©The Author(s) 2025.
World J Clin Oncol. Jul 24, 2025; 16(7): 107007
Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.107007
Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.107007
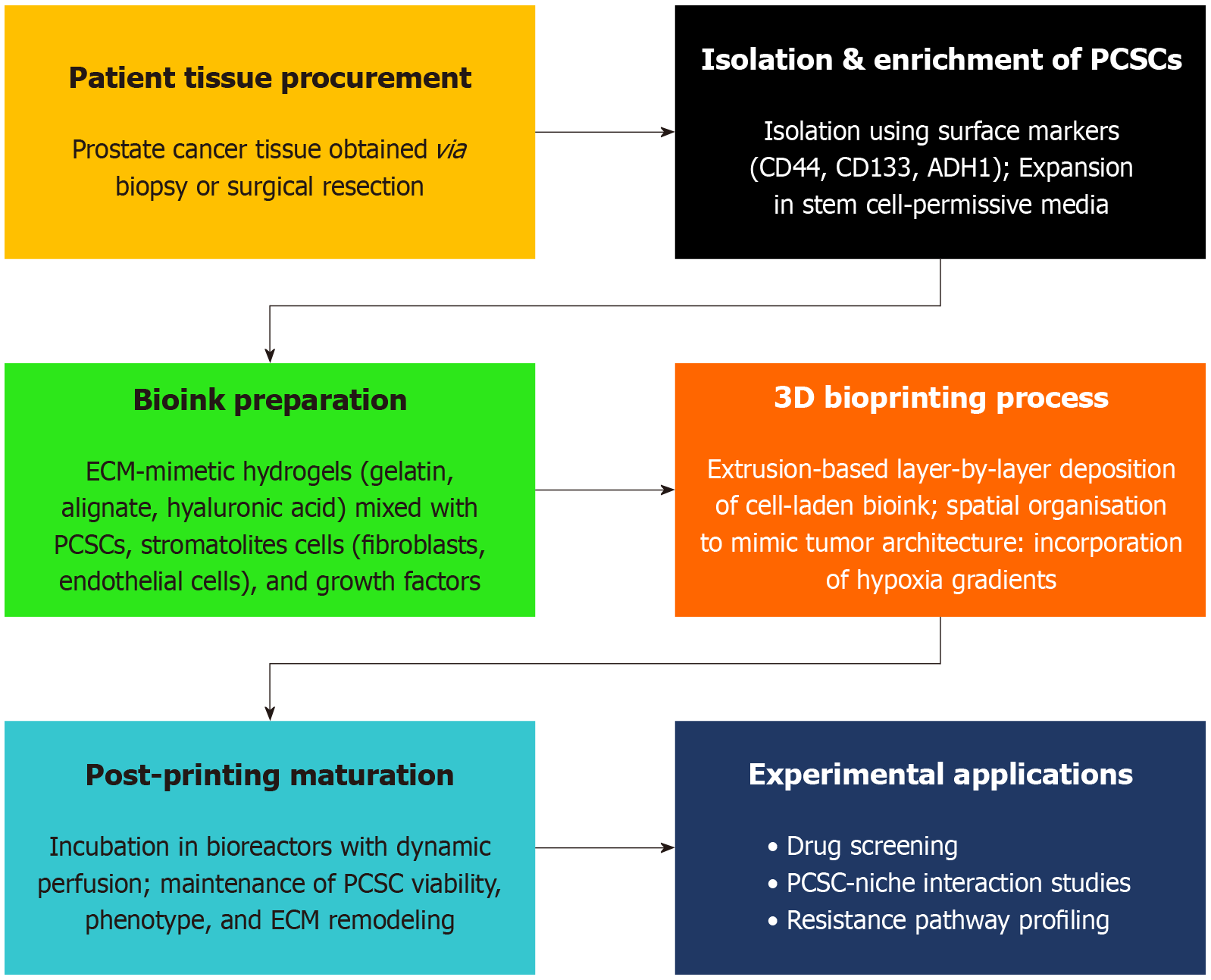
Figure 1 Workflow of patient-specific 3D bioprinting of prostate cancer stem cells: The schematic illustrates the stepwise workflow involved in generating patient-specific 3D bioprinted models of prostate cancer stem cells.
The process begins with tissue collection from prostate cancer patients, followed by cell isolation and enrichment of prostate cancer stem cells (PCSCs). Patient imaging data (e.g., magnetic resonance imaging/computed tomography scans) are used to design anatomically relevant 3D models. Using optimized bioinks, the PCSCs are bioprinted into spatially organized constructs that replicate the native tumor microenvironment. A brief maturation phase allows the construct to stabilize and develop key structural and functional properties, enabling applications in drug screening, mechanistic studies, and personalized therapy design.
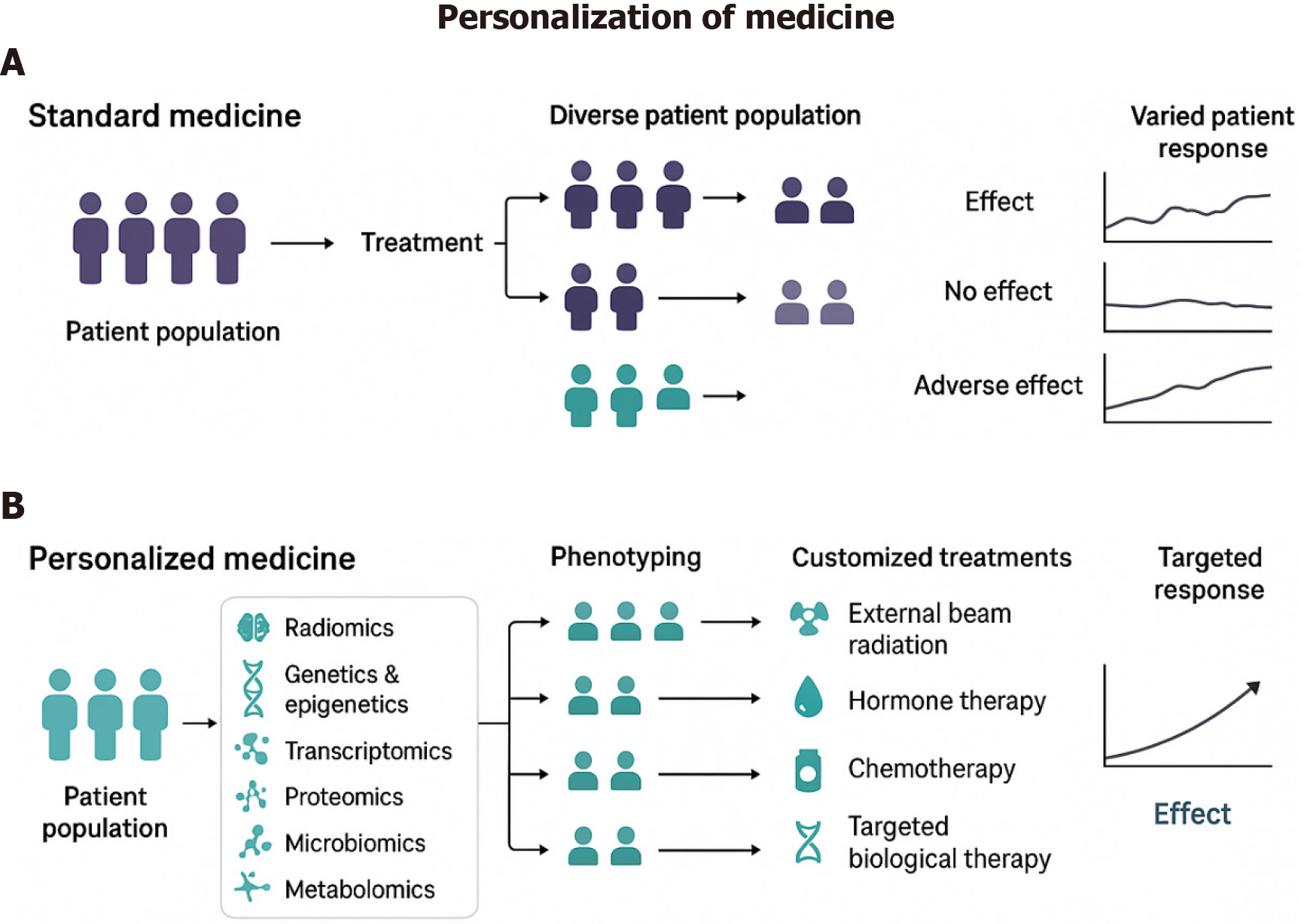
Figure 2 Standard vs personalized medicine in cancer treatment: The figure illustrates the distinction between standard and personalized medicine approaches in cancer treatment.
A: Standard medicine: Patients receive generalized treatment based on broad clinical guidelines, often leading to variable responses due to tumor heterogeneity; B: Personalized medicine: Patient-derived tumor cells are used to create bioprinted tumor models, enabling preclinical drug testing tailored to the individual’s cancer profile. This approach enhances treatment efficacy, minimizes adverse effects, and optimizes therapeutic outcomes.
- Citation: Gharia J, Pimplaskar S, Prajapati A. Revolutionizing cancer care: Bioprinting prostate cancer stem cells for targeted treatments. World J Clin Oncol 2025; 16(7): 107007
- URL: https://www.wjgnet.com/2218-4333/full/v16/i7/107007.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i7.107007