Published online Sep 28, 2020. doi: 10.4329/wjr.v12.i9.204
Peer-review started: April 19, 2020
First decision: June 5, 2020
Revised: June 7, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: September 28, 2020
Processing time: 162 Days and 1.5 Hours
Congenital heart disease (CHD) is a cardiovascular malformation caused by abnormal heart and/or vascular development in the fetus. In children with CHD, abnormalities in the development and function of the nervous system are common. At present, there is a lack of research on the preoperative neurological development and injury in young children with non-cyanotic CHD.
To determine the changes in white matter, gray matter, and cerebrospinal fluid (CSF) by magnetic resonance imaging (MRI) in children with non-cyanotic CHD as compared with healthy controls.
Children diagnosed with non-cyanotic CHD on ultrasonography (n = 54) and healthy control subjects (n = 35) were included in the study. All the subjects were aged 1–3 years. Brain MRI was performed prior to surgery for CHD. The SPM v12 software was used to calculate the volumes of the gray matter, white matter, CSF, and the whole brain (sum of the gray matter, white matter, and CSF volumes). Volume differences between the two groups were analyzed. Voxel-based morphometry was used to compare specific brain regions with statistically significant atrophy.
Compared with the control group, the study group had significantly reduced whole-brain white matter volume (P < 0.05), but similar whole-brain gray matter, CSF, and whole-brain volumes (P > 0.05). As compared with the healthy controls, children with non-cyanotic CHD had mild underdevelopment in the white matter of the anterior central gyrus, the posterior central gyrus, and the pulvinar.
Children with non-cyanotic CHD show decreased white matter volume before surgery, and this volume reduction is mainly concentrated in the somatosensory and somatic motor nerve regions.
Core Tip: As research on the preoperative development and injury of the nervous system in patients with non-cyanotic congenital heart disease (CHD) is currently lacking, the present study aimed to determine the changes in whole-brain white matter, whole-brain gray matter, and the cerebrospinal fluid in children with CHD relative to healthy controls, by using a voxel-based morphometry technique. We hope that the results will help facilitate early clinical intervention in these children, and reduce or prevent the occurrence and progression of nervous system damage.
- Citation: Jia X, Ma XH, Liang JW. Application of voxel-based morphometric method to detect brain changes in children with non-cyanotic congenital heart disease. World J Radiol 2020; 12(9): 204-212
- URL: https://www.wjgnet.com/1949-8470/full/v12/i9/204.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i9.204
Congenital heart disease (CHD) is a cardiovascular malformation caused by abnormal cardiac development during the fetal period. CHD is the most common type of congenital malformation[1], accounting for 28% of all congenital deformities. Globally, the incidence rate of CHD is over 100000/year[2,3]. Non-cyanotic CHDs are a group of congenital diseases with the clinical manifestations of a left-to-right shunt, including ventricular septal defect (VSD), atrial septal defect (ASD), and patent ductus arteriosus (PDA). Children with CHD frequently show delayed neurological development and neurological dysfunction[4,5].
The fastest period of neurological development, especially gray matter formation, myelination, and cerebrospinal fluid (CSF) secretion, occurs during infancy and early childhood[6]. By the age of 2 to 3 years, brain development is nearly complete, the emergence of self-awareness and the breakthrough of language are particularly significant milestones that occur during this period, and the connections between various parts of the brain are strengthened at this time[7]. Thus far, research on the neurological dysplasia associated with CHD has focused on the impacts of extracorporeal circulation technology during cardiac surgery and the intraoperative cardiac arrest time on the nervous system as well as the efficacy of postoperative neuroprotective measures. However, it was recently found that brain damage may even occur prior to cardiac surgery in children with CHD[8]. Moreover, various brain injuries before and after surgery in children with CHD are associated with potentially reversible clinical risk factors[9], whose timely management may potentially protect the developing central nervous system of the child.
As research on the preoperative development and injury of the nervous system in patients with non-cyanotic CHD is currently lacking, the present study aimed to determine the changes in whole-brain white matter, whole-brain gray matter, and the CSF in children with CHD relative to healthy controls, by using a voxel-based morphometry (VBM) technique. We hope that the results will help facilitate early clinical intervention in these children, and reduce or prevent the occurrence and progression of nervous system damage.
A total of 54 children who were diagnosed with non-cyanotic CHD and underwent concurrent interventional surgery at our hospital between May 2019 and September 2019 were selected as a study group. In addition, 35 age- and sex-matched healthy children were selected as a control group. The inclusion criteria for the study group were as follows: (1) Diagnosis of non-cyanotic CHD on ultrasonography, (2) age between 1.1 years and 3.0 years, (3) Interventional surgery, and (4) Limb partial pressure of oxygen 98% or above. At the same time, cyanotic CHD, open chest surgery, and sedation failure were excluded.
Magnetic resonance imaging (MRI) acquisitions were performed using a 3.0-T scanner (Achieva 3.0T Rex, Philips, Netherlands) with an 8-channel head coil. Because the children could not cooperate with the examination, they were administered a 10% chloral hydrate enema at a dose of 0.5 mL/kg body weight 30–40 min before the examination. The examination was carried out only after confirming that the child was asleep. Each child was given a special ear-protective device that included earplugs and earmuffs. In addition, a self-contained electrocardiographic monitoring device was used to monitor the child in real time throughout the MRI examination. A routine head scan was first obtained using a fast spin echo sequence to rule out organic brain disease. The scanning parameters were as follows: T2-weighted imaging, repetition time (TR)/echo time (TE) = 1858 ms/80 ms; T1-weighted imaging, TR/TE = 190 ms/2.3 ms; and T2-weighted imaging/fluid-attenuated inversion recovery, TR/TE = 6000 ms/120 ms. Next, the fast gradient echo sequence was used to perform three-dimensional T1-weighted imaging of the whole brain. The scanning parameters were as follows: Layer, 180; TR, 7.9 ms; TE, 3.9 ms; interval time, 0; layer thickness, 2 mm; field of view, 256 mm × 256 mm; matrix, 256 × 227; number of excitations, 1; and total scanning time, 2 min 58 s.
The original data were transferred to a personal computer and analyzed using the VBM toolbox of the Statistical Parameter Map software (SPM, version 12, http://www.fil.ion.ucl.ac.uk/spm). The operating environment was MATLAB (version R2018a, MathWorks Inc, Natick, MA, USA). We used a VBM method based on pixel analysis. First, three-dimensional T1-weighted images were segmented into natural-space gray matter, white matter, and CSF using a standard segmentation model, and the volume of each of these three segments as well as the total intracranial volume (TIV) was calculated. Second, we used the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) module to generate accurate templates of the segmented gray and white matter from the entire image dataset. Finally, an 8-mm half-Gauss full-width isotropic Gaussian kernel was used in the Montreal Institute of Neurology (MNI) space to spatially normalize the brain structure images of all individuals studied into an identical stereoscopic space.
Statistical comparisons were performed between the healthy control group and the CHD group by using a two-samples t-test and the general linear model of the SPM v12 software. The results were evaluated using a random-effect analysis to obtain accurate brain regions with statistical differences. The statistical threshold was P < 0.05. According to the familywise error rate correction (FEW), the region of the differential brain region containing more than 20 consecutive voxels was considered a meaningful brain region. The difference in white matter density between the two groups was calculated separately. Demographic analysis was performed using parametric (t-test) and non-parametric (χ2 test) tests, depending on the data characteristics. Differences with P < 0.05 were considered statistically significant. The parents/legal guardians of all children provided written informed consent before their participation in this study, and the study protocol was approved by the ethics committee of the Children's Hospital of Zhejiang University School of Medicine (No. 2019-IRB-047).
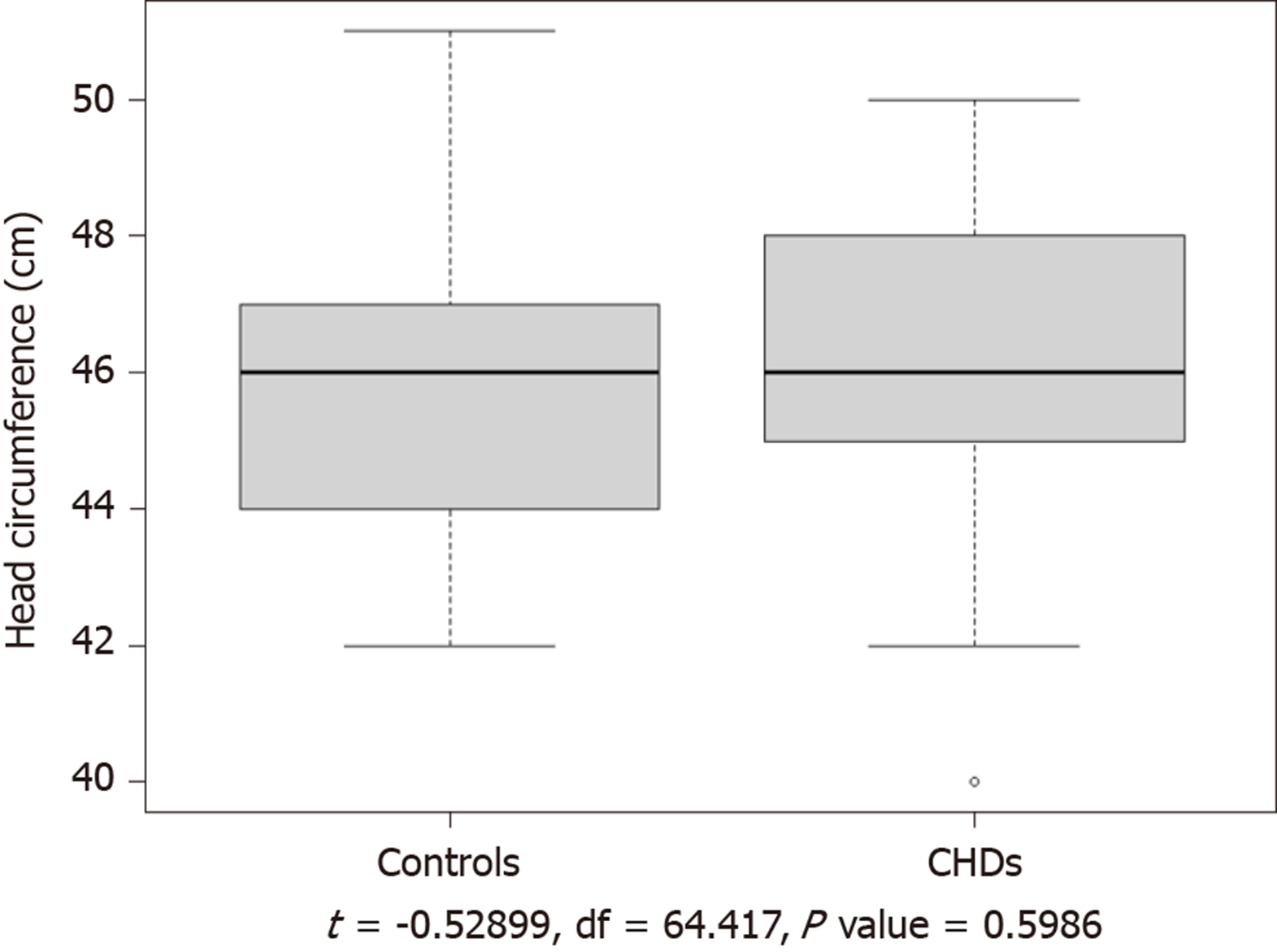
The study group consisted of 26 boys and 28 girls, aged 1.1 to 3.0 years (median age, 1.9 years). Of the 54 children in the study group, 43 were full-term and 11 were premature; 15 had ASD, 36 had PDA, 1 had VSD, and 2 had ASD as well as PDA. Except that 1 had malnutrition and 4 lagged behind in the same period of growth, the other 49 children have no obvious abnormalities. There were no significant differences in age, sex, or head circumference between the study and control groups (P > 0.05; Table 1 and Figure 1). The CHD-related data of the patients in the study group are shown in Table 2.
| CON | CHD | P value | |
| Gender, female/male | 19/16 | 28/26 | > 0.05 |
| Age, range (median) | 1-3Y (1.8Y) | 1-3Y (1.9Y) | > 0.05 |
| Type of CHD | PDA | ASD | VSD | ASD+PDA | Total |
| Number of cases | 36 | 15 | 1 | 2 | 54 |
The volumes of the gray matter, white matter, and CSF were determined using image segmentation. The TIV was calculated as follows: TIV = gray-matter volume + white-matter volume + CSF volume. All volume measurements were performed in triplicate, and average values were calculated (Table 3). The white matter volume was significantly lower (P < 0.05) in children with non-cyanotic CHD than in the control children. Thus, mild underdevelopment of the white matter was found in the study group. In contrast, the gray matter and CSF volumes were normal in the study group and did not significantly differ from those in the control group.
| GM (mean ± SD) | WM (mean ± SD) | CSF (mean ± SD) | TIV (mean ± SD) | WM% | |
| CON | 586.05 ± 17.08 | 275.06 ± 16.10 | 138.88 ± 21.87 | 1003.58 ± 100.56 | 27.41 |
| CHD | 566.88 ± 113.25 | 257.67 ± 50.23 | 175.45 ± 158.58 | 987.44 ± 124.16 | 26.09 |
| P value | > 0.05 | < 0.05 | > 0.05 | > 0.05 | < 0.05 |
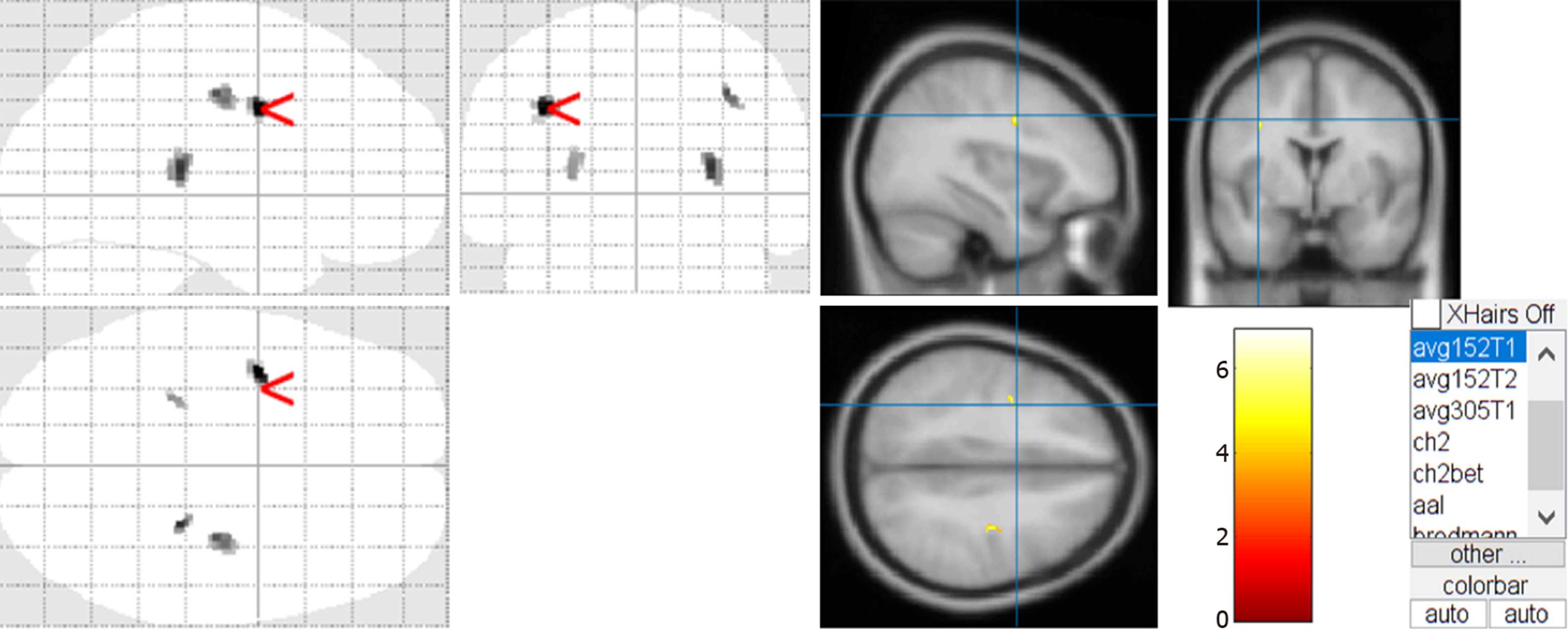
Two children with CHD had obvious encephalomalacia, which led to significant abnormalities in the two-samples t-test. After eliminating the data of these two children, we extracted the t-test results by using the xjView toolkit (http://www.alivelearn.net/xjview/download/). Figure 2 shows the white matter changes in children with non-cyanotic CHD. The maximum density transparent map and the cluster of the active region obtained by the two-samples t-test were superimposed on the avg152T1 template to generate a pseudo color map.
According to the xjView report, combined with the automatic anatomical labeling (AAL)[10], only regions with a cluster size > 20 were selected to evaluate white matter atrophy in non-cyanotic CHD, including the anterior central gyrus, the posterior central gyrus, and the pulvinar (Table 4). Regions with a cluster size < 20 were considered to be artefacts.
| Brain region | MNI coordinates | Cluster size | Peak intensity | ||
| Pulvinar | 27 | -31.5 | 9 | 33 | 6.0857 |
| Sub-lobar | 33 | ||||
| Right cerebrum | 33 | ||||
| White matter | 32 | ||||
| Extra-nuclear | 32 | ||||
| Precentral gyrus | -37.5 | -1.5 | 33 | 52 | 6.9196 |
| Left cerebrum | 52 | ||||
| Frontal lobe | 52 | ||||
| White matter | 49 | ||||
| Precentral gyrus | 31 | ||||
| Precentral_L (AAL) | 28 | ||||
| Postcentral gyrus | 33 | -18 | 40.5 | 34 | 5.6201 |
| Right cerebrum | 34 | ||||
| Frontal lobe | 34 | ||||
| White matter | 33 | ||||
| Sub-gyral | 32 | ||||
Among the 54 patients with non-cyanotic CHD in our study, except that 1 patient had malnutrition, 2 had poor motor development, and 2 had poor language development on routine physical examination, no significant growth abnormalities were found in the remaining 49 children. At the same time, there was no difference in head circumference statistics between the two groups. However, we found that children with non-cyanotic CHD showed mild white matter atrophy prior to undergoing surgery for CHD. Using the VBM DARTEL technique, we found a significant decrease in whole-brain white matter volume in the study group as compared to the control group, but the whole-brain gray matter and CSF volumes did not differ between the two groups. The most obvious atrophic changes in the white matter were observed in the anterior central gyrus, followed by the posterior central gyrus and the pulvinar. This suggested that although there was no obvious abnormality in these children, the white matter damage may already exist, and it will gradually appear in the process of growth and development in the future. Thus, early intervention is necessary.
As early as 2002, Mahle et al[11] first reported the fact that brain damage occurs in children with CHD. The study subjects included 24 children with CHD, all of whom had various complex CHDs. Miller et al[12] also found that neonates with CHD are at increased risk of newly acquired brain injury including focal white matter injury (WMI) and small focal strokes. The mechanism of WMI is thought to be secondary to hypoxic-ischemic and inflammatory injury to susceptible immature premyelinating oligodendrocytes. Moreover, Peyvandi et al[13] have proved that all brain areas were affected with complex CHD prior to any corrective operation as compared to controls, including cortical and deep grey matter, white matter, and cerebellar volumes. Severe hypoxic-ischemic episodes triggered by CHD can initiate a “brain-protective effect” via a series of stress responses such as redistribution of the cardiac output, heart rate regulation, and elevated aortic pressure[14-16]. Despite these responses, the probability of developing neurodevelopmental disorders is as high as 50% in these patients[17]. Moreover, because the initial symptoms are mild in children with non-cyanotic CHD, the changes in the nervous system are often gradually manifested only after the child reaches school age, and the optimal window period for intervention is missed. Despite the lack of early severe symptoms, abnormal brain imaging findings are common in children with CHD during the prenatal and postpartum preoperative periods. In the prenatal period, brain developmental delay is the most common manifestation. After birth, the condition often manifests as white matter damage or periventricular leukomalacia[18]. In our study, we did not find similar gray and white matter damage in children with non-cyanotic CHD because the oxygen saturation was within the normal range from 98% to 100%. There was no evidence of hypoxia, but the white matter of the brain is still partially under development. McDonald-McGinn et al[19] revealed that up to half of patients with Down syndrome and 75% of patients with 22q.11 deletions have CHD, most commonly in the form of atrioventricular septal defects and conotruncal abnormalities, respectively. It was confirmed that the brain damage caused by CHD is determined from the beginning of gene. Simultaneously, different types of CHD can cause changes in cerebrovascular resistance (CVR) between the brain and placenta and fetal cerebrovascular blood flow[20,21]. Early investigations of fetuses with single ventricle (SV) defects have suggested that these flow alterations may have an impact on early neurodevelopmental outcomes[22]. These studies illustrated that children with non-cyanotic CHD have partial white matter damage even without evidence of significant hypoxia.
Ortinau et al[23] confirmed that infants with cyanotic CHD have reduced white matter in the parietal lobe, cerebellum, and brainstem. Our study found a slight decrease in the white matter volume, but this was mainly concentrated in the anterior central gyrus, the posterior central gyrus, and the pulvinar, which are inconsistent with the above report. Cordina et al[24] confirmed that the area of white matter damage in adults with cyanotic CHD involves the anterior central gyrus and temporal gyrus, which are partially consistent with the areas of white matter damage in our study. Therefore, we believe that although there was no hypoxia or significant impact on the whole brain gray matter and cerebrospinal fluid volume in the children with non- cyanotic CHD in the study group, some white matter regions still had atrophy, which indicated that even with normal blood oxygen saturation, the anterior central gyrus, posterior central gyrus, and the pulvinar would still be damaged.
There are some limitations to this study. The sample size of VSD patients in the study group was too small. It was therefore impossible to analyze the brain MRI data according to the type of non-cyanotic CHD and determine whether the area of white matter damage is consistent between different CHDs. Furthermore, a controlled follow-up study is required to analyze the changes in the area of white matter damage after the improvement of hypoxia following interventional surgery for CHD.
In summary, this study has demonstrated that although there is no significant hypoxia, children with non-cyanotic CHD could have a decrease in white matter volume before surgery, and this reduction in white matter volume is mainly concentrated in the somatosensory and somatic motor nerve areas.
In children with congenital heart disease (CHD), abnormalities in the development and function of the nervous system are common. At present, there is a lack of research on the preoperative neurological development and injury in young children with non-cyanotic CHD.
Most children with non-cyanotic congenital heart disease have a lack of assessment of preoperative brain development.
The objective of the current study was to determine the changes in white matter, gray matter, and cerebrospinal fluid (CSF) by magnetic resonance imaging (MRI) in children with non-cyanotic CHD as compared with healthy controls.
Children diagnosed with non-cyanotic CHD and healthy control subjects aged 1–3 years were included in the study. Brain MRI was performed prior to surgery for CHD. The SPM v12 software was used to calculate the volumes of the gray matter, white matter, CSF, and the whole brain. Volume differences between the two groups were analyzed. Voxel-based morphometry was used to compare specific brain regions with statistically significant atrophy.
The study group had significantly reduced whole-brain white matter volume. Children with non-cyanotic CHD had mild underdevelopment in the white matter of the anterior central gyrus, the posterior central gyrus, and the pulvinar.
Children with non-cyanotic CHD show decreased white matter volume before surgery, and this volume reduction is mainly concentrated in the somatosensory and somatic motor nerve regions.
A controlled follow-up study is required to analyze the changes in the area of white matter damage after the improvement of hypoxia following interventional surgery for CHD.
The authors would like to thank Yi Zhang, MD for his help.
| 1. | Jing W, Xiaohong Z. The value of color Doppler ultrasound in the diagnosis of congenital heart disease in children. Zhongguo Shequ Yishi. 2012;14:252-253. [DOI] [Full Text] |
| 2. | Liu JF. New technology and new progress in the treatment of congenital heart disease in children. Linchuang Erke Zazhi. 2005;23:839-840. [DOI] [Full Text] |
| 3. | Chen X. The cause of congenital heart disease in children and its countermeasures. Yixue Xinxi. 2013;26:111-112. [DOI] [Full Text] |
| 4. | Massaro AN, El-Dib M, Glass P, Aly H. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev. 2008;30:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Tabbutt S, Nord AS, Jarvik GP, Bernbaum J, Wernovsky G, Gerdes M, Zackai E, Clancy RR, Nicolson SC, Spray TL, Gaynor JW. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics. 2008;121:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Aubert-Broche B, Fonov V, Leppert I, Pike GB, Collins DL. Human brain myelination from birth to 4.5 years. Med Image Comput Comput Assist Interv. 2008;11:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Bloom L. Language development and emotional expression. Pediatrics. 1998;102:1272-1277. [PubMed] |
| 8. | Khalil A, Suff N, Thilaganathan B, Hurrell A, Cooper D, Carvalho JS. Brain abnormalities and neurodevelopmental delay in congenital heart disease: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2014;43:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Dimitropoulos A, McQuillen PS, Sethi V, Moosa A, Chau V, Xu D, Brant R, Azakie A, Campbell A, Barkovich AJ, Poskitt KJ, Miller SP. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11627] [Cited by in RCA: 12879] [Article Influence: 536.6] [Reference Citation Analysis (0)] |
| 11. | Mahle WT, McBride MG, Paridon SM. Exercise performance in tetralogy of Fallot: the impact of primary complete repair in infancy. Pediatr Cardiol. 2002;23:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 361] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 13. | Peyvandi S, Latal B, Miller SP, McQuillen PS. The neonatal brain in critical congenital heart disease: Insights and future directions. Neuroimage. 2019;185:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 515] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Arbeille P, Maulik D, Fignon A, Stale H, Berson M, Bodard S, Locatelli A. Assessment of the fetal PO2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med Biol. 1995;21:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 95] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Yan B. Prenatal ultrasound measurement of fetal lateral ventricle to evaluate fetal brain malformation. Yatai Chuantong Yiyao. 2011;7:117-118. [DOI] [Full Text] |
| 17. | Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Li J, Smith SE, Bellinger DC, Mahle WT; American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 1185] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 18. | Mebius MJ, Kooi EMW, Bilardo CM, Bos AF. Brain Injury and Neurodevelopmental Outcome in Congenital Heart Disease: A Systematic Review. Pediatrics. 2017;140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, Scambler PJ, Bassett AS. 22q11.2 deletion syndrome. Nat Rev Dis Primers 1. 2015;1-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 805] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 20. | Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Williams IA, Fifer C, Jaeggi E, Levine JC, Michelfelder EC, Szwast AL. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle congenital heart disease. Am Heart J. 2013;165:544-550.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Ortinau C, Beca J, Lambeth J, Ferdman B, Alexopoulos D, Shimony JS, Wallendorf M, Neil J, Inder T. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J Thorac Cardiovasc Surg. 2012;143:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Cordina R, Grieve S, Barnett M, Lagopoulos J, Malitz N, Celermajer DS. Brain volumetric, regional cortical thickness and radiographic findings in adults with cyanotic congenital heart disease. Neuroimage Clin. 2014;4:319-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pan SL S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH