Published online Apr 28, 2023. doi: 10.4329/wjr.v15.i4.127
Peer-review started: February 19, 2023
First decision: March 15, 2023
Revised: March 28, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: April 28, 2023
Processing time: 65 Days and 18.5 Hours
Prominent leptomeningeal contrast enhancement (LMCE) in the brain is observed in some pediatric patients during sedation for imaging. However, based on clinical history and cerebrospinal fluid analysis, the patients are not acutely ill and do not exhibit meningeal signs. Our study determined whether sevoflurane inhalation in pediatric patients led to this pattern of ‘pseudo’ LMCE (pLMCE) on 3 Tesla magnetic resonance imaging (MRI).
To highlight the significance of pLMCE in pediatric patients undergoing enhanced brain MRI under sedation to avoid misinterpretation in reports.
A retrospective cross-sectional evaluation of pediatric patients between 0-8 years of age was conducted. The patients underwent enhanced brain MRI under inhaled sevoflurane. The LMCE grade was determined by two radiologists, and interobserver variability of the grade was calculated using Cohen’s kappa. The LMCE grade was correlated with duration of sedation, age and weight using the Spearman rho rank correlation.
A total of 63 patients were included. Fourteen (22.2%) cases showed mild LMCE, 48 (76.1%) cases showed moderate LMCE, and 1 case (1.6%) showed severe LMCE. We found substantial agreement between the two radiologists in detection of pLMCE on post-contrast T1 imaging (kappa value = 0.61; P < 0.001). Addi
pLMCE is relatively common on post-contrast spin echo T1-weighted MRI of pediatric patients sedated by sevoflurane due to their fragile and immature vasculature. It should not be misinterpreted for meningeal pathology. Knowing pertinent clinical history of the child is an essential prerequisite to avoid radiological overcalling and the subsequent burden of additional investigations.
Core Tip: Prominent leptomeningeal contrast enhancement (LMCE) in the brain is seen in some pediatric patients during sedation for imaging, but they do not exhibit meningeal disease. This pattern of pseudo LMCE is relatively common on post-contrast spin echo-T1-weighted magnetic resonance imaging of pediatric patients sedated by sevoflurane due to their fragile and immature vasculature. Knowing pertinent clinical history is an essential prerequisite to avoid radiological overcalling and additional investigations. Our study determined whether sevoflurane inhalation in pediatric patients led to pseudo LMCE on 3 Tesla magnetic resonance imaging.
- Citation: Hilal K, Khandwala K, Rashid S, Khan F, Anwar SSM. Does sevoflurane sedation in pediatric patients lead to “pseudo” leptomeningeal enhancement in the brain on 3 Tesla magnetic resonance imaging? World J Radiol 2023; 15(4): 127-135
- URL: https://www.wjgnet.com/1949-8470/full/v15/i4/127.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i4.127
Contrast material for cross-sectional magnetic resonance imaging (MRI) has been used since the mid-1980s. Since then, leptomeningeal contrast enhancement (LMCE) has been detected in pathological states such as infection and carcinomatosis. Its detection is crucial for disease management, particularly in pediatric patients[1]. Because MRI acquisition is a lengthy process, general anesthesia is frequently required in pediatric patients younger than 8 years of age. Approximately 90% of the pediatric population requires deep sedation or anesthesia for reducing anxiety and movement[2]. Over 35 years, scientific societies have proposed guidelines to perform pediatric non-operating room anesthesia to minimize complications. Subsequently, each center has developed its own protocol[3]. Different pharmacological agents have specific disadvantages, such as a high failure rate, poor predictability and prolonged awakening for midazolam[4] and chloral hydrate[5], and respiratory drive depression for propofol[6].
Sevoflurane is an inhalational anesthetic and has excellent outcomes in success of sedation, safety and manageability[7]. It has a minimal potential for hepatotoxicity and nephrotoxicity and a comparatively low risk for cardiac arrhythmias. Its effects on cerebral vasodilation, increased cerebral blood flow and increased intracranial pressure are less than propofol[8-10]. In our institution we prefer to induce sedation with sevoflurane due to its rapid induction and rapid recovery. It is primarily administered in pediatric patients undergoing MRI as an outpatient procedure.
A recent study carried out in 2018 revealed that “pseudo” LMCE (pLMCE) in the brain is frequently observed on 3 Tesla (3T) post-contrast spin echo (SE) T1-weighted imaging (WI) in younger pediatric patients anesthetized with propofol and should not be misinterpreted for meningeal pathology[11]. Propofol results in reduction in the tidal volume, a mild decrease in the partial pressure of oxygen in the blood and a decrease in the fraction of inspired oxygen. These changes may lead to the pathophysiology of pLMCE secondary to cerebral vasodilation by an increase in the partial pressure of carbon dioxide as a result of smooth muscle relaxation[12,13]. The suggested mechanism of pLMCE is the retention of carbon dioxide, a potent vasodilator, resulting in increased vessel visibility, and this effect could be amplified in young pediatric patients with sensitive leptomeningeal vessels resulting in leakiness.
Based on aforementioned rationale and pathophysiology of propofol, we aimed to determine whether sevoflurane sedation leads to a similar pattern of pLMCE in the brain on 3T MRI and to determine the interobserver agreement of detecting pLMCE in patients receiving sevoflurane. We also evaluated the degree of pLMCE and determined whether this phenomenon was correlated with sedation duration or patient age or weight.
This was a retrospective cross-sectional study conducted in the Radiology Department of a Joint Commission International-accredited university hospital after ethical review board approval. The period of data collection was from January 2020 to June 2020.
All studies were conducted on a single 3T MR unit (Canon Medical Systems Corp., Otawara, Tochigi, Japan) after administration of intravenous gadolinium. Sedation was performed by a pediatric anesthetist. The imaging parameters using 32 channel head coil for post-contrast SE T1WI were NAQ = 1, FOV = 22 cm × 22 cm, Matrix = 224 × 400, slice thickness 4.5 mm and acquisition time 1 min 30 s. The imaging parameters for post-contrast coronal fluid attenuated inversion recovery (FLAIR) were NAQ = 1, FOV = 22 cm × 22 cm, Matrix = 224 × 320, slice thickness 5 mm and acquisition time 1 min 10 s. Per our departmental protocol, FLAIR images were acquired in the coronal plane. The time interval between contrast injection and image acquisition was 10-30 s for post-contrast SE T1WI and 100-120 s for post-contrast FLAIR sequence. Axial SE T1WI, T2WI, FLAIR, susceptibility weighted imaging and diffusion weighted imaging were also performed in each patient before contrast administration. A standard weight based intravenous dose of gadolinium-based contrast was used in all patients [0.1 mL/kg of body weight of gadobutrol (Gadavist; Bayer HealthCare Pharmaceuticals, Berlin, Germany)][14].
Standard monitoring was used with MRI compatible equipment, and general anesthesia was induced with sevoflurane 8%. Anesthesia was maintained with sevoflurane at a minimum alveolar concentration of 2%-3% along with oxygen (40%) and nitrous oxide (60%)[15,16]. This ratio of oxygen and nitrous oxide was maintained in all cases, while SpO2 was monitored for adequacy throughout the procedure. There were no complications for any case. Laryngeal masks were used to maintain the airway except for in a few patients who were already intubated or had indications for a definitive airway[3].
The following inclusion criteria were used: (1) Pediatric patients between the ages of 0-8 years who underwent brain MRI on a 3T system; (2) Patients who received sevoflurane for sedation purposes before the procedure; and (3) Patients who had no clinical signs of meningism and/or a negative cerebrospinal fluid (CSF) analysis report, if performed.
The following exclusion criteria were used: (1) Patients with any known or clinically suspected meningeal inflammatory or infectious condition; (2) Patients who were undergoing radiation therapy or chemotherapy; (3) Patients with known central nervous system tumors; and (4) Patients who were sedated with chloral hydrate, midazolam and propofol.
A total of 130 patients (0–8 years of age) underwent brain 3T MRI with contrast over a period of 6 months at our institution. Out of the 69 pediatric patients sedated by sevoflurane for 3T MRI, 2 cases were excluded due to the lack of post-contrast imaging. Four cases were excluded due to positive CSF analysis for meningitis.
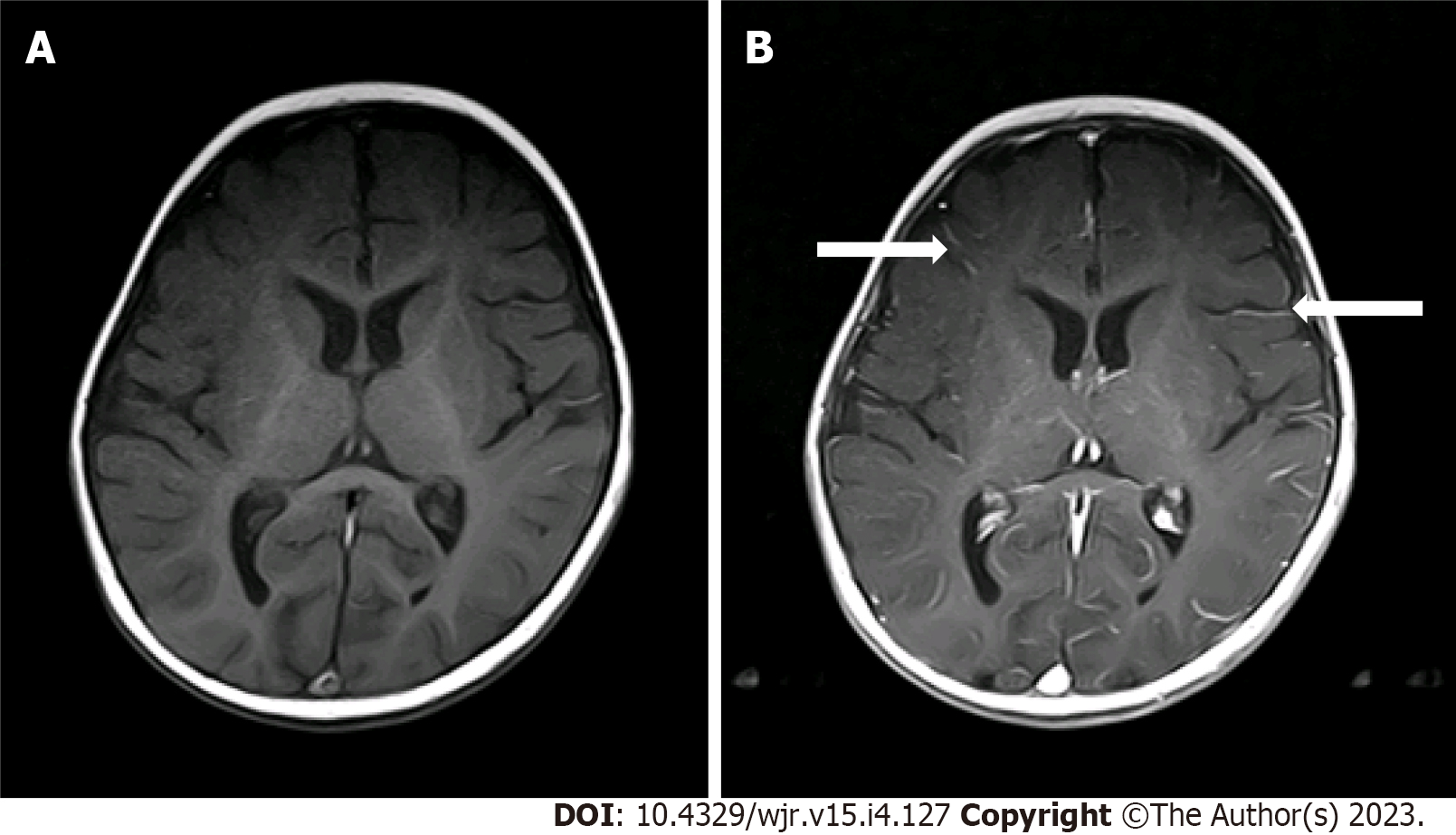
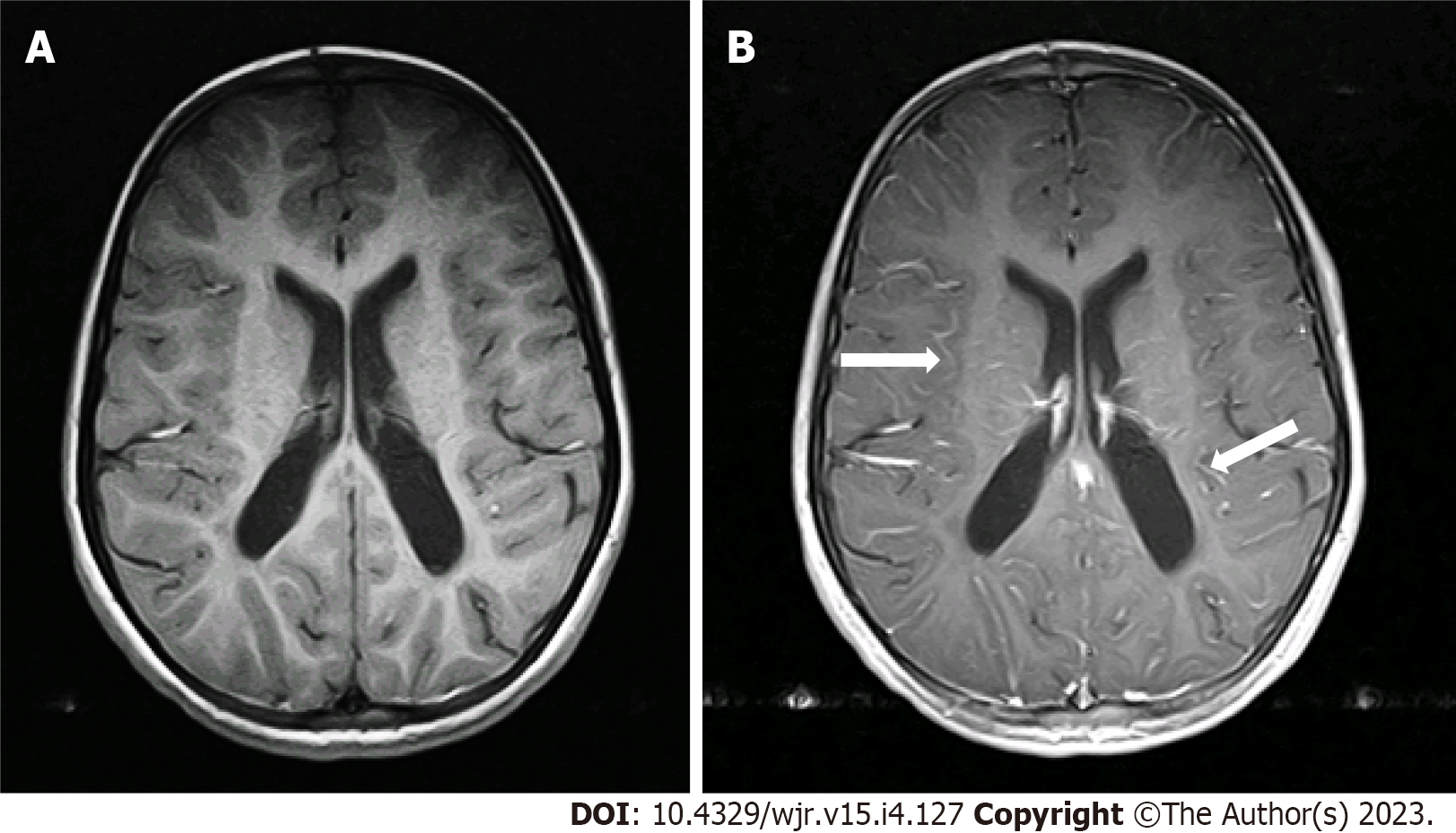
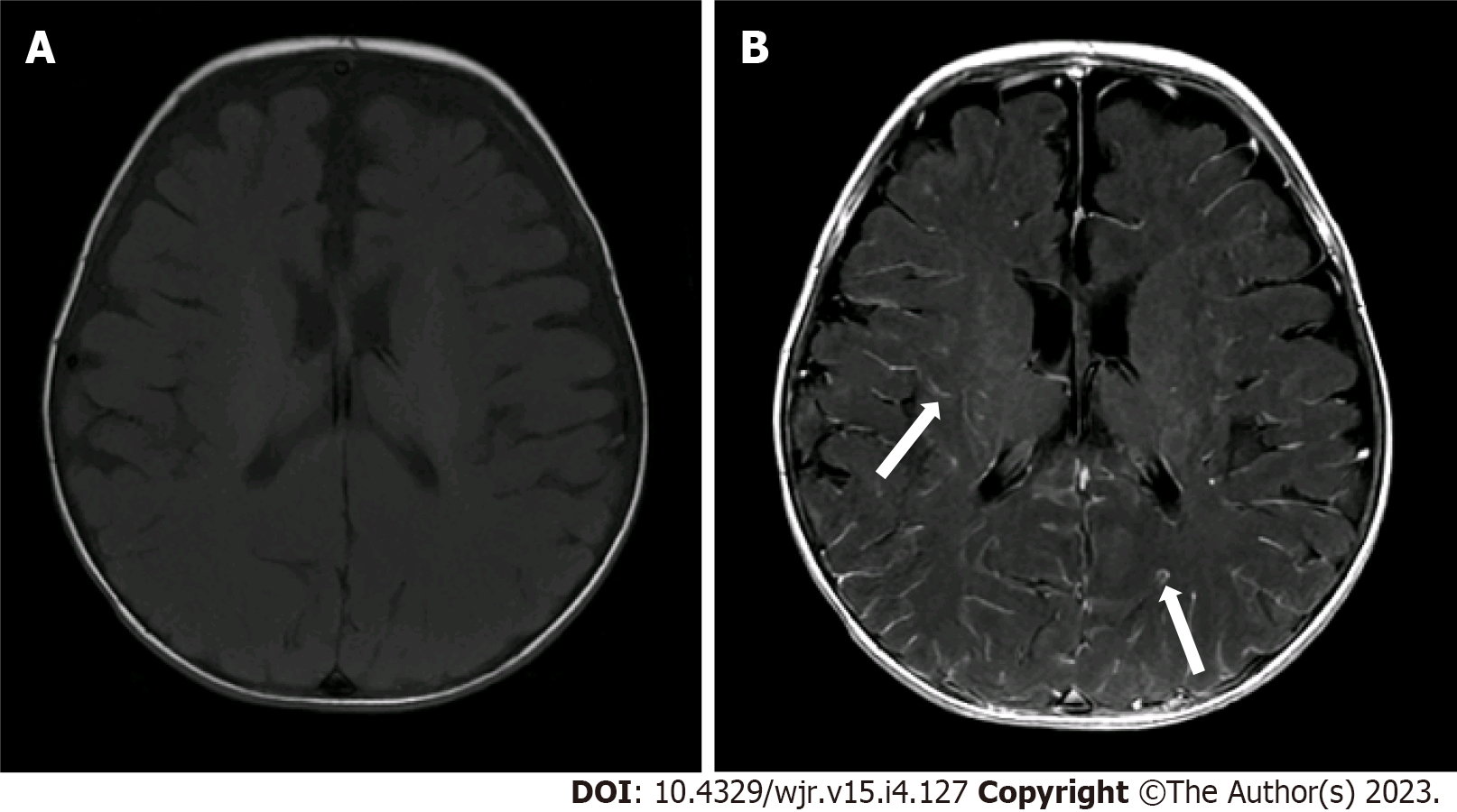
Two radiologists (one pediatric radiologist and one neuroradiologist) with more than 5 years of experience reviewed the scans and independently graded the degree of pLMCE on post-contrast SE T1WI in comparison to precontrast SE T1WI. The grades were: (1) None: Minimal thin vascular structures barely visible around the sulci; (2) Mild: Linear enhancement along the depths of the sulci (Figure 1); (3) Moderate: Smooth and slightly thickened LMCE (Figure 2); and (4) Severe: Almost nodular, diffusely thickened LMCE (Figure 3). Confirmation of true meningeal enhancement or disease was also confirmed on precontrast and post-contrast FLAIR images[11,17]. The disagreements were resolved on a consensus basis.
Data entry was performed in Statistical Package for Social Sciences version 20.0 software (IBM Corp., Armonk, NY, United States). Demographic data, such as age, were expressed as mean ± SD. The effect of age, weight and sex on LMCE was evaluated. The interobserver variability was calculated using Cohen’s kappa statistic. The LMCE grade was correlated with the duration of sedation and patient age and weight using the Spearman rho rank correlation. Correlation coefficients 0.1 < Ρ < 0.3, 0.3 < Ρ < 0.5 and > 0.5 were used as indications of weak, moderate and substantial associations, respectively. P values less than 0.05 were considered statistically significant.
A total of 63 patients were ultimately included for MRI review. All patients underwent post-contrast imaging by SE T1WI. Table 1 lists the mean patient age, weight and sedation duration. CSF studies were ordered for 7 patients (11.0%) and were negative for meningitis for all. Underlying conditions of the included patients are shown in Table 2.
| Parameter | Mean | SD | Minimum | Maximum | n | % | |
| Age, mo | 29.95 | 25.42 | 1.00 | 96.00 | |||
| Sex | Male | 31 | 49.2 | ||||
| Female | 32 | 50.8 | |||||
| Weight, kg | 12.03 | 6.25 | 1.30 | 26.50 | |||
| Duration of sedation, min | 57.78 | 24.76 | 28.00 | 196.00 | |||
| Diagnosis | n | % |
| Atrial septal defect | 1 | 1.6 |
| Autism | 1 | 1.6 |
| Bilateral congenital cataracts | 1 | 1.6 |
| Cerebral palsy | 2 | 3.1 |
| Congenital deafness | 1 | 1.6 |
| Developmental delay | 7 | 11.1 |
| Double aortic arch | 1 | 1.6 |
| Headache | 1 | 1.6 |
| Hypoxic ischemic encephalopathy | 2 | 3.1 |
| Hydrocephalus | 3 | 4.7 |
| Hypotonia | 1 | 1.6 |
| Infantile spasms | 1 | 1.6 |
| Metabolic disorder | 2 | 3.1 |
| Metachromatic leukodystrophy | 2 | 3.1 |
| Not available/none | 14 | 7.9 |
| Nystagmus | 1 | 1.6 |
| Retinal detachment/vitreous hemorrhage | 1 | 1.6 |
| Severe combined immunodeficiency | 1 | 1.6 |
| Seizure disorder | 20 | 31.7 |
The results showed that 14 cases (22.2%) had mild LMCE, 48 cases (76.1%) had moderate LMCE, and 1 case (1.6%) had severe LMCE. There were no cases that were negative for pLMCE. Overall, there was concordance with a kappa value of 0.61 (P < 0.001), which indicated substantial agreement between the two radiologists in detection of pLMCE on post-contrast T1 imaging. On precontrast FLAIR, no sulcul hyperintensity was noted in any patient. On post-contrast FLAIR, LMCE was absent in 63 (100%) cases, indicating the absence of true LMCE in all cases.
There were statistically significant, inverse, moderate correlations between patient weight and pLMCE grade and patient age and pLMCE grade (Table 3). The duration of sedation showed no significant association with pLMCE grade (P = 0.14).
| Parameter | Spearman’s rank coefficient, P | P value |
| Age | -0.41 | 0.011 |
| Weight | -0.42 | 0.030 |
| Duration of sedation | -0.18 | 0.142 |
We used 3T MRI in our study because it is universally approved for imaging in pediatric patients[18]. All patients underwent SE labelling sequences, which are routinely recommended for pediatric imaging due to their quick acquisition time. MRI specifically has advantageous effects in pediatric neuroimaging due to its use of non-ionizing radiation.
The duration of sedation with sevoflurane did not appear to statistically correlate with the degree of pLMCE in our study. This is in contrast to previously published studies advocating that propofol typically results in changes in perfusion and vascular hemodynamics leading to an altered blood brain barrier and possibly pLMCE[11,13,19]. Propofol has been shown to reduce cerebral blood flow, whereas sevoflurane has been shown to reduce cerebral blood flow less than propofol. Propofol and sevoflurane have been shown to reduce the metabolic rate of oxygen to a similar extent[20]. According to our literature search, no study has assessed the effect of sevoflurane sedation on pLMCE.
We found statistically significant, inverse, moderate correlations between the LMCE grade and patient age and weight. This indicates that the younger and smaller the child, the more immature the blood brain barrier, vasculature and dynamic perfusion changes leading to pLMCE[13,19]. We hypothesize that pLMCE represents prominent venous vasculature of the subarachnoid space in younger pediatric patients and is not true meningeal enhancement. Similar results were also obtained by McKinney et al[11], who also found pLMCE predominantly in SE sequences on 3T MRI using propofol. This indicates a similar mechanism of action by the two anesthetic agents. Radiologists should therefore be aware of this phenomenon and consider the patient’s clinical history before labelling meningeal enhancement. This would ultimately avoid unnecessary investigations such as lumbar punctures, which are invasive and incur a financial burden on patients, especially in developing countries.
We evaluated precontrast and post-contrast FLAIR images to exclude true LMCE, which was not performed in previous studies[11,21]. No LMCE was identified on post-contrast FLAIR sequence in the 63 cases included in our study, indicating the absence of true meningeal disease and indirectly supporting pLMCE.
Our study has a few limitations of note. First, general limitations of retrospective studies apply to our study. The major limitation of the study was the use of supplemental oxygen (40%) and nitrous oxide (60%), which could not be modified due to the retrospective nature of the study. Therefore, the effect of these agents could not be separated from that of sevoflurane, making them crucial confounding factors. Sevoflurane as an anesthetic acts by vasodilation and maintains the tissue oxygen concentration in the brain. However, the tissue may suffer from hypoxia when the patient breaths room oxygen. By giving oxygen at a low concentration, such as 40%, the oxygen level is optimized and will not cause hyperoxic injury[22]. Nitrous oxide is also a vasodilator, which further helps in sustaining regional cerebral blood flow and regional cerebral blood volume when used in conjunction with sevoflurane[20]. We hypothesize that by keeping the concentrations of oxygen and nitrous oxide constant the effects of sevoflurane on vasodilation can be assessed.
Since we have included patients below 8 years of age, a control group undergoing MRI examination without anesthesia was not possible, since sedation cannot be avoided in this age group. We did not compare the sevoflurane-sedated pediatric patients with other groups of patients who underwent sedation with intravenous midazolam, chloral hydrate or propofol because of the limited number of these patients. There may be a difference in pLMCE or the degree of pLMCE between these groups; however, this was not the objective of our study. We did not have a cohort of sedated patients that underwent MRI on a 1.5T MRI machine. Therefore the effect of magnetic strength on pLMCE could not be assessed. We also did not compare our results with juvenile or adolescent groups of pediatric patients who generally do not require anesthesia or sedation for their imaging. We could not determine the correlation of sevoflurane dose with pLMCE due to the retrospective nature of the study. These objectives can be explored in a future large scale prospective study with different cohorts of these patients.
The phenomenon of pLMCE is relatively common on post-contrast SE T1-weighted sequences of younger pediatric patients sedated by sevoflurane. However, these imaging results should not be misinterpreted for meningeal pathology. Our results showed that pLMCE may occur more frequently in younger and smaller pediatric patients due to their fragile and immature vasculature. However, further prospective studies are warranted to elucidate the exact relationship and evaluate any effect of magnetic strength and resolution on this finding.
Prominent leptomeningeal contrast enhancement (LMCE) in the brain is seen in some pediatric patients during sedation for imaging. However, they are not acutely ill and do not exhibit meningeal signs. Our study determined whether inhaled sevoflurane anesthesia in pediatric patients led to pseudo LMCE (pLMCE) using 3 Tesla (3T) magnetic resonance imaging (MRI).
pLMCE on brain MRI in pediatric patients undergoing sedation with propofol has been studied. However, pLMCE due to sedation by sevoflurane (inhalation anesthetic) has not been studied so far. We therefore undertook this study in a small cohort of patients to establish our hypothesis.
The aim of this study was to highlight the significance of pLMCE in pediatric patients undergoing enhanced brain MRI under sedation to avoid misinterpretation in radiology reports.
This was a retrospective cross-sectional study. Data analysis was performed by Statistical Package for Social Sciences version 20.0 software (IBM Corp.). Demographic data, such as age, were expressed as mean ± SD. The effect of age, weight and sex on LMCE was evaluated. The interobserver variability was calculated using Cohen’s kappa statistic. The LMCE grade was correlated with the duration of sedation and patient age and weight using the Spearman rho rank correlation. Correlation coefficients 0.1 < P < 0.3, 0.3 < P < 0.5 and > 0.5 were used as indications of weak, moderate and substantial associations, respectively. P < 0.05 was considered statistically significant.
There was substantial agreement between the two radiologists in detection of pLMCE on post-contrast T1 imaging (kappa = 0.61; P < 0.001). Our results show that this pattern may occur in younger and smaller pediatric patients due to their fragile and immature vasculature. Additionally, we found statistically significant inverse and moderate correlations between patient weight and age with pLMCE grade but no correlation between pLMCE and duration of sedation.
Results of our study revealed that this pattern of pLMCE is relatively common on post-contrast spin echo T1-weighted sequences of younger pediatric patients sedated by sevoflurane, on 3T MRI and should not be misinterpreted for meningeal pathology.
Future prospective studies with a larger cohort and controls are warranted to elucidate the exact relationship and evaluate any effect of magnetic strength and resolution on this finding.
| 1. | Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Bhargava R, Hahn G, Hirsch W, Kim MJ, Mentzel HJ, Olsen OE, Stokland E, Triulzi F, Vazquez E. Contrast-enhanced magnetic resonance imaging in pediatric patients: review and recommendations for current practice. Magn Reson Insights. 2013;6:95-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Mongodi S, Ottonello G, Viggiano R, Borrelli P, Orcesi S, Pichiecchio A, Balottin U, Mojoli F, Iotti GA. Ten-year experience with standardized non-operating room anesthesia with Sevoflurane for MRI in children affected by neuropsychiatric disorders. BMC Anesthesiol. 2019;19:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth. 2000;84:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 296] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg. 1997;85:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Lubisch N, Roskos R, Berkenbosch JW. Dexmedetomidine for procedural sedation in children with autism and other behavior disorders. Pediatr Neurol. 2009;41:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Sury MR, Harker H, Thomas ML. Sevoflurane sedation in infants undergoing MRI: a preliminary report. Paediatr Anaesth. 2005;15:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Palanca BJA, Avidan MS, Mashour GA. Human neural correlates of sevoflurane-induced unconsciousness. Br J Anaesth. 2017;119:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Aksenov DP, Miller MJ, Dixon CJ, Wyrwicz AM. The effect of sevoflurane and isoflurane anesthesia on single unit and local field potentials. Exp Brain Res. 2019;237:1521-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Stachnik J. Inhaled anesthetic agents. Am J Health Syst Pharm. 2006;63:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | McKinney AM, Chacko Achanaril A, Knoll B, Nascene DR, Gawande RS. Pseudo-Leptomeningeal Contrast Enhancement at 3T in Pediatric Patients Sedated by Propofol. AJNR Am J Neuroradiol. 2018;39:1739-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Machata AM, Willschke H, Kabon B, Kettner SC, Marhofer P. Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging. Br J Anaesth. 2008;101:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Szabó EZ, Luginbuehl I, Bissonnette B. Impact of anesthetic agents on cerebrovascular physiology in children. Paediatr Anaesth. 2009;19:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Saunders DE, Thompson C, Gunny R, Jones R, Cox T, Chong WK. Magnetic resonance imaging protocols for paediatric neuroradiology. Pediatr Radiol. 2007;37:789-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Serafini G, Zadra N. Anaesthesia for MRI in the paediatric patient. Curr Opin Anaesthesiol. 2008;21:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Jung SM. Drug selection for sedation and general anesthesia in children undergoing ambulatory magnetic resonance imaging. Yeungnam Univ J Med. 2020;37:159-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Dietemann JL, Correia Bernardo R, Bogorin A, Abu Eid M, Koob M, Nogueira T, Vargas MI, Fakhoury W, Zöllner G. [Normal and abnormal meningeal enhancement: MRI features]. J Radiol. 2005;86:1659-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Zimmerman RA, Bilaniuk LT, Pollock AN, Feygin T, Zarnow D, Simon EM, Harris C. 3T Pediatric Brain Imaging. Rivista di Neuroradiologia. 2006;19:19-27. [DOI] [Full Text] |
| 19. | Harreld JH, Helton KJ, Kaddoum RN, Reddick WE, Li Y, Glass JO, Sansgiri R, Ji Q, Feng T, Parish ME, Gajjar A, Patay Z. The effects of propofol on cerebral perfusion MRI in children. Neuroradiology. 2013;55:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kaisti KK, Långsjö JW, Aalto S, Oikonen V, Sipilä H, Teräs M, Hinkka S, Metsähonkala L, Scheinin H. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Ahmad A, Azad S, Azad R. Differentiation of Leptomeningeal and Vascular Enhancement on Post-contrast FLAIR MRI Sequence: Role in Early Detection of Infectious Meningitis. J Clin Diagn Res. 2015;9:TC08-TC12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Aksenov DP, Dmitriev AV, Miller MJ, Wyrwicz AM, Linsenmeier RA. Brain tissue oxygen regulation in awake and anesthetized neonates. Neuropharmacology. 2018;135:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Noncommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nagamine T, Japan; Yarmahmoodi F S-Editor: Ma YJ L-Editor: A P-Editor: Zhao S