Published online Apr 28, 2024. doi: 10.4329/wjr.v16.i4.82
Revised: March 19, 2024
Accepted: April 16, 2024
Published online: April 28, 2024
Processing time: 136 Days and 11.7 Hours
Currently, the differentiation of jaw tumors is mainly based on the lesion’s morphology rather than the enhancement characteristics, which are important in the differentiation of neoplasms across the body. There is a paucity of literature on the enhancement characteristics of jaw tumors. This is mainly because, even though computed tomography (CT) is used to evaluate these lesions, they are often imaged without intravenous contrast. This study hypothesised that the enhancement characteristics of the solid component of jaw tumors can aid in the differentiation of these lesions in addition to their morphology by dual-energy CT, therefore improving the ability to differentiate between various pathologies.
To evaluate the role of contrast enhancement and dual-energy quantitative parameters in CT in the differentiation of jaw tumors.
Fifty-seven patients with jaw tumors underwent contrast-enhanced dual-energy CT. Morphological analysis of the tumor, including the enhancing solid com
Ameloblastoma was the most common pathology (n = 20), followed by CGCG (n = 11) and OKC. CGCG showed a higher mean concentration of all quantitative parameters than ameloblastomas (P < 0.05). An IC threshold of 31.35 × 100 μg/cm3 had the maximum sensitivity (81.8%) and specificity (65%). Between ameloblastomas and OKC, the former showed a higher mean concentration of all quantitative parameters (P < 0.001), however when comparing unilocular ameloblastomas with OKCs, the latter showed significantly higher WC. Also, ameloblastoma had a higher IC and lower WC compared to “other jaw tumors” group.
Enhancement characteristics of solid components combined with dual-energy parameters offer a more precise way to differentiate between jaw tumors.
Core Tip: Quantitative dual-energy computed tomography (DECT) parameters provide a reliable way of characterizing morphologically similar jaw lesions and can serve as a single modality to differentiate jaw lesions based on their appearance and material density concentrations. In addition to providing fast imaging and material decomposition algorithms at about comparable dosage equivalency as compared to traditional computed tomography, contrast-enhanced DECT can potentially alleviate the challenge of discriminating jaw lesions without a biopsy.
- Citation: Viswanathan DJ, Bhalla AS, Manchanda S, Roychoudhury A, Mishra D, Mridha AR. Characterization of tumors of jaw: Additive value of contrast enhancement and dual-energy computed tomography. World J Radiol 2024; 16(4): 82-93
- URL: https://www.wjgnet.com/1949-8470/full/v16/i4/82.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i4.82
Various imaging modalities are available for the evaluation of jaw lesions, the most important being panoramic radio
Here comes the role of contrast-enhanced CT, which makes characterization of the solid component possible and gives important information about the nature and extent of a particular tumor, helping the radiologist to give a possible range of differential diagnoses. Dual-energy CT (DECT) is an innovative technique that operates based on differential attenuation of tissues when penetrated with higher (140 kVp) and lower (80/100 kVp) energy and combines the CT attenuation-based imaging with material-specific or spectral imaging[3]. This in turn gives the added advantage of characterizing lesions based on the quantitative parameters touted to be material-specific, which can further increase the diagnostic confidence with which the radiologist conveys the possible diagnoses. The hypothesis of this study is that the enhancement characteristics of the solid component of jaw tumors is important for the differentiation of these lesions and evaluation of the same in addition to its morphology by DECT, therefore, improving the ability to differentiate between various pathologies[4-6].
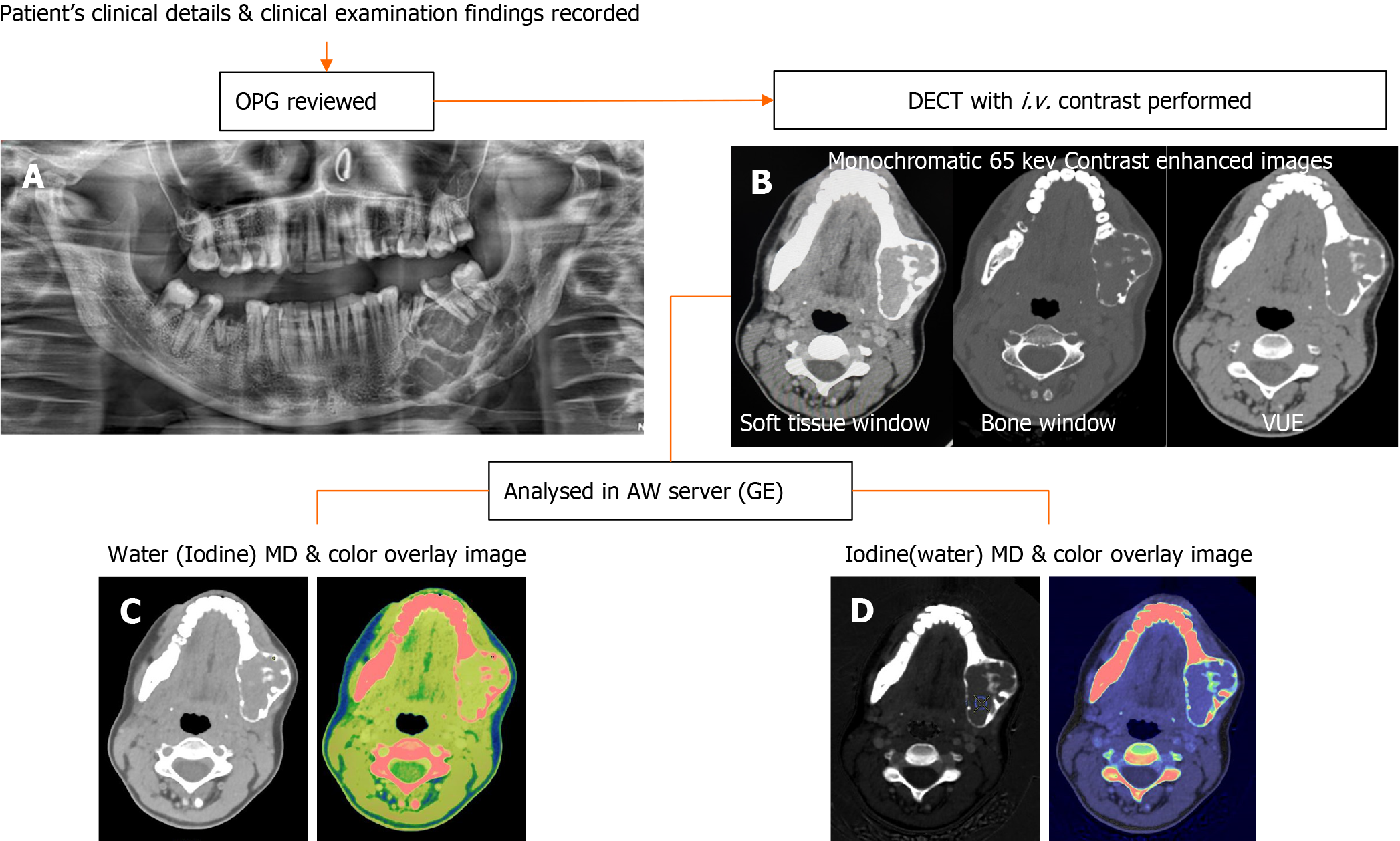
This observational study was conducted prospectively from July 2020 to April 2022 after obtaining approval by the Institutional Ethics Committee (IECPG-354/22.07.2020, RT-2/26.08.2020). The study subjects were patients who presented with complaints of swelling in the maxilla or mandible. Patients were first screened with panoramic radiographs. Those who were found to have any lytic or sclerotic lesions in the panoramic radiographs were included. Patients without histopathological confirmation, those with uncomplicated, typical benign cysts on orthopantomography (such as radicular cysts and dentigerous cysts), clinically insignificant lesions, patients who were unwilling to participate in the study, and those who were diagnosed with other infectious conditions like osteomyelitis, traumatic lesions, or primary tumors in the oral cavity invading the jaw, were excluded. After giving their full informed consent, all patients underwent a contrast-enhanced DECT. Blood investigations were done to evaluate the renal status before administering intravenous contrast agents.
The clinical information collected was patient demographic data (age and sex) through a proforma filled out by the patient, symptomatology (including swelling, pain, bleeding, fever, tooth mobility, trismus, or any other complaints), and their duration.
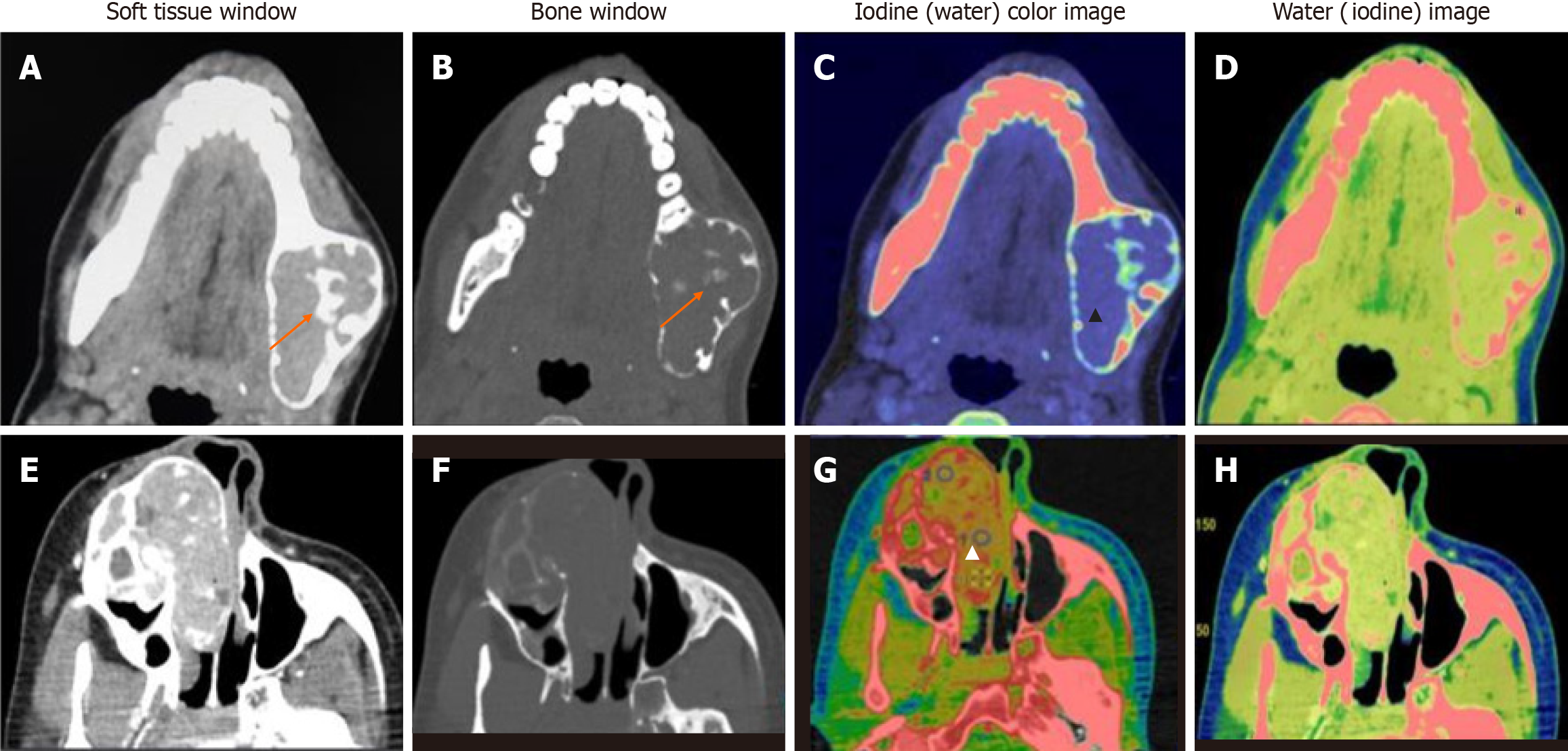
Data acquisitions were performed using single-source DECT in gemstone spectral imaging (GSI) mode with a fast tube voltage switching between 80 and 140 kVp (Revolution CT, GE Healthcare, Waukesha, WI, United States). Intravenous non-ionic contrast was given at 1.0 mL/kg. Routine soft tissue and bone windows were read. Standard multiplanar reconstructions and panoramic reconstructions were made. In addition, two types of images were obtained from the reconstruction of DECT imaging automatically with GSI viewer software (GE Healthcare) for each patient: The iodine-based and water-based material decomposition (MD) images (Figure 1).
Morphological parameters: The location of the lesion was recorded according to the bone in which it was seen. The para
DECT parameters: The regions of enhancement on soft tissue windows were selected in comparison to virtual non-contrast images, and an elliptical region of interest (ROI) was placed on the most enhancing parts as assessed on monochromatic (65 kev) and iodine images. The measurements included the mean value and area of measurement (mm2). To ensure consistency, all measurements were performed three times at different image levels, and the average values were calculated. For all measurements, the size, shape, and position of the ROI were consistent between the soft tissue images and the iodine-based MD images, as confirmed using the copy-and-paste function. Lesions with at least a soft tissue component of 1 mm2 were selected for analysis. The iodine concentration (IC) of the lesions was measured (expressed in multiples of 100 μg/cm3) from the iodine-based MD image, and the water concentration (WC) from the water-based MD image (expressed in multiples of 1000 mg/cm3) along with the overlay colormap to increase the assessed lesion contrast. The normalized IC (NIC) was calculated from the ratio of the measured IC of the lesion (ICL) and the IC of the ipsilateral common carotid artery (CCA) proximal to its bifurcation (ICA) via the insertion of two ROIs—one in the assessed lesion and the other in the CCA. In addition to the above, an analysis of the cystic component was also made in the unilocular ameloblastomas (UA) and odontogenic keratocysts (OKCs). The parameters recorded were IC and WC.
Histopathology data-gold standard: Post-biopsy, excision, or curettage, the sampled tissue specimens were reviewed by two consultant pathologists with 10 years of experience in oral pathology. Sections from routine tissue blocks were examined using hematoxylin and eosin staining. The results were documented as ameloblastoma and non-amelo
The statistical analysis for this study was done using SPSS version 28.0 software. Continuous variables (age, tumor volume, quantitative DECT, and IHC parameters) were all summarized as mean ± SD, and categorical values were summarized as proportions. The comparison of the mean ± SD between the two groups was done using an independent sample t-test. Categorical variables (histopathology data, patient symptomatology, and morphological parameters) were summarized as percentages. A comparison of proportions between the two groups was done using the chi-square test. Since we compared more than two independent groups for the analysis of DECT quantitative parameters, a one-way ANOVA test was performed for variables that showed a normal parametric distribution (mean HU at 65 kev, ICL, WCL) and a Kruskal-Wallis H test for non-parametric variables (NIC). If significant differences were discovered, we conducted a series of independent t-tests and Mann-Whitney U tests to determine the source of the difference. The value of P < 0.05 was considered statistically significant. The diagnostic performance was evaluated by calculating the area under the receiver operating characteristic curve (AUC).
The statistical methods of this study were reviewed by Mr. Hem Sati from the Department of Biostatistics, All India Institute of Medical Sciences, New Delhi.
Fifty-seven patients (mean age, 37 ± 17 years, 26 males, and 31 females) were included in the study. The maximum number of patients was in the age group of 31-40 years (n = 14). The most common presenting complaint was swelling, which was seen in 96% of patients (n = 55), followed by local pain in 39% of patients (n = 22). The majority of the lesions (44%) were present for more than 6 months.
In our study, histopathology was used as the gold standard for diagnosing jaw lesions. Twenty (35.09%) of the 57 patients had ameloblastomas, and 37 (64.91%) had non-ameloblastomas. With 11 cases, central giant cell granulomas (CGCG) were the most common lesions amongst non-ameloblastomas (29.7%). Table 1 summarizes the histopathological diagnosis of the lesions.
| Final diagnosis | Number of lesions | Percentage (%) |
| Ameloblastomas | 20 | 35 |
| Central giant cell granuloma | 11 | 19 |
| Odontogenic keratocyst | 6 | 10 |
| Ossifying fibroma | 5 | 8 |
| Salivary gland tumors | 2 | 4 |
| Malignancy | 3 | 5 |
| Chondromyxoid fibroma | 1 | 2 |
| Non-tumorous | 5 | 9 |
| Sclerotic lesions | 2 | 4 |
| Ameloblastic fibroma | 1 | 2 |
| Odontogenic myxoma | 1 | 2 |
| Total | 57 | 100 |
Of the 57 patients, 42 (73%) had lesions involving the mandible, and 13 (23%) had maxillary lesions, with thirteen patients having two lesions and two of them having three lesions. The morphological parameters were summarized for both ameloblastoma and non-ameloblastoma groups (Table 2). The ameloblastoma group showed a higher median volume (73.6 cm3), more necrosis, a higher percentage of inferior alveolar canal involvement, retromolar trigone (RMT) involvement, and cortical involvement in the form of expansion or thinning. All these were statistically significant.
| Variable | Ameloblastoma (n = 20) | Non-ameloblastoma (n = 37) | P value |
| Volume, cm3 | 73.6 (7.6–1014) | 39.12 (0.6–1296) | < 0.05 |
| Cystic/necrotic areas | 100 | 51 | < 0.05 |
| Cortical expansion/thinning | 100 | 78 | < 0.05 |
| Mandibular canal involvement | 60 | 32 | < 0.05 |
| Retromolar trigone involvement | 45 | 18 | < 0.05 |
Aggressive features evaluated in the case of mandibular tumors included mandibular canal involvement (n = 12), involvement of RMT (n = 16), condyle (n = 2), and coronoid process (n = 3). In cases of lesions in the maxilla, six cases showed aggressive features in the form of extension into the infratemporal fossa/orbit/pterygoid plates. Overall, locally aggressive features were seen in 19 cases (33%).
On a broad comparison between the ameloblastoma and non-ameloblastoma groups, the ameloblastomas had a higher mean IC, a higher mean HU at 65 kev, a lower average NIC, and a lower WC compared to the non-ameloblastomas.
The ameloblastomas mostly had IC s in the 16–30 (moderate) mmol/mm3 range and mean attenuation in the range of 50–150 HU. In contrast, 90% of CGCGs showed ICs greater than 31 mmol/mm3 and mean attenuation > 150 HU. The OKCs had low values in all the parameters, distinctly different from others. The rest of them did not show any significant difference between them in their respective groups (Table 3). This could be attributed to the heterogeneous sample within the non-ameloblastoma group, which included cystic lesions with virtually no enhancing solid component and avidly enhancing masses. Statistical analysis revealed that the values of DECT parameters in OKCs and CGCCs were on the extreme opposite spectrum, with other lesions having values in between. Hence, we further subdivided the non-ameloblastoma group into three sub-groups and compared ameloblastomas with these three subgroups: OKCs, CGCG, and other jaw tumors.
| Iodine concentration (μg/mL) | 0-15 (low) | 16-30 (moderate) | 31-45 (high) | ≥ 46 (extreme) |
| Ameloblastoma | 2 | 11 | 4 | 1 |
| CGCG | 0 | 1 | 8 | 0 |
| OKC | 5 | 0 | 0 | 0 |
| OF | 0 | 3 | 0 | 0 |
| Salivary gland tumor | 1 | 1 | 0 | 0 |
| Chondromyxoid fibroma | 0 | 0 | 0 | 1 |
| Others | 4 | 6 | 2 | 1 |
Comparison between ameloblastoma and three major subgroups within the non-ameloblastoma group (Table 4):
| Parameters | Amelo, mean ± SD | OKC, mean ± SD | CGCG, mean ± SD | Other JT, mean ± SD | 1P value | 2P value | 3P value |
| Mean HU | 122 ± 28.3 | 33 ± 12.4 | 151 ± 24.3 | 117 ± 37.9 | 0.007 | < 0.001 | 0.616 |
| IC | 29 ± 9.3 | 7.2 ± 5.8 | 36.1 ± 6.8 | 24.8 ± 11.5 | 0.036 | < 0.001 | 0.232 |
| WC | 1032.5 ± 13 | 1010 ± 11 | 1043 ± 11.6 | 1040 ± 11.4 | 0.036 | 0.044 | 0.056 |
| NIC | 0.35 ± 0.15 | 0.10 ± 0.1 | 0.59 ± 0.25 | 0.39 ± 0.24 | 0.011 | 0.007 | 0.501 |
| WC (cystic) | 997 ± 5.6 | 1020 ± 5 | - | - | - | < 0.001 | - |
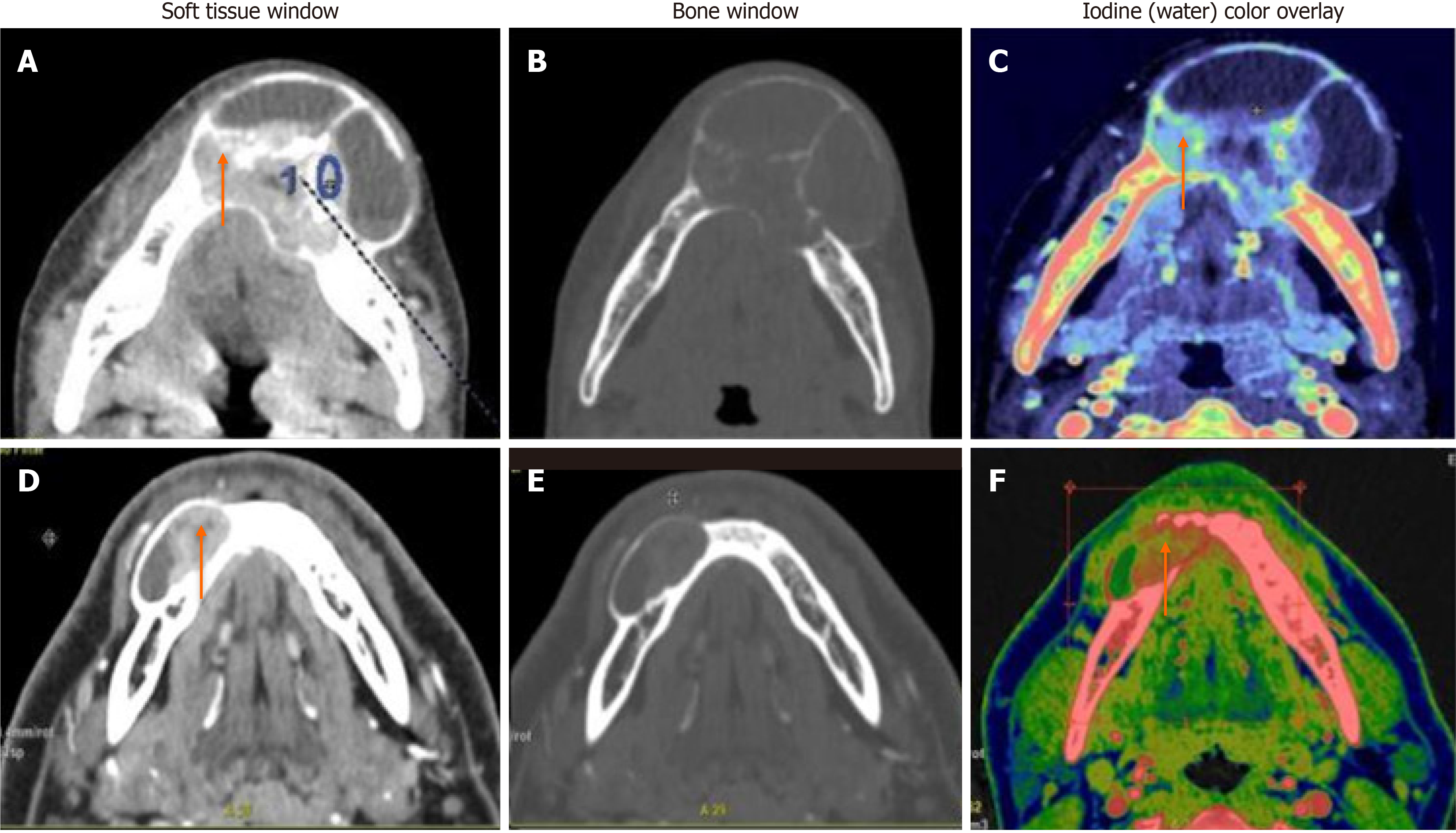
When we compared ameloblastoma and central giant cell granuloma lesions (n = 31), significant differences were found in all quantitative DECT parameters (P < 0.05). CGCGs showed a higher average iodine content (36.1 × 100 vs 29.8 × 100 μg/cm3), higher average WC (1042 × 1000 vs 1032 × 1000 mg/cm3), a higher mean HU at 65 Kev (151 vs 122 HU), and a higher NIC (0.59 vs 0.34) compared to ameloblastomas (Figure 3).
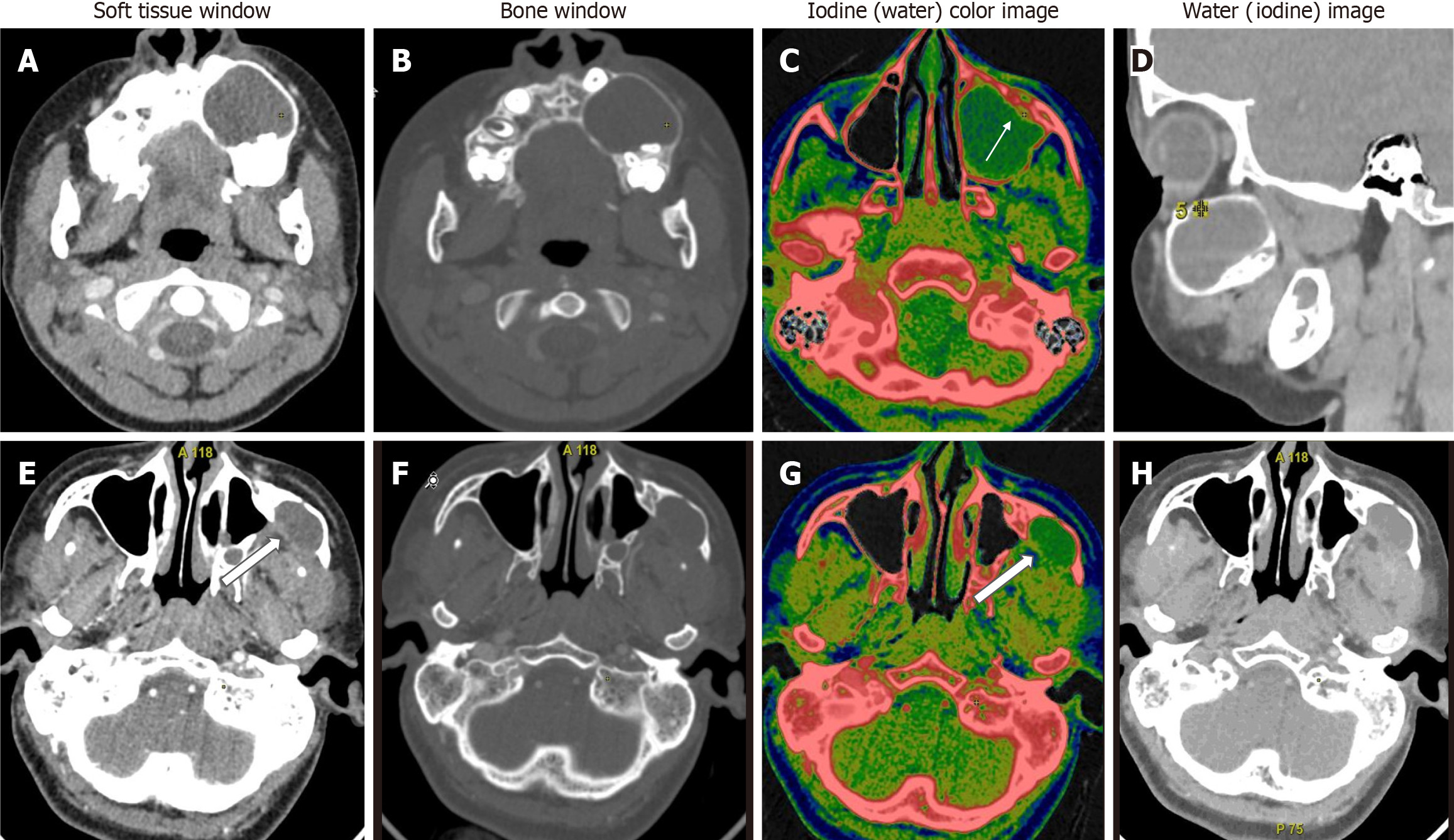
In comparison between ameloblastomas and OKCs (n = 26), both groups showed significant differences in all the DECT parameters. However, the diagnostic dilemma lies in the distinction between UA and OKCs, which appear similar in morphology on conventional CT. Hence, to make this comparison impactful, we compared the WC of the cystic component in addition to the DECT parameters mentioned above between UA and OKCs. Interestingly, in addition to the above quantitative parameters, which were statistically significant, the WC of the cystic component also showed statistically significant differences between the two subgroups (Figure 4). In the OKCs, significantly higher water content within the cystic component was observed compared to ameloblastomas. When the ameloblastomas were compared with the “other jaw tumor” group, the former showed a higher average iodine content, although not statistically significant, and a lower WC, which was marginally significant compared to the latter (Figure 5).
The comparison of ameloblastomas and CGCG yielded statistically significant differences and satisfied the sample size for receiver operating characteristic (ROC) analysis. Hence, ROC analysis for all the DECT parameters was performed, and based on the AUC values, we selected a threshold for each parameter with the largest areas under the ROC curves (Table 5).
| Variable | Cutoff value | Sensitivity, % | Specificity, % | AUC | SE |
| IC | 32.1 | 81.82 | 65.00 | 0.727 | 0.0955 |
| Mean HU | 134 | 81.82 | 65.00 | 0.789 | 0.0832 |
| WC | 1036 | 72.73 | 50.00 | 0.6659 | 0.1 |
| NIC | 0.4 | 81.82 | 70.00 | 0.795 | 0.0970 |
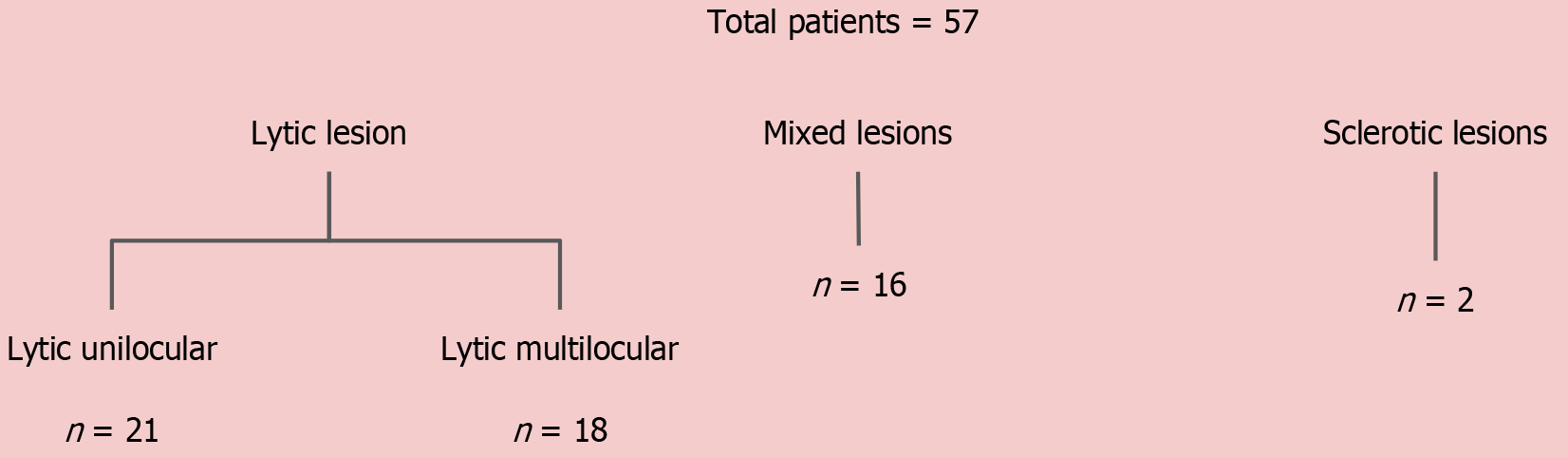
Because the majority of jaw lesions are diagnosed using a systematic approach based on morphological appearance, we attempted to categorize the lesions based on density and locularity as described above and then studied their DECT parameters, except for the sclerotic lesions. The mean values of the DECT parameters of the lesions in the different morphological subgroups are summarized in Table 6.
| Lytic unilocular (n = 21) | Amelo, n = 7 (mean ± SD) | OKC, n = 6 (mean ± SD) | CGCG, n = 2 (mean ± SD) | OF, n = 2 (mean ± SD) | Non-tumorous, n = 4 (mean ± SD) |
| Mean HU | 115 ± 18.3 | 33 ± 12.4 | 135 ± 24 | 87 ± 17.9 | 67 ± 17.9 |
| IC | 28 ± 8.3 | 7.2 ± 5.8 | 32.9 ± 6.8 | 18.8 ± 9.5 | 8.8 ± 9.5 |
| WC | 1030.5 ± 12.8 | 1010 ± 11.3 | 1043 ± 11.6 | 1038 ± 11.4 | 1018 ± 11.4 |
| NIC | 0.37 ± 0.15 | 0.10 ± 0.1 | 0.72 ± 0.3 | 0.45 ± 0.24 | 0.25 ± 0.24 |
| Lytic multilocular (n = 18) | Amelo, n = 8 (mean ± SD) | CGCG, n = 4 (mean ± SD) | SG tumors, n = 2 (mean ± SD) | Malignancy, n = 3 (mean ± SD) | Non-tumorous, n = 1 (mean ± SD) |
| Mean HU | 123 ± 21.3 | 152 ± 20.4 | 94.2 ± 24.3 | 120 ± 37.9 | 69 ± 18.9 |
| IC | 29 ± 9.3 | 35.1 ± 9.8 | 17.85 ± 6.8 | 24.8 ± 11.5 | 10 ± 2.5 |
| WC | 1032.5 ± 12.8 | 1048 ± 11.3 | 1038 ± 11.6 | 1040 ± 11.4 | 1011 ± 11.4 |
| NIC | 0.35 ± 0.15 | 0.54 ± 0.19 | 0.18 ± 0.05 | 0.39 ± 0.24 | 0.29 ± 0.1 |
| Mixed-sclerotic (n = 16) | Amelo, n = 5 (mean ± SD) | CGCG, n = 5 (mean ± SD) | OFs, n = 4 (mean ± SD) | CMF, n = 1 (mean ± SD) | Non-tumorous, n = 1 (mean ± SD) |
| Mean HU | 129 ± 20.3 | 158 ± 20.4 | 143.8 ± 24.3 | 164 ± 37.9 | 77 ± 16 |
| IC | 29.5 ± 9.3 | 38.2 ± 9.8 | 31.1 ± 6.8 | 49.8 ± 11.5 | 11.8 ± 9 |
| WC | 1032.5 ± 12.8 | 1048 ± 11.3 | 1043 ± 11.6 | 1037 ± 11.4 | 1028 ± 20.4 |
| NIC | 0.35 ± 0.15 | 0.60 ± 0.19 | 0.66 ± 0.25 | 0.37 ± 0.24 | 0.19 ± 0.14 |
We performed contrast-enhanced DECT with a predetermined split bolus contrast protocol in 57 patients with suspected maxillary and/or mandibular tumors or neoplasms after obtaining proper written informed consent, reviewing the clinical details, physical examination findings, and orthopantomogram. The morphological and quantitative spectral parameters obtained from DECT imaging were evaluated for the differentiation of various tumors of the jaw. The primary goal of the study was to identify qualitative and quantitative parameters for distinguishing ameloblastomas from non-ameloblastomas.
There was a slight female predominance and the majority, i.e., 77% of non-ameloblastomas comprised females compared to 35% in the ameloblastoma group. We studied the morphological features of lesions, and a comparison was made between the ameloblastoma and non-ameloblastoma groups. Median volume, degree of necrosis, inferior alveolar canal involvement, RMT involvement, and cortical involvement in the form of expansion or thinning were significantly higher in the ameloblastoma group. Our study agrees with these characteristics of ameloblastomas in other studies done previously in larger populations[7-10]. However, when the location was maxilla, there was no significant difference between the two groups. The rest of the variables, i.e., margins, relation to teeth, and soft tissue extension, showed no statistically significant difference between the two groups.
In this study, we also investigated the potential of using quantitative information provided by both the virtual monochromatic images and MD images in dual-energy spectral CT imaging for the differentiation of ameloblastomas and non-ameloblastomas. Iodine, as the main component of a contrast medium, allows the assessment of vascular beds and intercellular spaces, and it facilitates the differentiation of lesions at various locations in the body based on the assumption that malignant, aggressive, or vascular lesions exhibit a higher degree of contrast enhancement[11]. DECT allows the quantitative assessment of the concentration of iodine accumulated in a unit of tissue volume. The degree of angiogenesis indicates the degree of viability, the degree of malignancy, and the vascularization sources[5,12]. Although there were no studies evaluating the role of DECT in jaw tumors, various studies done elsewhere in the head and region showed MD images, especially IC images, can be used for the differentiation of various pathologies[4]. This was because it is now known that the IC value is more accurate than the CT value in assessing the blood supply to a lesion.
The higher IC in ameloblastomas can be attributed to the fact that these are slow-growing, locally invasive tumors with an explicit biologic pattern. Multiple stromal factors, including growth and angiogenic factors, extracellular matrix components, and proteinases, are overexpressed and linked to the development of this tumor, where they play critical roles in invasion, growth, and progression with aggressive behavior. This could explain the rise in metabolic activity in ameloblastoma connective tissue[13-16]. The non-ameloblastomas included a heterogeneous sample within the group that ranged from cystic lesions with enhancing wall/septae and virtually no enhancing mural component like OKCs to avidly enhancing solid lesions like CGCGs. This was also supported by the fact that statistical analysis revealed that the values of DECT parameters in OKCs and CGCCs were on the extreme opposite spectrum, with other lesions having values ranging in between. This led to further classification of the non-ameloblastomas and their comparison with amelo
On the first comparison between ameloblastomas and CGCG, the CGCGs had higher mean iodine, water, mean HU at 65 Kev, and NIC compared to ameloblastomas. This was in accordance with the earlier studies, which showed that central giant cell lesions had significantly higher angiogenetic potential compared to ameloblastomas[17,18]. The differential analysis based on the calculated threshold IC value showed that a value of 32.1 × 100 μg/cm3, best represented the differences based on the AUC values on the ROC curves, with a sensitivity and specificity of 81.8% and 65%, respectively.
In a comparison of ameloblastomas with OKCs, similar to morphological features, all quantitative parameters showed significant differences between the two lesions in our study[19]. Interestingly, in addition to the DECT quantitative parameters of enhancing components, the WC of the cystic component also showed a statistically significant difference between the two subgroups. In the OKCs, significantly higher water content within the cystic component was observed compared to ameloblastomas (1020 vs 997 μg/cm3). Our study showed that UA and OKCs could be effectively differentiated on the basis of the IC and WC measurements of the cystic component, as these lesions are often purely cystic (Figure 4). Our finding that the WC of the cystic areas differs significantly between the ameloblastomas and OKCs indicates that the density of the cystic components with suppressed iodine information varies between these odontogenic tumors. Cystic spaces in the ameloblastomas usually contain slightly proteinaceous fluids, occasionally associated with colloidal materials[20]. The cyst lumen of OKCs often contains desquamated keratin. This desquamated keratin accumulates in such large quantities that it influences the attenuations on CT images, which was even proven in an experimental study by Yoshiura et al[21]. Therefore, it is plausible that such desquamated keratin increased the viscosity of fluids in the lumen, thereby increasing the value of WC in the water (iodine) images compared with ameloblastomas, in which increases in viscosity may be minimal.
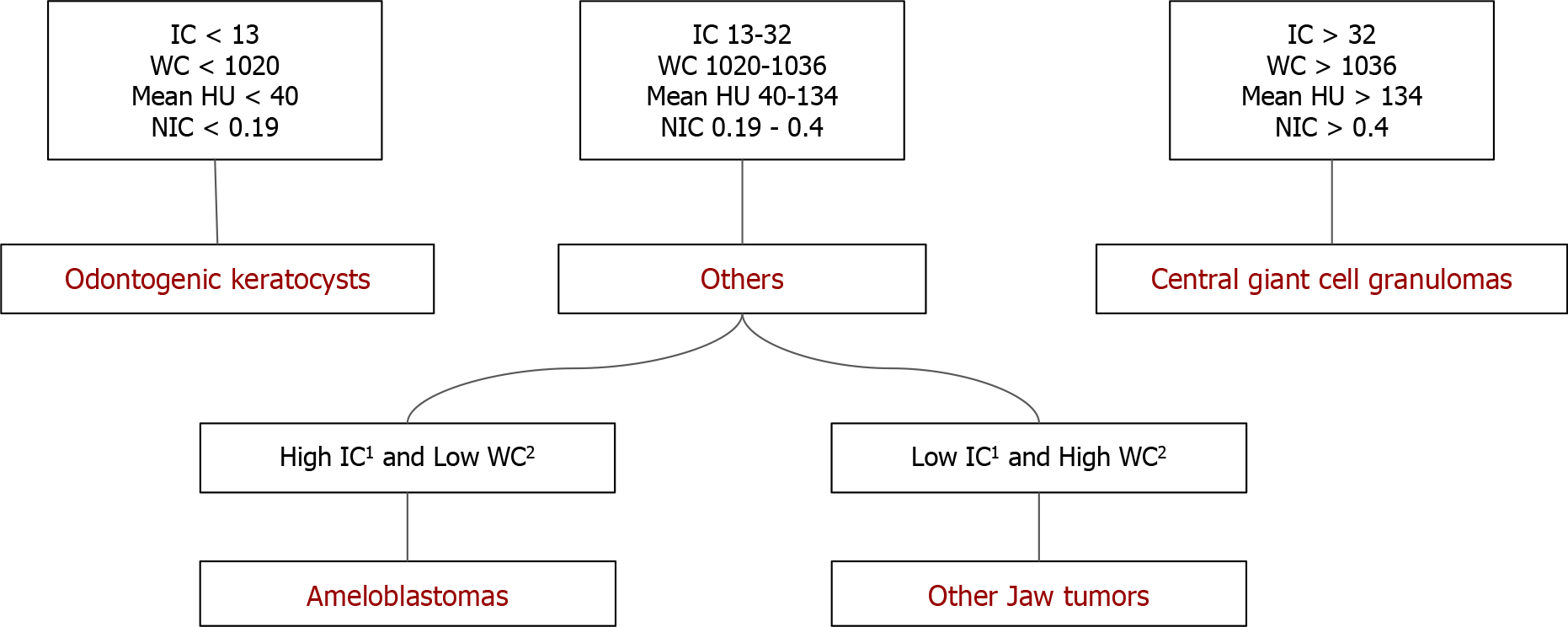
The above comparisons yielded an interesting fact: Ameloblastomas showed significantly increased values of DECT parameters, which were indirect markers of vascularity, compared to non-ameloblastomas except for the CGCG. As we can see, the latter has a significantly increased IC, mean HU value, WC, and NIC compared to ameloblastomas. The flowchart (Figure 6) presents an algorithmic approach to classifying jaw lesions based on differences in DECT quantitative parameters in our study.
The major limitation of the present study was the heterogeneous sample within the “other jaw tumor” group, which resulted in a limited comparison of separate pathological lesions. Another limitation was the inability to compare the DECT parameters based on the morphological subgroups due to the limited sample size.
We propose that DECT can help with both morphological and functional classification of jaw tumors, as well as dis
Faculty, residents, and radiographers of the Department of Radiodiagnosis and Interventional Radiology, All India Institute of Medical Sciences, New Delhi for their support and assistance. Mr. Hem Sati, Department of Biostatistics, AIIMS, New Delhi, for biostatistical guidance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: India
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Zhang Z, China S-Editor: Li L L-Editor: A P-Editor: Zhao S
| 1. | Soluk-Tekkeşin M, Wright JM. The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2017 (4th) Edition. Turk Patoloji Derg. 2018;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Dunfee BL, Sakai O, Pistey R, Gohel A. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics. 2006;26:1751-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 3. | McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology. 2015;276:637-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 1142] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 4. | Roele ED, Timmer VCML, Vaassen LAA, van Kroonenburgh AMJL, Postma AA. Dual-Energy CT in Head and Neck Imaging. Curr Radiol Rep. 2017;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Tawfik AM, Razek AA, Kerl JM, Nour-Eldin NE, Bauer R, Vogl TJ. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol. 2014;24:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Luo S, Sha Y, Wu J, Lin N, Pan Y, Zhang F, Huang W. Differentiation of malignant from benign orbital tumours using dual-energy CT. Clin Radiol. 2022;77:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | MacDonald-Jankowski DS, Yeung R, Lee KM, Li TK. Odontogenic myxomas in the Hong Kong Chinese: clinico-radiological presentation and systematic review. Dentomaxillofac Radiol. 2002;31:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Wu PC, Chan KW. A survey of tumours of the jawbones in Hong Kong Chinese: 1963-1982. Br J Oral Maxillofac Surg. 1985;23:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B:86-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 440] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Siar CH, Lau SH, Ng KH. Ameloblastoma of the jaws: a retrospective analysis of 340 cases in a Malaysian population. J Oral Maxillofac Surg. 2012;70:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Sanghavi PS, Jankharia BG. Applications of dual energy CT in clinical practice: A pictorial essay. Indian J Radiol Imaging. 2019;29:289-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Li L, Zhao Y, Luo D, Yang L, Hu L, Zhao X, Wang Y, Liu W. Diagnostic value of single-source dual-energy spectral computed tomography in differentiating parotid gland tumors: initial results. Quant Imaging Med Surg. 2018;8:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Chi AC, Neville BW. Odontogenic Cysts and Tumors. Surg Pathol Clin. 2011;4:1027-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ribeiro BF, Iglesias DP, Nascimento GJ, Galvão HC, Medeiros AM, Freitas RA. Immunoexpression of MMPs-1, -2, and -9 in ameloblastoma and odontogenic adenomatoid tumor. Oral Dis. 2009;15:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, Wang J, Pan C. Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro. BMC Cancer. 2008;8:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Jamshidi S, Zargaran M, Baghaei F, Shojaei S, Zare Mahmoodabadi R, Dehghan A, Moghimbeigi A. An Immunohistochemical Survey to Evaluate the Expression of CD105 and CD34 in Ameloblastoma and Odontogenic Keratocyst. J Dent (Shiraz). 2014;15:192-198. [PubMed] |
| 17. | Ali K, Zeb Khan S, Sultana N, Alghamdi O, Muhammad S, Mokeem SA, Ali S, Abduljabbar T, Vohra F. Assessment of Tumor Angiogenesis by Expression of CD 105 in Ameloblastoma, Odontogenic Keratocyst and Central Giant Cell Lesion. Asian Pac J Cancer Prev. 2020;21:3373-3379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Ghosh A, Lakshmanan M, Manchanda S, Bhalla AS, Kumar P, Bhutia O, Mridha AR. Contrast-enhanced multidetector computed tomography features and histogram analysis can differentiate ameloblastomas from central giant cell granulomas. World J Radiol. 2022;14:329-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 19. | Hayashi K, Tozaki M, Sugisaki M, Yoshida N, Fukuda K, Tanabe H. Dynamic multislice helical CT of ameloblastoma and odontogenic keratocyst: correlation between contrast enhancement and angiogenesis. J Comput Assist Tomogr. 2002;26:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Smoker WR, Harnsberger HR. Differential diagnosis of head and neck lesions based on their space of origin. 2. The infrahyoid portion of the neck. AJR Am J Roentgenol. 1991;157:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Yoshiura K, Higuchi Y, Ariji Y, Shinohara M, Yuasa K, Nakayama E, Ban S, Kanda S. Increased attenuation in odontogenic keratocysts with computed tomography: a new finding. Dentomaxillofac Radiol. 1994;23:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |