Published online Feb 6, 2016. doi: 10.4292/wjgpt.v7.i1.112
Peer-review started: June 1, 2015
First decision: October 14, 2015
Revised: October 20, 2015
Accepted: December 3, 2015
Article in press: December 4, 2015
Published online: February 6, 2016
Processing time: 245 Days and 15 Hours
Idiopathic inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), are multifactorial diseases that are manifested after disruption of a genetic predisposed individual and its intestinal microflora through an environmental stimulus. Urbanization and industrialization are associated with IBD. Epidemiological data, clinical observations and family/immigrants studies indicate the significance of environmental influence in the development of IBD. Some environmental factors have a different effect on the subtypes of IBD. Smoking and appendectomy is negatively associated with UC, but they are aggravating factors for CD. A westernized high fat diet, full of refined carbohydrates is strongly associated with the development of IBD, contrary to a high in fruit, vegetables and polyunsaturated fatty acid-3 diet that is protective against these diseases. High intake of nonsteroidal antiinflammatory drug and oral contraceptive pills as well as the inadequacy of vitamin D leads to an increased risk for IBD and a more malignant course of disease. Moreover, other factors such as air pollution, psychological factors, sleep disturbances and exercise influence the development and the course of IBD. Epigenetic mechanism like DNA methylation, histone modification and altered expression of miRNAS could explain the connection between genes and environmental factors in triggering the development of IBD.
Core tip: Epidemiological data, clinical observations and family/ immigrants studies indicate the significance of environmental influence in the development of inflammatory bowel diseases (IBD). A westernized high fat diet, full of refined carbohydrates is strongly associated with the development of IBD, contrary to a high in fruit, vegetables and polyunsaturated fatty acid-3 diet that is protective against these diseases. Additional factors such as air pollution, psychological factors, sleep disturbances and exercise influence the development and the course of IBD. Epigenetic mechanism like DNA methylation, histone modification and altered expression of miRNAS could explain the connection between genes and environmental factors in triggering the development of IBD.
- Citation: Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther 2016; 7(1): 112-125
- URL: https://www.wjgnet.com/2150-5349/full/v7/i1/112.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i1.112
Idiopathic inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC) are characterized by chronic relapsing inflammation without a particular infectious or environmental cause. IBD are heterogeneous, multifactorial diseases that are manifested after disruption of a genetic predisposed individual and its intestinal microflora through an environmental stimulus, as this leads to faulty response of both the innate (macrophages, neutrophils) and the acquired (T and B cells) immune system. This results in an intense recruitment of immune cells with prolonged survival due to the reduced cell apoptosis. These cells infiltrate the intestinal membrane, enhancing an ongoing inflammatory process[1-4]. IBD are called a disease of developed countries or a disease of the West as they occur more frequently in America and Europe compared with Asia. The incidence of IBD used to be rare in developing countries, but it is rising as these countries are industrialized[5-12]. Furthermore the incidence of IBD varies in different age groups and is primarily a disease of young ages. The pediatric IBD show an increasing trend worldwide, with more references to CD[13-18]. The maximum prevalence of CD is observed in the age group of 16-25 years while UC appears more at ages 30-40 years. The incidence gradually decreases with age for both diseases and present a new peak at the age of 76-85 years. The IBD pediatric cases are estimated at 7%-20% of all cases according to demographic studies[19,20]. Various incidences are observed between different sex and different nationalities. Generally, there is a higher incidence of 20%-30% in women for Crohn's disease, while there is a slight predominance of the male gender in UC appearance.
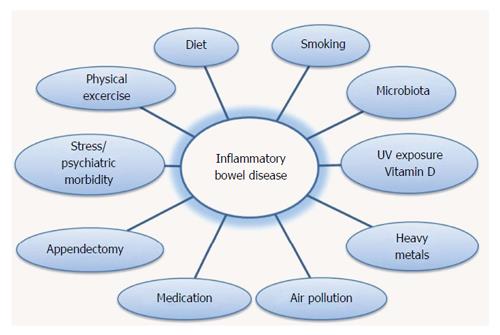
Urbanization and industrialization are associated with lifestyle changes. Epidemiological data, clinical and laboratory observations indicate the significance of environmental influence in the development of IBD. Family studies, mostly twin studies, provide an important tool for the identification of hereditary and environmental contribution in IBD pathogenesis. Family studies records increased prevalence in first degree relatives[21-23]. In large European studies conducted in Sweden, Denmark and the United Kingdom, the rate of CD in monozygotic twins was estimated to range between 20% and 50%, while the rate in dizygotic twins, who were brought up in the same environment, was less than 10%. The corresponding difference in monozygotic and dizygotic twins shows the relative effect of genes, however, the low rates highlight the most significant environmental effect on the pathogenesis of IBD[24-27]. Studies of immigrant populations suggest that ethnic and racial differences in the incidence of IBD may be more related to lifestyle and environmental influence rather than actual genetic differences[28]. Groups of immigrants who moved from areas with low incidence of IBD to areas with high incidence provide information on the environmental effects on the development of the disease. Migration from a low-incidence to a higher incidence region increases the risk of disease, particularly in the first generation children. The arrival in high risk areas at a younger age increases the risk of developing IBD in immigrants. For example, until recently, IBD thought to be rare in the Indian subcontinent. However, South Asians who moved to the United Kingdom, and their descendants, are at increased risk for UC compared to whites[29-38]. The changing epidemiology of IBD chronically and geographically suggests that environmental factors play an important role in modifying the development and the activity of disease. The rising incidence in developing countries, that have traditionally presented low incidence, shows that IBD is associated with both westernization of lifestyle and industrialization (Figure 1)[6,39].
Smoking is one of the most important and well-characterized environmental risk factors for IBD, but its pathogenic mechanism is not clear. Much evidence from studies suggests smoking is a causative agent in CD while it supports the protective role against UC. Smoking cessation dramatically changes the composition and increases the variety of the intestinal microbiome[40-43]. There is a dose-dependent relationship between smoking and IBD. Ex-smokers have a higher risk for UC development, while quitting smoking in UC patients aggravates the clinical outcome of the disease. Similarly, a reduced risk is observed in smokers, where patients tend to a more benign course as flares, hospitalization, need for steroids and colectomy are experienced rarely[44,45], and there is an improvement of disease activity in former smokers who started to smoke again[46,47]. Cigarette smoking appears to have a different impact on men and women with UC, with the beneficial effects appear mostly in men[48,49]. It is remarkable that 52% of patients developed UC in the first three years after quitting smoking[50], while UC patients experienced flares during the first years after smoking cessation[46]. A population study confirms the protective role of smoking in UC, concluding that the prevalence of UC was raised 5 times in the Mormon Church population in England and Ireland than in the rest of the population, where smoking is strongly discouraged[51]. Additional pilot studies indicate that nicotine could effectively induce remission in active UC, although its use provokes various mild side-effects such as nausea, headache and sleep disturbance[52,53].
Conversely, smoking doubles the risk of CD compared to that of non-smokers[47,54] and leads to a worse clinical outcome and to a more aggressive disease[43,55,56]. Smoking has been associated with a higher risk of severe relapse, a more complicated disease with development of strictures or fistulae and a higher need for steroids and surgery[45,57-59]. Smoking cessation is a therapeutic strategy for the CD[60]. A study has shown that patients who stop smoking for at least six months have a lower risk of relapse for the next 12-18 mo. Smoking has a greater effect on women[61]. A meta-analysis showed that CD patients who smoke have 2.5 times increased risk of postsurgical recurrence and a double risk of recurrence than nonsmokers[62]. There is not much data for passive smokers, however a study showed that CD patients who are passive smokers needed immunosuppressants and infliximab more often than non-passive smokers. Therefore, secondhand smoke appears to show a similar effect as active smoking, but with weaker results[63]. In addition CD patients are more likely to have been prenatally exposed to tobacco smoke[59].
Appendectomy also appears to have a different effect in UC and CD. Most studies show a strong negative association between appendectomy and UC suggesting that it can improve the course of disease and the need for colectomy[64-67], whereas a recent work in China found no significant association[68]. Children and adolescents experiencing appendicitis have a reduced risk for UC, as opposed to those who experience appendicitis during adulthood[69]. A population based cohort study of Sweden and Denmark concluded that the incidence of UC was 26% and 13% lower, respectively, in patients who had undergone appendectomy[70]. Also, a study from Spain showed that appendectomy was less common not only in patients with UC but also in their relatives[71]. In three different experimental mice models of colitis, removing the appendix prevented the development of colitis[72]. However, it is believed that appendicitis provides a protective role against UC, not its resection[73,74]. A meta-analysis of studies showed an increased risk for CD development in the first year after appendectomy, whereas five years later the risk for CD is no longer important[75].
Many studies propose that high frequency use of non-steroid anti-inflammatory medicines, in a large dose and for a long time period increases the risk for UC or CD and leads to disease relapse[76-81]. A study based on the European population suggested that the risk for CD is 6 times increased in those who take aspirin, with a higher incidence in women and young people[82]. Since 1980, many studies have indicated an association between consumption of contraceptive pills and developing of IBD[83-87]. A major recent study confirmed that, recording a greater association with the risk of CD. Women with a history of smoking present a significant association between oral contraceptive pills and UC[88]. Furthermore, early exposure to antibiotics is associated with development of pediatric IBD in a dose dependent relationship. Specifically, antianaerobic antibiotic use during childhood could alter gut flora and promote inflammation[89,90]. Virta el al[91] showed that there is higher risk using antibiotics in childhood for CD development than UC. A meta-analysis study confirmed that antibiotic exposure increases the risk of new- onset CD with a greater risk for children[92]. Two nested case-control analysis of the population-based University of Manitoba Inflammatory Bowel Disease Epidemiologic Database by Shaw et al[93] concluded that pediatric IBD patients are more likely to have been exposed to antibiotic use in their first year of life and that IBD patients may have been prescribed with antibiotics 2-5 years before their diagnosis[93,94].
A Western diet, a diet with high amount of fat and carbohydrates and low amount of fiber, is implicated in the increasing incidence of IBD[95]. Change in human nutritional standards has a great result in shaping the microbiome[96]. Children in Africa, whose diet is rich in fiber have a really different gut-microbial community to European children whose diet contains a high amount of sugar, fat and proteins[97].
Meat consumption has been associated with increased risk of developing IBD, and induce relapse[98,99]. A review of case-control studies and epidemiological data by Asakura et al[100] presented significant correlation between animal meat and CD. Likewise, meta-analyses of case studies show a positive correlation between consumption of animal protein or whole protein intake and CD[101]. A recent study population in middle-aged French women showed that high total protein intake, especially animal protein was associated with a significantly increased risk for IBD, while the consumption of eggs and dairy products were respectively associated with IBD[102]. Fish/tone consumption is negatively associated with both colonic and ileal CD[103]. In a study of pediatric patients whose CD was diagnosed before the age of 20 years, children who consumed a greater amount of fruit and vegetables had a lower risk for developing CD, with a significant dose-dependent manner[104].
A larger prospective study of adults also indicates a strong inverse association between fiber intake and risk for IBD, with a weaker effect on UC[105]. Many studies concluded in similar results with a negative association between both fruits and vegetables and development of IBD[103]. Russel et al[106] reported that consuming more than five citrus per week was significantly associated with decreased risk of UC. Low intake of raw fruits and vegetables is common in IBD patients. The meta-analysis of Hou et al[101] has shown that intake of high- fiber diet and fruits is associated with reduced risk for CD. The protective effect of fiber, however, appears to be related to the source of fiber. Dietary fiber from fruits and vegetables were associated with a reduced risk for CD in the population of Nurses’ Health Study, but insoluble fiber from whole grains and bran have not the same significant effect[105]. A study in Japanese population indicated the role of fiber in suppressing patients’ inflammation and recommends patients to consume more fiber, such as fruits, vegetables, seaweed, dried mushrooms and dried Japanese radish[107].
In 1976, both groups, Martini and Brandes[108] and Mayberry et al[109], were the first to report that CD patients consume excess amount of sugar and products containing refined carbohydrates. The increased consumption of refined sugar and processed carbohydrates can be a risk factor for CD and has also been demonstrated in some UC patients. Intake of refined carbohydrates, fizzy drinks, soft drinks cola, commercial desserts with added sugar, chocolate and/or pastry has been shown in several studies to affect the appearance of IBD. Intake of refined carbohydrates, fizzy drinks, soft drinks cola, commercial desserts with added sugar, chocolate and/or pastry has been implicated in the development of IBD[110-112].
High consumption of rice and pasta has been reported to increase but not significantly the risk for UC, while potato consumption reduces the risk for IBD[103]. High fat diet (HFD) prolongs and exacerbates inflammatory manifestations of chronic UC. In an experimental DSS-colitis model, colon analyses showed mild inflammation in DSS colitis group, which became more serious when HFD was administered[113]. Devkota et al[114] demonstrated that consumption of dietary fat can dramatically reshape the gut microflora, and trigger the initiation of colitis. The intake of long chain omega-6 polyunsaturated fatty acids, especially linoleic and arachidonic acid, may contribute to IBD development with UC incidence increased by two- and four-fold, respectively[115-117], in contrary n-3PUFA presents a protective role against IBD[118]. A prospective United Kingdom study showed that the total dietary intake of omega-3 PUFAs, eicosapentaenoic and docosahexaenoic acid, was associated with reduced risk for UC[119]. Similar results were presented in a North American study where it was demonstrated that higher intake of omega-3 long-chain PUFAs is associated with a lower risk for UC and a high long-term intake of trans unsaturated fatty acids is associated with an increased frequency of IBD development[120].
Meta-analysis studies in the role of breastfeeding in the development of IBD during childhood and adulthood presents a statistically significant protective effect for CD[121] and the early onset IBD[122]. Improved sanitary conditions are associated with increased risk of IBD. There is a negative association between IBD risk and family size, showing that many siblings are a protective factor against IBD with a graded manner, supporting the “‘old friends’ hypothesis”, means the exposure to pathogenic microorganisms during childhood[123-125]. Another hygienic protective factor is the presence of a pet at home[126]. Children living in rural crowded homes, consuming unpasteurized milk are at lower IBD risk, mainly CD[127].
Supporting the case of hygiene, negative association exists between some microorganisms such as Helicobacter pylori[128-130] and colonization of parasitic worms (i.e., helminths)[131-137] and development of IBD. The Mycobacterium Avium Paraturbeculosis spp (MAP) is a pathogen that may be a causative agent for IBD. A study indicate that a high percentage of both CD and UC patients have been contaminated with MAP[138-140] and a meta-analysis of 28 case-control studies showed a positive correlation between MAP and CD[141]. Furthermore, other pathogens such as Salmonella, Escherichia coli, Clostridium difficile and Campylobacter appear to be involved in the pathogenesis of IBD[142-144]. Moreover the case of cold chain, the correlation of refrigerating food and IBD, mainly CD[145] implicates psychotrofic bacteria with pathogenic properties such as Listeria monocytogenes, Yersinia enterocolitica, Clostridium botulinum and Bacillus cereus identified in CD patients[134,146,147].
The human gastrointestinal tract contains approximately 10-100 trillion microorganisms, the majority of which are anaerobic bacteria. It is estimated that there are more than 500 different species of bacteria in the intestine whose number and composition varies along the gastrointestinal tract. The most commonly found bacteria in normal intestinal flora are Firmicutes (49%-76%), Bacteroidetes (16%-23%), followed to a less extent by Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia[148]. The intestinal microbial community plays an important role for the host, as it carries out many useful functions including the digestion of substrates that host enzymes are unable to digest; the production of vitamins and short chain fatty acid; the formation of enteric immune system; and the protection of enteric homoeostasis repressing the growth of harmful microorganisms[149,150]. Although the diversity of microbes is huge, it appears from recent post-genomic studies that there is a common core of microbial genes which are common for at least 50% of people[151]. A westernized diet and overexposure to drugs such as antibiotics, mainly during childhood, could alter the intestinal microbial composition and affect the number ratios between protective and pathogen microorganisms[152,153]. Patients with IBD present a different composition in their intestine characterized by a reduction in their microbial diversity, specifically reduction of the dominant members of the gut microbiota. This altered balance in the gut microbiota constituents, called dysbiosis, causes functional changes that seem to be involved in the pathophysiology of many diseases, including IBD[154-156]. The reduced abundance of the Firmicutes phyla, and the decrease in their diversity, are the most well studied changes in IBD patients. Faecalibacterium prausnitzii, Butyricoccus pullicaecorum and Roseburia hominis are members of the Firmicutes where a reduction has been found in IBD patients in comparison to controls[157-160]. The other important anaerobic phylum also found depleted in patients with IBD are Bacteriodetes[158]. The bacteria in these phyla are known for their anti-inflammatory role in the gut by producing short-chain fatty acid metabolites, such as butyrate and acetate, and inducing the expansion of Treg cells that suppress intestinal inflammation[161-163]. Although gut microbiota in healthy populations shows temporal change, IBD patients present an unstable gut microbiota even during remission. Ott et al[164] noticed that, normal anaerobic bacteria such as Bacteroides, Escherichia, Eubacterium, Lactobacillus, and Ruminococcus are decreased and the diversity of the gut microbiota is also reduced before a relapse of UC. On the other hand, as a result of this dysbiosis, pathogenic microorganisms are increased in IBD patients showing a preference for inflammatory environments. High levels of Enterobacteriacae, including adherent invasive Escherichia coli, Klebsiella pneumonia and Proteus mirabilis have been detected in IBD patients, indicating their provocative role in enteric inflammation[165-168]. Moreover, an increase in Fusobacteria has been reported in patients with UC compared to healthy individuals. Of note, when a rectal enema of Fusobacterium isolates from humans was administered in mice, colonic mucosa erosions were induced. Thus, a positive correlation between Fusobacterium and the IBD status of the host indicates that invasive Fusobacterium may have an influence on IBD pathology[169].
Many references support the important role of vitamin D in both the pathogenesis and therapy of IBD[170,171]. Vitamin D appears to play an important role in innate and adaptive immunity and influences autophagy participating in IBD pathogenesis[172-177]. Several studies indicate a high rate of vitamin D deficiency in IBD patients[178,179]. Several groups have examined the geographic variability of IBD even within a given country and suggests a greater frequency in regions associated with reduced exposure to ultraviolet radiation[180,181]. In contrary, a high intake of vitamin D was associated with a reduced risk for IBD suggesting its pathophysiological role in IBD development, with a significant association to CD (increase 1 ng/mL of 25(OH)D plasma leads to a relative risk reduction of 6% for CD and 100 IU/d increase in total vitamin D intake was associated with a 10% relative risk reduction for UC[182]. A large study with 3217 IBD patients proved that lower 25(OH)D plasma levels are associated with an increased risk of surgery and hospitalization for both CD and UC, compared to those with adequate levels of vitamin[183]. Its role is also supported by animal experiments where administration of 1,25(OH)2D3 improves colitis through suppression of genes associated with TNF-a in the colon of mice[184,185]. Increased hospitalization rates and higher disease severity are recorded in regions with limited exposure to UV radiation. The precise mechanism of the effect of UV remains unknown but it is likely to be related to vitamin D[186]. Additionally, studies have associated the month of birth with the emergence of various inflammatory diseases including IBD. Shaw et al[187] recorded a small but significant increase in spring births among IBD patients, specifically CD patients. Respectively, Disanto et al[188] indicated that people born in spring are 1.06 more likely to develop UC. The effect of the birth month on inflammatory diseases incidence is possibly related to the UV intake and the adequacy of vitamin D during pregnancy.
Young people living in areas with high concentrations of SO2 show a greater tendency to develop UC and young people living in areas with high levels of NO2 are more likely to develop CD. This association appeared to be dose and age-dependent and was strengthened when the study was restricted to urban areas[189]. Another study showed association of IBD patients hospitalizations with overall concentration of pollutants, registered in the US Wisconsin. Total emission was associated with a 40% increase in hospitalization per each registered increase of contaminants[190,191].
Heavy metals are also environmental compounds that could contribute to inflammatory diseases like IBD. Ingested mercury causes various disturbances in the intestinal track such as abdominal pain, IBD, ulcers and bloody diarrhea[192]. Several studies have proved the association between major life stressors, anxiety, depression or psychiatric morbidity and onset IBD risk[193-200]. Stress reduces mucus secretion and increases the permeability of mice colon, both characteristics of IBD[201]. Levenstein et al[202] firstly, and Bitton et al[203] showed higher recruited stress associated with relapse of UC and CD, respectively. Bernstein et al[204] in their 704-patients study displayed stress as the only independent predictor of increased risk for disease flare. Also, the presence of anxiety or depression has been associated with increased disease activity and an increased risk of surgery in CD patients[205-207]. There is only a little data on whether anxiety and depression management leads to a more benign disease course. Results of these studies are controversial, however, it could improve the quality of life, particularly in UC patients[208].
Regular low intensity exercise seems constructive to the patients’ health reducing both anxiety and depression, and generally improves the quality of life[209,210]. Employment requiring outdoor physical activity has been associated with a lower IBD incidence. Active women seem to have a 44% reduced risk of CD compared to sedentary women[211]. An interesting environmental influence with emerging data is sleep. Mainly reduced, but also increased sleep has been associated with health problems. IBD patients in clinical remission who have sleep disorders are twice more likely to experience flare at 6 mo and are more likely to subclinical disease activity compared to those without sleep disturbances[207,212].
Epigenetics provides a connection between environmental exposure and the onset and continuation of the disease. Epigenetic modifications, including DNA methylation, are considered as the basis for Th cells differentiation and cytokines regulation. Consequently, methylation has emerged as a research priority for IBD pathogenesis. Nimmo et al[213] defined a global methylation profile for ileal CD and identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. DNA methylation changes in the colonic epithelial cells, normally occurred with aging, are accelerated in IBD because of higher cells recycling in inflammation. Increased DNA methylation is shown in dysplastic and surrounding non-dysplastic colonic tissue in UC patients. Four of the 15 loci related to cancer development (Cdh1, GDNF, HPP1 and MYOD1) were differently methylated in surgical resection specimens from patients with active UC compared to those with normal mucosa[214]. Genes showing strongest evidence for hypermethylation in CD compared to healthy controls were ATF2, CXCL5 and IL12B whereas CCL25, CXCL14, CXCL3, CXCL6, IL12A, INHA, IL15, IL17RA, IL4R, IL6R, IL6ST, FADD, GATA3, IL7, TYK2 were found to be hypomethylated. Regarding UC, methylation status of CXCL6 and IL13RA1 in peripheral blood samples did not differ significantly from the methylation status of healthy individuals, whereas most of the genes (ATF2, CXCL14, CXCL5, GATA3, IL12B, IL17C, IL4R, IL6R and IL6ST) were found to be significantly hypermethylated in UC patients compared to healthy individuals. CCL25, CXCL3, FADD, IL10RA, IL12A, IL13, IL15, IL17RA, INHA, TYK2 and IL7 were hypomethylated in UC. Additionally, the genes IL13, IL17C, CXCL6, IL10RA, CXCL14, GATA3, IL6ST, IL4R and IL6R show different methylation profiles between UC and CD. Methylation profile in intestinal tissue and peripheral blood are in concordance[215].
Increased acetylation of H4 (the lysine residues 8 and 12) has been found in inflamed tissues and Peyer patches from patients and rats with colitis. Several mechanisms have been proposed to link histone modification with inflammation, involving the innate immune response to microbiota[216,217]. Deregulation of intestinal inflammatory response can occur through disruption in the balance between miRNA activity and threshold levels of specific target mRNAS[218]. Several studies have investigated the different expression of miRNAs in IBD patients. Altered expression patterns of miRNAs in IBD patients were first described in 2008. In biopsy samples of patients with sigmoid active UC, 8 miRNAs levels were significantly increased and 3 were decreased compared with normal. MiR-192, which is expressed in normal colonic epithelial cells, was significantly reduced in tissues of patients with active UC[219]. Increased expression of miR-21 and -155, which promotes inflammation, has been reported in patients with active UC and colonic CD. The miR-196 is upregulated in inflamed epithelium of CD patients and can reduce the IRGM-mediated autophagy. Otherwise, different miR expression patterns have been identified in peripheral blood samples from IBD patients compared to controls and from CD patients compared to those with UC. Several miRs have been indicated to have negative or positive regulation, including miRs -16, -21, -28-5p, -149, -151-5p, -199-A, and -532-3p. Eleven miRs have also been found to be differently expressed in serum samples from pediatric CD patients and healthy children[220].
Environment plays a major role in the development and activity of IBD. The clarification of the pathophysiological mechanisms in relation with the environmental effect on the incidence of IBD can lead to more effective prevention and/or treatment of disease. More clinical studies could indicate if avoiding some drugs and a westernized diet followed by an intake of vitamin D, would lead to a remission even to colonic healing in IBD patients. Connection between environmental and genetic factors, through epigenetic alterations, may lead to a better understanding of IBD. The recent advances in our understanding of IBD-associated epigenetic mechanisms underlie many promising clinical applications such as molecular biomarkers for diagnosis and prognosis of the disease as well as prediction of treatment outcomes.
| 1. | Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Büschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol. 1995;102:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 635] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2259] [Article Influence: 132.9] [Reference Citation Analysis (10)] |
| 3. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1581] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 4. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1659] [Article Influence: 118.5] [Reference Citation Analysis (6)] |
| 5. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2175] [Article Influence: 98.9] [Reference Citation Analysis (2)] |
| 6. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3604] [Article Influence: 257.4] [Reference Citation Analysis (6)] |
| 7. | Zhao J, Ng SC, Lei Y, Yi F, Li J, Yu L, Zou K, Dan Z, Dai M, Ding Y. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis. 2013;19:1839-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol. 2014;20:11525-11537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 9. | Ng SC. Emerging leadership lecture: Inflammatory bowel disease in Asia: emergence of a “Western” disease. J Gastroenterol Hepatol. 2015;30:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Rehman A, Rausch P, Wang J, Skieceviciene J, Kiudelis G, Bhagalia K, Amarapurkar D, Kupcinskas L, Schreiber S, Rosenstiel P. Geographical patterns of the standing and active human gut microbiome in health and IBD. Gut. 2016;65:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 11. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 616] [Article Influence: 47.4] [Reference Citation Analysis (3)] |
| 12. | Vogel H, Halpert D, Horoupian DS. Hypoplasia of posterior spinal roots and dorsal spinal tracts with arthrogryposis multiplex congenita. Acta Neuropathol. 1990;79:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 13. | Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 726] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 14. | El Mouzan MI, Saadah O, Al-Saleem K, Al Edreesi M, Hasosah M, Alanazi A, Al Mofarreh M, Asery A, Al Qourain A, Nouli K. Incidence of pediatric inflammatory bowel disease in Saudi Arabia: a multicenter national study. Inflamm Bowel Dis. 2014;20:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Benchimol EI, Manuel DG, Guttmann A, Nguyen GC, Mojaverian N, Quach P, Mack DR. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis. 2014;20:1761-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Benchimol EI, Mack DR, Nguyen GC, Snapper SB, Li W, Mojaverian N, Quach P, Muise AM. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. 2014;147:803-813.e7; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 17. | El-Matary W, Moroz SP, Bernstein CN. Inflammatory bowel disease in children of Manitoba: 30 years’ experience of a tertiary center. J Pediatr Gastroenterol Nutr. 2014;59:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Jakobsen C, Paerregaard A, Munkholm P, Faerk J, Lange A, Andersen J, Jakobsen M, Kramer I, Czernia-Mazurkiewicz J, Wewer V. Pediatric inflammatory bowel disease: increasing incidence, decreasing surgery rate, and compromised nutritional status: A prospective population-based cohort study 2007-2009. Inflamm Bowel Dis. 2011;17:2541-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274-1282. [PubMed] |
| 20. | Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14 Suppl 2:S9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Binder V. Genetic epidemiology in inflammatory bowel disease. Dig Dis. 1998;16:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Laharie D, Debeugny S, Peeters M, Van Gossum A, Gower-Rousseau C, Bélaïche J, Fiasse R, Dupas JL, Lerebours E, Piotte S. Inflammatory bowel disease in spouses and their offspring. Gastroenterology. 2001;120:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Freeman HJ. Familial Crohn’s disease in single or multiple first-degree relatives. J Clin Gastroenterol. 2002;35:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 524] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Thompson NP, Driscoll R, Pounder RE, Wakefield AJ. Genetics versus environment in inflammatory bowel disease: results of a British twin study. BMJ. 1996;312:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 222] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Orholm M, Binder V, Sørensen TI, Rasmussen LP, Kyvik KO. Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scand J Gastroenterol. 2000;35:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Halfvarson J, Bodin L, Tysk C, Lindberg E, Järnerot G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology. 2003;124:1767-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (4)] |
| 29. | Pinsk V, Lemberg DA, Grewal K, Barker CC, Schreiber RA, Jacobson K. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. Am J Gastroenterol. 2007;102:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Odes HS, Fraser D, Krawiec J. Inflammatory bowel disease in migrant and native Jewish populations of southern Israel. Scand J Gastroenterol Suppl. 1989;170:36-38; discussion 50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Probert CS, Jayanthi V, Pinder D, Wicks AC, Mayberry JF. Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut. 1992;33:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Carr I, Mayberry JF. The effects of migration on ulcerative colitis: a three-year prospective study among Europeans and first- and second- generation South Asians in Leicester (1991-1994). Am J Gastroenterol. 1999;94:2918-2922. [PubMed] |
| 33. | Goh K, Xiao SD. Inflammatory bowel disease: a survey of the epidemiology in Asia. J Dig Dis. 2009;10:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Li X, Sundquist J, Hemminki K, Sundquist K. Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: a nationwide follow-up study. Inflamm Bowel Dis. 2011;17:1784-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Ko Y, Kariyawasam V, Karnib M, Butcher R, Samuel D, Alrubaie A, Rahme N, McDonald C, Cowlishaw J, Katelaris P. Inflammatory Bowel Disease Environmental Risk Factors: A Population-Based Case-Control Study of Middle Eastern Migration to Australia. Clin Gastroenterol Hepatol. 2015;13:1453-63.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Benchimol EI, Mack DR, Guttmann A, Nguyen GC, To T, Mojaverian N, Quach P, Manuel DG. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. 2015;110:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 37. | Barreiro-de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez-Muñoz JE. Emigration to western industrialized countries: A risk factor for developing inflammatory bowel disease. J Crohns Colitis. 2011;5:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Benchimol EI, Manuel DG, To T, Mack DR, Nguyen GC, Gommerman JL, Croitoru K, Mojaverian N, Wang X, Quach P. Asthma, type 1 and type 2 diabetes mellitus, and inflammatory bowel disease amongst South Asian immigrants to Canada and their children: a population-based cohort study. PLoS One. 2015;10:e0123599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Tysk C, O’Morain C, Moum B, Colombel JF. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 40. | Tuvlin JA, Raza SS, Bracamonte S, Julian C, Hanauer SB, Nicolae DL, King AC, Cho JH. Smoking and inflammatory bowel disease: trends in familial and sporadic cohorts. Inflamm Bowel Dis. 2007;13:573-579. [PubMed] |
| 41. | Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C, Frei A, Frei P, Scharl M. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8:e59260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 42. | Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohns Colitis. 2014;8:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 43. | Naito T, Kida H, Yokoyama H, Koshino Y, Tomosugi N, Hattori N, Kobayashi K. A case of diffuse panbronchiolitis (DPB) with benign monoclonal IgA gammopathy and IgA nephropathy with monoclonal IgA deposition. Jpn J Med. 1989;28:503-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18:481-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (3)] |
| 45. | Lakatos PL, Szamosi T, Lakatos L. Smoking in inflammatory bowel diseases: good, bad or ugly? World J Gastroenterol. 2007;13:6134-6139. [PubMed] [DOI] [Full Text] |
| 46. | Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre JP, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol. 2001;96:2113-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 540] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 48. | Cosnes J, Nion-Larmurier I, Afchain P, Beaugerie L, Gendre JP. Gender differences in the response of colitis to smoking. Clin Gastroenterol Hepatol. 2004;2:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Jha P, Ranson MK, Nguyen SN, Yach D. Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health. 2002;92:1002-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Motley RJ, Rhodes J, Ford GA, Wilkinson SP, Chesner IM, Asquith P, Hellier MD, Mayberry JF. Time relationships between cessation of smoking and onset of ulcerative colitis. Digestion. 1987;37:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Penny WJ, Penny E, Mayberry JF, Rhodes J. Prevalence of inflammatory bowel disease amongst Mormons in Britain and Ireland. Soc Sci Med. 1985;21:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Thomas GA, Rhodes J, Ragunath K, Mani V, Williams GT, Newcombe RG, Russell MA, Feyerabend C. Transdermal nicotine compared with oral prednisolone therapy for active ulcerative colitis. Eur J Gastroenterol Hepatol. 1996;8:769-776. [PubMed] |
| 53. | Bastida G, Beltrán B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J Gastroenterol. 2011;17:2740-2747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci. 1989;34:1841-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 378] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 55. | Nos P, Domènech E. Management of Crohn’s disease in smokers: is an alternative approach necessary? World J Gastroenterol. 2011;17:3567-3574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 57. | Lunney PC, Kariyawasam VC, Wang RR, Middleton KL, Huang T, Selinger CP, Andrews JM, Katelaris PH, Leong RW. Smoking prevalence and its influence on disease course and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2015;42:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (7)] |
| 58. | Picco MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn’s disease. Am J Gastroenterol. 2003;98:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Mahid SS, Minor KS, Stevens PL, Galandiuk S. The role of smoking in Crohn’s disease as defined by clinical variables. Dig Dis Sci. 2007;52:2897-2903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Johnson GJ, Cosnes J, Mansfield JC. Review article: smoking cessation as primary therapy to modify the course of Crohn’s disease. Aliment Pharmacol Ther. 2005;21:921-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Cosnes J, Beaugerie L, Carbonnel F, Gendre JP. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 279] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 62. | Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 63. | van der Heide F, Dijkstra A, Weersma RK, Albersnagel FA, van der Logt EM, Faber KN, Sluiter WJ, Kleibeuker JH, Dijkstra G. Effects of active and passive smoking on disease course of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Koutroubakis IE, Vlachonikolis IG, Kouroumalis EA. Role of appendicitis and appendectomy in the pathogenesis of ulcerative colitis: a critical review. Inflamm Bowel Dis. 2002;8:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Kurina LM, Goldacre MJ, Yeates D, Seagroatt V. Appendicectomy, tonsillectomy, and inflammatory bowel disease: a case-control record linkage study. J Epidemiol Community Health. 2002;56:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Radford-Smith GL, Edwards JE, Purdie DM, Pandeya N, Watson M, Martin NG, Green A, Newman B, Florin TH. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut. 2002;51:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | de Saussure P, Clerson P, Prost PL, Truong Tan N, Bouhnik Y. Appendectomy, smoking habits and the risk of developing ulcerative colitis: a case control study in private practice setting. Gastroenterol Clin Biol. 2007;31:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Wang YF, Ou-Yang Q, Xia B, Liu LN, Gu F, Zhou KF, Mei Q, Shi RH, Ran ZH, Wang XD. Multicenter case-control study of the risk factors for ulcerative colitis in China. World J Gastroenterol. 2013;19:1827-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Frisch M, Gridley G. Appendectomy in adulthood and the risk of inflammatory bowel diseases. Scand J Gastroenterol. 2002;37:1175-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Kaplan GG, Pedersen BV, Andersson RE, Sands BE, Korzenik J, Frisch M. The risk of developing Crohn’s disease after an appendectomy: a population-based cohort study in Sweden and Denmark. Gut. 2007;56:1387-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | López Ramos D, Gabriel R, Cantero Perona J, Moreno Otero R, Fernández Bermejo M, Maté Jiménez J. Association of MALTectomy (appendectomy and tonsillectomy) and inflammatory bowel disease: a familial case-control study. Rev Esp Enferm Dig. 2001;93:303-314. [PubMed] |
| 72. | Cheluvappa R, Luo AS, Palmer C, Grimm MC. Protective pathways against colitis mediated by appendicitis and appendectomy. Clin Exp Immunol. 2011;165:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Andersson RE, Olaison G, Tysk C, Ekbom A. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;344:808-814. [PubMed] |
| 74. | Beaugerie L, Sokol H. Appendicitis, not appendectomy, is protective against ulcerative colitis, both in the general population and first-degree relatives of patients with IBD. Inflamm Bowel Dis. 2010;16:356-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Kaplan GG, Jackson T, Sands BE, Frisch M, Andersson RE, Korzenik J. The risk of developing Crohn’s disease after an appendectomy: a meta-analysis. Am J Gastroenterol. 2008;103:2925-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | Evans JM, McMahon AD, Murray FE, McDevitt DG, MacDonald TM. Non-steroidal anti-inflammatory drugs are associated with emergency admission to hospital for colitis due to inflammatory bowel disease. Gut. 1997;40:619-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Felder JB, Korelitz BI, Rajapakse R, Schwarz S, Horatagis AP, Gleim G. Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2000;95:1949-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | Gleeson MH, Davis AJ. Non-steroidal anti-inflammatory drugs, aspirin and newly diagnosed colitis: a case-control study. Aliment Pharmacol Ther. 2003;17:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Ananthakrishnan AN, Higuchi LM, Huang ES, Khalili H, Richter JM, Fuchs CS, Chan AT. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 80. | Meyer AM, Ramzan NN, Heigh RI, Leighton JA. Relapse of inflammatory bowel disease associated with use of nonsteroidal anti-inflammatory drugs. Dig Dis Sci. 2006;51:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Philpott HL, Nandurkar S, Lubel J, Gibson PR. Drug-induced gastrointestinal disorders. Postgrad Med J. 2014;90:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Chan SS, Luben R, Bergmann MM, Boeing H, Olsen A, Tjonneland A, Overvad K, Kaaks R, Kennedy H, Khaw KT. Aspirin in the aetiology of Crohn’s disease and ulcerative colitis: a European prospective cohort study. Aliment Pharmacol Ther. 2011;34:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Rhodes JM, Cockel R, Allan RN, Hawker PC, Dawson J, Elias E. Colonic Crohn’s disease and use of oral contraception. Br Med J (Clin Res Ed). 1984;288:595-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Lesko SM, Kaufman DW, Rosenberg L, Helmrich SP, Miller DR, Stolley PD, Shapiro S. Evidence for an increased risk of Crohn’s disease in oral contraceptive users. Gastroenterology. 1985;89:1046-1049. [PubMed] |
| 85. | Sandler RS, Wurzelmann JI, Lyles CM. Oral contraceptive use and the risk of inflammatory bowel disease. Epidemiology. 1992;3:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2394-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 87. | Dubeau MF, Iacucci M, Beck PL, Moran GW, Kaplan GG, Ghosh S, Panaccione R. Drug-induced inflammatory bowel disease and IBD-like conditions. Inflamm Bowel Dis. 2013;19:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 88. | Khalili H, Higuchi LM, Ananthakrishnan AN, Richter JM, Feskanich D, Fuchs CS, Chan AT. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;62:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 89. | Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 90. | Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130:e794-e803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 91. | Virta L, Auvinen A, Helenius H, Huovinen P, Kolho KL. Association of repeated exposure to antibiotics with the development of pediatric Crohn’s disease--a nationwide, register-based finnish case-control study. Am J Epidemiol. 2012;175:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 92. | Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, Shaw S, Van Kruiningen H, Colombel JF, Atreja A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109:1728-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 93. | Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105:2687-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 94. | Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2011;106:2133-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 95. | Chapman-Kiddell CA, Davies PS, Gillen L, Radford-Smith GL. Role of diet in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 96. | Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1591] [Cited by in RCA: 1404] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 97. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691-14696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3584] [Cited by in RCA: 4143] [Article Influence: 258.9] [Reference Citation Analysis (0)] |
| 98. | Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, Welfare MR. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 343] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 99. | D’Souza S, Levy E, Mack D, Israel D, Lambrette P, Ghadirian P, Deslandres C, Morgan K, Seidman EG, Amre DK. Dietary patterns and risk for Crohn’s disease in children. Inflamm Bowel Dis. 2008;14:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Asakura H, Suzuki K, Kitahora T, Morizane T. Is there a link between food and intestinal microbes and the occurrence of Crohn’s disease and ulcerative colitis? J Gastroenterol Hepatol. 2008;23:1794-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 101. | Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 722] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 102. | Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol. 2010;105:2195-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 103. | Maconi G, Ardizzone S, Cucino C, Bezzio C, Russo AG, Bianchi Porro G. Pre-illness changes in dietary habits and diet as a risk factor for inflammatory bowel disease: a case-control study. World J Gastroenterol. 2010;16:4297-4304. [PubMed] |
| 104. | Amre DK, D’Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, Israel D, Mack D, Ghadirian P, Deslandres C. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol. 2007;102:2016-2025. [PubMed] |
| 105. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145:970-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 544] [Cited by in RCA: 473] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 106. | Russel MG, Engels LG, Muris JW, Limonard CB, Volovics A, Brummer RJ, Stockbrügger RW. Modern life’ in the epidemiology of inflammatory bowel disease: a case-control study with special emphasis on nutritional factors. Eur J Gastroenterol Hepatol. 1998;10:243-249. [PubMed] |
| 107. | Kowal-Vern A, McFadden J. Pseudomonas aeruginosa pneumonia as a presenting entity in an AIDS patient. Clin Pediatr (Phila). 1989;28:403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | Martini GA, Brandes JW. Increased consumption of refined carbohydrates in patients with Crohn’s disease. Klin Wochenschr. 1976;54:367-371. [PubMed] |
| 109. | Mayberry JF, Rhodes J, Newcombe RG. Increased sugar consumption in Crohn’s disease. Digestion. 1980;20:323-326. [PubMed] |
| 110. | Bianchi Porro G, Panza E. Smoking, sugar, and inflammatory bowel disease. Br Med J (Clin Res Ed). 1985;291:971-972. [PubMed] |
| 111. | Hansen TS, Jess T, Vind I, Elkjaer M, Nielsen MF, Gamborg M, Munkholm P. Environmental factors in inflammatory bowel disease: a case-control study based on a Danish inception cohort. J Crohns Colitis. 2011;5:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 112. | Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease -- a population based study 2007-2009. J Crohns Colitis. 2013;7:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 113. | Teixeira LG, Leonel AJ, Aguilar EC, Batista NV, Alves AC, Coimbra CC, Ferreira AV, de Faria AM, Cara DC, Alvarez Leite JI. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011;10:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 114. | Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1477] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 115. | Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741-745. [PubMed] |
| 116. | Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, Berglund G, Lindgren S, Grip O, Key T. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion. 2008;77:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 117. | de Silva PS, Olsen A, Christensen J, Schmidt EB, Overvaad K, Tjonneland A, Hart AR. An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Gastroenterology. 2010;139:1912-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 118. | Kono H, Fujii H, Ogiku M, Tsuchiya M, Ishii K, Hara M. Enteral diets enriched with medium-chain triglycerides and N-3 fatty acids prevent chemically induced experimental colitis in rats. Transl Res. 2010;156:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 119. | John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:602-606. [PubMed] |
| 120. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, Willett WC, Richter JM, Chan AT. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2014;63:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 381] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 121. | Klement E, Cohen RV, Boxman J, Joseph A, Reif S. Breastfeeding and risk of inflammatory bowel disease: a systematic review with meta-analysis. Am J Clin Nutr. 2004;80:1342-1352. [PubMed] |
| 122. | Barclay AR, Russell RK, Wilson ML, Gilmour WH, Satsangi J, Wilson DC. Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J Pediatr. 2009;155:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 123. | Blanchard JF, Bernstein CN, Wajda A, Rawsthorne P. Small-area variations and sociodemographic correlates for the incidence of Crohn’s disease and ulcerative colitis. Am J Epidemiol. 2001;154:328-335. [PubMed] |
| 124. | Klement E, Lysy J, Hoshen M, Avitan M, Goldin E, Israeli E. Childhood hygiene is associated with the risk for inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2008;103:1775-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 125. | Castiglione F, Diaferia M, Morace F, Labianca O, Meucci C, Cuomo A, Panarese A, Romano M, Sorrentini I, D’Onofrio C. Risk factors for inflammatory bowel diseases according to the “hygiene hypothesis”: a case-control, multi-centre, prospective study in Southern Italy. J Crohns Colitis. 2012;6:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 126. | Bernstein CN, Rawsthorne P, Cheang M, Blanchard JF. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol. 2006;101:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 127. | Hampe J, Heymann K, Krawczak M, Schreiber S. Association of inflammatory bowel disease with indicators for childhood antigen and infection exposure. Int J Colorectal Dis. 2003;18:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 128. | Wu XW, Ji HZ, Yang MF, Wu L, Wang FY. Helicobacter pylori infection and inflammatory bowel disease in Asians: a meta-analysis. World J Gastroenterol. 2015;21:4750-4756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 129. | Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 130. | Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20:6374-6385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 131. | Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 132. | Szkudlapski D, Labuzek K, Pokora Z, Smyla N, Gonciarz M, Mularczyk A, Maluch P, Okopien B. The emering role of helminths in treatment of the inflammatory bowel disorders. J Physiol Pharmacol. 2014;65:741-751. [PubMed] |
| 133. | Mohammadi R, Hosseini-Safa A, Ehsani Ardakani MJ, Rostami-Nejad M. The relationship between intestinal parasites and some immune-mediated intestinal conditions. Gastroenterol Hepatol Bed Bench. 2015;8:123-131. [PubMed] |
| 134. | Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: a critical review of the literature. World J Gastroenterol. 2008;14:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 135. | Hunter MM, McKay DM. Review article: helminths as therapeutic agents for inflammatory bowel disease. Aliment Pharmacol Ther. 2004;19:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Ottow MK, Klaver EJ, van der Pouw Kraan TC, Heijnen PD, Laan LC, Kringel H, Vogel DY, Dijkstra CD, Kooij G, van Die I. The helminth Trichuris suis suppresses TLR4-induced inflammatory responses in human macrophages. Genes Immun. 2014;15:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 137. | Sun S, Wang X, Wu X, Zhao Y, Wang F, Liu X, Song Y, Wu Z, Liu M. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit Vectors. 2011;4:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 138. | Bernstein CN, Blanchard JF, Rawsthorne P, Collins MT. Population-based case control study of seroprevalence of Mycobacterium paratuberculosis in patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2004;42:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 139. | Momotani E, Ozaki H, Hori M, Yamamoto S, Kuribayashi T, Eda S, Ikegami M. Mycobacterium avium subsp. paratuberculosis lipophilic antigen causes Crohn’s disease-type necrotizing colitis in Mice. Springerplus. 2012;1:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 140. | Nazareth N, Magro F, Machado E, Ribeiro TG, Martinho A, Rodrigues P, Alves R, Macedo GN, Gracio D, Coelho R. Prevalence of Mycobacterium avium subsp. paratuberculosis and Escherichia coli in blood samples from patients with inflammatory bowel disease. Med Microbiol Immunol. 2015;204:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 141. | Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, Pfyffer GE, Jemmi T, Baumgartner A, Egger M. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 378] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 142. | Wang ZK, Yang YS, Chen Y, Yuan J, Sun G, Peng LH. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol. 2014;20:14805-14820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 143. | Liang J, Sha SM, Wu KC. Role of the intestinal microbiota and fecal transplantation in inflammatory bowel diseases. J Dig Dis. 2014;15:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | Bosca-Watts MM, Tosca J, Anton R, Mora M, Minguez M, Mora F. Pathogenesis of Crohn’s disease: Bug or no bug. World J Gastrointest Pathophysiol. 2015;6:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Forbes A, Kalantzis T. Crohn’s disease: the cold chain hypothesis. Int J Colorectal Dis. 2006;21:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 146. | Korzenik JR. Past and current theories of etiology of IBD: toothpaste, worms, and refrigerators. J Clin Gastroenterol. 2005;39:S59-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 147. | Hugot JP, Alberti C, Berrebi D, Bingen E, Cézard JP. Crohn’s disease: the cold chain hypothesis. Lancet. 2003;362:2012-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 148. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5700] [Cited by in RCA: 5689] [Article Influence: 270.9] [Reference Citation Analysis (3)] |
| 149. | O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1839] [Article Influence: 92.0] [Reference Citation Analysis (3)] |
| 150. | Lotz M, Ménard S, Hornef M. Innate immune recognition on the intestinal mucosa. Int J Med Microbiol. 2007;297:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 151. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8120] [Article Influence: 507.5] [Reference Citation Analysis (4)] |
| 152. | Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 453] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 153. | Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, Aguilera M, Khanna S, Gil A, Edwards CA. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 154. | Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2470] [Article Influence: 205.8] [Reference Citation Analysis (1)] |
| 155. | Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1756] [Cited by in RCA: 2125] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 156. | Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 602] [Article Influence: 50.2] [Reference Citation Analysis (1)] |
| 157. | Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1704] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 158. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [PubMed] |
| 159. | Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 931] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 160. | Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 879] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 161. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 4064] [Article Influence: 312.6] [Reference Citation Analysis (0)] |
| 162. | Ohkusa T, Koido S. Intestinal microbiota and ulcerative colitis. J Infect Chemother. 2015;21:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 163. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 2218] [Article Influence: 170.6] [Reference Citation Analysis (3)] |
| 164. | Ott SJ, Plamondon S, Hart A, Begun A, Rehman A, Kamm MA, Schreiber S. Dynamics of the mucosa-associated flora in ulcerative colitis patients during remission and clinical relapse. J Clin Microbiol. 2008;46:3510-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 165. | Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 166. | Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 654] [Article Influence: 40.9] [Reference Citation Analysis (2)] |
| 167. | Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 168. | Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 169. | Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1355] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 170. | Garg M, Lubel JS, Sparrow MP, Holt SG, Gibson PR. Review article: vitamin D and inflammatory bowel disease--established concepts and future directions. Aliment Pharmacol Ther. 2012;36:324-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 171. | Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 172. | Cantorna MT, Mahon BD. D-hormone and the immune system. J Rheumatol Suppl. 2005;76:11-20. [PubMed] |
| 173. | Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004;229:1136-1142. [PubMed] |
| 174. | Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S-1720S. [PubMed] |
| 175. | Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 176. | Palmer MT, Weaver CT. Linking vitamin d deficiency to inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2245-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 177. | Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 178. | Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, Day AS. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 179. | Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 180. | Nerich V, Jantchou P, Boutron-Ruault MC, Monnet E, Weill A, Vanbockstael V, Auleley GR, Balaire C, Dubost P, Rican S. Low exposure to sunlight is a risk factor for Crohn’s disease. Aliment Pharmacol Ther. 2011;33:940-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 181. | Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, Chan AT. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 182. | Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 183. | Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1921-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 184. | Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 185. | Zhu T, Liu TJ, Shi YY, Zhao Q. Vitamin D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis by inhibiting intestinal epithelial apoptosis. Int J Mol Med. 2015;35:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 186. | Limketkai BN, Bayless TM, Brant SR, Hutfless SM. Lower regional and temporal ultraviolet exposure is associated with increased rates and severity of inflammatory bowel disease hospitalisation. Aliment Pharmacol Ther. 2014;40:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 187. | Shaw SY, Nugent Z, Targownik LE, Singh H, Blanchard JF, Bernstein CN. Association between spring season of birth and Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 188. | Disanto G, Chaplin G, Morahan JM, Giovannoni G, Hyppönen E, Ebers GC, Ramagopalan SV. Month of birth, vitamin D and risk of immune-mediated disease: a case control study. BMC Med. 2012;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 189. | Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, Ghosh S, Wheeler AJ, Villeneuve PJ. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol. 2010;105:2412-2419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 190. | Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis. 2011;17:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 191. | Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. 2014;5:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 192. | Rice KM, Walker EM, Wu M, Gillette C, Blough ER. Environmental mercury and its toxic effects. J Prev Med Public Health. 2014;47:74-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 591] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 193. | Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 194. | Reber SO. Stress and animal models of inflammatory bowel disease--an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 195. | Ennaifer R, Elleuch N, Cheikh M, Hefaiedh R, Romdhane H, Ben Nejma H, Belhadj N. Risk factors of psychological disorders in inflammatory bowel disease in a tunisian survey. Results of a cross-sectional study. Tunis Med. 2014;92:723-726. [PubMed] |
| 196. | Bannaga AS, Selinger CP. Inflammatory bowel disease and anxiety: links, risks, and challenges faced. Clin Exp Gastroenterol. 2015;8:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 197. | Goodhand JR, Wahed M, Mawdsley JE, Farmer AD, Aziz Q, Rampton DS. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis. 2012;18:2301-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 198. | Long MD, Kappelman MD, Martin CF, Chen W, Anton K, Sandler RS. Risk factors for depression in the elderly inflammatory bowel disease population. J Crohns Colitis. 2014;8:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 199. | Szigethy EM, Youk AO, Benhayon D, Fairclough DL, Newara MC, Kirshner MA, Bujoreanu SI, Mrakotsky C, Bousvaros A, Srinath AI. Depression subtypes in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 200. | Ananthakrishnan AN, Gainer VS, Perez RG, Cai T, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL. Psychiatric co-morbidity is associated with increased risk of surgery in Crohn’s disease. Aliment Pharmacol Ther. 2013;37:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 201. | Collins SM. Stress and the Gastrointestinal Tract IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G315-G318. [PubMed] |
| 202. | Levenstein S, Prantera C, Varvo V, Scribano ML, Andreoli A, Luzi C, Arcà M, Berto E, Milite G, Marcheggiano A. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2000;95:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 300] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 203. | Bitton A, Dobkin PL, Edwardes MD, Sewitch MJ, Meddings JB, Rawal S, Cohen A, Vermeire S, Dufresne L, Franchimont D. Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut. 2008;57:1386-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 204. | Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. 2010;105:1994-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 205. | Kaname S, Uchida S, Ogata E, Kurokawa K. Autocrine secretion of transforming growth factor-beta in cultured rat mesangial cells. Kidney Int. 1992;42:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 206. | Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 416] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 207. | Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 208. | Boye B, Lundin KE, Jantschek G, Leganger S, Mokleby K, Tangen T, Jantschek I, Pripp AH, Wojniusz S, Dahlstroem A. INSPIRE study: does stress management improve the course of inflammatory bowel disease and disease-specific quality of life in distressed patients with ulcerative colitis or Crohn’s disease? A randomized controlled trial. Inflamm Bowel Dis. 2011;17:1863-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 209. | Ng V, Millard W, Lebrun C, Howard J. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin J Sport Med. 2007;17:384-388. [PubMed] |
| 210. | Packer N, Hoffman-Goetz L, Ward G. Does physical activity affect quality of life, disease symptoms and immune measures in patients with inflammatory bowel disease? A systematic review. J Sports Med Phys Fitness. 2010;50:1-18. [PubMed] |
| 211. | Khalili H, Ananthakrishnan AN, Konijeti GG, Liao X, Higuchi LM, Fuchs CS, Spiegelman D, Richter JM, Korzenik JR, Chan AT. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ. 2013;347:f6633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 212. | Ali T, Madhoun MF, Orr WC, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2440-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 213. | Nimmo ER, Prendergast JG, Aldhous MC, Kennedy NA, Henderson P, Drummond HE, Ramsahoye BH, Wilson DC, Semple CA, Satsangi J. Genome-wide methylation profiling in Crohn’s disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis. 2012;18:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 214. | Saito S, Kato J, Hiraoka S, Horii J, Suzuki H, Higashi R, Kaji E, Kondo Y, Yamamoto K. DNA methylation of colon mucosa in ulcerative colitis patients: correlation with inflammatory status. Inflamm Bowel Dis. 2011;17:1955-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 215. | Karatzas PS, Mantzaris GJ, Safioleas M, Gazouli M. DNA methylation profile of genes involved in inflammation and autoimmunity in inflammatory bowel disease. Medicine (Baltimore). 2014;93:e309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 216. | Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 217. | Tsaprouni LG, Ito K, Powell JJ, Adcock IM, Punchard N. Differential patterns of histone acetylation in inflammatory bowel diseases. J Inflamm (Lond). 2011;8:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 218. | Stylianou E. Epigenetics: the fine-tuner in inflammatory bowel disease? Curr Opin Gastroenterol. 2013;29:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 219. | Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624-1635.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 412] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 220. | Wu F, Guo NJ, Tian H, Marohn M, Gearhart S, Bayless TM, Brant SR, Kwon JH. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2011;17:241-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Efthymiou A, Kopylov U, Spisni E, Tsai HH S- Editor: Qi Y L- Editor: A E- Editor: Wang CH