Published online Apr 28, 2024. doi: 10.4329/wjr.v16.i4.94
Revised: April 4, 2024
Accepted: April 16, 2024
Published online: April 28, 2024
Processing time: 135 Days and 21.2 Hours
The goal of therapy for traumatic carotid-cavernous fistula (TCCF) is the elimi
To determine the occurrence and long-term follow-up of pseudoaneurysm after transarterial detachable balloon for TCCF.
Between January 2009 and December 2019, 79 patients diagnosed with TCCF were treated using detachable latex balloons (GOLDBAL) of four sizes. Pseudoaneurysm sizes were stratified into five grades for analysis. Initial and follow-up assessments involved computed tomography angiography at 1 month, 6 month, 1 year, and longer intervals for significant cases. Clinical follow-ups occurred semi-annually for 2 years, then annually. Factors analyzed included sex, age, fistula size and location, and balloon size.
In our cohort of 79 patients treated for TCCF, pseudoaneurysms formed in 67.1%, with classifications ranging from grade 0 to grade 3; no grade 4 or giant pseudoaneurysms were observed. The majority of pseudoaneurysms did not progress in size, and some regressed spontaneously. Calcifications developed in most large pseudoaneurysms over 5-10 years. Parent artery occlusion occurred in 7.6% and recurrent fistulas in 16.5%. The primary risk factors for pseudoaneurysm formation were identified as the use of specific balloon sizes, with balloon SP and No. 6 significantly associated with its occurrence (P = 0.005 and P = 0.002, respectively), whereas sex, age, fistula size, location, and the number of balloons used were not significant predictors.
Pseudoaneurysm formation following detachable balloon embolization for TCCF is common, primarily influenced by the size of the balloon used. Despite this, all patients with pseudoaneurysms remained asymptomatic during long-term follow-up.
Core Tip: This study investigated the incidence and determinants of pseudoaneurysm formation following transarterial detachable balloon embolization in treating traumatic carotid-cavernous fistula over a decade. It highlighted balloon size as a significant risk factor, with larger balloons notably increasing pseudoaneurysm occurrence. Despite the high incidence of pseudoaneurysms, all affected patients remained asymptomatic through long-term follow-up, underscoring the procedure’s overall safety. These findings emphasize the critical role of selecting appropriate balloon sizes to mitigate risk and optimize outcomes, offering valuable insights for clinicians in managing this complex condition effectively.
- Citation: Iampreechakul P, Wangtanaphat K, Chuntaroj S, Wattanasen Y, Hangsapruek S, Lertbutsayanukul P, Puthkhao P, Siriwimonmas S. Pseudoaneurysm formation following transarterial embolization of traumatic carotid-cavernous fistula with detachable balloon: An institutional cohort long-term study. World J Radiol 2024; 16(4): 94-108
- URL: https://www.wjgnet.com/1949-8470/full/v16/i4/94.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i4.94
Traumatic carotid-cavernous fistulas (TCCFs) are high-flow shunts directly communicated between the cavernous segment of the internal carotid artery (ICA) and the cavernous sinus, resulting from trauma[1]. TCCFs were categorized as Type A by the Barrow classification of CCFs[2]. Spontaneous resolution of TCCFs is extremely rare[3]. The typical clinical manifestations, which depend on the venous drainage of the cavernous sinus, include pulsatile proptosis, conjunctival chemosis, red eye, and/or audible bruit. Rarely, aggressive neurological symptoms, especially deterioration of consciousness, may develop due to cortical venous reflux, leading to cerebral ischemia and/or hemorrhage[4].
The management of TCCFs has evolved significantly, transitioning from surgical to endovascular methods, utilizing detachable balloons or coils through arterial or venous approaches[5]. The first case of endovascular occlusion of TCCF using a fixed balloon catheter was described by Prolo and Hanberry[6] in 1971. Later, Serbinenko[7], a Russian neu
The pseudoaneurysm formation following endovascular treatment of TCCF with detachable balloon has rarely been observed, especially in long-term follow-up[12,22]. We conducted a retrospective cohort study to ascertain the occurrence and long-term follow-up of pseudoaneurysm after transarterial embolization of TCCF with detachable balloons. Risk factors for the occurrence of pseudoaneurysm were also investigated.
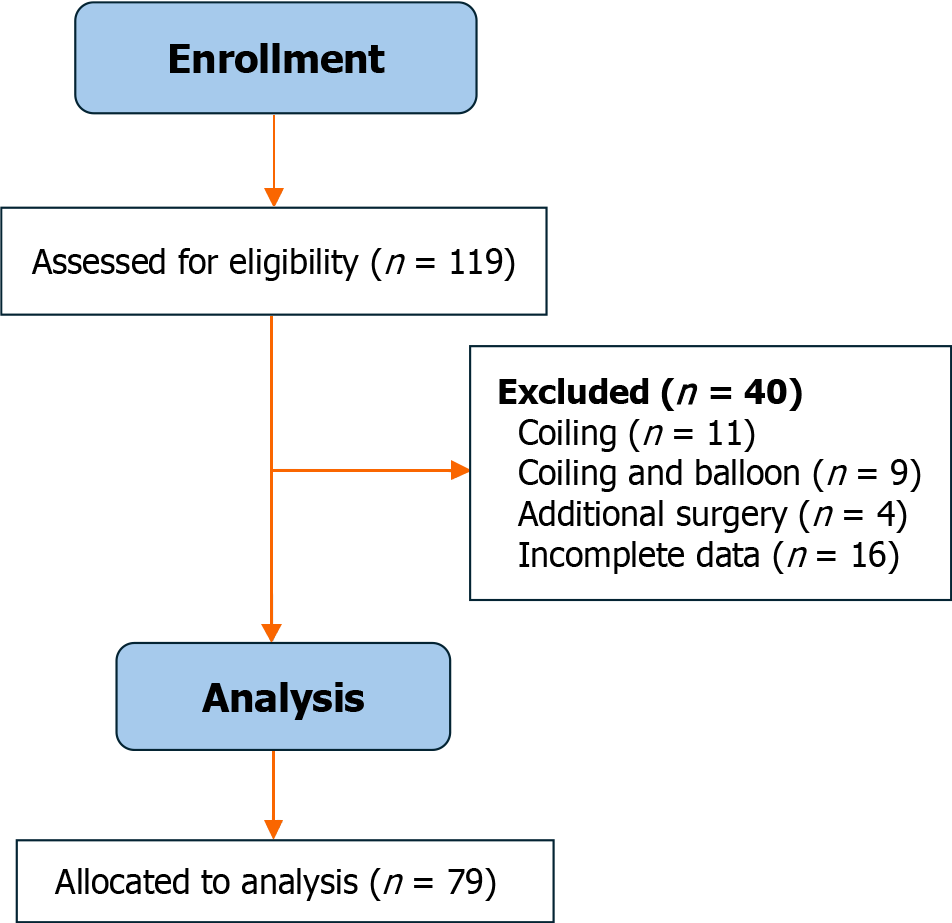
This retrospective cohort study was conducted at a tertiary neurosurgical center, with ethics committee approval from our institute. From January 2009 to December 2019, 119 consecutive patients with TCCF were recruited. Inclusion criteria were patients with TCCF treated by transarterial embolization using detachable balloons. The exclusion criteria were patients with TCCF treated by other options, including coiling (n = 11), coiling and balloon (n = 9), and combined endovascular and surgical treatment (n = 4). In addition, 16 patients with incomplete data were excluded from enrollment. The final cohort of our study included 79 patients (56 males and 71 females). Their age ranged from 11 years to 68 years, with a mean age of 34.82 ± 13.01 years. The flow chart of our study is illustrated in Figure 1.
Diagnosis was based on symptoms, trauma history, medical examination, computed tomography (CT) scan, and cerebral angiography. Demographic data collected included age, sex, cause of injury, and clinical symptoms at pre
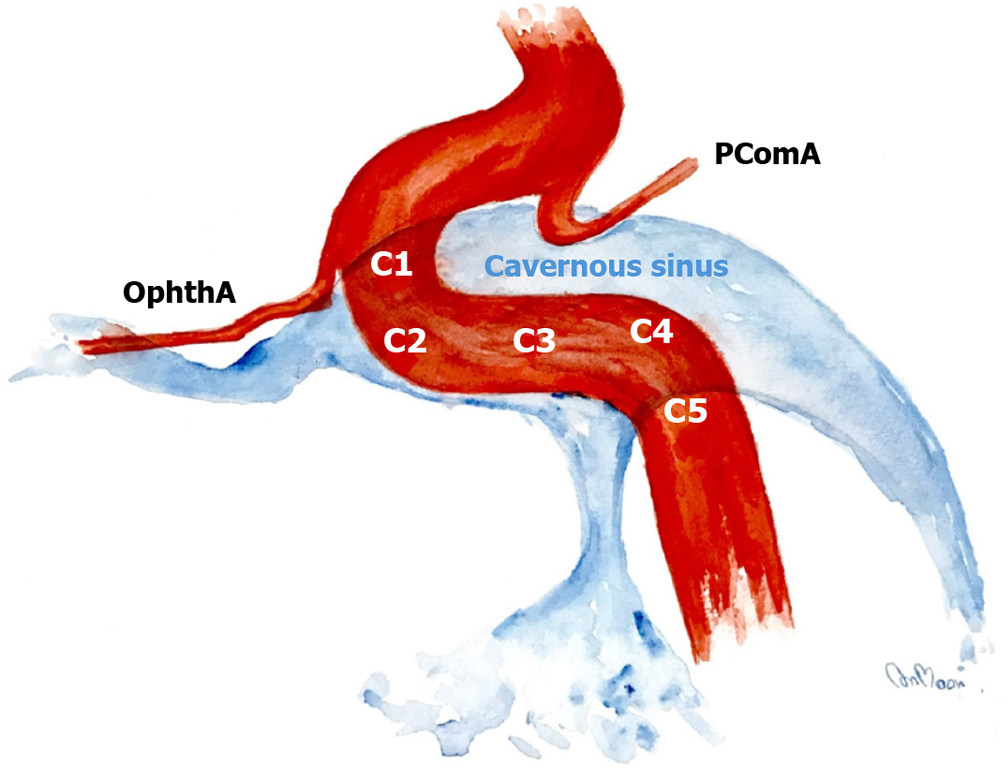
The fistula location was classified into five regions according to the cavernous ICA segmentation as described by Debrun et al[12]. These segments of ICA included C1 (anterior ascending segment), C2 (junction between the anterior ascending and horizontal segment), C3 (horizontal segment), C4 (junction between the horizontal and posterior ascending segment), and C5 (posterior ascending segment; Figure 2).
Based on the study of Chi et al[20], we classified the size of the fistula as small, medium, and large. Small-sized fistulas had good antegrade ipsilateral ICA flow, represented by opacification of both the anterior cerebral artery (ACA) and middle cerebral artery (MCA). Medium-sized fistulas had fair antegrade flow, represented by opacification of either the ACA or MCA. Large-sized fistulas had poor antegrade flow, represented by opacification of neither the ACA nor MCA (Figure 3).
The size of pseudoaneurysms was stratified by five grades including: Grade 0, no pseudoaneurysm; grade 1, small pseudoaneurysm (< 5 mm); grade 2, medium (5–10 mm); grade 3, large (11–25 mm); and grade 4, giant (> 25 mm).
The detachable latex balloon (GOLDBAL; Balt Extrusion, Montmorency, France) with four different sizes, including balloon No. 4, No. 5, No 6., and SP, was used in our study (Table 1). SP is abbreviated from the name and surname of Professor Sirintara Pongpech, Thai neurointerventionist, who designed this balloon size. Balloon SP contains a maximum volume of 0.9 mL and expands to 12 mm × 13 mm.
| Balloon size | Maximum volume in mL | Inflated dimensions in mm, diameter × length |
| GOLDBAL 4 | 0.75 | 9 × 16 |
| GOLDBAL 5 | 2.50 | 12 × 28 |
| GOLDBAL 6 | 3.00 | 15 × 25 |
| GOLDBAL SP | 0.90 | 12 × 13 |
Following transarterial balloon embolization, all patients underwent CT angiography (CTA) at 1 mo. If pseudoaneurysms occurred, CTA follow-up was performed at 6 month and 1 year. For large-sized pseudoaneurysm (grade 3) or giant (grade 4) pseudoaneurysm, CTA follow-up was carried out every 1 year to 3 years. The clinical follow-up was conducted every 3 month to 6 month for 2 years after the procedure and annually thereafter. Factors including sex, age, size and location of fistula, number of balloons, and the size of balloon, were statistically analyzed. All images were evaluated independently by the experienced neuroradiologist (SH) using Picture Archiving and Communication System (PACS; FUJIFILM, Stamford, CT, United States).
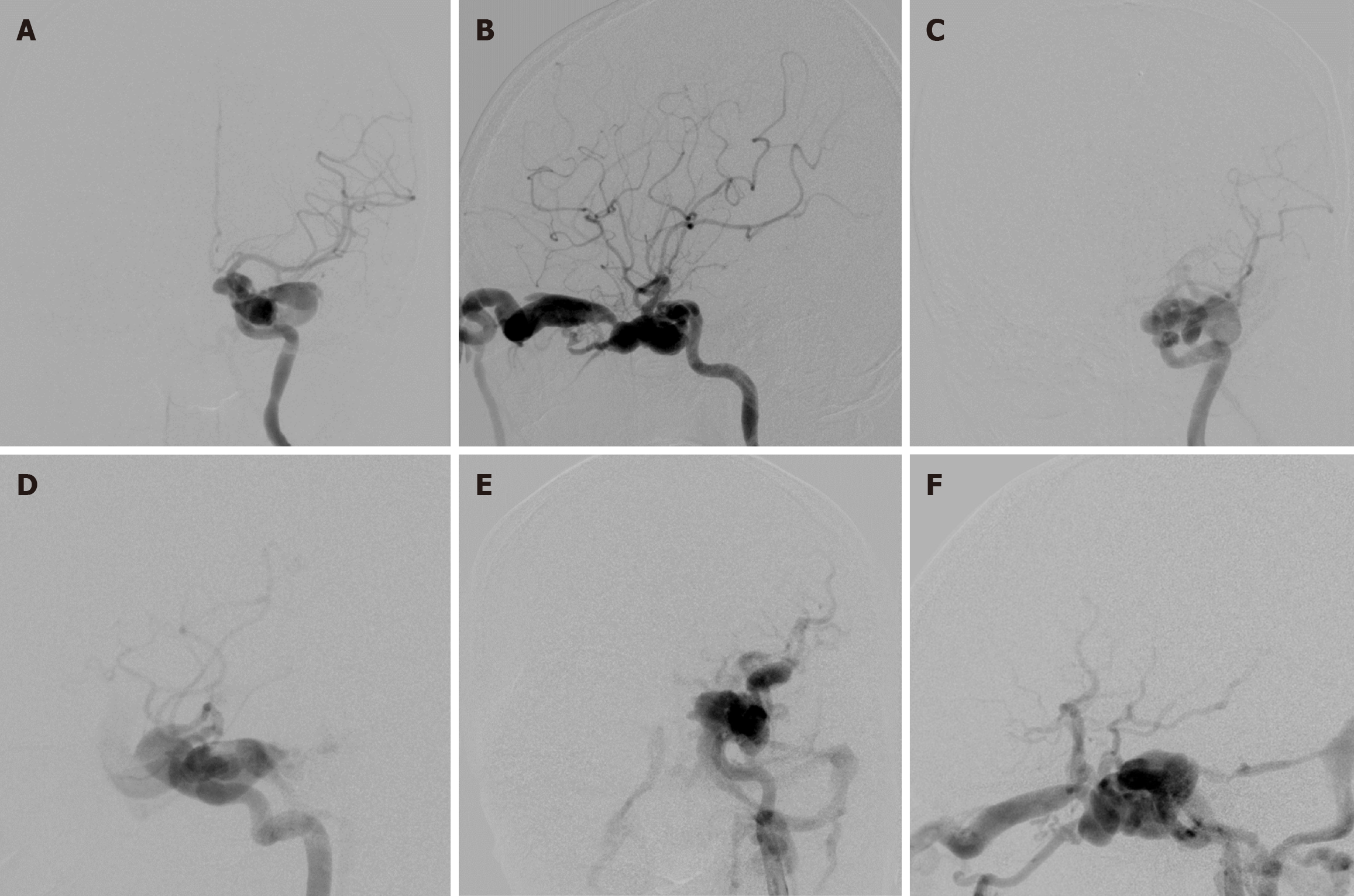
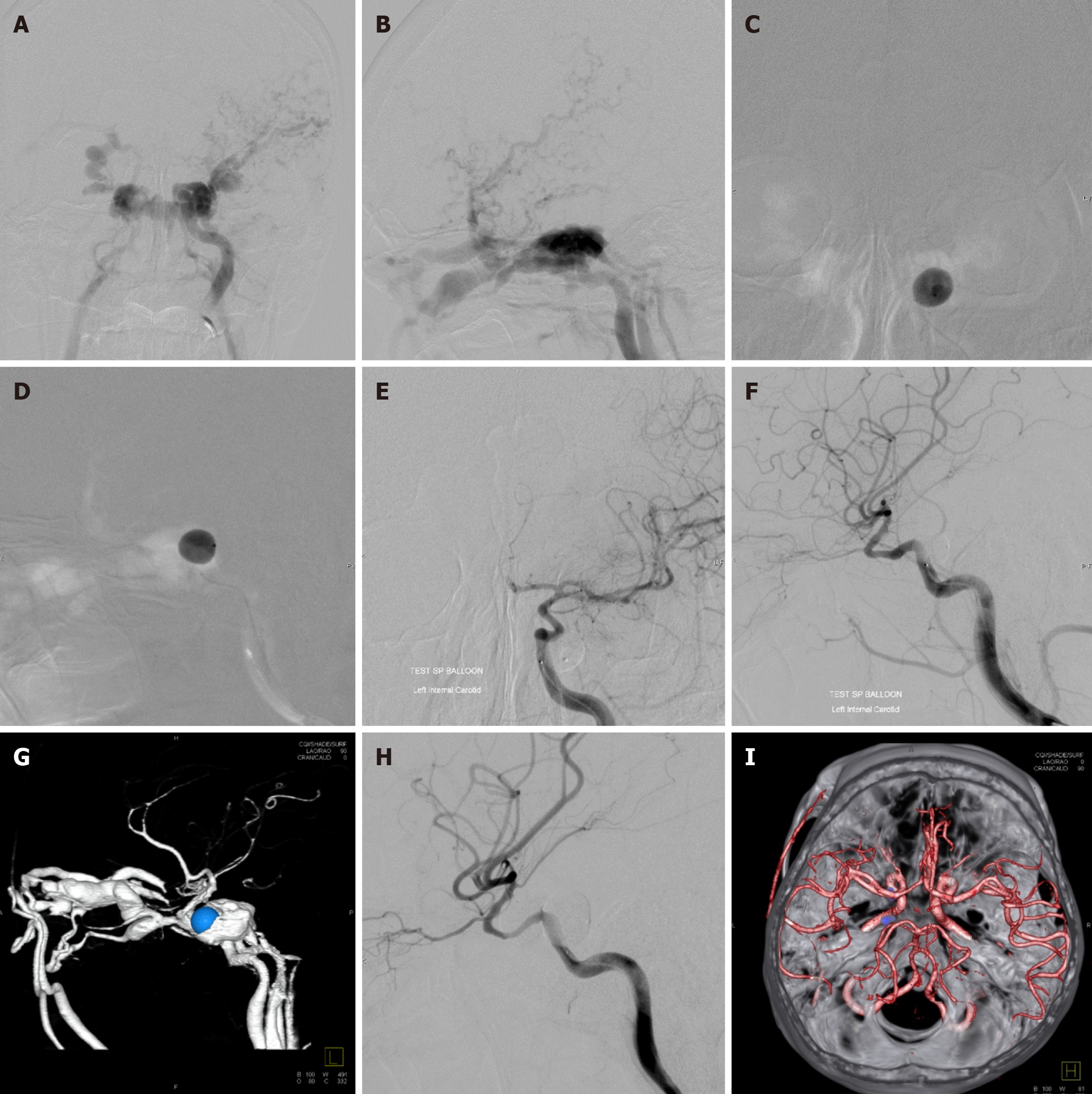
All endovascular procedures were performed under general anesthesia in a specialized biplane neuroangiography suite. Using the transfemoral approach, a 9 F introducing catheter was positioned into the cervical ICA. Subsequently, cerebral angiography was performed to assess the location and size of fistula, presence of any collateral supply through the circle of Willis, and venous drainage pattern. Additionally, contralateral carotid angiography during compression of the ipsilateral carotid artery was carried out to evaluate function of the anterior communicating artery. The precise fistula site was delineated by vertebral artery injection with simultaneous ipsilateral carotid compression. Following systemic heparinization, the balloon mouthed delivery microcatheter (Baltacci; Balt, Montmorency, France) was cautiously navigated by flow guidance into the orifice of the fistula under fluoroscopic road-mapping (Figures 4 and 5). The balloon was gradually inflated with iodinated contrast media, ensuring it did not exceed the maximum volume specified by the manufacturer for each balloon size (Table 1). Following the obliteration of the fistula by confirmation through a contrast media injection via the guiding catheter, the balloon was detached by gently pulling the microcatheter. Immediate post-embolization angiography was then performed to verify complete closure of the fistula. Multiple detachable balloons might be required if a single balloon failed to completely occlude TCCF. Post-procedure, all patients were advised absolute bed rest for 72 h to prevent balloon migration.
Data analysis was performed using the SPSS 16.0 software package (SPSS Inc, Chicago, IL, United States). Continuous data were expressed as mean (standard deviation) or median (interquartile range), and categorical variables were presented as numbers and corresponding percentages. Differences between groups were assessed using the Kruskal-Wallis test for continuous variables and Pearson Chi-square or Fisher’s exact tests for categorical variables. A P value < 0.05 was considered statistically significant.
Patient and fistula characteristics are summarized in Table 2. The causes of injury were predominantly motor vehicle accidents (75 patients, 94.9%), followed by assault (2 patients, 2.5%), falling from height (1 patient, 1.27%), and gunshot injury (1 patient, 1.27%). Clinical manifestations included pulsating proptosis (61 patients, 77.2%), conjunctival chemosis (39 patients, 49.4%), red eye (49 patients, 62.0%), audible bruit (28 patients, 35.4%), cranial nerve palsy (8 patients, 10.1%), and hemiparesis (2 patients, 2.5%).
| Patient characteristics | Number of patients, n (%) |
| Sex | |
| Male | 56 (70.9) |
| Female | 23 (29.1) |
| Cause of injury | |
| Motor vehicle accident | 75 (94.9) |
| Assault | 2 (2.5) |
| Falling from height | 1 (1.27) |
| Gunshot wound | 1 (1.27) |
| Symptoms | |
| Pulsatile proptosis | 61 (77.2) |
| Conjunctival chemosis | 39 (49.4) |
| Red eye | 49 (62.0) |
| Tinnitus | 28 (35.4) |
| Visual impairment | 9 (11.4) |
| Cranial nerve palsy | 8 (10.1) |
| Hemiparesis | 2 (2.5) |
| Fistula characteristics | |
| Side | |
| Right | 40 (50.6) |
| Left | 39 (49.4) |
| Location | |
| C1 | 1 (1.3) |
| C2 | 7 (8.9) |
| C3 | 26 (32.9) |
| C4 | 25 (31.6) |
| C5 | 20 (25.3) |
| Size | |
| Small | 14 (17.7) |
| Medium | 31 (39.2) |
| Large | 34 (43.1) |
| Result of treatment | |
| Number of balloons used | |
| One | 51(64.6) |
| Two | 20 (25.3) |
| Three | 8 (10.1) |
| Recurrence of fistula | 13 (16.5) |
| ICA preservation | 73 (92.4) |
| The occurrence of pseudoaneurysm | |
| Grade 0-No pseudoaneurysm | 24 (32.9) |
| Grade 1-Small pseudoaneurysm | 22 (30.1) |
| Grade 2-Medium pseudoaneurysm | 18 (24.7) |
| Grade 3-Large pseudoaneurysm | 9 (12.3) |
| Grade 4-Giant pseudoaneurysm | 0 (0) |
The cohort consisted of 79 fistulas including 14 (17.7%) small, 31 (39.2%) medium, and 34 (43.1%) large fistulas. Fistula locations were right-sided in 40 cases (50.6%) and left-sided in 39 cases (49.4%). The orifice of the fistula was located at C1 in 1 patient (1.3%), C2 in 7 patients (8.9%), C3 in 26 patients (32.9%), C4 in 25 patients (31.6%), and C5 in 20 patients (25.3%).
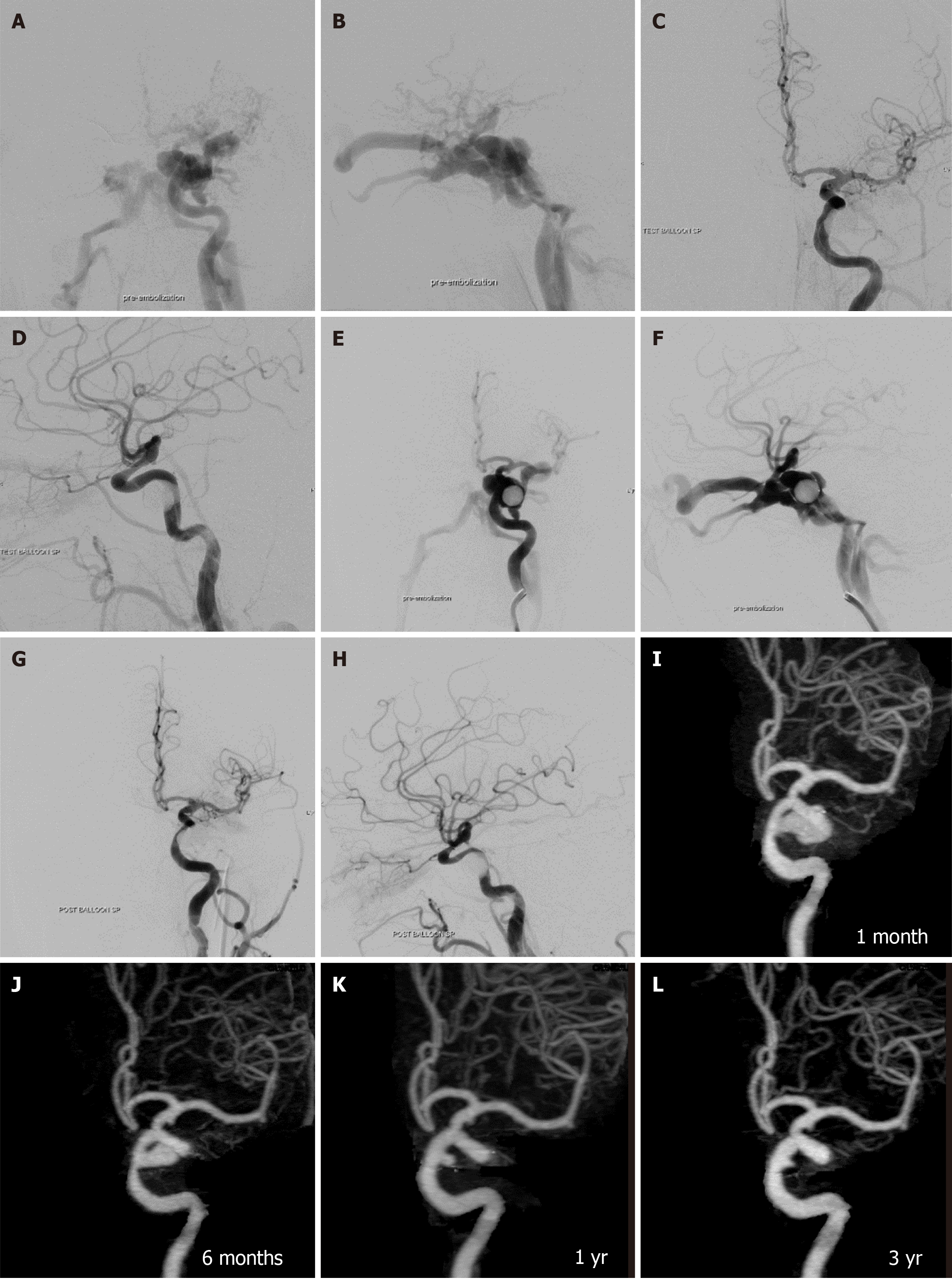
Treatment outcomes, detailed in Table 2, showed complete obliteration of the fistula in 78 patients (98.8%) and nearly complete obliteration in 1 patient (1.3%). One balloon was used in 51 patients (64.6%), two balloons in 20 patients (25.3%), and three balloons in 8 patients (10.1%). Recurrent fistulas occurred in 13 patients (16.5%; Figures 5 and 6). Parent artery occlusion after treatment or during follow-up was observed in 6 patients (7.6%).
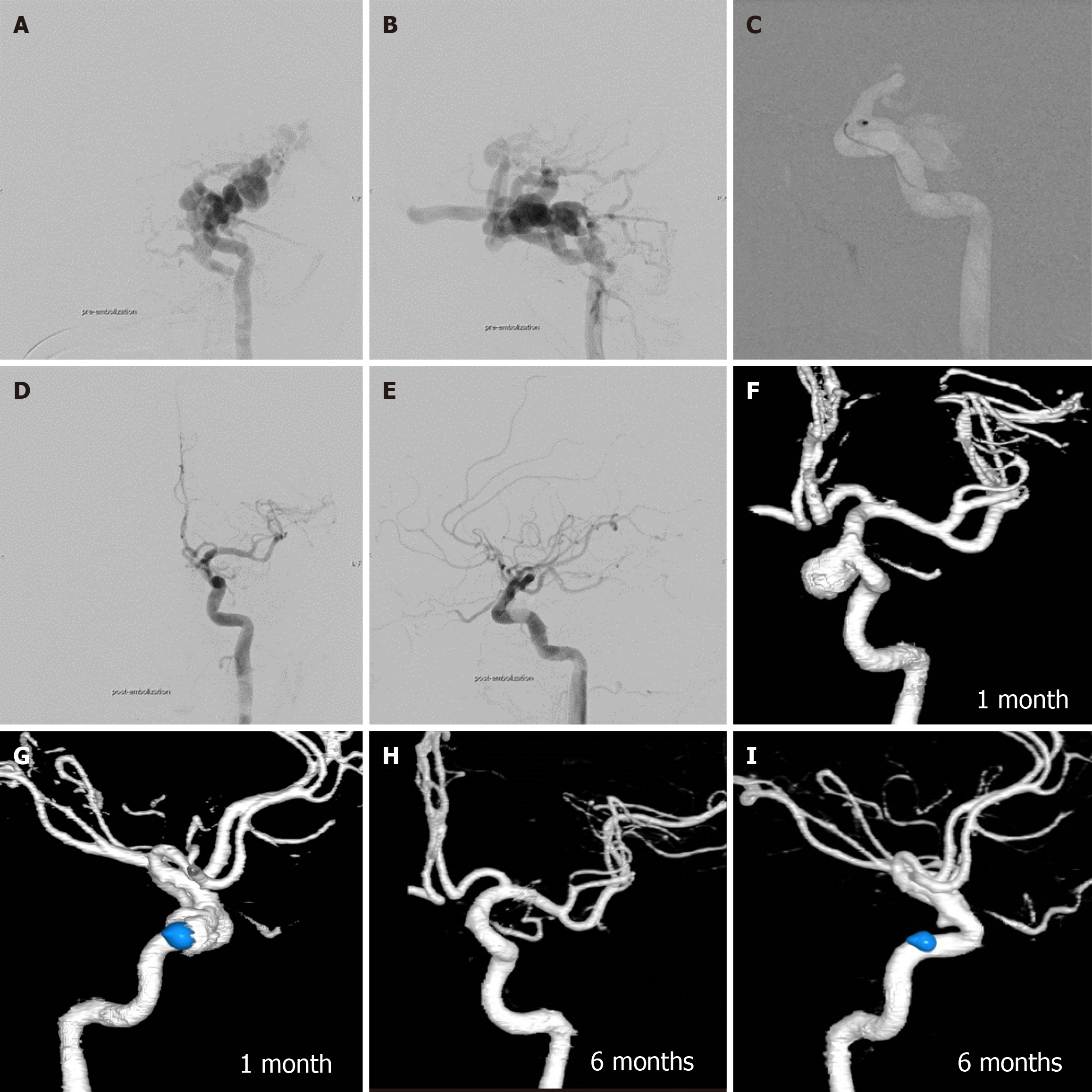
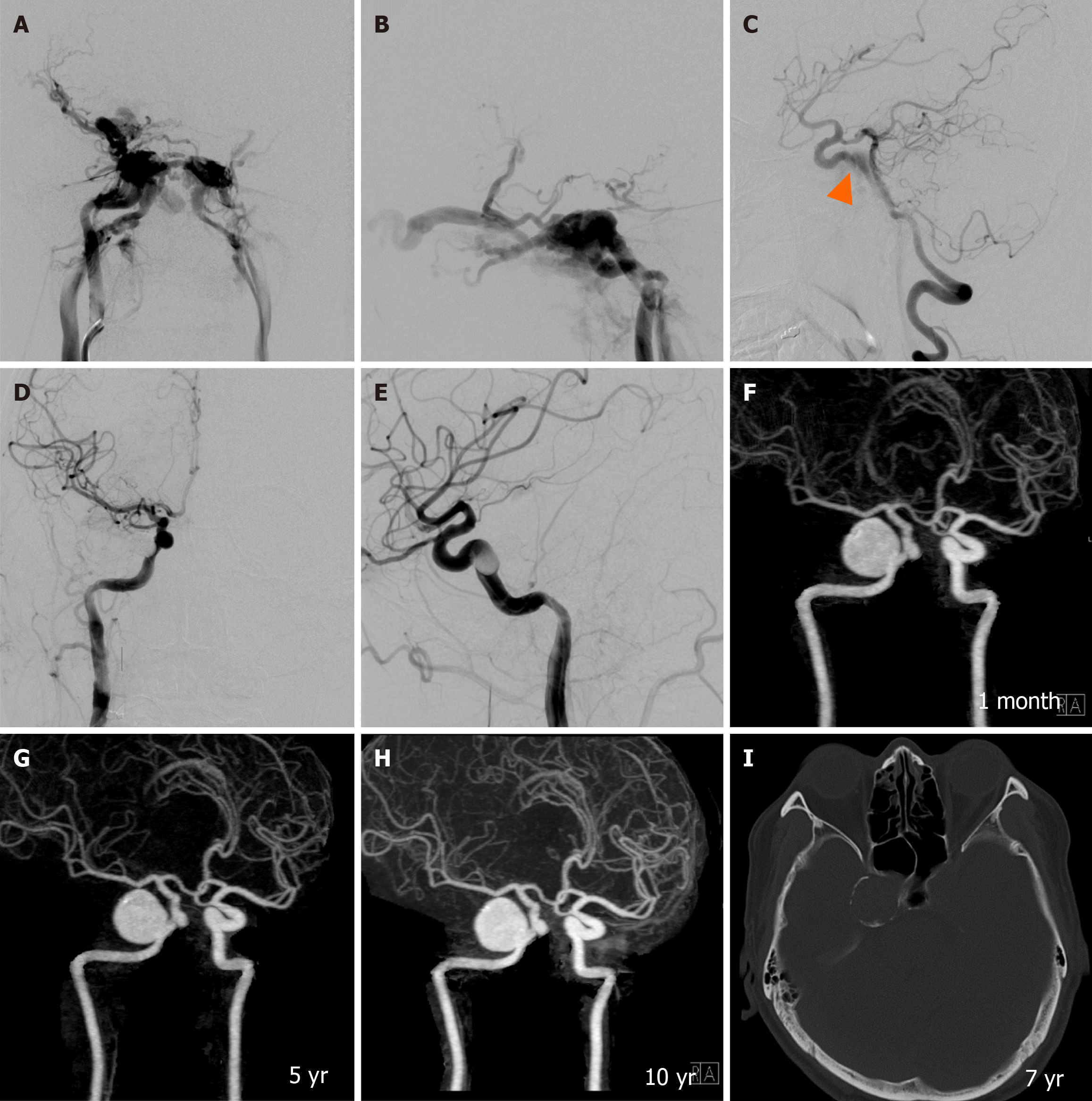
During a follow-up period of 2-10 years, grade 0 pseudoaneurysm were detected at final CTA follow-up in 24 patients (32.9%), grade 1 in 22 patients (30.1%), grade 2 in 18 patients (24.7%), and grade 3 in 9 patients (12.3%). There were no grade 4 or giant pseudoaneurysms in our study. Spontaneous regression of pseudoaneurysm size occurred in 10 patients (13.7%), with no progression in size observed. Most large pseudoaneurysms developed calcifications around the aneurysmal wall during follow-up at 5 years to 10 years (Figure 7). All patients harboring pseudoaneurysms were asymptomatic during long-term follow-up.
Univariate analysis indicated that sex, age, size, location of fistula, and number of balloons used were not related to the occurrence of pseudoaneurysm. The main factor for pseudoaneurysm formation was the size of the balloon including the use of balloon SP (P = 0.005) and balloon No. 6 (P = 0.002; Table 3). The use of balloon SP was associated with higher rates of complete obliteration of fistula without pseudoaneurysm formation, whereas balloon No. 6 developed large-sized pseudoaneurysms compared with other sizes.
Other balloon-related complications were found in 4 patients. Early detachment and migration of the detachable balloon occurred in 1 patient, but the balloon fragment was successfully retrieved with a snare without further ischemic symptoms. Three patients developed transient oculomotor nerve palsy after balloon embolization.
The goal of treatment of TCCF using a detachable balloon is to occlude the fistula while maintaining the carotid flow by the balloon placed into the cavernous sinus (venous side)[23,24]. In our study, we compared therapeutic outcomes of patients with TCCFs treated with transarterial detachable balloon embolization from different centers as shown in Table 4[12-14,19-22,25-32]. Two principal complications identified in balloon embolization for TCCF are the recurrence of the fistula and the formation of a pseudoaneurysm. Numerous studies have explored and identified risk factors contributing to the recurrence of TCCF following balloon embolization[21,29,32]. However, our study primarily concentrated on investigating the relevant factors associated with the occurrence of pseudoaneurysm, as this aspect has been less extensively covered in the existing literature.
| Ref. | Total cases | Total fistula treated with DB | Balloon type | Preservation of ICA (%) | Recurrence (%) | Pseudoaneurysm (%) | Other balloon-related complications (n) |
| Debrun et al[12], 1981 | 54 | 54 | Latex | 59.0 | 9.3 | 44.0 | Transient oculomotor nerve palsy (11) |
| Tsai et al[13], 1983 | 58 | 43 | Latex | N/A | 2.3 | 32.5 | Migration of deflated balloon into the ICA (2); delayed stroke (1) |
| Berthelsen and Svendsen[14], 1987 | 14 | 10 | Latex | N/A | 21.4 | N/A | Transient ischemic attack (1); migration of balloon into MCA (1) |
| Higashida et al[26], 1989 | 206 | 206 | Silicone | 88.0 | 0 | 2.4 | Oculomotor nerve palsy (1); ischemic stroke (5) |
| Lewis et al[27], 1995 | 100 | 88 | Latex/Silicone | 75.0 | 5.7 | N/A | Transient ischemia episodes (3); death (1) |
| Wu et al[28], 2000 | 482 | 471 | Latex | 84.0 | 3.3 | N/A | Cerebral infarction (2); vision loss (1) |
| Luo et al[29], 2004 | 143 | 143 | Latex | 81.0 | 11.2 | N/A | N/A |
| Szkup and Beningfield[30], 2005 | 34 | 34 | Latex | 53.0 | N/A | N/A | Transient hemiparesis (1) |
| Gupta et al[31], 2006 | 89 | 79 | Latex | 98.0 | 0 | N/A | Transient cranial palsy (1) |
| Wang et al[22], 2011 | 51 | 44 | Latex | 85.0 | 11.4 | 18.2 | None |
| Malan et al[25], 2012 | 32 | 17 | Latex | 66.0 | 15.6 | N/A | Balloon displacement (3); balloon rupture or deflation (8) |
| Xu et al[32], 2013 | 58 | 58 | Latex | 87.9 | 12.1 | N/A | None |
| Chi et al[20], 2014 | 172 | 138 | Latex | 70.0 | 9.8 | N/A | Transient hemiparesis (1); transient oculomotor nerve palsy (1); vagal shock, death (1) |
| Gao et al[21], 2018 | 188 | 182 | Latex | 85.7 | 13.9 | N/A | Cerebral embolism (1); abducent nerve paralysis (2) |
| Niu et al[19], 2020 | 24 | 21 | Latex | 90.4 | 8.3 | 4.2 | Oculomotor and abducens nerve palsy (1); abducens nerve palsy (1) |
| Present study | 79 | 79 | Latex | 92.4 | 16.5 | 67.1 | Early detachment and migration of balloon (1); transient oculomotor nerve palsy (3) |
Materials for the detachable balloons are made of either natural rubber (latex) or silicone rubber[31]. Under an electron microscope, the silicone detachable balloons display a smooth surface, whereas latex balloons exhibit an irregular surface with numerous large deep craters. These surface irregularities in latex balloons cause turbulent flow, promoting thrombosis and a more pronounced inflammatory response. Consequently, latex balloons are more thrombogenic than their silicone counterparts[30,33]. In vivo, if the tail of latex balloons protruded into the parent artery, it may increase thrombus formation around the balloons, more likely leading to parent artery occlusion as found in 7.6% of our patients[34]. Latex balloons are less expensive than silicone balloons and offer greater elasticity, allowing for larger inflation sizes. Detachable latex balloons can be more firmly attached to delivery catheters than silicone balloons and therefore should offer appreciably less risk of premature detachment. Latex balloons have a variety of sizes and shapes and require a hand ligation attachment, and they were reported as more prone to early deflation[27].
Silicone balloons are softer than latex and tend to conform to the shape of the vessel or aneurysm, reducing the risk of vessel rupture. Silicone balloons are also biocompatible, with their shell not degrading over time inside the vessel unlike latex[35]. Detachable silicone balloons are semipermeable and must be inflated with isotonic solutions. To prevent early deflation of the balloon, detachable silicone balloons require filling with iso-osmolar iodinated contrast agent before detachment[36-38]. On the other hand, latex balloons are not semipermeable, so the osmotic gradient between the balloon contents and the surrounding plasma has no influence on the role of premature deflation[39,40].
Balloons can be inflated with polymerizing substances, such as hydroxyethyl methacrylate, silicone fluid, or iodinated contrast material. A balloon filled with a polymerizing substance will produce better anatomic results and reduce the occurrence of a pseudoaneurysm. But the polymerizing substance may result in poor or incomplete recovery from oculomotor nerve palsy due to the remaining permanently solid balloon. In contrast, an iodine-inflated balloon may progressively deflate, possibly causing a pseudoaneurysm. However, when oculomotor nerve palsy occurs, balloon deflation will usually result in complete recovery from the palsy[24,28,31]. Currently, detachable silicone balloons are not available, and detachable latex balloons have been used by inflating with iodinated contrast material.
Pseudoaneurysms are remnants of the wall defect of the cavernous ICA at the orifice of the fistula that form following early deflation or migration of the detachable balloon[26,41]. The balloons usually become progressively deflated in 3 wk or 4 wk[10]. It is crucial for the balloons to stay inflated or retain their size and shape for approximately 2 wk to ensure a secure fibrous attachment to the vascular wall[11]. Premature deflation of the balloon often leads to the recurrence of fistulas and the subsequent development of pseudoaneurysms[14]. Balloons that deflate too early tend to migrate forward, ending up retained in the cavernous sinus[29]. The early deflation of balloons is caused primarily by relaxation or insufficiency of the ligature[10,12,20].
According to our review (Table 4), the incidence of pseudoaneurysm formation ranges from 2.4% to 44.0%. To our knowledge, there has been no study focusing on the long-term follow-up of pseudoaneurysm post-transarterial balloon embolization in TCCF. Our study found the highest incidence of pseudoaneurysm formation at 67.1%, including small-sized pseudoaneurysms in 30.1% of cases, medium-sized in 24.7%, and large-sized in 12.3%. The size of pseudoaneurysm spontaneously regressed in 13.7% during follow-up CTA, and there was no progression of pseudoaneurysms in our series. Interestingly, most large pseudoaneurysms developed rim calcification during follow-up at 5 years to 10 years.
Currently, GOLDBAL is the only detachable latex balloon available in our institute. Its deployment requires a single microcatheter, and the balloon is secured over a coaxial microcatheter using a latex thread. This thread then forms a self-sealing valve when the balloon is detached. Proper preparation and deployment of this system demands significant experience and skill[36,42].
In our study, univariate analysis showed that factors such as sex, age, fistula size and location, and the number of balloons used were not significant contributors in pseudoaneurysm development. Instead, the main contributing factor was the size of the balloon, specifically the use of balloon SP (P = 0.005) and balloon No. 6 (P = 0.002). The SP balloon was linked to higher rates of complete fistula obliteration without pseudoaneurysm formation, while the balloon No. 6 was associated with the development of large pseudoaneurysms.
Detachable balloon SP and No. 6 have different shapes and sizes. The balloon SP is spherical, holding a maximum volume of 0.9 mL, and expands to dimensions of 12 mm × 13 mm when fully inflated. In contrast, the balloon No. 6 is cylindrical, with a maximum volume of 3 mL, and expands to 15 mm × 25 mm. Detachable balloon No. 6 is the largest size and the most difficult to advance through a 9 F guiding catheter with some friction. We speculate that the ligature might relax, or the balloon may be damaged during prolonged manipulation, leading to early deflation and the formation of large pseudoaneurysms.
Recent advances in coil and flow divertor technologies have significantly impacted the treatment of intracranial aneurysms. In contrast, the development of detachable balloons, specifically latex detachable balloons, has not had similar progress. Although not approved by the Food and Drug Administration for use in the United States, these balloons are utilized in certain Southeast Asian countries, reflecting regional differences in medical practice and regulation. The market for detachable latex balloons is notably small, leading to a decrease in their manufacturing[36]. Despite this, detachable balloons maintain a role in treating TCCF and occluding vessels. It is anticipated that future developments in detachable balloon technology could potentially address current limitations, such as the risk of pseudoaneurysm formation, thereby enhancing the efficacy and safety of these devices in clinical practice.
Pseudoaneurysms, following the treatment of TCCF with detachable balloons, may either enlarge, causing a mass effect, or remain asymptomatic and decrease in size. The incidence of clinically symptomatic pseudoaneurysms is relatively low[13]. Small pseudoaneurysms typically remain asymptomatic and stable in size, while larger pseudoaneurysms can cause symptoms such as cranial nerve palsy and severe retroorbital pain[14,24,41]. Spontaneous regression of pseudoaneurysms after detachable balloon occlusion of TCCF has rarely been reported[43].
In most cases, pseudoaneurysms tend to be asymptomatic and do not necessitate further intervention[12]. However, some authors have suggested more aggressive treatment due to potential complications, which include mass effect on adjacent structures, recurrent TCCF from rupture of the pseudoaneurysm, and cerebral embolism resulting from thrombus formation[22].
For large symptomatic pseudoaneurysms, treatment options may include permanent occlusion of the carotid artery or the use of covered stents to preserve the integrity of the ICA[12,22,24]. In selected patients, pseudoaneurysms can be safely and effectively treated by embolization with detachable coils[44]. In our study, all patients with pseudoaneurysms remained asymptomatic, and no additional therapy was required during the long-term follow-up.
Our study showed that pseudoaneurysm formation was a common complication of detachable balloon embolization for treatment of TCCF. The main risk factor influencing this outcome was identified as the size of the detachable balloon used in treatment. Specifically, the balloon SP was associated with a higher success rate in completely obliterating the fistula without leading to the formation of pseudoaneurysms. In contrast, the largest balloon, No. 6, was more prone to result in the development of large-sized pseudoaneurysms. We speculate that the ligature might relax or the balloon may be damaged during prolonged manipulation, leading to early deflation and the formation of large pseudoaneurysms. Despite the high incidence of pseudoaneurysm formation, it is noteworthy that all patients with pseudoaneurysms remained asymptomatic throughout the long-term follow-up period. This observation underscores the complexity of managing TCCF and the need for careful consideration of the tools and techniques employed in treatment.
STROBE Statement: The authors have read the STROBE Statement – checklist of items, and the manuscript was prepared and revised according to the STROBE Statement – checklist of items.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Thailand
Peer-review report’s classification
Scientific Quality: Grade D
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Lei T, China S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Iampreechakul P, Wangtanaphat K, Lertbutsayanukul P, Siriwimonmas S. Revascularization of the internal carotid artery through the hypertrophied vasa vasorum in traumatic carotid-cavernous fistula previously treated by ligation of cervical carotid arteries: A case report. Surg Neurol Int. 2022;13:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 744] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 3. | Iampreechakul P, Tirakotai W, Tanpun A, Wattanasen Y, Lertbusayanukul P, Siriwimonmas S. Spontaneous resolution of direct carotid-cavernous fistulas: case series and literature review. Interv Neuroradiol. 2019;25:71-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 4. | Iampreechakul P, Tanpun A, Lertbusayanukul P, Siriwimonmas S. Contralateral extensive cerebral hemorrhagic venous infarction caused by retrograde venous reflux into the opposite basal vein of Rosenthal in posttraumatic carotid-cavernous fistula: A case report and literature review. Interv Neuroradiol. 2018;24:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Iampreechakul P, Liengudom A, Tirakotai W, Wangtanaphat K, Lertbutsayanukul P, Hangsapruek S, Siriwimonmas S. Combined endovascular and microsurgical management of complex traumatic carotid-cavernous fistula: Three case reports. Surg Neurol Int. 2022;13:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Prolo DJ, Hanbery JW. Intraluminal occlusion of a carotid-cavernous sinus fistula with a balloon catheter. Technical note. J Neurosurg. 1971;35:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 68] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 526] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Debrun G, Lacour P, Caron JP, Hurth M, Comoy J, Keravel Y. Inflatable and released balloon technique experimentation in dog -- application in man. Neuroradiology. 1975;9:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Debrun G, Lacour P, Caron JP, Hurth M, Comoy J, Kéravel Y, Loisance D. [Treatment of arteriovenous fistulas and of aneurysms using an inflatable and releasable balloon. Experimental principles. Application to man]. Nouv Presse Med. 1975;4:2315-2318. [PubMed] |
| 10. | Debrun G, Lacour P, Caron JP, Hurth M, Comoy J, Keravel Y. Detachable balloon and calibrated-leak balloon techniques in the treatment of cerebral vascular lesions. J Neurosurg. 1978;49:635-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 199] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Leipzig TJ, Mullan SF. Deflation of metrizamide-filled balloon used to occlude a carotid-cavernous fistula. Case report. J Neurosurg. 1983;59:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Debrun G, Lacour P, Vinuela F, Fox A, Drake CG, Caron JP. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55:678-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 237] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Tsai FY, Hieshima GB, Mehringer CM, Grinnell V, Pribram HW. Delayed effects in the treatment of carotid-cavernous fistulas. AJNR Am J Neuroradiol. 1983;4:357-361. [PubMed] |
| 14. | Berthelsen B, Svendsen P. Treatment of direct carotid-cavernous fistulas with detachable balloons. Acta Radiol. 1987;28:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Barrow DL, Fleischer AS, Hoffman JC. Complications of detachable balloon catheter technique in the treatment of traumatic intracranial arteriovenous fistulas. J Neurosurg. 1982;56:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Chalif DJ, Flamm ES, Berenstein A, Choi IS. Microsurgical removal of a balloon embolus to the internal carotid artery. Case report. J Neurosurg. 1983;58:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Graeb DA, Robertson WD, Lapointe JS, Nugent RA. Avoiding intraarterial balloon detachment in the treatment of posttraumatic carotid-cavernous fistulae with detachable balloons. AJNR Am J Neuroradiol. 1985;6:602-605. [PubMed] |
| 18. | Ducruet AF, Albuquerque FC, Crowley RW, McDougall CG. The evolution of endovascular treatment of carotid cavernous fistulas: a single-center experience. World Neurosurg. 2013;80:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Niu Y, Chen T, Tang J, Jiang Z, Zhu G, Chen Z. Detachable balloon embolization as the preferred treatment option for traumatic carotid-cavernous sinus fistula? Interv Neuroradiol. 2020;26:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Chi CT, Nguyen D, Duc VT, Chau HH, Son VT. Direct traumatic carotid cavernous fistula: angiographic classification and treatment strategies. Study of 172 cases. Interv Neuroradiol. 2014;20:461-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Gao BL, Wang ZL, Li TX, Xu B. Recurrence risk factors in detachable balloon embolization of traumatic direct carotid cavernous fistulas in 188 patients. J Neurointerv Surg. 2018;10:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Wang W, Li YD, Li MH, Tan HQ, Gu BX, Wang J, Zhang PL. Endovascular treatment of post-traumatic direct carotid-cavernous fistulas: A single-center experience. J Clin Neurosci. 2011;18:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Debrun GM. Treatment of traumatic carotid-cavernous fistula using detachable balloon catheters. AJNR Am J Neuroradiol. 1983;4:355-356. [PubMed] |
| 24. | Sbeih IA, O'Laoire SA. Traumatic carotid-cavernous fistula due to transection of the intracavernous carotid artery. Case report. J Neurosurg. 1984;60:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Malan J, Lefeuvre D, Mngomezulu V, Taylor A. Angioarchitecture and treatment modalities in posttraumatic carotid cavernous fistulae. Interv Neuroradiol. 2012;18:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Higashida RT, Halbach VV, Tsai FY, Norman D, Pribram HF, Mehringer CM, Hieshima GB. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases. AJR Am J Roentgenol. 1989;153:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 177] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Lewis AI, Tomsick TA, Tew JM Jr. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:239-44; discussion 244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 204] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Wu Z, Zhang Y, Wang C, Yang X, Li Y. Treatment of traumatic carotid-cavernous fistula. Interv Neuroradiol. 2000;6:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Luo CB, Teng MM, Yen DH, Chang FC, Lirng JF, Chang CY. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma. 2004;56:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Szkup P, Beningfield S. Endovascular treatment of post-traumatic carotid-cavernous fistulae using latex detachable balloons. S Afr J Radiol. 2005;9:4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 31. | Gupta AK, Purkayastha S, Krishnamoorthy T, Bodhey NK, Kapilamoorthy TR, Kesavadas C, Thomas B. Endovascular treatment of direct carotid cavernous fistulae: a pictorial review. Neuroradiology. 2006;48:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Xu XQ, Liu S, Zu QQ, Zhao LB, Xia JG, Zhou CG, Zhou WZ, Shi HB. Follow-up of 58 traumatic carotid-cavernous fistulas after endovascular detachable-balloon embolization at a single center. J Clin Neurol. 2013;9:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Heilman CB, Kwan ES, Wu JK. Aneurysm recurrence following endovascular balloon occlusion. J Neurosurg. 1992;77:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Miyachi S, Negoro M, Handa T, Terashima K, Keino H, Sugita K. Histopathological study of balloon embolization: silicone versus latex. Neurosurgery. 1992;30:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Higashida RT, Halbach VV, Dowd C, Barnwell SL, Dormandy B, Bell J, Hieshima GB. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990;72:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Alaraj A, Wallace A, Dashti R, Patel P, Aletich V. Balloons in endovascular neurosurgery: history and current applications. Neurosurgery. 2014;74 Suppl 1:S163-S190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | White RI Jr, Ursic TA, Kaufman SL, Barth KH, Kim W, Gross GS. Therapeutic embolization with detachable balloons. Physical factors influencing permanent occlusion. Radiology. 1978;126:521-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | DiTullio MV Jr, Rand RW, Frisch E. Detachable balloon catheter. Its application in experimental arteriovenous fistulae. J Neurosurg. 1978;48:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Tomsick TA. Osmotic effects upon long term inflation of latex detachable balloons. Neurosurgery. 1985;17:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Hawkins TD, Szaz KF. The permeability of detachable latex rubber balloons. An in vitro study. Invest Radiol. 1987;22:969-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Lewis AI, Tomsick TA, Tew JM Jr, Lawless MA. Long-term results in direct carotid-cavernous fistulas after treatment with detachable balloons. J Neurosurg. 1996;84:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Beaujeux RL, Reizine DC, Casasco A, Aymard A, Rüfenacht D, Khayata MH, Riché MC, Merland JJ. Endovascular treatment of vertebral arteriovenous fistula. Radiology. 1992;183:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Lee CH, Kim MS, Lee GJ. Spontaneous Regression of the Pseudoaneurysm Developed after Balloon Occlusion of the Direct Carotid-cavernous Fistula. J Korean Neurosurg Soc. 2007;41:323-326. |
| 44. | Lempert TE, Halbach VV, Higashida RT, Dowd CF, Urwin RW, Balousek PA, Hieshima GB. Endovascular treatment of pseudoaneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol. 1998;19:907-911. [PubMed] |