©2014 Baishideng Publishing Group Inc.
World J Clin Oncol. Dec 10, 2014; 5(5): 990-1001
Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.990
Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.990
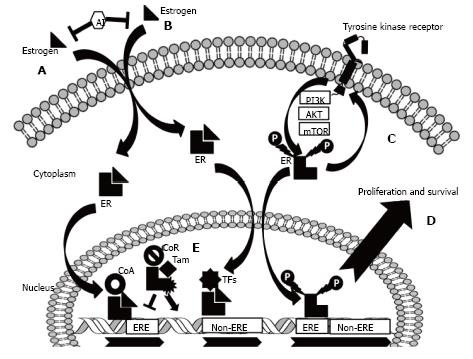
Figure 1 The biology of the estrogen receptor and a schematic representation of the key mechanisms of endocrine resistance.
A: Estrogen induces gene regulation via the “classical” pathway. Estrogen passively diffuses through cell membranes and binds to the estrogen receptor (ER), inducing receptor dimerization. This complex recruits co-activators (CoA) and binds regions of DNA known as estrogen response elements (EREs), promoting transcription. Aromatase inhibitors (AIs) negatively regulate ER activity by reducing circulating estrogen levels; B: The ER can also cooperate with other transcription factors (TFs) and regulate the transcription of genes not harbouring EREs via the “non-classical” pathway; C: ER strictly interacts with receptor tyrosine kinases (RTKs) via their downstream effectors. ER can, in fact, be directly phosphorylated and activated, the final result being gene expression and a cascade of second intracellular effectors (the non-nuclear activity of ER); D: This strict and bi-directional crosstalk between ER and RTKs and downstream effectors is responsible for endocrine resistance; E: In breast cancer cells, SERMs [such as tamoxifen (Tam)] bind ER and induce the recruitment of co-repressors (CoR) that negatively regulate the activity of ER. Mutated forms of ER are able to enhance gene expression in spite of the presence of Tam.
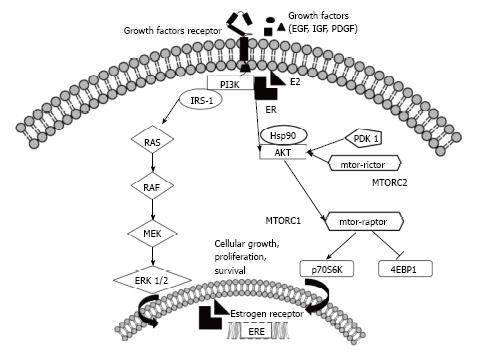
Figure 2 A representation of the molecular crosstalk between estrogen receptor and the receptor tyrosine kinases and PI3K-Akt-mTOR axes.
In breast cancer, the PI3K-Akt-mTOR pathway modulates responses to signals communicated through growth factor receptors and the estrogen receptor (ER), and this crosstalk is important for sensitivity to anti-endocrine therapy. In particular, Akt and ERK1/2 phosphorylate ER on key residues involved in the induction of ligand-independent activation of DNA transcription. Furthermore, the converse occurs: estradiol, bound to membrane ER, interacts with and activates a regulatory subunit of PI3K. The mammalian target of rapamycin (mTOR) signaling cascade is another key regulatory pathway that controls proliferation and survival in cancer cells and plays an important role in the molecular crosstalk with the ER pathway. Two mTOR-interacting proteins, raptor and rictor, define distinct branches of the mTOR pathway: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Both active mTORC1 (via the phosphorylation of downstream targets, such as 4E-BP1 and p70S6 Kinase) and active mTORC2 contribute to promoting cellular survival and proliferation. EGF: Epidermal growth factor; IGF: Insulin-like growth factor; PDGF: Platelet derived growth factor; PI3K: Phosphatidylinositol-3-phosphate kinase; E2: Estradiol; IRS-1: Insulin receptor substrate-1; RAS–RAF–MEK–ERK: Mitogen activated protein kinase pathway; HSP90: Heat shock protein 90; PDK-1: Pyruvate dehydrogenase lipoamide kinase isozyme 1; p70S6K: Protein 70S6 kinase; 4EBP1: Eukaryotic translation initiation factor 4E-binding protein 1; ERE: Estrogen response element.
- Citation: Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: Current evidence and future directions. World J Clin Oncol 2014; 5(5): 990-1001
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/990.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.990