Published online Nov 6, 2014. doi: 10.4292/wjgpt.v5.i4.209
Revised: April 5, 2014
Accepted: July 27, 2014
Published online: November 6, 2014
Processing time: 346 Days and 22 Hours
Protein kinases play a crucial role in the pathogenesis of inflammatory bowel disease (IBD), the two main forms of which are ulcerative colitis and Crohn’s disease. In this article, we will review the mechanisms of involvement of protein kinases in the pathogenesis of and intervention against IBD, in terms of their effects on genetics, microbiota, mucous layer and tight junction, and the potential of protein kinases as therapeutic targets against IBD.
Core tip: The roles of protein kinases in the pathogenesis and intervention of inflammatory bowel diseases (IBD) are emerging. In this article, we will review the specific roles of different protein kinases in the pathogenesis of IBD, classify these protein kinases into different categories based on their fundamental functions in IBD, and describe substantial new mechanistic insights into the pathogenesis of IBD, highlighting protein kinases as potential intervention targets against IBD.
- Citation: Yang L, Yan Y. Protein kinases are potential targets to treat inflammatory bowel disease. World J Gastrointest Pharmacol Ther 2014; 5(4): 209-217
- URL: https://www.wjgnet.com/2150-5349/full/v5/i4/209.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v5.i4.209
Ulcerative colitis (UC) and Crohn’s disease (CD), two main forms of inflammatory bowel disease (IBD), are relapsing, idiopathic intestinal inflammatory conditions, caused by inappropriate and continuing immunologic responses to aberrant intestinal microorganisms in genetically susceptible individuals under certain environmental conditions[1].
UC and CD differ[2] with each other dramatically in different respects. UC is confined to the superficial area of the intestinal wall, whereas CD is transmurally distributed throughout the entire digestive tract but in a discontinuous way. The lesion is patchy with “lead pipe sign” in UC, but many polyps with “string sign” are often observed in CD. UC displays a Th2-like immune response, while CD shows a Th1 dominant response. Antineutrophil cytoplasmic antibodies were found in 65% of UC cases and 5%-10% of CD cases, and antibodies to yeast S. cerevisiae were found in 60%-70% of CD cases and 10%-15% of UC cases[3]. Meanwhile, UC and CD share many similarities, such as neutrophil infiltration and epithelial barrier dysfunction. Despite the fact that there is no cure for IBD thus far, enormous progress about the pathogenic mechanisms of this inflammatory disorder has been around the corner in different aspects, such as genetics, regulatory immunology and microbiome.
The signaling pathways mediated by protein kinases have drawn much attention for connecting external stimuli including hostile environmental stresses with internal biological responses, such as intestinal inflammation. Protein kinases can be defined as enzymes which add phosphate (called phosphorylation) to the side chain of serine, threonine or tyrosine of substrate molecules. This modification alters the biological function of the substrate, such as changing enzyme activity, cellular distribution, and even causing diseases[4,5]. In this review, we will shed light on the roles of protein kinases in the pathogenesis of intestinal inflammation and describe some new mechanistic insights into the intervention of IBD, which targets at protein kinases.
Genome-wide association studies demonstrated that genetic factors are very crucial in the individual susceptibility to IBD, for example, relatives of UC patients including twins display almost ten times greater risk of UC than non-relatives[6,7]. As shown in Table 1, major IBD susceptibility regions on chromosomes 16 and 6 contain some genes encoding protein kinases like extracellular signals-regulated kinase 1 (ERK1)[8] and p38[9]. Several single-nucleotide polymorphisms in tyrosine kinase 2[10] and Janus kinase 2[11] were identified in IBD patients. Glucokinase regulator has also been associated with the risk of CD[12]. The cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like plays an important role in susceptibility to CD, psoriasis and type II diabetes[13,14]; leucine-rich repeat kinase 2 is identified to be related to the pathogenesis of CD[15].
| Kinase | IBD | Ref. |
| ERK1 | CD | [8] |
| p38 | CD and UC | [9] |
| TYK2 | CD and UC | [10] |
| JAK2 | CD and UC | [11] |
| GCKR | CD | [12] |
| CDKAL1 | CD | [13] |
| LRRK2 | CD | [15] |
Up to 1014 individual bacteria in the human gastrointestinal (GI) tract[16], together with the mucous layer where the microbiome lives in, constitute the first line of defense in host against hostile external environment, modulating GI tract development, maintaining immune homeostasis, and regulating host metabolism rate. The bacterial abnormality plays a dominant role in the onset and development of IBD.
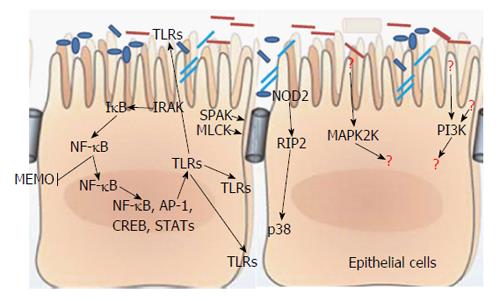
Commensal bacteria and host innate immune system evolve together and thus maintain mucosal immune homeostasis by balancing inflammatory responses and regulating a variety of bacteria-triggering signal transduction pathways[17], such as uncoupling nuclear factor (NF)-κB or mitogen activated protein kinase (MAPK) dependent target genes in a negative feedback manner[18,19]. The host’s innate immune system is poised to be triggered by signs of bacterial challenge, specially, some pathogen-associated molecules such as flagellin, peptidoglycan, lipoteichoic acid, or lipopolysaccharide, together called pathogen-associated molecular patterns which can wake up the host innate immune system[20,21] and be further sensed by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) or the nucleotide-binding oligomerization domain containing protein (NOD)-like receptors[22] (Figure 1). These PRRs would then induce the activation of signaling cascades, mostly MAPK and NF-κB pathways. In terms of MAPK pathways, it follows MAP4K-MAP3K-MAP2K-MAPK pattern, and then, the activated MAPK undergoes translocation to the nucleus to activate molecules required for gene transcription, including inflammatory molecules[23,24]. For example, anthrax toxin can induce macrophage death by inhibiting the p38 signaling pathway[25,26], and MAPK-activated protein kinase 2 plays an important role in the pathogenesis of Clostridium difficile-associated intestinal inflammation[27]. For the NF-κB pathway, after being activated by IκB kinase kinase complex, it phosphorylates α subunit of IκB, the inhibitor of NF-κB. Phosphorylation of IκB, accompanied by its ubiquitination and proteolytic degradation, results in exposure of the nuclear localization signal (NLS) on the now unbound NF-κB[28], which will further facilitate nuclear translocation of NF-κB and be followed by transcriptional activation of many genes. In addition, even being regarded as an molecule which can promote inflammatory responses, an anti-inflammatory effect of NF-κB was noticed; absence of NF-κB essential modulator kinase causes spontaneous severe colitis, but commensal bacteria can stimulate the NF-κB pathway to protect the host from exacerbating consequence[29]. Blockage of epithelial NF-κB pathway will deteriorate this colitis by increasing the translocation of bacterial to the mucosa[30]. Besides the MAPK and NF-κB pathways, some other signaling pathways are also very important, for example, after recognition of Salmonella enterica serovar Typhimurium curli fibrils in the gut, the TLR2-phosphatidylinositol 3 (PI3)-kinase pathway will be stimulated to tight the epithelial barrier[31]. However, PI3 kinase signaling promotes Campylobacter jejunum-induced colitis through neutrophil recruitment in mice[32]. RIP2 tyrosine kinase activity is required for NOD2-dependent autophagy process, but plays a dual role in this process. RIP2 sends a positive autophagy signal through activation of p38 MAPK and further relieves repression of autophagy mediated by the phosphatase PP2A[33]. Not like NOD2 whose signaling induces cryptdins, MyD88-mediated TLR signaling induces RegIIIg and α-defensins, and more importantly, regulates bacterial infection-related mucosal immunity[34-36]. In parallel, protein kinase C (PKC) can mediate the function of MyD88 adaptor-like (Mal) molecule in the maintenance of epithelial barrier integrity[37].
Basically, IBD is characterized by passive leaky diarrhea and compromised intestinal barrier function. Except for the fact that commensal bacteria function as primary line of defense, protein kinases are also important in regulating the intestinal barrier function.
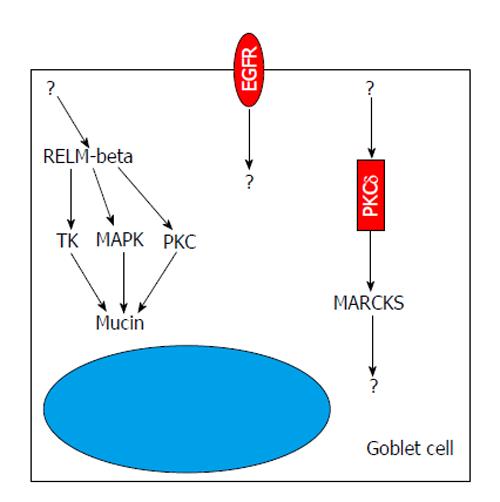
The luminal side of the intestine is covered by a mucus layer which provides protection to the mucosa from mechanical damage and invasion of pathogens, and, together with commensal bacteria, constitutes a physical barrier between the epithelium and luminal contents including pathogenic bacteria, viruses, and parasites[38,39]. This gel-like mucus layer can be divided by two distinguished layers-the outer and inner layers. The vast majority of intestinal bacteria, viruses and even parasites live in the flowing outer mucus layer; the inner layer is, however, an unstirred and relatively sterile layer adjacent to epithelial surface. The sterility of the inner layer accredits to the preservation of huge amounts of defensins, cathelicidins, and cryptidens with important function of anti-intestinal pathogens. Mucin coding gene muc2-/- mice demonstrated spontaneous colitis because of increased transepithelial permeability[40], in which bacteria can stick to the surface of the intestinal mucosa, which facilitates the translocation of bacteria into lower crypts and epithelial cells, thus triggering an inflammatory response[39,41]. Protein kinases are involved in the integrity and maintenance of these mucus layers (Figure 2). Epidermal growth factor receptor (EGFR), harboring tyrosine kinase (TK) activity, has critical functions in development, growth, differentiation, proliferation and repair of epithelial cells[42,43]. After stimulation by EGFR ligands such as transforming growth factor-alpha and epidermal growth factor, epithelial cells can develop into a mucous phenotype[44,45]. However, inhibition of EGFR tyrosine kinase activity can abolish the effects of EGFR ligands on mucus production both in vivo and in vitro. PKCδ stimulates the secretion of mucin in the epithelium via regulation of myristoylated alanine-rich protein kinase C substrate pathway[46]. Treatment of epithelial cells with PD98059 (MEK inhibitor) can inhibit MAPK activity and block the expression of terminal differentiation markers, such as sucrase-isomaltase, ITF, and MUC2, thereby interfering with the production of mucin[47]. Some kinases like ERKs, TK, and PKC[48] can regulate the production of mucin by mediating the activity of resistin and resistin-like molecule-beta; cathelicidin up-regulates MUC1 and MUC2 expression through MAPK pathway to modulate mucus synthesis[49].
The intestinal monolayer is characterized by polarization of apical and basolateral sides. The apical membrane is generally impermeable to hydrophilic solutes and contributes predominantly to mucosal barrier[41]. Among the most important structures to determine paracellular permeability of the intestinal barrier are the epithelial tight junctions (TJs), which are made up of multiple proteins such as occludin and claudins[50]. Occludin as the first identified TJ[51], plays an important role in epithelial/endothelial barrier integrity, and disruption of occludin regulation is an important aspect of a number of diseases[52-54]. The claudins, as a group of TJ proteins with approximately 24 members, interact with numbers of other cell structures and affects junctional function[55-58]. Claudins are expressed in a tissue-specific manner and may show distinct functions, for example, in the colon are expressed the claudins-1, 2, 3, 4, 5, 7, and 8; the claudin-2 is a pore-forming TJ protein, but claudins-1 and 4 are barrier tightening proteins[59-63]. 12-O-tetradecanoylophorbol-13-acetate can increase transepithelial electrical resistance by activating different isoforms of PKC and enhancing the expression of TJ proteins ZO-1, 2, occludin and claudin-1[64,65]. Ca2+/calmodulin-dependent protein kinase II can compromise endothelial barrier function[66]. Ras-transfected epithelial cells demonstrated compromised barrier function; however, inhibition of the MAPK signaling pathway can restore the morphology of epithelial cells and the TJ assembly. Further, the phosphorylation of tyrosine residues in occludin and ZO-1 may be crucial for the formation of TJ[67]. cAMP-dependent protein kinases regulate epithelial barrier function by phosphorylation of claudin-3[68,69].
Generally, at least two relatively independently routes known thus far are responsible for communication between host and external environment through paracellular pathway, both of which can be regulated by protein kinases[70-72]. The size-selectivity related paracellular pathway is one of the two routes, which facilitates transepithelial passage of different size of molecules, such as lipopolysaccharides[71,72], and can be regulated by protein kinases, such as MAPKs, Ste20 like proline/alanine rich kinase (SPAK)[73], PKC[64,65] and myosin light chain kinase (MLCK)[74]. Another route, also called charge-selectivity route, is composed of pore-forming proteins claudins[75-77]. Dysfunction of these two routes, either size-dependent or charge-dependent pathway, may result in the abnormality of overall epithelial TJ, which provides an even more leaky gut. This situation will facilitate the contact of intestinal microorganisms including bacteria, viruses and parasites with the host’s immune system, resulting in altered production of inflammatory mediators that contribute to the compromised barrier function.
Mucosal permeability is influenced by many different factors in there distinct ways. Except the mucus layer, microbiota and epithelial cells themselves mentioned above, genetic factors play crucial roles in the regulation of intestinal barrier function[6]; innate and adaptive immune systems can interfere with epithelial permeability in a dramatic manner[78]; autonomic nerves, like enteric glial nerve ablation, can perish epithelial permeability to develop fulminant jejunoileitis[79]. However, barrier dysfunction itself, like in MLCK[74] and SPAK[73] gene modified mice, does not necessarily mean that the mice are destined to develop intestinal inflammation, implying formidable compensation in host.
Notably, protein kinases play very crucial roles in many aspects of pathogenesis of IBD, highlighting their emerging roles as potential therapeutic targets against IBD. Besides the NF-κB pathway, the MAPK signaling pathway is another highlighted pathway involved in many different diseases including IBD[80]. The activation of MAPK-ERK1/2 phosphorylates the downstream proinflammatory proteins such as cytosolic phospholipase A2 and some transcription factors such as activated proteins, Ets-1, Elk and c-myc. Interestingly, ERK1/2, by a study using an ERK1/2 inhibitor, was found to play an important role in the function of immune cells and other cell types during IBD, by regulating some pro-inflammatory mediators [such as interleukin-1 (IL-1)] related signaling transduction[81,82], evidenced by their enhanced expression and phosphorylation status during IBD[83,84]. Furthermore, the “tightening” junction protein claudin-4, which plays an important role in epithelial barrier function, is regulated by protein kinase ERK[85]. By inducing Akt but blocking p38 signaling, Lactobacillus GG prevents cytokine-induced apoptosis of intestinal epithelial cells, indicating p38 and Akt as key mediators of epithelial barrier function[86,87]. p38 activity is increased significantly in tissues from IBD patients and in mouse models of colitis[83,84,88], in which inhibition of p38 lowers KC (IL-8) and IL-6 production. A similar result was reported that heat-killed Lactobacillus brevis phosphorylates p38 kinase to regulate the expression of proinflammatory cytokines such as TNF-α, and to improve intestinal integrity[89]. JNK1/2 kinase activity was enhanced in IBD inflamed tissue and blockage of JNK1/2 in experimental colitis reduced the production of proinflammatory cytokines[84,90,91].
SPAK: SPAK is a serine/threonine kinase containing an N-terminal series of proline and alanine repeats (PAPA box) followed by a kinase domain, an NLS, a consensus caspase cleavage motif, and a C-terminal regulatory region[92]. Colonic SPAK presents as a unique isoform that lacks the PAPA box and F-helix loop in the N-terminus[93]. The diversity of domains in SPAK might be associated with a variety of biological roles. For example, SPAK was reported to play roles in cell differentiation, transformation and proliferation, and regulation of chloride transport[94,95]. More importantly, a linkage has been established between SPAK and inflammation. SPAK as an upstream kinase to Na+-K+-2Cl-co-transporter 1 (NKCC1), can phosphorylate NKCC1 and play an important role in inflammation[96]. Further, we have demonstrated that SPAK can activate the p38 pathway[93]. Decreased expression of SPAK contributes to enhanced intestinal barrier, and thus SPAK knockout mice were more tolerant to experimental colitis induced by dextran sodium sulphate (DSS) with decreased intestinal microorganism translocation into the mucosa and inhibition of the production of inflammatory mediators[97].
MLCK: MLCK is named after its phosphorylation of MLC to induce contraction of the perijunctional actomyosin ring, and it is indispensable for tumor necrosis factor (TNF) related barrier dysfunction. In turn, TNF can induce the phosphorylation and transcription of MLCK[98,99]. Constitutive MLCK activation in the intestinal epithelium increases intestinal paracellular permeability and aggravates the severity of colitis in mouse models. However, blockage of MLCK activation can increase significantly the intestinal barrier function and ameliorate DSS-induced colitis[100].
PKC: PKC has a variety of isoforms that are involved in the pathogenesis of IBD by their effect on the mucus layer[101], microbiota[34-37], cell junction[64,65] and immune system. Specially, PKCθ plays an important role in T cell receptor activation and signaling[102], and PKCδ is crucial for B cell tolerance[103,104]. PKCη can control CTLA-4-mediated regulatory T cell (Treg) function[105]; however, PKC-θ inhibits Treg function, implying its blocking of Treg-mediated suppression. Inhibition of PKC-θ stimulates Treg, resumes compromised Treg function in rheumatoid arthritis patients, and enhances protection against experimental colitis in mice. As a result, PKC-θ mediates negative feedback on Treg cell function[106].
Protein kinases and the related signaling transduction pathways are involved in many physiological and pathological processes such as development, inflammation (for example, intestinal inflammation) and tumorigenesis. In this review, we shed some light on the roles of protein kinases in terms of their effect on IBD-related genetic factors, microbiota, mucus layer, epithelial cell and the tight junction. Further studies are needed to explore the feasibility and application of these signaling pathways in the control of IBD.
We would thank Miss Ijeoma Okoro, a PhD candidate at Georgia State University, Atlanta, GA, for critically reading the manuscript.
| 1. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1575] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 2. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2261] [Article Influence: 133.0] [Reference Citation Analysis (10)] |
| 3. | Kiliç ZM, Tunç B, Ayaz S, Filik L, Aktaş S, Parlak E, Ulker A. Antineutrophil cytoplasmic autoantibodies and anti-Saccharomyces cerevisiae antibodies in inflammatory bowel diseases. Turk J Gastroenterol. 2004;15:238-242. [PubMed] |
| 4. | Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5931] [Cited by in RCA: 6095] [Article Influence: 254.0] [Reference Citation Analysis (6)] |
| 5. | Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol. 2011;195:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 7. | Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, Zhang CK, Boucher G, Ripke S, Ellinghaus D, Burtt N. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 607] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 8. | Hugot JP, Laurent-Puig P, Gower-Rousseau C, Olson JM, Lee JC, Beaugerie L, Naom I, Dupas JL, Van Gossum A, Orholm M. Mapping of a susceptibility locus for Crohn’s disease on chromosome 16. Nature. 1996;379:821-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 600] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Hampe J, Shaw SH, Saiz R, Leysens N, Lantermann A, Mascheretti S, Lynch NJ, MacPherson AJ, Bridger S, van Deventer S. Linkage of inflammatory bowel disease to human chromosome 6p. Am J Hum Genet. 1999;65:1647-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 162] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1982] [Article Influence: 132.1] [Reference Citation Analysis (2)] |
| 11. | Polgar N, Csongei V, Szabo M, Zambo V, Melegh BI, Sumegi K, Nagy G, Tulassay Z, Melegh B. Investigation of JAK2, STAT3 and CCR6 polymorphisms and their gene-gene interactions in inflammatory bowel disease. Int J Immunogenet. 2012;39:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2035] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 13. | Quaranta M, Burden AD, Griffiths CE, Worthington J, Barker JN, Trembath RC, Capon F. Differential contribution of CDKAL1 variants to psoriasis, Crohn’s disease and type II diabetes. Genes Immun. 2009;10:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Wolf N, Quaranta M, Prescott NJ, Allen M, Smith R, Burden AD, Worthington J, Griffiths CE, Mathew CG, Barker JN. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J Med Genet. 2008;45:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2114] [Cited by in RCA: 2067] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 16. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8122] [Article Influence: 507.6] [Reference Citation Analysis (4)] |
| 17. | Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560-1563. [PubMed] |
| 18. | Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265-1276. [PubMed] |
| 19. | Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 754] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 20. | Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973-983. [PubMed] |
| 21. | Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1472] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 22. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3900] [Cited by in RCA: 3839] [Article Influence: 132.4] [Reference Citation Analysis (11)] |
| 23. | Zheng CF, Guan KL. Cytoplasmic localization of the mitogen-activated protein kinase activator MEK. J Biol Chem. 1994;269:19947-19952. [PubMed] |
| 24. | Yan Y, Merlin D. Ste20-related proline/alanine-rich kinase: a novel regulator of intestinal inflammation. World J Gastroenterol. 2008;14:6115-6121. [PubMed] |
| 25. | Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 386] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 26. | Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Bobo LD, El Feghaly RE, Chen YS, Dubberke ER, Han Z, Baker AH, Li J, Burnham CA, Haslam DB. MAPK-activated protein kinase 2 contributes to Clostridium difficile-associated inflammation. Infect Immun. 2013;81:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599-10603. [PubMed] |
| 29. | Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 957] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 30. | Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 31. | Oppong GO, Rapsinski GJ, Newman TN, Nishimori JH, Biesecker SG, Tükel Ç. Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica serovar Typhimurium curli fibrils in the gut. Infect Immun. 2013;81:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Sun X, Liu B, Sartor RB, Jobin C. Phosphatidylinositol 3-kinase-γ signaling promotes Campylobacter jejuni-induced colitis through neutrophil recruitment in mice. J Immunol. 2013;190:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Homer CR, Kabi A, Marina-García N, Sreekumar A, Nesvizhskii AI, Nickerson KP, Chinnaiyan AM, Nuñez G, McDonald C. A dual role for receptor-interacting protein kinase 2 (RIP2) kinase activity in nucleotide-binding oligomerization domain 2 (NOD2)-dependent autophagy. J Biol Chem. 2012;287:25565-25576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1497] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 35. | Frantz AL, Rogier EW, Weber CR, Shen L, Cohen DA, Fenton LA, Bruno ME, Kaetzel CS. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 2012;5:501-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 36. | Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1336] [Article Influence: 63.6] [Reference Citation Analysis (2)] |
| 37. | Corr SC, Palsson-McDermott EM, Grishina I, Barry SP, Aviello G, Bernard NJ, Casey PG, Ward JB, Keely SJ, Dandekar S. MyD88 adaptor-like (Mal) functions in the epithelial barrier and contributes to intestinal integrity via protein kinase C. Mucosal Immunol. 2014;7:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Khan WI. Physiological changes in the gastrointestinal tract and host protective immunity: learning from the mouse-Trichinella spiralis model. Parasitology. 2008;135:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064-15069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1602] [Article Influence: 89.0] [Reference Citation Analysis (1)] |
| 40. | Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 41. | Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Dig Dis. 2009;27:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184-189. [PubMed] |
| 44. | Takeyama K, Dabbagh K, Lee HM, Agustí C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA. 1999;96:3081-3086. [PubMed] |
| 45. | Shatos MA, Gu J, Hodges RR, Lashkari K, Dartt DA. ERK/p44p42 mitogen-activated protein kinase mediates EGF-stimulated proliferation of conjunctival goblet cells in culture. Invest Ophthalmol Vis Sci. 2008;49:3351-3359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Park JA, Crews AL, Lampe WR, Fang S, Park J, Adler KB. Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein. Am J Pathol. 2007;171:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Taupin D, Podolsky DK. Mitogen-activated protein kinase activation regulates intestinal epithelial differentiation. Gastroenterology. 1999;116:1072-1080. [PubMed] |
| 48. | Krimi RB, Kotelevets L, Dubuquoy L, Plaisancié P, Walker F, Lehy T, Desreumaux P, Van Seuningen I, Chastre E, Forgue-Lafitte ME. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis. 2008;14:931-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, Cho CH. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem. 2008;104:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 520] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 51. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [PubMed] |
| 52. | Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231-C1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 53. | Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA. 2009;106:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 55. | Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078-F1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 56. | Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda). 2004;19:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 57. | Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 870] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 58. | Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 59. | Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433-443. [PubMed] |
| 60. | Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol. 2005;203:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Szakál DN, Gyorffy H, Arató A, Cseh A, Molnár K, Papp M, Dezsofi A, Veres G. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch. 2010;456:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Wang N, Yu H, Ma J, Wu W, Zhao D, Shi X, Tian H, Jiang H. Evidence for tight junction protein disruption in intestinal mucosa of malignant obstructive jaundice patients. Scand J Gastroenterol. 2010;45:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 975] [Article Influence: 51.3] [Reference Citation Analysis (1)] |
| 64. | Koizumi J, Kojima T, Ogasawara N, Kamekura R, Kurose M, Go M, Harimaya A, Murata M, Osanai M, Chiba H. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol. 2008;74:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Banan A, Zhang LJ, Shaikh M, Fields JZ, Choudhary S, Forsyth CB, Farhadi A, Keshavarzian A. theta Isoform of protein kinase C alters barrier function in intestinal epithelium through modulation of distinct claudin isotypes: a novel mechanism for regulation of permeability. J Pharmacol Exp Ther. 2005;313:962-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 66. | Wang Z, Ginnan R, Abdullaev IF, Trebak M, Vincent PA, Singer HA. Calcium/Calmodulin-dependent protein kinase II delta 6 (CaMKIIdelta6) and RhoA involvement in thrombin-induced endothelial barrier dysfunction. J Biol Chem. 2010;285:21303-21312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Chen Yh, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:849-862. [PubMed] |
| 68. | D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233-26240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143:1872-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 70. | Fihn BM, Sjöqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology. 2000;119:1029-1036. [PubMed] |
| 71. | Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 72. | Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118:5221-5230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 73. | Yan Y, Laroui H, Ingersoll SA, Ayyadurai S, Charania M, Yang S, Dalmasso G, Obertone TS, Nguyen H, Sitaraman SV. Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice. J Immunol. 2011;187:1496-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 453] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 75. | Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969-4976. [PubMed] |
| 76. | Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346-C1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 77. | Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103-106. [PubMed] |
| 78. | Fuss IJ. Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S110-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189-201. [PubMed] |
| 80. | Coskun M, Olsen J, Seidelin JB, Nielsen OH. MAP kinases in inflammatory bowel disease. Clin Chim Acta. 2011;412:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, Caprioli F, Del Vecchio Blanco G, Paoluzi OA, Macdonald TT. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells. Gastroenterology. 2007;132:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Kwon KH, Ohigashi H, Murakami A. Dextran sulfate sodium enhances interleukin-1 beta release via activation of p38 MAPK and ERK1/2 pathways in murine peritoneal macrophages. Life Sci. 2007;81:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342-5351. [PubMed] |
| 84. | Dahan S, Roda G, Pinn D, Roth-Walter F, Kamalu O, Martin AP, Mayer L. Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology. 2008;134:192-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Pinton P, Braicu C, Nougayrede JP, Laffitte J, Taranu I, Oswald IP. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J Nutr. 2010;140:1956-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 86. | Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 236] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 87. | Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959-50965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 88. | Garat C, Arend WP. Intracellular IL-1Ra type 1 inhibits IL-1-induced IL-6 and IL-8 production in Caco-2 intestinal epithelial cells through inhibition of p38 mitogen-activated protein kinase and NF-kappaB pathways. Cytokine. 2003;23:31-40. [PubMed] |
| 89. | Ueno N, Fujiya M, Segawa S, Nata T, Moriichi K, Tanabe H, Mizukami Y, Kobayashi N, Ito K, Kohgo Y. Heat-killed body of lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm Bowel Dis. 2011;17:2235-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 90. | Assi K, Pillai R, Gómez-Muñoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Mitsuyama K, Suzuki A, Tomiyasu N, Tsuruta O, Kitazaki S, Takeda T, Satoh Y, Bennett BL, Toyonaga A, Sata M. Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory bowel disease. Int J Mol Med. 2006;17:449-455. [PubMed] |
| 92. | Johnston AM, Naselli G, Gonez LJ, Martin RM, Harrison LC, DeAizpurua HJ. SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene. 2000;19:4290-4297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 93. | Yan Y, Nguyen H, Dalmasso G, Sitaraman SV, Merlin D. Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform. Biochim Biophys Acta. 2007;1769:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem. 2002;277:50812-50819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 313] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 95. | Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 96. | Topper JN, Wasserman SM, Anderson KR, Cai J, Falb D, Gimbrone MA. Expression of the bumetanide-sensitive Na-K-Cl cotransporter BSC2 is differentially regulated by fluid mechanical and inflammatory cytokine stimuli in vascular endothelium. J Clin Invest. 1997;99:2941-2949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Zhang Y, Viennois E, Xiao B, Baker MT, Yang S, Okoro I, Yan Y. Knockout of Ste20-like proline/alanine-rich kinase (SPAK) attenuates intestinal inflammation in mice. Am J Pathol. 2013;182:1617-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409-419. [PubMed] |
| 99. | Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205-26215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 100. | Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 101. | Larivée P, Levine SJ, Martinez A, Wu T, Logun C, Shelhamer JH. Platelet-activating factor induces airway mucin release via activation of protein kinase C: evidence for translocation of protein kinase C to membranes. Am J Respir Cell Mol Biol. 1994;11:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 747] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 103. | Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 104. | Mecklenbräuker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 105. | Kong KF, Fu G, Zhang Y, Yokosuka T, Casas J, Canonigo-Balancio AJ, Becart S, Kim G, Yates JR, Kronenberg M. Protein kinase C-η controls CTLA-4-mediated regulatory T cell function. Nat Immunol. 2014;15:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Zanin-Zhorov A, Ding Y, Kumari S, Attur M, Hippen KL, Brown M, Blazar BR, Abramson SB, Lafaille JJ, Dustin ML. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science. 2010;328:372-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
P- Reviewer: Catania VA, Hou WH, Lambrecht NW S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Liu SQ