Published online Oct 28, 2025. doi: 10.4329/wjr.v17.i10.108804
Revised: June 16, 2025
Accepted: September 17, 2025
Published online: October 28, 2025
Processing time: 187 Days and 19.9 Hours
Imaging plays a crucial role in the evaluation of hepatocellular carcinoma (HCC) treatment response. Contrast enhanced computed tomography and magnetic resonance imaging with extra-cellular or hepatobiliary contrast agents are the imaging techniques of choice. Contrast enhanced ultrasound is a promising tech
Core Tip: Computed tomography and magnetic resonance imaging play a crucial role in the evaluation of hepatocellular carcinoma (HCC) treatment response. Contrast en
- Citation: Agnello F, Taibbi A, Galia M, Orlando A, Gagliardo C, Bartolotta TV. Hepatocellular carcinoma treatment response: Imaging findings and criteria. World J Radiol 2025; 17(10): 108804
- URL: https://www.wjgnet.com/1949-8470/full/v17/i10/108804.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i10.108804
Hepatocellular carcinoma (HCC) is the most common primary tumor, and the second liver tumor after metastasis. HCC usually occurs in cirrhotic patients. Imaging plays a crucial role in the diagnosis of HCC, in the selection of therapeutic options, and in the evaluation of treatment response[1]. Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) with extra-cellular or hepatobiliary contrast agents are commonly used.
CT and MRI show no statistical differences in their diagnostic accuracy except for HCC treated with lipiodol-based trans-arterial chemoembolization (TACE)[2].
Contrast enhanced ultrasound (CEUS) is a relatively novel and promising technique, although its use is found primarily in academic centers. Scientific societies have proposed several imaging criteria to evaluated HCC treatment response, including various versions of Response Evaluation Criteria in Solid Tumors (RECIST) and, more recently, Liver Imaging Reporting and Data System (Li-RADS) criteria.
The purpose of this paper is to describe radiological techniques, imaging findings after HCC treatment, the criteria of response evaluation and their challenges. The utility of the structured report is also analyzed.
Several treatment options for HCC are currently available, and can be broadly divided into surgical, locoregional, and systemic therapies[1]. The choice depends on tumor burden, liver function, and patient performance status[1,2].
In Europe and North America, the Barcelona Clinic Liver Cancer classification is a universally accepted system to stratify the survival of patients with HCC into five stages (0, A, B, C, and D) and select treatment[1]. This system takes into account multiple factors, including liver function, patient performance status, and tumor-specific factors (e.g., size and number)[1].
Image-based artificial intelligence models can help to select therapeutic options by extracting quantitative imaging features and evaluating the relationships between these features and clinical outcomes[3].
Hepatic resection is a curative treatment, and is the treatment of choice for localized HCC without underlying cirrhosis[1].
A pre-operative multidisciplinary evaluation of tumor characteristics and nontumor factors is recommended in cirrhotic patients; the former include tumor number and size, location, and vascular invasion; the latter include hepatic function, portal hypertension, remnant hepatic volume, and function and prediction of early post-operative patient outcome[1,4].
Advances in minimally invasive liver surgery via laparoscopic/robotic approaches have improved perioperative morbidity and mortality after hepatic resection, compared to open surgery[4-6].
Locoregional therapies include local ablative therapies and trans-arterial therapies.
Local ablative therapies include thermal ablation (e.g., radiofrequency ablation, microwave ablation, and cryoablation), radiation segmentectomy [or selective trans-arterial radioembolization (TARE)], and external beam radiation therapy.
Local ablative therapies are recommended in patients with single < 3 cm HCC who are ineligible for or refuse surgery[1]. Ablation and embolization can also be used to downstage HCCs exceeding Milan criteria, and bridge to liver transplantation[1,7]. Patients within Milan criteria after HCC downstaging become potential candidates for liver transplantation[1].
Ablation should be favored over hepatic resection and intra-arterial therapies in patients with poor hepatic function, or centrally located tumors[4].
Moreover, irreversible electroporation, a recently introduced ablative technique, can be used to treat HCCs close to major biliary structures or vessels since it is not affected by the heat-sink effect[2].
HCC ablation and resection achieve have a 5-year survival rates above 50%[8].
Trans-arterial therapies [TACE, trans-arterial embolization (TAE) and TARE] are non-curative treatments, and are recommended in patients with intermediate BCLC stage[1].
TACE is the primary treatment option and consists of trans-arterial administration of embolizing particles and a mixture of chemotherapy (Doxorubicin or Cisplatin in most cases) and iodized oil (Lipiodol, Guerbert, France)[2].
TAE induces tumor necrosis thought occlusion of tumor-feeding arteries.
TAE should be performed in patients with poor liver function or contraindications to chemotherapy, such as heart failure[2].
Transarterial (chemo) embolization and radiation therapy (n = 659) have a 5-year survival rates of approximately 30% and 18% respectively[7].
Systemic therapies are indicated for patients with unresectable HCCs (BCLC C and B stage), and those with earlier stage HCCs progressing despite locoregional therapy[1]. Systemic therapies are divided into two groups: Antiangiogenic targeted therapies and immune checkpoint inhibitors[1]. The former includes multi-target tyrosine kinase inhibitors (sorafenib, lenvatinib, cabozantinib, and regorafenib) and monoclonal antiangiogenic antibodies (ramucirumab and bevacizumab).
The SHARP trial have reported that patients with unresectable HCC on sorafenib had a nearly 3 months longer median survival compared with patients on placebo[9].
The latter include inhibitors of programmed death 1 (pembrolizumab and nivolumab) or its ligand (PD-L1) (dur
Combination immunotherapies (e.g., atezolizumab plus bevacizumab, durvalumab plus tremelimumab) have im
The IMbrave trial have shown that atezolizumab combined with bevacizumab resulted in better median progression-free survival outcomes than sorafenib (6.8 months vs 4.3 months)[11].
Combination therapies and transitions between different therapies during follow-up due to tumor progression or response can be considered in some patients and require multidisciplinary discussion[1]. Perioperative or preoperative neoadjuvant therapy can increase tumor resectability and reduce postoperative recurrence[1].
At this time, there is no consensus on the timing for imaging follow-up to evaluate treatment response and imaging modality (CT, MRI or CEUS). Differences between imaging techniques are summarized in Table 1.
| CT | MRI | CEUS | |
| Availability | Broadly available | Limited accessibility | Limited accessibility |
| Costs | Less expensive | High | Less expensive |
| Radiation exposure | Moderate | None | None |
| Time taken | Fast | Long | Fast |
| Advantages | Good soft tissue contrast | High soft tissue contrast; Problem-solving technique | High temporal resolution; No nephrotoxicity of contrast agents |
| Limits | Nephrotoxicity of contrast agents; Radiation exposure | Claustrophobia; Metal and medical implants (e.g., peace-makers) due to magnetic field pulses | High level of operator expertise; Deep lesion location Patient obesity |
The first follow-up is useful to detect residual tumor and postprocedural complications. Subsequent follow-up is essential to detect tumor recurrence or occurrence of new foci of HCC[12,13].
Imaging follow-up response after locoregional therapies is commonly evaluated first at one month, then at 3 months and every 3 months afterwards[13]. CEUS can be performed immediately following ablation to detect residual tumor and the need for re-treatment within the same interventional session[14].
Imaging follow-up after TARE and radiation segmentectomy is commonly performed at 3 months after treatment, and every 3-6 months thereafter[1]. On first imaging follow-up treated HCC and surrounding hepatic parenchyma can show an increased hepatic arterial phase (HAP) hyperenhancement, which may be misinterpreted as residual viable tumor[13].
Assessment of treatment response requires a comparison not only between the current and prior examination, but also with older examinations to avoid misinterpretations, as in the case of slowly growing HCCs[15].
CT is a fundamental imaging technique for the study of cirrhotic patients. It is accurate in the detection of viable tissue in treated HCC and post-procedural complications, and is quick, broadly available, and safe[16-18]. Concerns are related to CT radiation dose and nephrotoxicity of contrast agents[19-23].
CT protocol includes unenhanced phase, late HAP, portal venous phase (PVP), and 3-minute delayed phase (DP)[24,25]. HAP is obtained 35 seconds after start of contrast injection or, when bolus-tracking technique is used, 18 seconds after the trigger threshold (100 HU-120 HU) is reached at the level of the suprarenal abdominal aorta. PVP is obtained 60-70 seconds after the start of contrast injection. The unenhanced phase helps differentiate normal posttreatment changes (e.g., spontaneous hyperattenuating areas in HCC treated with radiofrequency ablation) from abnormal enhancement of viable tumor. Contrast material should have a minimum iodine concentration of 300 mg per millimeter[24,25]. The injection of a saline solution is strongly recommended to decrease the dose of contrast material remaining in the dead space, and the arrival time of contrast material in the liver[26]. Eight-detector row CT scanner and 5-mm slice thickness are the minimal technical requirements for obtaining high-quality images[24,25]. The current international mainstream trend is to recommend the application of 64-detector row CT scanner combined with a slice thickness of 1-5 mm for abdominal examination.
MRI is an accurate, problem-solving technique for the evaluation of treatment response, though it has limited accessibility and high costs. MRI is recommended in patients with questionable areas of residual enhancement on CT after treatment, and in patients treated with lipiodol-based TACE since beam hardening artifacts can impair detection of arterially enhancing areas at CT. A 1.5-T or a 3-T MRI scanner is recommended[24,25].
MRI protocol of the liver is designed to evaluate hepatic parenchyma and vasculature, and detect and characterize focal liver lesions. Thus, standard protocol includes T2-weighted two-dimensional turbo/fast spin-echo sequences (with and without fat suppression), diffusion-weighted (DW) sequences with at least three b values (e.g., 150, 600 and
Contrast materials differ in the approved dose: 1 mmol/kg body weight of an extracellular contrast agent or
The acquisition of a triple arterial phase may improve the detection of arterial hyperenhancement of hepatic lesions, with increased lesion contrast noise ratio, compared to standard single-phase arterial phase[28].
T2-weighted and DW sequences can be obtained between the transitional and hepatobiliary phase to reduce the total examination time[29-31].
CEUS is a safe, well-tolerated and cost-effective imaging technique to evaluate treatment response[31-34]. The advantages of CEUS include no ionizing radiation, no nephrotoxicity, and higher temporal resolution compared to CT and MRI[31-33]. However, CEUS requires a high level of operator expertise. CEUS quality and diagnostic confidence can be nega
Usually, CEUS is performed with purely intravascular microbubble contrast agents, which allow real-time visualization of blood flow in the level of tissue perfusion[33]. There are three contrast agents for CEUS of the liver: Sonovue (sulfur hexafluoride), Bracco SpA, Milan, Italy; sonazoid (perfluorobutane), Daiichi-Sankyo, GE Tokyo, Japan, and definity/Luminity (octafluoropropane), Lantheus Medical, Billerica, MA, United States[35]. Of note, unlike sonovue and lantheus, sonazoid is uptaken by Kupffer cells, resulting in a liver specific phase named post-vascular phase (6-10 minutes to over 60 minutes after contrast injection)[34]. At time of this writing, sonazoid is commercially available only in Japan and Norway[34,36]. United States scanners must be provided with low mechanical index imaging, which promotes microbubble oscillation and minimizes bubble disruption[35].
Digital cine-loops are stored during the hepatic arterial (5-40 seconds from the start of contrast injection), hepatic venous (55-90 seconds), and late (delayed) phase (> 120 seconds). Wash-in phase is monitored for 30-40 seconds to evaluate the peak enhancement.
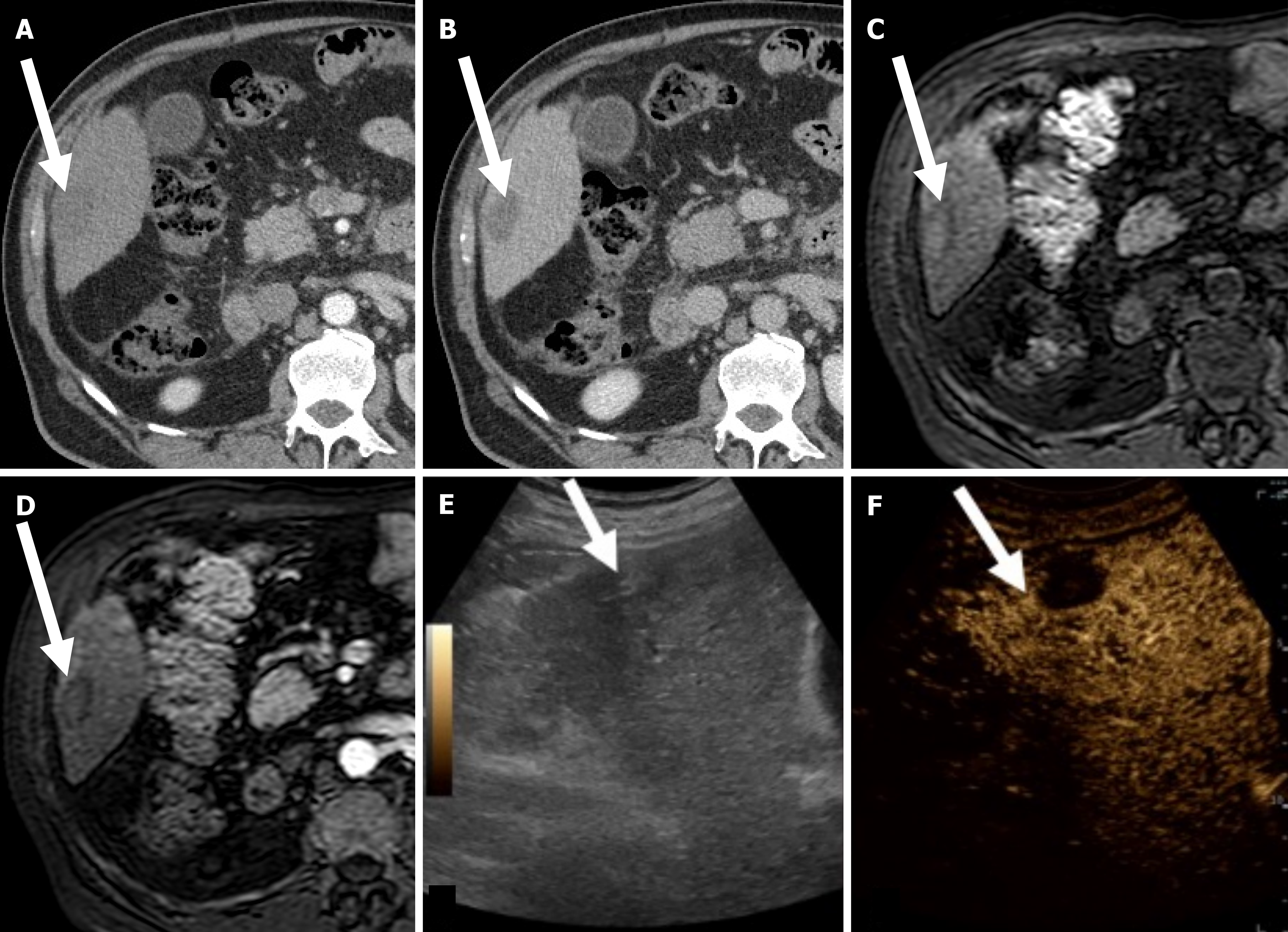
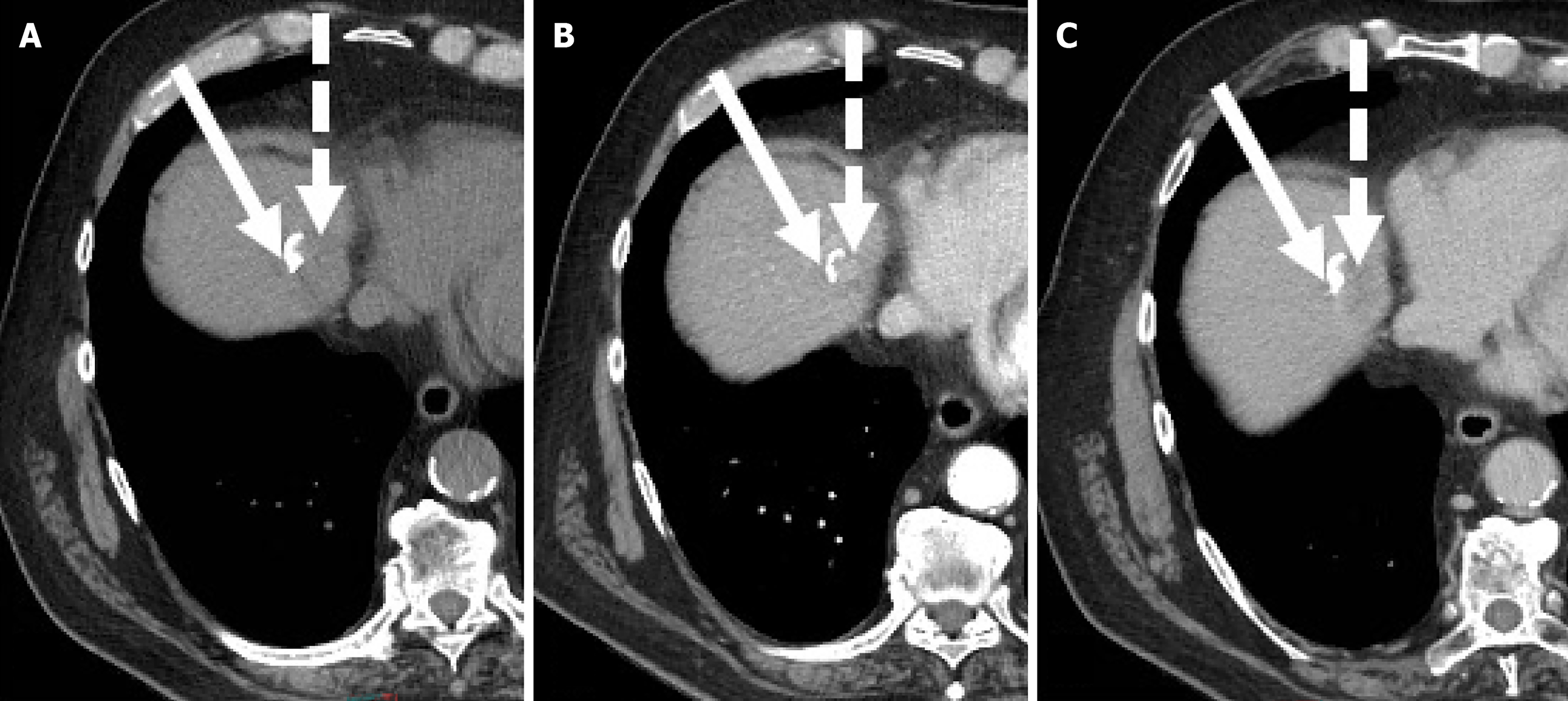
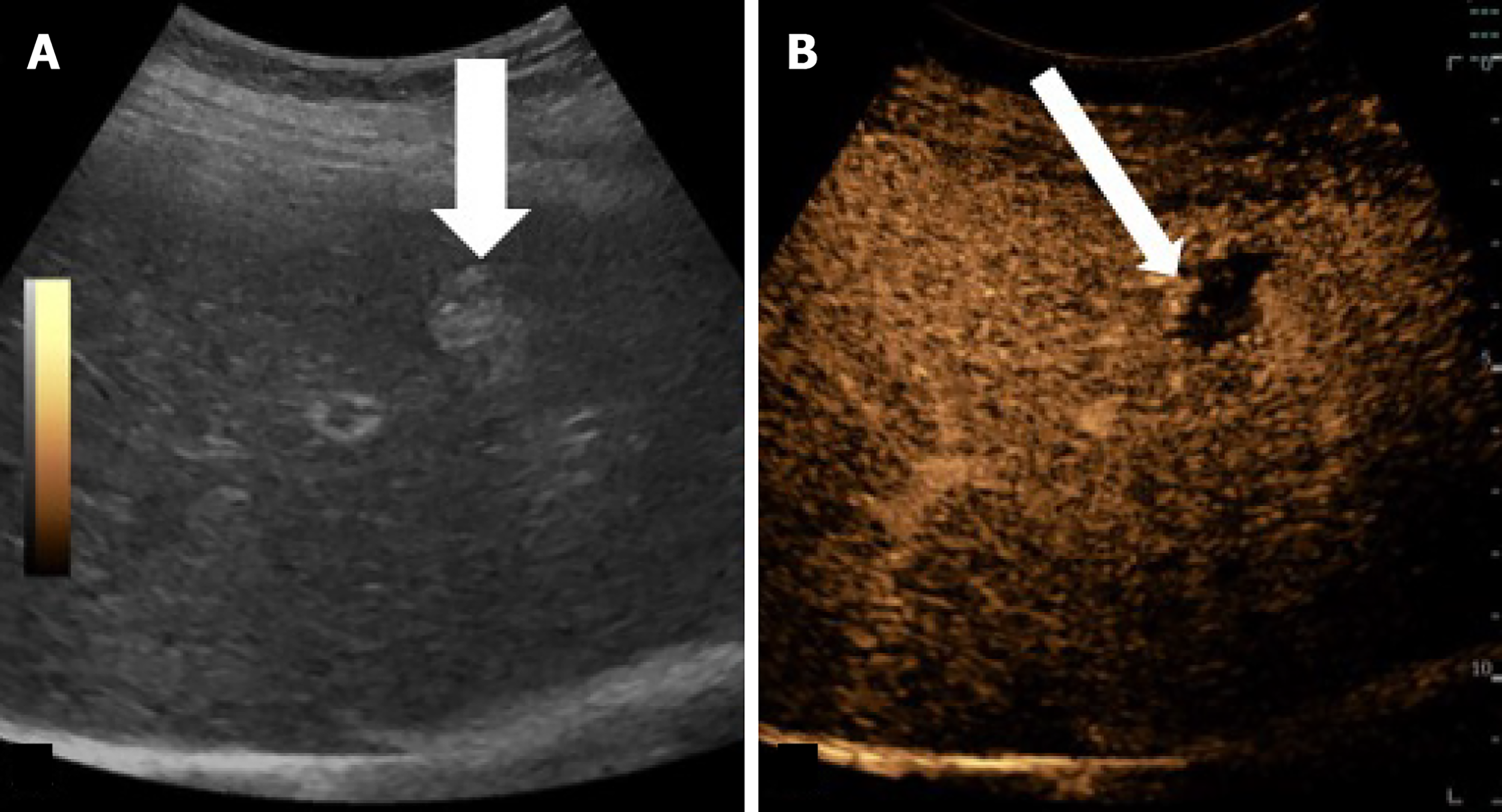
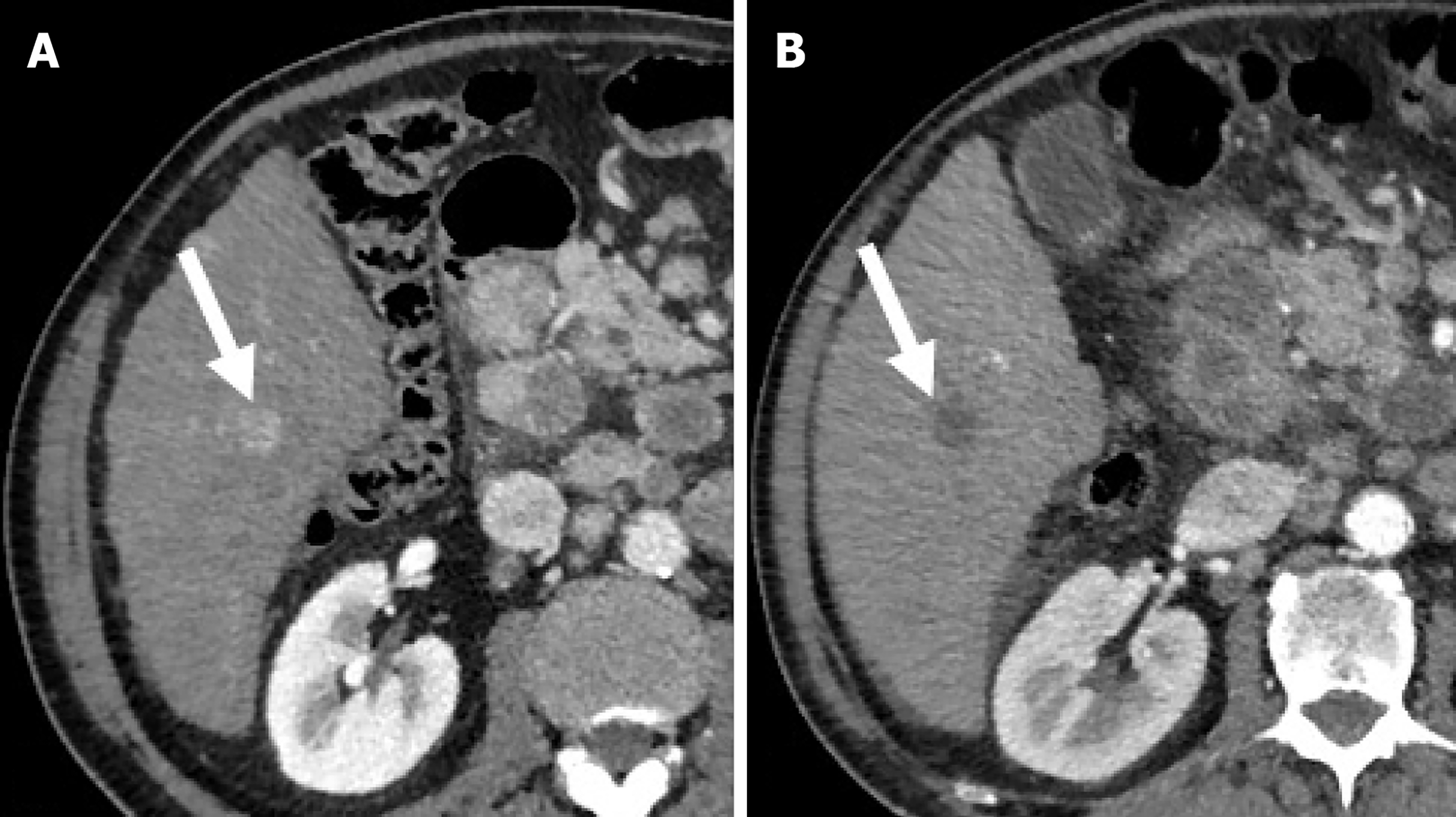
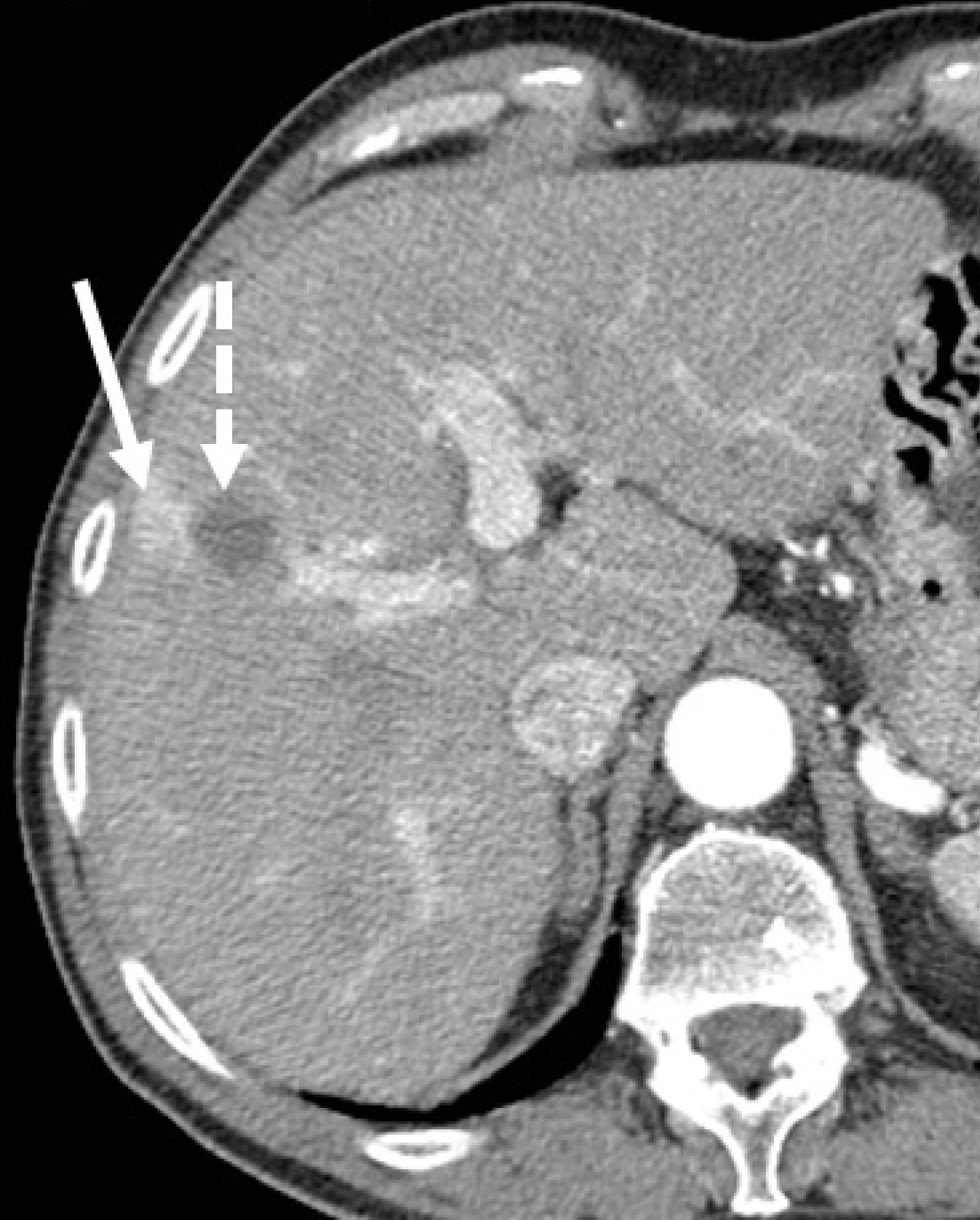
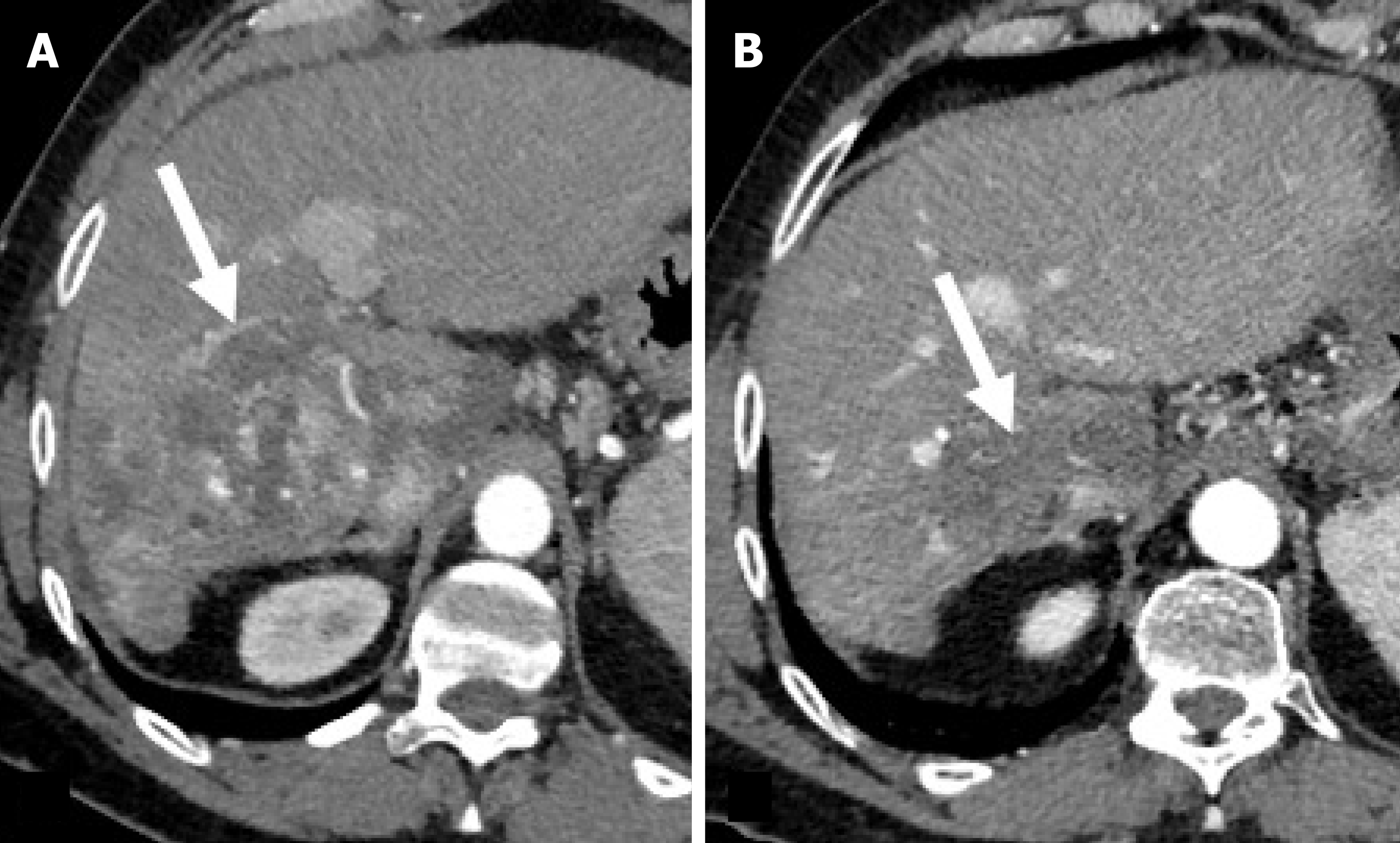
Imaging evaluation of treatment response is crucial for management decisions in clinical care. As a general rule, absence of arterial enhancement suggests a successful HCC treatment (Figure 1), whereas any nodular arterially enhancing area is suspicious for viable tissue (Figures 2, 3 and 4).
Several treatment response criteria have been proposed over time. Traditionally, RECIST are used to evaluate tumor response to therapy. RECIST criteria were first published in 2000 (version 1.0), and were upgraded in 2009 (version 1.1)[37]. These criteria analyze tumor change in size of target lesions as an indicator of treatment response as they are designed to evaluate response to cytotoxic agents. A target lesion must be measurable (e.g., the longest diameter > 1 cm) and suitable for repeated measurement.
RECIST 1.1 updated version defines four response categories: Complete response (disappearance of all target lesions), partial response (> 30% decrease in sum of longest diameters of target lesions), progressive disease (> 20% increase in sum of longest diameters of target lesions with an absolute increase of ≥ 5 mm or the appearance of one or more new lesions), and stable (none of the above). The pretreatment sum of longest diameters of target lesions is the reference for defining the partial response category, while the smallest sum of longest diameters of target lesions since the start of therapy is the reference for evaluating disease progression[37].
However, RECIST 1.1 does not always represent an appropriate tool for HCC treatment response assessment because the goal of most HCC treatments is to destroy tumor vascularity and induce tumor necrosis with rare tumor shrinkage[37-40]. For instance, in patients undergoing thermal ablation, a successfully treated zone should be 5-10 mm larger compared to the preexisting HCC, and decrease in size on delayed follow-up[13].
To overcome these limitations, Modified RECIST (mRECIST) has been proposed. Unlike RECIST, mRECIST measures the largest diameter of the viable portion of a target lesion. The viable portion is defined as an area that shows en
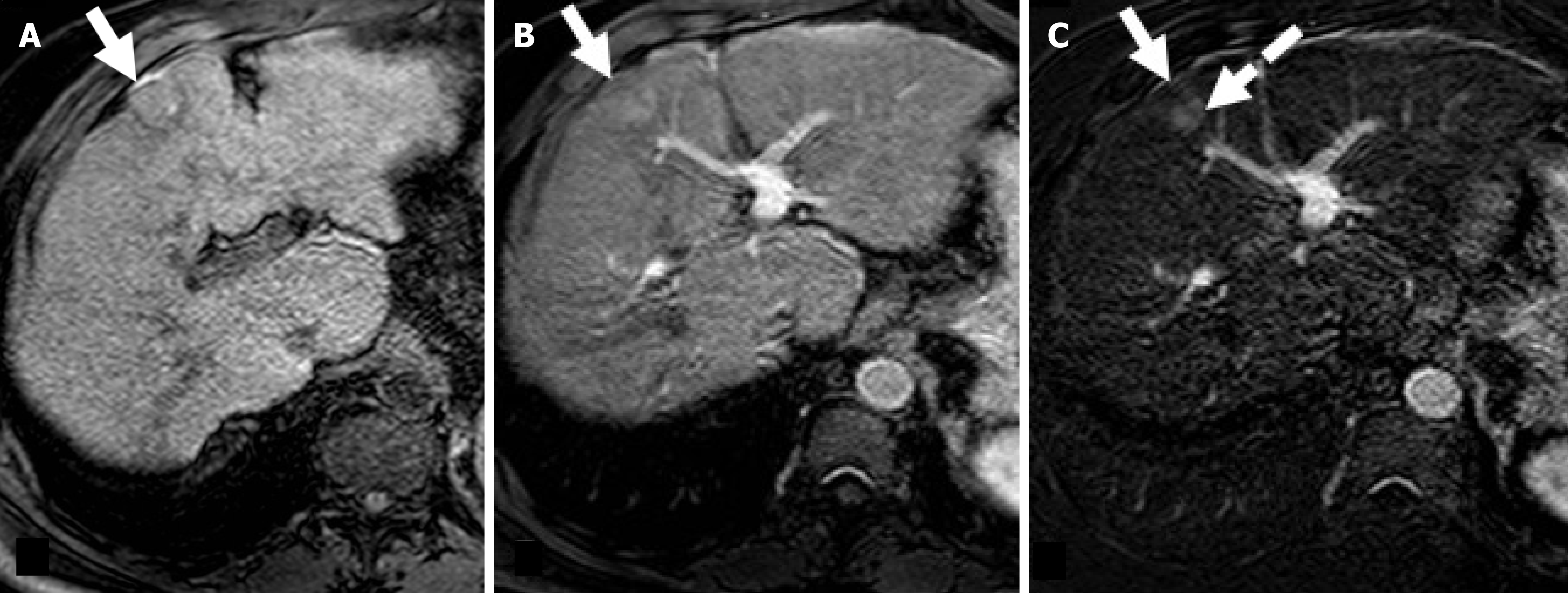
Of note, current ASSLD and EASL guidelines recommend applying mRECIST to evaluate tumor response to locoregional therapies[1,41]. By contrast, RECIST 1.1 and mRECIST are recommended to evaluate response after systemic therapy. This can be surprising because mRECIST better detects HCC response to target therapies compared to RECIST 1.1[42-46]. Indeed, target therapies are cytostatic and primarily decrease tumor vascularity rather than tumor size, as well as induce tissue necrosis or cavitation[43-45] (Figure 5). However, RECIST 1.1 and mRECIST have excellent concordance in the assessment of the disease progression, and in the differentiation between progressors and non-progressors; the latter represents the most relevant parameter for clinical decision making in clinical practice[39]. A possible explanation of this finding is that increase in vascularization leads to an increase in in lesion size[39]. Progressive disease on therapy suggests treatment failure[46]. In other words, current therapy is terminated, and alternative therapies are initiated[46].
EASL criteria classifies HCC treatment response in four categories thought a measure of the largest axial bidimensional diameters or the enhanced area of the lesion in HAP or PVP[47].
Thus, different partial response (≥ 50% decrease in cross-product of 2 Largest diameters of all measurable lesion) and progressive disease (≥ 25% increase in size of one or more measurable lesion)[47].
Choi criteria incorporate tumor size and attenuation changes after systemic therapies and TARE[48].
Four response categories are defined: Complete response (disappearance of target lesions); partial response (10% decrease in tumor size or a 15% decrease in tumor attenuation), stable disease (no criteria for partial response or pro
Choi criteria showed better correlation with clinical outcomes and identified a higher rate of early responders com
Chun criteria, also known as MD Anderson's morphological criteria, highlight the importance of morphological changes in addition to size to lesion attenuation ed rim enhancement, in assessing response to bevacizumab in patients with metastatic colorectal cancer[49]. However, these criteria can not be used in patients with HCC[49].
Both RECIST 1.1 and mRECIST cannot accurately evaluate the response to immunotherapies[50]. Because of their peculiar mechanisms, immunotherapies can cause unusual response patterns on imaging. Some patients can exhibit a transient progression of target lesions and/or the appearance of new lesions followed by shrinkage of tumor burden (pseudoprogression)[46]. These findings can be misinterpreted as progressive disease using RECIST 1.1 and mRECIST. The principal clinical implication of pseudoprogression is that therapies are continued. Thus, several immune-related response criteria, including immune-modified RECIST, and immune-related RECIST have been proposed[51,52]. In these, the definition of immune progressive disease is modified, and requires confirmation of progression on a 4-8 weeks imaging follow-up[51,52]. The definitions of immune complete response, immune partial response, and immune stable disease are identical to those in RECIST 1.1[53-55].
The LI-RADS had proposed a treatment response algorithm (Treatment Response [LI-RADS TRA]) to local-regional therapies and surgical resection[25,53]. LI-RADS-TRA was first published on 2017 and uploaded on 2024[25].
LI-RADS Treatment Response (LR-TRA) cannot be used after systemic therapies.
Unlike other systems, LI-RADS TRA v2024 evaluates response to therapy at a lesion-level; in other words, each treated HCC is evaluated separately[25,53].
Since the type of therapy influences imaging post-treatment appearance, LI-RADS TRA v2024 provides two separate cores: Nonradiation TRA for assessing TRA after nonradiation-based LRT or surgical resection, and Radiation TRA for assessing TRA after radiation-based LRT (i.e., transarterial radioembolization and external beam radiation therapy[53].
Based on the probability of residual or recurrent disease at imaging, non-radiation LR-TRA defines three categories: LR-nonviable, LR-equivocal and LR-viable[55]. LR-TR non evaluable is assigned when treatment response cannot be evaluated because of imaging low quality or inadequate technique (e.g., absence of HAP)[53].
Mass-like enhancement (any degree, any phase) within or along the margins of a treated HCC favors LR-viable category while absent enhancement favors LR-nonviable category[55]. Mass-like enhancement can be nodular, smooth, or irregular[55]. LR-nonviable HCC can also show smooth, perilesional enhancement; or perilesional perfusion changes[53] (Figure 6). For instance, at first follow-up after radiofrequency ablation, treated HCC can show a thin and peripheral arterially enhancing rim, which is due inflammatory reaction to thermal necrosis and resolve with time[12].
If enhancement is unsure, a treated HCC is categorized as LR-equivocal.
Wash-out appearance is not a criteria for LR-viable category because of its low sensitivity to detect viable tumor[53].
Ancillary features (i.e., restricted diffusion and mild-moderate T2-hyperintensity) can be used to upgrade LR-equivocal to LR-viable, and reduces the use of the LR-TR equivocal category[53-57].
DW imaging is a functional imaging technique that analyzes post-treatment tumor changes by evaluating water molecules diffusion which is restricted in residual or recurrent tissue[58]. Successful HCC treatment produces an increase in the apparent diffusion coefficient value[58].
Hepatobiliary phase hypointensity helps to characterize an arterially enhancing area at the periphery of a treated HCC: Hypointensity suggests viable tissue while isointensity suggests a perfusion abnormality[58]. However, hepatobiliary phase hypointensity can not be used as an ancillary feature because no sufficient data are currently available[59].
Radiation LR-TRA defines three categories: LR-viable, LR-non progressing, LR-nonviable, and LR-TR non evaluable[53].
Any new or increasing in size mass-like enhancement (any degree, any phase) within or along the margins of a treated HCC favors LR-viable category while any residual (stable or decreasing in size) mass-like enhancement favors LR-stable category[53].
The rationale is that radiation therapies can induce delayed HCC necrosis and post-treatment enhancement can persist for months[56,57] (Figure 7).
Pseudoprogression indicates an increase in tumor size after TARE and persist for months. In this case, the absence of enhancement indicates tumor necrosis[59].
Tumor enhancement should not be confused with radiation induced-changes that occur in liver parenchyma surrounding the treated area[59].
No enhancement within or along the margins of a treated HCC favors LR-nonviable category[53]. Differences between RECIST 1.1, mRECIST, and LR-TRA are summarized in Table 2.
| RECIST 1.1 | mRECIST | LR-TRA | |
| Evaluation targets | Largest diameter of target lesions | Largest diameter of the viable portion of target lesions | Mass-like enhancement; Ancillary Li-RADS features |
| Advantages | - | Evaluation of post-treatment changes in lesion vascularity | Response to therapy is evaluated at a lesion-level; Pseudoprogression is not taken into account; Evaluation of response to radiotherapies |
| Limits | Post-treatment changes in lesion vascularity are not evaluated; Evaluation of response to immunotherapies | Evaluation of response to immunotherapies | Evaluation of response to systemic or immunotherapy; Hepatobiliary phase is not an ancillary feature |
m-RECIST and LR-TR criteria were developed for CT and MRI but were successfully applicable to CEUS[35,60].
Finally, changes in uptake of fluorodeoxyglucose at Positron Emission Tomography (PET) CT can be used to evaluate HCC treatment response[61]. PET Response Criteria in Solid Tumors (PERCIST 1.0) classifies treatment response into four categories: Complete metabolic response, partial metabolic response, stable metabolic disease, or progressive metabolic disease[61].
Radiological reports are crucial to plan patient management and evaluate treatment response[62-64]. Traditional non structured, free narrative reports contain variable information and terminology, and are sometimes difficult to interpret for the referring physicians[65]. An unclear report can impair the selection of appropriate treatment although imaging diagnosis is correct[63,64]. Feinberg has reported that narrative reports were associated with an overestimation of treatment response in comparison to RECIST 1.1, with the largest overestimation among patients with complete responses[65].
Structured reporting is a method of communicating imaging data with the use of standardized language and for
Its usage improves both the evaluation of clinical question and the confidence of referring physicians. Scientific societies have created templates for cirrhotic patients[23,64]. Template should contain the following paragraphers clinical history, technique, comparison with prior imaging studies, findings and impression[62-64]. The impression should clearly explain imaging findings to referring physicians and provide a diagnosis or differential diagnosis and recommendations, without use of radiology jargon[64,65]. For each HCC, radiologists should describe location according to Couinaud classification, maximum diameter, imaging features (e.g., arterial phase enhancement), and changes from previous examinations[66-68]. The use of a standardized report is a more time-consume compared with narrative report.
Nowadays, several treatment options for HCC are available. Imaging plays a crucial role in the evaluation of HCC response to treatment. Contrast enhanced CT and MRI are the main imaging techniques. CEUS is a promising technique. Several imaging response criteria have been proposed by scientific societies, including RECIST, mRECIST, and Li-RADS. The choice of imaging criteria depends on treatment modality. Radiologists should be familiar with the HCC appearance after treatment and should be able to differentiate normal post-treatment changes from residual or recurrent tumor. However, imaging criteria have high specificity and low sensitivity for the depiction of residual or recurrent tumor[12]. Thus, evaluation of post-treatment pathological response (e.g., post-TACE or radiofrequency ablation) in patients undergoing resection or liver transplant is important to evaluate treatment efficacy[12]”.
Changes in serological markers levels such as α-Fetoprotein can also be useful to evaluate treatment response[69].
The use of structured reporting must be increased to improve both the evaluation of clinical questions and the confidence of referring physicians.
| 1. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 900] [Cited by in RCA: 1119] [Article Influence: 373.0] [Reference Citation Analysis (23)] |
| 2. | Horvat N, de Oliveira AI, Clemente de Oliveira B, Araujo-Filho JAB, El Homsi M, Elsakka A, Bajwa R, Martins GLP, Elsayes KM, Menezes MR. Local-Regional Treatment of Hepatocellular Carcinoma: A Primer for Radiologists. Radiographics. 2022;42:1670-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Hsieh C, Laguna A, Ikeda I, Maxwell AWP, Chapiro J, Nadolski G, Jiao Z, Bai HX. Using Machine Learning to Predict Response to Image-guided Therapies for Hepatocellular Carcinoma. Radiology. 2023;309:e222891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of autoimmune hepatitis. J Hepatol. 2025;83:453-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 5. | Coletta D, De Padua C, Parrino C, De Peppo V, Oddi A, Frigieri C, Grazi GL. Laparoscopic Liver Surgery: What Are the Advantages in Patients with Cirrhosis and Portal Hypertension? Systematic Review and Meta-Analysis with Personal Experience. J Laparoendosc Adv Surg Tech A. 2020;30:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Molina V, Sampson-Dávila J, Ferrer J, Fondevila C, Díaz Del Gobbo R, Calatayud D, Bruix J, García-Valdecasas JC, Fuster J. Benefits of laparoscopic liver resection in patients with hepatocellular carcinoma and portal hypertension: a case-matched study. Surg Endosc. 2018;32:2345-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Gamblin TC, Geller DA. Downstaging hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1466-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Cassinotto C, Nogue E, Durand Q, Panaro F, Assenat E, Dohan A, Malafaye N, Guiu B, Molinari N. Life expectancy of patients with hepatocellular carcinoma according to the upfront treatment: A nationwide analysis. Diagn Interv Imaging. 2023;104:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10504] [Article Influence: 583.6] [Reference Citation Analysis (9)] |
| 10. | Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1:EVIDoa2100070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 835] [Article Influence: 208.8] [Reference Citation Analysis (0)] |
| 11. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 1143] [Article Influence: 285.8] [Reference Citation Analysis (0)] |
| 12. | Agnello F, Salvaggio G, Cabibbo G, Maida M, Lagalla R, Midiri M, Brancatelli G. Imaging appearance of treated hepatocellular carcinoma. World J Hepatol. 2013;5:417-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Mendiratta-Lala M, Masch WR, Shampain K, Zhang A, Jo AS, Moorman S, Aslam A, Maturen KE, Davenport MS. MRI Assessment of Hepatocellular Carcinoma after Local-Regional Therapy: A Comprehensive Review. Radiol Imaging Cancer. 2020;2:e190024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Mauri G, Porazzi E, Cova L, Restelli U, Tondolo T, Bonfanti M, Cerri A, Ierace T, Croce D, Solbiati L. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Cappello G, Romano V, Neri E, Fournier L, D'Anastasi M, Laghi A, Zamboni GA, Beets-Tan RGH, Schlemmer HP, Regge D. A European Society of Oncologic Imaging (ESOI) survey on the radiological assessment of response to oncologic treatments in clinical practice. Insights Imaging. 2023;14:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Marin D, Nelson RC, Rubin GD, Schindera ST. Body CT: technical advances for improving safety. AJR Am J Roentgenol. 2011;197:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to Common Questions About the Use and Safety of CT Scans. Mayo Clin Proc. 2015;90:1380-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | McCollough CH, Guimarães L, Fletcher JG. In defense of body CT. AJR Am J Roentgenol. 2009;193:28-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | ACR Committee on Drugs and Contrast Media (2018). ACR manual on contrast media, v10.3. American College of Radiology. Available from: https://www.acr.org/Clinical-Resources/Clinical-Tools-and-Reference/Contrast-Manual. |
| 20. | Orlacchio A, Guastoni C, Beretta GD, Cosmai L, Galluzzo M, Gori S, Grassedonio E, Incorvaia L, Marcantoni C, Netti GS, Passamonti M, Porta C, Procopio G, Rizzo M, Roma S, Romanini L, Stacul F, Casinelli A. SIRM-SIN-AIOM: appropriateness criteria for evaluation and prevention of renal damage in the patient undergoing contrast medium examinations-consensus statements from Italian College of Radiology (SIRM), Italian College of Nephrology (SIN) and Italian Association of Medical Oncology (AIOM). Radiol Med. 2022;127:534-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 21. | van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, Thomsen HS. Post-contrast acute kidney injury - Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2018;28:2845-2855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 334] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 22. | Davenport MS, Perazella MA, Yee J, Dillman JR, Fine D, McDonald RJ, Rodby RA, Wang CL, Weinreb JC. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 336] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 23. | Smith-Bindman R, Chu PW, Azman Firdaus H, Stewart C, Malekhedayat M, Alber S, Bolch WE, Mahendra M, Berrington de González A, Miglioretti DL. Projected Lifetime Cancer Risks From Current Computed Tomography Imaging. JAMA Intern Med. 2025;185:710-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 118] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 24. | Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 25. | Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 857] [Article Influence: 107.1] [Reference Citation Analysis (1)] |
| 26. | Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256:32-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 762] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 27. | Tanimoto A, Lee JM, Murakami T, Huppertz A, Kudo M, Grazioli L. Consensus report of the 2nd International Forum for Liver MRI. Eur Radiol. 2009;19 Suppl 5:S975-S989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Ikram NS, Yee J, Weinstein S, Yeh BM, Corvera CU, Monto A, Hope TA. Multiple arterial phase MRI of arterial hypervascular hepatic lesions: improved arterial phase capture and lesion enhancement. Abdom Radiol (NY). 2017;42:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Tamada T, Ito K, Yoshida K, Sone T, Murakami K, Kanki A, Watanabe S, Higashi H, Yamashita T. T2-weighted magnetic resonance imaging of the liver: evaluation of the effect in signal intensity after Gd-EOB-DTPA enhancement. J Comput Assist Tomogr. 2010;34:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Muhi A, Ichikawa T, Motosugi U, Sou H, Sano K, Araki T. Diffusion- and T₂-weighted MR imaging of the liver: effect of intravenous administration of gadoxetic acid disodium. Magn Reson Med Sci. 2012;11:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Agnello F, Dioguardi Burgio M, Picone D, Vernuccio F, Cabibbo G, Giannitrapani L, Taibbi A, Agrusa A, Bartolotta TV, Galia M, Lagalla R, Midiri M, Brancatelli G. Magnetic resonance imaging of the cirrhotic liver in the era of gadoxetic acid. World J Gastroenterol. 2016;22:103-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Watanabe Y, Ogawa M, Kumagawa M, Hirayama M, Miura T, Matsumoto N, Nakagawara H, Yamamoto T, Moriyama M. Utility of Contrast-Enhanced Ultrasound for Early Therapeutic Evaluation of Hepatocellular Carcinoma After Transcatheter Arterial Chemoembolization. J Ultrasound Med. 2020;39:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257:24-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 34. | Chiorean L, Tana C, Braden B, Caraiani C, Sparchez Z, Cui XW, Baum U, Dietrich CF. Advantages and Limitations of Focal Liver Lesion Assessment with Ultrasound Contrast Agents: Comments on the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines. Med Princ Pract. 2016;25:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 36. | Salvatore V, Borghi A, Piscaglia F. Contrast-enhanced ultrasound for liver imaging: recent advances. Curr Pharm Des. 2012;18:2236-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22675] [Article Influence: 1333.8] [Reference Citation Analysis (1)] |
| 38. | Yaghmai V, Miller FH, Rezai P, Benson AB 3rd, Salem R. Response to treatment series: part 2, tumor response assessment--using new and conventional criteria. AJR Am J Roentgenol. 2011;197:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3420] [Article Influence: 213.8] [Reference Citation Analysis (43)] |
| 40. | Horger M, Lauer UM, Schraml C, Berg CP, Koppenhöfer U, Claussen CD, Gregor M, Bitzer M. Early MRI response monitoring of patients with advanced hepatocellular carcinoma under treatment with the multikinase inhibitor sorafenib. BMC Cancer. 2009;9:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6391] [Article Influence: 798.9] [Reference Citation Analysis (9)] |
| 42. | Yu H, Bai Y, Xie X, Feng Y, Yang Y, Zhu Q. RECIST 1.1 versus mRECIST for assessment of tumour response to molecular targeted therapies and disease outcomes in patients with hepatocellular carcinoma: a systematic review and meta-analysis. BMJ Open. 2022;12:e052294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Salvaggio G, Furlan A, Agnello F, Cabibbo G, Marin D, Giannitrapani L, Genco C, Midiri M, Lagalla R, Brancatelli G. Hepatocellular carcinoma enhancement on contrast-enhanced CT and MR imaging: response assessment after treatment with sorafenib: preliminary results. Radiol Med. 2014;119:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 45. | Takada J, Hidaka H, Nakazawa T, Kondo M, Numata K, Tanaka K, Matsunaga K, Okuse C, Kobayashi S, Morimoto M, Ohkawa S, Koizumi W. Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma. BMC Res Notes. 2015;8:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 701] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 47. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3255] [Article Influence: 130.2] [Reference Citation Analysis (1)] |
| 48. | Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond E, Faivre S. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 49. | Gregory J, Dioguardi Burgio M, Corrias G, Vilgrain V, Ronot M. Evaluation of liver tumour response by imaging. JHEP Rep. 2020;2:100100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Kim DH, Min EJ, Kim B, Choi JY, Jang JW, Sung PS, Han JW, Kim H, Choi JI. RECIST 1.1, mRECIST, and Choi criteria for evaluating treatment response and survival outcomes in hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab. Eur Radiol. 2025;35:684-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, Powles T, Smith D, Hoos A, McKenna C, Beyer U, Rhee I, Fine G, Winslow N, Chen DS, Wolchok JD. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol. 2018;36:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 52. | Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1820] [Article Influence: 202.2] [Reference Citation Analysis (0)] |
| 53. | American College of Radiology. LI-RADS CT/MRI Treatment Response Assessment. Available from: https://www.acr.org/Clinical-Resources/Clinical-Tools-and-Reference/Reporting-and-Data-Systems/LI-RADS. |
| 54. | Park S, Joo I, Lee DH, Bae JS, Yoo J, Kim SW, Lee JM. Diagnostic Performance of LI-RADS Treatment Response Algorithm for Hepatocellular Carcinoma: Adding Ancillary Features to MRI Compared with Enhancement Patterns at CT and MRI. Radiology. 2020;296:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Kim SW, Joo I, Kim HC, Ahn SJ, Kang HJ, Jeon SK, Lee JM. LI-RADS treatment response categorization on gadoxetic acid-enhanced MRI: diagnostic performance compared to mRECIST and added value of ancillary features. Eur Radiol. 2020;30:2861-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Do RK, Mendiratta-Lala M. Moving Away from Uncertainty: A Potential Role for Ancillary Features in LI-RADS Treatment Response. Radiology. 2020;296:562-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Aslam A, Chernyak V, Tang A, Miller FH, Bashir M, Do R, Sirlin C, Lewandowski RJ, Kim CY, Kielar AZ, Kambadakone AR, Yarmohammadi H, Kim E, Owen D, Charalel RA, Shenoy-Bhangle A, Burke LM, Mendiratta-Lala M. CT/MRI LI-RADS 2024 Update: Treatment Response Assessment. Radiology. 2024;313:e232408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 58. | Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 617] [Article Influence: 38.6] [Reference Citation Analysis (2)] |
| 59. | Mastrocostas K, Fischer S, Munoz-Schuffenegger P, Jang HJ, Dawson LA, Liu ZA, Sapisochin G, Kim TK. Radiological tumor response and histopathological correlation of hepatocellular carcinoma treated with stereotactic body radiation therapy as a bridge to liver transplantation. Abdom Radiol (NY). 2021;46:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Miller FH, Lopes Vendrami C, Gabr A, Horowitz JM, Kelahan LC, Riaz A, Salem R, Lewandowski RJ. Evolution of Radioembolization in Treatment of Hepatocellular Carcinoma: A Pictorial Review. Radiographics. 2021;41:1802-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 61. | O JH, Lodge MA, Wahl RL. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 371] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 62. | Poullos PD, Tseng JJ, Melcher ML, Concepcion W, Loening AM, Rosenberg J, Willmann JK. Structured Reporting of Multiphasic CT for Hepatocellular Carcinoma: Effect on Staging and Suitability for Transplant. AJR Am J Roentgenol. 2018;210:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Herts BR, Gandhi NS, Schneider E, Coppa CP, Mody RN, Baker ME, Remer EM. How We Do It: Creating Consistent Structure and Content in Abdominal Radiology Report Templates. AJR Am J Roentgenol. 2019;212:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Cannella R, Taibbi A, Pardo S, Lo Re G, La Grutta L, Bartolotta TV. Communicating with the hepatobiliary surgeon through structured report. BJR Open. 2019;1:20190012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Ganeshan D, Duong PT, Probyn L, Lenchik L, McArthur TA, Retrouvey M, Ghobadi EH, Desouches SL, Pastel D, Francis IR. Structured Reporting in Radiology. Acad Radiol. 2018;25:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 66. | Feinberg BA, Zettler ME, Klink AJ, Lee CH, Gajra A, Kish JK. Comparison of Solid Tumor Treatment Response Observed in Clinical Practice With Response Reported in Clinical Trials. JAMA Netw Open. 2021;4:e2036741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 67. | RSNA RadReport Template. Radiological Society of North America. Available from: https://www.rsna.org/practice-tools/data-tools-and-standards/radreport-reporting-templates. |
| 68. | Hartung MP, Bickle IC, Gaillard F, Kanne JP. How to Create a Great Radiology Report. Radiographics. 2020;40:1658-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 69. | Cox DRA, Chung W, Grace J, Wong D, Kutaiba N, Ranatunga D, Khor R, Perini MV, Fink M, Jones R, Goodwin M, Dobrovic A, Testro A, Muralidharan V. Evaluating treatment response following locoregional therapy for hepatocellular carcinoma: A review of the available serological and radiological tools for assessment. JGH Open. 2023;7:249-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |