INTRODUCTION
Precise characterization of any renal mass is of paramount importance for clinicians to decide the most appropriate treatment and thereby improve the survival outcome. Being safe, cost-effective, and non-invasive, ultrasound is the current screening modality for the evaluation of renal masses for malignancy with a sensitivity of 82%-83% and specificity of 98%-99%[1-3]. However, the sensitivity is low for masses less than 3cm in size[4]. Multidetector computed tomography (MDCT) forms the mainstay for diagnosis and is the imaging modality of choice for characterization of renal masses[5]. In this technique, a non-contrast scan is performed, followed by a corticomedullary phase at 25-70 s, a nephrographic phase at 80-180 s, and an excretory phase at 4-8 min[5]. A non-contrast scan is essential to detect any hemorrhage or calcification within the mass. Identification of pseudotumor is best done in the corticomedullary phase. A nephrographic phase is ideal for the detection of renal tumors. An excretory phase is acquired to detect pelvicalyceal system involvement. Conventional and dynamic contrast-enhanced magnetic resonance imaging (MRI) serves as a problem-solving tool[6]. MRI can also be done in cases when contrast-enhanced computed tomography (CT) is contraindicated.
Current imaging methods for the evaluation of renal tumors suffer from a few major drawbacks. As multiphasic MDCT involves the acquisition of multiple scans at different time intervals for the characterization of renal tumors, the risk of radiation is high with this repeated and multiple scanning. Contrast administered for CT or MRI can lead to various allergic reactions as well as impairment of renal function. Even after all these investigations, no conclusive diagnosis can be established in a few lesions like Bosniak 3 lesions and indeterminate renal masses. Masses like oncocytoma or lipopenic angiomyolipoma cannot be confidently diagnosed as they have no specific imaging criterion. Ruling out malignancy in pseudolesions at times becomes difficult. Focal pyelonephritis and evolving or resolving abscess may sometimes simulate malignancy; hence, more advanced imaging techniques are required for a definitive answer as the management would significantly differ in these groups.
To overcome the deficiency in imaging techniques, newer/advanced imaging methods have been devised and are being tested. This review will cover contrast-enhanced ultrasonography (CEUS), shear wave elastography (SWE), dual-energy CT (DECT), CT perfusion, MR perfusion, diffusion-weighted MRI, blood oxygen level-dependent (BOLD) MRI, MR spectroscopy (MRS), and positron emission tomography (PET)/prostate-specific membrane antigen (PSMA)-PET.
ADVANCED IMAGING TECHNIQUES
Contrast-enhanced ultrasound
CEUS has recently gained popularity in the last decade as it has become a problem-solving tool in many areas including renal diseases. The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) has incorporated renal applications of CEUS in 2012[7] and updated the recommendations in a paper published in 2017[8]. Being safe and quick, it has augmented the sensitivity and specificity of ultrasound in the characterization of renal masses. It can fairly differentiate small isoechoic or small solid lesions, better characterize complex cystic lesions, and differentiate tumors vs pseudotumors and renal cell carcinoma (RCC) vs angiomyolipoma, and can be utilized for detection and follow-up of renal infections.
Ultrasound contrast agents (USCA) are made up of microbubbles surrounded by a shell. This shell is composed of lipid, protein, or polymer. As these microbubbles are very fragile, the shell provides them with stability[9]. Two principles play a role in CEUS, one is enhancing the echogenicity of the lesion that is imaged and the other is the suppression of the background signal. Contrast agents markedly increase the backscatter due to a large difference in acoustic impedance at gas fluid/tissue surface interface. The second effect of background suppression is achieved by a technique called pulse inversion. For this, two similar signals with opposite phases which are mirror images of each other are sent through the same scan line and echoes from both are collected and added by the transducer. Normal tissues which act like linear reflectors produce no net signal due to the cancellation of echoes whereas the ones having microbubbles act like non-linear reflectors that produce some net signal which stands out against a dark background. When ultrasound waves strike these molecules (tissues with microbubbles), they strongly backscatter and increase the echoes by a factor of 500-1000, thus resulting in enhancement. Microvascular flow rate can also be calculated by calculating the rate at which microbubbles are in the imaging plane.
USCA evaluates both the macrovascular and microvascular systems. As soon as the contrast agent is injected, there is an avid and rapid enhancement of the kidney. Initially, the main renal artery and its branches are enhanced, followed by segmental, interlobular, arcuate, and intralobular branches. This is followed by enhancement of the cortex, then the outer medulla, and finally the pyramids. Only two phases are seen, cortical from 15 to 30s and parenchymal from 25 s to 4 min[10]. The point to note is that there will be no excretory phase as the contrast agents are not excreted in the kidneys, thus allowing it to use safely in patients with deranged renal function[8]. Current applications of CEUS in renal mass is as follows.
Differentiating renal tumors from pseudolesions: B-mode ultrasound and Doppler ultrasound fail to differentiate the solid renal tumors from pseudolesions. CEUS can make an apt and confident differential between the two, especially in patients with chronic kidney disease (CKD). Pseudolesions will show the same enhancement pattern as the normal renal parenchyma, whereas renal tumors will show different enhancement patterns in at least one of the phases[11]. In 5% of the cases when the tumor is isoenhancing, renal vascular anatomy should be studied in the early arterial phase which will show normal arteries passing through pseudolesions and aberrant deviation of arteries by the renal tumor[8].
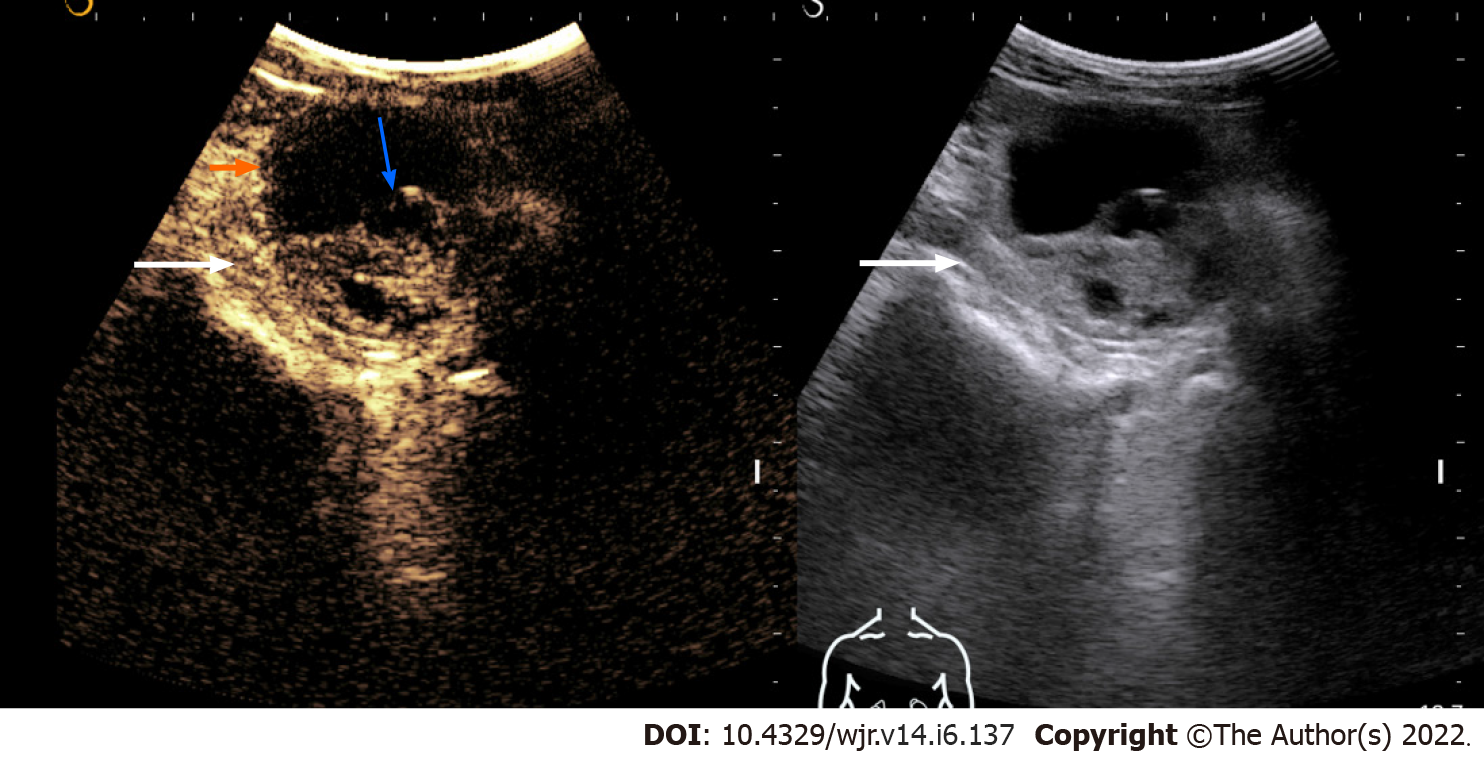
Evaluation of renal cystic lesions: Several studies have shown CEUS to be better than CT in detecting any solid component, septa, and thickening in the wall within the cystic renal mass and thus can classify it according to Bosniak classification[12-16](Figure 1). Follow-up of complex renal cysts can be easily and quickly done by CEUS instead of expensive and high radiation exposure modalities like CT.
Figure 1 Contrast-enhanced ultrasonography images of a solid-cystic lesion in the left kidney showing thick nodular septal enhancement (blue arrow) and enhancement of solid component (long arrow).
Consistent with Bosniak category 4 lesion/malignant lesion. The lesion was resected and histology revealed clear cell renal cell carcinoma.
Characterization of indeterminate renal tumors: CEUS proves useful in determining even minimal vascularity in hypovascularized tumors to differentiate complex renal lesions from solid mass, which are indeterminate on cross-sectional imaging[12,17,18]. This is especially advantageous in CKD patients where both complex cysts and tumors have a high incidence[19].
Renal vein invasion: Renal vein invasion by tumor thrombus can be reliably detected by CEUS in which an enhancing thrombus can be seen within the renal vein and a diagnosis of malignancy can thus be confidently made[20].
Renal infections: Early detection of renal abscesses in a case of acute pyelonephritis can be done by CEUS which shows avascular areas in renal parenchyma in the parenchymal phase. Also, follow-up can be done by CEUS to look for progression or resolution of the disease[21].
The major advantages of CEUS are that it is extremely safe with no radiation exposure and can be done in CKD or patients with contrast allergy. It is quick and inexpensive. Major limitations are its operator dependence and poor visualization at times due to bowel gas or ribs.
SWE
SWE is a quantitative elastography technique that evaluates the stiffness of the tissue. The EFSUMB laid down guidelines and recommendations for its use in non-hepatic areas in the year 2013 and has updated it in 2018[8]. In the kidney, its use is limited to assess stiffness in CKD or transplant cases. The EFSUMB recommends its use as an additional tool for the diagnosis of chronic allograft nephropathy and does not recommend its use in native kidneys[8] as they are deep-seated and beyond 40mm depth which is usually the depth of the region of interest (ROI) box at which measurements are done. Only a few studies are available which have tried to differentiate benign from malignant renal masses based on their stiffness[22].
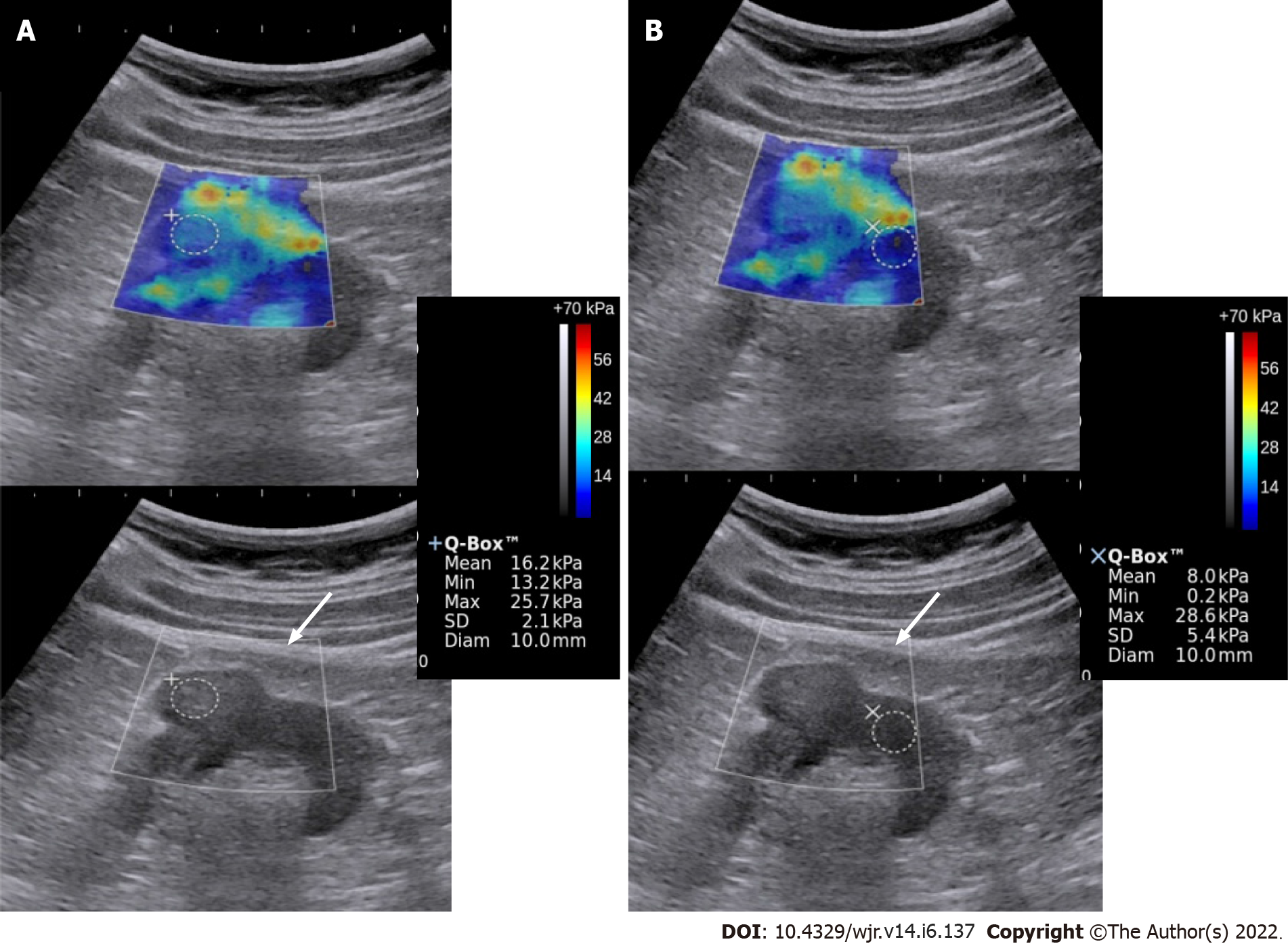
Shear wave propagation speed in tissues varies depending upon the tissue stiffness. Acoustic radiation force impulse transmits longitudinal forces which result in deformation of the tissue and generation of transverse waves called the shear waves. These are then captured by the transducer and their propagation speed is calculated. A color-coded, real-time SWE map is generated which shows local tissue stiffness in kilopascals (quantitative assessment). ROI is selected and values for maximum, minimum, mean stiffness, and standard deviation are produced. Less than ten studies have been published which have tried to differentiate benign from malignant renal mass based on elasticity. Amongst these, several have done strain elastography[23,24] and several shear wave elastography[22,25]. No standardized values have been obtained yet. One of the studies postulates elasticity values in the range of 4.5-4.7 ± 1.5-1.7 kPa of the normal renal cortex[22]. A study by Aydin et al[25] evaluated 40 renal masses and found the highest elasticity value in the malignant and benign groups to be 27.27 ± 25.66 kPa and 16.13 ± 8.89 kPa, respectively. However, more studies with a larger number of patients are required to authenticate these findings and establish a nomogram and cut-off for elasticity values of benign and malignant lesions. Figure 2 depicts a case of RCC having greater stiffness as compared to normal renal parenchyma.
Figure 2 Elastography & gray-scale images of the renal mass (A) and the normal kidney (B).
The mass showed greater stiffness relative to the surrounding kidney. Biopsy showed high-grade renal cell carcinoma.
This is a rapid, non-invasive, radiation-free, repeatable, and inexpensive technique that has no major side effects[22]. The only caution is advised in sensitive areas and fetuses as it can increase local temperature like Doppler ultrasound.
DECT
DECT works on the principle of differences in absorption of photons at different photon energies which also varies with differences in material composition. Dual energy is produced either by two tubes with different peak voltages (dual-source scanner) or using a single tube with alternating peak voltage (single source)[26]. Atoms with large atomic number, like iodine, shows attenuation differences at two different peak voltages. Post-processing software is available which can subtract iodine from all images, resulting in the generation of virtual non-contrast images. Iodine overly maps can be generated which can precisely determine areas of iodine uptake[27]. Hence, many masses which are termed indeterminate on CECT of the abdomen can be classified based on iodine uptake.
Non-contrast scans are not routinely included in abdominal CT protocol[26]. As most of the renal masses are incidentally detected, it becomes very difficult at times to detect post-contrast enhancement, hence many times the masses are labeled as indeterminate and advised for further imaging, either by multiphasic CT or MRI. If DECT is routinely done, virtual non-contrast scans can be generated and any iodine uptake can be confidently identified[28]. This will preclude the need for additional scans, thereby resulting in radiation dose reduction. Also, the cost and time of doing additional investigations can be markedly cut down.
Iodine quantification can also be done with iodine maps generated by DECT, which is given in milligram/milliliter (mg/mL). This is an indirect measure of the vascularity of the region of interest. Several studies have shown values greater than 0.5 mg/mL in tissue are indicative of enhancement[29,30]. Measurement of iodine concentration within a lesion (instead of attenuation value) solves the problem of pseudo enhancement of cysts and differentiating hyperdense cysts from hypovascular tumours[31] (Figure 3). Obtaining iodine levels from a single ROI is another advantage as it avoids the error of keeping ROI in multiple scans which can vary in position due to respiration or motion artifact[32]. Also, when the mass is large and heterogeneous, fallacious attenuation values can come if a hemorrhagic or necrotic component is included within the ROI. Measuring net total iodine concentration will not vary if such areas get included in the ROI. Hence, a large ROI covering the entire mass can be kept[33]. According to a systematic analysis and meta-analysis by Salameh, the pooled sensitivity and specificity of DECT were to be 96.6% and 95.1%, respectively, in renal masses using quantitative iodine concentration[34].
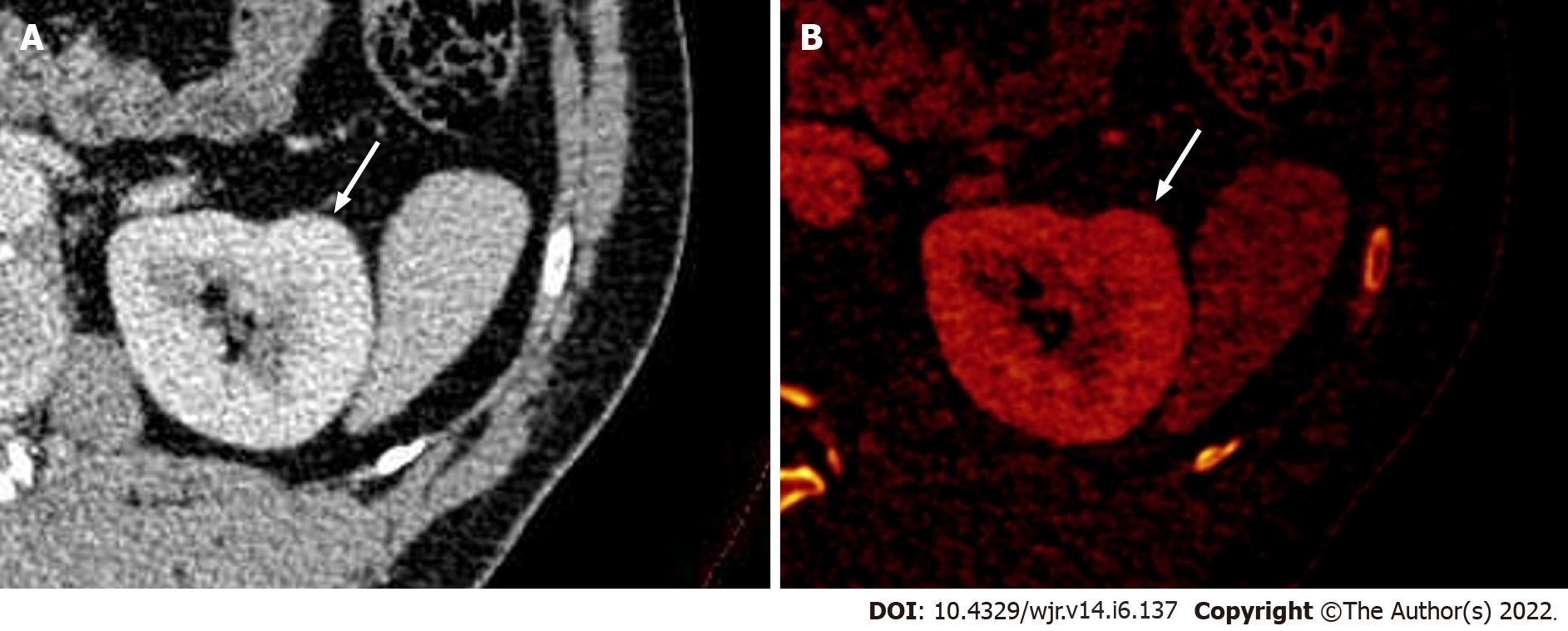
Figure 3 Dual-energy computed tomography images.
A: Contrast-enhanced axial dual-energy computed tomography image showing contour bulge from the lateral cortex of the interpolar region of the left kidney, enhanced similar to background parenchyma; B: Iodine overlay image confirming the absence of any differential iodine distribution, suggesting the lesion to be a dromedary hump.
CT perfusion
CT perfusion imaging detects the temporal changes in tissue attenuation after iodinated contrast is administered intravenously. It can detect changes at the molecular level and assess tissue perfusion and vascular permeability. Both qualitative and quantitative measurements can be done. Blood flow (BF) and blood volume (BV) can be obtained from the initial intravascular phase and vascular permeability (PMB) from the second phase.
Perfusion studies are an indirect predictor of neoangiogenesis. In renal cell carcinoma, which is a highly vascular tumor, multiple factors like vascular endothelial growth factor and tyrosine kinase are recruited, which result in neoangiogenesis. This neoangiogenesis is responsible for the growth of the tumor and metastasis. Targeted chemotherapy stops the proliferation of new vessels and reduces the perfusion parameters; hence, the response to treatment can be assessed. As the size of the mass decreases much later than the reduction in vascular parameters, early response evaluation is possible with perfusion technique[35] (Figures 4 and 5).
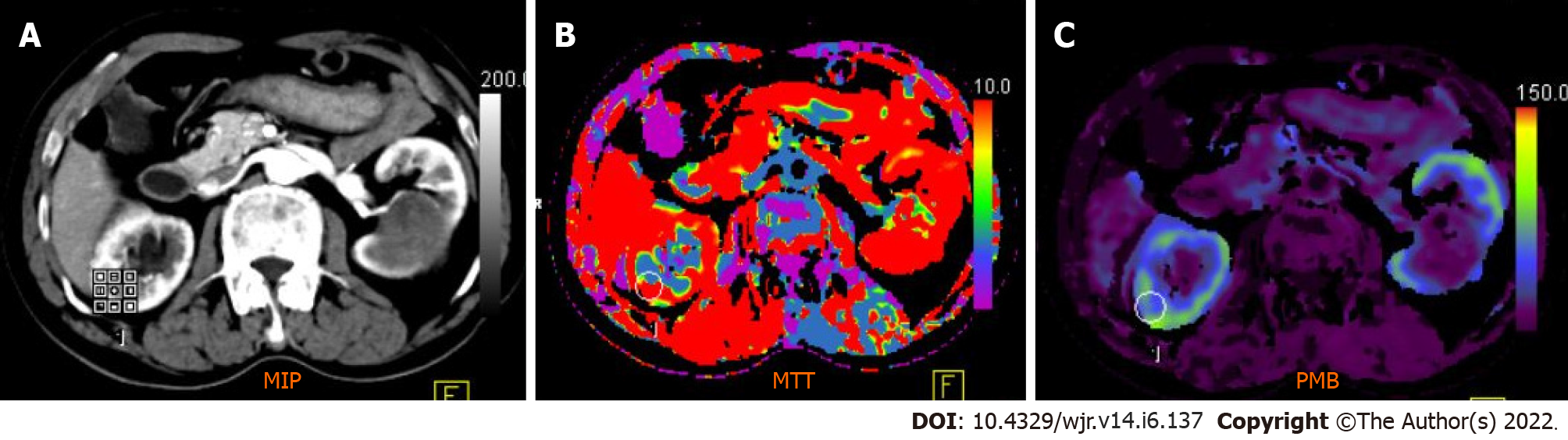
Figure 4 Computed tomography perfusion images (A-C) reveal a significant difference in permeability and mean transit time values between normal renal cortex and malignant lesions.
Mean transit time (MTT) and permeability (PMB) in normal renal cortex were 10.48 s and 55.56 mL/100 mL/min, respectively, which were significantly different from those of renal cell carcinoma (RCC) (MTT: 9.06 s; PMB: 237.09 mL/100 mL/min). A cut-off of 2.5 mL/100 g/min yielded a 100% sensitivity and 95.92% accuracy to predict RCC.
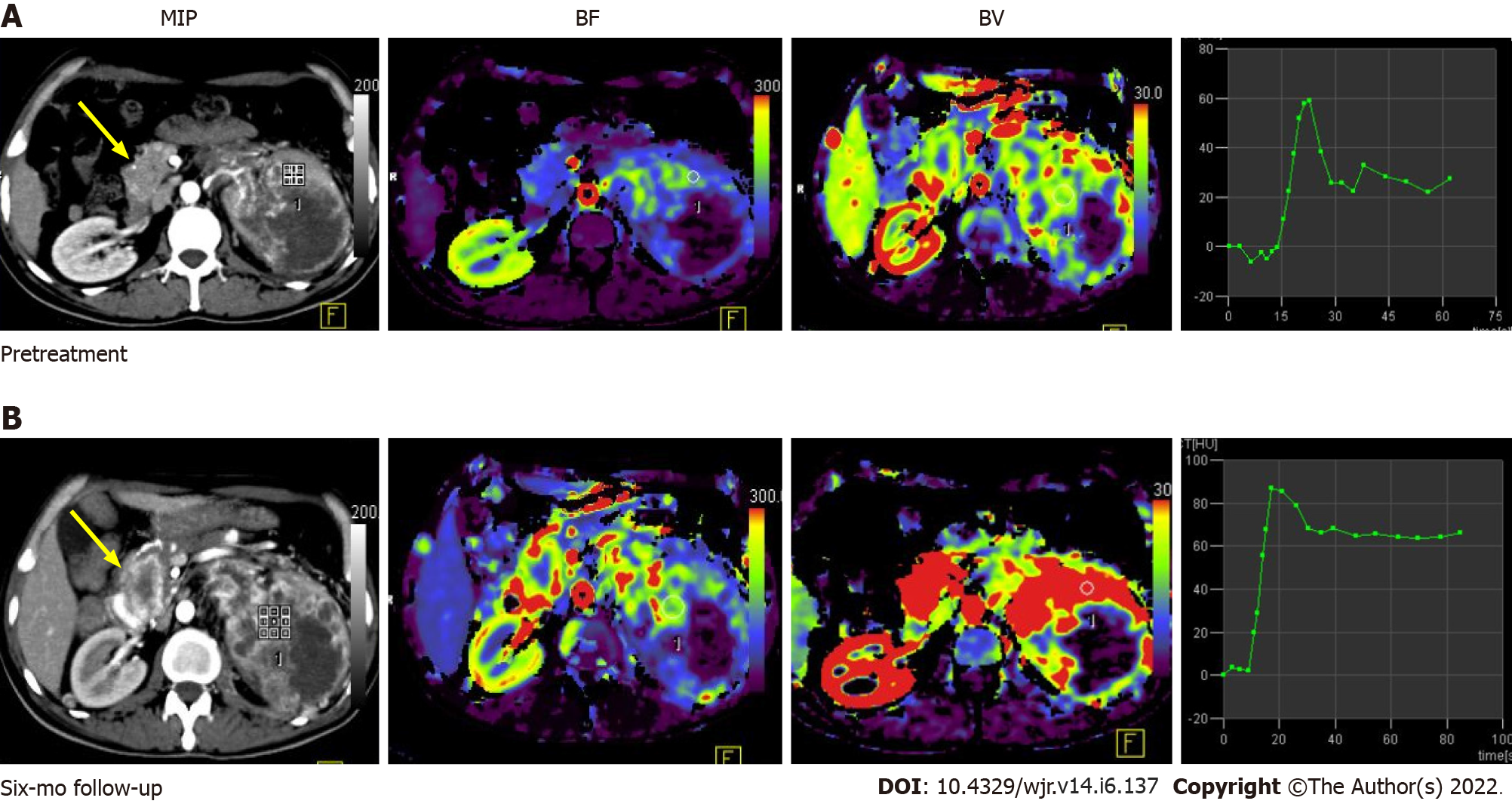
Figure 5 Pre-treatment (A) and post-treatment (B) computed tomography perfusion images in a case of large left renal cell carcinoma with metastasis to the uncinate process of the pancreas (yellow arrow).
Comparison of perfusion parameters at the 6-mo follow-up after anti-angiogenic therapy showed that there was an increase in BF and BV, which was suggestive of progressive disease. No significant change in lesion size would have qualified this case as a stable disease as per size criteria.
MR perfusion
MR perfusion is the technique developed to measure perfusion or vascularity of tissue after injection of a contrast medium. Multiphasic MRI is routinely done to assess the enhancement of the tissue. However, quantitative parameters can be derived only with MR perfusion. In this signal intensity, curves are generated which are placed against time and many post-processing techniques are done to obtain perfusion parameters[36]. Tissue perfusion can also be measured without administering a contrast medium through arterial spin labeling technique[37-40]. In this technique, the red blood cells (RBCs) behave as endogenous contrast agents. They are labeled non-invasively with MR gradient and radiofrequency. Tissue perfusion is calculated by estimating the inflow of the labeled RBCs to the tissue of interest. The major advantage of non-contrast MR perfusion is as endogenous contrast is used, erroneous calculation due to vascular permeability is not problematic here. Second, it can be done in patients where MR contrast medium is contraindicated. But it has a low sensitivity in hypovascular masses.
Tumor grading of RCC is also possible by perfusion studies. Higher grade RCC shows higher perfusion values than low-grade RCC. One of the studies by Palmowski obtained mean perfusion values of high-grade RCC to be 1.59 ± 0.44 (mL/g/min) vs low-grade RCC 1.08 ± 0.38 (mL/g/min)[41]. Another advantage of MR perfusion is in the evaluation of antiangiogenic drug therapy commonly administered for metastatic RCC. Changes in vascularity occur much before the change in the size of the mass. Hence, for early response assessment, several studies[42-44] have demonstrated the potential role of this technique.
Diffusion-weighted imaging
Diffusion-weighted imaging (DWI) is a type of functional MR technique that quantitatively assesses the free Brownian motion of water molecules and thus derives the image contrast based on the restriction of this free motion. This in turn is dependent on the tissue cellularity, organization, cell membrane integrity, and extracellular space tortuosity[45,46]. Abundant work is available in establishing its usefulness in the central nervous system. Many studies have also come forth evaluating its role in the kidneys in the last two decades. DWI has shown promise in differentiating benign vs malignant renal lesions, in differentiating clear vs non-clear cell carcinoma, and in further subtyping the grade of RCCs[47-53].
Benign masses show higher apparent diffusion coefficient (ADC) than malignant lesions. According to a study by Sandrasegaran et al[53], the mean ADC of benign lesions is [mean (Standard deviation) 2.76 (0.32) × 10-3 mm2/s] vs malignant lesions [2.02 × 10-3 mm2/s]. Amongst the malignant masses, clear cell RCCs show significantly higher ADC values than papillary and chromophobe RCCs, which have a better prognosis than clear cell RCCs. Low-grade clear cell RCCs have significantly higher ADC values than high-grade RCC, i.e., an inverse relation is seen, the higher the grade, the lower the ADC[46]. According to a study by Agnello et al[54], mean ADC was significantly different between RCC (1.2 ± 0.01 mm2/s), angiomyolipoma(1.07 ± 0.3 mm2/s), metastases (1.25 ± 0.04 mm2/s), and oncocytomas (1.56 ± 0.08 mm2/s; P < 0.05).
DWI is particularly helpful in patients with contrast allergy or renal impairment when the iodinated control is contraindicated. In such situations, diffusion enhances the confidence of making the distinction between benign and malignant masses on a plain scan.
As the treatment of focal renal lesions could be variable ranging from ablative procedures to complete or partial nephrectomy and radiological follow-up to chemotherapy depending upon the aggressiveness of the lesion, clear demarcation and characterization of renal mass are of utmost importance, which can be optimally done by DWI. Besides, diffusion is also done in renal parenchymal disease and renal infections. It is especially helpful in assessing the response to treatment and in follow-up scans to look for any recurrence (Figure 6). In postoperative scans due to marked post-operative/chemo inflammatory changes, it is hard to detect early recurrence in plain scans.
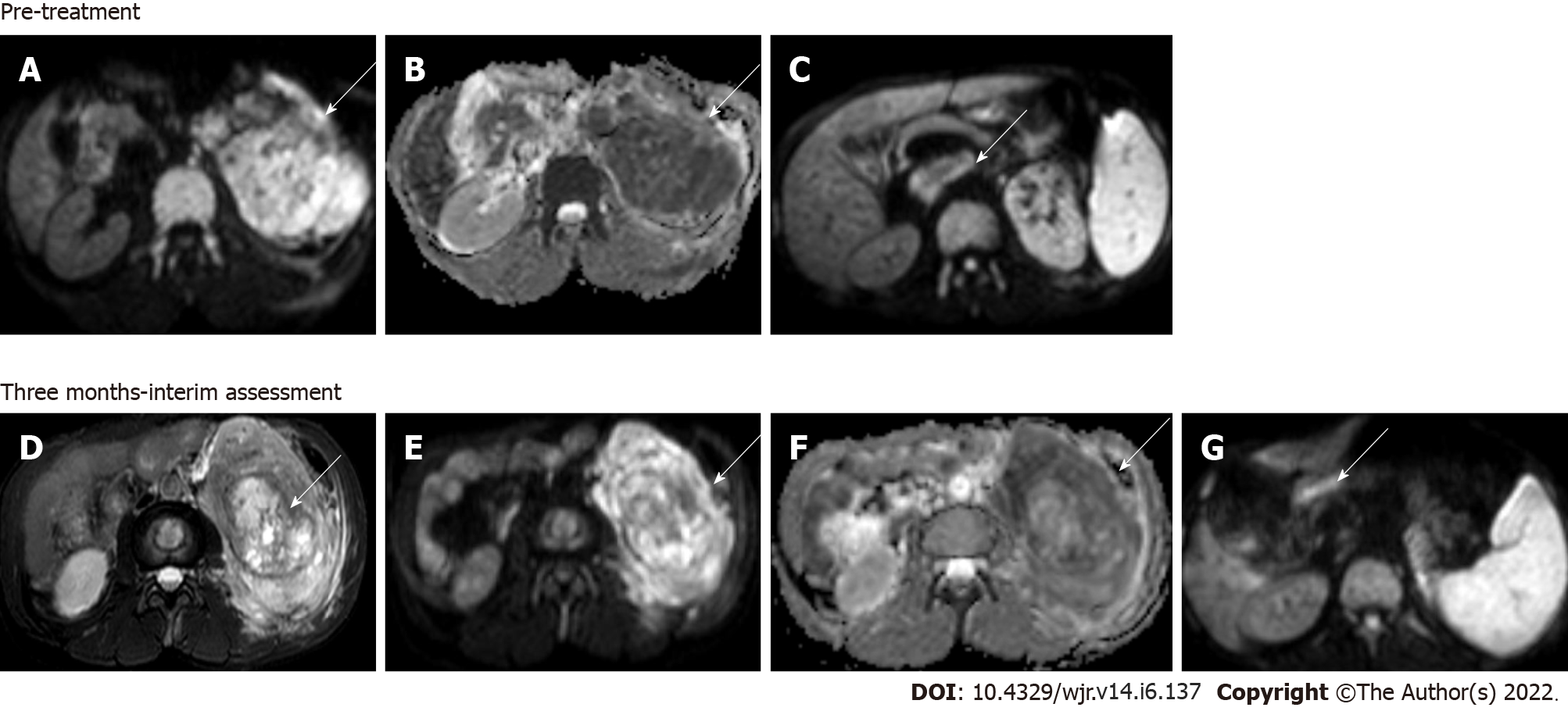
Figure 6 Magnetic resonance images.
Diffusion-weighted imaging (DWI) at b = 800 s/mm2 (A) and apparent diffusion coefficient (ADC) map (B) showed large clear cell renal cell carcinoma replacing the left kidney showing markedly restricted diffusion; DWI image (C) showing a malignant thrombus extending to the inferior vena cava (arrow) Axial T2W FS image (D) showing that 3 mo after treatment with sorafenib (a tyrosine kinase inhibitor), there was no significant change in the size of the lesion; however, there was increased necrosis in the mass; Resultant increase in ADC value on the corresponding DWI at b = 800 s/mm2 (E) and ADC map (F); DWI (G) also revealed partial recanalization of the malignant thrombus (arrow)—overall features suggestive of partial response.
The main advantage of this sequence is that it is a quick sequence without much extending the total span of MRI examination and hence has been routinely incorporated in standard abdominal MRI. No contrast administration is required. It is quite specific and has proved more specific when evaluated along with basic MRI than CT or MRI alone. However, caution needs to be taken with regard to the area from which the values are calculated. If ROI is kept over the hemorrhagic or necrotic component of the mass, the values can be misleading. The scanner needs to be optimized and appropriate b values (400-800) should be used to improve the accuracy of results.
Many researchers are showing interest in evaluating the role of intravoxel incoherent motion (IVIM) and diffusion kurtosis in differentiating benign from malignant renal masses and also in the grading of renal cell carcinoma. IVIM is a biexponential model which includes both true and pseudo diffusion, which is predominantly driven by perfusion[55]. Parameters like diffusivity (D), pseudo diffusion coefficient (D*), and perfusion index (F) can be calculated. Jenson et al[56] in the year 2005 gave the concept of diffusion kurtosis which follows non-Gaussian diffusion, and parameters like mean diffusivity, mean kurtosis, kurtosis anisotropy, and radial kurtosis can be measured. A significant difference in Diffusion kurtosis parameters is seen in different subtypes and grades of RCC. A study by Ding et al[57] obtained superior results with IVIM in differentiating benign from malignant renal masses than diffusion or kurtosis parameters.
BOLD
BOLD is a quick MRI sequence that non-invasively evaluates deoxyhemoglobin levels in the kidney. In most human organs, oxygen tension is relatively constant and varies with the regional blood flow. The kidney is one of the organs where the oxygen tension varies both with the blood flow and the need for filtration. As tubular filtration is an active process requiring energy, this results in a variation of oxygen tension. The cortex of the kidney is well perfused and high in oxygen whereas the medulla is relatively less perfused with and low in oxygen tension. The medulla also has a counter-current arrangement of vessels which further contributes to lower oxygen tension in this region. This results in higher production of deoxyhemoglobin. Deoxyhaemoglobin has a paramagnetic effect and results in the rapid dephasing of protons. A higher amount of deoxyhemoglobin leads to a decrease in T2* relaxation time[58].
BOLD MRI has wide applications in the brain[59-62]. Many studies are being done on the kidneys, justifying their utility in renal pathologies. The echo-planar imaging (EPI) sequence or more sequences are used. The multiple gradient-recalled echo sequence is better than the EPI sequence in terms of SNI, spatial distortion, and spatial resolution. It calculates 1/R2* and generates various maps. After the excitation pulse, multiple gradient echoes are acquired. The higher the strength of the magnetic field, the better the results obtained. BOLD MRI proves useful in the detection of ischemia in the kidneys as in renal artery stenosis, renal artery occlusion, etc. as proven by a few studies[63-65]. Early initiation of treatment results in a better outcome. It is also useful in diabetics, hypertension, allograft rejection, etc. As various renal lesions would result in a change in perfusion of the kidney, this can be a reliable tool to differentiate benign from malignant renal lesion (Figures 7 and 8).
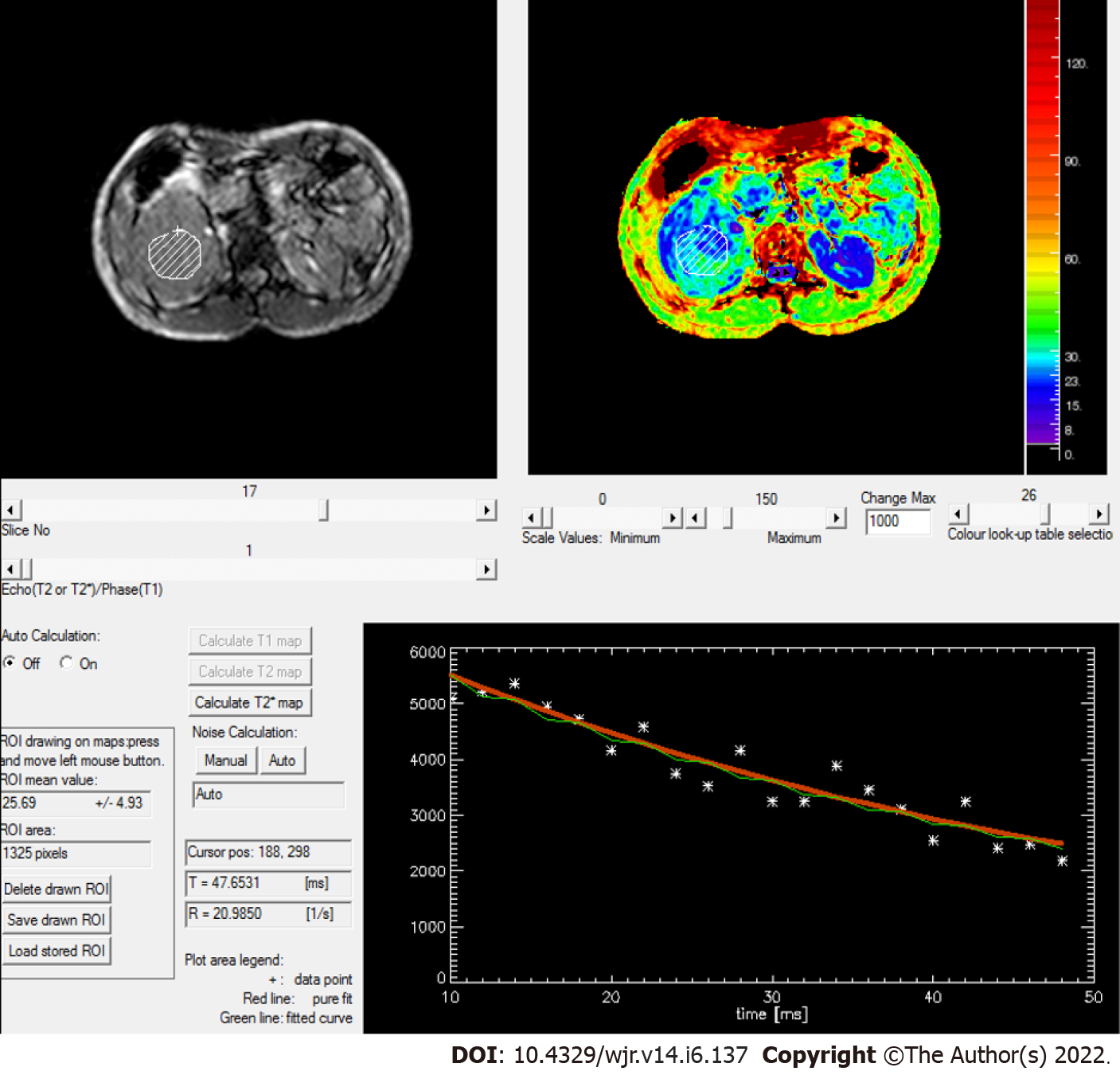
Figure 7 Rate of spin dephasing (R2* map) showing R2* value of right renal cell carcinoma (20.9/s), which was lower than that of a normal kidney (25.5/s).
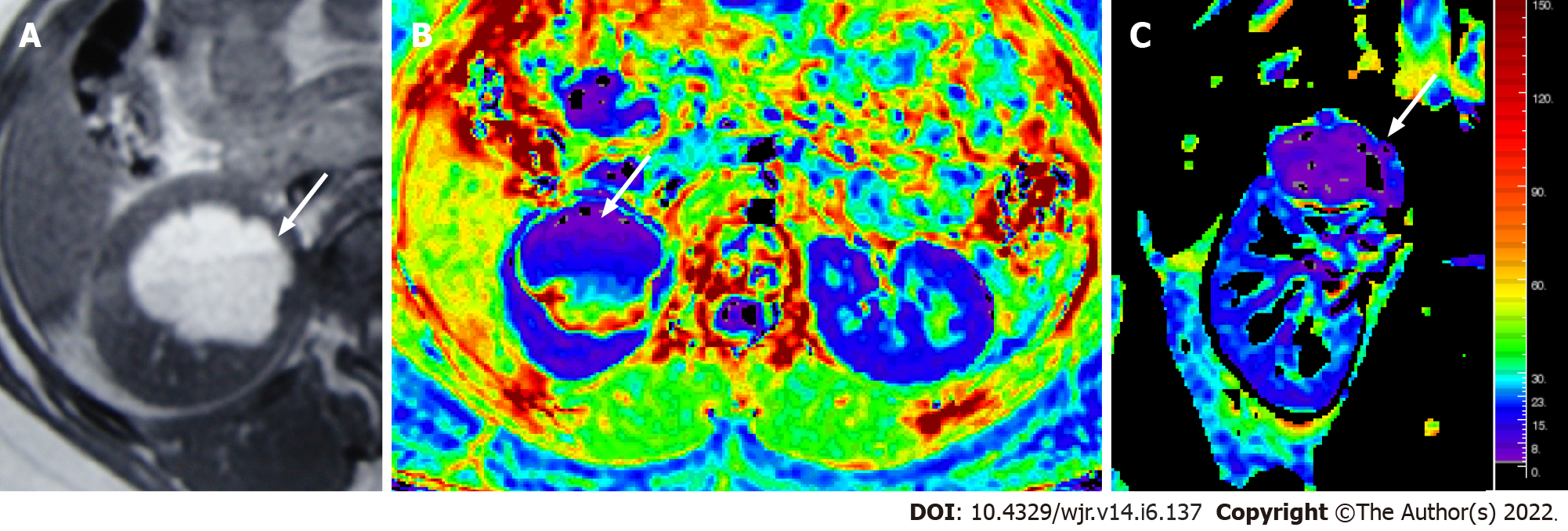
Figure 8 Magnetic resonance images.
T1W axial MR image (A) showing a hyperintense lesion in the upper pole of the right kidney, with fluid-fluid level, suggestive of a hemorrhagic cyst; Axial & coronal R2* maps (B and C) showing an R2* value of 6.1/s.
Proton spectroscopy
Proton spectroscopy (H1MRS) is an advanced MR technique that studies the variation in chemical metabolites in determining various pathologies. It is a powerful, non-invasive tool to quantitatively study the chemical compositions and metabolic processes in vivo.
Whenever there is an application of magnetic field, there is a generation of small magnetic fields by the circulating protons which interact with the main magnetic field and bring about a change in Larmor frequency. This change in frequency due to differing chemical composition is called “chemical shift[66]”. Proctor and Yu, for the first time, proposed the concept of chemical shift in 1951, and the first in vivo proton MR spectrum was published in 1985[67].
Proton MR spectroscopy has well-known applications in the central nervous system, breast, and prostate. Its role in renal masses is the current area of interest. Promising results of a few in vitro and in vivo studies are available in the differentiation of different renal masses[68].
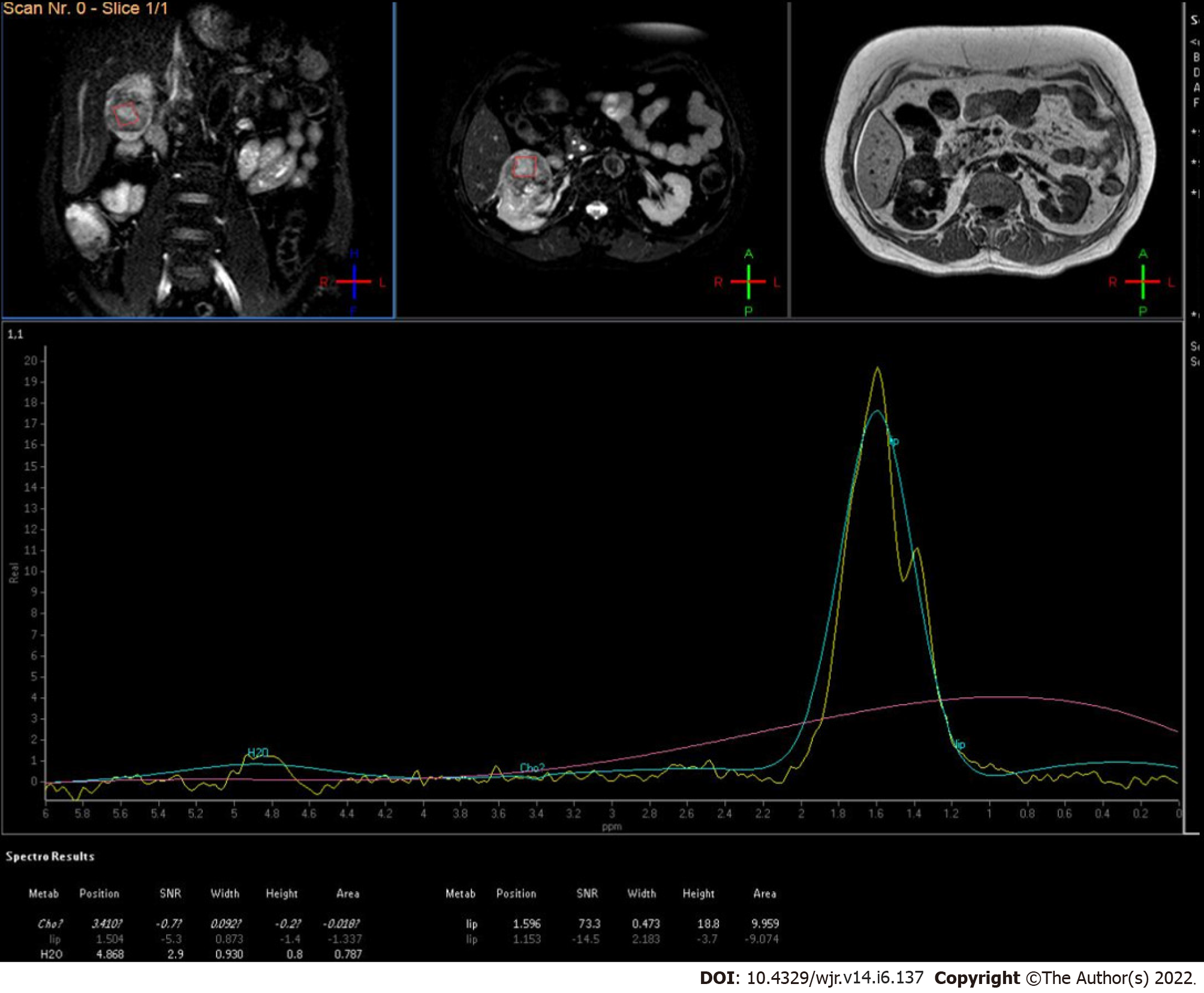
The first in vivo study was conducted by Kim et al[69] who recruited five patients with biopsy-proven cases of RCC and found the difference of metabolite lipid and choline as per the grade of the tumour(Figure 9).
Figure 9
MR spectroscopy of low-grade renal cell carcinoma showed increased lipid-lactate peak.
MRS is limited by the complexity of its acquisition, processing, and data interpretation[70]. It suffers from a low sensitivity and poor spatial resolution. It can only act as a complementary tool that supplements the results of basic MRI examination.
PET scan: Fluorodeoxyglucose and PSMA PET
Fluorodeoxyglucose (FDG) PET scan plays a limited role in primary RCC due to low sensitivity[71] but can be useful in case of aggressive or advanced RCC[72], recurrent disease[73], and metastatic RCC[74], and for post-treatment evaluation[75]. As FDG is excreted by the kidneys, FDG PET is not suitable for local staging of primary RCC. However, it has a crucial role in overall staging of the disease, differentiating malignant vs bland thrombosis in the renal vein/inferior vena cava, detecting metastasis to distant sites, detecting recurrent/residual disease in postoperative/chemotherapy evaluation, monitoring response to therapy, and restaging of the disease. Quantitative measurement of maximum standardized uptake values(SUVs) can be done for objective assessment. The higher the SUV, the more dismal the prognosis. A cut-off of 3 is seen to be optimal to differentiate low grade vs high-grade RCC[75].
Although FDG is the most common tracer to be used for PET, other new tracers like 68Ga-PSMA, 18F-fluoroethylcholine, 11C-acetate, 18F-fluoromisonidazole, and 18F-fluorothymidine are being investigated[76]. Prostate-specific membrane antigen (PSMA) is a molecule that is expressed in prostatic cells and has established application in the prostatic tumor. Further research showed its expression on neovasculature of renal cancer cells and hence its potential role in RCC has been explored by researchers with encouraging results[77]. Several studies have shown a change in the management of RCC when gallium PSMA PET was used for primary staging compared with CECT scan due to detection of small areas of metastasis and synchronous malignancies[78]. Gallium PSMA PET is proven to be better than FDG PET for oligometastatic RCC[79].