacizumab[21-25]. This biological advantage of aflibercept may explain its superior antitumor activity when compared with bevacizumab in patient-derived xenograft models of CRC[21]. In addition, studies in tumor xenografts have demonstrated that switching to aflibercept during disease progression following bevacizumab therapy resulted in a higher tumor response than the cases receiving continued bevacizumab-based therapy[26].
The phase III VELOUR trial was designed to evaluate the effectiveness of aflibercept in combination with FOLFIRI regimen during the second-line chemotherapy of patients with mCRC who had developed disease progression either during or after completion of oxaliplatin-based chemotherapy without a biologic agent[27]. Moreover, patients who relapsed within 6 mo of the completion of adjuvant oxaliplatin-based chemotherapy were also included in this study. Patients with prior exposure to irinotecan were not eligible, although those previously treated with bevacizumab were included. Patients were randomized to receive either FOLFIRI plus aflibercept (4 mg/kg i.v. every 2 wk) (n = 612) or FOLFIRI plus placebo (n = 614), and stratified according to ECOG performance status (0 vs 1 vs 2), prior bevacizumab exposure (approximately 30.5% of patients in both treatment arms had received first-line bevacizumab-based therapy), age, sex, anatomic location of primary tumor, number of involved organs, hepatic metastasis, prior hypertension, and geographical region. Treatment was continued until the development of disease progression or intolerable toxicity. The primary endpoint was OS.
After a median follow-up of 22.3 mo, patients receiving FOLFIRI plus aflibercept demonstrated a significantly longer PFS (median, 6.90 mo vs 4.67 mo; HR 0.758; 95%CI: 0.661-0.869; P < 0.0001) and OS (median, 13.5 mo vs 12 mo; HR 0.817; 95.34%CI: 0.713-0.937; P = 0.0032) than those receiving placebo plus FOLFIRI. The aflibercept group had a higher ORR than the placebo group (28% vs 18.7%)[28,29]. Subsequent subgroup analyses revealed that patients previously exposed to bevacizumab also benefited from a longer OS (albeit less pronounced) through the application of aflibercept; the median OS values were 12.5 and 11.7 mo with the aflibercept and placebo groups, respectively (HR 0.862). However, the most significant benefit from aflibercept treatment was observed among patients with liver-only metastases and among those with no previous exposure to bevacizumab[30,31].
Compared with the placebo group, the aflibercept group were found to experience more grade ≥ 3 anti-VEGF class-specific side effects, which included hypertension (19.5% vs 1.5%), hemorrhage (2.9% vs 1.7%), arterial thromboembolic events (1.8% vs 0.5%), and venous thromboembolic events (7.9% vs 6.3%). In addition, aflibercept administration led to an increase in the incidence of chemotherapy-related toxicities such as neutropenia, diarrhea, asthenia, stomatitis, infections, and palmar-plantar erythrodysesthesia. Patients aged ≥ 65 years appeared to be particularly vulnerable to these adverse events[32,33].
Ramucirumab is another inhibitor of the VEGF/VEGFR axis. It selectively targets VEGFR-2 and induces conformational changes in the extracellular domain of the receptor, which prevents the binding of all VEGF ligands and receptor activation[34]. Several preclinical studies suggest that the inhibition of VEGFR-2 using monoclonal antibodies, such as DC101, inhibits the growth of CRC cells that are resistant to other angiogenesis inhibitors[35,36]. Therefore, the use of potent and selective VEGFR-2 inhibitors, such as ramucirumab, provides a rational therapeutic option for patients with mCRC who developed disease progression despite receiving first-line bevacizumab-based therapy.
The multicenter, randomized, double-blind, phase III RAISE trial compared the effectiveness of ramucirumab versus placebo, both in combination with second-line FOLFIRI regimen[37]. The study included patients with mCRC who developed disease progression within 6 mo after the final dose of first-line oxaliplatin-based chemotherapy plus bevacizumab. Patients who had received bevacizumab (within 28 d) or chemotherapy (within 21 d) before randomization were excluded. Overall, 1072 patients were randomized to receive ramucirumab (8 mg/kg every 2 wk) plus FOLFIRI or placebo plus FOLFIRI (n = 536 in each group). Stratification variables included the geographical location (North America vs Europe vs all other regions), KRAS exon 2 status (mutant vs wild-type), and time to disease progression after first-line therapy (< 6 mo vs ≥ 6 mo). Of the patients, 83% had received at least 3 mo of first-line bevacizumab-based therapy. Treatment continued until the development of disease progression or intolerable toxicity. The primary endpoint of the study was OS.
After a median follow-up of 21.7 mo, OS was significantly longer in the ramucirumab group than the placebo group (13.3 mo vs 11.7 mo; HR 0.844; 95%CI: 0.730–0.976; P = 0.0219). An improved PFS also was detected in patients receiving ramucirumab (5.7 mo vs 4.5 mo; HR 0.793; 95%CI: 0.697-0.903; P = 0.0005). The survival benefit was consistent across all patient subgroups that received ramucirumab plus FOLFIRI. However, the response rates in the ramucirumab and placebo groups were comparable (ORR 13.4% vs 12.5%; P = 0.63).
The addition of ramucirumab to chemotherapy was associated with higher rates of neutropenia, hypertension, diarrhea, and fatigue. Despite the transient deterioration in the quality of life of these patients, the adverse events were manageable.
In a prospective biomarker analysis of the RAISE trial, the efficacy of ramucirumab was compared with pretreatment plasma levels of several angiogenic cytokines[38]. In particular, ramucirumab plus FOLFIRI therapy was found to be more beneficial in patients with elevated plasma VEGF-D levels, with an improvement of 2.4 mo in OS (13.9 mo vs 11.5 mo). However, this therapy was associated with reduced OS in patients with low VEGF-D levels, compared with the placebo group (12.6 mo vs 13.1 mo).
The data presented above shows that the maintenance of angiogenesis inhibition using bevacizumab, aflibercept, or ramucirumab beyond the initial development of disease progression is an effective and tolerable strategy with a consistent and significant improvement in OS (approximately 1.4 mo) observed in patients with mCRC. In fact, no notable differences between these three drugs were found in terms of their contribution to survival and safety profile. The estimated HR for OS values were similar in the ML18147 (0.81), BEBYP (0.77), VELOUR (0.82), and RAISE (0.84) studies. Accordingly, the most recent version of the European Society of Medical Oncology consensus guidelines for the management of mCRC recommended either the continuation of bevacizumab or switching to aflibercept or ramucirumab (only in combination with FOLFIRI and in irinotecan-naïve patients) for the second-line chemotherapy of patients in whom first-line bevacizumab-based therapy was ineffective (category 1A)[39].
At present, a head-to-head randomized clinical study comparing the efficacy of these three angiogenesis inhibitors in this setting has not been undertaken. Moreover, useful biomarkers that could be integrated into an ideal treatment protocol are not available. Although the measurement of pretreatment plasma levels of angiogenic cytokines (particularly VEGF-D) is a promising approach in this setting, the process is inconvenient for routine clinical use.
The clinical course of patients during first-line therapy may assist clinicians in their decision-making. In this context, patients who exhibit rapid progression (i.e., within 3 mo) following the initiation of first-line bevacizumab-based therapy are usually good candidates for treatment with aflibercept or ramucirumab. It should be noted that such patients were not included in the ML18147 study, and it is possible that they have an intrinsic resistance to bevacizumab.
Cost-effectiveness is also a factor that influences the clinician’s decision. Goldstein and El-Rayes calculated the costs of these agents for the treatment of mCRC based on average US prices[40]. They estimated that ramucirumab leads to a more than two-fold increase in the cost of treatment compared with bevacizumab and aflibercept. Morlock et al[41] indirectly compared the total cost and clinical outcomes of using bevacizumab plus chemotherapy and aflibercept plus chemotherapy as second-line chemotherapeutic strategies for mCRC using Butcher’s method. Bevacizumab plus chemotherapy was found to be more cost-effective than aflibercept plus chemotherapy ($39104 less per treated patient), with similar effectiveness (OS 13.3 mo vs 12.5 mo; HR 0.94). Therefore, the use of bevacizumab beyond disease progression appears to be the most reasonable therapeutic approach in selected patients.
For patients with RAS wild-type mCRC in whom the first-line bevacizumab-based treatment was ineffective, the optimal second-line chemotherapy remains controversial. The data from two small phase II studies, the SPIRITT and PRODIGE 18, suggests that switching from bevacizumab to an epidermal growth factor inhibitor (panitumumab or cetuximab) in the second-line chemotherapy of patients with KRAS wild-type mCRC does not provide a survival benefit that is superior to the continuation of bevacizumab[42,43]. However, the SPIRITT study demonstrated that a switch from bevacizumab to panitumumab might be associated with increased tumor response (19% vs 32%)[42]. Therefore, when a rapid response is desired, the continuation of treatment with an EGFR inhibitor may be more appropriate.
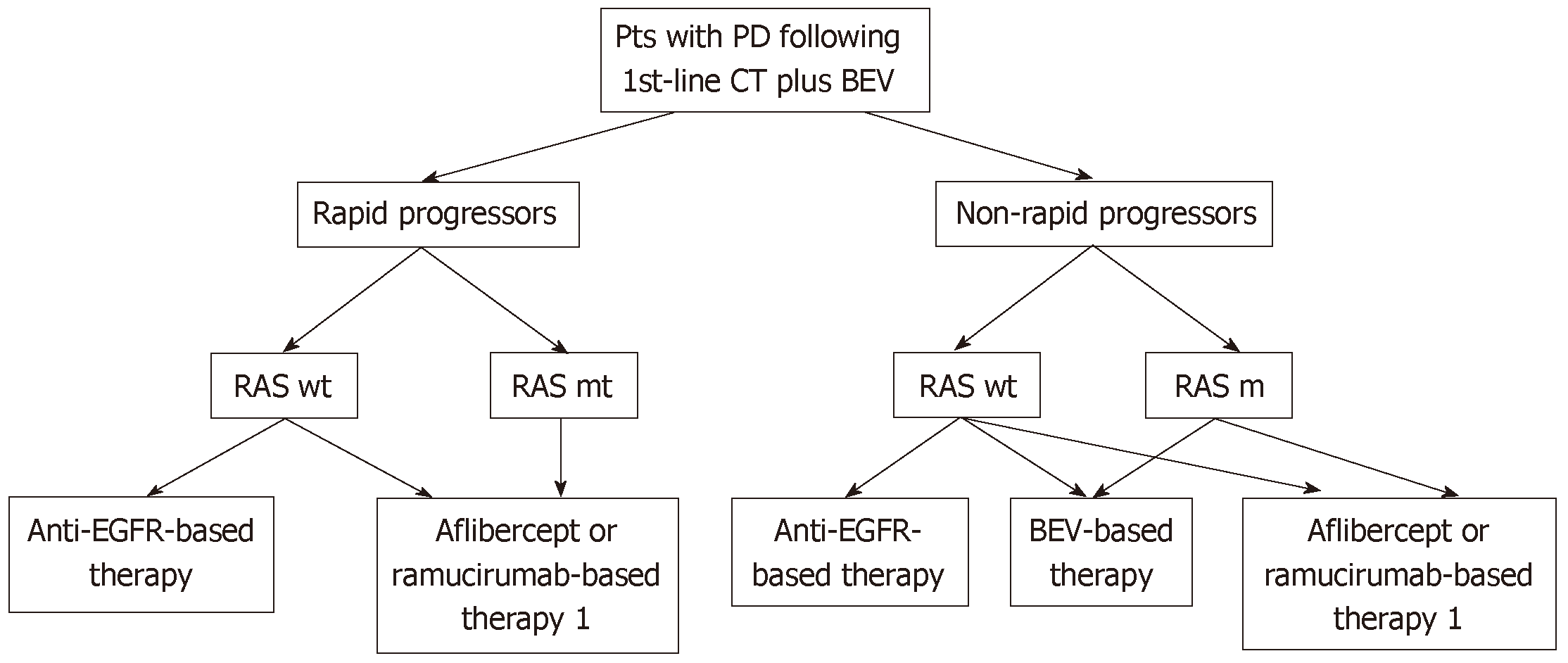
In conclusion, based on current evidence, we propose a simple algorithm for the management of patients with mCRC who developed disease progression following first-line bevacizumab-based therapy (Figure 2). The identification of clinically useful predictive markers reflecting tumor sensitivity to a specific antiangiogenic agent would improve the effectiveness of treatment and reduce costs.
| 1. | Ohhara Y, Fukuda N, Takeuchi S, Honma R, Shimizu Y, Kinoshita I, Dosaka-Akita H. Role of targeted therapy in metastatic colorectal cancer. World J Gastrointest Oncol. 2016;8:642-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Sanz-Garcia E, Argiles G, Elez E, Tabernero J. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol. 2017;28:2648-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, Srock S, Bardelli A, Trusolino L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29:1108-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 4. | Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev. 2016;51:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. 2013;4:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 6. | Clarke JM, Hurwitz HI, Rangwala F. Understanding the mechanisms of action of antiangiogenic agents in metastatic colorectal cancer: a clinician's perspective. Cancer Treat Rev. 2014;40:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Konda B, Shum H, Rajdev L. Anti-angiogenic agents in metastatic colorectal cancer. World J Gastrointest Oncol. 2015;7:71-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Shibuya M. Vascular endothelial growth factor receptor-2: its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2003;94:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Sun W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J Hematol Oncol. 2012;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Peach CJ, Mignone VW, Arruda MA, Alcobia DC, Hill SJ, Kilpatrick LE, Woolard J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int J Mol Sci. 2018;19:pii: E1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 372] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 11. | Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11:1000-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 12. | Cébe-Suarez S, Zehnder-Fjällman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63:601-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Jang HJ, Kim BJ, Kim JH, Kim HS. The addition of bevacizumab in the first-line treatment for metastatic colorectal cancer: an updated meta-analysis of randomized trials. Oncotarget. 2017;8:73009-73016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Chebib R, Verlingue L, Cozic N, Faron M, Burtin P, Boige V, Hollebecque A, Malka D. Angiogenesis inhibition in the second-line treatment of metastatic colorectal cancer: A systematic review and pooled analysis. Semin Oncol. 2017;44:114-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Giampieri R, Caporale M, Pietrantonio F, De Braud F, Negri FV, Giuliani F, Pusceddu V, Demurtas L, Restivo A, Fontanella C, Aprile G, Cascinu S, Scartozzi M. Second-line angiogenesis inhibition in metastatic colorectal cancer patients: Straightforward or overcrowded? Crit Rev Oncol Hematol. 2016;100:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 513] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 17. | Hurwitz HI, Bekaii-Saab TS, Bendell JC, Cohn AL, Kozloff M, Roach N, Mun Y, Fish S, Flick ED, Grothey A; ARIES Study Investigators. Safety and effectiveness of bevacizumab treatment for metastatic colorectal cancer: final results from the Avastin(®) Registry - Investigation of Effectiveness and Safety (ARIES) observational cohort study. Clin Oncol (R Coll Radiol). 2014;26:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Cartwright TH, Yim YM, Yu E, Chung H, Halm M, Forsyth M. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US community oncology. Clin Colorectal Cancer. 2012;11:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, Steffens CC, Alonso-Orduña V, Schlichting C, Reyes-Rivera I, Bendahmane B, André T, Kubicka S; ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 20. | Masi G, Salvatore L, Boni L, Loupakis F, Cremolini C, Fornaro L, Schirripa M, Cupini S, Barbara C, Safina V, Granetto C, Fea E, Antonuzzo L, Boni C, Allegrini G, Chiara S, Amoroso D, Bonetti A, Falcone A; BEBYP Study Investigators. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol. 2015;26:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Chung C, Pherwani N. Ziv-aflibercept: a novel angiogenesis inhibitor for the treatment of metastatic colorectal cancer. Am J Health Syst Pharm. 2013;70:1887-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Tang PA, Moore MJ. Aflibercept in the treatment of patients with metastatic colorectal cancer: latest findings and interpretations. Therap Adv Gastroenterol. 2013;6:459-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Falcon BL, Chintharlapalli S, Uhlik MT, Pytowski B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol Ther. 2016;164:204-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Ricci V, Ronzoni M, Fabozzi T. Aflibercept a new target therapy in cancer treatment: a review. Crit Rev Oncol Hematol. 2015;96:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, Lin E, Eng C, Morris J, Ellis L, Heymach JV, Kopetz S. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One. 2013;8:e77117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Chiron M, Bagley RG, Pollard J, Mankoo PK, Henry C, Vincent L, Geslin C, Baltes N, Bergstrom DA. Differential antitumor activity of aflibercept and bevacizumab in patient-derived xenograft models of colorectal cancer. Mol Cancer Ther. 2014;13:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1055] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 28. | Van Cutsem E, Joulain F, Hoff PM, Mitchell E, Ruff P, Lakomý R, Prausová J, Moiseyenko VM, van Hazel G, Cunningham D, Arnold D, Schmoll HJ, Ten Tije AJ, McKendrick J, Kröning H, Humblet Y, Grávalos C, Le-Guennec S, Andria M, Dochy E, Vishwanath RL, Macarulla T, Tabernero J. Aflibercept Plus FOLFIRI vs. Placebo Plus FOLFIRI in Second-Line Metastatic Colorectal Cancer: a Post Hoc Analysis of Survival from the Phase III VELOUR Study Subsequent to Exclusion of Patients who had Recurrence During or Within 6 Months of Completing Adjuvant Oxaliplatin-Based Therapy. Target Oncol. 2016;11:383-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Tabernero J, Van Cutsem E, Lakomý R, Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR, McKendrick JJ, Soussan-Lazard K, Chevalier S, Allegra CJ. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. 2014;50:320-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Chau I, Joulain F, Iqbal SU, Bridgewater J. A VELOUR post hoc subset analysis: prognostic groups and treatment outcomes in patients with metastatic colorectal cancer treated with aflibercept and FOLFIRI. BMC Cancer. 2014;14:605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Joulain F, Proskorovsky I, Allegra C, Tabernero J, Hoyle M, Iqbal SU, Van Cutsem E. Mean overall survival gain with aflibercept plus FOLFIRI vs placebo plus FOLFIRI in patients with previously treated metastatic colorectal cancer. Br J Cancer. 2013;109:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Ruff P, Ferry DR, Lakomỳ R, Prausová J, Van Hazel GA, Hoff PM, Cunningham D, Arnold D, Schmoll HJ, Moiseyenko VM, McKendrick JJ, Ten Tije AJ, Vishwanath RL, Bhargava P, Chevalier S, Macarulla T, Van Cutsem E. Time course of safety and efficacy of aflibercept in combination with FOLFIRI in patients with metastatic colorectal cancer who progressed on previous oxaliplatin-based therapy. Eur J Cancer. 2015;51:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Ruff P, Van Cutsem E, Lakomy R, Prausova J, van Hazel GA, Moiseyenko VM, Soussan-Lazard K, Dochy E, Magherini E, Macarulla T, Papamichael D. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol. 2018;9:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Verdaguer H, Tabernero J, Macarulla T. Ramucirumab in metastatic colorectal cancer: evidence to date and place in therapy. Ther Adv Med Oncol. 2016;8:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Tonra JR, Deevi DS, Corcoran E, Li H, Wang S, Carrick FE, Hicklin DJ. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res. 2006;12:2197-2207. [PubMed] |
| 36. | Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, Bucana CD, Hicklin DJ, Ellis LM. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausová J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 689] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 38. | Tabernero J, Hozak RR, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Prausová J, Muro K, Siegel RW, Konrad RJ, Ouyang H, Melemed SA, Ferry D, Nasroulah F, Van Cutsem E. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann Oncol. 2018;29:602-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 39. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2540] [Article Influence: 254.0] [Reference Citation Analysis (41)] |
| 40. | Goldstein DA, El-Rayes BF. Considering Efficacy and Cost, Where Does Ramucirumab Fit in the Management of Metastatic Colorectal Cancer? Oncologist. 2015;20:981-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Morlock R, Yu E, Ray J. A cost-effectiveness analysis of bevacizumab (BV) plus chemotherapy (CT) versus aflibercept (AFLI) plus CT in patients with metastatic colorectal cancer (mCRC) previously treated with BV. J Clın Oncol. 2013;31:417-417. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline-Burkhardt M, Tian Y, Go WY. SPIRITT: A Randomized, Multicenter, Phase II Study of Panitumumab with FOLFIRI and Bevacizumab with FOLFIRI as Second-Line Treatment in Patients with Unresectable Wild Type KRAS Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2015;14:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Bennouna J, Hiret S, Borg C, Bertaut A, Bouche O, Deplanque G, Francois E, Conroy T, Ghiringhelli F, des Guetz G, Seitz JF, Artru P, Stanbury T, Charpentier S, Denis M, Adenis A. 477O Bevacizumab (Bev) or cetuximab (Cet) plus chemotherapy after progression with bevacizumab plus chemotherapy in patients with wild-type (WT) KRAS metastatic colorectal cancer (mCRC): Final analysis of a French randomized, multicenter, phase II study (PRODIGE 18). Ann Oncol. 2017;28:mdx393.004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cao D, Kim HS, Morelli F, Yuan Y S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ