Published online Nov 22, 2021. doi: 10.4291/wjgp.v12.i6.106
Peer-review started: March 16, 2021
First decision: May 6, 2021
Revised: May 14, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: November 22, 2021
Processing time: 244 Days and 10.1 Hours
Bacteria of the human intestinal microflora have a dual role. They promote digestion and are part of a defense mechanism against pathogens. These bacteria could become potential pathogens under certain circumstances. The term “bacterial translocation” describes the passage of bacteria of the gastrointestinal tract through the intestinal mucosa barrier to mesenteric lymph nodes and other organs. In some cases, the passage of bacteria and endotoxins could result in blood stream infections and in multiple organ failure. Open elective abdominal surgery more frequently results in malfunction of the intestinal barrier and subsequent bacterial translocation and blood stream infections than laparoscopic surgery. Postoperative sepsis is a common finding in patients who have undergone non-elective abdominal surgeries, including trauma patients treated with laparotomy. Postoperative sepsis is an emerging issue, as it changes the treatment plan in surgical patients and prolongs hospital stay. The association between bacterial translocation and postoperative sepsis could provide novel treatment options.
Core Tip: Increased intestinal permeability can potentially induce intestinal flora dysbiosis. Bacterial translocation, attributed to intestinal barrier impairment, may lead to systematic infection in the postoperative period. The definitive correlation between translocation and postoperative sepsis is yet to be proven, but the latter is an emerging issue for patients undergoing major gastrointestinal surgeries.
- Citation: Doudakmanis C, Bouliaris K, Kolla C, Efthimiou M, Koukoulis GD. Bacterial translocation in patients undergoing major gastrointestinal surgery and its role in postoperative sepsis. World J Gastrointest Pathophysiol 2021; 12(6): 106-114
- URL: https://www.wjgnet.com/2150-5330/full/v12/i6/106.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v12.i6.106
The incidence of postoperative sepsis has increased in the past decades, with the proportion of severe sepsis cases rising to unprecedented levels. Cases of sepsis are noted both after elective and emergency surgeries, but in the cases of elective surgeries, mortality is not respectively affected[1]. Gastrointestinal perforation is the most common surgical condition requiring immediate surgical intervention. More specifically, colonic perforation may cause peritonitis through the spread of bacteria from the intestines, and, therefore, there is a high risk for further bacterial spread via blood flow[2].
Gut microbiota affects the host decisively in both states of health and illness. The human gut microbiota consists of numerous bacteria that coexist and play a beneficial role in normal functions of the intestine. In normal conditions, bacteria of the gut assist in the absorption of nutrients. In illness, there are vast changes that alter the balance of these bacteria, leading to proliferation of potentially dangerous bacteria, capable of causing infections[3]. Diseases like colorectal cancer, inflammatory bowel disease, and diseases of the liver could alter the relationship between bacteria of the gut and the host.
In addition to bacterial dissemination due to mechanical disruption of the continuity of the intestinal barrier, as in the case of perforation, another potential mechanism proposed is bacterial translocation. Bacterial translocation is the movement of bacteria or their products from the intestinal lumen through the mucosa layer to a normally sterile tissue[4]. The most common routes for bacterial passage from the intestine to the systemic circulation and eventually to distant organs are the lymphatic route and the vascular route[5].
Major abdominal surgeries are procedures that promote an imbalance in intestinal bacteria. Patients undergoing major abdominal surgery are considered at high risk of developing postoperative infections as a result of bacterial translocation. Those undergoing emergency surgery are at even higher risk[6]. An increase in morbidity and mortality has been shown in cases of ascertained translocation to locoregional mesenteric lymph nodes[7,8].
The intestinal barrier interacts with the contents of the intestinal lumen at immunological and chemical levels, besides being a physical barrier. It is composed of a single layer of columnar epithelial cells, which have diverse functions, such as absorptive, secretory and immune functions. The majority of intestinal epithelial cells are absorptive enterocytes. Other types of intestinal epithelial cells are secretory goblet cells, Paneth cells, and enteroendocrine cells. All these cells are under constant renewal by intestinal epithelial stem cells located in the bases of mucosal crypts[9].
Commensal bacteria found in the intestinal lumen prevent the proliferation of potential pathogens through regulating intestinal pH and decreasing the nutrients required by those pathogens. On the surface of the lumen, a layer of water, the glycocalyx, and the mucus layer containing immunoglobulin A (IgA) create a first defensive line, preventing adhesion of pathogenic bacteria to the epithelium and diminishing interaction between pathogen and epithelial cells. In addition, antimicrobial agents secreted by epithelial cells attract monocytes and assist in the opsonization of macrophages. Immunoglobulins and cytokines are secreted by cells of the lamina propria, as those cells are part of the innate and acquired immune system and play a vast role in immunological regulation in the intestine[10]. Besides having a role as a physical barrier, the mucus layer of the intestine contains an abundance of secretory IgA and antimicrobial proteins. There is a substantial difference in the composition of the mucus layer between the small and large intestine. This layer in the small intestine is penetrable by bacteria, while the large intestine has both a penetrable outer mucus layer and an impenetrable inner mucus layer. Intestinal epithelial cells create a defense barrier below the layers of mucous inside the lumen of the intestine[11]. A barrier formed by mucins between the intestinal lumen and intestinal epithelial cells can regulate expression of tolerogenic and inflammatory cytokines[12].
Intestinal epithelial cells are connected to each other with tight junctions[9,10]. Tight junctions are an assembly of multiple proteins located on the apical part of neighboring epithelial cells and affect paracellular permeability, as they selectively regulate permeability. Tight junctions are fundamental in maintaining intestinal barrier function. They act as adhesive and mechanical mediators, maintaining barrier function, but do not seal the paracellular space. There are two functional protein categories, namely integral transmembrane proteins that form a network between adjacent cell membranes and peripheral membranes. Four integral transmembrane proteins are occludin, claudin, junctional adhesion molecule, and tricellulin[13,14]. In certain conditions of intestinal inflammation, it is shown that these tight junctions dysfunction, increasing permeability. The repair process of the epithelial cells affects intestinal motility and is considered an important factor in intestinal barrier function[9,10,15].
Intestinal permeability is the condition during which soluble molecules and fluids are exchanged between the intestinal lumen and tissues. In normal conditions, intestinal barrier homeostasis acts to prevent this exchange, but both permeability and barrier function are dynamic states[16]. Dysfunction of the mucosal barrier can be found in both stress-associated conditions and in a diverse group of conditions in otherwise healthy people. It has been shown that there is increased intestinal permeability in patients with gastroenterological diseases correlated with intestinal inflammation, especially in those with inflammatory bowel disease. In addition, healthy relatives of these patients are at high risk of developing increased intestinal permeability[17,18]. Use of non-steroidal anti-inflammatory drugs may alter the structural normality of the intestinal lumen, thereby impairing the barrier and potentially increasing permeability[19]. More importantly, studies have shown that in a number of patients undergoing abdominal surgery, bacterial DNA was detected as early as a few hours postoperatively, indicating a relation between surgery and translocation[6].
The human intestinal microbiota plays a main role in intestinal metabolism and in immunological response of the intestines[20]. Balance of the intestinal microbiota is a prerequisite for a healthy intestinal environment. Imbalance of microbiota and of the host immune system is present in intestinal diseases. Altered concentrations of commensal intestinal bacteria depends on disease activity, and this can easily be noted when patients are compared to healthy individuals[21].
Studies in patients with colorectal cancer have shown that alterations in microbiota are also associated with tumorigenesis. These alterations are characterized by the dominance of certain bacteria species. In the spotlight are Fusobacterium nucleatum, Escherichia coli, and Bacteroides fragilis[22]. Analyses of intestinal microbiota are performed using 16S ribosomal RNA techniques. When the aforementioned bacteria species are increased, other bacteria are depleted. Gram-positive bacteria are vastly affected, and Clostridia species are also decreased in these patients[23-26]. Bacteroides fragilis colonizes the intestine and has a prominent place in the microbiota. Although Escherichia coli is considered a commensal bacteria, some of its species are potential pathogens, promoting intestinal inflammation and producing oncogenic toxins. This phenomenon, when there is an imbalance in intestinal flora, is called dysbiosis. Dysbiosis may characterize inflammatory gastrointestinal diseases and colorectal cancer but may also be explained by the changes in dietary habits that have occurred over the past decades. The importance of metabolites and their products to intestinal inflammation have led to increased concern for the impact of metabolic diseases on microbiota[23,27].
Bowel obstruction has both local and systemic effects. Fecal retention promotes bacterial overgrowth. Besides changes in bowel motility, moderate inflammation is a probable finding. This inflammatory response may lead to systemic responses, with sepsis and septic shock being the most serious. The causative factor for these systemic responses is bacterial translocation[28].
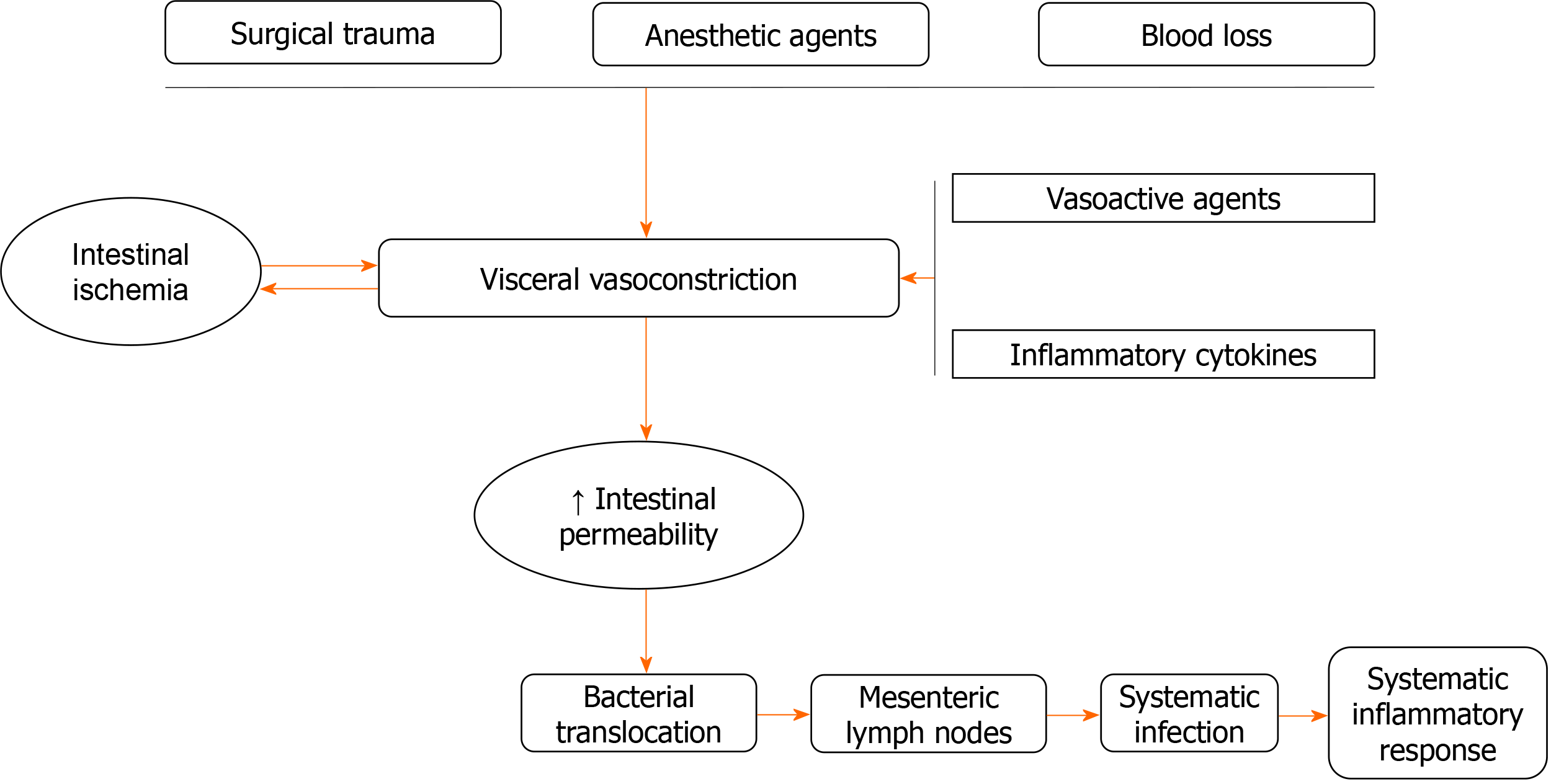
Bacterial translocation, attributed to increased intestinal permeability, can be present as early as 2 h after abdominal surgery. The grade and the prognosis depend on the severity of surgical trauma and the presence of intestinal ischemia. Studies have proposed that the potential mechanism involves visceral vasoconstriction due to surgical trauma, anesthetic agents, intestinal ischemia, and blood loss. In addition, production of vasoactive agents and release of inflammatory cytokines promote visceral vasoconstriction and therefore intestinal ischemia. Postoperative bacterial translocation is associated with systematic infection and systematic inflammatory response[6] (Figure 1).
In order to assess and confirm bacterial translocation, cultures from mesenteric lymph nodes are taken. Furthermore, blood cultures are collected from patients in the postoperative period. These samples are assessed using real-time polymerase chain reaction techniques to identify bacteria. In the case of bacterial translocation, positive cultures of samples from mesenteric lymph nodes have been reported to have slightly higher specificity[29-31]. The most common isolated bacterium associated with translocation is Escherichia coli[32]. A feasible method proposed to assess and monitor the progress of bacterial translocation is the evaluation of levels of D-lactate. D-lactate is a product of bacteria normally found in the intestinal lumen and is not metabolized by the human body. Levels of plasma D-lactate are used as a postoperative indicator of dissemination of these bacteria from the intestinal tract to the mesenteric lymph nodes, liver, spleen, and bloodstream[33].
Infections in the postoperative period are found to be more common in patients with identified bacterial translocation. A positive result in cultures taken from mesenteric lymph nodes is a more accurate prognostic factor than cultures from surgical site, intra-abdominal fluid collection, or peripheral blood samples. In other words, mesenteric lymph nodes act as beacons for progression of the infection[34]. This fact raises concerns regarding prophylactic use of antibiotics in patients undergoing abdominal surgery. Elective surgeries are performed under better circumstances and with better precautions taken. Emergency surgeries and surgeries for trauma are considered high-risk for the development of bacterial translocation, thus requiring use of antibiotics in the perioperative period[35]. Patients with advanced colorectal and gastric cancer, potentially associated with cachexia, are also in need of prophylactic use of antibiotics due to immunological imbalance induced by the progressed disease[36,37]. Prophylaxis against bacterial translocation seems to be associated with better survival rates in cancer patients who undergo surgery[7]. However, while gastric and colon resections are correlated with augmented rates of translocation, the use of antibiotics does not seem to prevent the occurrence of translocation[38,39].
The definite significance of bacterial translocation is yet to be determined, although there is evidence suggesting a causative role for sepsis. In some cases of sepsis, the causative factor was determined to be bacteria found in the intestine. In critically ill and frail patients undergoing major abdominal surgeries, those bacteria cause sepsis and even septic morbidity[40]. Sepsis is a diverse syndrome of varying severity. Late diagnosis and treatment could lead to more severe illness, even septic shock. In some cases, it may cause multi-organ failure. Severe sepsis is characterized by the presence of hypoperfusion or hypotension and by the failure of at least one organ[41]. However, this is hard to verify in most cases, as in cases of multi-organ failure occurring early postoperatively, and it is probably due to the inflammatory response causing endothelial cell activation. In contrast, late-onset multi-organ failure may be attributed to bacterial translocation, as it creates an imbalance between proinflammatory and anti-inflammatory cytokines[42]. When the septic condition in surgical patients is so severe that it causes a state of immunosuppression, multi-organ failure is responsible, with high mortality rates (reaching 50%-80%). This fact supports the theory of gut-induced sepsis[43].
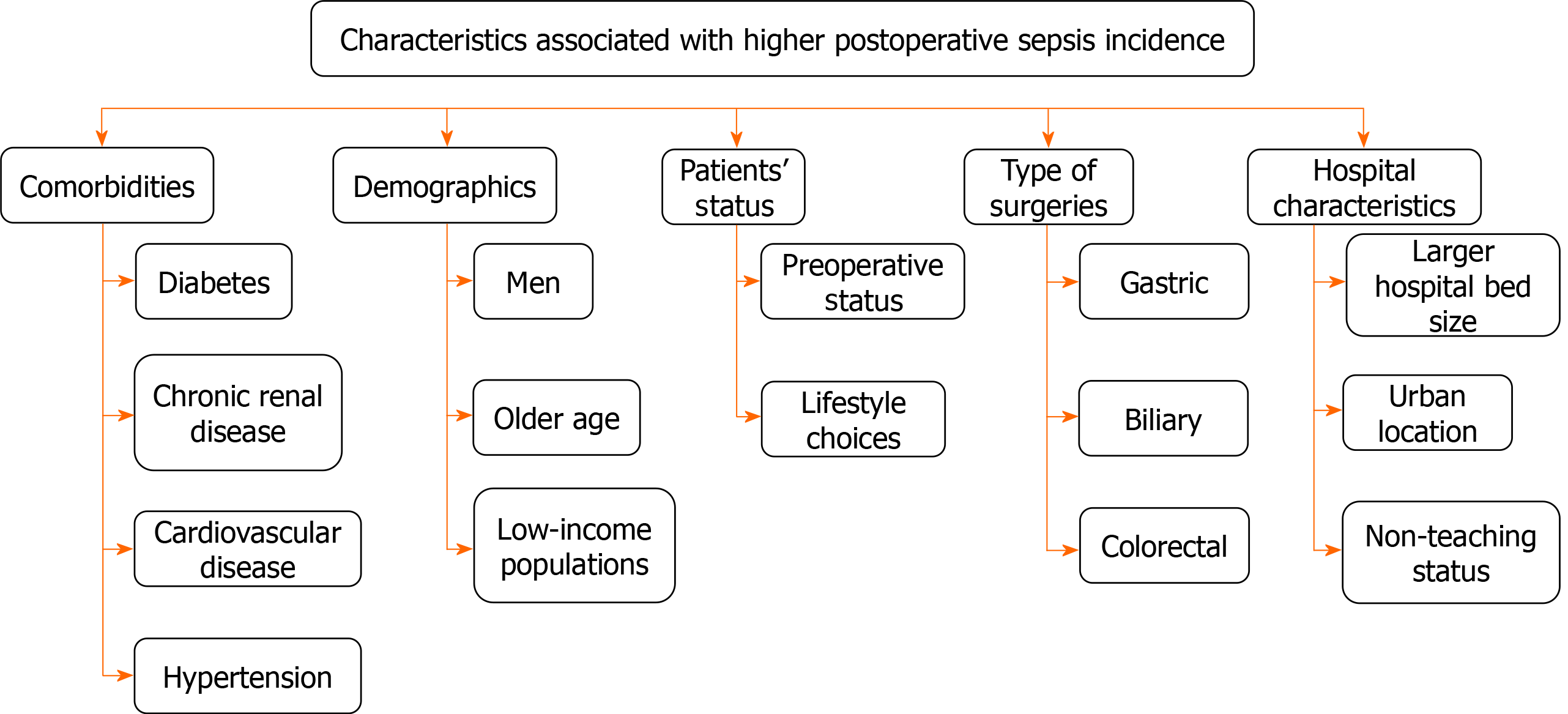
Postoperative sepsis was found to be most common among men and among older and low-income populations. Besides these characteristics, other factors regarding hospitalization are also crucial. Larger hospital bed size, urban hospital location, and non-teaching status were associated with higher postoperative sepsis rates. Comorbidities like diabetes, chronic renal disease, cardiovascular disease, and hypertension increase the risk for postoperative sepsis. Patients’ preoperative status and lifestyle choices contribute to modifying the relative risk. In addition, the type of surgery also has an impact, as gastric, biliary and colorectal surgeries were associated with relatively higher rates of postoperative sepsis, when at the same time esophageal surgery had the lowest risk of postoperative sepsis[44-46] (Figure 2). Although the incidence is rising, especially in elderly patients, mortality rates are decreasing[47].
As sepsis progresses, the release of proinflammatory cytokines triggers the production of toxic mediators that damage the endothelium, thus leading to increased capillary leakage. In addition, the release of agents that act as vasodilators, resulting in hypotension, indicates that evolution of sepsis to septic shock and subsequently to multi-organ failure requires vigilance. Early detection and therapeutic intervention could improve outcome and prognosis. Diagnosis is based on both clinical assessment and taking into consideration other factors, such as impaired consciousness and severe underlying diseases. Hypotension, oliguria, and acute altered mental status are indicative signs of severe sepsis[48]. As this condition continues to cause concern, efforts are being made to create a predictive score that will help physicians to assess probability of postoperative sepsis and mortality and to intervene sooner[49].
The cornerstone of treatment is fluid resuscitation to address hypovolemia, hypotension, and hypoperfusion. Hemodynamic stability could be restored using vasopressors when fluids alone are not adequate to maintain blood pressure. Furthermore, broad-spectrum intravenous antibiotics should be administered within the first hour. The choice of antibiotics should be guided by the suspected causative factors. Response of patients to treatment must be monitored closely, because in cases where there is no improvement, surgical intervention may be needed[50]. Novel treatments have been proposed for postoperative sepsis due to bacterial translocation, such as the use of probiotics and prebiotics. These are considered living microorganisms, which can be beneficial. Lactobacillus and Bifidobacterium are the most commonly used. They act through competition with pathogens for binding sites and nutrients. Probiotics also induce immunological response and reduce inflammation. Prebiotics are non-digestible food ingredients that promote the growth and the increase in activity of certain intestinal bacteria. These treatments have been studied well in patients with sepsis in intensive care units, with results being promising, as prophylactic use of probiotics has been shown to reduce infections, sepsis, and mortality. Another potential treatment is fecal microbiota transplantation. This is a technique that attempts to restore commensal bacteria in the intestinal epithelium. It also acts as an immunomodulatory tool, as it assists intestinal crypts to express immunological pathways. This being said, this technique prevents severe inflammation and dysregulation of intestinal lumen homeostasis[51].
The effect of increased intra-abdominal pressure on bacterial translocation has been under investigation. Abdominal surgeries are associated with increased intra-abdominal pressure. Studies have shown that bacterial translocation usually occurs at pressure levels above 14 mmHg[52]. Patients undergoing laparoscopic surgeries should be monitored, as pneumoperitoneum significantly increases intra-abdominal pressure. Randomized control trials regarding patients with colorectal cancer have concluded that there is an increase in intra-abdominal pressure, systemic endotoxemia, and bacterial translocation during both open and laparoscopic resection but without a statistically significant difference between the two groups[53]. The effect of pneumoperitoneum in translocation was also studied in animal models. It was found to provoke alterations in the inflammatory response, with milder inflammation and quicker restoration. However, there was no evidence supporting the premise that laparoscopic surgery is related to higher incidence of bacterial translocation[54-56].
Postoperative sepsis is an emerging issue that can be present as soon as a few hours postoperatively and requires immediate treatment. It may cause severe disease and result in high mortality rates, especially in frail and elderly surgical patients. Bacterial translocation is proposed as a causative factor of postoperative sepsis. This fact suggests that intestinal microbiota combined with altered homeostasis in the intestinal barrier could create a chain of events leading to sepsis, as commensal bacteria translocate to usually sterile tissues. Bacterial translocation has been noted both in laparotomy and in laparoscopic surgeries, with no significant differences regarding incidence. Proper management and early intervention are needed, based on the fundamentals of sepsis treatment. Over the past few years, data regarding novel treatments using probiotics, which assist classic treatments, have been developed. More randomized studies will be needed to clarify the role of these treatments in postoperative sepsis in the years to come.
| 1. | Fried E, Weissman C, Sprung C. Postoperative sepsis. Curr Opin Crit Care. 2011;17:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Xu X, Dong HC, Yao Z, Zhao YZ. Risk factors for postoperative sepsis in patients with gastrointestinal perforation. World J Clin Cases. 2020;8:670-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Meng M, Klingensmith NJ, Coopersmith CM. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care. 2017;23:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Komatsu S, Yokoyama Y, Nagino M. Gut microbiota and bacterial translocation in digestive surgery: the impact of probiotics. Langenbecks Arch Surg. 2017;402:401-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Qiao Z, Li Z, Li J, Lu L, Lv Y. Bacterial translocation and change in intestinal permeability in patients after abdominal surgery. J Huazhong Univ Sci Technolog Med Sci. 2009;29:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Chin KF, Kallam R, O'Boyle C, MacFie J. Bacterial translocation may influence the long-term survival in colorectal cancer patients. Dis Colon Rectum. 2007;50:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Sedman PC, Macfie J, Sagar P, Mitchell CJ, May J, Mancey-Jones B, Johnstone D. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 235] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1682] [Cited by in RCA: 2275] [Article Influence: 189.6] [Reference Citation Analysis (5)] |
| 10. | Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Coates M, Lee MJ, Norton D, MacLeod AS. The Skin and Intestinal Microbiota and Their Specific Innate Immune Systems. Front Immunol. 2019;10:2950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 13. | Buckley A, Turner JR. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb Perspect Biol. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 521] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 14. | Lee B, Moon KM, Kim CY. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J Immunol Res. 2018;2018:2645465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 15. | Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 669] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 16. | Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 17. | Katzka DA, Geno DM, Blair HE, Lamsam JL, Alexander JA, Camilleri M. Small intestinal permeability in patients with eosinophilic oesophagitis during active phase and remission. Gut. 2015;64:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 542] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 747] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 20. | Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Kataoka K. The intestinal microbiota and its role in human health and disease. J Med Invest. 2016;63:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 22. | Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 582] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 23. | Sittipo P, Lobionda S, Lee YK, Maynard CL. Intestinal microbiota and the immune system in metabolic diseases. J Microbiol. 2018;56:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Lucas C, Barnich N, Nguyen HTT. Microbiota, Inflammation and Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 25. | Mizutani S, Yamada T, Yachida S. Significance of the gut microbiome in multistep colorectal carcinogenesis. Cancer Sci. 2020;111:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Kim M, Vogtmann E, Ahlquist DA, Devens ME, Kisiel JB, Taylor WR, White BA, Hale VL, Sung J, Chia N, Sinha R, Chen J. Fecal Metabolomic Signatures in Colorectal Adenoma Patients Are Associated with Gut Microbiota and Early Events of Colorectal Cancer Pathogenesis. mBio. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28 Suppl 4:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 28. | Hegde S, Lin YM, Golovko G, Khanipov K, Cong Y, Savidge T, Fofanov Y, Shi XZ. Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Sci Rep. 2018;8:13044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Akashi K, Yokoyama Y, Mizuno T, Abe T, Fukaya M, Asahara T, Nagino M. Association Between Preoperative Muscle Mass and Intraoperative Bacterial Translocation in Patients Undergoing Hepatectomy, Pancreatoduodenectomy, and Esophagectomy. Ann Surg Oncol. 2019;26:4805-4813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Yokoyama Y, Fukaya M, Mizuno T, Ebata T, Asahara T, Nagino M. Clinical importance of "occult-bacterial translocation" in patients undergoing highly invasive gastrointestinal surgery: A review. Surg Today. 2021;51:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Schietroma M, Pessia B, Colozzi S, Carlei F, Amicucci G. Does bacterial translocation influence the postoperative infections in splenectomized patients after abdominal trauma? Surgeon. 2018;16:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | O'Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 273] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum D(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg. 1994;167:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Nieves E, Tobón LF, Ríos DI, Isaza A, Ramírez M, Beltrán JA, Garzón-Ospina D, Patarroyo MA, Gómez A. Bacterial translocation in abdominal trauma and postoperative infections. J Trauma. 2011;71:1258-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Saadia R. Trauma and bacterial translocation. Br J Surg. 1995;82:1243-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Mi L, Lin J, Zheng H, Xu X, Zhang J, Zhang D. Bacterial translocation contributes to cachexia from locally advanced gastric cancer. Hepatogastroenterology. 2012;59:2348-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Jiang Y, Lin J, Zhang D, Yu Z, Li Q, Jiang J, Li J. Bacterial translocation contributes to cachexia and its possible pathway in patients with colon cancer. J Clin Gastroenterol. 2014;48:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Koratzanis G, Giamarellos-Bourboulis EJ, Papalambros E, Giamarellou H. Bacterial translocation following intrabdominal surgery. Any influence of antimicrobial prophylaxis? Int J Antimicrob Agents. 2002;20:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Diepenhorst GM, van Ruler O, Besselink MG, van Santvoort HC, Wijnandts PR, Renooij W, Gouma DJ, Gooszen HG, Boermeester MA. Influence of prophylactic probiotics and selective decontamination on bacterial translocation in patients undergoing pancreatic surgery: a randomized controlled trial. Shock. 2011;35:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | MacFie J, O'Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 294] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 41. | Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3891] [Cited by in RCA: 4193] [Article Influence: 182.3] [Reference Citation Analysis (12)] |
| 42. | Lemaire LC, van Lanschot JJ, Stoutenbeek CP, van Deventer SJ, Wells CL, Gouma DJ. Bacterial translocation in multiple organ failure: cause or epiphenomenon still unproven. Br J Surg. 1997;84:1340-1350. [PubMed] |
| 43. | Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol. 2013;31:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Chen PY, Luo CW, Chen MH, Yang ML, Kuan YH. Epidemiological Characteristics of Postoperative Sepsis. Open Med (Wars). 2019;14:928-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Vogel TR, Dombrovskiy VY, Carson JL, Graham AM, Lowry SF. Postoperative sepsis in the United States. Ann Surg. 2010;252:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Blair LJ, Huntington CR, Cox TC, Prasad T, Lincourt AE, Gersin KS, Heniford BT, Augenstein VA. Risk factors for postoperative sepsis in laparoscopic gastric bypass. Surg Endosc. 2016;30:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Bouza C, López-Cuadrado T, Amate-Blanco JM. Characteristics, incidence and temporal trends of sepsis in elderly patients undergoing surgery. Br J Surg. 2016;103:e73-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Sartelli M, Catena F, Di Saverio S, Ansaloni L, Malangoni M, Moore EE, Moore FA, Ivatury R, Coimbra R, Leppaniemi A, Biffl W, Kluger Y, Fraga GP, Ordonez CA, Marwah S, Gerych I, Lee JG, Tranà C, Coccolini F, Corradetti F, Kirkby-Bott J. Current concept of abdominal sepsis: WSES position paper. World J Emerg Surg. 2014;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Gabriel RA, Trivedi S, Schmidt UH. A Point-Based Risk Calculator Predicting Mortality in Patients That Developed Postoperative Sepsis. J Intensive Care Med. 2020;885066620960991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3206] [Article Influence: 246.6] [Reference Citation Analysis (0)] |
| 51. | Bassetti M, Bandera A, Gori A. Therapeutic Potential of the Gut Microbiota in the Management of Sepsis. Crit Care. 2020;24:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Polat C, Aktepe OC, Akbulut G, Yilmaz S, Arikan Y, Dilek ON, Gokce O. The effects of increased intra-abdominal pressure on bacterial translocation. Yonsei Med J. 2003;44:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Schietroma M, Pessia B, Carlei F, Cecilia EM, Amicucci G. Intestinal permeability, systemic endotoxemia, and bacterial translocation after open or laparoscopic resection for colon cancer: a prospective randomized study. Int J Colorectal Dis. 2013;28:1651-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Horattas MC, Haller N, Ricchiuti D. Increased transperitoneal bacterial translocation in laparoscopic surgery. Surg Endosc. 2003;17:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Wang G, Wu R, Guo F, Liu W, Chen X, Yu Q. Effects of carbon dioxide pneumoperitoneum on the inflammatory response and bacterial translocation in intraabdominal infection. J Laparoendosc Adv Surg Tech A. 2014;24:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Shan CX, Ni C, Qiu M, Jiang DZ, Li M. Influence of laparoscopy vs. laparotomy on bacterial translocation and systemic inflammatory responses in a porcine model with peritonitis. J Invest Surg. 2014;27:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kordzaia D S-Editor: Fan JR L-Editor: A P-Editor: Li JH