Published online Jun 28, 2023. doi: 10.4329/wjr.v15.i6.201
Peer-review started: December 23, 2022
First decision: March 28, 2023
Revised: April 17, 2023
Accepted: May 31, 2023
Article in press: May 31, 2023
Published online: June 28, 2023
Processing time: 186 Days and 10.5 Hours
Aneurysmal subarachnoid hemorrhage is an emergency that can lead to a high mortality rate and many severe complications. It is critical to make a rapid radiological evaluation of ruptured intracranial aneurysms (RIAs) to determine the appropriate surgical treatment.
To assess the reliability of computed tomography angiography (CTA) in assessing different features of ruptured intracranial aneurysm and its impact on patient management.
The final cohort of this study consisted of 146 patients with RIAs (75 male and 71 female) who underwent cerebral CTA. Their age ranged from 25 to 80, and the mean age ± SD was 57 ± 8.95 years. Two readers were asked to assess different features related to the aneurysm and perianeurysmal environment. Inter-observer agreement was measured using kappa statistics. Imaging data extracted from non-contrast computed tomography and CTA were considered to categorize the study population into two groups according to the recommended therapeutic approach.
The inter-observer agreement of both reviewers was excellent for the detection of aneurysms (K = 0.95, P = 0.001), aneurysm location (K = 0.98, P = 0.001), and (K = 0.98, P = 0.001), morphology (K = 0.92, P = 0.001) and margins (K = 0.95, P = 0.001). There was an excellent interobserver agreement for the measurement of aneurysm size (K = 0.89, P = 0.001), neck (K = 0.85, P = 0.001), and dome-to-neck ratio (K = 0.98, P = 0.001). There was an excellent inter-observer agreement for the detection of other aneurysm-related features such as thrombosis (K = 0.82, P = 0.001), calcification (K = 1.0, P = 0.001), bony landmark (K = 0.89, P = 0.001) and branch incorporation (K = 0.91, P = 0.001) as well as perianeurysmal findings including vasospasm (K = 0.91, P = 0.001), perianeurysmal cyst (K = 1.0, P = 0.001) and associated vascular lesions (K = 0.83, P = 0.001). Based on imaging features, 87 patients were recommended to have endovascular treatment, while surgery was recommended in 59 patients. 71.2% of the study population underwent the recommended therapy.
CTA is a reproducible promising diagnostic imaging modality for detecting and characterizing cerebral aneurysms.
Core Tip: This study evaluated the interobserver reliability of computed tomography angiography (CTA) in the assessment of ruptured intracranial aneurysm features among 146 patients. Our results showed good to an excellent inter-observer agreement in imaging features related to the aneurysm, aneurysm characters, measurements, and perianeurysmal information. Imaging data extracted from non-contrast computed tomography and CTA guided the multidisciplinary neurovascular team to better treatment approach selection.
- Citation: Elmokadem AH, Elged BA, Abdel Razek A, El-Serougy LG, Kasem MA, EL-Adalany MA. Interobserver reliability of computed tomography angiography in the assessment of ruptured intracranial aneurysm and impact on patient management. World J Radiol 2023; 15(6): 201-215
- URL: https://www.wjgnet.com/1949-8470/full/v15/i6/201.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i6.201
Acute subarachnoid hemorrhage (SAH) is a medical emergency that can lead to a high mortality rate and many severe complications. The most frequent cause of non-traumatic SAH is a ruptured aneurysm. Ruptured aneurysms not only cause subarachnoid bleeding but can also cause subdural or intracerebral hematomas. It is critical to make a rapid radiological evaluation of ruptured intracranial aneurysms (RIAs) to determine the appropriate surgical treatment of SAH[1]. Different imaging modalities are used or have been used to assess intracranial vascular lesions. Digital subtraction angiography (DSA) has been considered the “gold standard” for detecting intracranial aneurysms and therapeutic decision-making. Additionally, intra-arterial cone beam computed tomography angiography (CTA) using a flat panel detector incorporates the high spatial vascular resolution of three-dimensional (3D) rotational angiography with computed tomography (CT) postprocessing techniques to increase the contrast resolution[2]. Nevertheless, it is an invasive and time-consuming technique with a reported 0.5% risk of neurological complications[3]. Magnetic resonance (MR) angiography enables visualization of intra-cranial arteries without ionizing radiation or intravenous contrast material. The performance of MR angiography in evaluating acute SAH also has been compared favorably with that of DSA. However, MR angiography can be technically challenging for acutely ill patients. Additionally, aneurysms less than 5 mm in diameter might be missed in MR angiography studies[4].
Compared to DSA, CTA is a safe, relatively inexpensive, and noninvasive imaging. CT angiography is not associated with significant patient risks other than those related to administering iodinated contrast media. Images can be relatively safely obtained without the need for arterial puncture or catheter manipulation that carries the possibility of acquiring a permanent neurologic deficit[5]. For these reasons, helical cerebral CT angiography has been widely used in detecting intracranial aneurysms, with a reported sensitivity of 70%–96%[6]. Currently, multidetector CT angiography has the ability to detect most intracranial aneurysms of 5 mm or larger. Multidetector row CT scanners provide increased spatial resolution and decreased scanning time, which should increase the sensitivity of the technique in depicting aneurysms of less than 5 mm in diameter. Currently, CT angiography is accepted as a first-line diagnostic imaging modality for evaluating intracranial aneurysms. Nevertheless, using CT angiography to depict intracranial aneurysms showed variable sensitivity. The reported sensitivity of CTA in detections of small intracranial aneurysms (< 3 mm) varied from 28%–43% in a study that included 99 small aneurysms[7] to 83%-92% in another study had 579 small aneurysms[8].
Deciding whether to perform surgical clipping or endovascular therapy for an intracranial aneurysm is vital. Treatment of intracranial aneurysms depends on clinical features, including age, Hunt and Hess grade, neurological or medical comorbidity, and characters of the aneurysm itself. Advances in imaging technologies have improved our comprehension of the 3D geometry of cerebral aneurysms, which has further affected treatment procedures, and helps neurosurgeons in the diagnosis, planning, and assessment of the therapeutic modalities. Exact information must be obtained preoperatively regarding the location, size, and morphology of the aneurysm and the property of the aneurysmal wall, its relationship with the parent vessel and adjacent branches, vessel incorporation, presence of calcification or thrombus[9]. Perianeurysmal information such as associated vasospasm, vascular variants, presence of vascular malformations, and the relationship of the aneurysm to the bony skull base is also crucial for proper treatment method selection.
This work aims to assess the inter-observer reliability of CTA in assessing different features of RIAs and their impact on patient management.
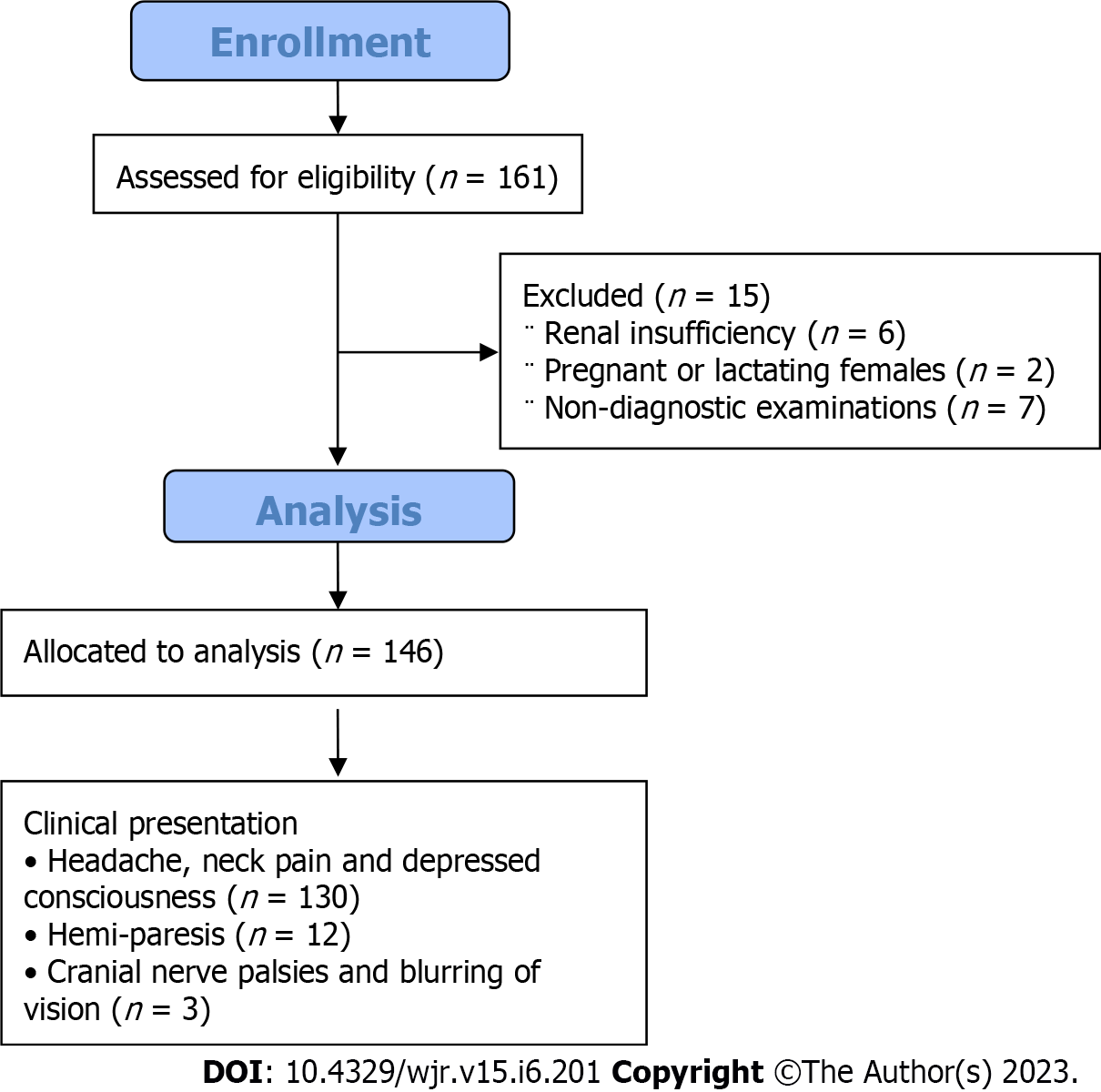
The Institutional review board approved this single-center prospective study. One hundred sixty-one consecutive patients with clinically suspected intracranial aneurysms were recruited. Inclusion criteria were adult patients with imaging signs of SAH by non-enhanced CT. The exclusion criteria were patients with renal insufficiency (n = 6), pregnant or lactating females (n = 2), and non-diagnostic examinations (n = 7). The final cohort of our study included 146 patients (75 male and 71 female). Their age ranged from 25 to 80 years, with a mean age was 57 ± 8.9 years. Once enrolled, all patients were submitted to a contrast-enhanced CTA scan for diagnosis of intracranial aneurysm. The commonest clinical presentation was a headache, neck pain, and depressed consciousness in 130 patients, hemiparesis in 12 patients, cranial nerve palsies, and blurring of vision being less common presenting symptoms in 3 patients. One patient presented in an accident. The flow chart of our study is illustrated in (Figure 1).
All examinations were carried out with 160-slice computed tomography (Toshiba Aquilion Prime 160, Tochigi, Japan). Examinations were performed in a supine position with the arms of the patients beside the body. We first performed a non-contrast CT (NCCT) scan of the whole brain in an axial plane tilted along the orbitomeatal line using the following parameters: tube voltage 100 kVp, field of view (FOV) 220 mm, collimation 0.75 mm, matrix size 512 × 512, slice thickness 3.0 mm, and rotation time 1.0 s. CTA examinations were achieved using the bolus triggering technique for the timing of contrast medium injection. A region of interest (ROI) was centered at the upper end of the common carotid artery and sized to include only the lumen of the artery. The bolus triggering technique started automatically after contrast-medium injection when an attenuation value of 100 HU was reached in ROI. Non-ionic contrast medium Ultravist 370 (370 mgI/mL, Bayer Healthcare Ltd, Guangzhou, China) at a dose of 1 mL/Kg and rate of 4mL/s followed by a 50 mL saline flush administered via automatic injector (Medrad Stellent, Warrendale, PA) through a 20 G intravenous cannula in the antecubital fossa of either upper extremity. The following parameters were used: Tube voltage 120 kVp, automatic tube current modulation, FOV 250 mm, collimation 0.75 mm, matrix size 512 × 512, slice thickness 1.5 mm, rotation time 0.3 s. Scanning was performed in the caudocranial direction and started at the level of the aortic arch up to the vertex. Axial images were stored and transferred into the workstation as source images after reconstruction at 0.625-mm intervals. Multiplanar reformats (MPR), maximum intensity projection (MIP), and 3D volume-rendered images were used for further analysis.
Image analysis was conducted on the PACS system (GE Healthcare, Waukesha, WI). All CTA examinations were revised and interpreted by two experienced neuroradiologists with 14 and 8 years of experience in neurovascular imaging (AHE, BE). They independently reviewed the images blinded to patient data and final diagnosis. The following features obtained at NCCT were individually evaluated for each patient: The degree of SAH using the Fisher scale, associated parenchymal hemorrhage, hydrocephalus, and ischemic changes. Both readers were asked to predict the location of the aneurysm based on the distribution of SAH and Intraparenchymal hematoma (IPH). At CTA, both readers were asked to evaluate the following aneurysm features’: (1) Number; (2) Size; (3) Location (anterior vs posterior circulation, parent artery, and affected segment); (4) Shape (saccular, fusiform, serpentine or blister); (5) Margin (sharp, irregular/daughter sacs); (6) Presence of calcification and/or thrombus; (7) The presence of branch incorporation; and (8) The orientation of the aneurysm in relation to the parent vessel (e.g., pointing superior/inferior, medial/Lateral, anterior/posterior). Aneurysm size was defined by the maximum measurement of aneurysm width, neck width (the largest cross-sectional diameter of the aneurysm neck), depth (the longest diameter between the neck and dome), and aneurysm neck-to-dome length. According to size, aneurysms were classified into: (1) Small aneurysms are less than 5 mm; (2) Medium-sized aneurysms are 6–15 mm; (3) Large aneurysms are 16–25 mm; and (4) Giant aneurysms are > 25 mm. The following features related to the perianeurysmal environment were evaluated by both readers: (1) Associated vasospasm; (2) Perianeurysmal cysts; (3) Associated vascular malformations (type and classification based on relation to aneurysm); (4) Vascular variants; and (5) The relationship of the aneurysm to the bony landmarks or related osseous changes (remodeling or fractures).
Imaging data extracted from NCCT and CTA were considered to categorize the study population into two groups according to the recommended therapeutic approach. The first group was recommended to proceed to endovascular therapy, and the second group was advised to undergo surgical management (clipping).
Features in favor of endovascular therapy (coiling) were: (1) Absence of parenchymal hematoma; (2) Posterior circulation aneurysm; (3) Small aneurysm neck < 4 mm; (4) Aneurysm diameter < 15 mm; (5) Unilobar shape; (6) Multiple aneurysms; (7) Calcification located at the aneurysm neck that might interfere with clipping; and (8) Presence of vasospasm. Coiling using balloon remolding technique or stent-assisted coiling was recommended with wide-neck aneurysms.
Features favored surgical management (clipping) Were (1) Presence of intra-parenchymal hematoma; (2) Middle cerebral and pericallosal arteries aneurysm; (3) Wide neck of the aneurysm > 4 mm; (4) Aneurysm diameter > 15 mm; (5) Abnormal configuration unsuitable for endovascular therapy; (6) Aneurysmal sac incorporating arterial branches; (7) Presence of intraluminal thrombus; and (8) Vascular variant interferes with endovascular management. Features considered in managing RIAs are listed in Table 1.
| Endovascular therapy | Surgical management | |
| Non-contrast computed tomography | Absence of parenchymal hematoma | Presence of intra-parenchymal hematoma |
| Aneurysm characters | Posterior circulation aneurysms | Middle cerebral and pericallosal arteries aneurysm |
| Small aneurysm neck < 4 mm | Aneurysm diameter > 15 mm Wide neck aneurysm > 4 mm | |
| Aneurysm diameter < 15 mm | Abnormal configuration | |
| Unilobar configuration | Aneurysmal sac incorporating arterial branches | |
| Multiple aneurysms | Presence of intraluminal thrombus | |
| Calcification located at the aneurysm neck | ||
| Perianeurysmal findings | Presence of vasospasm | Vascular variants interfere with endovascular management |
All statistical analyses were performed with software (SPSS, version 16.0; SPSS, Chicago, Ill). The Kolmogorov-Smirnov Z-test was used to test the normality of the continuous variable groups. The CTA image quality scores were ranked and listed as a mean and standard deviation. The kappa statistic (K), including a 95%CI with an intra-class correlation (r), was used to estimate the proportion of interobserver agreement beyond that expected by chance for the image quality grading and depiction of the aneurysm, its neck, parent artery, and associated anomalies. A P value less than 0.05 was considered to indicate a statistically significant difference. The K values were interpreted as follows: K values between 0.61 and 0.80 represented good; K values between 0.81 and 1.00 represented excellent agreement.
Both readers used the Fisher scale to assess SAH. The means scores were (3.47 ± 0.6 and 3.33 ± 0.4) with a good interobserver agreement (K = 0.79, P = 0.001). Excellent interobserver agreement between both readers in the detection of IPH (K = 0.1, P = 0.001) as well as in the detection of hydrocephalus (K = 0.92, P = 0.001). A statistically significant strong positive correlation was found between the location of SAH and IPH on NCCT scan and the eventual location of a ruptured cerebral aneurysm in single ones and as a differentiating point of culprit aneurysm in multiple aneurysms confirmed by CTA and surgical or endovascular intervention. The sensitivity of NCCT predicting aneurysms depends on blood distribution of more than 85% in our study. Table 2 shows the inter-observer results of CT and CTA findings.
| Signs | Reader 1 | Reader 2 | K | 95% CI | P value | Agreement, % | |
| NCCT findings | |||||||
| SAH Fisher scale | 3.47 ± 0.6 | 3.33 ± 0.4 | 0.84 | 0.78-0.85 | < 0.001 | 89.50 | |
| Hydrocephalus (HCP) | Negative (N) | 93 | 95 | 0.91 | 0.85-0.97 | < 0.001 | 95.90 |
| Positive (p) | 53 | 51 | |||||
| Intraparenchymal hematoma (IPH) | Negative (N) | 105 | 105 | 1 | 1.0-1.0 | < 0.001 | 100.00 |
| Positive (p) | 41 | 41 | |||||
| Aneurysm features | |||||||
| Number | Total | 166 | 169 | 0.95 | 0.89-1.0 | < 0.001 | 98.80 |
| Single | 126 | 127 | |||||
| Multiple | 40 | 42 | |||||
| Size | Small | 55 | 57 | 0.89 | 0.83-0.96 | < 0.001 | 94.70 |
| Medium | 101 | 102 | |||||
| Large | 5 | 5 | |||||
| Giant | 5 | 5 | |||||
| Site | Anterior | 156 | 157 | 0.94 | 0.83-1.0 | < 0.001 | 99.40 |
| Posterior | 10 | 12 | |||||
| Shape | Saccular | 160 | 162 | 0.92 | 0.76-1.0 | < 0.001 | 99.40 |
| Fusiform | 4 | 4 | |||||
| Serpentine | 2 | 2 | |||||
| Blister | 0 | 1 | |||||
| Margin | Sharp | 71 | 75 | 0.95 | 0.90-0.99 | < 0.001 | 97.60 |
| Lobulated | 95 | 94 | |||||
| Thrombus | 6 | 9 | 0.82 | 0.65-0.99 | < 0.001 | 97.60 | |
| Calcification | 9 | 9 | 1 | 1.0-1.0 | < 0.001 | 100.00 | |
| Branch incorporation | 50 | 55 | 0.9 | 0.83-0.97 | < 0.001 | 95.90 | |
| Orientation | 0.95 | 0.91-0.97 | < 0.001 | 96.50 | |||
| Perianeurysmal findings | |||||||
| Vasospasm | 21 | 25 | 0.9 | 0.81-0.99 | < 0.001 | 97.60 | |
| Perianeurysmal cyst | 1 | 1 | 1 | 1.0-1.0 | < 0.001 | 100.0 | |
| Other vascular lesions | 2 | 4 | 0.82 | 0.59-1.0 | < 0.001 | 98.80 | |
| Bony relations | 23 | 26 | 0.88 | 0.78-0.98 | < 0.001 | 97.00 | |
| Bone fracture | 1 | 1 | |||||
| Vascular variant | 84 | 88 | 0.89 | 0.83-0.96 | < 0.001 | 94.90 | |
| Overall agreement | 0.95 | 0.94-0.96 | < 0.001 | 97.80 | |||
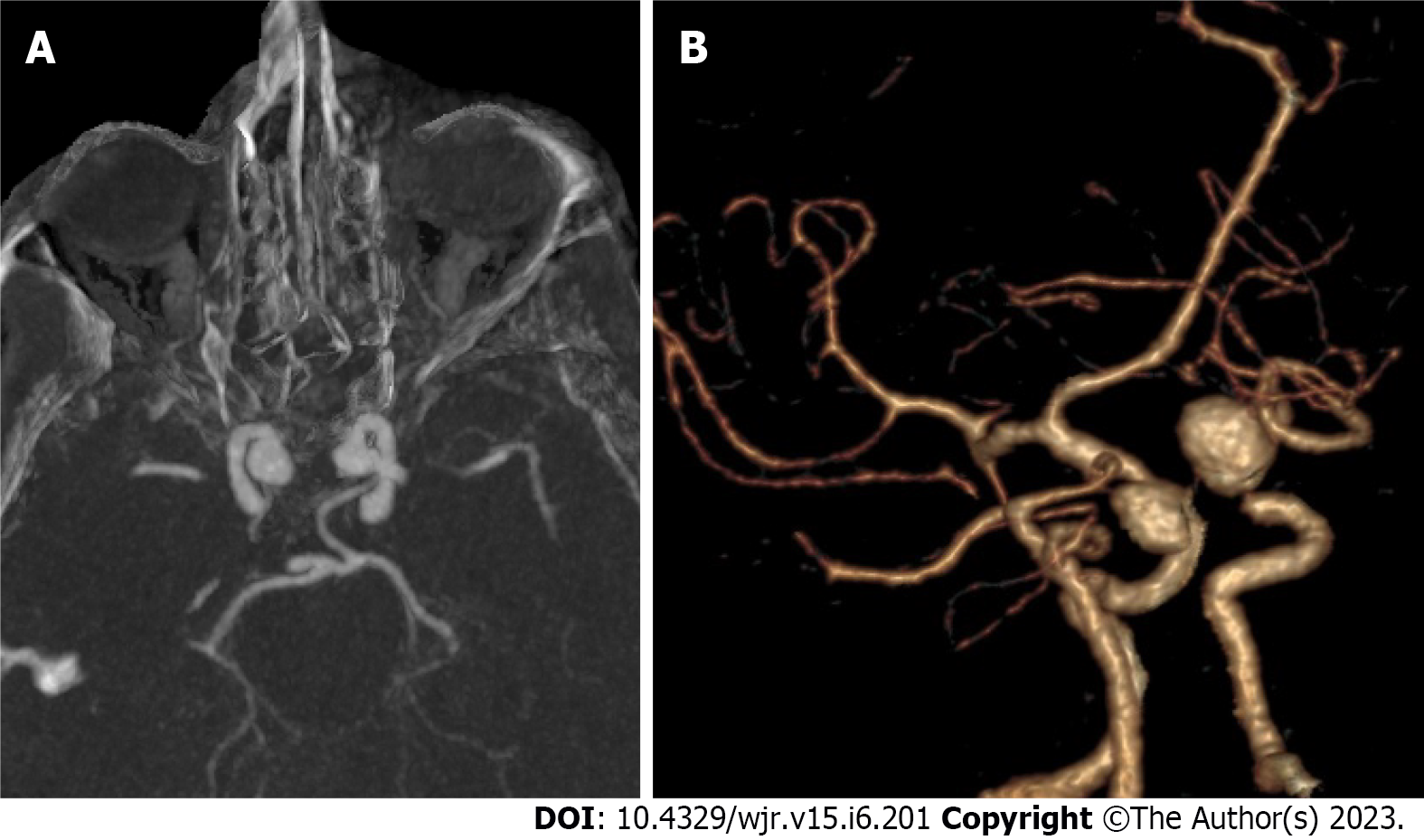
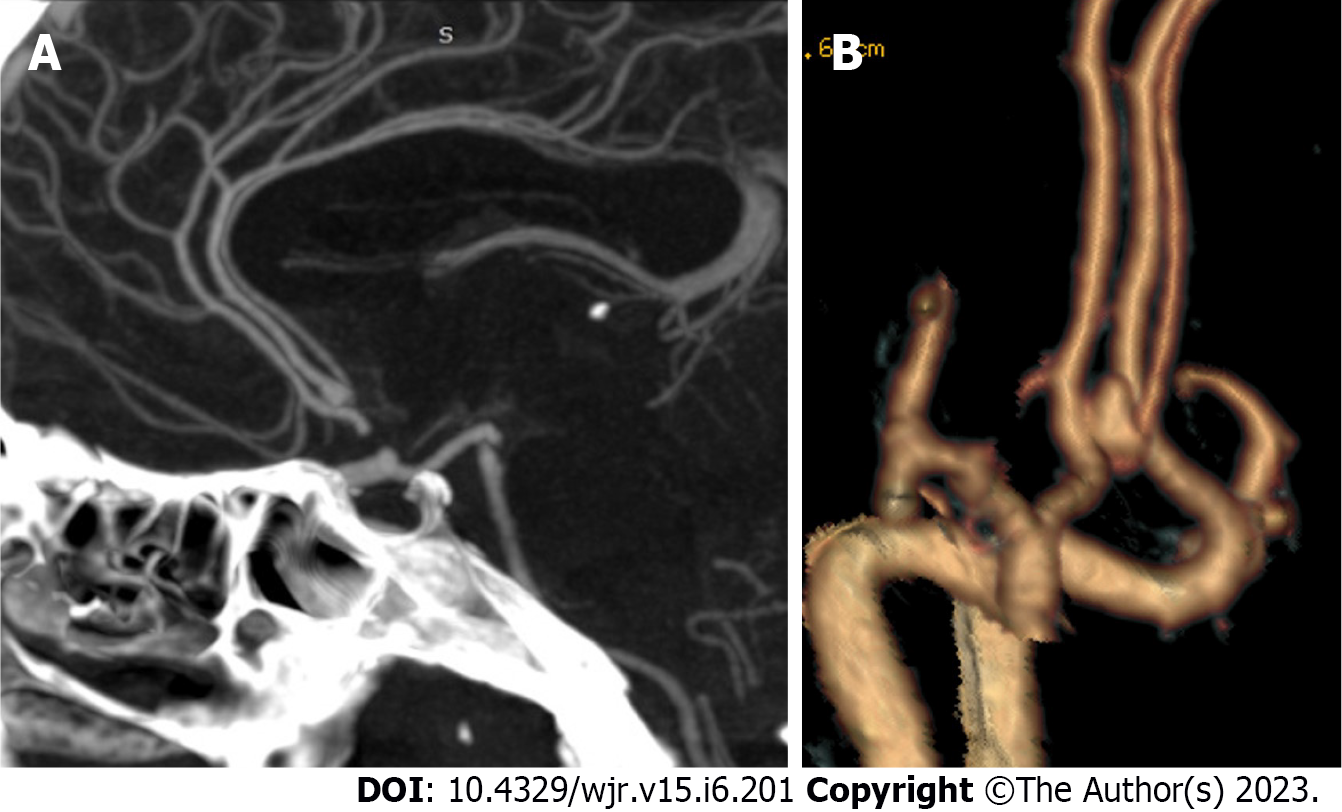
Detection: The first reader detected 166 aneurysms [126 single aneurysms (75.9%) and 40 multiple aneurysms (24.1%)], while 2nd reader detected 169 aneurysms [127 single aneurysms (75.1%) and 42 multiple aneurysms (24.9%)]. An excellent interobserver agreement was found between both readers in detecting an aneurysm [98.8% (K = 0.95, P = 0.001)]. Among multiple aneurysms, 14 were mirror image aneurysms (MA), and the remaining were non-mirror image (NMA). The commonest location of MA in our series was the M1 segment or at the middle cerebral artery (MCA) bifurcation (n = 3), followed by the cavernous portion of internal carotid artery (ICA) (n = 2) (Figure 2), the posterior communicating artery (PCOM) (n = 1), and ICA terminus (n = 1). The sensitivity of CTA compared to DSA in the detection of intracranial aneurysms was 98.8%. DSA identified 89 aneurysms in 80 patients. Among these aneurysms, CTA failed to detect one in a patient with three other aneurysms. The missed aneurysm was located in posterior cerebral artery (PCA) with diameters of 3 mm. In two cases, CTA depicted aneurysms, which DSA missed. An ICA blister and a distal MCA mycotic aneurysm were the two missed aneurysms.
Location: For most of the study, the population was harboring anterior circulation aneurysms (94%). Excellent interobserver agreement was found between both readers in the detection of aneurysms in the anterior and posterior circulation (K = 0.95, P = 0.001) as well as its site in parent artery and segment (K = 0.98, P = 0.001), and (K = 0.98, P = 0.001). In this study, Acom was the most affected artery, and Acom-left anterior cerebral artery (ACA) complex was the most affected segment. Both readers successfully detected intracranial aneurysms in relatively rare locations such as basilar perforators (2/L46), distal ACA (4/L46), pericallosal artery (2/L46), and azygous ACA (2/L46). Inter-observer results of the CTA location of the aneurysm are summarized in Table 3.
| Parent artery | Reader 1 | Reader 2 |
| ACOM | 73 | 73 |
| ACA | 5 | 6 |
| RT ICA | 20 | 20 |
| LT ICA | 16 | 16 |
| RT MCA | 21 | 22 |
| LT MCA | 20 | 20 |
| Basilar | 8 | 9 |
| LT PCA | 1 | 1 |
| RT Vertebral | 1 | 1 |
| LT SCA | 0 | 1 |
| K | 95%CI | P value |
| 0.97 | 0.87-1.0 | < 0.001 |
| Arterial segment | Reader 1 | Reader 2 |
| ACOM- Lt ACA complex | 36 | 36 |
| ACOM- Rt ACA complex | 34 | 34 |
| ACOM proper | 3 | 3 |
| A1 | 1 | 1 |
| RT A2 | 1 | 1 |
| LT A2 | 0 | 1 |
| Azygous A2 | 2 | 2 |
| A3 | 1 | 1 |
| RT MCA bifurcation | 14 | 14 |
| LT MCA bifurcation | 15 | 15 |
| RT M1 | 4 | 4 |
| LT M1 | 3 | 3 |
| RT M2 | 3 | 3 |
| LT M2 | 2 | 2 |
| M4 | 0 | 1 |
| RT PCOM | 12 | 12 |
| LT PCOM | 9 | 9 |
| RT ICA Terminus | 4 | 4 |
| LT ICA Terminus | 2 | 2 |
| RT Cavernous ICA | 4 | 4 |
| LT Cavernous ICA | 5 | 5 |
| Basilar tip | 4 | 4 |
| Mid Basilar | 2 | 3 |
| Basilar Perforators | 2 | 2 |
| Rt V4 | 1 | 1 |
| LT SCA | 0 | 1 |
| Lt P1 | 1 | 1 |
| K | 95%CI | P value |
| 0.97 | 0.87-1.0 | < 0.001 |
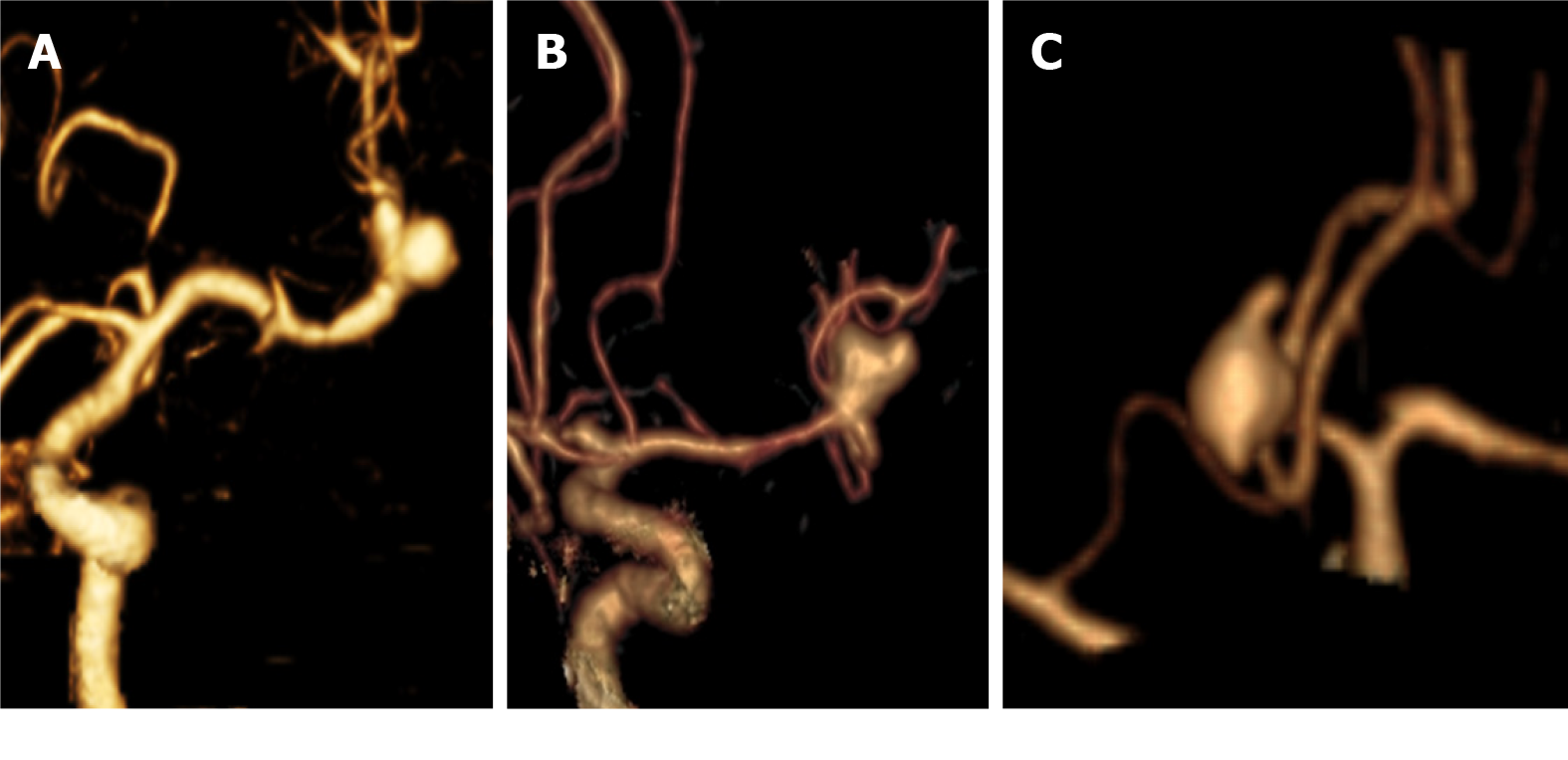
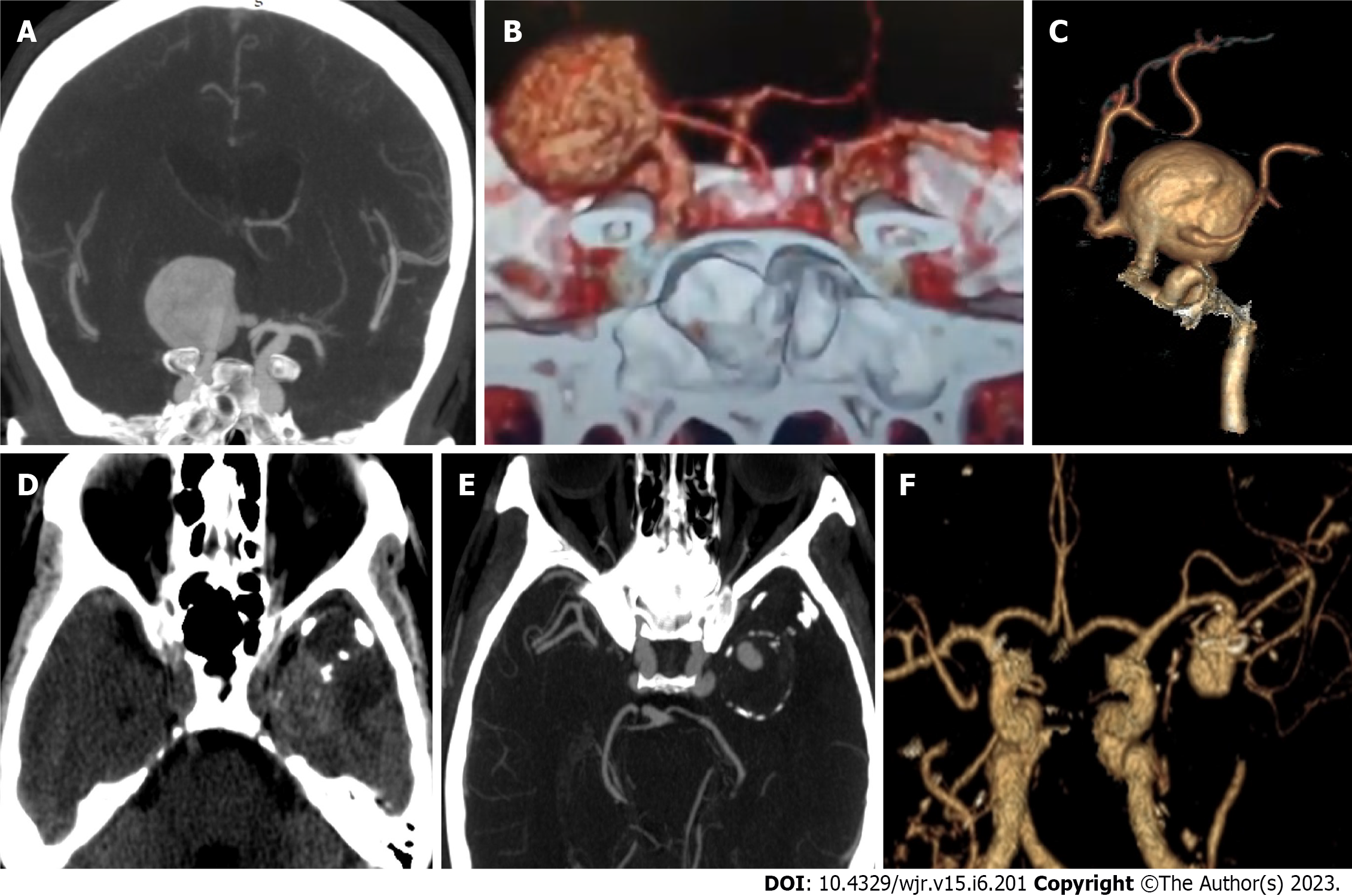
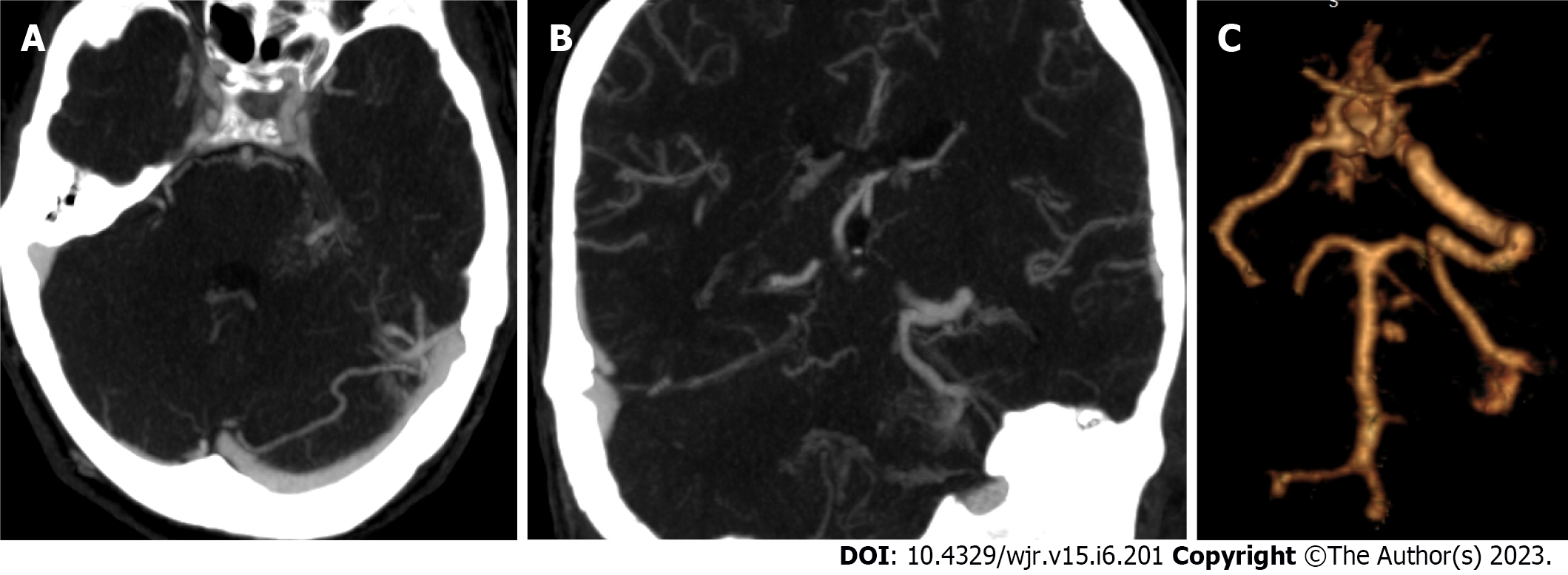
Morphology: Among the assessed aneurysms, (96.4%) were saccular, (2.4%) were fusiform, and (1.1%) were serpentine (Figure 3). Excellent interobserver agreement was found between both readers in the detection of aneurysm shape (K = 0.92, P = 0.001) and for margins (K = 0.95, P = 0.001). Other features such as calcification or thrombus, branch incorporation, and aneurysm orientation also show excellent inter-observer agreement. Calcification and thrombosis were detected in large and giant aneurysms (Figure 4). Branch incorporation was found in association with 55 aneurysms, one of them showed 3rd A2 segment incorporated Acom aneurysm dome (ACA trifurcation), to the best of the author’s knowledge this association is not reported before in the literature (Figure 5).
Measurements: Most cases were medium-sized aneurysms (60.8%), followed by small-sized (30.1%). Both readers measured aneurysm size, neck, and dome-to-neck ratio with an excellent interobserver agreement (K = 0.89, P = 0.001), (K = 0.85, P = 0.001), and (K = 0.98, P = 0.001). Inter-observer results of CTA measurements of an aneurysm are summarized in Table 4.
| Size | Dome | Neck | Dome/neck ratio | |||||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Mean | 7.4 | 7.4 | 3.7 | 3.6 | 2.7 | 2.7 | 1.3 | 1.3 |
| Median | 6 | 6 | 3 | 3 | 2.3 | 2.3 | 1.2 | 1.2 |
| SD | 5.44 | 5.47 | 3.2 | 3.1 | 2.1 | 2.1 | 0.8 | 0.8 |
| Minimum | 1.8 | 1.8 | 1 | 1 | 1 | 1 | 0.2 | 0.2 |
| Maximum | 40 | 42 | 22 | 22 | 20 | 20 | 4.7 | 5.6 |
| ICC | 0.97 | 0.97 | 0.96 | 0.98 | ||||
| 95%CI | (0.96-0.98) | (0.96-0.97) | (0.95-0.97) | (0.97-0.98) | ||||
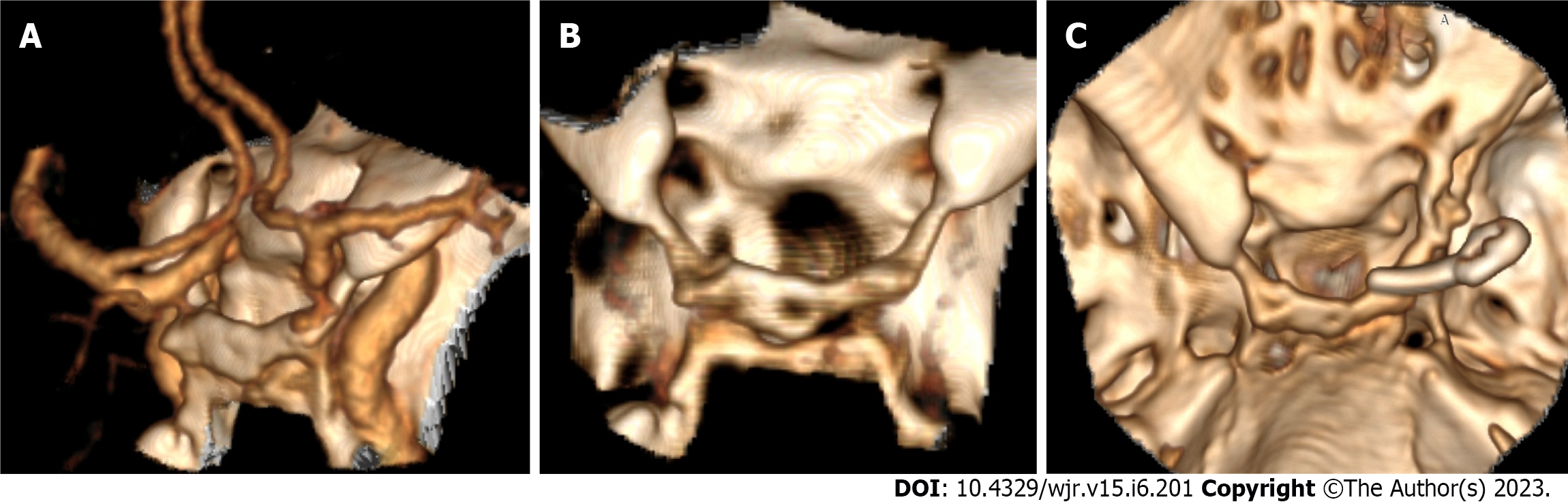
Peri-aneurysm findings: Excellent interobserver agreement was found between both readers in detecting vasospasm in the nearby arterial segment (K = 0.91, P = 0.001) and detection of the perianeurysmal cyst (K = 1.0, P = 0.001). Associated vascular lesions, including arterio-venous malformation (AVM) and developmental venous anomalies (DVA) (Figure 6), were detected by both readers with an excellent interobserver agreement (K = 0.83, P = 0.001). Vascular variations commonly affected the A1 segment, followed by PCA and MCA. Both readers detected an important bony variant in a case presented with a PCOM aneurysm in the form of a bridging sella turcica which was managed surgically by clitoridectomy (Figure 7).
Management: Based on imaging features, 87 patients were recommended to have endovascular treatment, while surgery was recommended in 59 patients. 71.2% of the study population underwent the recommended therapy, as 19 patients did not receive any therapy for the culprit, 66 underwent endovascular treatment, and 61 underwent surgical clipping. Only 15% of the study population needed external ventricular drain (EVD) to manage associated hydrocephalus.
This study evaluated the interobserver reliability of CTA in the assessment of ruptured intracranial aneurysm features among 146 patients. Our results showed good to an excellent inter-observer agreement in imaging features related to the aneurysm, aneurysm characters, measurements, and perianeurysmal information. Imaging data extracted from NCCT and CTA guided the multidisciplinary neurovascular team to better treatment approach selection.
Our results showed high sensitivity of NCCT (85%) in predicting aneurysms’ location depending on blood distribution with a good inter-observer agreement regarding the Fischer scale scores for SAH. The combination of NCCT images and CTA is a valuable tool for detecting intracranial aneurysms and predicting outcomes. Aneurysm detection could be expected by assessing the distribution of SAH, the site of the largest clot, the difference in attenuation, and the presence of mural calcification. A recent study performed on patients with SAH and multiple cerebral aneurysms found that NCCT can identify the source of bleeding with high accuracy for ACA, ACom, and MCA aneurysms, especially with the presence of intracranial hemorrhage (ICH)[10]. Our results showed excellent inter-observer agreement for detecting acute hydrocephalus with a variable degree of acute ventricular dilation, yet only 15% of our cases need management by EVD. Our results were close to those of the study performed on 389 patients with aneurysm-associated SAH[11].
We found that CTA has a high sensitivity for detecting intracranial aneurysms and avoids false-negative results. In a meta-analysis of extracted data from 8 studies, CTA had a pooled sensitivity of 99% and specificity of 94% for detecting and ruling out cerebral aneurysms[12]. Our study had an excellent interobserver agreement for detecting the aneurysms; the first reader detected 166 aneurysms, while the second reader detected 169 aneurysms. Yang et al[8] revealed a similar discrepancy in number detection by two observers, as one reviewer missed three aneurysms[8]. The inter-observer discrepancy usually occurs in a set of multiple small aneurysms in uncommon locations, such as the distal MCA. In this study, we detected mirror MA in 7 patients. Our results concord with the data obtained by a recent study by Rajagopal et al[13].
In our study, the anterior cerebral circulation aneurysms remained the predominant location of all aneurysms detected by CTA, constituting about 94% of the anterior and 6% of the posterior circulation. Acom aneurysm is the most common location of aneurysm in our results followed by ICA and MCA aneurysms, respectively. A similar distribution was reported by previous studies that assessed CTA vs DSA in the detection of intracranial aneurysms[14,15]. Imaging is vitally important to determine the location of the aneurysm and its relationship to the surrounding anatomy. Data from many studies suggest that surgical clipping may be superior in managing anterior circulation aneurysms with low recurrent rates. In contrast, posterior circulation aneurysms may be better treated through an endovascular approach[16]. Both readers successfully detected intracranial aneurysms in relatively rare locations, such as basilar perforators and azygous ACA. These findings aligned with prior studiesstating that basilar perforator aneurysms are sporadic lesions often not recognized on initial imaging[17,18]. Similarly, azygos ACA aneurysm has a low incidence among intracranial aneurysms in literature ranging from 0.38 to 3.7%[19].
In this study, non-saccular intracranial aneurysms were less frequent. The margins were sharp (42.7%), and the rest were lobulated. These results partially agree with Rai et al[15], who reported 91% of saccular aneurysms, with only 22% having lobulated margins[15]. An irregular margin or lobulation along the margin of an aneurysm suggests rupture. It is an important observation in the setting of diffuse SAH and multiple intracranial aneurysms when treatment is directed at the culprit aneurysm[20]. Excellent inter-observer agreement was found in the assessment of branch incorporation and aneurysm orientation. The location of a branch to the aneurysm is a critical finding, as inadvertent occlusion of this branch during aneurysm treatment could have devastating consequences[21]. Therefore, it is essential to have a basic understanding of cerebral circulation and anatomical variants when interpreting CTA studies. Aneurysm orientation, especially Acom aneurysm, is a crucial element in selecting surgical vs endovascular management. Aneurysms with a more complex surgical approach are often assessed for endovascular therapy rather than surgical management[22].
Excellent agreement was found in this study between both readers regarding aneurysm mea
The majority of our cases were medium-sized aneurysms (60.8%). This finding was in line with results reported by previous studies, which noted that medium-sized aneurysms were the majority of intracranial aneurysms[14,15]. We also observed that the presence of thrombosis and calcification was strongly tied to aneurysm size in large-sized and giant aneurysms compared to the corresponding intra-operative details of each case. Spontaneous thrombosis of intracranial aneurysms may occur in up to 40% of giant aneurysms, eventually becoming symptomatic due to mass effect or stroke. On the contrary, spontaneous thrombosis of a non-giant aneurysm (< 25 mm) is rarer[27]. Calcification appears to be an important pre-operative indicator of poor outcomes[28] and should be considered when deciding treatment options for a patient with a calcified aneurysm.
Perianeurysmal findings such as vasospasm, perianeurysmal cyst, associated vascular lesions or variants, and bony landmarks or pathologic changes are essential factors that might change the treatment decision. Cerebral vasospasm and resultant delayed cerebral ischemia consider a significant etiology of morbidity and mortality in patients with aneurysmal SAH (DCI)[29]. CTA is a fast and reliable tool for detecting decreases in vessel caliber, as demonstrated by excellent interobserver agreement in this study.
The rarity of a perianeurysmal cyst may lead to a false diagnosis of cystic neoplasm and missing the possibility of a ruptured cerebral aneurysm. Interestingly, a perianeurysmal cyst was described in one out of 146 patients in our study. Pedro and Sih[30] reported a case of a sizeable pontine cyst associated with an enhancing mural nodule that was misdiagnosed as a cystic neoplasm. During surgery, an aneurysm of the left PCA was identified and clipped successfully[30].
Our work included four patients with associated vascular anomalies, including AVMs and DVA. The risk of intracranial hemorrhage in patients with coexisted AVM and aneurysm has been reported to be 7% annually, compared with 2%–4% annually for those with AVM alone or isolated intracranial aneurysm[31,32]. To our knowledge, the association between intracranial aneurysms and DVA is infrequent, and few case reports have been published[33].
In our study, both observers noted a sphenoid bone fracture and a nearby aneurysm of paraclinoid ICA secondary to head trauma. It is in agreement with results done on 5532 patients with cerebral aneurysms over 30 years by Jung et al[34], who state that traumatic intracranial aneurysm (TIA) after blunt brain trauma is rare and represents (0.23%)[34]. Osseous landmarks such as the optic strut and the tuberculum sellae are considered the anatomical boundary between the intradural and extradural segments of the ICA in the paraclinoid region, which is an important factor in determining treatment strategy[35].
We recommended surgical clipping or endovascular management for the culprit aneurysms based on data extracted from NCCT and CTA. 71.2% of the study population underwent the recommended therapy. The ideal therapeutic approach for RIAs is still controversial. In general, the decision on whether to clip or coil depends on several factors related to the patient, such as age and comorbidities, burden, and location of hemorrhage, aneurysm characteristics such as size, number, location, and morphology, or related procedure such as competence, technical skills and availability[36]. En
There are a few limitations to this study. First, there is no correlation with conventional angiography in all cases. Second, the general drawbacks of CT include patient exposure to ionizing radiation and the risks of iodinated contrast medium. Further studies with applications of low-dose CTA, such as the use of automatic tube current modulation and a small amount of contrast medium, are recommended. Additionally, future studies using advanced CT techniques such as higher 320 slices CT scan[37], dual-energy[38] or source CTA, and time-resolved 4D CTA will improve temporal and spatial resolution and the image quality CTA. Also, applications of advanced vascular post-processing packages for analyzing CTA images will give comprehensive image interpretation in different planes and angles. We recommend further studies assessing the inter-observer agreement between neuroradiologists, neurologists, neurosurgeons, and neuro-interventionist.
We concluded that CTA is a promising diagnostic imaging modality for assessing intracranial aneurysm features that impact management and patient outcomes.
Aneurysmal subarachnoid hemorrhage (SAH) is an emergency that can lead to a high mortality rate and many severe complications. It is critical to make a rapid radiological evaluation of ruptured intracranial aneurysms (RIAs) to determine the appropriate surgical treatment.
The high morbidity and mortality of RIAs nicissitate rapid diagnosis and management decision. Compared to Digital subtraction angiography, computed tomography angiography (CTA) is a safe, relatively inexpensive, and noninvasive imaging. Computed tomography (CT) angiography is not associated with significant patient risks other than those related to administering iodinated contrast media. Images can be relatively safely obtained without the need for arterial puncture or catheter manipulation that carries the possibility of acquiring a permanent neurologic deficit. Our motivation is to assess the reliability of CTA in assessing different features of ruptured intracranial aneurysm and its impact on patient management.
To assess the reliability of CTA in assessing different features of ruptured intracranial aneurysm and its impact on patient management. We found out that CTA is a promising diagnostic imaging modality for assessing intracranial aneurysm features and help in management decision and patient outcomes. We recommend further studies assessing the inter-observer agreement between neuroradiologists, neurologists, neurosurgeons, and neuro-interventionist.
Helical cerebral CT angiography has been widely used in detecting intracranial aneurysms, with a reported sensitivity of 70%–96%. Currently, multidetector CT angiography has the ability to detect most intracranial aneurysms of 5 mm or larger. Multidetector row CT scanners provide increased spatial resolution and decreased scanning time, which should increase the sensitivity of the technique in depicting aneurysms of less than 5 mm in diameter. Currently, CT angiography is accepted as a first-line diagnostic imaging modality for evaluating intracranial aneurysms. Nevertheless, using CT angiography to depict intracranial aneurysms showed variable sensitivity. The reported sensitivity of CTA in detections of small intracranial aneurysms (< 3 mm) varied from 28%–43% in a study that included 99 small aneurysms to 83%-92% in another study had 579 small aneurysms. CTA examinations for 146 patients in the current study were revised and interpreted by two experienced neuroradiologists to assess the inter-observer agreement of CTA findings related to intracranial aneurysms. Data extracted from CT was considered in patient management decision.
This study evaluated the interobserver reliability of CTA in the assessment of ruptured intracranial aneurysm features among 146 patients. Our results showed good to an excellent inter-observer agreement in imaging features related to the aneurysm, aneurysm characters, measurements, and perianeurysmal information. Imaging data extracted from NCCT and CTA guided the multidisciplinary neurovascular team to better treatment approach selection. There are a few limitations to this study. First, there is no correlation with conventional angiography in all cases. Second, the general drawbacks of CT include patient exposure to ionizing radiation and the risks of iodinated contrast medium.
We concluded that CTA is a promising diagnostic imaging modality for assessing intracranial aneurysm features that impact management and patient outcomes. We recommend endovascular approach in a ruptured intracranial aneurysm with comorbidity, old age, narrow neck, and a posterior circulation aneurysm, and surgical clipping is warranted if the patient is young with an MCA aneurysm and interested in durability.
Further studies with applications of low-dose CTA, such as the use of automatic tube current modulation and a small amount of contrast medium, are recommended. Additionally, future studies using advanced CT techniques such as higher 320 slices CT scan, dual-energy or source CTA, and time-resolved 4D CTA will improve temporal and spatial resolution and the image quality CTA. Also, applications of advanced vascular post-processing packages for analyzing CTA images will give comprehensive image interpretation in different planes and angles.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Taheri M, Iran; uz Zaman M, Pakistan S-Editor: Li L L-Editor: A P-Editor: Zhao S
| 1. | Turan N, Heider RA, Roy AK, Miller BA, Mullins ME, Barrow DL, Grossberg J, Pradilla G. Current Perspectives in Imaging Modalities for the Assessment of Unruptured Intracranial Aneurysms: A Comparative Analysis and Review. World Neurosurg. 2018;113:280-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Al-Smadi AS, Elmokadem A, Shaibani A, Hurley MC, Potts MB, Jahromi BS, Ansari SA. Adjunctive Efficacy of Intra-Arterial Conebeam CT Angiography Relative to DSA in the Diagnosis and Surgical Planning of Micro-Arteriovenous Malformations. AJNR Am J Neuroradiol. 2018;39:1689-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Howard BM, Hu R, Barrow JW, Barrow DL. Comprehensive review of imaging of intracranial aneurysms and angiographically negative subarachnoid hemorrhage. Neurosurg Focus. 2019;47:E20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Elsherbini MM, Elmokadem AH, El-Morsy A, Sherif FM, Khalil1 AF. Inter-Observer Reliability of Fused Time-of-Flight MR Angiography and 3D Steady State Sequence vs 3D Contrast Enhanced Images in Evaluation of Neurovascular Compression. Open Journal of Modern Neurosurgery. 2022;12. [DOI] [Full Text] |
| 5. | Ares WJ, Jankowitz BT, Tonetti DA, Gross BA, Grandhi R. A comparison of digital subtraction angiography and computed tomography angiography for the diagnosis of penetrating cerebrovascular injury. Neurosurg Focus. 2019;47:E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Yoon NK, McNally S, Taussky P, Park MS. Imaging of cerebral aneurysms: a clinical perspective. Neurovascular Imaging. 2016;2:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bechan RS, van Rooij SB, Sprengers ME, Peluso JP, Sluzewski M, Majoie CB, van Rooij WJ. CT angiography versus 3D rotational angiography in patients with subarachnoid hemorrhage. Neuroradiology. 2015;57:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Yang ZL, Ni QQ, Schoepf UJ, De Cecco CN, Lin H, Duguay TM, Zhou CS, Zhao YE, Lu GM, Zhang LJ. Small Intracranial Aneurysms: Diagnostic Accuracy of CT Angiography. Radiology. 2017;285:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Kizilkilic O, Huseynov E, Kandemirli SG, Kocer N, Islak C. Detection of wall and neck calcification of unruptured intracranial aneurysms with flat-detector computed tomography. Interv Neuroradiol. 2016;22:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Sawicki M, Kościukiewicz K, Jeżewski D, Chełstowski K, Gołofit P, Skoczylas MM, Gębka M, Grabizna K, Kołaczyk K, Zwarzany Ł, Sagan L, Poncyljusz W. Diagnostic value of non-enhanced computed tomography in identifying location of ruptured cerebral aneurysm in patients with aneurysmal subarachnoid haemorrhage. Neurol Neurochir Pol. 2020;54:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Woernle CM, Winkler KM, Burkhardt JK, Haile SR, Bellut D, Neidert MC, Bozinov O, Krayenbühl N, Bernays RL. Hydrocephalus in 389 patients with aneurysm-associated subarachnoid hemorrhage. J Clin Neurosci. 2013;20:824-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Feng TY, Han XF, Lang R, Wang F, Wu Q. Subtraction CT angiography for the detection of intracranial aneurysms: A meta-analysis. Exp Ther Med. 2016;11:1930-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Rajagopal N, Balaji A, Yamada Y, Kawase T, Kato Y. Etiopathogenesis, clinical presentation and management options of mirror aneurysms: A comparative analysis with non-mirror multiple aneurysms. Interdisciplinary Neurosurgery. 2019;18:100535. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Maniharan S, Athukorala ADP, Pullaperuma DLN, Pirasath S, Lekamge IN. Role of digital subtraction angiography in detection of intracranial aneurysms compared with computed tomographic angiography. Sri Lanka Journal of Radiology. 2019;5. [DOI] [Full Text] |
| 15. | Rai SP, ChandRan P, Pai M, Kamath M, Bele K, Kumar A, Chakraborti S. Comparison of Morphology of Intracranial Aneurysms on Computed Tomography Angiography with Digital Subtraction Angiography and Intraoperative Findings-A Single Centre Experience. J Clin Diagn Res. 2019;13. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Abecassis IJ, Zeeshan Q, Ghodke BV, Levitt MR, Ellenbogen RG, Sekhar LN. Surgical Versus Endovascular Management of Ruptured and Unruptured Intracranial Aneurysms: Emergent Issues and Future Directions. World Neurosurg. 2020;136:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Daruwalla VJ, Syed FH, Elmokadem AH, Hurley MC, Shaibani A, Ansari SA. Large basilar perforator pseudoaneurysm: A case report. Interv Neuroradiol. 2016;22:662-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Enomoto N, Shinno K, Tamura T, Shikata E, Shono K, Takase K. Ruptured Basilar Artery Perforator Aneurysm: A Case Report and Review of the Literature. NMC Case Rep J. 2020;7:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Meguins LC, Hidalgo RC, Spotti AR, de Morais DF. Aneurysm of azygos anterior cerebral artery associated with falcine meningioma: Case Report and review of the literature. Surg Neurol Int. 2017;8:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Wang GX, Gong MF, Wen L, Liu LL, Yin JB, Duan CM, Zhang D. Computed Tomography Angiography Evaluation of Risk Factors for Unstable Intracranial Aneurysms. World Neurosurg. 2018;115:e27-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Kawabata Y, Nakazawa T, Fukuda S, Kawarazaki S, Aoki T, Morita T, Tsukahara T. Endovascular embolization of branch-incorporated cerebral aneurysms. Neuroradiol J. 2017;30:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Chen J, Li M, Zhu X, Chen Y, Zhang C, Shi W, Chen Q, Wang Y. Anterior Communicating Artery Aneurysms: Anatomical Considerations and Microsurgical Strategies. Front Neurol. 2020;11:1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R. Unruptured Cerebral Aneurysms: Evaluation and Management. ScientificWorldJournal. 2015;2015:954954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Kim HJ, Yoon DY, Kim ES, Lee HJ, Jeon HJ, Lee JY, Cho BM. Intraobserver and interobserver variability in CT angiography and MR angiography measurements of the size of cerebral aneurysms. Neuroradiology. 2017;59:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Al Kasab S, Nakagawa D, Zanaty M, Bathla G, Policeni B, Soni N, Allan L, Hudson J, Limaye K, Ortega-Gutierrez S, Samaniego EA, Hasan D. In vitro accuracy and inter-observer reliability of CT angiography in detecting intracranial aneurysm enlargement. J Neurointerv Surg. 2019;11:1015-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Waqas M, Chin F, Rajabzadeh-Oghaz H, Gong AD, Rai HH, Mokin M, Vakharia K, Dossani RH, Meng H, Snyder KV, Davies JM, Levy EI, Siddiqui AH. Size of ruptured intracranial aneurysms: a systematic review and meta-analysis. Acta Neurochir (Wien). 2020;162:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Vandenbulcke A, Messerer M, Starnoni D, Puccinelli F, Daniel RT, Cossu G. Complete spontaneous thrombosis in unruptured non-giant intracranial aneurysms: A case report and systematic review. Clin Neurol Neurosurg. 2021;200:106319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Bhatia S, Sekula RF, Quigley MR, Williams R, Ku A. Role of calcification in the outcomes of treated, unruptured, intracerebral aneurysms. Acta Neurochir (Wien). 2011;153:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Kolias AG, Sen J, Belli A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res. 2009;87:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Pedro KM, Sih IM. Perianeurysmal Parenchymal Cyst of the Pons: A Case Report and Review of Literature. Interdisciplinary Neurosurgery. 2020;100731. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Chen J, Wang Y, Li P, Chen W, Zhou J, Hu X, Zhu J, Jiang B. Treatment of a giant arteriovenous malformation associated with intracranial aneurysm rupture during pregnancy: A case report. Exp Ther Med. 2016;12:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Flores BC, Klinger DR, Rickert KL, Barnett SL, Welch BG, White JA, Batjer HH, Samson DS. Management of intracranial aneurysms associated with arteriovenous malformations. Neurosurg Focus. 2014;37:E11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Li J, Du S, Sun L, Shang F, Zhang H, Li G. Hybrid Operation of a Ruptured Aneurysm Associated with a Developmental Venous Anomaly. World Neurosurg. 2018;120:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Jung SH, Kim SH, Kim TS, Joo SP. Surgical Treatment of Traumatic Intracranial Aneurysms: Experiences at a Single Center over 30 Years. World Neurosurg. 2017;98:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Lefevre E, Apra C, Chodraui-Filho SF, Chauvet D, Smajda S, Piotin M, Fahed R. Reliability of Bony Landmarks to Predict Intradural Location of Paraclinoid Aneurysms. Clin Neuroradiol. 2020;30:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G; European Stroke Organization. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 823] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 37. | Kim HJ, Yoon DY, Kim ES, Yun EJ, Jeon HJ, Lee JY, Cho BM. 256-row multislice CT angiography in the postoperative evaluation of cerebral aneurysms treated with titanium clips: using three-dimensional rotational angiography as the standard of reference. Eur Radiol. 2020;30:2152-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Yun SY, Heo YJ, Jeong HW, Baek JW, Choo HJ, Shin GW, Kim ST, Jeong YG, Lee JY, Jung HS. Dual-energy CT angiography-derived virtual non-contrast images for follow-up of patients with surgically clipped aneurysms: a retrospective study. Neuroradiology. 2019;61:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |