Published online Nov 15, 2014. doi: 10.4291/wjgp.v5.i4.496
Revised: September 18, 2014
Accepted: October 1, 2014
Published online: November 15, 2014
Processing time: 175 Days and 10 Hours
This review is a current summary of the role that both zinc deficiency and zinc supplementation can play in the etiology and therapy of a wide range of gastrointestinal diseases. The recent literature describing zinc action on gastrointestinal epithelial tight junctions and epithelial barrier function is described. Zinc enhancement of gastrointestinal epithelial barrier function may figure prominently in its potential therapeutic action in several gastrointestinal diseases.
Core tip: This is an overview of the role that both zinc deficiency and zinc supplementation can play in the etiology and therapy of a wide range of gastrointestinal diseases.
- Citation: Skrovanek S, DiGuilio K, Bailey R, Huntington W, Urbas R, Mayilvaganan B, Mercogliano G, Mullin JM. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol 2014; 5(4): 496-513
- URL: https://www.wjgnet.com/2150-5330/full/v5/i4/496.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i4.496
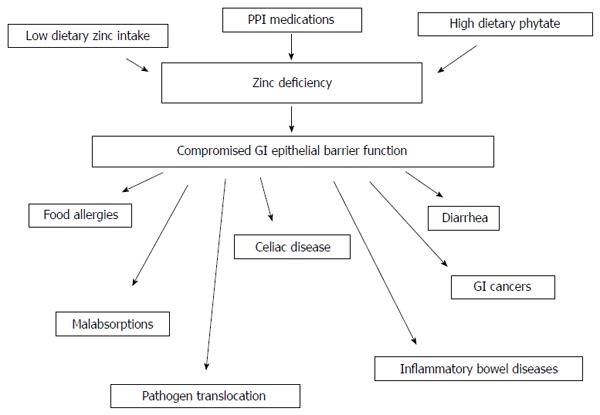
When considering the topic of zinc and gastrointestinal (GI) disease, several general biomedical and nutritional situations must be considered. The first, and perhaps most obvious, is that of dietary zinc deficiency (ZD) - whether this arises out of generalized diet insufficiency, genetically-based zinc malabsorption, or dietary interference with zinc absorption (e.g., phytates in the diet) - and resultant diseases from said deficiency. The second consideration is that diseases, such as the inflammatory bowel diseases (IBDs), whose morbidity generates GI malabsorption issues, ultimately giving rise to ZD. Finally, one must consider the supplementation of zinc to the diet, and the positive role that may play in certain GI diseases, especially those characterized by impairment of barrier function. The connection among all three situations is perhaps that ZD, from whatever source appears to lead to GI barrier compromise, an eventuality that is self perpetuating (Figure 1).
This is, then, a very broad topic, and one in which numerous excellent reviews have been written concerning the above individual situations. Duggan et al[1] (2002) did a thorough reporting of zinc and other “functional foods” for maintaining GI mucosal function. In terms of barrier function per se, Hering et al[2] (2009) have recently published on this from a more cellular perspective. Semrad[3] (1999) reported on the general role of zinc in intestinal function, particularly in diarrhea. Goh et al[4] (2003) deal with both ZD arising out of IBDs as well as the role zinc and other nutraceuticals may play in providing an alternative to the use of steroids and anti-tumor necrosis factor (TNF) modalities in IBD therapy. Treatment via zinc supplementation of GI disease incited by ZD may in fact be the first (though inadvertent) clinical summary of supplemental zinc effects on GI barrier compromise[5]. The very concept of ZD as well as the myriad roles played by zinc in cellular and systemic function, are discussed comprehensively by Tuerk et al[6] (2009) and Wapnir[7] (2000). The singular issue of zinc in parenteral feeding, an important medical area for which zinc (and epithelial barrier function) may be highly important, is something that we do not consider here in any depth, but has been well investigated by Jeejeebhoy[8] (2009). The critical area of zinc ‘‘physiology”, i.e., its transport and binding in cells in general, and enterocytes in particular [ZIPs, ZnTs, metallothioneins (MTs), etc.], as well as the issue of zinc homeostasis systemically is discussed thoroughly by King[9] (2010), King et al[10] (2000) and Cousins[11] (2010). Finally, a very comprehensive review of zinc’s role in disease generally has just recently been produced[12].
Our review generally does not address the enormous literature surrounding zinc finger proteins and zinc metalloproteinases, except where these proteins specifically deal with a deficiency or supplementation. There are numerous reviews covering these various topics touching on zinc[13-16]. Both protein classes have almost certain relevance toward all of the diseases we discuss in this review, but we largely avoid that literature because of its focus on the protein rather than specifically on zinc.
As alluded to above, one can be ZD for three reasons: (1) the diet is simply too low in zinc content, as can be true for certain diets poor in meat or fish protein; or (2) and (3) there can be two situations of ZD in the face of diets replete with zinc. The latter two reasons can be brought about through the chronic use of Proton Pump Inhibitor (PPI) medications, whose inhibition of gastric acid production (and elevation of gastroduodenal lumen pH) can render luminal zinc non-absorbable[17]. In addition, diets high in the form of phosphorous known as phytate (found significantly in whole grains, nuts and seeds), can result in zinc complexing with phytates and a resultant non-absorbable species[18,19]. It is worth considering that the average American, especially those of higher socioeconomic status, may be the victim of one or both outcomes, as PPIs are taken by over 20 million Americans yearly, and phytates exist at high levels in the very foods that are prominently featured in “healthy diet choices”. The now common use of PPIs to treat gastroesophageal reflux in neonates makes this group particularly worrisome in this regard. Additionally, the body has no cell/tissue zinc stores (unlike, e.g., iron or calcium) therefore its daily zinc needs are heavily dependent on satisfactory daily zinc intake and at least short-term states of zinc deficiency could be quite likely[20].
The government recommended daily intake of zinc is 11 mg/d[21], however studies have shown that, at least in the relatively short term, higher doses of zinc are safe. For example, our recent study in patients with Barrett’s esophagus showed no adverse effects on a total dose of 52 mg/d for 14 d, with positive changes on intracellular signal transduction in the Barrett’s epithelia[22]. It is clear that a dose of 150 mg/d over an extended period can be toxic, as is evidenced by the development of severe copper deficiency and anemia[23,24], but zinc lozenges are only effective in reducing the duration of the common cold at a minimum dose of 75 mg/d[25]. At daily doses above 50 mg/d, periodic measurements of blood copper levels (which can be driven lower by high zinc intake) and cholesterol levels would be prudent[26].
The use of zinc as a potential therapeutic in these various diseases and disorders should be treated realistically. No one would suggest, e.g., that zinc-induced potentiation of GI barrier function might “cure” any associated illnesses. However, considering the issue quantitatively rather than qualitatively, there is a quite reasonable possibility that zinc administration could reduce morbidity by a meaningful extent, and that would be highly useful. This is especially true given that zinc could readily be used together with existing medications for these conditions.
The role that zinc may exert in specific GI diseases is now discussed below, along with a final consideration of zinc’s role in maintaining GI epithelial barrier function. The compromise of that barrier function can be a catalyst for several of these diseases, and zinc-induced barrier enhancement may prove to be a means of reducing morbidity in some of these conditions (Table 1).
| Condition | Model used | Reversibility with Zn supplementation | Ref. |
| Esophageal cancer | Rodent | Yes | Fong et al[27], 2011 |
| Diarrhea | Human | Yes | Hambidge[72], 1992 |
| Inflammatory bowel diseases | Porcine | Yes | Sturniolo et al[82], 2002 |
| Celiac disease | Human | Not determined | Wierdsma et al[143], 2013 |
| Alcoholic liver disease | Mouse | Yes | Lambert et al[198], 2003 |
| Malnutrition | Guinea pig | Yes | Rodriguez et al[65], 1996 |
An important consideration in reading this review and interpreting the findings that are presented, is that one needs to always consider the chemical form of the administered zinc, its dosage/concentration, and its vehicle (tablet, capsule, emulsion, lozenge, etc.), in the interpretation of any given result/finding. Not all zinc salts are equally soluble and therefore permissive for zinc delivery to cells. In addition, in the discussion presented below on zinc-mediated inhibition of esophageal cancer in rodents, it is critical that the zinc was administered in drinking water[27]. If it was administered in capsule or tablet form, it would result in no topical delivery to the target tissue. This is further discussed in Valenzano et al[22] (2014) for delivery to humans. It is not really feasible in a review to discuss the mode of zinc administration in every citation, and so we encourage the reader to refer to specific references for chemistry, drug delivery and concentration information.
While there is a minimal amount of published literature regarding zinc’s effect on transformed cells in culture, zinc - at a concentration that is otherwise not seen to be toxic or a hindrance to normal cell growth - has been shown to negatively impact growth in transformed human tumor cells[28]. Further evidence of tumor growth inhibition by zinc was observed in mice inoculated with sarcoma 180 cells into the peritoneal cavity. Mice treated with zinc sulfate injections experienced a suppression in the number of tumors produced; however, the treatment was unable to prevent the subsequent growth of a tumor once it developed[29]. Similarly, when mice inoculated by intraperitoneal injection with L1210 leukemia cells were treated with zinc acetate injections, they exhibited a reduction in tumor cell growth[30]. The mechanism by which zinc is capable of producing this effect may relate to its ability to prevent oxidative stress that causes DNA damage. Additionally, it is noteworthy that ZD impairs DNA repair by compromising the DNA binding activity of the tumor suppressor protein, p53, disrupting its function[31,32]. In a study on the treatment of the human colon cancer cell line, HCT-116, with zinc chloride, zinc inhibited cell proliferation by stabilizing adenomatous polyposis coli (APC) protein and arresting cell growth[33]. Zinc dysregulation and its subsequent effect on the development of cancer has been suggested to be cell type specific. For example, breast tumor biopsies have higher zinc levels than normal tissue whereas cellular zinc levels in prostate cancer and ovarian cancer tumor tissue are significantly lower than benign tissue[34].
Esophageal and oral cancers are significant upper aerodigestive tract cancers. Esophageal cancer is the eighth most common cancer and the sixth most common cause of death from cancer worldwide, while oral cancers, particularly those of the tongue, have a high mortality rate in part due to the risk of developing a second primary tumor, usually in the esophagus[35,36]. Esophageal cancers can be either squamous cell carcinoma (SCC) or adenocarcinoma (AC). Abnet et al[37] (2005) performed a 16-year observational study on participants in a nutrition intervention trial and found that the subjects who had a high zinc concentration in their esophageal biopsy specimens also had a reduced risk of developing esophageal SCC, indicating that dietary ZD associates strongly with SCC. Although studies examining dietary zinc intake and its relationship to cancer risk have reported conflicting results, a meta-analysis of 19 studies involving an estimated 400000 participants found that the level of zinc intake was inversely associated with digestive tract cancers, especially colorectal cancer[38]. In addition to these cancers of the GI tract, low serum zinc levels are also associated with prostate, ovarian, lung, gallbladder, and head and neck cancers[34].
There is strong evidence that ZD in rats and mice enhances carcinogenesis. When control and ZD rats were intragastrically administered 2 mg/kg body weight doses of the carcinogen methylbenzylnitrosamine the ZD rats had a higher frequency of esophageal tumor development[39]. Similar outcomes ensued when ZD and zinc-sufficient (ZS) Sprague-Dawley rats were exposed to N-nitrosodimethylamine. The control rats did not suffer epithelial irregularities; however, 63% of the rats deficient in zinc developed squamous papillomas in the forestomach[40]. ZD and ZS Sprague-Dawley rats, exposed through their drinking water to precursors of the carcinogen N-nitro-N-benzylmethylamine (benzylmethylamine and NaNO2) manifested like results[41]. Similarly, when C57BL/6 mice were given N-nitrosomethylbenzylamine (NMBA), after 46 wk the ZD mice had significantly more esophageal and forestomach tumors than ZS mice[42]. In an oral cancer study, ZD and ZS rats were treated with the carcinogen 4-nitroquinoline 1-oxide (NQO) to induce lingual tumors. A greater incidence of lingual squamous cell carcinomas was found in the ZD rats[43]. This is not a phenomenon limited to squamous cell cancers, as zinc supplementation (in combination with aspirin or vitamin C) showed dramatic reduction of colon tumors in dimethylhydrazine-treated rodents[44].
The mechanisms by which ZD creates a pro-tumor environment may relate to its ability to induce cell proliferation. In a study on the relationship between cell proliferation and tumor incidence, ZD and ZS rats either received intragastric doses of NMBA or were left untreated. At various time points, in vivo bromodeoxyuridine (BrDU) labeling and immunohistochemical detection of cells in S-phase were used to assess esophageal cell proliferation. In both NMBA-treated and untreated rats, the ZD condition showed a significantly higher labeling index than the ZS condition. In NMBA-treated animals, 100% of the ZD ad libitum rats, 23% of the ZS ad libitum fed rats, and 6% of the ZS rats pair-fed to the ZD rats developed tumors. After about 10 wk of the ZD diet, two rats not exposed to NMBA developed esophageal papillomas[45]. In an alternate study, BrDU labeling of ZD and ZS mice given doses of NMBA intragastrically showed that the labeling index and number of labeled cells were also increased in the ZD mice[42].
Dietary ZD also alters gene expression. Liu et al[46] (2005) identified 33 genes that were differentially expressed in a hyperplastic ZD vs a ZS esophagus. Key factors are the upregulation of the cyclooxygenase (COX-2) inflammatory gene and the induction of an overexpression of the proinflammatory mediators, S100A8 and S100A9. In the hyperplastic esophagus and tongue of ZD rats, the expression levels of both COX-2 protein and mRNA were between 8 and 14.6 fold higher than their ZS counterparts[43]. Treating these rats with an inhibitor of the COX-2 pathway, celecoxib, led to a reduction in cell proliferation but not a prevention of carcinogenesis, suggesting that there must be an additional process involved[43,47]. Celecoxib was found not to be an efficient treatment because it did not show a real effect on S100A8 overexpression. The expression of S100A8 and S100A9 in hyperplastic ZD esophagi was upregulated 57 and 5 fold, respectively[48]. Combining ZD-induced inflammation with low levels of NMBA resulted in a 66.7% incidence of esophageal SCC[49].
ZD in collaboration with other factors, such as p53 deficiency and cyclin D1 overexpression, can produce an accelerated progression towards malignancy[50-52]. p53 is a tumor suppressor protein responsible for the prevention of uncontrolled cell proliferation. Both p53 deficiency (p53 -/-) and insufficiency (p53 +/-) in combination with ZD leaves mice more susceptible to carcinogens, increasing the tumor incidence in the esophagus and tongue[50,52]. This rapid rate of tumor progression was accompanied by nearly 20% of ZD and p53-deficient rats developing esophageal Barrett’s metaplasia[50]. Cyclin D1 overexpression in conjunction with ZD disrupts the cell cycle leading to uncontrolled cell proliferation and consequently a substantial increase in tumor incidence. At 77 d, 14% of mice with both cyclin D1 overexpression and ZD developed esophageal intestinal metaplasia[52].
Several experiments have investigated the ability of zinc replenishment (ZR) to prevent esophageal cancer. ZR was shown to begin reversing the inflammatory signature and reduce COX-2 overexpression to only 3-fold of that seen in ZS rats[43,49]. ZR also stimulated apoptosis, increased the expression of the proapoptotic Bax protein[53], returned 29 of the 33 altered genes back to normal expression levels[54], decreased cell proliferation[55], restored the expression of S100A8 and S100A9[48], and consequently began to reverse the pre-cancerous phenotype condition. Furthermore, animals with an already ZS diet were found to benefit from zinc supplementation. Additional zinc in the diet of these rats limited cell proliferation and stimulated apoptosis causing a reduced incidence of tumors and tumor progression induced by both low and high doses of NQO[27]. This finding is critical support for the value of zinc supplementation in cancer chemoprevention.
Dietary ZD may be creating a pro-tumor environment in the GI tract, enhancing carcinogenesis by inducing cell proliferation, altering gene expression, and promoting inflammation. Zinc as a dietary nutrient is essential because it plays a role in a variety of important functions, including DNA repair, apoptosis, cell cycle progression, p53 activation, and the prevention of oxidative stress that causes DNA damage. This in conjunction with several research studies suggesting zinc replenishment and supplementation results in a positive effect against carcinogens, supports the thesis that zinc supplementation has the potential to be efficacious in the prevention of several GI cancers.
It is well described that ZD can lead to diarrhea. Acrodermatitis enteropathica, a hereditary disorder of zinc metabolism, was reported in infants with skin lesions and diarrhea in the early 1970s[56], and shortly thereafter a similar syndrome was found in certain adult patients placed on total parenteral nutrition (TPN). These patients presented with diarrhea, depression and dermatitis, and were found to have acute ZD[57]. Zinc supplementation resulted in rapid improvement of diarrhea and dermatitis in both of the above groups[56-61]. Very recent meta analyses of multiple studies supports this conclusion, showing decreased diarrhea duration with zinc therapy[62,63]. A recent review on infectious diarrhea supports this[64]. It is noteworthy that even in control, non disease states, zinc supplementation can positively affect multiple aspects of GI mucosa (on a molecular and cellular level) that would likely act to enhance GI barrier function[46]. ZD can cause intestinal hyperpermeability (“leaky gut”), which itself can be secondary to increased nitric oxide and oxidative stress, thereby leading to diarrhea[65-67]. In rats, ZD has been shown to upregulate expression of intestinal uroguanylin (a peptide that triggers Cl- secretion and subsequent water secretion)[67,68], decrease absorption of triglycerides by altering chylomicron formation[69], and decrease absorption of proteins by altering enterocyte peptidase activity[70,71], all of which can potentiate diarrhea.
Later it was discovered that not only can ZD cause diarrhea but also chronic diarrhea conditions can cause ZD, thereby promoting even more diarrhea[72]. This has led to multiple studies of zinc supplementation in infants and children with diarrheal illnesses, mostly in developing nations where malnutrition often results in ZD. Children with acute diarrhea treated with zinc show a decrease in the rate and duration of diarrhea as well as decreased need for antibiotics when compared to controls (who were typically treated with oral rehydration and/or vitamin supplements)[73-76]. Zinc supplementation in healthy children in developing countries has decreased the prevalence, morbidity and mortality of diarrhea[77]. The World Health Organization currently recommends treating diarrhea in children with zinc tablets along with oral rehydration solutions as part of a first-line approach[78].
ZD can also decrease human immune function, increasing the risk for infection[74,79]. In infectious diarrhea there is typically increased intestinal permeability (seen as an increase in the lactulose/mannitol ratio)[80] which can sometimes be improved by zinc supplementation[81-83]. ZD leads to decreased electrolyte and water absorption, and can exacerbate diarrhea caused by Vibrio cholera (V. cholera)[84,85]. V. cholera causes diarrhea by increasing cyclic adenosine monophosphate (cAMP) production, inducing the intestine to secrete water and chloride, and inhibiting the absorption of sodium[85]. Interestingly, zinc supplementation actually impedes cAMP-regulated secretion of chloride via basal-lateral K+ channels, explaining its efficacy in reducing the duration of cholera-induced diarrhea, an effect that may involve basal-lateral zinc action on basal-lateral membrane K+ channels[86,87]. Zinc supplementation decreases expression of certain genes linked to immune function in piglets infected with enterotoxigenic E. coli (ETEC) as well decreasing ETEC-induced diarrhea and inflammation. This action is possibly due in part to a decrease in MUC4 expression, which might be an ETEC K88 receptor[88]. In treating ETEC-related diarrhea the mode of delivery in zinc supplementation can matter significantly[89]. In CACO-2 cells, zinc supplementation inhibits Ca++ and nitric oxide-mediated ion secretion, both of which are known pathways for pathogen-induced diarrhea. However the same may not be true for Ca++-mediated ion secretion in rat ileum[86,90]. It should be noted however that Bzik et al[87] (2012) did observe an inhibitory effect of zinc on carbachol-stimulated short circuit current. Zinc has also been shown to have a direct antimicrobial effect on infectious enteric bacteria such as E. coli, Shigella, and various strains of Salmonella in vitro[91].
In summary, zinc is useful in the treatment of diarrhea of various etiologies. Its role in decreasing fluid secretion to the intestinal lumen (directly or indirectly) requires further investigation but cannot be disputed. Its impact on intestinal paracellular leak, another potential source of diarrhea, will be addressed in a later section of this review.
ZD has been well established in Crohn’s disease (CD) and can arise in part due to poor zinc absorption in the small intestine (even if the jejunum appears normal) as well as from chronic dietary intolerances and restrictions[92-96]. In a large cohort of patients with IBD, it was estimated that 8.5% of patients had inadequate intake of zinc. The prevalence of low serum zinc levels was 29.3%[97]. ZD has even been documented in Crohn’s patients while in remission[98]. Crohn’s patients on TPN can develop acute ZD resulting in acrodermatitis enteropathica and decreased vision[99]. More commonly, ZD in CD contributes to stunted growth in children and manifests as decreased taste sensation, visual acuity, and immune function[100-102].
One very important element of CD pathophysiology is a defect in mucosal barrier function. Increased mucosal permeability correlates with disease activity as evidenced by increased uptake of large molecular markers (such as lactulose) from the GI lumen into the bloodstream[103]. While in remission, increased transmucosal permeability (leak) has been used as a marker for predicting relapse[104]. In the human intestinal cell line, CACO-2/T7, zinc depletion in conjunction with TNFα exposure (common in the inflamed mucosa in CD) increases apoptosis, ultimately compromising the organization of tight junctions (TJs) and epithelial barrier integrity[105]. This could explain why zinc supplementation has been reported to decrease transmucosal leak in CD[106], although this finding has been challenged[107]. CD pathophysiology involves a persistent recruitment of leukocytes into intestinal mucosa and submucosa followed by an unregulated granulomatous inflammatory response. Epithelial barrier dysfunction may facilitate this passage of leukocytes and allow for an unregulated leakage of luminal antigens across the epithelial barrier, a leak that normally does not take place. This is evidenced by a high frequency of neutrophils and crypt abscesses in the intestinal mucosa[108]. ZD may exacerbate CD morbidity by increasing gut permeability, allowing for increased neutrophil transmigration and luminal antigen permeation[109]. Biologic agents such as Natalizumab have taken advantage of this persistent recruitment mechanism by blocking leukocyte transmigration at the level of the vascular endothelium[110,111].
Zinc supplementation in CD, whether the Crohn’s is active or in remission, may be beneficial. It had been postulated that zinc supplementation in patients with IBD would increase serum and mucosal levels of the zinc-dependent free radical scavengers superoxide dismutase (SOD) and MT, however their rise in concentrations was not statistically significant[112]. According to Brignola et al[113] (1993), dietary supplementation with zinc in CD patients with active disease significantly improved serum zinc levels in addition to levels of zinc-dependent hormones like thymulin, which can potentiate T-cell differentiation and natural killer (NK) cell activity[113]. The effect of zinc supplementation on NK cell activity of IBD patients, varies in the literature. A small randomized trial by Van de Wal et al[114] (1993) showed a significantly reduced level of NK cell activity with zinc supplementation. Despite the current evidence in the literature for their benefits, zinc products have not undergone the scrutiny and validation of the Food and Drug Administration for this disease. For this reason, there are no specific guidelines for zinc supplementation beyond emphasizing the importance of adequate and balanced nutritional intake in CD. As described in the sections on zinc and GI cancers, and zinc and TJs, there may well be medical benefit of zinc daily intake above the current RDA.
Zinc is absorbed from the GI lumen principally in the small intestine at the distal duodenum and proximal jejunum[115]. This leads to obvious implications for zinc malabsorption in diseases targeting and damaging the small intestine, such as CD and Celiac sprue. However, ulcerative colitis (UC) does not typically manifest in the small bowel proximal to the ileum, and numerous studies have shown that well nourished UC patients are not ZD. In fact, when patients with moderate and severe (active) UC are compared to healthy controls and/or UC patients in remission, they have shown normal or even elevated serum zinc concentrations[112,116,117]. Similarly, serum zinc levels in children with UC were no different than controls[118].
In active UC flares, elevated serum zinc concentrations are correlated with increased levels of complement component C3C (part of the innate immune system) and elevated antinuclear antibodies, a known predictor of UC flares and a marker of steroid dependence in UC[119,120]. This may be indicative of zinc being released locally in response to the activated inflammatory cascade in active UC. However, an additional study showed no correlation between serum zinc and elevated erythrocyte sedimentation rate or C-Reactive protein, both standard predictors of high grade inflammation in UC[121]. Patients with refractory UC who underwent colectomies with ileal pouch anal anastomosis or a Brooke ileostomy had either elevated or normal serum zinc levels[122-124]. In fact, ZD in UC patients most likely stems from malnutrition deriving from poor oral intake due to acute (systemic) illness associated with active UC and not the damaged colonic tissue per se[125,126]. There is little evidence that the colon itself normally has any major role in zinc metabolism or homeostasis.
On a molecular level, the reactive oxygen species (ROS) produced from respiratory burst during neutrophilic invasion in UC cause significant mucosal damage[127]. SOD, vitamin E and ascorbic acid are all known to reduce ROS species produced in this process[128]. Zinc in various ionic forms and as a part of various moieties in proteins is also important in ROS reduction. For example, the copper/zinc isoform of SOD (Cu/Zn-SOD) removes ROS and indirectly prevents lipid peroxidation[129]. Interestingly, Cu/Zn-SOD is decreased in the epithelium of active UC[130]. Another family of zinc-cytoprotective enzymes are MTs. These are zinc-binding proteins that likely play a role in alleviating acute inflammation as ROS scavengers. The literature shows variability in MT expression in IBD. In ZD individuals, mucosal layers of active UC patients had decreased concentrations of MTs in their colonic mucosa and had increased concentrations of ROS intermediates[131-133]. Inducing ZD in animal models of UC increases the disease activity index (DAI) and serum TNFα levels, exacerbates weight loss, and leads to further shortening of colon length[134]. MT concentrations are increased in UC-associated colorectal carcinoma but are decreased in active UC flares at the inflamed mucosa[116,135]. In actively inflamed UC in both human and animal models, supplementing zinc has been shown to either slightly increase or have no effect on the tissue level of MTs[112,136-138]. There have been many studies on the effects of zinc supplementation on inflammation in UC. Di Leo et al[136] (2001) reported improvements in diarrhea and weight gain in a rat colitis model when supplemented with zinc but found no effect on neutrophil infiltration or visible inflammation. Mulder et al[112] (1994) reported no change in DAI or inflammation in human UC intestinal biopsies after zinc supplementation. More recently, however, Tran et al[137] (2007) found that zinc supplementation suppressed colitis in a mouse model as evidenced by decreased DAI and histological severity scores as well as reduced myeloperoxidase activity. Intrarectal zinc also has been found to be beneficial at the microscopic level with reduced inflammation in rat models in as little as 96 h, with other studies also reporting similar decreases in inflammation in both rat and mouse UC models[139-142].
In summary, in UC patients, systemic ZD does not arise in response to colonic tissue inflammation, but if a patient has decreased nutritional intake due to associated illness in ongoing UC, they may develop insufficient levels of serum zinc. This decreased serum zinc concentration does appear to have important implications at the cellular level and therefore may diminish the body’s ability to reduce active inflammation. More research is required to further examine the benefits of zinc supplementation in UC patients, specifically its effect, if any, on epithelial barrier function.
Celiac disease (CeD) is an immunologic enteropathy triggered by the intake of gluten (and more specifically gliadin) which causes, among other outcomes, the loss of brush border proteins, enzymes needed for both digestion and absorption. CeD patients are deficient in many vitamins and minerals, including zinc[143]. Decreased plasma zinc has been observed in both untreated CeD patients and as well as those in clinical remission[144]. The prevalence of ZD in newly diagnosed adult CeD patients has been reported to be as high as 67%, and up to 64% of children with CeD are also ZD[143,145-148]. Moreover, ZD is known to correlate with villous atrophy, with one study finding 60% of patients with partial villous atrophy, 80% of patients with subtotal villous atrophy, and 92% of patients with total villous atrophy to be deficient in zinc[149-152].
Adherence to a gluten free diet (GFD) typically induces clinical and histological remission of CeD. While serum zinc levels are significantly lower in children with untreated CeD with enteropathy, levels normalize after following a GFD[147,153,154]. Interestingly, Jones et al[155] (2010) found that supplementing zinc along with a GFD provided no additional rise in plasma zinc levels. A small study in patients with non-responsive CeD showed a rise in serum and plasma zinc after oral zinc supplementation as well as a slight increase in brush border enzyme activity, but there was no improvement in the patients’ clinical status[155]. Both Crofton et al[156] (1990) and Tran et al[157] (2011) found no difference in zinc absorption between untreated CeD patients and controls, however there was evidence of impaired zinc homeostasis. Crofton et al[156] (1990) reported an “increased turnover and loss of endogenous zinc” that improved on a GFD, and Tran et al[157] (2011) found the exchangeable zinc pool (EZP), i.e., the total of the zinc pools in the body that are able to exchange with serum zinc, to be significantly decreased in CeD patients. Since the size of the EZP directly correlates with zinc nutritional status, patients with CeD can be ZD despite normal zinc absorption[157-159].
Stenberg et al[160] (2008) suggest that ZD, specifically in the intestinal mucosa (perhaps due to recruitment of zinc to a site of inflammation elsewhere in the body), activates the enzyme transglutaminase-2 (TG2) (normally inhibited by the presence of zinc) in the intestine. They theorize that a TG2-thioester intermediate-deamidated gliadin complex acts as a “neo-antigen,” activating T-cells in persons genetically susceptible to CeD. These activated T-cells trigger B-lymphocytes to make antibodies against both TG2 and gliadin with the resultant inflammation causing villous atrophy (2008). Possibly contributing to this inflammatory phenomenon is an increased baseline level of paracellular permeability to gliadin via the TJs of the gut[161,162]. As the inflammation response intensifies, enterocyte apoptosis occurs and the TJs become increasingly disorganized, further amplifying paracellular permeability[163-165].
It remains to be investigated whether the known ability of zinc to enhance intestinal epithelial TJs[166] (see below) could be a therapeutic modality in CeD by way of reducing gluten and other luminal antigen permeation across the small bowel epithelium of the CeD patient.
The observation of zinc affording protection of the gastric mucosal surface was actually reviewed over 20 years ago[167]. Bandyopadhyay et al[168] (1997) reported in animal studies that orally-administered zinc protects against chemically-induced ulceration, presumably in part by inhibiting gastric H+ secretion. This followed published observations of zinc-mediated suppression of gastric acid output as well as zinc-derived improvement of gastric ulcer healing in the 1970s and 1980s[169-171]. Kirchhoff et al[172] (2011) more recently reported in humans that low-dose zinc administration abolishes secretagogue-induced gastric acid secretion, raising luminal pH as effectively as PPI medications. It remains to be determined if orally-administered zinc can be as effective as PPIs in treating, e.g., gastroesophageal reflux disease without at least some of the side effects (liver cytochrome P450 inhibition) of the omeprazole class of drugs. These actions of zinc may trace in part to zinc’s observed stabilizing effect on secretory cells and lysosomal organelles in general[173].
Omeprazole-induced inhibition of gastric acid production and elevation of gastroduodenal luminal pH has been observed to reduce small intestinal zinc absorption in humans[17,174], although not all studies agree[175]. Joshaghani et al[176] (2012) reported lower serum stores of zinc in males after 8 wk of omeprazole use and decreased zinc absorption was also observed with inhibition of gastric acid production by H-2 blockers[177]. The ability of omeprazole to increase pH only extends to the lumen of the stomach and upper small intestine[178]. It should be noted that the duodenum is not the only site for zinc absorption, as the cecum and colon have been observed to contribute significantly to zinc absorption by the GI tract if small intestine absorption is impaired, though the extent of absorption is not as great[179].
In most of the GI morbidities discussed in this review, the role of transepithelial barrier leak specifically at the site of the epithelial TJ, is very prominent. The recently documented ability of zinc to reduce TJ leak is very likely involved in zinc supplementation’s ability to alleviate these morbidities, and the exacerbation of the morbidities in periods of ZD. For this reason, we present here an expanded description of zinc action on the tight junctional complex.
Examining the impact of ZD or supplementation on epithelial barrier function requires one to consider the molecular structure of the barrier. Epithelial and endothelial TJs (zonula occludens) selectively seal the space between adjacent cells, preventing unregulated paracellular exchange across the epithelial or endothelial barrier. The TJs are comprised of various proteins including the tetraspan transmembrane proteins occludin, tricellulin, and the 24+ members of the claudin family, as well as the single span transmembrane protein JAM-A, scaffolding proteins zona occuldens (ZO)-1, ZO-2, ZO-3, and ZAK and various peripheral membrane proteins[180,181]. Additionally, the TJ is regulated by the actin cytoskeleton through its interactions with scaffolding proteins[182]. The TJ’s primary role is to regulate the paracellular permeability of the epithelial or endothelial barrier. Once thought to be a completely passive structure, it is now known that the TJ is actually quite dynamic, constantly adapting to stimuli[183]. Claudins are thought to interact in both homophilic and heterophilic patterns. Certain interactions are likely to have a sealing function while others can form pores that can be anion or cation specific[184,185]. Occludin likely plays a regulatory role at the TJ based on its phosphorylation state[186]. Exposure to different environmental stimuli such as pathogenic bacteria, foods or micronutrients can have a drastic positive or negative effect on the TJ’s ability to regulate permeation of nutrients, water, and electrolytes[183].
The presence or absence of zinc has been shown to impact the barrier on its own, in conjunction with other molecules, and in various disease states. Zinc is a divalent cation that plays an important role in many mechanisms in the body. It is involved in DNA replication and transcription to RNA (via zinc-finger transcription factors), metalloproteinase catalysis, protection from oxidative stress (when bound to MTs), regulation of apoptosis, cell homeostasis, and immune function[187-191]. Both zinc supplementation and zinc deprivation have been found to impact epithelial barrier function with some evidence indicating this effect is at least partially due to TJ modifications[109,192]. In addition to TJ modification however, epithelial barrier leak can be induced more simply and dramatically by induction of cell death and detachment. Zinc has been shown to be quite able to induce apoptosis at certain concentrations in certain cell types[193,194]. However it has also been shown that epithelial barrier function can withstand the onset of cellular apoptosis (on an individual cell basis) by phagocytosis of the apoptotic cell by its epithelial neighbors, a process that ensues throughout with preservation of tight junctional seals[195].
The following disease situations illustrate zinc’s ability to modify TJs in medically relevant scenarios.
Chronic alcohol exposure has drastic consequences for the liver. Alcohol induces leakage of endotoxins from naturally occurring Gram negative bacteria across the epithelial barrier of the gut into the bloodstream[196]. Alcohol-fed mice have impaired barrier function of the ileal epithelia (as measured by plasma endotoxin levels and increased leak of fluorescein-labeled dextrans) and a correlating decrease in levels of certain TJ proteins such as occludin and ZO-1[197]. Zinc supplementation has been shown to improve and even reverse alcohol induced epithelial barrier permeability and liver damage. Pre-treatment with zinc (prior to alcohol consumption) of a mouse model that mimics binge drinking in humans keeps serum levels of endotoxin at levels close to normal, resulting in significantly less elevation of serum ALT, AST and liver TNF-α, thereby inhibiting liver necrosis[198].
Alcohol exposure is also known to increase ROS in the ileum which is itself associated with decreased ileal zinc concentration. CACO-2 (human GI epithelia) cells exposed to alcohol display a time-dependent increase in barrier permeability (as shown by decreased Rt and increased flux of fluorescein-labeled dextrans) accompanied by an increase in ROS. When zinc deprivation is induced in CACO-2 cells by the zinc chelator, TPEN, there is significant downregulation of claudin-1, occludin and ZO-1. The combination of mild zinc deprivation and alcohol exposure to CACO-2 cells results in even greater decreases in expression of claudin-1, occludin, and ZO-1 as well as impairment of barrier function (as measured by increased dextran leak and decreased Rt)[192]. hepatic nuclear factor α (HNFα) is a zinc finger nuclear transcription factor regulating the expression of certain genes in the kidney, intestine, and liver that is highly expressed in the ileum. The alcohol-induced increase in ROS inactivates HNFα which negatively impacts CACO-2 epithelial barrier function by downregulation of claudin-1, occludin and ZO-1 at the transcriptional level[197].
Alcohol exposure also negatively affects alveolar epithelial barrier function in rats by decreasing expression of claudins -1 and -7, both known to be important in type I pneumocyte TJs in this animal model[199]. It also decreases claudin-3 expression as well as increasing expression of claudin-5, both known to be changes associated with leakier alveolar epithelia[199-201]. Supplementing alcohol-consumption with zinc acetate decreases alcohol-induced alveolar epithelial cell permeability to sucrose in a dose dependent manner and significantly increases expression and localization of occludin and ZO-1 at aveolar epithelial TJs.
Depleting zinc in alveolar epithelia in the presence of proinflammatory cytokines causes paracellular leak simultaneously with breakdown of E-cadherin and beta-catenin, induction of apoptosis, and activation of caspase-3 activation[202].
It is known that neutrophils can migrate across the TJ during immune response[203] which is initially beneficial, however accumulation of neutrophils causes prolonged inflammation[204]. In zinc-depleted CACO-2 cells, TJ barrier function was disrupted as evidenced by decreased Rt and disruption of occludin and ZO-1 expression (attributed to change in phosphorylation state). F-actin and beta-tubulin disorganization were also seen. This allowed increased transmigration of neutrophils across the cell layer[109].
There is evidence that a leaky gut is a factor in Chronic Fatigue Syndrome (CFS) since endotoxin levels in CFS patients (as measured by increased IgM and IgA serum levels against LPS) are elevated. A case study showed a complete remission of CFS symptoms after being put on a leaky gut diet (low carbohydrate, gluten and milk free), in conjunction with intravenous immunoglobulins (IVIGs) and NAIOs (natural anti-inflammatory and anti-oxidative substances, namely N-acetyl-cysteine, glutamine and zinc, among others) which resulted in normalization of IgM and IgA levels and normalization of LPS translocation[205]. Presumably this was due to a tightening of the intestinal barrier. In a follow up study, CFS patients followed only the leaky gut diet and took NAIOs without the addition of IVIGs, and had similar results, meaning that these two therapies alone tightened leaky TJs in the gut, reducing translocation of endotoxin into the bloodstream[206].
Cadmium is an environmental hazard found, for example, in fossil fuels and fertilizers, and it can accumulate in the human body causing nephrotoxicity and other morbidities[207]. When exposed to the renal epithelial cell culture, LLC-PK1, cadmium disrupted TJs, resulting in decreased Rt[208]. Zinc significantly inhibits changes to inulin U/P (urinary to plasma ratio), GFR (glomerular filtration rate), and urinary flow rate in cadmium-exposed rats and, given enough time, it can completely inhibit the negative effect of cadmium on renal function. Zinc treatment also protected against cadmium-induced disorganization of claudins -2 and -5 at the TJs in preparations of rat kidney tubules in a time-dependent manner[207].
Zinc likewise exerts a protective function against copper or iron toxicity. This can occur by both induction of metallothioneins as well as simply competition at the same ligand sites[209]. Zinc has been shown to prevent copper- and iron-related cirrhosis in rats[210] as well as iron accumulation in a simpler cell culture model[211].
Malnutrition is also known to negatively impact intestinal barrier function. Rodriguez et al[65] (1996) found that malnourishment causes increased ionic conductance, mannitol and Na+ permeability of the jejunal epithelium of guinea pigs, but zinc supplementation kept these permeability levels comparable to controls. Additionally, whereas freeze fracture electron microscopy showed malnourished guinea pigs’ jejunal epithelia had 10% less TJ strands, this was not the case when malnourished animals were supplemented with zinc (1996). Treatment with zinc appears to alleviate small intestine paracellular leak due to malnutrition and protects TJs from degradation.
When zinc is enterally delivered to rats with TNBS-induced colitis, the increase in paracellular permeability to Na+ and mannitol in the distal colon is partially ameliorated[212]. In a related study, rats treated with ZnSO4 prior to TNBS-induced colitis, weighed more, had less diarrhea, and showed increased MT synthesis, but ZnSO4 did not affect inflammation, neutrophil invasion of the colonic mucosa, distension of the colon, or zinc concentrations[136]. Interestingly, rats pre-treated for 3 d with a high dose of zinc (30 mg/kg) before colitis was induced via intrarectal DNBS (dinitrobenzene sulfonic acid) showed significantly fewer TJs that were leaky to lanthanum and gained more weight than controls, but a lower dose of zinc did not show this effect (despite still being 10 times the daily requirement for humans)[82]. The level at which zinc conveys the maximal benefit without detrimental effects must be investigated more thoroughly.
In human studies, CD patients who were in remission but displayed increased permeability of the small intestine had a significantly lower lactulose/mannitol ratio when given ZnSO4 supplements. Ten of the 12 patients reached normal permeability levels.
Further studies of zinc’s effect on barrier function in specific GI-related disease states have also been promising. When rats with methotrexate (MTX)-induced mucositis (a state mimicking intestinal mucosal injury in chemotherapy) were administered oral zinc alone or in conjunction with bovine whey-derived growth factor extract (WGFE) they showed decreased permeability of their intestine to 51Cr-EDTA[213]. The combination of zinc + WGFE was the most effective in recovery of the intestine post-MTX injury, providing further evidence that zinc can enhance the beneficial properties of other treatments. In a human study examining the effects of the easily available natural food supplement, zinc carnosine (ZnC), on NSAID-induced intestinal leakiness, ZnC protected against the typical increase in small intestine permeability from indomethacin as well as indomethacin-induced gut injury[214].
Bacterial infection can distinctly target epithelial barrier function. ETEC increases paracellular flux of inulin and decreases Rt in CACO-2 cells. While ZnO treatment of healthy CACO-2 cells did not affect TJ permeability, ZnO treatment of ETEC-infected cells inhibited the ETEC-induced increase in inulin paracellular leak while maintaining Rt comparable to control cell layers in a time and dose-dependent manner[215]. Given that TNFα is known to cause breakdown in epithelial barrier function[216] whereas TGFβ can enhance TJ barrier function[217], it is also of note that TNFα expression was elevated and TGFβ was drastically reduced in ETEC-infected cells. Increasing ZnO concentrations counteracted these effects[215]. It should be noted that this study chose to use ZnO rather than ZnSO4 because ZnO is more effective in skin epithelial wound healing[218]. Further investigation is warranted into the most effective zinc source, keeping in mind that it could be different depending on the tissue and physiology.
Ochratoxin A (OTA) is a mycotoxin that is both a food contaminant and potential human carcinogen. Although it takes over 24 h of exposure, OTA negatively affects barrier function in CACO-2/TC7 cell layers by reducing claudins -3 and -4 in TJs and increasing TJ permeability[219,220]. Zinc-depleted CACO-2/TC7 cells exposed to OTA showed increased TJ permeability (as measured by decreased Rt) and increased apoptosis. Zinc depletion significantly enhanced the deleterious effects of OTA on CACO-2/TC7 cells[219].
Hyperthermia increases intestinal mucosal permeability[221] and is known to affect the expression of TJ proteins[222,223]. Heat stress is known to negatively affect porcine growth and production during the warmer part of the year. When varying concentrations of zinc amino acid complex (ZnAA) were fed to growing pigs exposed to heat stress (HS), those exposed to ZnAA showed improved intestinal barrier function on day 7, although the lower level of ZnAA (+100 ppm over control levels) showed greater improvement than the higher level (+220 ppm over controls)[224]. This could be because higher concentrations are known to negatively affect the pancreas and other organs[225].
Post-weaning diarrhea is a common cause of death in piglets and the resulting increase in gut permeability - likely due at least in part to alterations in the TJs - could be the reason[226,227]. Piglets fed either tetrabasic zinc chloride or ZnO showed decreased lactulose/mannitol ratios in their urine as well as increased expression of the TJ proteins occludin and ZO-1 in the ileum[228]. Piglets fed diosmectite-zinc oxide (DS-ZnO) showed comparable results[229]. These studies suggest that diarrhea is improved by zinc specifically via alleviation of intestinal barrier compromise[228].
In epithelial cell culture studies, zinc’s effects on barrier function are promising. Zinc supplementation decreases expression of claudins -2 and -7 in CACO-2 cell layer TJs and improves barrier function to electrolytes (as shown via increased Rt), although paracellular permeability to small nonelectrolytes (mannitol) is actually increased[166]. In the renal epithelial cell culture line LLC-PK1 zinc increases Rt and significantly decreases transepithelial mannitol leak. Although changes in claudin levels in whole cell lysates were not seen with zinc supplementation, increases in cytosolic levels of claudins -2 and -4 were observed[166]. A combination of the micronutrients, zinc and quercetin, had an even stronger positive effect on Rt, but still did not significantly affect mannitol leak. The zinc/quercetin combination also seemed to further increase claudin-7 expression in the cells[230]. Apical exposure of the renal epithelial cell line MDCK II to zinc chloride, conveys high Rt to the normally leaky cell line[231]. MDCK cell layer conductance is predominantly paracellular, so the increase in Rt (i.e., decrease in conductance) due to zinc chloride treatment likely involves a paracellular pathway[232]. Claudin-2 confers cation-selective pores in leaky epithelia such as MDCK[233]. In zinc-treated cells, claudin-2 internalization and degradation was increased, quite possibly being the reason for the tighter barrier. It should be noted that application of zinc chloride to both the basal-lateral and apical sides of MDCKII cells (simultaneously) causes a drop in Rt[231]. The barrier properties of the colon epithelial cell line, T84, however, showed no change when incubated in Ussing chambers with zinc for short time periods (30 min or less)[234].
Zinc supplementation has been shown to have a protective effect on the epithelial barrier - specifically at the level of the TJ - in cell lines, animal models, and humans in a variety of pathologies including chronic alcohol exposure, heat stress, diarrhea, CFS, colitis, other GI ailments, and even some neurological conditions. Often it can enhance the effects of other beneficial molecules, such as WGFE and quercetin. Whereas in a biological system at homeostasis there is evidence that zinc can improve baseline barrier function, zinc can also protect against both pathophysiologically-generated TJ and general barrier leak. Zinc deprivation, on the other hand, can have catastrophic effects and aggravate disease states via induced barrier leak. It should be mentioned, however, that one electron microscopy-based study of severe ZD in rats did not show junctional leak (to the electron dense tracer, lanthanum ion)[235]. The optimal level of zinc and the best form for delivery to distinct target tissues still require further investigation, as do the precise mechanisms involved in its regulation of TJs. However, its positive impact on epithelial barrier function is undeniable.
The state of ZD is very favorable to the development of various GI diseases, deriving in large part through its negative effect on GI epithelial barrier function. ZD in the United States seems counterintuitive, but with extensive use of PPI drugs, diets abundant in phytate-rich foods, and decreasing consumption of meat and fish in general, lower zinc body stores are not out of the question. Zinc supplementation could be a highly inexpensive and, within well-described daily dosage limits, quite safe, prophylactic measure against several distinct classes of GI disease. Zinc could also serve to possibly reduce the morbidity of certain established diseases. The utility of zinc as an adjuvant to certain current pharmacologic treatments may have real merit. Ironically if one refrains from expecting unrealistic effects of zinc, i.e., out and out cures of certain GI diseases, its real merit and value comes into focus.
| 1. | Duggan C, Gannon J, Walker WA. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr. 2002;75:789-808. [PubMed] |
| 2. | Hering NA, Schulzke JD. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis. 2009;27:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Semrad CE. Zinc and intestinal function. Curr Gastroenterol Rep. 1999;1:398-403. [PubMed] |
| 4. | Goh J, O’Morain CA. Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther. 2003;17:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Scrimgeour AG, Condlin ML. Zinc and micronutrient combinations to combat gastrointestinal inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:653-660. [PubMed] |
| 6. | Tuerk MJ, Fazel N. Zinc deficiency. Curr Opin Gastroenterol. 2009;25:136-143. [PubMed] |
| 7. | Wapnir RA. Zinc deficiency, malnutrition and the gastrointestinal tract. J Nutr. 2000;130:1388S-1392S. [PubMed] |
| 8. | Jeejeebhoy K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137:S7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | King JC. Does zinc absorption reflect zinc status? Int J Vitam Nutr Res. 2010;80:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S-1366S. [PubMed] |
| 11. | Cousins RJ. Gastrointestinal factors influencing zinc absorption and homeostasis. Int J Vitam Nutr Res. 2010;80:243-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Maret W. Zinc and human disease. Met Ions Life Sci. 2013;13:389-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Bode W. Structural basis of matrix metalloproteinase function. Biochem Soc Symp. 2003;1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta. 2010;1803:72-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Urnov FD. A feel for the template: zinc finger protein transcription factors and chromatin. Biochem Cell Biol. 2002;80:321-333. [PubMed] |
| 16. | Pieler T, Bellefroid E. Perspectives on zinc finger protein function and evolution--an update. Mol Biol Rep. 1994;20:1-8. [PubMed] |
| 17. | Farrell CP, Morgan M, Rudolph DS, Hwang A, Albert NE, Valenzano MC, Wang X, Mercogliano G, Mullin JM. Proton pump inhibitors interfere with zinc absorption and zinc body stores. Gastroent Res. 2011;4:243-251. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4:176-190. [PubMed] |
| 19. | Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr. 2010;91:1478S-1483S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | King JC, Shames DM, Lowe NM, Woodhouse LR, Sutherland B, Abrams SA, Turnlund JR, Jackson MJ. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr. 2001;74:116-124. [PubMed] |
| 21. | Institute of Medicine. Dietary reference intakes: vitamin a, vitamin k, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Food and Nutrition Board. Washington, D.C. : National Academy Press 2002; . |
| 22. | Valenzano MC, Mercado JM, Wang X, Zurbach EP, Raines J, McDonnell E, Morgan M, Farrell C, Rudolph D, Hwang A. Drug delivery of zinc to Barrett’s metaplasia by oral administration to Barrett’s esophagus patients. Ther Deliv. 2014;5:257-264. [PubMed] |
| 23. | Porter KG, McMaster D, Elmes ME, Love AH. Anaemia and low serum-copper during zinc therapy. Lancet. 1977;2:774. [PubMed] |
| 24. | Prasad AS, Brewer GJ, Schoomaker EB, Rabbani P. Hypocupremia induced by zinc therapy in adults. JAMA. 1978;240:2166-2168. [PubMed] |
| 25. | Hemilä H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir Med J. 2011;5:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Fosmire GJ. Zinc toxicity. Am J Clin Nutr. 1990;51:225-227. [PubMed] |
| 27. | Fong LY, Jiang Y, Rawahneh ML, Smalley KJ, Croce CM, Farber JL, Huebner K. Zinc supplementation suppresses 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis. Carcinogenesis. 2011;32:554-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Epstein J. SV40-transformed human cells fail to grow in zinc concentrations which permit normal human fibroblast proliferation. J Cell Physiol. 1982;110:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Woster AD, Failla ML, Taylor MW, Weinberg ED. Zinc suppression of initiation of sarcoma 180 growth. J Natl Cancer Inst. 1975;54:1001-1003. [PubMed] |
| 30. | Phillips JL, Sheridan PJ. Effect of zinc administration on the growth of L1210 and BW5147 tumors in mice. J Natl Cancer Inst. 1976;57:361-363. [PubMed] |
| 31. | Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci USA. 2002;99:16770-16775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 295] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Song Y, Chung CS, Bruno RS, Traber MG, Brown KH, King JC, Ho E. Dietary zinc restriction and repletion affects DNA integrity in healthy men. Am J Clin Nutr. 2009;90:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Jaiswal AS, Narayan S. Zinc stabilizes adenomatous polyposis coli (APC) protein levels and induces cell cycle arrest in colon cancer cells. J Cell Biochem. 2004;93:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 2012;4:875-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249-258. [PubMed] |
| 36. | Makuuchi H, Machimura T, Shimada H, Mizutani K, Chino O, Kise Y, Nishi T, Tanaka H, Mitomi T, Horiuchi M. Endoscopic screening for esophageal cancer in 788 patients with head and neck cancers. Tokai J Exp Clin Med. 1996;21:139-145. [PubMed] |
| 37. | Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, Dong ZW, Mark SD, Dawsey SM. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J, Wu Y. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin Nutr. 2014;33:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Fong LY, Sivak A, Newberne PM. Zinc deficiency and methylbenzylnitrosamine-induced esophageal cancer in rats. J Natl Cancer Inst. 1978;61:145-150. [PubMed] |
| 40. | Fong LY, Ng WL, Newberne PM. N-nitrosodimethylamine-induced forestomach tumours in male Sprague-Dawley rats fed a zinc-deficient diet. IARC Sci Publ. 1984;543-546. [PubMed] |
| 41. | Fong LY, Lee JS, Chan WC, Newberne PM. Zinc deficiency and the development of esophageal and forestomach tumors in Sprague-Dawley rats fed precursors of N-nitroso-N-benzylmethylamine. J Natl Cancer Inst. 1984;72:419-425. [PubMed] |
| 42. | Fong LY, Magee PN. Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Lett. 1999;143:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Fong LY, Zhang L, Jiang Y, Farber JL. Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats. J Natl Cancer Inst. 2005;97:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Christudoss P, Selvakumar R, Pulimood AB, Fleming JJ, Mathew G. Protective role of aspirin, vitamin C, and zinc and their effects on zinc status in the DMH-induced colon carcinoma model. Asian Pac J Cancer Prev. 2013;14:4627-4634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Fong LY, Li JX, Farber JL, Magee PN. Cell proliferation and esophageal carcinogenesis in the zinc-deficient rat. Carcinogenesis. 1996;17:1841-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Liu P, Pieper R, Rieger J, Vahjen W, Davin R, Plendl J, Meyer W, Zentek J. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PLoS One. 2014;9:e91091. [PubMed] |
| 47. | Fong LY, Jiang Y, Riley M, Liu X, Smalley KJ, Guttridge DC, Farber JL. Prevention of upper aerodigestive tract cancer in zinc-deficient rodents: inefficacy of genetic or pharmacological disruption of COX-2. Int J Cancer. 2008;122:978-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Taccioli C, Wan SG, Liu CG, Alder H, Volinia S, Farber JL, Croce CM, Fong LY. Zinc replenishment reverses overexpression of the proinflammatory mediator S100A8 and esophageal preneoplasia in the rat. Gastroenterology. 2009;136:953-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Taccioli C, Chen H, Jiang Y, Liu XP, Huang K, Smalley KJ, Farber JL, Croce CM, Fong LY. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene. 2012;31:4550-4558. [PubMed] |
| 50. | Fong LY, Ishii H, Nguyen VT, Vecchione A, Farber JL, Croce CM, Huebner K. p53 deficiency accelerates induction and progression of esophageal and forestomach tumors in zinc-deficient mice. Cancer Res. 2003;63:186-195. [PubMed] |
| 51. | Fong LY, Jiang Y, Farber JL. Zinc deficiency potentiates induction and progression of lingual and esophageal tumors in p53-deficient mice. Carcinogenesis. 2006;27:1489-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Fong LY, Mancini R, Nakagawa H, Rustgi AK, Huebner K. Combined cyclin D1 overexpression and zinc deficiency disrupts cell cycle and accelerates mouse forestomach carcinogenesis. Cancer Res. 2003;63:4244-4252. [PubMed] |
| 53. | Fong LY, Nguyen VT, Farber JL. Esophageal cancer prevention in zinc-deficient rats: rapid induction of apoptosis by replenishing zinc. J Natl Cancer Inst. 2001;93:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 54. | Liu CG, Zhang L, Jiang Y, Chatterjee D, Croce CM, Huebner K, Fong LY. Modulation of gene expression in precancerous rat esophagus by dietary zinc deficit and replenishment. Cancer Res. 2005;65:7790-7799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 55. | Fong LY, Farber JL, Magee PN. Zinc replenishment reduces esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in zinc-deficient rats. Carcinogenesis. 1998;19:1591-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329. [PubMed] |
| 57. | Kay RG, Tasman-Jones C, Pybus J, Whiting R, Black H. A syndrome of acute zinc deficiency during total parenteral alimentation in man. Ann Surg. 1976;183:331-340. [PubMed] |
| 58. | Kay RG, Tasman-Jones C. Acute zinc deficency in man during intravenous alimentation. Aust N Z J Surg. 1975;45:325-330. [PubMed] |
| 59. | Arakawa T, Tamura T, Igarashi Y, Suzuki H, Sandstead HH. Zinc deficiency in two infants during total parenteral alimentation for diarrhea. Am J Clin Nutr. 1976;29:197-204. [PubMed] |
| 60. | Der Kaloustian VM, Musallam SS, Sanjad SA, Murib A, Hammad WD, Idriss ZH. Oral treatment of acrodermatitis enteropathica with zinc sulfate. Am J Dis Child. 1976;130:421-423. [PubMed] |
| 61. | Leupold D, Poley JR, Meigel WN. Zinc therapy in acrodermatitis enteropathica. Helv Paediatr Acta. 1976;31:109-115. [PubMed] |
| 62. | Zou TT, Mou J, Zhan X. Zinc Supplementation in Acute Diarrhea. Indian J Pediatr. 2014;Epub ahead of print. [PubMed] |
| 63. | Lamberti LM, Walker CL, Chan KY, Jian WY, Black RE. Oral zinc supplementation for the treatment of acute diarrhea in children: a systematic review and meta-analysis. Nutrients. 2013;5:4715-4740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Dickinson B, Surawicz CM. Infectious diarrhea: an overview. Curr Gastroenterol Rep. 2014;16:399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Rodriguez P, Darmon N, Chappuis P, Candalh C, Blaton MA, Bouchaud C, Heyman M. Intestinal paracellular permeability during malnutrition in guinea pigs: effect of high dietary zinc. Gut. 1996;39:416-422. [PubMed] |
| 66. | Cui L, Takagi Y, Wasa M, Sando K, Khan J, Okada A. Nitric oxide synthase inhibitor attenuates intestinal damage induced by zinc deficiency in rats. J Nutr. 1999;129:792-798. [PubMed] |
| 67. | Blanchard RK, Cousins RJ. Upregulation of rat intestinal uroguanylin mRNA by dietary zinc restriction. Am J Physiol. 1997;272:G972-G978. [PubMed] |
| 68. | Blanchard RK, Cousins RJ. Regulation of intestinal gene expression by dietary zinc: induction of uroguanylin mRNA by zinc deficiency. J Nutr. 2000;130:1393S-1398S. [PubMed] |
| 69. | Koo SI, Turk DE. Effect of zinc deficiency on intestinal transport triglyceride in the rat. J Nutr. 1977;107:909-919. [PubMed] |
| 70. | Moran JR, Lyerly A. The effects of severe zinc deficiency on intestinal amino acid losses in the rat. Life Sci. 1985;36:2515-2521. [PubMed] |
| 71. | Ying AJ, Shu XL, Gu WZ, Huang XM, Shuai XH, Yang LR, Jiang MZ. [Effect of zinc deficiency on intestinal mucosal morphology and digestive enzyme activity in growing rat]. Zhonghua Erke Zazhi. 2011;49:249-254. [PubMed] |
| 72. | Hambidge KM. Zinc and diarrhea. Acta Paediatr Suppl. 1992;381:82-86. [PubMed] |
| 73. | Dutta P, Mitra U, Dutta S, Naik TN, Rajendran K, Chatterjee MK. Zinc, vitamin A, and micronutrient supplementation in children with diarrhea: a randomized controlled clinical trial of combination therapy versus monotherapy. J Pediatr. 2011;159:633-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 74. | Sazawal S, Jalla S, Mazumder S, Sinha A, Black RE, Bhan MK. Effect of zinc supplementation on cell-mediated immunity and lymphocyte subsets in preschool children. Indian Pediatr. 1997;34:589-597. [PubMed] |
| 75. | Faruque AS, Mahalanabis D, Haque SS, Fuchs GJ, Habte D. Double-blind, randomized, controlled trial of zinc or vitamin A supplementation in young children with acute diarrhoea. Acta Paediatr. 1999;88:154-160. [PubMed] |
| 76. | Awasthi S. Zinc supplementation in acute diarrhea is acceptable, does not interfere with oral rehydration, and reduces the use of other medications: a randomized trial in five countries. J Pediatr Gastroenterol Nutr. 2006;42:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Fischer Walker CL, Black RE. Micronutrients and diarrheal disease. Clin Infect Dis. 2007;45 Suppl 1:S73-S77. [PubMed] |
| 78. | Unger CC, Salam SS, Sarker MS, Black R, Cravioto A, El Arifeen S. Treating diarrhoeal disease in children under five: the global picture. Arch Dis Child. 2014;99:273-278. [PubMed] |
| 79. | Oleske JM, Westphal ML, Shore S, Gorden D, Bogden JD, Nahmias A. Zinc therapy of depressed cellular immunity in acrodermatitis enteropathica. Its correction. Am J Dis Child. 1979;133:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 62] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Zhang Y, Lee B, Thompson M, Glass R, Cama RI, Figueroa D, Gilman R, Taylor D, Stephenson C. Lactulose-mannitol intestinal permeability test in children with diarrhea caused by rotavirus and cryptosporidium. Diarrhea Working Group, Peru. J Pediatr Gastroenterol Nutr. 2000;31:16-21. [PubMed] |
| 81. | Alam AN, Sarker SA, Wahed MA, Khatun M, Rahaman MM. Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut. 1994;35:1707-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D’inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311-315. [PubMed] |
| 83. | Roy SK, Behrens RH, Haider R, Akramuzzaman SM, Mahalanabis D, Wahed MA, Tomkins AM. Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Pediatr Gastroenterol Nutr. 1992;15:289-296. [PubMed] |
| 84. | Roy SK, Tomkins AM, Ara G, Jolly SP, Khatun W, Chowdhury R, Chakrabarty B. Impact of zinc deficiency on vibrio cholerae enterotoxin-stimulated water and electrolyte transport in animal model. J Health Popul Nutr. 2006;24:42-47. [PubMed] |
| 85. | Qadir MI, Arshad A, Ahmad B. Zinc: Role in the management of diarrhea and cholera. World J Clin Cases. 2013;1:140-142. [PubMed] |
| 86. | Hoque KM, Rajendran VM, Binder HJ. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G956-G963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Bzik VA, Medani M, Baird AW, Winter DC, Brayden DJ. Mechanisms of action of zinc on rat intestinal epithelial electrogenic ion secretion: insights into its antidiarrhoeal actions. J Pharm Pharmacol. 2012;64:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Sargeant HR, McDowall KJ, Miller HM, Shaw MA. Dietary zinc oxide affects the expression of genes associated with inflammation: Transcriptome analysis in piglets challenged with ETEC K88. Vet Immunol Immunopathol. 2010;137:120-129. [PubMed] |
| 89. | Kwon CH, Lee CY, Han SJ, Kim SJ, Park BC, Jang I, Han JH. Effects of dietary supplementation of lipid-encapsulated zinc oxide on colibacillosis, growth and intestinal morphology in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim Sci J. 2014;85:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Berni Canani R, Secondo A, Passariello A, Buccigrossi V, Canzoniero LM, Ruotolo S, Puzone C, Porcaro F, Pensa M, Braucci A. Zinc inhibits calcium-mediated and nitric oxide-mediated ion secretion in human enterocytes. Eur J Pharmacol. 2010;626:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Surjawidjaja JE, Hidayat A, Lesmana M. Growth inhibition of enteric pathogens by zinc sulfate: an in vitro study. Med Princ Pract. 2010;13:286-289. [PubMed] |
| 92. | Sturniolo GC, Molokhia MM, Shields R, Turnberg LA. Zinc absorption in Crohn’s disease. Gut. 1980;21:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Nishida K, Yao T, Tsuzuki O. [A study on a low serum zinc level in Crohn’s disease]. Nihon Shokakibyo Gakkai Zasshi. 1985;82:424-433. [PubMed] |
| 94. | Matsui T. Zinc deficiency in Crohn’s disease. J Gastroenterol. 1998;33:924-925. [PubMed] |
| 95. | Hendricks KM, Walker WA. Zinc deficiency in inflammatory bowel disease. Nutr Rev. 1988;46:401-408. [PubMed] |
| 96. | McClain C, Soutor C, Zieve L. Zinc deficiency: a complication of Crohn’s disease. Gastroenterology. 1980;78:272-279. [PubMed] |
| 97. | Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 98. | Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis. 2006;12:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 99. | Myung SJ, Yang SK, Jung HY, Jung SA, Kang GH, Ha HK, Hong WS, Min YI. Zinc deficiency manifested by dermatitis and visual dysfunction in a patient with Crohn’s disease. J Gastroenterol. 1998;33:876-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Fauci AS. Harrison’s principles of internal medicine. New York, USA: McGraw-Hill Medical 2008; . |
| 101. | Ainley C, Cason J, Slavin BM, Wolstencroft RA, Thompson RP. The influence of zinc status and malnutrition on immunological function in Crohn’s disease. Gastroenterology. 1991;100:1616-1625. [PubMed] |
| 102. | Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 103. | Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn’s disease of the terminal ileum and colon. Digestion. 1983;27:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437-1439. [PubMed] |
| 105. | Ranaldi G, Ferruzza S, Canali R, Leoni G, Zalewski PD, Sambuy Y, Perozzi G, Murgia C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967-976. [PubMed] |
| 106. | Sturniolo GC, Di Leo V, Ferronato A, D’Odorico A, D’Incà R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm Bowel Dis. 2001;7:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 107. | El-Tawil AM. Zinc supplementation tightens leaky gut in Crohn’s disease. Inflamm Bowel Dis. 2012;18:E399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (4)] |
| 109. | Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr. 2008;138:1664-1670. [PubMed] |
| 110. | Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912-1925. [PubMed] |
| 111. | Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672-1683. [PubMed] |
| 112. | Mulder TP, van der Sluys Veer A, Verspaget HW, Griffioen G, Peña AS, Janssens AR, Lamers CB. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 1994;9:472-477. [PubMed] |
| 113. | Brignola C, Belloli C, De Simone G, Evangelisti A, Parente R, Mancini R, Iannone P, Mocheggiani E, Fabris N, Morini MC. Zinc supplementation restores plasma concentrations of zinc and thymulin in patients with Crohn’s disease. Aliment Pharmacol Ther. 1993;7:275-280. [PubMed] |
| 114. | Van de Wal Y, Van der Sluys Veer A, Verspaget HW, Mulder TP, Griffioen G, Van Tol EA, Peña AS, Lamers CB. Effect of zinc therapy on natural killer cell activity in inflammatory bowel disease. Aliment Pharmacol Ther. 1993;7:281-286. [PubMed] |
| 115. | Lee HH, Prasad AS, Brewer GJ, Owyang C. Zinc absorption in human small intestine. Am J Physiol. 1989;256:G87-G91. [PubMed] |
| 116. | Sturniolo GC, Mestriner C, Lecis PE, D’Odorico A, Venturi C, Irato P, Cecchetto A, Tropea A, Longo G, D’Inca R. Altered plasma and mucosal concentrations of trace elements and antioxidants in active ulcerative colitis. Scand J Gastroenterol. 1998;33:644-649. [PubMed] |
| 117. | Ringstad J, Kildebo S, Thomassen Y. Serum selenium, copper, and zinc concentrations in Crohn’s disease and ulcerative colitis. Scand J Gastroenterol. 1993;28:605-608. [PubMed] |
| 118. | Ojuawo A, Keith L. The serum concentrations of zinc, copper and selenium in children with inflammatory bowel disease. Cent Afr J Med. 2002;48:116-119. [PubMed] |
| 119. | Dalekos GN, Ringstad J, Savaidis I, Seferiadis KI, Tsianos EV. Zinc, copper and immunological markers in the circulation of well nourished patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 1998;10:331-337. [PubMed] |
| 120. | Barahona-Garrido J, Camacho-Escobedo J, García-Martínez CI, Tocay H, Cabiedes J, Yamamoto-Furusho JK. Antinuclear antibodies: a marker associated with steroid dependence in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 121. | Hocke M, Winnefeld K, Bosseckert H. Zinc concentration in serum and leucocytes in chronic inflammatory diseases. J Trace Elem Med Biol. 1995;9:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 122. | Whineray E, Inder WJ, Roche D, Dobbs BR, Frizelle FA. Comparison of micronutrients in patients having had panproctocolectomy and either ileal pouch anal anastomosis or Brooke ileostomy for chronic ulcerative colitis (UC). Colorectal Dis. 2000;2:351-354. [PubMed] |
| 123. | Ma X, Zhao K, Wei L, Song P, Liu G, Han H, Wang C. Altered plasma concentrations of trace elements in ulcerative colitis patients before and after surgery. Biol Trace Elem Res. 2013;153:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 124. | El Muhtaseb MS, Duncan A, Talwar DK, O’Reilly DS, McKee RF, Anderson JH, Finlay IG. Assessment of dietary intake and trace element status in patients with ileal pouch-anal anastomosis. Dis Colon Rectum. 2007;50:1553-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000;54:514-521. [PubMed] |
| 126. | Ainley CC, Cason J, Carlsson LK, Slavin BM, Thompson RP. Zinc status in inflammatory bowel disease. Clin Sci (Lond). 1988;75:277-283. [PubMed] |
| 127. | Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3068] [Cited by in RCA: 2977] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 128. | Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987;1:441-445. [PubMed] |
| 129. | Xia B, Deng CS, Chen DJ, Zhou Y, Xiao JQ. Role of copper zinc superoxide dismutase in the short-term treatment of acetic acid-induced colitis in rats. Acta Gastroenterol Latinoam. 1996;26:227-230. [PubMed] |
| 130. | Kruidenier L, Kuiper I, van Duijn W, Marklund SL, van Hogezand RA, Lamers CB, Verspaget HW. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J Pathol. 2003;201:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Mulder TP, Verspaget HW, Janssens AR, de Bruin PA, Peña AS, Lamers CB. Decrease in two intestinal copper/zinc containing proteins with antioxidant function in inflammatory bowel disease. Gut. 1991;32:1146-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 132. | Kruidenier L, van Meeteren ME, Kuiper I, Jaarsma D, Lamers CB, Zijlstra FJ, Verspaget HW. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic Biol Med. 2003;34:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078-2086. [PubMed] |
| 134. | Suwendi E, Iwaya H, Lee JS, Hara H, Ishizuka S. Zinc deficiency induces dysregulation of cytokine productions in an experimental colitis of rats. Biomed Res. 2012;33:329-336. [PubMed] |
| 135. | Bruewer M, Schmid KW, Krieglstein CF, Senninger N, Schuermann G. Metallothionein: early marker in the carcinogenesis of ulcerative colitis-associated colorectal carcinoma. World J Surg. 2002;26:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 136. | Di Leo V, D’Incà R, Barollo M, Tropea A, Fries W, Mazzon E, Irato P, Cecchetto A, Sturniolo GC. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig Liver Dis. 2001;33:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Tran CD, Ball JM, Sundar S, Coyle P, Howarth GS. The role of zinc and metallothionein in the dextran sulfate sodium-induced colitis mouse model. Dig Dis Sci. 2007;52:2113-2121. [PubMed] |
| 138. | Tran CD, Butler RN, Philcox JC, Rofe AM, Howarth GS, Coyle P. Regional distribution of metallothionein and zinc in the mouse gut: comparison with metallothionien-null mice. Biol Trace Elem Res. 1998;63:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 139. | Luk HH, Ko JK, Fung HS, Cho CH. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur J Pharmacol. 2002;443:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Chen BW, Wang HH, Liu JX, Liu XG. Zinc sulphate solution enema decreases inflammation in experimental colitis in rats. J Gastroenterol Hepatol. 1999;14:1088-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Yoshikawa T, Yamaguchi T, Yoshida N, Yamamoto H, Kitazumi S, Takahashi S, Naito Y, Kondo M. Effect of Z-103 on TNB-induced colitis in rats. Digestion. 1997;58:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 142. | Ohkawara T, Takeda H, Kato K, Miyashita K, Kato M, Iwanaga T, Asaka M. Polaprezinc (N-(3-aminopropionyl)-L-histidinato zinc) ameliorates dextran sulfate sodium-induced colitis in mice. Scand J Gastroenterol. 2005;40:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 143. | Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5:3975-3992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 144. | Solomons NW, Rosenberg IH, Sandstead HH. Zinc nutrition in celiac sprue. Am J Clin Nutr. 1976;29:371-375. [PubMed] |
| 145. | Kuloğlu Z, Kirsaçlioğlu CT, Kansu A, Ensari A, Girgin N. Celiac disease: presentation of 109 children. Yonsei Med J. 2009;50:617-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 146. | Henker J, Gabsch HC. [Serum zinc levels in children with celiac disease]. Helv Paediatr Acta. 1985;40:47-53. [PubMed] |
| 147. | Naveh Y, Lightman A, Zinder O. A prospective study of serum zinc concentration in children with celiac disease. J Pediatr. 1983;102:734-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 148. | Singhal N, Alam S, Sherwani R, Musarrat J. Serum zinc levels in celiac disease. Indian Pediatr. 2008;45:319-321. [PubMed] |
| 149. | Jameson S. Zinc deficiency in malabsorption states: a cause of infertility? Acta Med Scand Suppl. 1976;593:38-49. [PubMed] |
| 150. | Jameson S. Trace element metabolism in coeliac disease. Trace elements in health and disease. Stockholm: Almqvist and Wiksell International 1985; 242-252. |
| 151. | Jameson S. Villous atrophy and nutritional status in celiac disease. Am J Clin Nutr. 1999;69:573-575. [PubMed] |
| 152. | Jameson S, Björklund O, Lööf L, Ulfberg J. Zinc and folate malabsorption in coeliac disease. Trace element metabolism in man and animals. Canberra, Australia: Springer-Verlag 1981; 495-497. |
| 153. | Högberg L, Danielsson L, Jarleman S, Sundqvist T, Stenhammar L. Serum zinc in small children with coeliac disease. Acta Paediatr. 2009;98:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 154. | Rea F, Polito C, Marotta A, Di Toro A, Iovene A, Collini R, Rea L, Sessa G. Restoration of body composition in celiac children after one year of gluten-free diet. J Pediatr Gastroenterol Nutr. 1996;23:408-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 155. | Jones PE, Peters TJ. Oral zinc supplements in non-responsive coeliac syndrome: effect on jejunal morphology, enterocyte production, and brush border disaccharidase activities. Gut. 1981;22:194-198. [PubMed] |
| 156. | Crofton RW, Aggett PJ, Gvozdanovic S, Gvozdanovic D, Mowat NA, Brunt PW. Zinc metabolism in celiac disease. Am J Clin Nutr. 1990;52:379-382. [PubMed] |
| 157. | Tran CD, Katsikeros R, Manton N, Krebs NF, Hambidge KM, Butler RN, Davidson GP. Zinc homeostasis and gut function in children with celiac disease. Am J Clin Nutr. 2011;94:1026-1032. [PubMed] |
| 158. | Krebs NF, Miller LV, Naake VL, Lei S, Westcott JE, Fennessey PV, Michael Hambidge K. The use of stable isotope techniques to assess zinc metabolism. J Nutr Biochem. 1995;6:292-301. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 159. | Krebs NF, Hambidge KM, Westcott JE, Miller LV, Sian L, Bell M, Grunwald G. Exchangeable zinc pool size in infants is related to key variables of zinc homeostasis. J Nutr. 2003;133:1498S-1501S. [PubMed] |
| 160. | Stenberg P, Roth EB, Sjöberg K. Transglutaminase and the pathogenesis of coeliac disease. Eur J Intern Med. 2008;19:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 161. | Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol. 2006;176:2512-2521. [PubMed] |
| 162. | Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 163. | Ciccocioppo R, Finamore A, Ara C, Di Sabatino A, Mengheri E, Corazza GR. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol. 2006;125:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 164. | Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, Fromm M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res. 1998;43:435-441. [PubMed] |
| 165. | Ventura A, Magazzù G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297-303. [PubMed] |
| 166. | Wang X, Valenzano MC, Mercado JM, Zurbach EP, Mullin JM. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig Dis Sci. 2013;58:77-87. [PubMed] |
| 167. | D’Souza RS, Dhume VG. Gastric cytoprotection. Indian J Physiol Pharmacol. 1991;35:88-98. [PubMed] |
| 168. | Bandyopadhyay B, Bandyopadhyay SK. Protective effect of zinc gluconate on chemically induced gastric ulcer. Indian J Med Res. 1997;106:27-32. [PubMed] |
| 169. | Escolar G, Bulbena O. Zinc compounds, a new treatment in peptic ulcer. Drugs Exp Clin Res. 1989;15:83-89. [PubMed] |
| 170. | Frommer DJ. The healing of gastric ulcers by zinc sulphate. Med J Aust. 1975;2:793-796. [PubMed] |
| 171. | Alcala-Santaella R, Castellanos D, Velo JL, Gonzalez Lara V. Zinc acexamate in treatment of duocenal ulcer. Lancet. 1985;2:157. |
| 172. | Kirchhoff P, Socrates T, Sidani S, Duffy A, Breidthardt T, Grob C, Viehl CT, Beglinger C, Oertli D, Geibel JP. Zinc salts provide a novel, prolonged and rapid inhibition of gastric acid secretion. Am J Gastroenterol. 2011;106:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 173. | Pfeiffer CJ, Cho CH, Cheema A, Saltman D. Reserpine-induced gastric ulcers: protection by lysosomal stabilization due to zinc. Eur J Pharmacol. 1980;61:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 174. | Ozutemiz AO, Aydin HH, Isler M, Celik HA, Batur Y. Effect of omeprazole on plasma zinc levels after oral zinc administration. Indian J Gastroenterol. 1980;21:216-218. [PubMed] |
| 175. | Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, Russell RR. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr. 1995;14:364-368. [PubMed] |
| 176. | Joshaghani H, Amiriani T, Vaghari G, Besharat S, Molana A, Badeleh M, Roshandel G. Effects of omeprazole consumption on serum levels of trace elements. J Trace Elem Med Biol. 2012;26:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 177. | Sturniolo GC, Montino MC, Rossetto L, Martin A, D’Inca R, D’Odorico A, Naccarato R. Inhibition of gastric acid secretion reduces zinc absorption in man. J Am Coll Nutr. 1991;10:372-375. [PubMed] |
| 178. | Nakamura T, Arai Y, Tando Y, Terada A, Yamada N, Tsujino M, Imamura K, Machida K, Kikuchi H, Takebe K. Effect of omeprazole on changes in gastric and upper small intestine pH levels in patients with chronic pancreatitis. Clin Ther. 1991;17:448-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 179. | Hara H, Konishi A, Kasai T. Contribution of the cecum and colon to zinc absorption in rats. J Nutr. 2000;130:83-89. [PubMed] |
| 180. | Schulzke JD, Fromm M. Tight junctions: molecular structure meets function. Ann N Y Acad Sci. 2009;1165:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 181. | Walter JK, Rueckert C, Voss M, Mueller SL, Piontek J, Gast K, Blasig IE. The oligomerization of the coiled coil-domain of occludin is redox sensitive. Ann N Y Acad Sci. 2009;1165:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 182. | Tsukita S, Katsuno T, Yamazaki Y, Umeda K, Tamura A, Tsukita S. Roles of ZO-1 and ZO-2 in establishment of the belt-like adherens and tight junctions with paracellular permselective barrier function. Ann N Y Acad Sci. 2009;1165:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 183. | Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 878] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
| 184. | Krause G, Winkler L, Piehl C, Blasig I, Piontek J, Müller SL. Structure and function of extracellular claudin domains. Ann N Y Acad Sci. 2009;1165:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 185. | Yu AS. Molecular basis for cation selectivity in claudin-2-based pores. Ann N Y Acad Sci. 2009;1165:53-57. [PubMed] |
| 186. | Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009;1165:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 187. | Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol. 2006;80:1-9. [PubMed] |
| 188. | Falchuk KH. The molecular basis for the role of zinc in developmental biology. Mol Cell Biochem. 1998;188:41-48. [PubMed] |
| 189. | Kondoh M, Imada N, Kamada K, Tsukahara R, Higashimoto M, Takiguchi M, Watanabe Y, Sato M. Property of metallothionein as a Zn pool differs depending on the induced condition of metallothionein. Toxicol Lett. 2003;142:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 190. | Seve M, Chimienti F, Favier A. [Role of intracellular zinc in programmed cell death]. Pathol Biol (Paris). 2002;50:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 191. | Wellinghausen N, Kirchner H, Rink L. The immunobiology of zinc. Immunol Today. 1997;18:519-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 192. | Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625-G633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 193. | Chang KL, Hung TC, Hsieh BS, Chen YH, Chen TF, Cheng HL. Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells. Nutrition. 2006;22:465-474. [PubMed] |
| 194. | Weissgarten J, Berman S, Modai D, Rosenberg R, Rapoport M, Cohen M, Averbukh Z. Zn metabolism affects apoptosis rate and proliferative responsiveness of PBMC from patients on chronic hemodialysis. Metabolism. 2002;51:1392-1396. [PubMed] |
| 195. | Soler AP, Laughlin KV, Mullin JM. Effects of epidermal growth factor versus phorbol ester on kidney epithelial (LLC-PK1) tight junction permeability and cell division. Exp Cell Res. 1993;207:398-406. [PubMed] |
| 196. | Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 197. | Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor-4{alpha} mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2010;299:G643-G651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 198. | Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 199. | Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol. 2007;41:371-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 200. | Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166-L1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 201. | Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 202. | Bao S, Knoell DL. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1132-L1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 203. | Edens HA, Levi BP, Jaye DL, Walsh S, Reaves TA, Turner JR, Nusrat A, Parkos CA. Neutrophil transepithelial migration: evidence for sequential, contact-dependent signaling events and enhanced paracellular permeability independent of transjunctional migration. J Immunol. 2002;169:476-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 204. | Elliott SN, Wallace JL. Neutrophil-mediated gastrointestinal injury. Can J Gastroenterol. 2002;12:559-568. [PubMed] |
| 205. | Maes M, Coucke F, Leunis JC. Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuro Endocrinol Lett. 2007;28:739-744. [PubMed] |
| 206. | Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. 2008;29:902-910. [PubMed] |
| 207. | Jacquillet G, Barbier O, Cougnon M, Tauc M, Namorado MC, Martin D, Reyes JL, Poujeol P. Zinc protects renal function during cadmium intoxication in the rat. Am J Physiol Renal Physiol. 2006;290:F127-F137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 208. | Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol. 2003;189:180-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 209. | Santon A, Formigari A, Irato P. The influence of metallothionein on exposure to metals: an in vitro study on cellular models. Toxicol In Vitro. 2008;22:980-987. [PubMed] |
| 210. | Medici V, Sturniolo GC, Santon A, D’Incà R, Bortolami M, Cardin R, Basso D, Albergoni V, Irato P. Efficacy of zinc supplementation in preventing acute hepatitis in Long-Evans Cinnamon rats. Liver Int. 2005;25:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 211. | Formigari A, Santon A, Irato P. Efficacy of zinc treatment against iron-induced toxicity in rat hepatoma cell line H4-II-E-C3. Liver Int. 2007;27:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 212. | Rohweder J, Runkel N, Fromm M, Schulzke JD, Buhr HJ. [Zinc acts as a protective agent on the mucosal barrier in experimental TNBS colitis]. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:223-227. [PubMed] |
| 213. | Tran CD, Howarth GS, Coyle P, Philcox JC, Rofe AM, Butler RN. Dietary supplementation with zinc and a growth factor extract derived from bovine cheese whey improves methotrexate-damaged rat intestine. Am J Clin Nutr. 2003;77:1296-1303. [PubMed] |
| 214. | Mahmood A, FitzGerald AJ, Marchbank T, Ntatsaki E, Murray D, Ghosh S, Playford RJ. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. 2007;56:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 215. | Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J Nutr. 2003;133:4077-4082. [PubMed] |
| 216. | Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172-2176. [PubMed] |
| 217. | Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157: H7-induced increased permeability. Am J Pathol. 2005;167:1587-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 218. | Agren MS, Chvapil M, Franzén L. Enhancement of re-epithelialization with topical zinc oxide in porcine partial-thickness wounds. J Surg Res. 1991;50:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 219. | Lambert D, Padfield PJ, McLaughlin J, Cannell S, O’Neill CA. Ochratoxin A displaces claudins from detergent resistant membrane microdomains. Biochem Biophys Res Commun. 2007;358:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 220. | Ranaldi G, Mancini E, Ferruzza S, Sambuy Y, Perozzi G. Effects of red wine on ochratoxin A toxicity in intestinal Caco-2/TC7 cells. Toxicol In Vitro. 2007;21:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 221. | Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol (1985). 2002;92:1750-1761; discussion 1749. [PubMed] |
| 222. | Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 2013;8:e70215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 223. | Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290:G204-G212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 224. | Sanz Fernandez MV, Pearce SC, Gabler NK, Patience JF, Wilson ME, Socha MT, Torrison JL, Rhoads RP, Baumgard LH. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal. 2014;8:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 225. | Fujimura T, Matsui T, Funaba M. Regulatory responses to excess zinc ingestion in growing rats. Br J Nutr. 2012;107:1655-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 226. | Boudry G, Péron V, Le Huërou-Luron I, Lallès JP, Sève B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 2004;134:2256-2262. [PubMed] |
| 227. | Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139-G147. [PubMed] |
| 228. | Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 2009;102:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 229. | Hu C, Song J, Li Y, Luan Z, Zhu K. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br J Nutr. 2013;110:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 230. | Mercado J, Valenzano MC, Jeffers C, Sedlak J, Cugliari MK, Papanikolaou E, Clouse J, Miao J, Wertan NE, Mullin JM. Enhancement of tight junctional barrier function by micronutrients: compound-specific effects on permeability and claudin composition. PLoS One. 2013;8:e78775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 231. | Carr G, Wright JA, Simmons NL. Epithelial barrier resistance is increased by the divalent cation zinc in cultured MDCKII epithelial monolayers. J Membr Biol. 2010;237:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 232. | Gitter AH, Bertog M, Schulzke J, Fromm M. Measurement of paracellular epithelial conductivity by conductance scanning. Pflugers Arch. 1997;434:830-840. [PubMed] |
| 233. | Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 592] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 234. | Hoque KM, Sarker R, Guggino SE, Tse CM. A new insight into pathophysiological mechanisms of zinc in diarrhea. Ann N Y Acad Sci. 2009;1165:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 235. | Moran JR, Lewis JC. The effects of severe zinc deficiency on intestinal permeability: an ultrastructural study. Pediatr Res. 1985;19:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
P- Reviewer: Irato P, Yin JY S- Editor: Ji FF L- Editor: A E- Editor: Wang CH